Abstract
A synthetic acetone operon (ace4) composed of four Clostridium acetobutylicum ATCC 824 genes (adc, ctfAB, and thl, coding for the acetoacetate decarboxylase, coenzyme A transferase, and thiolase, respectively) under the control of the thl promoter was constructed and was introduced into Escherichia coli on vector pACT. Acetone production demonstrated that ace4 is expressed in E. coli and resulted in the reduction of acetic acid levels in the fermentation broth. Since different E. coli strains vary significantly in their growth characteristics and acetate metabolism, ace4 was expressed in three E. coli strains: ER2275, ATCC 11303, and MC1060. Shake flask cultures of MC1060(pACT) produced ca. 2 mM acetone, while both strains ER2275(pACT) and ATCC 11303(pACT) produced ca. 40 mM acetone. Glucose-fed cultures of strain ATCC 11303(pACT) resulted in a 150% increase in acetone titers compared to those of batch shake flask cultures. External addition of sodium acetate to glucose-fed cultures of ATCC 11303(pACT) resulted in further increased acetone titers. In bioreactor studies, acidic conditions (pH 5.5 versus 6.5) improved acetone production. Despite the substantial acetone evaporation due to aeration and agitation in the bioreactor, 125 to 154 mM acetone accumulated in ATCC 11303(pACT) fermentations. These acetone titers are equal to or higher than those produced by wild-type C. acetobutylicum. This is the first study to demonstrate the ability to use clostridial genes in nonclostridial hosts for solvent production. In addition, acetone-producing E. coli strains may be useful hosts for recombinant protein production in that detrimental acetate accumulation can be avoided.
Significant improvements in acetone-butanol (AB) fermentation by Clostridium acetobutylicum must be achieved before it can become an economically viable industrial process (8, 12). Key factors which contribute to the elevated costs of fermentative production of acetone and butanol are the low product titers and low product selectivity. Butanol inhibits cell growth even at relatively low concentrations, and its final titers are limited to ca. 13 g/liter. This and the low product selectivity (i.e., the production of more than one product) result in increased costs for product separation. In addition, continuous cultures have been of limited applicability because solventogenic clostridia degenerate under continuous-culture conditions; that is, they stop producing solvents.
Several approaches have been undertaken in the last 15 years to overcome current limitations in AB fermentation by reducing separation costs and improving bioreactor productivity (6, 7, 12, 21, 30). Metabolic engineering strategies may also prove beneficial towards improving the economics of the AB fermentation. Recent advances in the genetics of solventogenic clostridia (3, 17, 29) and early efforts to metabolically engineer clostridia (15, 16) are promising and may eventually lead to an industrially attractive AB fermentation. Both acetone and butanol are currently being produced from petrochemical processes (12). Specifically, acetone can be produced as a by-product of either phenol production from cumene or oxidation cracking of propane. It can also be produced from isopropanol reduction.
Heterologous expression of solvent formation clostridial genes in industrial organisms such as Saccharomyces cerevisiae, Escherichia coli, and Alcaligenes eutrophus may also prove attractive for solvent production. The possibility of producing only butanol or acetone at high titers (in cases where product tolerance is high) combined with established high-cell-density fermentation technologies is the main attraction of this alternative strategy. In addition, the availability of a wide variety of molecular biological techniques for these organisms allows chromosomal integration of genes and thus the construction of stable strains. Such strains can potentially be used in continuous cultures, thus resulting in further-reduced production costs.
We report here the heterologous expression of clostridial genes in E. coli for acetone production and the prevention of acetate accumulation. The clostridial acetone pathway uses acetyl coenzyme A (acetyl-CoA) as the initial substrate and subsequently converts it to acetone via reactions catalyzed by three enzymes. Thiolase catalyzes the condensation reaction of two acetyl-CoA molecules to generate acetoacetyl-CoA (20, 27). Acetoacetyl-CoA and one of the two acid products (acetic or butyric acid) are converted into acetoacetate and the corresponding acyl-CoA (acetyl-CoA or butyryl-CoA) via acetoacetyl-CoA:acetate/butyrate:CoA transferase (CoAT) (18, 28). Finally, the acetoacetate decarboxylase (AADC) catalyzes the conversion of acetoacetate to acetone and carbon dioxide (19).
