Abstract
Air pollution is one of the leading causes of death from noncommunicable diseases globally, and in Arizona, both mining activities and abandoned agriculture can generate erodible dust. This dust is transported via wind and can carry high amounts of toxic pollutants. Industry-adjacent communities, or “fenceline communities,” are generally closer to the pollution sources and are disproportionally impacted by pollution, or in this case, dust. The dust transported from the mine settles into nearby rivers, gardens, and homes, and increases the concentrations of elements beyond their naturally occurring amounts (i.e., enriched). This study was built upon previous community science work in which plant leaves were observed to collect similar concentrations to an accepted dust collection method and illustrated promise for their use as low-cost air quality monitors in these communities. This work investigated the concentration of Na, Mg, Al, K, Ca, Mn, Co, Cu, Zn, Mo, and Ba in dust from the leaves of community-collected backyard and garden plants (foliar dust), as well as if certain variables affected collection efficacy. This assessment evaluated (1) foliar concentration versus surface area for 11 elements, (2) enrichment factor (EF) values and ratios, (3) comparisons of foliar, garden, and yard samples to US Geological Survey data, and (4) what variable significantly affected dust collection efficacy. The EF results indicate that many of the samples were enriched (anthropogenically contaminated) and that the foliar samples were generally more contaminated than the yard and garden soil samples. Leaf surface area was the most influential factor for leaf collection efficiency (p < 0.05) compared to plant family or sampling location. Further studies are needed that standardize the plant species and age and include multiple replicates of the same plant species across partnering communities. This study has demonstrated that foliar dust is enriched in the participating partnering communities and that plant leaf samples can serve as backyard aerosol pollution monitors. Therefore, foliar dust is a viable indicator of outdoor settled dust and aerosol contamination and this is an adoptable monitoring technique for “fenceline communities.”
Keywords: Air monitor, Mining, Foliar, Fugitive dust, Co-created community science
Introduction
In 2019, the World Health Organization (WHO) identified air pollution as the second leading cause of death from noncommunicable diseases (NCDs) after tobacco smoking. NCDs are chronic diseases, like heart attacks, strokes, asthma, and diabetes, and account for 71% of annual global deaths, or 41 million people (WHO, 2021). They are also distributed inequitably, targeting communities in poverty and of color (Braveman, 2014; Bullard, 2008; Burwell-Naney et al., 2019; Gee et al., 2019; Gee & Payne-Sturges, 2004; Marmot & Allen, 2014; NASEM, 2017; Schulz et al., 2016). This includes communities near active and legacy mines.
The processes that occur at mines, like grinding, smelting, and refining, can be potential sources of contaminant-laden aerosols and atmospheric dust. Toxic species like arsenic (As) and lead (Pb) have an increased propensity to be transported via wind dispersion of dust (Johnson et al., 1994; Stovern et al., 2016). Even inactive, legacy, and/or unremediated mines can pose as long-term dust sources from waste-containment facilities like mine tailings (Alloway, 1995; Camacho et al., 2011; Corriveau et al., 2011; Csavina et al., 2014; Jung, 2001; Meza-Figueroa et al., 2009; Navarro et al., 2008). Another major reservoir of erodible dust in Arizona is active and abandoned agricultural lands, specifically in Pinal and Maricopa counties (Fig. 1) (Maricopa County Air Quality Department, 2017; Sierra Research and Arizona Department of Environmental Quality, 2014). Compared to other major contributors of windblown dust, abandoned agriculture receives no regulatory scrutiny, whereas active farms are subject to Agricultural Best Management Practices (BMPs; Hyde et al., 2018), like Agricultural PM10 or Agricultural Water Conservation BMPs. The effects of climate change, like increased temperatures and reduced precipitation, will exacerbate windblown dust and droughts in the Southwest (MacDonald, 2010). Further reductions in water available for agriculture (from reduced groundwater pumping and Central Arizona Project (CAP) canal water) will cause actively farmed lands to be abandoned at an increasing rate (Hyde et al., 2018).
Fig. 1.
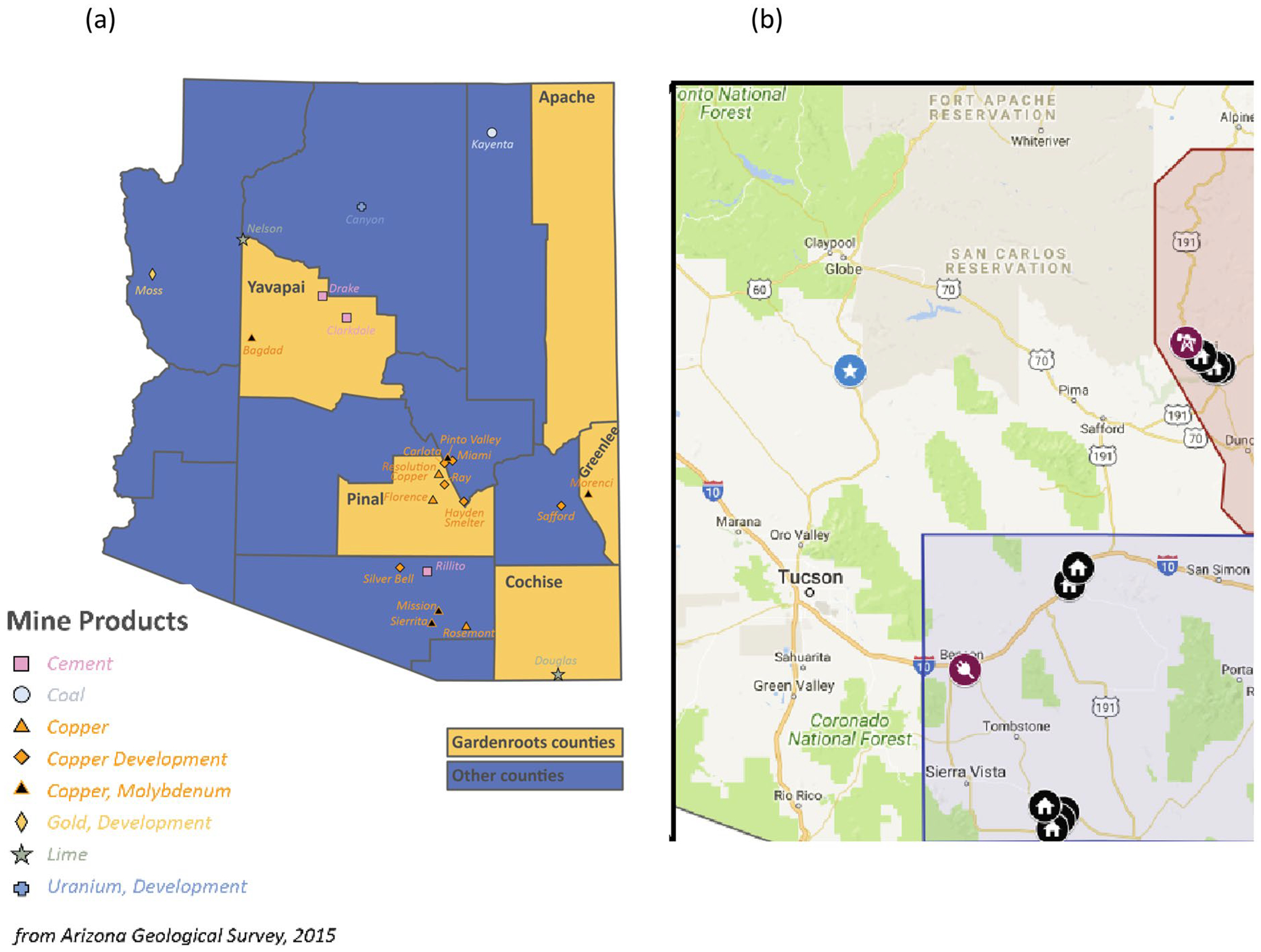
a Map of the state of Arizona with Gardenroots counties in yellow. Modified from Ramírez-Andreotta et al., 2021. b Regions included in this study: Greenlee (red) and Cochise (blue). Markers indicate representative sampling areas (black) and some major industries in each county (burgundy). Exact locations are not shown to protect the privacy of participants
Fugitive dust from mines, farms, and other sources of erodible dust, can affect nearby communities by polluting waterways and soils, and subsequently crops. A major contaminant exposure pathway for fenceline communities is inadvertent consumption of such crops, especially when contaminated soil and dust particles settle on and adhere to the crop surface (Cobb et al., 2000; Lee et al., 2005; Murray et al., 2009) or food from gardens (Brand et al., 2007; Manjón & Ramírez-Andreotta, 2020; Ramírez-Andreotta et al., 2013a, b, 2015). In 2014, 35% of American households grew their own food (National Gardening Association, 2014), and by 2021, 55% of American households were engaging in gardening activities (Miracle-Gro, 2021). There were 18.3 million new self-identified gardeners in 2021, with a suspected factor of increased participation and interest being the global pandemic, as two-thirds of surveyed gardeners tried a new gardening activity during the COVID-19 pandemic (National Gardening Association, 2021). The dust that collects on the leaves of these home-grown crops and even native plants can pose a risk if consumed, but can also be used to quantify contaminants in the air and soil.
