Abstract
Species of the widespread marine prokaryote Prochlorococcus exhibited ultradian growth (faster than 1 division per day) both in situ and in culture, even though cell division is strictly phased to the light-dark cycle. Under optimal conditions a second DNA replication and cell division closely followed, but did not overlap with, the first division. The timing of cell cycle events was not affected by light intensity or duration, suggesting control by a light-triggered timer or circadian clock rather than by completion of a light-dependent assimilation phase. This mode of ultradian growth has not been observed previously and poses new questions about the regulation of cellular rhythms in prokaryotes. In addition, it implies that conclusions regarding the lack of nutrient limitation of Prochlorococcus in the open ocean, which were based on the appearance that cells were growing at their maximal rate, need to be reconsidered.
Species of Prochlorococcus, the smallest known photosynthetic organism, account for a significant fraction of the autotrophic biomass and primary production in mid- and low-latitude oceans (3, 7, 17, 18, 23, 27). The cell cycle of Prochlorococcus is tightly phased to the light-dark cycle, with DNA replication during the afternoon, followed by binary fission after dusk (18, 24, 27). This division pattern, coupled with the unique light scattering and fluorescence signature of these cells, allows oceanographers to estimate the growth rate of Prochlorococcus, by using flow cytometry to monitor DNA frequency distributions in situ over the diel cycle. Such measurements are attractive because, in addition to singling out a specific organism, they provide information on the details of division patterns as well as the overall rate, and they avoid incubation artifacts.
Prochlorococcus follows the slow-growth mode of the prokaryotic cell cycle paradigm (10); DNA distributions reveal peaks corresponding to either one or two genome copies, implying that DNA replication rounds do not overlap. This has led to the use of the terms usually reserved for the eukaryotic cell cycle (G1, S, and G2 phases) to identify cells according to their DNA content (see, e.g., references 2, 18, 24, and 27). The maximum growth rate of Prochlorococcus is not yet well established. The tight coupling of the cell cycle phasing to the light-dark cycle, with a single division burst, suggested that Prochlorococcus could not grow faster than 1 division per day (div d−1), and when slightly higher rates were computed from cell cycle analyses (18, 27), they were attributed to uncertainties in estimating the duration of S plus G2 phases (a critical parameter in the growth rate computation) or left unexplained. However, a few growth rate estimates from changes in cell numbers, in the highly productive western Arabian Sea, have indicated that Prochlorococcus can grow significantly faster than 1 doubling per day (25, 28). In addition, growth rates slightly faster than 1 doubling per day have been observed in laboratory cultures (21).
We used diel measurements of DNA frequency distributions to estimate Prochlorococcus in situ growth rates, and to investigate the underlying cell division patterns, in the northwestern Arabian Sea during monsoon and intermonsoon seasons. Our observations indicated growth rates exceeding 1 doubling per day and suggested that this ultradian growth was occurring through a novel division pattern in which some cells divided twice in rapid succession. These findings were confirmed by subsequent laboratory culture studies.
MATERIALS AND METHODS
Prochlorococcus natural populations.
Seawater samples were collected in 1995 as part of the U.S. Joint Global Ocean Flux Study (Arabian Sea) aboard the R/V Thomas G. Thompson at two locations in August (southwest monsoon, during a period of intensive vertical mixing) and two locations in November (northeast monsoon, with surface waters well stratified). Surface water was sampled by using a conductivity-temperature-depth rosette and supplemented by bucket sampling to obtain high-frequency samples (0.5 to 1.5 h) for at least 24 h. Seawater samples were fixed immediately in 0.1% glutaraldehyde for 10 min and were frozen in liquid nitrogen until analysis in the laboratory.
Cultures.
Prochlorococcus strain MIT 9302 (nonaxenic) was isolated from the Sargasso Sea and provided to us by Lisa Moore, Massachusetts Institute of Technology. Strain AS 9601 (nonaxenic) was isolated from a water sample collected at 50 m in November 1995 in the Arabian Sea (19°12′N, 67°10′E). Batch cultures were maintained in modified K/10(−Cu) medium (6), enriched with 100 μM urea, 10 nM NiSO4, and 1 nM CuSO4, in 50-ml culture tubes at 26°C under cool white fluorescent lamps.
