Abstract
Background
Recently, several new treatment regimens have been approved for treating metastatic hormone-sensitive prostate cancer, building on androgen deprivation therapy alone. These include docetaxel androgen deprivation therapy, abiraterone acetate-prednisone androgen deprivation therapy, apalutamide androgen deprivation therapy, enzalutamide androgen deprivation therapy, darolutamide-docetaxel androgen deprivation therapy, and abiraterone-prednisone androgen deprivation therapy with docetaxel. There are no validated predictive biomarkers for choosing a specific regimen. The goal of this study was to conduct a health economic outcome evaluation to determine the optimal treatment from the US public sector (Veterans Affairs).
Methods
We developed a partitioned survival model in which metastatic hormone-sensitive prostate cancer patients transitioned between 3 health states (progression free, progressive disease to castrate resistance state, and death) at monthly intervals based on Weibull survival model estimated from published Kaplan–Meier curves using a Bayesian network meta-analysis of 7 clinical trials (7208 patients). The effectiveness outcome in our model was quality-adjusted life-years (QALYs). Cost input parameters included initial and subsequent treatment costs and costs for terminal care and for managing grade 3 or higher drug-related adverse events and were obtained from the Federal Supply Schedule and published literature.
Results
Average 10-year costs ranged from $34 349 (androgen deprivation therapy) to $658 928 (darolutamide-docetaxel androgen deprivation therapy) and mean QALYs ranged from 3.25 (androgen deprivation therapy) to 4.57 (enzalutamide androgen deprivation therapy). Treatment strategies docetaxel androgen deprivation therapy, enzalutamide androgen deprivation therapy docetaxel, apalutamide androgen deprivation therapy, and darolutamide-docetaxel androgen deprivation therapy were eliminated because of dominance (ie, they were more costly and less effective than other strategies). Of the remaining strategies, abiraterone acetate-prednisone androgen deprivation therapy was the most cost-effective strategy at a willingness-to-pay threshold of $100 000/QALY (incremental cost-effectiveness ratios = $21 247/QALY).
Conclusions
Our simulation model found abiraterone acetate-prednisone androgen deprivation therapy to be an optimal first-line treatment for metastatic hormone-sensitive prostate cancer from a public (Veterans Affairs) payer perspective.
Prostate cancer accounted for more than 34 000 deaths in US males (1) and 325 000 deaths world-wide (2) in 2021. Since 2015, substantial progress has been made in the therapeutic landscape of metastatic hormone-sensitive prostate cancer starting with the publication of the ChemoHormonal Therapy versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) study results (3). This trial’s results re-established a new standard of care for treating metastatic hormone-sensitive prostate cancer after decades of androgen deprivation therapy alone. Combination docetaxel chemotherapy and androgen deprivation therapy in CHAARTED improved overall survival and slowed progression to metastatic castrate-resistant prostate cancer, a cancer progressive state that occurs after metastatic hormone-sensitive prostate cancer. Subsequent studies using combinations of novel hormonal therapeutic drugs with androgen deprivation therapy in metastatic hormone-sensitive prostate cancer have reported similar benefit for prolonging survival and slowing down progression to metastatic castrate-resistant prostate cancer state for combination strategies compared with androgen deprivation therapy alone. Clinical outcomes for abiraterone acetate-prednisone with androgen deprivation therapy (4-6), apalutamide with androgen deprivation therapy (7), and enzalutamide with androgen deprivation therapy (8) compared with androgen deprivation therapy alone have consistently favored combination therapies. Recently, triple combinations of darolutamide with androgen deprivation therapy and docetaxel chemotherapy compared with androgen deprivation therapy alone (9), abiraterone acetate-prednisone with docetaxel chemotherapy and androgen deprivation therapy compared with androgen deprivation therapy alone (10) and enzalutamide with docetaxel and androgen deprivation therapy compared with androgen deprivation therapy (11) have shown similar benefits for combination therapies. Combination drug therapies are now the preferred interventions over androgen deprivation therapy alone in metastatic hormone-sensitive prostate cancer (12,13).
Despite emergence of combination treatment regimens, there is inevitable universal progression to metastatic castrate-resistant prostate cancer and death (14). Combination treatments also increase drug-related adverse events and toxicities compared with androgen deprivation therapy alone. There are also no validated predictive biomarkers to choose a particular combination for preferential use over the other.
We conducted a cost-effectiveness analysis of all currently approved drug combinations in metastatic hormone-sensitive prostate cancer using data from randomized clinical trials to determine which current treatment regimen is the optimal approach to enhance quality of life without compromising on longevity of life. All randomized trials used in this analysis combined androgen deprivation therapy with either novel hormonal agents or with docetaxel or combined all 3 drug therapeutics. The comparator study arm was androgen deprivation therapy alone, except in 1 study (Prostate Cancer Consortium in Europe-1 [PEACE-1] study) (10) in which the triplet of novel hormonal drug agent abiraterone acetate-prednisone was combined with androgen deprivation therapy and docetaxel and was compared with abiraterone acetate-prednisone plus androgen deprivation therapy. Based on the overwhelming use of androgen deprivation therapy alone as the comparator arm, we used androgen deprivation therapy as a reference for conducting the cost-effectiveness analysis.