Since in recombinant E. coli the acetone pathway can use only acetic acid as a substrate for the CoAT, acetone titers will probably depend on the production of acetic acid, unless acetic acid is externally supplied. In E. coli, acetic acid is produced both aerobically and anaerobically. In a pathway analogous to the clostridial one, acetyl-CoA is converted to acetyl phosphate by phosphotransacetylase. Acetyl phosphate can transfer its high-energy phosphate to ADP via acetate kinase to produce acetic acid and ATP (5). These enzymes are expressed under both aerobic and anaerobic conditions and can function in both directions. In fact, when E. coli grows under aerobic conditions and high glucose concentrations, the majority of the acetyl-CoA is converted to acetate via this pathway. Anaerobically, acetate production is used by E. coli to generate sufficient energy for growth (11).
MATERIALS AND METHODS
Bacterial strains and plasmids.
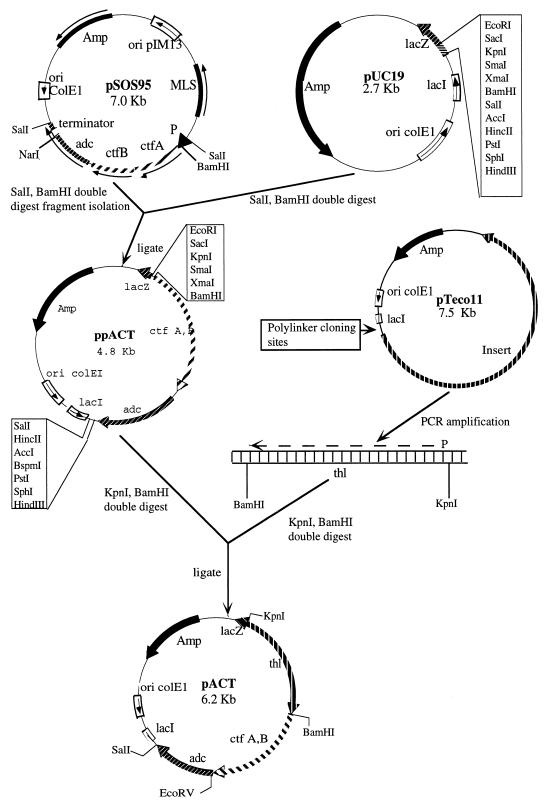
Table 1 lists all bacterial strains and plasmids used in this study. DNA was manipulated by standard molecular cloning techniques. The construction of plasmids pACT and ppACT is shown in Fig. 1. ppACT was constructed by cloning the 2.2-kb fragment from plasmid pSOS95 (containing the adc and ctfAB genes and the rho-independent transcriptional terminator of the clostridial adc gene) into pUC19 (Fig. 1). The clostridial thiolase promoter in front of the ctfA, ctfB, and adc genes in pSOS95 was deleted in ppACT. Blue-white colony selection was utilized to isolate colonies harboring the desired 4.8-kb vector (ppACT). The clostridial thiolase promoter and gene (thl) were amplified by PCR (4). The upstream primer KPNI-THS (5′-CAT GAT TTT AAG GGG GGT ACC ATA TGC A-3′) was generated by substituting T for G at nucleotide position 286 and G for C at nucleotide position 289 to provide an internal KpnI restriction site (underlined). The downstream primer BAMHI-TH (5′-GTT ATT TTT AAG GAT CCT TTA TAG CAC-3′) was designed by substituting C for G at position 1675, A for G at position 1676, and A for C at nucleotide position 1679 in order to introduce an internal BamHI site (underlined). The PCR-amplified fragment was ligated into the ppACT vector. The construction of pACT was verified by restriction enzyme analysis.
TABLE 1.
Bacterial strains and plasmids
| E. coli strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| B | Wild type | ATCC 11303b |
| ER2275 | trp31 his1 tonA2 rpsL104 supE44 xyl-7 mtl-2 metB1 e14+ Δ(lac)U169 endA1 recA1 R(zgb-210::Tn10) Tets Δ(mcr-hsd-mrr)114::IS10/F′ proAB lacI ZΔM15zz::min-Tn10 (Kmr) | NEBc |
| MC1060 | F− Δ(lacI-lacY)74 galE15 galK16 relA1 rpsL150 spoT1 hsdR2 λ− | CGSC 6648d |
| Plasmids | ||
| pUC19 | 2.6 kb; Ampr | 31 |
| pSOS95 | 7.0 kb; Ampr Emradc ctfA ctfB | 24a |
| pTECO11 | 7.4 kb; Amprthl | 20 |
| ppACT | 4.8 kb; Ampradc ctfA ctfB | This study |
| pACT | 6.2 kb; Amprthl adc ctfA ctfB | This study |
Abbreviations: adc, AADC gene; ctfA and ctfB, genes for the acetoacetyl-CoA:acetate/butyrate:CoAT subunits; recA, homologous recombination abolished; lacZ, β-galactosidase gene; mcrBC, methylcytosine-specific restriction system; thl, thiolase gene; Ampr, ampicillin resistant.