Laboratory experiments.
Semicontinuous batch cultures were acclimated to experimental light conditions for at least 3 weeks before data were collected. Transfers to fresh medium were made every 4th day before noon to keep the cultures in the exponential-growth phase. In vivo chlorophyll fluorescence was monitored at noon daily. Light intensities were measured with a Biospherical Instruments QSL-100 quantum scalar irradiance meter. Samples were taken every hour during the morning and late night and every 0.5 h during the afternoon and evening, when DNA replication and division occurred. Staining and flow-cytometric analysis were performed immediately without fixation or freezing.
Sample preparation and staining.
Experimental samples were diluted 10- or 20-fold with filtered seawater before staining. Diluted samples and thawed seawater samples were stained 15 to 25 min at room temperature in the dark with the DNA-specific fluorochrome Hoechst 33342 (Sigma) at a final concentration of 1 μg/ml (2, 20).
Flow cytometry.
Following the addition of 0.57-μm-diameter fluorescent beads (Polysciences, Inc.), stained samples were analyzed with a single-beam Coulter EPICS-753 flow cytometer modified for high sensitivity (23). An argon ion laser (Coherent, Inc.) provided 250-mW UV (365 nm) excitation. Forward light scattering, right-angle light scattering, and red, orange, and blue fluorescence data were collected and analyzed as described previously (27). Prochlorococcus cells were discriminated from bacteria, cyanobacteria, and picoeukaryotes according to their light-scattering and fluorescence characteristics (23). DNA frequency distributions were analyzed with MCYCLE software (Phoenix Flow Systems) in order to obtain cell fractions in G1, S, and G2 phases.
Growth rate computations.
In situ specific growth rates based on DNA distributions (μDNA, per day) were computed as described previously (18, 27), based on the equation (4, 19)
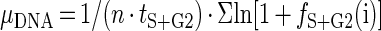 |
1 |
where n is the number of samples taken during the 24-h period, tS+G2 is the combined duration of S and G2 phases, and fS+G2(i) is the fraction of cells in S plus G2 for sample i. Our computations of tS+G2 as twice the distance between the peak of cells in S and the peak of cells in G2 yielded surprisingly low values of 2 to 3 h compared to the previous 6-h (18, 27) or 4.5-h (24) estimates. Therefore, we also estimated this duration as the time between the appearance of the first S cells and that of the first newborn cells. This approach yielded values of 3 to 4.5 h. We used the most conservative estimate of 4.5 h for all calculations; using shorter estimates of S plus G2 duration would result in proportionally higher growth rates. Specific growth rates (μ, per day) were converted to division rates (number of divisions per day) by dividing by the natural logarithm of 2.
In situ specific growth rates based on cell numbers (μcell, per day) were estimated as the difference between observed cell abundances at the beginning and the end of division, corrected for mortality rate (m, per day), as follows:
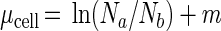 |
2 |
where Na is the cell number when division is complete (at ta [see Fig. 1]), Nb is the cell number just before the first division occurred (at tb), and m is the total mortality rate (per day) due to grazing and other losses. We estimated m for the time period when Prochlorococcus does not divide and computed it for 24 h (assuming m to be constant over the entire day) as
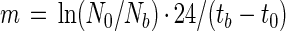 |
3 |
where N0 is the cell number at the beginning of the period without division (at t0), and tb − t0 is the duration of this period.
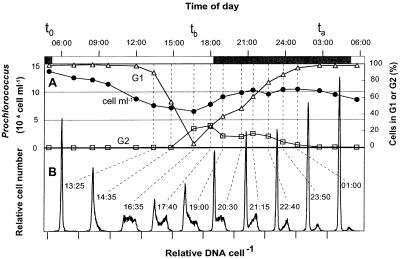
FIG. 1.
Prochlorococcus division pattern in situ (22 to 23 July 1995; 19°10′N, 67°10′E). (A) Cell concentrations and percentage of cells in G1 and G2 phases; t0, ta, and tb are the timing points used for growth rate calculations (equations 2 and 3). (B) DNA frequency distributions for selected samples (indicated by dashed lines) during the period of DNA replication and cell division. At other time points only G1 cells were present. Ticks on the x axis mark the origin for each distribution.