Methods
Study design overview
A decision analytic model was developed to illustrate the treatment option of patients diagnosed with metastatic hormone-sensitive prostate cancer using costs from the Department of Veterans Affairs (VA) taking a US public perspective. We estimated the cost and effectiveness of the 7 approved treatment strategies used for metastatic hormone-sensitive prostate cancer management: 1) docetaxel with androgen deprivation therapy (3); 2) abiraterone acetate-prednisone with androgen deprivation therapy (4,5); 3) apalutamide with androgen deprivation therapy (7); 4) enzalutamide with androgen deprivation therapy (8); 5) darolutamide with androgen deprivation therapy and docetaxel (9,15); 6) enzalutamide with androgen deprivation therapy and docetaxel (11); and 7) androgen deprivation therapy alone (see Table 1). The effectiveness measure was quality-adjusted life-years (QALYs), a measure that represents the degree to which a treatment extends life and improves quality of life. Costs included the direct costs of the drug and administration, monitoring and end-of-life, and a set of adverse drug events for each treatment. The time horizon of the study was 10 years with a cycle of 4 weeks, as the life expectancy of patients with metastatic hormone-sensitive prostate cancer rarely marks 10 years. Incremental cost-effectiveness ratios were calculated by dividing the difference in cost by the difference in effectiveness between nondominated treatment strategies. A half-cycle correction and a 3% annual discount rate were applied for both costs and effectiveness outcomes. The model was programmed in Tree-Age Pro 2023 (TreeAge Software, Williamstown, MA, USA), and Bayesian network meta-analyses were performed in R: The R Project for Statistical Computing (r-project.org).
Table 1.
List of treatment strategies in published randomized clinical trialsa
| Treatment (trial) | No. of patients | Median PFS, mo | Median OS, mo |
|---|---|---|---|
| Docetaxel with androgen deprivation therapy (CHAARTED, ENZAMET) | 646 | 25.6 | 61.0 |
| Abiraterone acetate-prednisone with androgen deprivation therapy (LATITUDE, STAMPEDE, PEACE) | 1452 | 38.2 | 58.8 |
| Apalutamide with androgen deprivation therapy (TITAN) | 525 | 37.0 | 81.5 |
| Enzalutamide with androgen deprivation therapy (ARCHES, ENZAMET) | 883 | 59.5 | 73.9 |
| Darolutamide with androgen deprivation therapy and docetaxel (ARANSENS) | 651 | 59.2 | 68.0 |
| Enzalutamide with androgen deprivation therapy and docetaxel (ENZAMET) | 254 | 43.4 | 63.3 |
| Androgen deprivation therapy (previous SOC) | 3922 | 23.4 | 48.9 |
OS = overall survival; PFS = progression-free survival.
Model structure
We constructed a decision analytic model to perform a partitioned survival analysis, which is a commonly used approach for cost-effectiveness analyses in oncology (16-18). The model included 3 health states: a progression-free, progression to metastatic castrate-resistant prostate cancer, and death (see Figure 1). The partitioned survival analysis simulates the probability that a patient is in each health state at each point in time, derived from the progression-free survival (PFS) and the overall survival (OS) curves from published clinical trials (see Table 2). All patients continued with the metastatic hormone-sensitive prostate cancer treatment strategy until disease progression to metastatic castrate-resistant prostate cancer or death. Our model performs cost analysis calculations for patients in the metastatic hormone-sensitive prostate cancer state until progression occurs based on the PFS curve estimated from the meta-analysis using published randomized clinical trials. On progression of the disease to the metastatic castrate-resistant prostate cancer state, we assumed patients would receive standard of care subsequent treatments with docetaxel (19,20), cabazitaxel (21), olaparib (22,23), lutetium-177 (24), and mitoxantrone (19,20) following the transition probabilities estimated using each of the PFS and OS curves. The duration for each metastatic castrate-resistant prostate cancer treatment was assumed to follow the median PFS of each treatment as published in the reported clinical trials that led to drug approvals in metastatic castrate-resistant prostate cancer: docetaxel for 6.3 months, cabazitaxel for 8 months, olaparib for 7.4 months, lutetium for 8.7 months, and 3.2 months for mitoxantrone.
Figure 1.
Health states with arrows depicting allowable transitions. *Input parameters including all costs, effectiveness, and utility scores are list in Table 3 and Supplementary Tables 2 and 3 (available online). mCRPC = metastatic castrate-resistant prostate cancer; mHSPC = metastatic hormone-sensitive prostate cancer.
Table 2.
Estimated parameters for Weibull distribution from network meta-analysis
| Treatment (trial) | PFSa |
OSa |
||
|---|---|---|---|---|
| Shape | Scale | Shape | Scale | |
| Docetaxel with androgen deprivation therapy (CHAARTED, ENZAMET) | 1.64 | 76.25 | 1.23 | 34.45 |
| Abiraterone Acetate-Prednisone with androgen deprivation therapy (LATITUDE, STAMPEDE, PEACE) | 1.40 | 76.47 | 1.26 | 51.13 |
| Apalutamide with androgen deprivation therapy (TITAN) | 1.27 | 108.66 | 1.46 | 47.65 |
| Enzalutamide with androgen deprivation therapy (ARCHES, ENZAMET) | 1.60 | 92.85 | 1.16 | 81.70 |
| Darolutamide with androgen deprivation therapy and docetaxel (ARANSENS) | 1.40 | 88.41 | 1.06 | 83.56 |
| Enzalutamide with androgen deprivation therapy and docetaxel (ENZAMET) | 1.92 | 76.67 | 1.72 | 53.78 |
| Androgen deprivation therapy (previous SOC) | 1.52 | 62.28 | 1.15 | 32.18 |
OS = overall survival; PFS = progression-free survival.