From American Type Culture Collection, Rockville, Md.
NEB, New England BioLabs, Beverly, Mass.
From the E. coli Genetic Stock Center.
FIG. 1.
Cloning steps for the construction of plasmids ppACT and pACT. For each plasmid, the location and direction of transcription for relevant genes are indicated (arrows). Relevant restriction sites are shown. Abbreviations: Amp, ampicillin resistance gene; MLS, erythromycin resistance gene; ori colE1, gram-negative origin of replication; ori pIM13, gram-positive origin of replication; P, thiolase promoter; lacZ, β-galactosidase gene; lacI, lac repressor gene; adc, AADC gene; thl, thiolase gene; and ctf A,B, units A and B, respectively, of the CoAT gene.
Growth conditions and maintenance.
E. coli strains were grown at 37 or 30°C as specified. Three media were used to grow cultures: Luria-Bertani (LB) and SD-7 and SD-8 (14) media. SD-7 and SD-8 media were utilized for shake flask experiments and bioreactor runs. SD-8 medium was modified as follows: yeast extract (5 g/liter), ampicillin (100 μg/ml), or carbenicillin (50 μg/ml). Both recombinant and wild-type strains were stored at −85°C in 15% (vol/vol) glycerol (23).
Shake flask cultures.
SD-7 medium (5 ml in 15- by 1.5-cm tubes) was inoculated with a single colony (from fresh LB medium plates) and grown overnight at 37°C. The resulting culture was utilized to inoculate 125 ml of SD-8 medium in 2-liter flasks. Selective pressure was maintained with ampicillin (100 μg/ml) unless otherwise specified. The cultures were incubated in a gyratory incubator-shaker (model G25; New Brunswick Scientific, Edison, N.J.) at 250 rpm and 37°C. The culture-to-flask volume ratio was maintained at 1:16 in order to ensure adequate oxygenation.
Bioreactor experiments.
Aerobic large-scale fed-batch fermentations of E. coli were performed in a BioFLo II fermentor (5.5 liters; New Brunswick Scientific) with a starting culture volume of 4.0 liters. Growth was monitored through A600 measurements made with a spectrophotometer (DU-65; Beckman Instruments, Fullerton, Calif.) (lightpath length, 1 cm). A 5-ml volume of SD-7 medium was inoculated with a single colony (from fresh LB medium plates) and grown overnight at 37°C. The resulting culture was utilized to inoculate 400 ml of SD-8 (pH 7.0) medium in a flask which was incubated at 30°C (reduced temperature was used in order to reduce acetone evaporation). When the flask culture reached mid-exponential growth phase (A600 of ca. 0.5), it was used to inoculate the bioreactor. The cultivation temperature was maintained at 30°C throughout the fermentation. The pH was continuously monitored with a pH Fermprobe (model F-620-B310-DH; Broadly James Corp., Santa Ana, Calif.). The pH was also measured off-line with an Accumet pH meter (model 925; Fisher Scientific, Pittsburgh, Pa.), and set-point adjustments were made in order to account for drifts in the readings of the Fermprobe. The pH was controlled as specified by using 6 M NH4OH or 3 M HCl. The dissolved oxygen concentration was monitored with a galvanic Phoenix probe (Phoenix Electrode Company, Houston, Tex.) and controlled at the desirable level. The reactor was sparged initially with 0.1 vvm (volume of gas per volume of medium per minute) of air regulated with a flow meter (Cole-Palmer, Niles, Ill.). As the culture progressed through the exponential phase, O2 sparging was used to maintain a dissolved oxygen concentration of 10%. During aerobic fermentations, the agitation speed was adjusted automatically (but never exceeded 250 rpm) throughout the fermentation to prevent cultures from becoming oxygen limited.
Glucose and fermentation product analysis.
