Abstract
Background
The National Surgical Adjuvant Breast and Bowel Project B-42 trial evaluated extended letrozole therapy (ELT) in postmenopausal breast cancer patients who were disease free after 5 years of aromatase inhibitor (AI)–based therapy. Seven-year results demonstrated a nonstatistically significant trend in disease-free survival (DFS) in favor of ELT. We present 10-year outcome results.
Methods
In this double-blind, phase III trial, patients with stage I-IIIA hormone receptor–positive breast cancer, disease free after 5 years of an AI or tamoxifen followed by an AI, were randomly assigned to 5 years of letrozole or placebo. Primary endpoint was DFS, defined as time from random assignment to breast cancer recurrence, second primary malignancy, or death. All statistical tests are 2-sided.
Results
Between September 2006 and January 2010, 3966 patients were randomly assigned (letrozole: 1983; placebo: 1983). Median follow-up time for 3923 patients included in efficacy analyses was 10.3 years. There was statistically significant improvement in DFS in favor of letrozole compared with placebo (hazard ratio [HR] = 0.85, 95% confidence interval [CI] = 0.74 to 0.96; P = .01; 10-year DFS: placebo = 72.6%, letrozole = 75.9%, absolute difference = 3.3%). There was no difference in the effect of letrozole on overall survival (HR = 0.97, 95% CI = 0.82 to 1.15; P = .74). Letrozole statistically significantly reduced breast cancer–free interval events (HR = 0.75, 95% CI = 0.62 to 0.91; P = .003; absolute difference in cumulative incidence = 2.7%) and distant recurrences (HR = 0.72, 95% CI = 0.55 to 0.92; P = .01; absolute difference = 1.8%). The rates of osteoporotic fractures and arterial thrombotic events did not differ between treatment groups.
Conclusions
The beneficial effect of ELT on DFS persisted at 10 years. Letrozole also improved breast cancer–free interval and distant recurrences without improving overall survival. Careful assessment of potential risks and benefits is necessary for selecting appropriate candidates for ELT.
Hormone receptor–positive, early-stage breast cancer is associated with persistent risk of late recurrence and death after 5 years of adjuvant endocrine therapy (ET) (1,2). Thus, extending the duration of adjuvant ET beyond 5 years has been evaluated as a strategy for improving long-term outcomes. Extended endocrine therapy (EET) with either tamoxifen or an aromatase inhibitor (AI) after 5 years of tamoxifen has been shown to improve disease-free survival (DFS) (3-6) and, in one large trial (5), breast cancer–specific mortality and overall survival (OS).
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-42 trial evaluated whether 5 years of extended letrozole therapy (ELT) improves DFS in patients who had completed 5 years of adjuvant ET (with either an AI or with tamoxifen for ≤3 years followed by an AI for a total of 5 years) and remained recurrence free. Outcome results with 6.9 years of median follow-up demonstrated a beneficial ELT effect on DFS that did not reach statistical significance (hazard ratio [HR] = 0.85; P = .048) because the significance level was set to 0.0418 to adjust for interim analyses (7). Letrozole did not improve OS but provided a statistically significant 29% reduction in breast cancer–free interval (BCFI) event rate (HR = 0.71; P = .003) and 28% reduction in distant recurrences (DRs) (HR = 0.72; P = .03). ELT did not increase risk of osteoporotic-related fractures (OF) or arterial thrombotic (AT) events. Although this 10-year update analysis was not prespecified in the protocol, NRG Oncology/NSABP has the practice of updating long-term follow-up in adjuvant breast cancer studies usually at 10 and/or 15 years of follow-up. In this report, we provide updated 10-year outcome data including updated data on OF and AT events.
Methods
Details of the B-42 study have been previously published (7). In short, B-42 was a randomized, double-blind, placebo-controlled, phase III trial in postmenopausal women with hormone receptor–positive breast cancer, stage I-IIIA, who had either breast-conserving therapy or mastectomy plus axillary lymph-node staging and were disease free after 5 years of ET with either an AI or tamoxifen for no more than 3 years followed by an AI (NCT00382070).
The primary endpoint was DFS, defined as time from random assignment to invasive or in situ local breast cancer recurrence, regional recurrence, second primary malignancy, or death. Secondary endpoints were OS (time from random assignment to death from any cause), BCFI (time from random assignment to local, regional, or distant recurrence or contralateral breast cancer as a first event), DR (time from random assignment to DR), incidence of OF (defined as Colles, hip, or spinal fractures), and incidence of AT events as defined by Common Terminology Criteria for Adverse Events v4.0 (grade ≥1 stroke or transient ischemic attack; grade ≥2 acute coronary syndrome or cerebrovascular ischemia; grade ≥3 myocardial infarction or peripheral ischemia or visceral arterial ischemia; and grade ≥4 selected thromboembolic events [cerebrovascular event, arterial insufficiency]). For this update, we also present the results of the analysis of the recurrence-free interval (RFI), defined as time from random assignment to any recurrence, which was not prespecified as one of the secondary endpoints but is a primary endpoint for several ongoing correlative science projects. For all endpoints, event-free patients were censored at the date of last follow-up. Second primary cancers and death without evidence of recurrence were treated as censored events for BCFI. Clinical assessment was required for determining patients’ status for all endpoints except OS.