This study builds off previous work (Ramírez-Andreotta et al., 2013a, b, 2015; Zeider et al., 2021), in which plant leaves (foliar) were statistically shown to be reliable air quality monitors in a mining-adjacent community. The goal of this study was to collect dust on various backyard and garden plants and determine if plant family or leaf surface area affected how much dust a plant leaf collected, or if there was a collection difference between counties. Though plants are known to accumulate elements via uptake (Manjón et al., 2019; Manjón & Ramírez-Andreotta, 2020; Ramírez-Andreotta et al., 2013a, b), this study was solely examining dust settling on plant leaves. Therefore, plant uptake will likely have minimal influence on dust collection or enrichment from a leaf surface. Foliar, garden soil, and yard soil community science samples were analyzed for contaminant enrichment. The following elements were reported: Na, Mg, Al, K, Ca, Mn, Co, Cu, Zn, Mo, and Ba. The results of this study can inform any community-science projects or further academic studies on important foliar analysis characteristics.
Methods
Study and site description
Gardenroots (https://gardenroots.arizona.edu/) was established in 2010 to evaluate the environmental quality of gardens in underserved, rural communities (Ramirez-Andreotta et al., 2013a, b, 2015). Community members chose to participate in Gardenroots because of concerns regarding the safety of their home-grown produce due to their proximity to legacy mining operations and extraction sites (Sandhaus et al., 2019). Samples for this study were taken from home gardens in the cities of Saint Johns in Apache County, Arizona, Bisbee and Willcox in Cochise County, Arizona, and in the towns of Clifton and Morenci in Greenlee County, Arizona (Fig. 1). Apache County is located in the northeast corner of Arizona with approximately 66,021 people (US Census Bureau, 2020a) and 44 mine employees (CDC, 2020). Only soil samples were collected from Apache County, so this work used the foliar and soil samples from Cochise and Greenlee counties. Cochise County is located at the southeast corner of Arizona with an estimated population of 125,447 people (US Census Bureau, 2020b) and approximately 81 mine operation employees as of 2020 (CDC, 2020). According to the Arizona Department of Environmental Quality (ADEQ) Non-attainment Areas eMap, sections of Cochise County have been classified as a “nonattainment area” for particulate matter with diameter ≤ 10 μm (PM10), which are associated with long-term and short-term adverse respiratory effects when inhaled (Xing et al., 2016). Greenlee County is located just north of Cochise County with a population of approximately 9563 people (US Census Bureau, 2020c) with around 3231 people employed by nearby mining operations (CDC, 2020). Greenlee County is home to the Morenci open-pit copper mine, which is one of Arizona’s leading producers of copper (Hoffman et al., 2011).
Sample collection
Forty-two individuals from Cochise County (C) and 30 individuals from Greenlee County (G) were trained and given sample-collecting kits, which included the needed materials and an instruction manual with steps for proper sample collection. The participants were instructed to collect leaf samples from two different plants in their own home garden, either a leafy vegetable and/or an ornamental plant. Participants collected adult leaves that were parallel to the ground and located on the upper portion of the plant. They were asked to collect five leaves each from the two plants. Each sample set (i.e., all the leaves from one plant) was placed in separate one-gallon Ziploc bags with the air removed from the inside before sealing or a trace metal-free 50-mL polypropylene vial. The participants were then instructed to place their samples in a refrigerator until they were ready to deliver their sample to their county’s University of Arizona (UA) cooperative extension office (Sierra Vista Cooperative Extension office for participants in Cochise County and UA Greenlee Cooperative Extension office for participants living in Greenlee County). The foliar samples were collected from September to October 2015, and 15 individuals submitted leaves for analysis (ten and five participants from each county, respectively) with a total of 27 leaves. Although participants were asked to submit five leaves per plant, most submitted one to two leaves per plant. Samples were stored and refrigerated at the designated cooperative extension office, and then transported on ice to the Integrated Environmental Science and Health Risk Laboratory at the UA in Tucson, Arizona for analysis.
At the same time and in addition to leaf samples, Gardenroots participants also collected soil samples. Participants were instructed to collect a composite soil sample from their yard and garden soils. The participants first selected six spots in a grid-like pattern in both their yard and garden areas. Then, using a provided hand trowel, they loosened the top 15 cm of soil (the approximate length of the hand trowel) within each spot and collected one full scoop of soil from all six spots. Participants then composited and mixed the soil samples thoroughly (bulk sampling) in two clean, two-gallon plastic buckets, one designated for yard soil and the other for garden soil. The participants were instructed to place their samples in a refrigerator until they were ready to deliver their sample to their county’s UA cooperative extension office.
Laboratory processing
Leaf samples were refrigerated and stored upon arrival at the laboratory. A metal-free, trace clean polypropylene 50-mL vial and its cap were weighed. The sample (unless already in a vial) was then transferred to a vial from its original Ziploc bag without rinsing the bag and then the vial was capped. The leaf specimen, vial, and cap were then weighed before adding 40 mL of Nanopure water to the vial. To not break down or harm the plant leaf, the vials with the specimen and water were lightly agitated at room temperature using a VWR® Hybridization Oven (Model 5400) for 24 hours at 55.0 rpm, and then the entire leaf sample was gently removed from the vial without any further rinsing, and photographed. The photograph contained a ruler lying horizontally next to the sample to calculate the surface area of the sample specimen.
The 40-mL extract was then split in half into two separate metal-free, trace-clean polypropylene 50-mL vials. Twenty milliliters were filtered through a sieve and categorized as the filtered sample, and the remaining, unsieved 20 mL served as the unfiltered sample. For this study, only the unfiltered foliar samples were used for data analysis. For each sample, a 1-mL aliquot of the extract was treated with 1 mL of concentrated nitric acid in a polytetrafluoroethylene vial to pre-digest the dust material in the solution. Then 1 mL of ultra-pure water was added to the digested solution to dilute the nitric acid to 2%. The vials were then capped, sealed, and then microwave-digested (CEM Corporation, MARS 6). Finally, the samples were analyzed for trace metal analysis via inductively-coupled plasma mass spectrometry (Elan DRC-II ICP-MS) by the Arizona Laboratory for Emerging Contaminants (ALEC). Laboratory blanks were prepared and also analyzed by ICP-MS. The elemental method detection limits are given in Table S1 for elements toxic to plants and Al.
Garden and yard soil samples from the specific sampling locations were digested as per US EPA Method 3051A (US EPA SW-846, 1986), and then also analyzed via ICP-MS by ALEC (for additional methodological details see Ramírez-Andreotta et al., 2013a,b, Manjón & Ramírez-Andreotta, 2020). Yard soil samples were used for this study unless only garden soil was submitted by the participant.
Data analysis
Adobe Photoshop CS5 Extended was used to measure the surface area of each leaf. All leaf samples were photographed alongside a ruler, and the leaves were outlined using the Lasso Tool. The Paint Bucket Tool was then used to fill in the entire outline with a solid color. Using the Histogram window, the number of pixels within the colored area was recorded. Then, the Rectangular Marquee Tool was used to outline a square inch using the ruler image. The pixels were also recorded using the Histogram window. The surface area (SA) of the selected leaf was calculated using the expression,
| (1) |
where pxleaf is the total number of pixels found within the leaf outline and pxsq in is the total number of pixels in the known squared inch. These values were then converted to cm2. The (C) gourd, (G) red potato, and (G) okra leaves were more deteriorated than the other samples, so those surface areas were compared to published surfaces areas (cm2): gourd, 3.20 versus 96 (Bemis et al., 1978); red potato, 2.81 versus 100 (Charles et al., 1992); and okra, 6.26 versus 168 (Dehigaspitiya et al., 2016). The surface areas from the sample set were kept for this study because smaller surface areas are more common for backyard gardens and fit the spread of the other calculated surface areas.