Glucose and fermentation product concentrations in supernatant fluids were determined by using a glucose analyzer and gas chromatography, respectively, as described previously (15).
RESULTS
Strain evaluation.
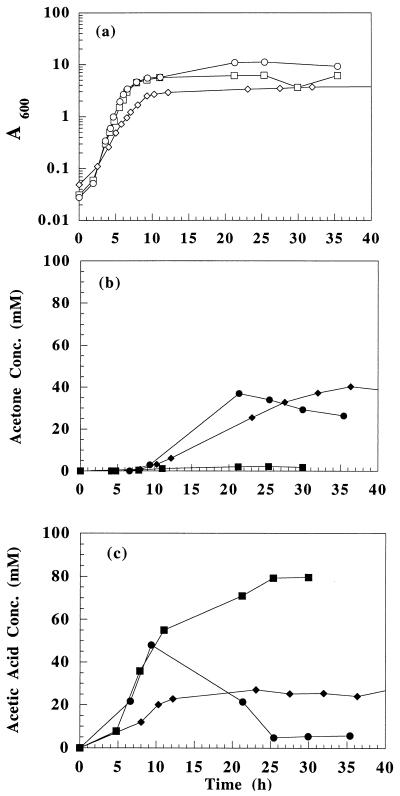
The acetone pathway from C. acetobutylicum was introduced into three E. coli strains via the multicopy-number vector pACT (Fig. 1). Plasmid pACT contains the clostridial synthetic acetone operon (ace4) composed of four C. acetobutylicum ATCC 824 genes (adc, ctfA, ctfB, and thl) under the control of the thl promoter. Plasmid isolation and restriction enzyme analysis were used to confirm the integrity of all vectors in each strain. The heterologous expression of the thl, adc, and ctfAB genes (for the production of the thiolase, AADC, and CoAT enzymes, respectively) resulted in acetone production (Fig. 2), indicating that the clostridial metabolic pathway is functional in E. coli and capable of converting glucose into acetone by acetate uptake.
FIG. 2.
Evaluation of E. coli ER2275(pACT) (◊ and ⧫), MC1060(pACT) (□ and ▪), and ATCC 11303(pACT) (○ and •) in batch shake flask experiments. Time profiles of cell growth (a), acetone concentration (b), and acetic acid concentration (c) are shown.
Strain ER2275 grows relatively slowly and produces moderate amounts of acetate. Strain MC1060 grows fast and accumulates high concentrations of acetate. Strain B (ATCC 11303) grows fast and produces moderate amounts of acetate (Fig. 2 and reference 14). Following a period of acetate accumulation throughout the exponential phase, all three strains initiated acetone production when reaching the late exponential phase and maintained acetone production during the stationary phase. Upon initiation of acetone production, acetic acid levels decreased, and a relatively constant concentration was reached. Strain ER2275(pACT) produced approximately 40 mM acetone before exhausting the glucose supply. The acetone levels produced by ATCC 11303(pACT) were similar to those of ER2275(pACT), but the volumetric acetone production rate was approximately twice that of ER2275(pACT). Strain MC1060(pACT) produced approximately 80 mM acetic acid and 2 mM acetone. Even though high levels of acetic acid were produced, the carbon flow was not diverted towards acetone production in this strain. Strain ATCC 11303(pACT) was able to grow to an optical density at 600 nm (A600) of approximately 11, the highest among the three strains tested. Strain ER2275(pACT) achieved an A600 of 4.1, and strain MC1060(pACT) achieved an A600 of 6.1. Since strains ATCC 11303(pACT) and ER2275(pACT) were able to accumulate the highest levels of acetone, they were selected for further studies.
Strain ER2275(ppACT), which carries ppACT, with only three of the four genes in the acetone pathway (adc and ctfAB), but without a promoter, could not produce acetone. Cultures of E. coli ER2275(pACT) and ER2275(ppACT) (control strain) differed in growth and pH characteristics. The final optical densities of these two cultures were virtually the same, although the specific growth rate of the non-acetone-producing culture was slightly higher (data not shown). Because ER2275(ppACT) was incapable of diverting acetic acid production towards acetone formation, the culture pH dropped to significantly lower levels than in the ER2275(pACT) culture. In addition, the ER2275(pACT) culture accumulated lower levels of acetate than the ER2275(ppACT) culture. These results suggest that the introduction of the acetone pathway enables E. coli to detoxify the medium (in a manner similar to that of clostridia) by converting acetate to acetone, and as a result, to prevent the decrease in culture pH.