Drug effectiveness: model survival and progression risk estimates
Kaplan–Meier curves for OS and radiographic PFS by intervention arm were extracted using WebPlotDigitizer (25). If data on radiographic PFS were not available, then data on clinical PFS or PFS were used as a proxy. The extracted survival curves, along with corresponding numbers of events and numbers at risk over follow-up, were converted into individual patient outcome data using an approach by Guyot et al. (26). Weibull survival models (26) were fit by maximum likelihood to OS and PFS data by intervention arm, using a fixed effect maximum likelihood approach to meta-analysis (27). The fixed effect approach was used given the limited replication of treatment comparisons across included studies.
Drug toxicities
We included adverse drug events for each treatment that were grade 3 or higher reported in clinical trials. Total treatment costs for adverse drug events were obtained from published literature (2,28-37) and were applied in the first cycle of the decision model. The total treatment cost of adverse drug events reflecting frequencies for each treatment strategy is summarized in Table 3, and a full list of adverse drug events and their frequencies included are attached in Supplementary Table 1 (available online).
Table 3.
Estimated input parameters: monthly costs and utility scores
| Treatment strategy (trial) | Total cost |
Annual utility weight |
|||
|---|---|---|---|---|---|
| Monthly FSS costa | Monthly average wholesale price costa | Total costs for adverse event (Reference) | Progression free (Reference) | Progressive (Reference) | |
| Metastatic hormone-sensitive prostate cancer state | |||||
| Docetaxel with androgen deprivation therapy (CHAARTED) | $506 | $1305 | $1745 | 0.830 | 0.725 |
| (34,36,37) | (38) | (39) | |||
| Abiraterone Acetate-Prednisone with androgen deprivation therapy (LATITUDE) | $562 | $6591 | $2923 | 0.830 | 0.725 |
| (2,32-35,37) | (38) | (39) | |||
| Apalutamide with androgen deprivation therapy (TITAN) | $11 504 | $16 773 | $1755 | 0.800 | 0.630 |
| (33-36) | (40) | (40) | |||
| Enzalutamide with androgen deprivation therapy (ARCHES) | $8063 | $16 142 | $5204 | 0.839 | 0.754 |
| (31,33-35,37) | (41) | (41) | |||
| Darolutamide with androgen deprivation therapy and docetaxel (ARASENS) | $10 953 | $16 008 | $2538 | 0.830 | 0.725 |
| (28,30-32,34,35,37) | (42) | (42) | |||
| Enzalutamide with androgen deprivation therapy and docetaxel (ENZAMET) | $8415 | $16 905 | $3124 | 0.830 | 0.725 |
| (2,28-37) | (41) | (41) | |||
| Androgen deprivation therapy (Reference arm) | $154 | $542 | $6965 | 0.830 | 0.600 |
| (2,28-37) | (41) | (43) | |||
| Castrate-resistant prostate cancer state treatments | |||||
| Docetaxel (TAX327) | $352 | ||||
| Cabazitaxel (TROPIC) | $13 910 | ||||
| Olaparib (PROFOUND) | $20 147 | ||||
| Lutetium (VISION) | $23 459 | ||||
| Mitoxantrone (TAX327) | $400 | ||||
Calculations based on 90.63 kg patient. FFS = Federal Supply Schedule.
Utility inputs
QALYs were calculated in the model by weighting the time spent in health states by a utility value, a health-related quality of life that ranges from 0 (death) to 1 (perfect health). Utility scores for each treatment strategy were derived from published literature that established these treatment strategies (38-43). Among the treatment strategies, the utility values ranged from 0.800 for apalutamide with androgen deprivation therapy (40) to 0.839 for enzalutamide with androgen deprivation therapy (41) for the progression-free health state and 0.600 for androgen deprivation therapy alone (43) to 0.754 for enzalutamide with androgen deprivation therapy (41) for the progression to metastatic castrate-resistant prostate cancer health state (Table 3).
Direct drug cost
Direct drug costs of the treatment strategies for metastatic hormone-sensitive prostate cancer and metastatic castrate-resistant prostate cancer were derived from the Federal Supply Schedule (FSS) pharmaceutical pricing data. FSS provides negotiated prices between drug companies and the VA, one of the largest consumers of pharmaceuticals in the United States. Drug costs from FSS would capture the actual burden of the treatments better than the list price of the drug. All direct drug costs were estimated on the basis of a 90.63 kg patient weight. Average administration costs of $208, derived from the Center of Medicare and Medicaid Services physician fee schedule, were added to the total direct costs for parenterally administered drugs. Monthly direct costs of each treatment are summarized in Table 3, and the unit costs and dosages of each drug for the 7 treatments are summarized in Supplementary Table 2 (available online).
Monitoring, postprogression, and end-of-life costs
Costs included costs for monthly monitoring of disease state, costs for administration of parenterally administered therapies and oral therapies (44), costs for performing bone scintigraphy studies and computed tomography scans of the abdomen and pelvis with contrast, and serial prostate-specific antigen tests. Costs for mitoxantrone were also included for patients who had progression of the disease following a series of metastatic castrate-resistant prostate cancer treatments, and the cost of the final month of life was assigned at death (45). Monitoring, postprogression, and end-of-life costs are summarized in Supplementary Table 3 (available online). All costs were adjusted to 2021 US dollars using the Personal Consumption Expenditures Price Index for health-care services.