ALEC reported the dust concentrations in μg L−1 and the method limit of detection (MLOD) for each element. The data were converted to μg by multiplying the concentrations (in μg L−1) by the volume of the sample-HNO3-water solution (3 mL) and a dilution factor of 3, and then by dividing by the surface area of the respective leaf. Values below the MLOD were omitted from the dataset to ensure unique and representative enrichment factor values and to aid in identifying a clear trend. This study breaks down the reported elements into plant macronutrients and elements associated human toxicity. The macronutrients include Ca, Mg, and K (White & Brown, 2010), and the toxic elements to humans are Mn, Cu, Zn (which are also considered plant micronutrients), and Ba (ATSDR, 2004). Na and Al were also investigated and are considered potentially toxic elements to plants and Al does have associated toxicity to humans. A preliminary analysis of collection date versus concentration was conducted, and it was determined that collection date did not significantly affect concentration.
Enrichment factors (EF) are widely used to quantitatively determine if the concentration of metals in dust are from an anthropogenic source (Avila et al., 2017; Bian et al., 2015; Gajbhiye et al., 2016; Yang et al., 2016). The EF of a contaminant is calculated using the expression,
| (2) |
where Cn is the concentration of the contaminant in units of μg cm−2. We consider Al to be the reference species, Cref, as in previous work (Taghavi et al., 2019), with concentrations that are assumed to be uncontaminated by anthropogenic sources. Concentrations in the numerator of the EF ratio in Eq. (2), with the subscript “sample,” are from the foliar and soil datasets. Concentrations in the denominator, with the subscript “baseline,” are previously reported crustal values (Goldschmidt, 1937). An important EF threshold value is 10: ratios less than that indicate species with crustal origins, and ratios greater than that indicate non-crustal origins, such as anthropogenic sources (Liu et al., 2002) and are considered “enriched.” Furthermore, EF values greater than 100 signify more significant contamination (Li et al., 2015).
United States Geological Survey (USGS) data were used for comparison to the calculated EF values of the Gardenroots soil samples (Smith et al., 2013). Appendix 2a data were used, which reports geochemical and mineralogical data of surface soils to a depth of 5 cm for different types of land cover at various latitudes and longitudes, collected from 21–29 June 2010 (Smith et al., 2013). It is important to note that Gardenroots collected soil samples to a depth of 15 cm. The coordinate boundaries of Cochise and Greenlee counties were determined and used to select the soil samples that fell within the county lines. Since there was not a site-specific local soil for each participant, the USGS data were used as a proxy. To compare this study’s soils most accurately to the enrichment of the USGS soils, the “shrubland” land type was selected, since its definition most closely resembles the environment of this study: a vegetated area dominated by shrubs and often including grasses and herbs (Earth Observatory, n.d.). Equation (2) was used to calculate the enrichment of the USGS soil, and then the difference of the calculated and reported values was taken and reported.
The Kruskal-Wallis test was performed to determine if the concentration of trace metals had any relationship to plant family, leaf surface area, or county. It is a non-parametric ANOVA test that uses ranking and does not assume a normal distribution. The null hypothesis was that each concentration in a categorical group would have no difference and had come from the same distribution at a significance level of 0.05. This study erred on the side of Type I error by not performing any ad hoc tests, however, it was more important for this study to not remove any data due to the low volume of samples.
Results
Enrichment factor analysis
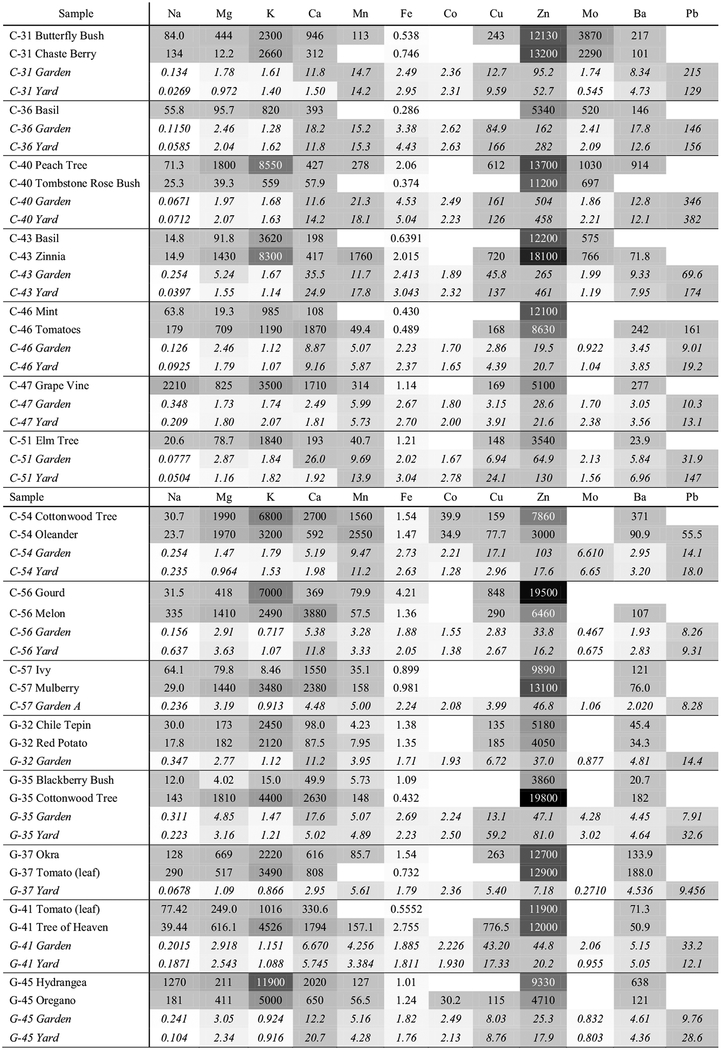
The magnitude of the EF values for foliar, garden, and yard samples (Table 1) varied both element-wise and sample-wise. Generally, the yard and garden samples were less enriched than the foliar samples, and sometimes differed by one or two orders of magnitude. All foliar samples (27) were linked to crustal origins for Fe, followed by three samples for Mn, and one sample for each Mg and K. The remainder of the foliar samples were enriched, with the majority being significantly contaminated for all analytes except for Na and Co. There were only two foliar concentrations with Pb above the MLOD.
Table 1.
Enrichment factor values for foliar (non-italicized), garden, and yard samples. Aluminum is used as the reference species. White shades indicate no to low anthropogenic enrichment, and grey to black shades indicate moderate to significant contamination. Refer to the “Data analysis” section for enrichment threshold values. Blank cells are samples with elemental concentrations below the MLOD
|
Half of the elements tested for the yard/garden soil samples (values in parenthesis) were not linked to anthropogenic contamination. Calcium (5/8), Mn (6/4), and Ba (2/2) (yard/garden, respectively) had several samples that were moderately enriched. Copper (4/5), Zn (8/10), and Pb (6/6) had a mix of samples that were moderately contaminated and a couple of samples that were significantly contaminated (1/3, 4/4, and 5/3, respectively). Iron had no anthropogenic enrichment and no samples had significant Co contamination. With the exception of Na, between 50 and 96% of the samples for each element were significantly enriched.