Patients
Patients were ineligible if they had history of nontraumatic OF, bilateral breast cancer (including ductal carcinoma in situ), or other malignancies (except carcinoma in situ of the colon or cervix, melanoma in situ, or squamous or basal cell carcinoma of the skin) unless they were disease free for at least 5 years before random assignment and deemed at low risk of recurrence by their physician. Use of hormonal therapy for osteoporosis had to be discontinued before random assignment. The study was approved by local human investigations committees or institutional review boards in accordance with assurances by the Department of Health and Human Services. Written informed consent was required.
Eligible patients were stratified by pathological node status at diagnosis (negative vs positive), prior tamoxifen use (no vs yes), and lowest bone mineral density T-score in the lumbosacral spine, total hip, or femoral neck (≤-2.0 or >-2.0 SD), then randomly assigned to receive either letrozole 2.5 mg orally daily for 5 years or matching placebo.
A total of 3966 patients were randomly assigned to either letrozole or placebo between September 2006 and January 2010.
Statistical analysis
Differences in primary and secondary endpoints between treatment groups were assessed using stratified log-rank tests, controlling for stratification variables (8). Hazard ratios and corresponding 95% confidence intervals (CIs) were computed on the basis of a stratified Cox proportional hazards model for all time-to-event endpoints (9). The Cox model was used to control for the effect of additional prognostic variables. Stratification factors (nodal status, tamoxifen use, and T-score), as well as age group and surgery type (identified in the primary analysis) (7), were considered. Those that reached an identified statistical significance level were included in the final multivariable model. When the proportional hazards assumption was not satisfied (10), an optimal time point was identified to divide the time interval into regions with proportionality (8). Distribution of DFS and OS endpoints was estimated using the Kaplan–Meier method (11). Cumulative incidence function was used to estimate the proportions of BCFI, RFI, DR, OF, and AT events over time to account for competing risks of death as first event (12). In addition, second primary cancers (other than breast) and death without evidence of recurrence were considered competing events in estimating cumulative incidence of BCFI events. All analyses were based on the intention-to-treat principle. Patients with no follow-up and those not at risk for the primary endpoint (metastases at time of random assignment or first nondeath event within 30 days from random assignment) were excluded from all analyses. All reported P values are 2-sided, using P values less than .05 to indicate statistical significance. All statistical analyses were performed using SAS(v9.4). Data cutoff for reported analyses was April 30, 2020.
Results
Patient entry and characteristics
Distributions of patient and tumor characteristics were well balanced between treatment groups (Table 1). Seven patients randomly assigned to letrozole were excluded from analyses (not at risk for the primary endpoint), and 36 (placebo = 19, letrozole = 17) were excluded because of no follow-up (Supplementary Figure 1, available online). Among 3923 patients with follow-up, 20 (placebo = 11, letrozole = 9) were excluded from analyses of all disease-related endpoints except for OS because of no clinical assessment. Median follow-up time for 3923 patients included in efficacy analyses was 10.3 years (interquartile range [IQR] = 9.5-11.1 years). Median treatment duration was 59.8 months in both groups. Details for treatment information have been previously reported (7).
Table 1.
Patient and tumor characteristics: NSABP B-42 10-year update
| Characteristic | Placebo (n = 1983) | Letrozole (n = 1983) |
|---|---|---|
| No. (%) | No. (%) | |
| Age at random assignment, y | ||
| Younger than 60 | 675 (34.0) | 685 (34.5) |
| 60 and older | 1308 (66.0) | 1298 (65.5) |
| Race | ||
| Asian | 39 (2.0) | 39 (2.0) |
| Black or African American | 81 (4.1) | 70 (3.5) |
| Other or unknowna | 23 (1.2) | 26 (1.3) |
| White | 1840 (92.8) | 1848 (93.2) |
| Ethnicity | ||
| Hispanic or Latino | 39 (2.0) | 53 (2.7) |
| Not Hispanic or Latino | 1864 (94.0) | 1849 (93.2) |
| Unknown | 80 (4.0) | 81 (4.1) |
| Pathologic nodal status | ||
| Negative | 1134 (57.2) | 1145 (57.7) |
| Positive | 849 (42.8) | 838 (42.3) |
| Lowest BMD T-score | ||
| ≤ -2.0 | 493 (24.9) | 489 (24.7) |
| > -2.0 | 1490 (75.1) | 1494 (75.3) |
| Surgery type | ||
| Lumpectomy | 1208 (60.9) | 1201 (60.6) |
| Mastectomy | 775 (39.1) | 782 (39.4) |
| HER2 status | ||
| Positive | 278 (14.0) | 287 (14.5) |
| Negative | 1547 (78.0) | 1546 (78.0) |
| Not done or unknown | 158 (8.0) | 150 (7.6) |
| Duration of tamoxifen prior to randomization, mo | ||
| 0 | 1212 (61.1) | 1207 (60.9) |
| 1-12 | 164 (8.3) | 150 (7.6) |
| 13-24 | 254 (12.8) | 259 (13.1) |
| 25-36 | 353 (17.8) | 367 (18.5) |
| Duration of AI prior to randomization, mo | ||
| ≤36b | 412 (20.8) | 399 (20.1) |
| 37-48 | 192 (9.7) | 207 (10.4) |
| 49-60 | 992 (50.0) | 970 (48.9) |
| >60 | 387 (19.5) | 407 (20.5) |
Race and ethnicity categories were based on the report submitted by accruing sites upon enrollment. Other race categories include Native Hawaiian or Other Pacific Islander, American Indian or Alaska Native, and multiracial. AI = aromatase inhibitor; BMD = bone mineral density; NSABP = National Surgical Adjuvant Breast and Bowel Project.