Since the mortality rate was negligible during laboratory experiments, the specific growth rate was computed directly from cell numbers as
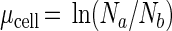 |
4 |
RESULTS AND DISCUSSION
Phasing of cell cycle events in situ was remarkably tight, as observed in previous studies in the equatorial Pacific (18, 27) and tropical Atlantic (24); more than 95% of the cells entered the DNA replication phase S early in the afternoon. The cells progressed through S phase in about 3 h and began dividing shortly before dusk (Fig. 1). DNA distributions at 1435, 1635, and 1740 demonstrate a cohort of cells entering, progressing through, and completing DNA replication, respectively. By 1740 some cells had already divided, as evident from the newly enlarged G1 peak; some of these cells apparently then entered DNA replication again, widening the G1 peak to the right. Progression of this smaller cohort through S phase is reflected in distributions at 1900 to 2115 and resulted in a second peak in the fraction of cells in G2 at 2115. All the cells divided by 0300.
This second wave of division, which has not previously been observed, could in these natural samples result from two populations of cells with different temporal patterns of DNA replication. The division rate estimates, however, suggest rather that some of the cells divided twice in rapid succession: computations of growth rate from DNA distributions (equation 1) yielded rates well in excess of 1 div d−1 (mean = 1.42 div d−1; standard deviation [SD] = 0.09) at all four locations where high-frequency diel sampling was performed.
Independent estimates of division rates were made from observations of changes in in situ cell concentrations over the diel cycle, corrected for cell losses (equations 2 and 3). These values were similar in magnitude (mean = 1.34 div d−1; SD = 0.36) to the cell cycle-based values, though more variable. Our assumption of constant mortality over the diel cycle may have resulted in underestimation of the true growth rates, since some observations have suggested that grazing on Prochlorococcus is highest at night (18), when the cells are dividing. Even if we assumed grazing to be zero during the division period, however, the cell number data indicate growth rates in excess of 1 div d−1.
These data, though suggestive, are subject to uncertainty because of the necessary assumptions about population homogeneity, S plus G2 phase durations, and mortality rates in the field. Therefore, we carried out similar diel experiments using cultures isolated from the Sargasso Sea (MIT 9302) and the Arabian Sea (AS 9601), in which samples could be taken with higher time resolution, and in which growth rates could be precisely determined by direct cell counts. The results of these measurements serve to confirm and clarify the field observations.
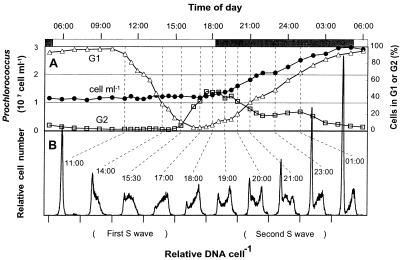
On a light-dark cycle similar to that of the field studies (13 h of light and 11 h of darkness), both Prochlorococcus isolates grew at rates well in excess of 1 div d−1 over a wide range of light intensities (Table 1). The pattern of cell cycle events was virtually the same at all light intensities. The first cells entered S phase 6 h after the onset of light and G2 phase about 4 h later, with newborn cells appearing about 2 h afterward (1 h before the lights went off) (Fig. 2, DNA distributions at 1100 to 1800). These observations are in general agreement with those for natural populations (18, 24, 27) (Fig. 1), although the durations of S and G2 phases were longer in culture. At light intensities of 40 microeinsteins m−2 s−1 and higher, however, a second cohort of cells could clearly be seen in S phase by 2000 (Fig. 2B). These cells reached G2 phase by 2300 and divided before the end of the dark period. The fact that the division rate was significantly higher than 1 div d−1 (Table 1) indicated that the second wave constituted division of newly released daughter cells rather than a delayed division of some of the cells. When the division rate was less than 1 div d−1 (i.e., not all the cells divided), the second wave was absent (MIT 9302 at 30 microeinsteins m−2 s−1 [Fig. 3]). The second wave of division differed from the first in the smaller number of cells involved (at most, only about one-third of the cells divided twice [Table 1]) and in the dramatically reduced duration of the G1 phase. The time gap between the release of the first newborn cells and the beginning of the second S phase suggests that G1 phase for some cells in the second division is less than 1 h.