Sensitivity analyses
The primary partitioned survival analysis was conducted using the point estimates for each input parameter. To examine the sensitivity of these results to drug costs, we also analyzed our model using drug costs obtained from the average wholesale price. We also performed a one-way sensitivity analysis on the drug cost parameter to identify the threshold at which treatments that are more effective at higher costs would become cost-effective at a willingness-to-pay threshold of US$100 000/QALY. Probabilistic sensitivity analysis was performed to assess the impact of uncertainty in drug, adverse drug events, and maintenance treatment costs and utilities. Utility scores were assumed to follow a beta distribution, and cost estimates were assumed to follow a gamma distribution, each with 50% variation (46).
Results
A total of 7208 patients were included from the network meta-analysis for which the cost-effectiveness analysis was performed. Estimated median OS and PFS rates, as well as estimated Weibull parameters from the meta-analysis, are provided in Tables 1 and 2. The results from the primary partitioned survival analysis using transition probabilities determined by the estimated survival functions showed that the estimated 10-year total costs ranged from $34 349 (androgen deprivation therapy) to $658 928 (darolutamide with androgen deprivation therapy and docetaxel) (Table 4). Estimated 10-year QALYs were highest for enzalutamide with androgen deprivation therapy (QALY = 4.57) and the lowest for androgen deprivation therapy (QALY = 3.25). Docetaxel with androgen deprivation therapy, enzalutamide with androgen deprivation therapy and docetaxel, apalutamide with androgen deprivation therapy, and darolutamide with androgen deprivation therapy and docetaxel were eliminated from consideration because they were less effective and more costly than either androgen deprivation therapy, abiraterone acetate-prednisone with androgen deprivation therapy, or enzalutamide with androgen deprivation therapy (dominance) or their linear combination (extended dominance). The incremental cost-effectiveness ratio for abiraterone acetate-prednisone with androgen deprivation therapy compared with androgen deprivation therapy was $21 247/QALY, and hence, abiraterone acetate-prednisone with androgen deprivation therapy was the most cost-effective treatment strategy for metastatic hormone-sensitive prostate cancer at a willingness-to-pay threshold of $100 000/QALY.
Table 4.
Results of base case cost-effectiveness analyses stratified by perspective
| Treatment strategy | Cost (2021 US$) | Effectiveness (QALYs) | Incremental cost-effectiveness ratios (US$/QALY) |
|---|---|---|---|
| Veterans Affairs perspective | |||
| Androgen deprivation therapy alone | 34 349 | 3.25 | |
| Abiraterone acetate-prednisone with androgen deprivation therapy | 46 560 | 3.83 | 21 247 |
| Enzalutamide with androgen deprivation therapy | 488 445 | 4.57 | 597 089 |
| Docetaxel with androgen deprivation therapy | 48 471 | 3.79 | Dominateda |
| Enzalutamide with androgen deprivation therapy and docetaxel | 402 930 | 3.99 | Dominatedb |
| Apalutamide with androgen deprivation therapy | 483 540 | 4.16 | Dominatedb |
| Darolutamide with androgen deprivation therapy and docetaxel | 658 928 | 4.29 | Dominateda |
| Average wholesale price perspective | |||
| Androgen deprivation therapy alone | 45 439 | 3.25 | |
| Docetaxel with androgen deprivation therapy | 72 471 | 3.79 | 50 389 |
| Enzalutamide with androgen deprivation therapy | 955 785 | 4.57 | 1 134 913 |
| Abiraterone acetate-prednisone with androgen deprivation therapy | 301 030 | 3.83 | Dominatedb |
| Apalutamide with androgen deprivation therapy | 692 614 | 4.16 | Dominatedb |
| Enzalutamide with androgen deprivation therapy and docetaxel | 777 484 | 3.99 | Dominateda |
| Darolutamide with androgen deprivation therapy and docetaxel | 950 169 | 4.29 | Dominatedb |
This treatment strategy is more costly and less effective than another treatment strategy (ie, absolute dominance). QALYs = quality-adjusted life-years.
This treatment strategy is more costly and less effective than a linear combination of other treatment strategies (ie, extended dominance).
One-way sensitivity analysis on the drug cost for enzalutamide showed that enzalutamide with androgen deprivation therapy became the most cost-effective treatment strategy at willingness-to-pay of $100 000/QALY if its monthly price is reduced from $7909 to $1550 (Supplementary Figure 1.1, available online). Docetaxel with androgen deprivation therapy was eliminated from consideration because its 10-year total QALY (3.79) was lower at a higher cost ($48 471) than abiraterone acetate-prednisone with androgen deprivation therapy (QALY = 3.83; $46 560), however, docetaxel with androgen deprivation therapy could become a cost-effective option if the drug and administration cost for docetaxel decreased from $352 to $161 while all other input parameters remained the same (Supplementary Figure 1.2, available online). Finally, when considering a range of costs for androgen deprivation therapy ($0-$1500), docetaxel with androgen deprivation therapy becomes the most cost-effective treatment strategy at a willingness-to-pay of $100 000/QALY if the drug cost of androgen deprivation therapy is $626, which is considerably higher than its baseline value ($154) (Supplementary Figure 1.3, available online).