Duration was unknown for 1 placebo patient in this category.
Primary endpoint: DFS
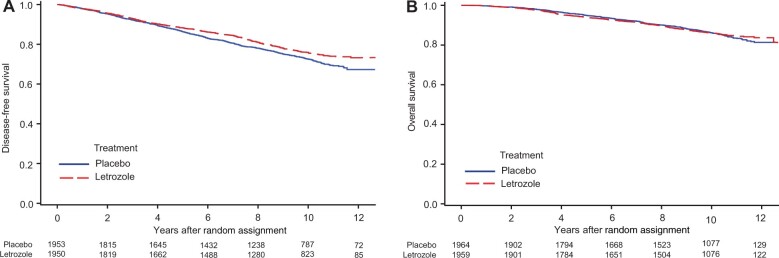
A total of 940 DFS events were recorded (placebo = 505, letrozole = 435) (Table 2). Treatment with letrozole resulted in an overall statistically significant DFS increase (10-year absolute benefit = 3.3%; HR = 0.85, 95% CI = 0.74 to 0.96; P = .01). The 10-year DFS were placebo at 72.6% and letrozole at 75.9% (Figure 1, A).
Table 2.
Type of DFS events by treatment group: NSABP B-42 10-year updatea
| First event | Placebo (n = 1953) | Letrozole (n = 1950) |
|---|---|---|
| No. (%) | No. (%) | |
| Distant recurrence | 115 (5.9) | 85 (4.4) |
| Local-regional recurrence | 47 (2.4) | 47 (2.4) |
| Second primary | 242 (12.4) | 193 (9.9) |
| Breast | 84 (4.3) | 57 (2.9) |
| Other | 158 (8.1) | 136 (7.0) |
| Death | 101 (5.2) | 110 (5.6) |
| Total first event | 505 (25.9) | 435 (22.3) |
| Alive, event free | 1448 (74.1) | 1515 (77.7) |
DFS = disease-free survival; NSABP = National Surgical Adjuvant Breast and Bowel Project.
Figure 1.
Effect of extended letrozole therapy on disease-free survival (A) and overall survival (B): National Surgical Adjuvant Breast and Bowel Project B-42 10-year update.
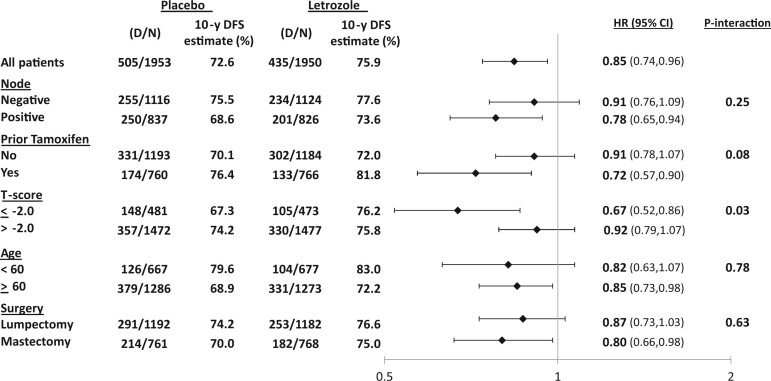
In the multivariable Cox model, younger age at diagnosis, negative pathologic nodal status, and prior tamoxifen use were independently associated with better DFS (Table 3). The ELT effect was statistically significantly different for patients with T-score no more than -2.0 (HR = 0.67, 95% CI = 0.52 to 0.86) vs those with more than -2.0 (HR = 0.91, 95% CI = 0.79 to 1.06) (Ptreatment-by-T-score interaction = .04). The ELT effect on DFS was not statistically significantly different according to other factors (Figure 2).
Table 3.
Results of the multivariable analyses of DFS: NSABP B-42 10-year update
| Characteristic | Treatment group | Hazard ratio (95% CI) | P |
|---|---|---|---|
| Lowest BMD T-score | .04a | ||
| ≤ -2.0 | Placebo | 1.00 | |
| Letrozole | 0.67 (0.52 to 0.86) | ||
| > -2.0 | Placebo | 1.00 | |
| Letrozole | 0.91 (0.79 to 1.06) | ||
| Age, y | <.001 | ||
| Younger than 60 | 1.00 | ||
| 60 and older | 1.67 (1.44 to 1.95) | ||
| Pathologic nodal status | <.001 | ||
| Negative | 1.00 | ||
| Positive | 1.38 (1.21 to 1.57) | ||
| Received tamoxifen | <.001 | ||
| No | 1.00 | ||
| Yes | 0.76 (0.66 to 0.87) |
P interaction between treatment and T-score (for placebo, lowest T-score above -2.0 compared with no more than -2.0: HR = 0.74, 95% CI = 0.61 to 0.90; for letrozole lowest T-score: above -2.0 compared with no more than -2.0: HR = 1.01, 95% CI = 0.81 to 1.26). BMD = bone mineral density; DFS = disease-free survival; NSABP = National Surgical Adjuvant Breast and Bowel Project.
Figure 2.