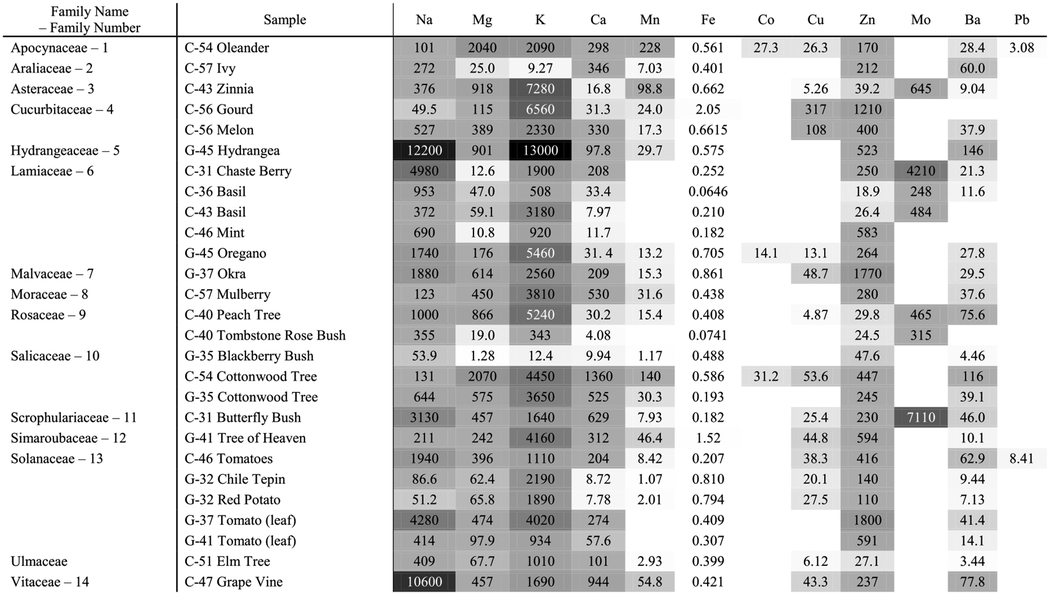
To compare the enrichment of the sample to the surrounding sample area, the foliar EF value was normalized by its corresponding yard sample (Table 2). The yard sample was chosen over the garden sample since it is not amended and because enrichment does not take into account plant uptake, but rather what is surrounding the plant. Most of the yard and garden samples agreed on the severity of contamination (i.e., none, moderate, or severe) except for a handful of cases for Ca, Mn, Cu, and Pb (Table 1). Table 2 illustrates that Fe was the only element with yard EFs greater than foliar EFs, followed by Mn that had several samples with relatively similar yard and foliar concentrations. Additionally, Mn, Cu, Zn, and Ba had much higher foliar to yard enrichment compared to the Ca, Mg, K, Na, and Al, on average.
Table 2.
Ratio of foliar to yard enrichment factors (i.e., ratio of foliar and yard concentrations). White shades indicate higher yard enrichment and black shades indicate higher foliar enrichment, with a value of 1 meaning equivalent yard and foliar enrichment. Blank cells are samples with elemental concentrations below the MLOD. Family Numbers are given for Groups* in Table 4
|
The soil EFs observed in this study were compared to a 2010 USGS Soil Report (Smith et al., 2013) (Table 3). While several elements from this study were enriched, the EFs derived from the USGS Soil Report were higher than this study’s EF values for all the elements except for Ca and Fe. The EF values of Ca, Mg, K, Na, and Al from this study differed from the Soil Report EF values by −0.56 to 8.5, with a negative value indicating the USGS samples were more enriched, and vice versa. Manganese, Cu, Zn, and Ba had a larger range of differences between this study and the USGS report, ranging from −3800 to −27,000. Also, the EF values for the harmful elements from this study, Mn, Cu, Zn, and Ba, were consistently less than the USGS Report by a factor of at least 2.
Table 3.
Comparison of enrichment factor values between this study and a 2010 USGS Soil Report
| Counties (study) | Na | Mg | K | Ca | Mn | Fe | Co | Cu | Zn | Mo | Ba | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average Cochise (yard samples) | 0.16 | 1.8 | 1.5 | 8.8 | 12 | 3.1 | 2.1 | 53 | 160 | 2.0 | 6.4 | 120 |
| Cochise–Shrubland (USGS) | 0.63 | 0.23 | 1.9 | 0.32 | 7000 | 0.58 | 2100 | 3900 | 19000 | 910 | 19000 | 29000 |
| Difference | −0.47 | 1.5 | −0.41 | 8.5 | −7000 | 2.6 | −2100 | −3800 | −19000 | −900 | −19000 | −29000 |
| Average Greenlee (yard samples) | 0.15 | 2.3 | 1.0 | 8.6 | 4.5 | 1.9 | 2.2 | 23 | 32 | 1.3 | 4.7 | 21 |
| Greenlee–Shrubland (USGS) | 0.71 | 0.65 | 1.1 | 0.74 | 11000 | 1.0 | 6000 | 12000 | 27000 | 1200 | 24000 | 16000 |
| Difference | −0.57 | 1.6 | −0.062 | 7.9 | −11000 | 0.90 | −6000 | −12000 | −27000 | −1200 | −24000 | −16000 |
Concentration versus surface area and Kruskal-Wallis analysis
The species that collected the highest foliar concentrations of multiple elements were the red potato, gourd, and oregano leaves (Table S1). Figure 2 graphs foliar concentrations for Ca, Mg, and K versus sample site distance from the major industry of its respective county (shown in Fig. 1). Magnesium, Al, and K generally had higher concentrations for leaves with lower surface areas. Ca and Mg had high concentrations for a surface area of 12.76 cm2 (melon) and Na had a peak concentration at 27.64 cm2 (grape vine). Surface areas of 2.81 cm2 (red potato) and 5.21 cm2 (oregano) generally had the two highest concentrations in the plant nutrient category. Cu, Zn, and Ba had a similar trend in foliar concentrations (Fig. 3) to Mg, Al, and K: as surface area increased, concentration decreased. The spread of the concentration versus distance graph of Mn was similar to that of Na, with the greatest concentrations of Mn at 13.1 cm2 (zinnia) and 113.5 cm2 (oleander).
Fig. 2.
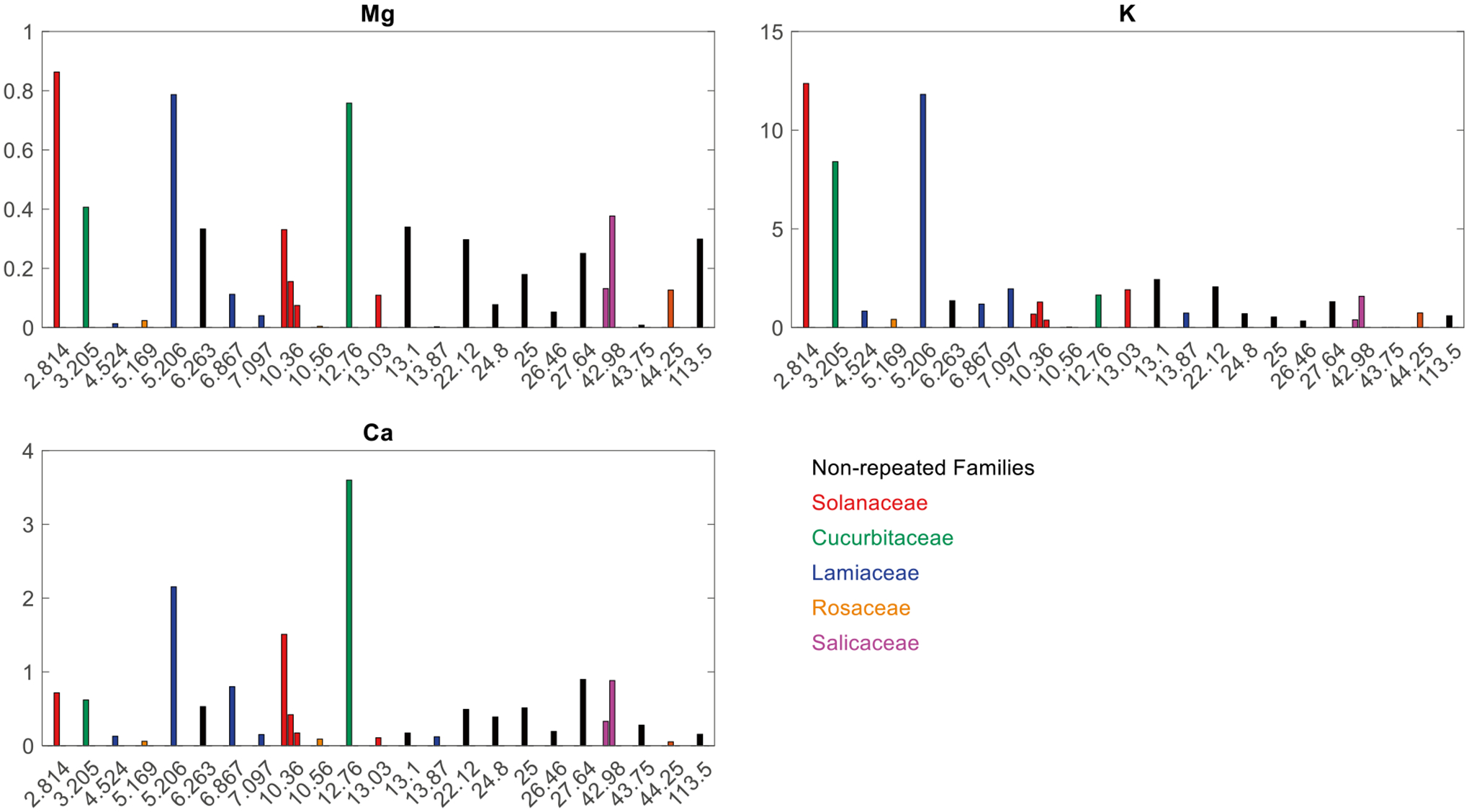
Concentration (y-axis; μg cm−2) versus surface area (x-axis; cm−2) of selected plant macronutrients. The colors represent different family types repeated (i.e., more than one representative sample) in the dataset (see legend). The black bars signify different, non-repeating families. Refer to Table S1 for numerical concentration results
Fig. 3.