In models using average wholesale price for drug costs, docetaxel with androgen deprivation therapy was the most cost-effective strategy with an incremental cost-effectiveness ratio of $50 389/QALY compared with androgen deprivation therapy (Table 4). All other treatment strategies including abiraterone acetate-prednisone with androgen deprivation therapy were eliminated from consideration because they were less effective and more costly than androgen deprivation therapy, docetaxel with androgen deprivation therapy, enzalutamide with androgen deprivation therapy, or their linear combination. In one-way sensitivity analyses, the monthly cost of abiraterone acetate-prednisone would need to decrease from $6049 to $724 for abiraterone acetate-prednisone with androgen deprivation therapy to be the most cost-effective treatment strategy compared with androgen deprivation therapy (Supplementary Figure 2, available online).
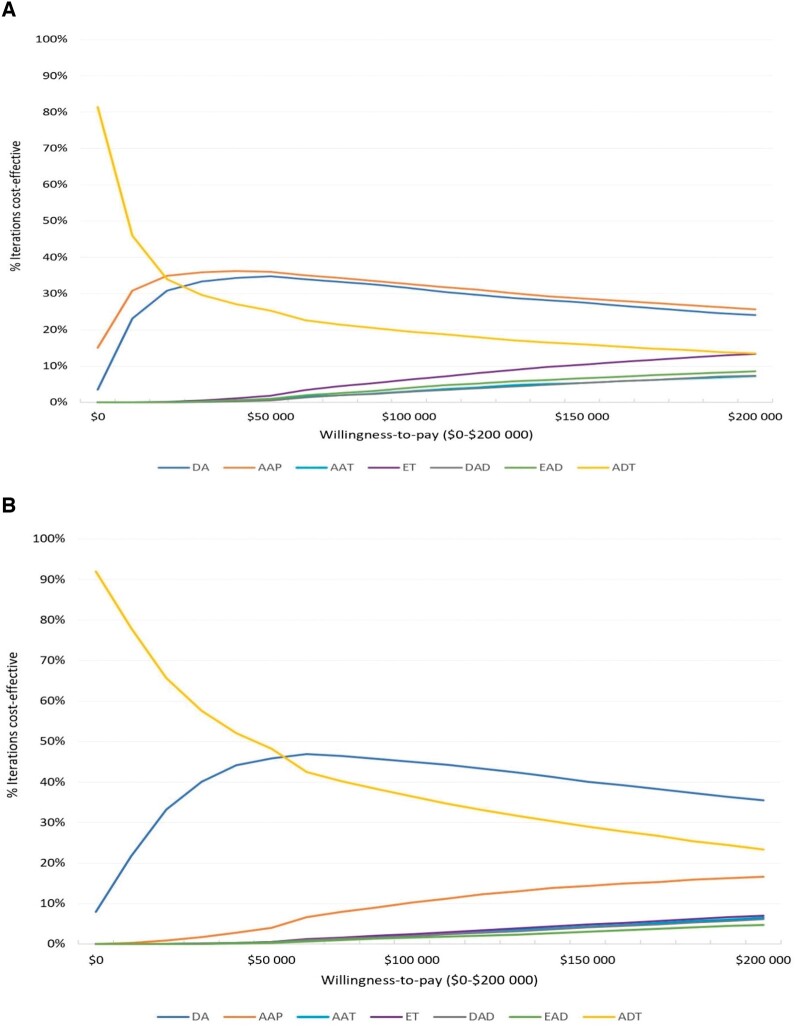
Results from our probabilistic sensitivity analysis are presented in cost-effectiveness acceptability curves (Figures 2, A and B). These results show that abiraterone acetate-prednisone with androgen deprivation therapy was most frequently the cost-effective treatment strategy for willingness-to-pay thresholds of $100 000/QALY (in 26% of the iterations) and $200 000/QALY (in 33% of the iterations), followed closely by docetaxel with androgen deprivation therapy (Figure 2, A). Probabilistic sensitivity analysis from the model using the average wholesale price shows that docetaxel with androgen deprivation therapy was most frequently the cost-effective treatment strategy (in 36%-45% of the iterations) for willingness-to-pay thresholds of $100 000/QALY and $200 000/QALY (Figure 2, B).
Figure 2.
A) Cost-effectiveness acceptability curve from probabilistic sensitivity analysis (Veterans Affairs Federal Supply Schedule costing). B) Cost-effectiveness acceptability curve from probabilistic sensitivity analysis (average wholesale price costing). AAP = abiraterone acetate-prednisone with androgen deprivation therapy; AAT = apalutamide with androgen deprivation therapy; ADT = androgen deprivation therapy; DA = docetaxel with androgen deprivation therapy; DAD = darolutamide with androgen deprivation therapy and docetaxel; EAD = enzalutamide with androgen deprivation therapy and docetaxel; ET = enzalutamide with androgen deprivation therapy.