TABLE 1.
Growth parameters of Prochlorococcus strains MIT 9302 and AS 9601 in laboratory experiments
| Strain | Light intensity (microeinsteins m−2 s−1) | Light-dark cycle (h:h) | Specific growth rate (μ d−1) | Division rate (div d−1) | % of cells that divided twicea |
|---|---|---|---|---|---|
| MIT 9302 | 30 | 13:11 | 0.48 | 0.69 | 0 |
| 40 | 13:11 | 0.73 | 1.05 | 4 | |
| 60 | 13:11 | 0.79 | 1.14 | 10 | |
| 100 | 13:11 | 0.97 | 1.40 | 32 | |
| 120 | 13:11 | 0.93 | 1.34 | 26.5 | |
| 40 | 14.5:9.5 | 0.99 | 1.43 | 34.5 | |
| AS 9601 | 60 | 13:11 | 0.82 | 1.18 | 13.5 |
| 120 | 13:11 | 0.84 | 1.21 | 16 | |
| 40 | 14.5:9.5 | 0.92 | 1.33 | 25.5 |
Computed from observed growth rate by assuming that, during ultradian growth, every cell divided at least once and some divided twice, producing four daughter cells.
FIG. 2.
Prochlorococcus strain MIT 9302 division pattern in laboratory culture on a light-dark cycle (light intensity, 120 microeinsteins m−2 s−1). (A) Cell concentrations and percentage of cells in G1 and G2 phases. Symbols are as explained for Fig. 1. (B) DNA frequency distributions for selected samples (indicated by dashed lines).
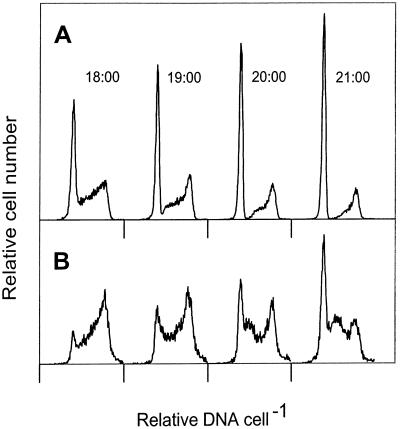
FIG. 3.
DNA frequency distributions of Prochlorococcus strain MIT 9302 at 1800 to 2100 at division rates of 0.69 div d−1 (30 microeinsteins m−2 s−1) (A) and 1.34 div d−1 (120 microeinsteins m−2 s−1) (B). Note that when the division rate is less than 1 div d−1 (panel A), there is no sign of new G1 cells entering S phase, in contrast to the clearly visible cohort of cells reentering S phase during ultradian growth (panel B).
In two experiments in which the light period was longer than 13 h, all cell cycle events (including the second wave) occurred at the same time as in the shorter light regimens with respect to the onset of light intensity (data not shown). This, together with the lack of dependence of event timing on light intensity, suggests control of cell cycle events by a light-triggered timer or the recently demonstrated prokaryotic circadian clock (14, 15, 22) rather than by completion of a light-dependent assimilation phase. When the light period was longer, more cells completed division before darkness, and more cells divided twice, even when the total amount of light received was lower (Table 1), suggesting that a newborn cell must experience some light to divide.
The strict timing of both DNA replication and cell division events and the very short G1 phase before a second division makes Prochlorococcus quite different from other phytoplankton. The closest known relative of Prochlorococcus, the cyanobacterium Synechococcus spp., can grow much faster than 1 div d−1 but has continuous DNA replication and cell divisions occurring all through the day and into the night (1, 22). Ultradian growth in many centric diatoms and in the coccolithophore Hymenomonas carterae is characterized by the presence of some dividing cells at all times of the day and night, and the peaks in cell division rate are separated by about 12 h (5, 8). The freshwater chlorophytes Chlamydomonas spp. and Scenedesmus armatus carry out multiple mitoses before dividing into four, eight, or more cells at once (9, 26). Another chlorophyte, Euglena gracilis, when grown at high light intensity on a cycle of 14 h of light and 10 h of darkness, exhibited phased ultradian growth, with cell division confined to the dark period (12). Although Euglena’s DNA replication and cell division patterns under ultradian growth conditions have not been studied, they might be similar to those of Prochlorococcus.