Batch versus glucose-fed cultures.
In batch cultures, strains ER2275(pACT) and ATCC 11303(pACT) completely exhausted the glucose supply. Thus, glucose-fed cultures were used to examine whether higher acetone titers could be obtained under conditions in which the carbon source is not limiting. This resulted in high cell densities and an extended stationary phase and acetone production period. Glucose-fed shake flask cultures of strains ER2275(pACT) and ATCC 11303(pACT) were conducted by adding glucose and magnesium when the glucose concentration dropped to 20 mM (Table 2). During the late stationary phase, trace minerals were also added to the medium. ATCC 11303(pACT) exhausted the initial glucose supply after ca. 15 h of fermentation. Strain ER2275(pACT) exhausted the initial carbon supply after ca. 30 h. The pH of the ATCC 11303(pACT) culture was maintained above 4.5 through base addition. The pH of the ER2275(pACT) culture, however, did not drop significantly below 5.0. Glucose-fed cultures of strain ER2275(pACT) accumulated relatively high levels of acetic acid due to glucose catabolism, but acid production was not diverted towards acetone formation, and consequently, acetone concentrations were equivalent to those in batch cultures. In contrast, strain ATCC 11303(pACT) maintained a fairly constant level of acetic acid and accumulated higher levels of acetone than in batch cultures. Nutrient (glucose and magnesium) addition to the cultures resulted in a 150% increase in acetone levels (to over 90 mM) with respect to those in batch cultures. Acetone titers for glucose-fed cultures of strain ATCC 11303(pACT) were equivalent to concentrations routinely produced by wild-type C. acetobutylicum. In these experiments and the bioreactor experiments reported below, the acetone yield on glucose (moles of acetone produced per mole of glucose consumed) under stationary-phase growth conditions was typically below 0.50 but was transiently 0.7 to 0.8 (these values should be higher in view of the evaporative loss of acetone; see below). The theoretical yield under stationary-phase growth conditions is 1, since 1 mol of acetone results from 1 mol of acetoacetyl-CoA which is the result of the condensation reaction of 2 mol of acetyl-CoA, which can be produced from 1 mol of glucose through glycolysis.
TABLE 2.
Summary of glucose-fed shake flask experiments
| Strain | Maximum concn (mM)
|
Final A600a | μb (h−1) | Doubling time (h) | Qacnc (mM/h) | qacnd (mM/h/A600) | ||
|---|---|---|---|---|---|---|---|---|
| Acetone | Acetate | Ethanol | ||||||
| ATCC 11303(pACT) | 93 | 23 | 3 | 16.0 | 0.91 | 0.76 | 2.7–3.0 | 0.20 |
| ER2275(pACT) | 37 | 63 | 4 | 5.1 | 0.53 | 1.3 | 1.7 | 0.37 |
A600, optical density at 600 nm.
μ, specific growth rate.
Qacn, acetone formation rate throughout the exponential and stationary phases.
qacn, specific acetone formation rate during the stationary phase.
Glucose-fed cultures of ATCC 11303(pACT) with acetate addition.
In order to examine whether the acetate supply is a limiting factor in the production of acetone, strain ATCC 11303(pACT) shake flask cultures were grown as described above until the cells reached the stationary phase. We prepared a 1.2 M (100 g/liter) sodium acetate stock solution from which 2.4 mmol (2 ml) was added to the cultures every ca. 10 h after the stationary phase was reached. Sodium acetate was not added to the control culture. Glucose was periodically added to the cultures as before. The results from these parallel cultures are shown in Table 3. The control culture achieved a maximum acetone concentration of ca. 66 mM. Pulse additions of 2 ml of 1.2 M sodium acetate solution during the stationary phase at approximately 22, 32, and 42 h resulted in increased levels of acetone. This culture achieved a maximum acetone concentration of 109 mM. As expected, the acetate levels of the two cultures differed significantly. Acid levels in the control culture were maintained below 35 mM, with a significant decrease between hours 60 and 80. The culture with acetate addition reached a maximum acetic acid concentration of ca. 60 mM. In this culture, acetate concentration decreased after 60 h in the same manner as in the control culture. Both cultures completely exhausted the glucose supply by h 60. These findings were confirmed by additional experiments and suggest that acetate is a limiting factor for acetone production.