Discussion
The treatment landscape for metastatic hormone-sensitive prostate cancer with androgen deprivation therapy alone in 2015 has rapidly evolved to combination androgen deprivation therapy–based treatment with several novel hormonal therapies and/or chemotherapy doublets or triplet combinations. This evolution pace has been rapid between 2015 and 2022, and at the same time, none of the treatment regimens have been compared head-to-head for superiority in a structured randomized controlled trial. There are also no validated predictive molecular biomarkers for choosing a specific treatment regimen. In this environment, providers have no other option but to base treatment decisions on empiric clinical judgements and expert opinions. Because several of these new combinations are costly and have associated treatment toxicity, our goal was to quantify these trade-offs to identify the optimal combination that preserves quality of life without compromising longevity of life. Although there are some differences in the inclusion criteria among the published metastatic hormone-sensitive prostate cancer state trials, with some trials having a higher percentage of high-volume metastatic hormone-sensitive prostate cancer patients, clinical practice for treating metastatic hormone-sensitive prostate cancer is based on the National Comprehensive Cancer Network guidelines. These guidelines include as category 1 (prostate.pdf [nccn.org]), all of the listed combinations that were analyzed in our cost-effectiveness analysis. Our analyses thus assumes that the decision to use any of the systemic combination therapy choices at present in an individual patient will be in line with the guidelines considered to be standard of care. We used a public payer perspective in the United States and found that the combination abiraterone acetate-prednisone with androgen deprivation therapy was the most cost-effective treatment strategy in this trade-off with the highest QALY and the most optimum incremental cost-effectiveness ratio (dollars per QALY). However, we also observed that in a probabilistic sensitivity analysis at a willingness-to-pay of $100 000/QALY to $200 000/QALY apart from abiraterone acetate-prednisone with androgen deprivation therapy combination, other combinations including androgen deprivation therapy combinations with docetaxel could also be a candidate for optimal cost-effective treatment strategies.
Of note, these results differ to some extent from other published studies investigating cost effectiveness in metastatic hormone-sensitive prostate cancer state performed with a 30-year time horizon in other countries including Canada and Europe. In a cost effectiveness study conducted in Europe on metastatic hormone-sensitive prostate cancer treatments, which took into account doublets with docetaxel, abiraterone, apalutamide, or enzalutamide and also costs for only 1-2 metastatic castrate-resistant prostate cancer state downstream treatments (47), different results were observed. This study found that the combination of androgen deprivation therapy with abiraterone-acetate and prednisone to be most cost-effective, although the input parameters in this study for reaching the derived cost effectiveness differs from our study when we use average wholesale price costs and also from the perspective of costs and number of downstream treatments. Yet, we do find similarities in conclusions when we consider using VA-FSS costs with this published report, which includes only 1-2 lines of downstream metastatic castrate-resistant prostate cancer treatment costs, while we considered more numbers of sequential metastatic castrate-resistant prostate cancer treatments in our analysis. In another cost effectiveness study, using a cost analysis with the Canadian health-care system comparing only androgen deprivation therapy with apalutamide with androgen deprivation therapy in metastatic hormone-sensitive prostate cancer, androgen deprivation therapy alone was found to be more cost effective at a willingness-to-pay $100 000, unless there were substantial cost reductions in the price for apalutamide (40). We also observe that in a study conducted in the United States with VA-FSS cost structures, a different conclusion from ours was observed after analyzing cost-effectiveness for only 5 doublets in metastatic hormone-sensitive prostate cancer, which had been published at the time of that analysis. This study concluded that the most cost-effective treatment was docetaxel with androgen deprivation therapy when using a willingness-to-pay threshold of $50 000/QALY and abiraterone acetate with androgen deprivation therapy when using a willingness-to-pay threshold of $200 000/QALY (48), although this is likely because metastatic castrate-resistant prostate cancer treatment inputs were not considered as extensively as in our study. In our study, we purposefully included metastatic castrate-resistant prostate cancer drug costs of subsequent antineoplastic standard of care treatments that are now widely available and used, because most metastatic castrate-resistant prostate cancer patients undergo treatment intensification with sequential drug regimens (49,50).
In fact, the variation in input parameters as well as differences in the configurations of treatment combination studied may explain the differences in results across these and other cost-effectiveness approaches. Our study is the largest cost effectiveness analysis in metastatic hormone-sensitive prostate cancer state and includes all available and recent drug combinations approved to treat metastatic hormone-sensitive prostate cancer, including triplet combinations (6,9,15). We also include costs of multiple downstream treatments following progression to castrate-resistant prostate cancer state including recently approved Lu-177 (24) and poly(adenosine diphosphate-ribose) polymerase inhibitor (22) regimens along with costs of monitoring treatment effects and end-of-life care. Many of these input variables were lacking in previous reports (40,47,48). Finally, additional factors to consider in cost effectiveness analyses is the price disparities in different countries and insurer and/or payment systems, not only for drug acquisition costs but also for the management of toxicities, and the number of downstream castrate-resistant prostate cancer treatments, which can impact effectiveness results.
A limitation of our study is that drug costs vary substantially across payers in the United States, particularly between private and public payers, therefore, the results of these analyses are not generalizable across the United States and are largely restricted to VA-FSS. Although this study did not consider the private payer perspective, the drug cost estimates from VA-FSS reflects actual burden in public sector better than the average wholesale price, which is the most commonly used data source for drug costs in cost-effectiveness analyses in oncology. The optimal combination can indeed be different while applying nonpublic payer systems, which in the United States is difficult to estimate as multiple private payer systems exist, and cost figures are not always available for analyses.
Nevertheless, economic evaluation is a critical decision-making tool in the management of cancer therapeutics when multiple therapeutic options are available and no biomarkers exist for choosing a regimen and provide better guidance than clinical estimates alone or if head-to-head trials between treatment strategies are missing. In that context, our results may aid in deciding how to choose individualized treatments without compromising standards of care established for treatment metastatic hormone-sensitive prostate cancer state.