Although the phased ultradian growth of Prochlorococcus is the first such pattern to be observed in a marine species (as well as the first observed in situ), it is probably not unique, and it may represent a common mode of growth in natural communities of marine phytoplankton. It is consistent with estimates by a variety of methods of in situ division rates for the whole phytoplankton community being faster than 1 div d−1 (13, 16), coupled with recent observations that cell size in populations of pico- and nanophytoplankton typically increases steadily over the day to reach a maximum shortly before dusk (11). Such a division pattern appears to combine the efficient use of daylight hours for photosynthesis with the potential for maximum cell division during the night (cells which have fixed enough carbon during the day may then divide more than once).
Accurately measuring in situ growth rates by cell cycle analysis requires high sampling frequency (hourly), since uncertainty in the growth rate estimate is proportional to the error in estimated cell cycle phase durations. Our observations of ultradian growth suggest that the maximum achievable growth rate for Prochlorococcus has been underestimated in the past and complicate the use of this organism as an indicator of environmental growth conditions. For example, the conclusion that Prochlorococcus in the equatorial Pacific Ocean is not nutrient limited, which was based on the appearance that cells in situ were growing at their maximal rate (i.e., near 1 div d−1) (2, 18, 27), now appears questionable.
ACKNOWLEDGMENTS
We thank chief scientists R. Barber and B. Balch and the dedicated technical staff and crew of R/V Thomas G. Thompson for their help during the cruises; L. Moore for providing Prochlorococcus strain MIT 9302; and S. Chisholm, H. Sosik, and J. Waterbury for critical reading of the manuscript.
This work was supported by National Science Foundation grant OCE 9311113.
REFERENCES
- 1.Binder B J, Chisholm S W. Cell cycle regulation in marine Synechococcus sp. strains. Appl Environ Microbiol. 1995;61:708–717. doi: 10.1128/aem.61.2.708-717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binder B J, Chisholm S W, Olson R J, Frankel S L, Worden A Z. Dynamics of picophytoplankton, ultraphytoplankton and bacteria in the central equatorial Pacific. Deep-Sea Res Part II. 1996;43:907–931. [Google Scholar]
- 3.Campbell L, Nolla H A, Vaulot D. The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnol Oceanogr. 1994;39:954–961. [Google Scholar]
- 4.Carpenter E J, Chang J. Species-specific phytoplankton growth rates via diel DNA synthesis cycles. I. Concept of the method. Mar Ecol Prog Ser. 1988;32:139–148. [Google Scholar]
- 5.Chisholm S W, Costello J C. Influence of environmental factors and population composition on the timing of cell division in Thalassiosira fluviatilis (Bacillariophyceae) grown on light/dark cycles. J Phycol. 1980;16:375–383. [Google Scholar]
- 6.Chisholm S W, Frankel S L, Goericke R, Olson R J, Palenik B, Waterbury J B, West-Johnsrud L, Zettler E R. Prochlorococcus marinus nov. gen. nov. sp.: an oxyphototrophic marine prokaryote containing divinil chlorophyll a and b. Arch Microbiol. 1992;157:297–300. [Google Scholar]
- 7.Chisholm S W, Olson R J, Zettler E R, Waterbury J, Goericke R, Welschmeyer N. A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature (London) 1988;334:340–343. [Google Scholar]
- 8.Chisholm S W, Vaulot D, Olson R J. Cell cycle controls in phytoplankton. Comparative physiology and ecology. In: Edmunds L N, editor. Cell cycle clocks. New York, N.Y: Marcel Dekker; 1984. pp. 365–394. [Google Scholar]
- 9.Coleman A W. The nuclear cell cycle in Clamydomonas (Chlorophyceae) J Phycol. 1982;18:192–195. [Google Scholar]
- 10.Cooper S. Bacterial growth and division. San Diego, Calif: Academic Press Inc.; 1991. [Google Scholar]