TABLE 3.
Final optical density, maximum product concentration, and acetone production rates in ATCC 11303(pACT) glucose-fed shake flask experiments with sodium acetate pulse additions
| Sodium acetate pulse addition | Final A600a | Maximum product concn (mM)
|
Qacnb (mM/h) | qacnc (mM/h/A600) | |
|---|---|---|---|---|---|
| Acetone | Acetate | ||||
| None (control) | 14.0 | 81 | 35 | 0.90 | 0.06 |
| 2 ml of 1.2 M solutiond | 13.0 | 109 | 66 | 1.45 | 0.11 |
A600, optical density at 600 nm.
Qacn, acetone formation rate during the stationary phase.
qacn, specific acetone formation rate during the stationary phase.
Three pulse additions at h 22, 32, and 42.
Bioreactor experiments. (i) Oxygen requirements.
Experiments were conducted to evaluate if acetone could be produced under anaerobic conditions. ATCC 11303(pACT) cultures in which the pH was maintained above 5.0 and which were allowed to become anaerobic at the onset of the stationary phase accumulated only small quantities of acetone (less than 12 mM). Cultures that were maintained under strict anaerobic conditions produced ethanol as the main fermentation product and very little acetone (less than 7 mM). These experiments suggest that in order to accumulate significant levels of acetone, aerobic conditions are necessary.
(ii) Effect of pH.
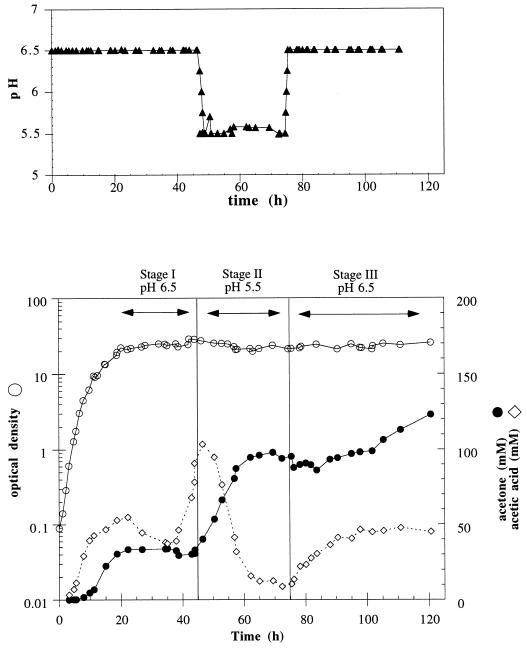
To further evaluate pH effects on acetone formation, glucose-fed bioreactor experiments were conducted under aerobic conditions (Fig. 3). During the fermentation, the culture pH was maintained at either 6.5 or 5.5. Glucose additions were used to prevent the culture from becoming carbon limited. Culture growth progressed adequately when the pH was maintained at 6.5 during the first 48 h (stage I). Acetone was produced at a rate of 2.5 mM/h until the acetone concentration leveled off at ca. 35 mM. Afterwards, the culture pH was switched to 5.5 for approximately 30 h (stage II). In stage II, a significant reduction in the glucose consumption was observed (from ca. 13 to 4 mM/h), which suggests a decrease in cell metabolism. Acetic acid concentration decreased continuously to 10 mM, while acetone concentration increased (at an average rate of 3.7 mM/h), leveling off at ca. 100 mM. Subsequently, the pH of the culture was adjusted and maintained at 6.5 (stage III). During this period, the levels of acetic acid increased and acetone production resumed at a rate of ca. 1 mM/h, reaching ca. 125 mM at the time the experiment was terminated. These and other experiments (data not shown) suggest that the higher pH promotes cell growth and glucose catabolism, while the lower pH favors acetate reuptake and acetone production.
FIG. 3.
Effect of culture pH on acetone production and acetate utilization in a bioreactor E. coli ATCC 11303(pACT) aerobic fermentation.
Acetone evaporation.
Acetone evaporation is quite substantial, especially in the bioreactor cultures, due to agitation and gas sparging. Acetone evaporation remained substantial despite the fact that we have used experimental conditions (lower temperature [30°C], agitation rates below 250 rpm, and sparging of pure O2, thus avoiding aeration above 0.1 vvm) to minimize it. We carried out experiments to estimate the rate of acetone evaporation in the bioreactor (under the experimental conditions used) as ca. 1 mM/h. If this acetone evaporation rate is taken into account, the final titers of acetone in the experiment of Fig. 3 would have been at least 200 mM. In another bioreactor experiment, acetone accumulated to 154 mM, which would correspond to over 250 mM acetone if evaporation is taken into account.