Effect of extended letrozole therapy on disease-free survival in subgroups: National Surgical Adjuvant Breast and Bowel Project B-42 10-year update. CI = confidence interval; D = number of events; DFS = disease-free survival; HR = hazard ratio; N = number of patients.
Similar to our original report, as part of the post hoc analysis, the ELT effect was evaluated by subgroups of patients defined by T-score and bisphosphonate (BSP) use at the time of random assignment. There were 1393 (35.7%) patients (placebo = 701, letrozole = 692) who reported BSP use at baseline. BSP use at baseline varied according to T-score with 577 of 954 (60.5%) patients with a T-score of no more than -2 and 816 of 2949 (27.7%) patients with a T-score of more than -2. Among BSP users, 99% planned to continue BSP use after random assignment. There was no differential ELT effect on DFS between BSP users and not users for patients with a T-score of no more than -2 and those with more than -2 (Supplementary Figure 2, available online).
Secondary endpoints
A total of 534 deaths occurred during the study (placebo = 272, letrozole = 262). There was no difference in OS between groups (HR = 0.97, 95% CI = 0.82 to 1.15; P = .74). The 10-year OS was placebo at 86.2% and letrozole at 86.1% (Figure 1, B). There were 142 breast cancer deaths (placebo = 77, letrozole = 65). Cause of death was unknown for 130 patients (placebo = 63, letrozole = 67).
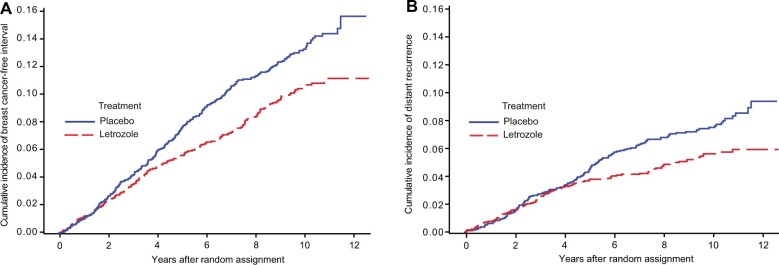
A total of 435 BCFI events have been observed (placebo = 246, letrozole = 189). Letrozole resulted in a statistically significant decrease in BCFI events (10-year absolute benefit = 2.7%; HR = 0.75, 95% CI = 0.62 to 0.91; P = .003). The 10-year BCFI risk was placebo at 13.2% and letrozole at 10.5% (Figure 3, A).
Figure 3.
Effect of extended letrozole therapy on cumulative incidence of breast cancer–free interval event (A) and distant recurrence (B): National Surgical Adjuvant Breast and Bowel Project B-42 10-year update.
A total of 240 distant recurrences were observed (placebo = 139, letrozole = 101). This resulted in an overall 28% reduction in DR rate for letrozole vs placebo (10-year absolute benefit = 1.8%, HR = 0.72, 95% CI = 0.55 to 0.92; P = .01). A nonproportionality of the hazards in 2 treatment groups was detected (P = .029). Two change points for the relative risk were identified (4.0 and 7.4 years). No difference in the risk of DR events was evident before 4.0 years (HR = 1.03, 95% CI = 0.72 to 1.47; P = .88) and after 7.4 years (HR = 1.05, 95% CI = 0.58 to 1.90; P = .88) with statistically significant benefit between 4 and 7.4 years (HR = 0.28, 95% CI = 0.17 to 0.49; P < .001). The DR risk through 4, 7, and 10 years was 3.2%, 6.1%, and 7.4%, in the placebo group and 3.2%, 4.1%, and 5.6% in the letrozole group, respectively (Figure 3, B).
There were 229 OF reported (placebo = 109, letrozole = 120). Time to development of the fractures did not differ between the 2 groups (HR = 1.12, 95% CI = 0.86 to 1.45; P = .40). The 10-year risk of OF was placebo at 6.1% (95% CI= 5.0% to 7.4%) and letrozole at 6.6% (95% CI= 5.4% to 7.8%).
Treatment with letrozole did not result in an increase in AT events reported (placebo = 78, letrozole = 86; HR = 1.11, 95% CI = 0.82 to 1.51; P = .50) with the 10-year cumulative incidence of 4.1% (95% CI = 3.2% to 5.1%) for placebo and 4.7% (95% CI = 3.8% to 5.8%) for the letrozole group.
A total of 314 RFI events have been observed (placebo = 174, letrozole = 140), resulting in statistically significant decrease in the rate of RFI events (10-year absolute benefit = 1.5%; HR = 0.80, 95% CI = 0.64 to 0.99; P = .043). The 10-year cumulative incidence of RFI was placebo at 9.3% and letrozole at 7.8%.
Toxicity
Other toxicity information was presented previously, and there were no notable differences between the groups (7).
Discussion
NSABP B-42 is the largest trial to date to investigate extended adjuvant AI therapy in patients who were disease free after 5 years of ET, most of whom had been treated with an AI. The study was designed to detect a 20% reduction in risk of DFS events with ELT (HR = 0.80) based on 631 events. Our initial findings of the ELT effect on DFS with approximately 7 years of median follow-up (HR = 0.85; P = .048) did not reach a protocol-defined level of statistical significance, which accounted for 4 interim analyses (P < .0418). Our 10-year results, with 940 DFS events, demonstrated similar magnitude of benefit (HR = 0.85, 95% CI = 0.74 to 0.96) to the 7-year results. This was also observed for other disease-outcome endpoints (BCFI, DR) (Supplementary Figure 3, available online). We also continue to see no benefit in OS at 10 years. In addition, there continues to be no significant increase in the risk of OF and AT events. No additional toxicity signals were identified with more follow-up.