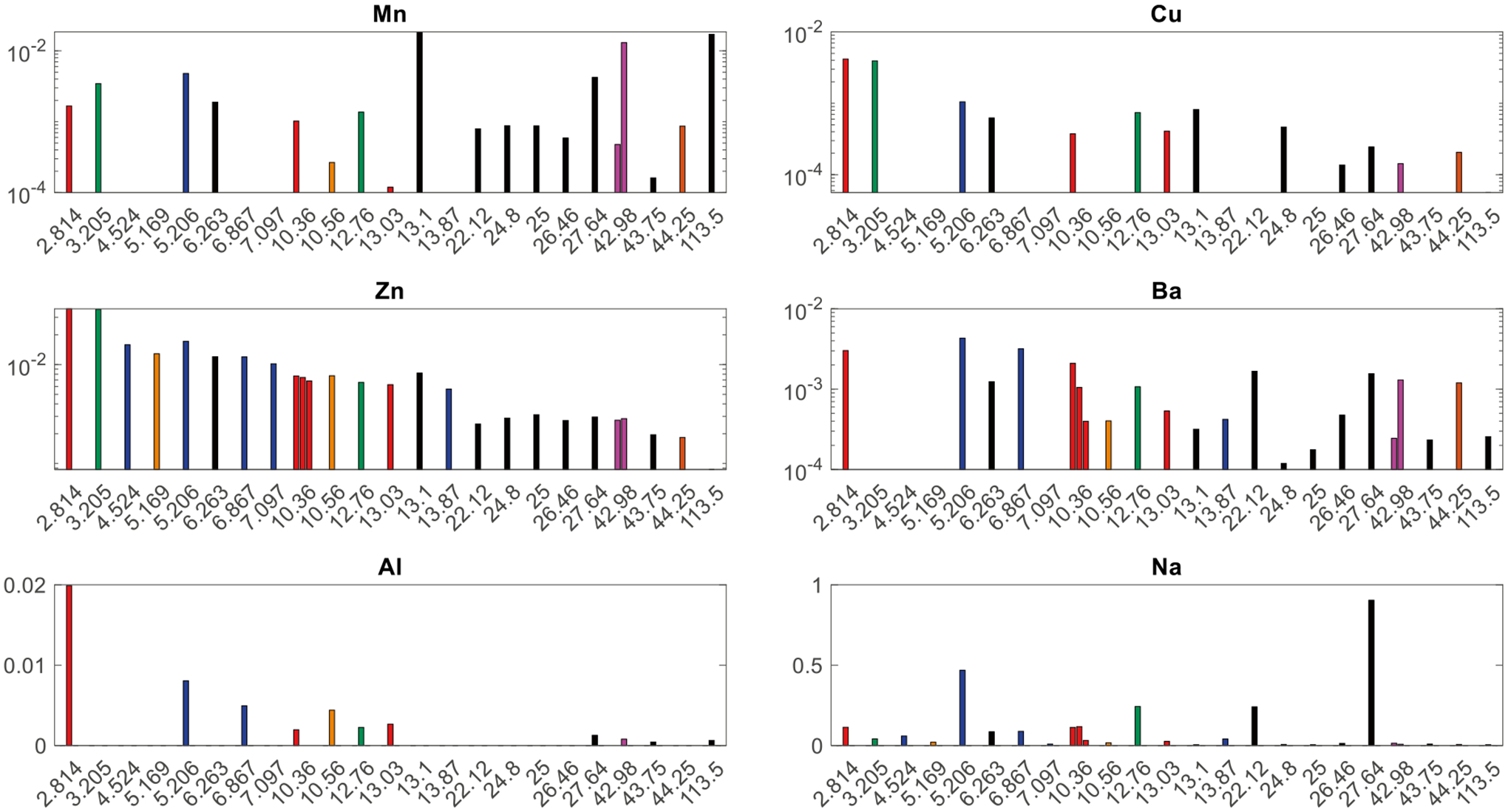
Same as Fig. 2, but for elements toxic to humans and Na. Refer to Table S1 for numerical concentration results
An abbreviated summary of cumulative probabilities (p-values) from the Kruskal-Wallis tests is given in Table 4 and the full results for all elements are provided in Tables S2–S11. No plant families were found to be statistically different from one another, with the lowest p-value of 0.44 corresponding to Mg for the Cucurbitaceae (e.g., gourd, melon) and Rosaceae (e.g. peach, blackberry) families. The results for differentiating between surface area (cm2) groups yielded statistically significant results for four of the elements—Al, Cu, Ba, and two size groups for Zn—mostly between the smallest and largest groups. Al had the only statistically significant result for concentration difference between the two counties.
Table 4.
Results of the Kruskal-Wallis test. Reporting the lowest p-value of the element analyzed per test group. Groups* (family numbers) are listed in Table 2. Groups** correspond to surface area categories of (1) 2.000–10.00 cm2, (2) 10.01–20.00 cm2, and (3) 20.01–50.00 cm2. Refer to Tables S2–S11 for a complete list of p-values from the Kruskal-Wallis tests
| Na | Mg | Al | K | Ca | Mn | Cu | Zn | Mo | Ba | |
|---|---|---|---|---|---|---|---|---|---|---|
| Families | 0.55 | 0.44 | 0.42 | 0.64 | 0.96 | 0.68 | 0.61 | 0.63 | 0.48 | 0.65 |
| Groups* | 3:14 | 4:9 | 2:4 | 2:4 | 1:4 | 2:3 | 1:3 | 1:6 | 2:3 | 6:12 |
| Sizes | 0.17 | 0.78 | 0.013 | 0.091 | 0.74 | 0.18 | 0.010 | 0.0000/0.046 | 0.68 | 0.027 |
| Groups** | 1:3 | 1:2 | 1:3 | 1:3 | 1:2 | 1:3 | 1:3 | 1:3/2:3 | 1:3 | 1:3 |
| Counties | 0.17 | 0.64 | 0.023 | 0.56 | 0.87 | 0.19 | 0.096 | 0.53 | N/A | 0.84 |
Discussion
Enrichment
The intent of this study was to build off the results of Zeider et al. (2021) and had a double focus: to determine foliar and soil contamination within industry-adjacent communities and to investigate if there was a particular factor that helped the plant samples collect more dust. The dust collected from the backyard plant leaves were more anthropogenically contaminated (enriched) than the yard and garden soils. Because the yard and garden samples collected were from the top 15 cm of soil, this could indicate that only the surface layer was enriched. This may explain why the USGS Soil Report samples were notably more enriched, since the USGS field sampling was one-third of the depth of the Gardenroots study.
The ratio of enrichment factors (Table 2) and, correspondingly, the ratio of foliar to yard concentrations help to determine local versus non-local sources of dust. Local and transported soils will have different particle sizes, with larger particles coming from closer sources. Local movement is recorded in meters from a sampling site (Csavina et al., 2012) and is usually from within the same region/state where the sample is taken, whereas transported soils come from adjacent to far-off states and are recorded in kilometers (Miller et al., 2021). When dust is transported, the larger particles settle out, and there is a smaller size distribution at the sample site. This is a general trend that is observed at an increasing rate (i.e., more large particles settle out) with increased distance from the dust source. As an air mass with aerosol particles moves from a source to another location, it can be diluted in the atmosphere by mixing with surrounding, cleaner air. Therefore, the concentrations of the samples can be much lower and less contaminated than dust coming from a neighbor’s house. Calcium, Mg, and K have higher concentrations and a higher ratio of EFs compared to the toxic elements, indicating that they are more likely transported from nearby soils and not from a long-range source.