Supplementary Material
Contributor Information
Minkyoung Yoo, Division of Epidemiology, Department of Internal Medicine, University of Utah (UT) School of Medicine, Salt Lake City, UT, USA.
Richard E Nelson, Division of Epidemiology, Department of Internal Medicine, University of Utah (UT) School of Medicine, Salt Lake City, UT, USA.
Benjamin Haaland, Department of Population Health Sciences, University of UT, Salt Lake City, UT, USA.
Maura Dougherty, Department of Economics, University of UT, Salt Lake City, UT, USA.
Zachary A Cutshall, University of UT School of Medicine, Salt Lake City, UT, USA.
Rhea Kohli, Case Western Reserve University, Cleveland, OH, USA.
Rylee Beckstead, School of Medicine, University of UT, Salt Lake City, UT, USA.
Manish Kohli, Division of Oncology-Department of Medicine, University of UT/Huntsman Cancer Institute, Salt Lake City, UT, USA.
Data availability
All data are incorporated into the article and its online supplementary material (available online).
Author contributions
Minkyoung Yoo, PhD (Data curation; Formal analysis; Funding acquisition; Investigation; Writing—original draft; Writing—review & editing), Richard E. Nelson, PhD (Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing—review & editing), Benjamin Haaland, PhD (Formal analysis; Investigation; Methodology; Writing—review & editing), Maura Doughtery, BS (Formal analysis; Investigation; Writing—review & editing), Zachary Cutshall, BS (Data curation; Investigation; Writing—review & editing), Rhea Kohli, MPH (Data curation; Formal analysis; Writing—review & editing), Rylee Beckstead, BS (Data curation; Formal analysis; Investigation; Writing—review & editing), and Manish Kohli, MD (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing).
Funding
This work was supported by the University of Utah Matheson Center for Health Care Studies and the University of Utah Clinical & Translational Science Institute with funding from the National Cancer Institute/National Center for Advancing Translational Sciences (NCATS) (grant number 1UM1TR004409-01).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
No funder/agency played a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
References
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7-33. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 3. Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fizazi K, Tran N, Fein L, et al. ; for the LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360. [DOI] [PubMed] [Google Scholar]
- 5. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686-700. [DOI] [PubMed] [Google Scholar]
- 6. Attard G, Murphy L, Clarke NW, et al. ; for the Systemic Therapy in Advancing or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022;399(10323):447-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chi KN, Agarwal N, Bjartell A, et al. ; for the TITAN Investigators. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13-24. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith MR, Hussain M, Saad F, et al. ; for the ARASENS Trial Investigators. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fizazi K, Foulon S, Carles J, et al. ; for the PEACE-1 investigators. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 x 2 factorial design. Lancet. 2022;399(10336):1695-1707. [DOI] [PubMed] [Google Scholar]
- 11. Davis ID, Martin AJ, Stockler MR, et al. ; for the ENZAMET Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131. [DOI] [PubMed] [Google Scholar]
- 12. Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243-262. [DOI] [PubMed] [Google Scholar]
- 13. Ciccarese C, Iacovelli R, Sternberg CN, Gillessen S, Tortora G, Fizazi K.. Triplet therapy with androgen deprivation, docetaxel, and androgen receptor signalling inhibitors in metastatic castration-sensitive prostate cancer: a meta-analysis. Eur J Cancer. 2022;173:276-284. [DOI] [PubMed] [Google Scholar]
- 14. Tan J-L, Sathianathen N, Geurts N, Nair R, Murphy DG, Lamb AD.. Androgen receptor targeted therapies in metastatic castration-resistant prostate cancer - the urologists’ perspective. Urol Sci. 2017;28(4):190-196. [Google Scholar]
- 15. Smith MR. Darolutamide in metastatic prostate cancer. Reply. N Engl J Med. 2022;386(24):2345. [DOI] [PubMed] [Google Scholar]
- 16. Woods BS, Sideris E, Palmer S, Latimer N, Soares M.. Partitioned survival and state transition models for healthcare decision making in oncology: where are we now? Value Health. 2020;23(12):1613-1621. [DOI] [PubMed] [Google Scholar]
- 17. Smare C, Lakhdari K, Doan J, Posnett J, Johal S.. Evaluating partitioned survival and Markov decision-analytic modeling approaches for use in cost-effectiveness analysis: estimating and comparing survival outcomes. Pharmacoeconomics. 2020;38(1):97-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ball G, Levine M, Thabane L, Tarride JE.. Onwards and upwards: a systematic survey of economic evaluation methods in oncology. Pharmacoecon Open. 2021;5(3):397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351(15):1513-1520. [DOI] [PubMed] [Google Scholar]
- 20. Tannock IF, de Wit R, Berry WR, et al. ; for the TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351(15):1502-1512. [DOI] [PubMed] [Google Scholar]
- 21. de Bono JS, Oudard S, Ozguroglu M, et al. ; for the TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376(9747):1147-1154. [DOI] [PubMed] [Google Scholar]
- 22. de Bono J, Mateo J, Fizazi K, et al. ; for the PROfound Trial Investigators. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 2020;383(24):2345-2357. [DOI] [PubMed] [Google Scholar]
- 23. Hussain M, Mateo J, Fizazi K, et al. ; for the PROfound Trial Investigators. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med 2020;383(24):2345-2357. [DOI] [PubMed] [Google Scholar]
- 24. Sartor O, de Bono J, Chi KN, et al. ; for the VISION Investigators. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 2021;385(12):1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rohatgi A. WebPlotDigitizer, Version 4.5. https://automeris.io/WebPlotDigitizer. Accessed June 12, 2022.