The lack of OS benefit in B-42 is in concordance with all other reported trials of EET in which no differences in OS have been observed (3-6,13-17). However, one exception was the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial (5), which demonstrated an OS and a breast cancer–specific survival improvement with 10 vs 5 years of adjuvant tamoxifen. Furthermore, recent results from the Early Breast Cancer Trials Collaborative Group (EBCTCG) (18) on benefits of prolonging AI therapy after 5 years of ET confirmed lack of OS benefit except for statistically significant improvement when the extended AI therapy followed 5 years of tamoxifen (18).
Other trials that have examined the benefit of extended AI therapy have shown somewhat diverse results. The MA.17R trial (13), which enrolled 1918 postmenopausal women with primary breast cancer who were free of recurrent disease after receiving 4.5-6 years of adjuvant AI therapy (preceded in most patients by 5 years of tamoxifen) then randomly assigned to receive 5 years of placebo or letrozole within 2 years of completion of AI therapy, showed a statistically significant reduction in DFS events in favor of letrozole (HR = 0.66; P = .01; 5-year DFS placebo = 91%, letrozole = 95%). However, when deaths as first events were included in the DFS endpoint, the MA.17R trial did not show a significant improvement with ELT (HR = 0.80; P = .06; 5-year DFS placebo = 88%, letrozole = 90%).
Four recently reported phase III trials have compared different durations of extended adjuvant AI therapy (14-17). None have demonstrated a statistically significant improvement in DFS or OS. The DATA trial (14) compared 3 vs 6 years of extended adjuvant anastrozole in 1860 postmenopausal women with hormone receptor–positive early stage breast cancer and no disease recurrence after 2-3 years of adjuvant tamoxifen. The 5-year adapted DFS was 83.1% (95% CI = 80.0 to 86.3) in the 6-year group and 79.4% (95% CI = 76.1 to 82.8) in the 3-year group (HR = 0.79, 95% CI = 0.62 to 1.02; P = .066). A recent 10-year follow-up of the DATA trial continues to show modest benefit from the EET (HR = 0.86, 95% CI = 0.72 to 1.01; P = .073) (19). The IDEAL trial (15) compared 2.5 vs 5 years of ELT in 1824 postmenopausal patients with hormone receptor–positive breast cancer who had received 5 years of any ET. With a median follow-up of 6.6 years, there was no statistically significant difference in DFS between the 2 groups (HR = 0.92, 95% CI = 0.74 to 1.16) and no statistically significant differences in OS or distant metastasis-free survival. The ABCSG-16 (Austrian Breast and Colorectal Cancer Study Group) trial (16) compared 2 vs 5 years of anastrozole in 3484 postmenopausal women with stage I-III, hormone receptor–positive breast cancer who had completed 5 years of ET with tamoxifen, an AI, or tamoxifen followed by an AI. With median follow-up of 118 months, there were no significant differences in DFS between the 2 groups (HR = 0.99, 95% CI = 0.85 to 1.15; P = .90). Lastly, the SOLE (The Study of Letrozole Extension) trial (17) compared 5 years of continuous vs intermittent letrozole in 4884 postmenopausal women with hormone receptor–positive, lymph-node positive operable breast cancer who had completed 4-6 years of adjuvant ET. With a median follow-up of 60 months, there was no significant difference in DFS between the 2 groups (HR = 1.08, 95% CI = 0.93 to 1.26; P = .31).
When the results of these 4 trials are taken together with the updated results of B-42, they suggest that extended adjuvant AI therapy confers an improvement in DFS. In addition, these results indirectly suggest that a similar benefit might be achieved with shorter (or intermittent) extended AI therapy (approximately 2-3 years). A meta-analysis of these datasets will likely shed more light on this question.
Recent results from the EBCTCG analysis (18) that examined the benefits of prolonging AI therapy after 5 years of ET (with either tamoxifen, AI, or a sequence of both) demonstrated larger benefit in reducing recurrence when the AI followed 5 years of tamoxifen vs when the AI followed 5 years of the sequence of tamoxifen followed by AI or 5 years of an AI. Furthermore, a slight improvement in OS was shown only when the extended therapy with the AI followed 5 years of tamoxifen (18).
In the initial results of B-42 (which were included in the above EBCTCG overview), as well as in these updated results, we observed a similar trend of larger benefit from ELT in patients who were treated with tamoxifen followed by an AI vs those who were treated with only an AI.
Our observation that the ELT effect was statistically significantly greater for patients with baseline T-score of no more than -2.0 compared with those with T-score above -2.0 is of interest. As outlined in our results, it does not appear that BSP use before random assignment can explain this difference. Because the relationship between serum estrogen levels and bone mineral density is well-established (20), it is important to further explore if serum estrogen levels in postmenopausal women can be used as surrogate markers of the effect of EET. Unfortunately, blood collection did not occur in the B-42 trial, so that question cannot be addressed in our study.