- 11.DuRand M D, Olson R J. Contributions of phytoplankton light scattering and cell concentration changes to diel variations in beam attenuation in the equatorial Pacific from flow cytometric measurements of pico-, ultra- and nanoplankton. Deep-Sea Res Part II. 1996;43:891–906. [Google Scholar]
- 12.Edmunds L N, Jr, Funch R. Effects of “skeleton” photoperiods and high frequency light-dark cycles on the rhythm of cell division in synchronized cultures of Euglena. Planta (Berlin) 1969;87:134–163. doi: 10.1007/BF00386972. [DOI] [PubMed] [Google Scholar]
- 13.Furnas M J. In situ growth rates of marine phytoplankton: approaches to measurement, community and species growth rates. J Plankton Res. 1990;12:1117–1151. [Google Scholar]
- 14.Johnson C H, Golden S S, Ishiura M, Kondo T. Circadian clocks in prokaryotes. Mol Microbiol. 1997;21:5–11. doi: 10.1046/j.1365-2958.1996.00613.x. [DOI] [PubMed] [Google Scholar]
- 15.Kondo T, Mori T, Lebedeva N V, Aoki S, Ishiura M, Golden S S. Circadian rhythms in rapidly dividing cyanobacteria. Science. 1997;275:224–227. doi: 10.1126/science.275.5297.224. [DOI] [PubMed] [Google Scholar]
- 16.Landry M R, Constantinou J, Kirshtein J. Microzooplankton grazing in the central equatorial Pacific during February and August, 1992. Deep-Sea Res Part II. 1995;42:657–671. [Google Scholar]
- 17.Li W K W, Dickie P M, Irwin B D, Wood A M. Biomass of bacteria, cyanobacteria, prochlorophytes and photosynthetic eukaryotes in the Sargasso Sea. Deep-Sea Res Part I. 1992;39:501–519. [Google Scholar]
- 18.Liu H, Nolla H A, Campbell L. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat Microb Ecol. 1997;12:39–47. [Google Scholar]
- 19.McDuff R E, Chisholm S W. The calculation of in situ growth rates of phytoplankton populations from fractions of cells undergoing mitosis: a clarification. Limnol Oceanogr. 1982;27:783–788. [Google Scholar]
- 20.Monger B C, Landry M R. Flow cytometric analysis of marine bacteria with Hoechst 33342. Appl Environ Microbiol. 1993;59:905–911. doi: 10.1128/aem.59.3.905-911.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore L R. Physiological ecology of Prochlorococcus: a comparison of isolates from diverse oceanographic regimes. Ph.D. thesis. Cambridge: Massachusetts Institute of Technology; 1997. [Google Scholar]
- 22.Mori T, Binder B, Johnson C H. Circadian gating of cell division in cyanobacteria growing with average doubling times of less than 24 hours. Proc Natl Acad Sci USA. 1996;93:10183–10188. doi: 10.1073/pnas.93.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson R J, Chisholm S W, Zettler E Z, Altabet M A, Dusenberry J A. Spatial and temporal distributions of prochlorophyte picoplankton in the North Atlantic Ocean. Deep-Sea Res. 1990;37:1033–1051. [Google Scholar]
- 24.Partensky F, Blanchot J, Lantoine F, Neveux J, Marie D. Vertical structure of picophytoplankton at different trophic sites of the tropical northeastern Atlantic Ocean. Deep-Sea Res Part I. 1996;43:1191–1213. [Google Scholar]
- 25.Reckermann M, Veldhuis M J W. Trophic interactions between picophytoplankton and micro- and nanozooplankton in the western Arabian Sea during the NE monsoon 1993. Aquat Microb Ecol. 1997;12:263–273. [Google Scholar]
- 26.Tukaj Z, Kubinova A, Zachleder V. Effect of irradiance on growth and reproductive processes during the cell cycle in Scenedesmus armatus (Chlorophyta) J Phycol. 1996;32:624–631. [Google Scholar]
- 27.Vaulot D, Marie D, Olson R J, Chisholm S W. Growth of Prochlorococcus, a photosynthetic prokaryote, in the Equatorial Pacific Ocean. Science. 1995;268:1480–1482. doi: 10.1126/science.268.5216.1480. [DOI] [PubMed] [Google Scholar]
- 28.Veldhuis M J V, Kraay G W, van Bleijswijk J D L, Baars M A. Seasonal and spatial variability in phytoplankton biomass, productivity and growth in the northwestern Indian Ocean: the southwest and northeast monsoon, 1992–1993. Deep-Sea Res Part I. 1997;44:425–449. [Google Scholar]