This is further supported by Table 3, which shows that this study’s EF values are not far off from the Ca, Mg, and K EF values from the USGS samples. Moreover, the toxic elements were present in lower concentrations than Ca, Mg, and K and had smaller foliar to yard EF ratios, indicating that these elements likely came from long-range sources. Transport from local sources could account for some of the range in differences (−3800 to −27,000) between this study and the USGS data for Mn, Cu, Zn, and Ba. Additionally, the USGS study collected soil only to a depth of 5 cm, whereas this study went to a depth of 15 cm, where topsoil enrichment from could be diluted by deeper, less enriched soil.
There is a general trend among both the nutrients and toxic elements that element concentration decreases as leaf size increases. This could be because smaller surfaces are better at concentrating objects than larger ones due to particle agglomeration and removal forces (Hinds, 1999), or the density of trichomes and stomata for plants (Watts & Kariyat, 2021). There are some data points that do not fit within the trend, which could indicate that certain elements may have additional characteristics that influence their depository and adhesive tendencies.
Influential factors
The results of the Kruskal-Wallis tests indicate that surface area size is a better differentiating factor than plant family or county of origin. Additionally, there was general agreement that the smallest surface area group of 2.000–10.00 cm2 had the most statistically different concentrations than the 20.01−50.00 cm2 group. One explanation for this could be a phenomenon mentioned earlier: that smaller surfaces are better at collecting and concentrating objects. Smaller surfaces collect more aerosol particles than larger surfaces due to particle agglomeration and the associated removal force. Leaves may act in a similar capacity. Given two freshly washed leaves, one small and one large, they collect the same size and number of particles. However, the particles on the smaller leaf are closer together, so more likely to agglomerate and create a stronger adhesive force to the surface. Meanwhile, the particles on the larger leaf are farther apart and are more likely to be re-suspended or re-entrained. Another explanation for this could be plant physiology, namely trichomes and stomata. Trichomes, which are bristle-like hairs on plant leaves, and stomata, or small pores on the leaves and stalks of plants, can also aid in retention ability. These plant features, which primarily help to avoid excess transpiration and regulate the flow of gases in an out of plants, could trap smaller particles and prevent them from leaving the leaf surface.
Within plant families and counties, there is a wide range of leaf sizes, which may explain why there are very few concentrations that are statistically different from each other for those groups. More contaminated dust is associated with higher EF, which could also explain the significant difference in EF values of foliar to yard samples in Table 2.
Limitations
There are some limitations with this study design. This study did not have a control leaf (uncontaminated) sample, since, due to the community science approach, it was challenging to know what plants would be submitted. Based on Zeider et al. (2021) and this work, future efforts will include a control plant from a greenhouse study and standardizing the plant species and age and including multiple replicates of the same plant species across partnering communities. There are small changes that can be made for sample preparation too; for example, one could rinse out the bag the leaf was transported in, and after light agitation, one could then fully wash the leaf surfaces. This would provide more confidence in the reported numbers. There are also several potentially confounding factors, such as weather, shading, and method of collection, that could influence dust collection. With respect to weather, researchers could perform the study in a different time of year, although the original collection period of September to October had very little rain. The investigators could also request that participants check the daily forecast and harvest leaves before any rain or high winds. However, it was found that the period of collection (even with some rain) did not influence the overall trend of the data, nor did it greatly affect the relative concentrations of each analyzed element. Shading or plants being separated from overhanging trees and shedding plants is another factor linked to the study design that could be modified. However, based on the community science approach, these were not plants that the participants were given—these were plants they already had in their backyards and gardens. Regarding the method of collection, although participants may have collected samples in different ways, it has been shown that there are no significant differences between community and “expert” collection (Aceves-Bueno et al., 2017; Bowser et al., 2020; Danielsen et al., 2014). Finally, 27 foliar samples are a small quantity, compared to how many individuals were trained to collect leaves. That was part of the intention of utilizing the Kruskal-Wallis test, which does well with small sample sets in determining statistical significance.
Conclusions
This study examined community contamination via foliar dust and yard and garden soil samples, as well as determining what influences foliar collection. It built upon previous studies (Ramírez-Andreotta et al., 2013a, b, 2015; Zeider et al., 2021) in which plant leaves (foliar) were statistically shown to be reliable air quality monitors in a mining-adjacent community. The major goal of this study was to understand additional contributing factors to leaf collection efficacy. Future work would involve modifying the original plant air monitor using the results of this study to further improve dust collection in fenceline communities.
The EF results indicate that foliar dust was moderately or significantly contaminated for many of the measured elements, with lower contamination values for associated yard and garden soils. Additionally, based on magnitude of element concentration and the ratio of foliar to yard EF values, it was determined that toxic elements were likely to have long-range sources whereas nutritional elements were locally sourced. This is supported by the USGS Soil Report, which had similar EF magnitudes for nutrients and significantly different magnitudes for toxic elements. Based on the Kruskal-Wallis test, it was determined that leaf surface area had more of an influence on leaf collection than plant family or county location.
Given that there was contamination in both Cochise and Greenlee counties and leaf surface area influences leaf collection efficacy, this study should be repeated but with some study design elements amended, as highlighted in the “Limitations” section. For example, one change would be to reduce the number of plant types examined, but increase the quantity and include multiple replicates of the same plant species across partnering communities. In that case, the influence of the location may be clearer and there would be fewer plant families to compare. However, what a community scientist is growing/what is present is their backyards is a limiting factor in this design modification. As with this study and Gardenroots efforts overall, emphasis is placed on co-design and community participation in the research process and to inform residents of their environmental quality. In addition, and specific to this study, efforts were co-designed to determine the efficacy of foliar dust as a dust and aerosol pollution monitor and improve upon the understanding of leaf collection and air quality monitoring efficacy, which was determined in Zeider et al. (2021).
Supplementary Material
Acknowledgements
Massive and special thank you to all the Gardenroots participants and the following University of Arizona Cooperative Extension County offices: Cochise (Susan Pater, Josh Sherman), Greenlee (Kim McReynolds, Bill Cook), and Apache (Mike Hauser). We would like to thank Shana Sandhaus, Jon Mainhagu, and Mely Bohlman for support which enabled this research and our colleagues in the Integrated Environmental Science and Health Risk Laboratory for their support throughout this process.
Funding
We would like to thank the National Institute of Environmental Health Sciences Superfund Research Program (P42ES04940) and the Center for Environmentally Sustainable Mining initiated by the Water, Environmental, and Energy Solutions—Technology Research Initiative Fund Water Sustainability Program.
Footnotes
Competing interests The authors declare no competing interests.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10661-023-11752-2.
Contributor Information
Kira Zeider, Department of Chemical and Environmental Engineering, University of Arizona, Tucson, AZ, USA.
Iliana Manjón, Department of Environmental Science, University of Arizona, Tucson, AZ, USA.
Eric A. Betterton, Department of Hydrology and Atmospheric Sciences, University of Arizona, 1177 E Fourth Street, Rm. 429, Tucson, AZ 85721, USA
A. Eduardo Sáez, Department of Chemical and Environmental Engineering, University of Arizona, Tucson, AZ, USA.
Armin Sorooshian, Department of Chemical and Environmental Engineering, University of Arizona, Tucson, AZ, USA; Department of Hydrology and Atmospheric Sciences, University of Arizona, 1177 E Fourth Street, Rm. 429, Tucson, AZ 85721, USA.
Mónica D. Ramírez-Andreotta, Department of Environmental Science, University of Arizona, Tucson, AZ, USA Mel and Enid Zuckerman College of Public Health’s Division of Community, Environment & Policy, University of Arizona, Tucson, AZ, USA.
Data availability
Select datasets generated during and/or analyzed during the current study are available in the Supplemental Materials. The remaining datasets can be requested from the corresponding author. The corresponding author is working to make the data available in a public repository.