In the current analysis, we continue to see the statistically significant nonproportionality in the DR rates between the 2 treatment groups, which was initially noted in the primary B-42 results (7). We have previously hypothesized that lack of ELT benefit in the first 4 years, but significant benefit after 4 years, was due to a carryover effect of the AI in the control group. Similar findings have been noted with extended tamoxifen in the ATLAS and Adjuvant Tamoxifen-To Offer More? trials (5,6). We also observed that the rates of DR events were similar between the 2 treatment groups after 7.4 years. However, estimates might be unreliable because of heavy censoring toward the end of follow-up. Extended follow-up will be needed to confirm these results.
It is reassuring that with additional follow-up, the B-42 trial does not demonstrate an increase in AT events. A systematic review of randomized controlled trials that compared AIs and tamoxifen as primary adjuvant ET in postmenopausal women (21) showed that longer duration of AI use was associated with increased odds of developing cardiovascular disease (odds ratio [OR] = 1.26; P < .001) and bone fractures (OR = 1.47; P < .001) but decreased odds of venous thrombosis (OR = 0.55; P < .001) and endometrial carcinoma (OR = 0.34; P < .001). Given the B-42 results, it is possible that the effect seen in the meta-analysis might be more indicative of a protective effect from tamoxifen rather than a detrimental effect from the AIs on cardiovascular disease risk. Also reassuring is that, with additional follow- up, ELT did not significantly increase OF risk as reported in other trials of EET with AIs (13-15,21). However, fracture prevalence is likely underreported in major adjuvant trials.
Given the small absolute benefit from extended AI therapy on DFS, it is important to identify patient subgroups at increased risk of recurrence and subgroups who might receive greater proportional benefit from such therapy. During the past few years, there have been several attempts to refine risk of late recurrence after 5 years of ET. These include development of clinicopathological algorithms such as CTS5 (22), assessment of circulating tumor cell counts (23), and assessment of several commercially available genomic classifiers, some of which might also predict which patients could benefit from EET (24-31). Incorporating such approaches into the clinical decision-making algorithm for recommending EET could improve patient selection and optimize the risk-to-benefit ratio. Correlative science studies evaluating such biomarkers using B-42 tumor–archived tissue are currently being conducted.
Our long-term findings continue to suggest that careful assessment of the risks and potential benefits of ELT is required to identify optimal candidates for extended adjuvant AI therapy. This assessment should include patient and tumor characteristics, existing comorbidities, information about bone mineral density, and tolerance of AI treatment in the initial 5 years of treatment for breast cancer.
Supplementary Material
Acknowledgements
The authors acknowledge the contributions of Christine I. Rudock, publications and graphics specialist and Wendy L. Rea, BA, editorial associate, both of whom are employees of NSABP. They were not compensated beyond their normal salaries for this work.
The authors would also like to acknowledge the contributions of Mark Graham, MD, and Thomas E. Seay, MD.
Role of the funder: The funder played no role in the study design, study conduct, data analysis, interpretation of results, or preparation or approval of the manuscript. The decision to submit the manuscript for publication was made without input from the funder.
The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Previous, related works: Mamounas EP, Bandos H, Lembersky BC, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial [Erratum in: Lancet Oncol. 2019;20(1):e10]. Lancet Oncol. 2019; 20(1):88-99. https://pubmed.ncbi.nlm.nih.gov/30509771/.
Contributor Information
Eleftherios P Mamounas, Department of Surgical Oncology, Orlando Health Cancer Institute, Orlando, FL, USA.
Hanna Bandos, NRG Oncology SDMC, and the Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, USA.
Priya Rastogi, University of Pittsburgh Medical Center Hillman Cancer Center, Department of Oncology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Department of Oncology, University of Pittsburgh Magee-Womens Hospital, Pittsburgh, PA, USA.
Barry C Lembersky, University of Pittsburgh Medical Center Hillman Cancer Center, Department of Oncology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Jong-Hyeon Jeong, NRG Oncology SDMC, and the Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, USA.
Charles E Geyer, Jr, University of Pittsburgh Medical Center Hillman Cancer Center, Department of Oncology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Louis Fehrenbacher, Department of Medical Oncology, Kaiser Permanente Oncology Clinical Trials Northern California, Novato, CA, USA.
Stephen K Chia, Department of Medical Oncology, British Columbia Cancer Agency (BCCA), Vancouver, British Columbia, Canada.
Adam M Brufsky, Department of Oncology, University of Pittsburgh Magee-Womens Hospital, Pittsburgh, PA, USA.
Janice M Walshe, Department of Oncology, Cancer Trials Ireland (formerly known as Irish Clinical Oncology Research Group—ICORG), Dublin, Ireland.
Gamini S Soori, Department of Oncology, Florida Cancer Specialists, Fort Myers, FL, USA.
Shaker R Dakhil, Department of Oncology, Community Clinical Oncology Program, Wichita via Christi Regional Medical Center, Wichita, KS, USA.
James L Wade, III, Department of Oncology, Decatur Memorial Hospital, Cancer Care Specialists of Illinois, Heartland National Cancer Institute Community Oncology Research Program, Decatur, IL, USA.
Edward C McCarron, Department of Surgical Oncology, MedStar Franklin Square Medical Center at Weinberg Cancer Institute, Baltimore, MD, USA.
Sandra M Swain, Department of Surgical Oncology, Georgetown Lombardi Comprehensive Cancer Center, MedStar Health, Washington, DC, USA.