- 26. Guyot P, Ades AE, Ouwens MJ, Welton NJ.. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JP, Thompson SG, Spiegelhalter DJ.. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172(1):137-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costanzo MR, Fonarow GC, Rizzo JA.. Ultrafiltration versus diuretics for the treatment of fluid overload in patients with heart failure: a hospital cost analysis. J Med Econ. 2019;22(6):577-583. [DOI] [PubMed] [Google Scholar]
- 29. Strilciuc Ș, Grad DA, Radu C, et al. The economic burden of stroke: a systematic review of cost of illness studies. J Med Life. 2021;14(5):606-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eguia E, Bunn C, Kulshrestha S, et al. Trends, cost, and mortality from sepsis after trauma in the United States: an evaluation of the national inpatient sample of hospitalizations, 2012-2016. Crit. Care Med. 2020;48(9):1296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnston SS, Curkendall S, Makenbaeva D, et al. The direct and indirect cost burden of acute coronary syndrome. J Occup Environ Med. 2011;53(1):2-7. [DOI] [PubMed] [Google Scholar]
- 32. Silver SA, Long J, Zheng Y, Chertow GM.. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12(2):70-76. [DOI] [PubMed] [Google Scholar]
- 33. Jayasekera J, Onukwugha E, Bikov K, Mullins CD, Seal B, Hussain A.. The economic burden of skeletal-related events among elderly men with metastatic prostate cancer. Pharmacoeconomics. 2014;32(2):173-191. [DOI] [PubMed] [Google Scholar]
- 34. Bui C, O’Day K, Flanders S, et al. Budget impact of enzalutamide for chemotherapy-naive metastatic castration-resistant prostate cancer. J Manag Care Spec Pharm 2016;22(2):163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perrin A, Sherman S, Pal S, et al. Lifetime cost of everolimus vs axitinib in patients with advanced renal cell carcinoma who failed prior sunitinib therapy in the US. J Med Econ. 2014;18(3):200-209. [DOI] [PubMed]
- 36. Swallow E, Messali A, Ghate S, McDonald E, Duchesneau E, Perez JR.. The additional costs per month of progression-free survival and overall survival: an economic model comparing everolimus with cabozantinib, nivolumab, and axitinib for second-line treatment of metastatic renal cell carcinoma. J Manag Care Spec Pharm. 2020;24(4):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roy A, Kish JK, Bloudek L, et al. Estimating the costs of therapy in patients with relapsed and/or refractory multiple myeloma: a model framework. Am Health Drug Benef. 2015;8(4):204-215. [PMC free article] [PubMed] [Google Scholar]
- 38. Chi KN, Protheroe A, Rodriguez-Antolin A, et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol. 2018;19(2):194-206. [DOI] [PubMed] [Google Scholar]
- 39. Zhong L, Pon V, Srinivas S, et al. Therapeutic options in docetaxel-refractory metastatic castration-resistant prostate cancer: a cost-effectiveness analysis. PLoS One. 2013;8(5):e64275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parmar A, Timilshina N, Emmenegger U, et al. A cost-utility analysis of apalutamide for metastatic castration sensitive prostate cancer. Can Urol Assoc J. 2022;16(3):E126-E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saad F, Chilelli A, Hui B, et al. Cost-effectiveness of enzalutamide versus apalutamide versus androgen deprivation therapy alone for the treatment of metastatic castration-sensitive prostate cancer in Canada. J Med Econ. 2022;25(1):583-590. [DOI] [PubMed] [Google Scholar]
- 42. Ramamurthy C, Handorf EA, Correa AF, Beck JR, Geynisman DM.. Cost-effectiveness of abiraterone versus docetaxel in the treatment of metastatic hormone naïve prostate cancer. Urol Oncol. 2019;37(10):688-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heijnsdijk EA, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst. 2015;107(1):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beca J, Majeed H, Chan KKW, Hotte SJ, Loblaw A, Hoch JS.. Cost-effectiveness of docetaxel in high-volume hormone-sensitive metastatic prostate cancer. Can Urol Assoc J. 2019;13(12):396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Casciano R, Chulikavit M, Di Lorenzo G, et al. Economic evaluation of everolimus versus sorafenib for the treatment of metastatic renal cell carcinoma after failure of first-line sunitinib. Value Health. 2011;14(6):846-851. [DOI] [PubMed] [Google Scholar]
- 46. Briggs AH, Claxton K, Sculpher MJ.. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 47. Barbier MC, Tomonaga Y, Menges D, et al. Survival modelling and cost-effectiveness analysis of treatments for newly diagnosed metastatic hormone-sensitive prostate cancer. PLoS One 2022;17(11):e0277282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang L, Hong H, Alexander GC, Brawley OW, Paller CJ, Ballreich J.. Cost-effectiveness of systemic treatments for metastatic castration-sensitive prostate cancer: an economic evaluation based on network meta-analysis. Value Health. 2022;25(5):796-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ryan CJ, Smith MR, Fizazi K, et al. ; for the COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16(2):152-160. [DOI] [PubMed] [Google Scholar]
- 50. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71(2):151-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are incorporated into the article and its online supplementary material (available online).