References
- Aceves-Bueno E, Adeleye AS, Feraud M, Huang Y, Tao M, Yang Y, & Anderson SE (2017). The Accuracy of citizen science data: A quantitative review. The Bulletin of the Ecological Society of America, 98(4), 278–290. 10.1002/bes2.1336 [DOI] [Google Scholar]
- Alloway BJ (1995). The origins of heavy metals in soils. In Alloway BJ (Ed.), Heavy metals in soils (p. 368). Blackie Academic and Professional Publ. 10.1007/978-94-011-1344-1 [DOI] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). (2004). Lead, manganese, zinc, copper. Retrieved from https://www.atsdr.cdc.gov/interactionprofiles/ip06.html [PubMed]
- Avila PF, da Silva EF, & Candeias C (2017). Health risk assessment through consumption of vegetables rich in heavy metals: The case study of the surrounding villages from Panasqueira mine. Central Portugal Environ Geochem Health, 39(3), 565–589. 10.1007/s10653-016-9834-0 [DOI] [PubMed] [Google Scholar]
- Bemis WP, Curtis LD, Weber CW, & Berry J (1978). The feral buffalo gourd, Cucurbita foetidissima. Economic Botany, 32, 87–95. [Google Scholar]
- Bian B, Zhou LJ, Li L, Lv L, & Fan YM (2015). Risk assessment of heavy metals in air, water, vegetables, grains, and related soils irrigated with biogas slurry in Taihu Basin, China. Environmental Science and Pollution Research. 10.1007/s11356-015-4292-2 [DOI] [PubMed] [Google Scholar]
- Brand E, Otte PF, Lijzen JPA (2007). CSOIL 2000 An exposure model for human risk assessment of soil contamination. A model description. Accessed from https://www.rivm.nl/bibliotheek/rapporten/711701054.html. Accessed 26 Sept 2022 [Google Scholar]
- Braveman P (2014). What are health disparities and health equity? We need to be clear. Public Health Reports, 129(suppl 2), 5–8. 10.1177/00333549141291S203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser A, Cooper C, de Sherbinin A, Wiggins A, Brenton P, Chuang T-R, Faustman E, Haklay M, & (Muki), & Meloche M (2020). Still in need of norms: The state of the data in citizen science. Citizen Science: Theory and Practice, 5(1), 18. 10.5334/cstp.303 [DOI] [Google Scholar]
- Bullard RD (2008). Dumping in Dixie: Race, class, and environmental quality (3rd ed.). Routledge. [Google Scholar]
- Burwell-Naney K, Wilson SM, Whitlock ST, & Puett R (2019). Hybrid resiliency-stressor conceptual framework for informing decision support tools and addressing environmental injustice and health inequities. International Journal of Environmental Research and Public Health, 16(8), 1466. 10.3390/ijerph16081466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho LM, Gutierrez W, Alarcon-Herrera MT, Villalba MD, & Deng SG (2011). Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and southwestern USA. Chemosphere, 83(3), 211–225. 10.1016/j.chemosphere.2010.12.067 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2020). Mine operator employees. Retrieved from https://wwwn.cdc.gov/NIOSH-Mining/MMWC/EmployeeMap.
- Charles G, Rossignol L, & Rossignol M (1992). Environmental effects on potato plants in vitro. Journal of Plant Physiology, 139(6), 708–713. 10.1016/S0176-1617(11)81715-3 [DOI] [Google Scholar]
- Cobb GP, Sands K, Waters M, Wixson BG, & Dor-ward-King E (2000). Accumulation of heavy metals by vegetables grown in mine wastes. Environmental Toxicology and Chemistry: An International Journal, 19(3), 600–607. 10.1002/etc.5620190311 [DOI] [Google Scholar]
- Corriveau MC, Jamieson HE, Parsons MB, Campbell JL, & Lanzirotti A (2011). Direct characterization of airborne particles associated with arsenic-rich mine tailings: Particle size, mineralogy and texture. Applied Geochemistry, 26(9−10), 1639–1648. 10.1016/j.apgeochem.2011.04.021 [DOI] [Google Scholar]
- Csavina J, Field J, Taylor MP, Gao S, Landázuri A, Betterton EA, & Sáez AE (2012). A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Science of the Total Environment, 433, 58–73. 10.1016/j.scitotenv.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csavina J, Taylor MP, Felix O, Rine KP, Saez AE, & Betterton EA (2014). Size-resolved dust and aerosol contaminants associated with copper and lead smelting emissions: implications for emission management and human health. Science of the Total Environment, 493, 750–756. 10.1016/j.scitotenv.2014.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen F, Jensen PM, Burgess ND, Altamirano R, Alviola PA, Andrianandrasana H, Brashares JS, Burton AC, Coronado I, Corpuz N, Enghoff M, Fjeldså J, Funder M, Holt S, Hübertz H, Jensen AE, Lewis R, Massao J, Mendoza MM, et al. (2014). A multicountry assessment of tropical resource monitoring by local communities. BioScience, 64(3), 236–251. 10.1093/biosci/biu001 [DOI] [Google Scholar]
- Dehigaspitiya DDPBD, Mayuri Dhananjanie AG, Dahanayake N, Atapattu AGKMWS, & Perera PCD (2016). Identification of the effect of sulfur spray on okra (abelmoschus esculentus L. var MI 5) seedlings. Journal of AgriSearch, 3(4), 217–219. 10.21921/jas.v3i4.6704 [DOI] [Google Scholar]
- Earth Observatory. (n.d.). Shrubland. Retrieved from https://earthobservatory.nasa.gov/biome/bioshrubland.php
- Gajbhiye T, Pandey SK, Kim KH, Szulejko JE, & Prasad S (2016). Airborne foliar transfer of PM bound heavy metals in Cassia siamea: a less common route of heavy metal accumulation. Science of the Total Environment, 573, 123–130. 10.1016/j.scitotenv.2016.08.099 [DOI] [PubMed] [Google Scholar]
- Gee GC, Hing A, Mohammed S, Tabor DC, & Williams DR (2019). Racism and the life course: taking time seriously. American Journal of Public Health, 109(S1), S43–S47. 10.2105/AJPH.2018.304766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, & Payne-Sturges DC (2004). Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environmental Health Perspectives, 112(17), 1645–1653. 10.1289/ehp.7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt VM (1937). The principles of distribution of chemical elements in minerals and rocks. The seventh hugo müller lecture, delivered before the chemical society on March 17th, 1937. Journal of the Chemical Society (Resumed), 655–673. 10.1039/JR9370000655 [DOI] [Google Scholar]
- Hinds WC (1999). Aerosol technology: properties, behavior, and measurement of airborne particles. John Wiley & Sons. [Google Scholar]
- Hoffman D, McPheters L, Rex T (2011). Florence copper project: Economic impact study. Retrieved from https://www.florencecopper.com/assets/docs/reports/florence-copper-project-final-report-42612.pdf.