Norman Wolmark, NRG Oncology SDMC, and the Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, USA.
Data availability
Individual participant data that underlie the results reported in this article, after deidentification, will generally be available within 1 year after publication and will be accessible through the National Clinical Trials Network Data Archive.
Author contributions
Eleftherios P. Mamounas, MD (Conceptualization; Investigation; Methodology; Visualization; Writing - original draft; Writing - review & editing); Hanna Bandos, PhD (Conceptualization; Formal analysis; Methodology; Software; Validation; Visualization; Writing - original draft; Writing - review & editing); Priya Rastogi, MD (Investigation; Writing - review & editing); Barry C. Lembersky, MD (Conceptualization; Investigation; Research); Jong-Hyeon Jeong, PhD (Conceptualization; Investigation; Methodology; Writing - review & editing); Charles E. Geyer, Jr., MD (Conceptualization; Investigation; Writing - review & editing); Louis Fehrenbacher, MD (Conceptualization; Investigation; Writing - review & editing); Stephen K. Chia, MD (Resources; Writing - review & editing); Adam M. Brufsky, MD, PhD (Investigation; Resources; Writing - review & editing); Janice M. Walshe, MD (Investigation; Resources; Validation; Writing - review & editing); Gamini S. Soori, MD (Conceptualization; Data curation; Funding acquisition; Investigation; Resources; Validation; Writing - review & editing); Shaker R. Dakhil, MD (Investigation; Resources; Writing - review & editing); James L. Wade, III, MD (Investigation; Resources; Writing - review & editing); Edward C. McCarron, MD (Investigation; Resources; Writing - review & editing); Sandra M. Swam, MD (Conceptualization; Investigation; Methodology; Supervision; Writing - original draft; Writing - review & editing); Norman Wolmark, MD (Conceptualization; Funding acquisition; Investigation; Project administration; Resources; Supervision; Visualization; Writing - review & editing).
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health, US Department of Health and Human Services, Public Health Service grants U10-CA180868, U10-CA180822, UG1CA189867, and U24-CA196067, The Korea Health Technology R&D Project through the Korean Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI13C2162; SP), and Novartis.
Conflicts of interest
ALL: NCI U10CA180868, -180822; UG1CA189867; Novartis: Directly to institution only.
Eleftherios P. Mamounas, MD—Consulting: Genentech/Roche, Exact Sciences, Biotheranostics, Merck; Honoraria, etc: Genentech/Roche, Exact Sciences, Merck; DSMB/Advisory Board(s): Genentech/Roche, Exact Sciences, Biotheranostics, Agendia, Puma Biotechnology; Stocks: Moderna.
Charles E. Geyer, Jr, MD—Grants: Institutional—AstraZeneca, Genentech/Roche, Daiichi Sankyo, Abbvie; Consulting: Athenex; Honoraria, etc: Medical writing—Genentech/Roche, Abbvie; Travel: AstraZeneca, Genentech/Roche, Daiichi Sankyo; DSMB/Advisory Board(s): Advisory Board compensated—Exact Sciences; Advisory Board uncompensated—Genentech/Roche, SeaGen, Daiichi-Sankyo.
Stephen K. Chia, MD—Grants: Novartis, Hoffmann LaRoche, Merck, AstraZeneca, Pfizer (All to institution); Honoraria/speakers bureaus: Novartis, Eli Lilly, Pfizer, Merck; Data Safety/Advisory Board: Hoffmann LaRoche, Eli Lilly.
Adam M. Brufsky, MD, PhD—Honoraria, etc: Roche/Genentech: Personal consulting fee.
Janice M. Walshe, MD—Honoria- Novartis, Pfizer; Travel- Novartis.
Sandra M. Swain, MD—Grants: Genentech/Roche, Kailos Genetics, BCRF; Consulting: Roche/Genentech, Molecular Therapeutics; Honoraria, etc: Genentech/Roche, Daiichi Sankyo; Travel: Genentech/Roche Travel to Boston 11/2019; DSMB/Advisory Board(s): DSMB: AstraZeneca; Ad Board AstraZeneca, Daiichi Sankyo, Exact Sciences, Biotheranostics, Natera, Merck, Silverback Therapeutics, Athenex, Lilly; scientific advisory Board Inivata; Leadership role: NSABP Vice chairman; CCF, ASCO Director; Receipt of gifts/other: Third party writing Genentech/Roche and AstraZeneca.
Norman Wolmark, MD—Leadership role: NSABP Foundation, Inc: Chairman, Board of Directors, paid position.
All other authors declare no other conflicts of interest.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group. Early breast cancer trialists’ collaborative group: tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451-1467. [PubMed] [Google Scholar]
- 2. Pan H, Gray R, Braybrooke J, et al. ; for the EBCTCG. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793-1802. [DOI] [PubMed] [Google Scholar]
- 4. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol. 2008;26(12):1965-1971. [DOI] [PubMed] [Google Scholar]
- 5. Davies C, Pan H, Godwin J, et al. ; for the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gray RG, Rea DW, Handley K, et al. ; for the aTTom Collaborators. aTTom: randomized trial of 10 versus 5 years of adjuvant tamoxifen among 6,934 women with estrogen receptor-positive (ER+) or ER untested breast cancer–preliminary results. J Clin Oncol. 2008;26(suppl 15):513.18202431 [Google Scholar]
- 7. Mamounas EP, Bandos H, Lembersky BC, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial [Erratum in: Lancet Oncol. 2019;20(1):e10]. Lancet Oncol. 2019;20(1):88-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. In: Dietz K, Gail M, Krickeberg K, Samet J, Tsiatis A. Statistics for Biology and Health. New York: Springer; 2003:1-542. http://sistemas.fciencias.unam.mx/~ediaz/Cursos/Estadistica3/Libros/0a9X.pdf. Accessed May 23, 2023. [Google Scholar]
- 9. Cox DR. Regression models and life-tables (with discussion). J Royal Stat Soc B. 1972;34(2):187-202. [Google Scholar]
- 10. Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557-572. doi: 10.2307/2337177. [DOI] [Google Scholar]
- 11. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Asscoc. 1958;53(282):457-481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 12. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. Hoboken, NJ: J. Wiley & Sons, Inc; 2002. ISBN 978-0-471-36357-6. https://www.worldcat.org/title/statistical-analysis-of-failure-time-data/oclc/981397941?referer=di&ht=edition+. [Google Scholar]
- 13. Goss PE, Ingle JN, Pritchard K, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, et al. ; for the Dutch Breast Cancer Research Group (BOOG) for the DATA Investigators. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 2017;18(11):1502-1511. [DOI] [PubMed] [Google Scholar]
- 15. Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. ; on behalf of the IDEAL Study Group. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL Trial (BOOG 2006-05). J Natl Cancer Inst. 2018;110(1):40-48. doi: 10.1093/jnci/djx134. [DOI] [PubMed] [Google Scholar]
- 16. Gnant M, Fitzal F, Rinnerthaler G, et al. ; for the Austrian Breast and Colorectal Cancer Study Group. Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N Engl J Med. 2021;385(5):395-405. [DOI] [PubMed] [Google Scholar]
- 17. Colleoni M, Luo W, Karlsson P, et al. ; for the SOLE Investigators. Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(1):127-138. [DOI] [PubMed] [Google Scholar]
- 18. Gray R, Early Breast Cancer Trialists' Collaborative Group. Effects of prolonging adjuvant aromatase inhibitor therapy beyond five years on recurrence and cause-specific mortality: An EBCTCG meta-analysis of individual patient data from 12 randomised trials including 24,912 women [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; Dec 4-8, 2018; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2019;79(Suppl 4):Abstract nr GS3-03.
- 19. Tjan-Heijnen CGL, Lammers SWM, Geurts SME, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy: final results of the phase III DATA trial. Ann Oncol. 2022;33(suppl 7):S55-S84. doi: 10.1016/annonc/annonc1038. [DOI] [Google Scholar]
- 20. Rapuri PB, Gallagher JC, Haynatzki G. Endogenous levels of serum estradiol and sex hormone binding globulin determine bone mineral density, bone remodeling, the rate of bone loss, and response to treatment with estrogen in elderly women. J Clin Endocrinol Metab. 2004;89(10):4954-62. doi: 10.1210/jc.2004-0434. Erratum in: J Clin Endocrinol Metab. 2005;90(3):1711. https://pubmed.ncbi.nlm.nih.gov/15472191/. [DOI] [PubMed] [Google Scholar]
- 21. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299-1309. https://pubmed.ncbi.nlm.nih.gov/21743022/. [DOI] [PubMed] [Google Scholar]
- 22. Dowsett M, Sestak I, Regan MM, et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol. 2018;36(19):1941-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sparano JA, O'Neill A, Alpaugh K, et al. Circulating tumor cells (CTCs) five years after diagnosis are prognostic for late recurrence in operable stage II-III breast cancer [abstract]. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium; Dec 5-9, 2017; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2018;78(Suppl 4):Abstract nr GS6-03.
- 24. Dubsky P, Brase JC, Jakesz R, et al. ; for the Austrian Breast and Colorectal Cancer Study Group (ABCSG). The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109(12):2959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wolmark N, Mamounas EP, Baehner FL, et al. Prognostic impact of the combination of recurrence score and quantitative estrogen receptor expression (ESR1) on predicting late distant recurrence risk in estrogen receptor-positive breast cancer after 5 years of tamoxifen: results from NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-28 and B-14. J Clin Oncol. 2016;34(20):2350-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14(11):1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Filipits M, Nielsen TO, Rudas M, et al. ; for the Austrian Breast and Colorectal Cancer Study Group. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clinical Cancer Res. 2014;20(5):1298-1305. [DOI] [PubMed] [Google Scholar]
- 28. Blok EJ, Bastiaannet E, van den Hout WB, et al. Systematic review of the clinical and economic value of gene expression profiles for invasive early breast cancer available in Europe. Cancer Treat Rev. 2018;62:74-90. [DOI] [PubMed] [Google Scholar]
- 29. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst. 2013;105(14):1036-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bartlett JMS, Sgroi DC, Treuner K, et al. Breast Cancer Index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen-To Offer More? (aTTom) trial. Ann Oncol. 2019;30(11):1776-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noordhoek I, Treuner K, Putter H, et al. Breast cancer index predicts extended endocrine benefit to individualize selection of patients with HR+ early-stage breast cancer for 10 years of endocrine therapy. Clin Cancer Res. 2021;27(1):311-319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification, will generally be available within 1 year after publication and will be accessible through the National Clinical Trials Network Data Archive.