- Hyde P, Mahalov A, & Li J (2018). Simulating the meteorology and PM10 concentrations in Arizona dust storms using the Weather Research and Forecasting model with Chemistry (Wrf-Chem). Journal of the Air & Waste Management Association, 68(3), 177–195. 10.1080/10962247.2017.1357662 [DOI] [PubMed] [Google Scholar]
- Johnson MS, Cooke JA, & Stevenson JKW (1994). Revegetation of metalliferous wastes and land after metal mining. In Hester RE & Harrison RM (Eds.), Mining and Its Environmental Impact (p. 164). Royal Society of Chemistry. 10.1039/9781847551467-00031 [DOI] [Google Scholar]
- Jung MC (2001). Heavy metal contamination of soils and waters in and around the Imcheon Au–Ag mine, Korea. Applied Geochemistry, 16(11–12), 1369–1375. 10.1016/S0883-2927(01)00040-3 [DOI] [Google Scholar]
- Lee J-S, Chon H-T, & Kim K-W (2005). Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environmental Geochemistry and Health, 27, 185–191. 10.1007/s10653-005-0131-6 [DOI] [PubMed] [Google Scholar]
- Li H, Wang J, Wang Q, Qian X, Qian Y, Yang M, Li F, Lu H, & Wang C (2015). Chemical fractionation of arsenic and heavy metals in fine particle matter and its implications for risk assessment: A case study in Nanjing, China. Atmospheric Environment, 103, 339–346. 10.1016/j.atmosenv.2014.12.065 [DOI] [Google Scholar]
- Liu CL, Zhang J, & Shen ZB (2002). Spatial and temporal variability of trace metals in aerosol from the desert region of China and the Yellow Sea. Journal of Geophysical Research: Atmospheres, 107(D14). 10.1029/2001JD000635 [DOI] [Google Scholar]
- MacDonald GM (2010). Water, climate change, and sustainability in the southwest. Proceedings of the National Academy of Sciences, 107(50), 21256–21262. 10.1073/pnas.0909651107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjón I, & Ramírez-Andreotta MD (2020). A dietary assessment tool to estimate arsenic and cadmium exposures from locally grown foods. Environmental Geochemistry and Health, 42(7), 2121–2135. 10.1007/s10653-019-00486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjón I, Ramírez-Andreotta MD, Sáez AE, Root RA, Hild J, Janes MK, & Alexander-Ozinskas A (2019). Ingestion and inhalation of metal(loid)s through preschool gardening: an exposure and risk assessment in legacy mining communities. Science of the Total Environment, 718, 134639. 10.1016/j.scitotenv.2019.134639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricopa County Air Quality Department. (2017). 2014 periodic emissions inventory for PM10 for the Maricopa County, Arizona, PM10 nonattainment area. Accessed from https://www.maricopa.gov/DocumentCenter/View/20711/2014-Periodic-Emissions-Inventory-PM10-PDF. Accessed 26 Sept 2022
- Marmot M, & Allen JJ (2014). Social determinants of health equity. American Journal of Public Health, 104(suppl4), S517–S519. 10.2105/AJPH.2014.302200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Figueroa D, Maier RM, & de la O-Villanueva M, Gómez-Alvarez A, Moreno-Zazueta A, Rivera J, Campillo A, Grandlic CJ, Anaya R, Palafox-Reyes J (2009). The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere, 77(1), 140–147. 10.1016/j.chemosphere.2009.04.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, McFarquhar GM, Rauber RM, O’Brien JR, Gupta S, Segal-Rozenhaimer M, Dobracki AN, Sedlacek AJ, Burton SP, Howell SG, Freitag S, & Dang C (2021). Observations of supermicron-sized aerosols originating from biomass burning in South Central Africa. Atmospheric Chemistry and Physics, 21(19), 14815–14831. 10.5194/acp-21-14815-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle-Gro. (2021). Americans are growing more than ever. Retrieved from https://www.miraclegro.com/en-us/library/americans-are-growing-more-ever.
- Murray H, Thompson K, & Macfie SM (2009). Site-and species-specific patterns of metals bioavailability in edible plants. Botany, 87, 702–711. 10.1139/B09-019 [DOI] [Google Scholar]
- NASEM (National Academies of Sciences, Engineering, and Medicine). (2017). Health and Medicine Division; Board on Population Health and Public Health Practice. In Baciu A, Negussie Y, Geller A, & Weinstein JN (Eds.), Communities in action: Pathways to health equity. National Academies Press. [PubMed] [Google Scholar]
- National Gardening Association. (2014). Garden to table: A 5-year look at food gardening in America. https://garden.org/special/pdf/2014-NGA-Garden-to-Table.pdf.
- National Gardening Association. (2021). National gardening survey 2021 Edition. Retrieved from https://gardenresearch.com/view/national-gardening-survey-2021-edition/.
- Navarro MC, Perez-Sirvent C, Martinez-Sanchez MJ, Vidal J, Tovar PJ, & Bech J (2008). Abandoned mine sites as a source of contamination by heavymetals: a case study in a semi-arid zone. Journal of Geochemical Exploration, 96(2–3), 183–193. 10.1016/j.gexplo.2007.04.011 [DOI] [Google Scholar]
- Ramírez-Andreotta MD, Brusseau ML, Artiola J, & Maier RM (2013a). A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Science of the Total Environment, 443, 299–306. 10.1016/j.scitotenv.2012.10.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Andreotta MD, Brusseau ML, Beamer P, & Maier RM (2013b). Home gardening near a mining site in an arsenic-endemic region of Arizona: assessing arsenic exposure dose and risk via ingestion of home garden vegetables, soils, and water. Science of the Total Environment, 454–455, 373–382. 10.1016/j.scitotenv.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Andreotta MD, Brusseau ML, Artiola J, Maier RM, & Gandolfi AJ (2015). Building a co-created citizen science program with gardeners neighboring a superfund site: The Gardenroots case study. International Public Health Journal, 7(1), 13 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4420190/ [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Andreotta MD, Walls RL, Youens-Clark K, Blumberg K, Isaacs K, Kaufmann DB, & Maier RM (2021). Alleviating environmental health disparities through community science and data integration. Frontiers in Sustainable Food Systems , 5 https://www.frontiersin.org/article/10.3389/fsufs.2021.620470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhaus S, Kaufmann D, & Ramírez-Andreotta MD (2019). Public participation, trust and data sharing: gardens as hubs for citizen science and environmental health literacy efforts. International Journal of Science Education, Part B, 9(1), 54–71. 10.1080/21548455.2018.1542752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra Research, & Arizona Department of Environmental Quality. (2014). Appendix B: Pinal County PM10 nonattainment area emissions inventories for 2008 and 2018 base years and design days.
- Schulz AJ, Mentz GB, Sampson N, Ward M, Anderson R, et al. (2016). Race and the distribution of social and physical environmental risk: a case example from the Detroit metropolitan area. Du Bois Review, 13(2), 285–304. 10.1017/S1742058X16000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Cannon WF, Woodruff LG, Solano F, Kil-burn JE, Fey DL (2013). Geochemical and mineralogical data for soils of the conterminous United States: U.S. Geological Survey Data Series 801, p. 19. https://pubs.usgs.gov/ds/801/.
- Stovern M, Guzmán H, Rine KP, Felix O, King M, Ela WP, Betterton EA, & Sáez AE (2016). Windblown dust deposition forecasting and spread of contamination around mine tailings. Atmosphere, 7(2), 16. 10.3390/atmos7020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi SN, Kamani H, Dehghani MH, Nabizadeh R, Afshari N, & Mahvi AM (2019). Assessment of heavy metals in street dusts of Tehran using enrichment factor and geo-accumulation index. Health Scope, 8(1), e57879. 10.5812/jhealthscope.57879 [DOI] [Google Scholar]
- U.S. Census Bureau. (2020a). Census Redistricting Data (Public Law 94–171). Retrieved from https://data.census.gov/cedsci/table?q=apache%20county,%20arizona.
- U.S. Census Bureau. (2020b). Census Redistricting Data (Public Law 94–171). Retrieved from https://data.census.gov/cedsci/table?q=cochise%20county,%20arizona.
- U.S. Census Bureau. (2020c). Census Redistricting Data (Public Law 94–171). Retrieved from https://data.census.gov/cedsci/table?q=greenlee%20county,%20arizona.
- Watts S, & Kariyat R (2021). Morphological characterization of trichomes shows enormous variation in shape, density and dimensions across the leaves of 14 Solanum species. AoB PLANTS, 13(6). 10.1093/aobpla/plab071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, & Brown PH (2010). Plant nutrition for sustainable development and global health. Annals of botany, 105(7), 1073–1080. 10.1093/aob/mcq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2021). Noncommunicable diseases. Retrieved from https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases.
- Xing Y-F, Xu Y-H, Shi M-H, & Lian Y-X (2016). The impact of PM2.5 on the human respiratory system. Journal of Thoracic Disease, 8(1), E69–E74. 10.3978/j.issn.2072-1439.2016.01.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Teng YG, Song LT, & Zuo R (2016). Tracing sources and contamination assessments of heavy metals in road and foliar dusts in a typical Mining City, China. PLo-SOne, 11(12), e0168528. 10.1371/journal.pone.0168528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeider K, Van Overmeiren N, Rine KP, Sandhaus S, Eduardo SA, Sorooshian A, Munoz HC, & Ramirez-Andreotta MD (2021). Foliar surfaces as dust and aerosol pollution monitors: An assessment by a mining site. Science of the Total Environment, 790(148164). 10.1016/j.scitotenv.2021.148164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Select datasets generated during and/or analyzed during the current study are available in the Supplemental Materials. The remaining datasets can be requested from the corresponding author. The corresponding author is working to make the data available in a public repository.