DISCUSSION
Since E. coli strains differ significantly in their acetate metabolism (14, 24), the abilities of three E. coli strains to produce acetone were evaluated (Fig. 2). The strains were selected on the basis of their growth rate and acetic acid production characteristics. Strain ATCC 11303(pACT) had the most promising characteristics of the three. Of the two strains cultivated in glucose-fed cultures, only E. coli ATCC 11303(pACT) accumulated acetone levels substantially higher than those in batch culture. The specific acetone production rate of strain ER2275(pACT) was higher than that of strain ATCC 11303(pACT), but the maximum acetone concentration of the ATCC 11303(pACT) cultures was 2.5 times higher (Table 2). E. coli ATCC 11303(pACT) cultures achieved significantly higher cell densities and were capable of further growth (at low rates) during the stationary phase. The concentration of acetic acid throughout the ATCC 11303(pACT) fermentation was consistently lower than that of strain ER2275(pACT).
The experimental observation that significant levels of acetone are not produced under anaerobic conditions is consistent with E. coli biochemistry. Under anaerobic conditions, E. coli generates a combination of reduced fermentation products in ratios necessary to generate ATP and reoxidize the NAD(P)H generated by the glycolytic production of pyruvate. Acetic acid production is the main means for ATP generation, while ethanol production is necessary to reoxidize NAD(P)H. Under anaerobic and no-growth conditions, E. coli generates 1 mol each of ethanol and acetate and 2 mol of formic acid per mol of glucose utilized. Formic acid does not usually accumulate to significant amounts because it is further catabolized into hydrogen and carbon dioxide. The acetone formation pathway cannot recycle reducing equivalents [NAD(P)H] and as a consequence is unable to functionally substitute for the ethanol formation pathway. It serves only as a detoxification mechanism, since it converts acetic acid to acetone via the net utilization of an additional mole of acetyl-CoA. Thus, acetone production under anaerobic conditions would result in the consumption of acetyl-CoA generated during glycolysis without NAD(P)H reoxidation and eventually would lead to cessation of glycolysis and thus cell metabolism. The situation is radically different under aerobic conditions, whereby NAD(P)H oxidation via the electron transport chain produces additional ATP and makes sustained acetone production possible.
The data of Fig. 3 (and other data not presented here) suggest that lower pH (here, 5.5 versus 6.5) is beneficial for acetone production, although the lower pH is detrimental for glucose catabolism and thus acetate formation. A possible explanation may be based on the fact that the rate of acetic acid transport and the effective species of acetic acid (its undissociated form) are higher at lower pH values. As the culture pH decreases, the amount of undissociated acetic acid increases. Since only the undissociated form can permeate the cell membrane and serve as a substrate in enzymatic reactions, a lower pH would promote a faster acetate transport and possibly also a faster rate of acetate use by the CoAT reaction, the latter due to a higher concentration of the undissociated form of acetic acid resulting from a lower intracellular pH.
The importance of relatively high acetate concentrations for acetone formation by recombinant E. coli was demonstrated by the data of Table 3 and data from additional experiments (not shown). This requirement for high acetic acid concentrations is probably due to the properties of the clostridial CoAT, which has a Km for acetate of 1,200 mM (28). Assuming that the E. coli CoAT does not contribute significantly to the uptake of acetate to form acetoacetate, this clostridial CoAT is clearly suboptimal for acetone production in E. coli precisely because of this very large Km. Thus, a possible improvement of the ace4 construct would be the use of a CoAT with a low Km for acetate. This is likely to prove beneficial for acetate detoxification and acetone production by driving the uptake of acetate towards acetone formation at much lower acetate concentrations. In addition, it will likely make acetone formation less pH dependent, a practically desirable characteristic.
We have not examined what is the minimum number of genes required for acetone production in E. coli. If all three clostridial enzyme activities are necessary for acetone production, it is quite remarkable that E. coli can express the genes of, and effectively utilize, a multistep clostridial pathway, given the vastly different genetic and physiological characteristics of the two organisms. It is possible, however, that the autologous thiolase and CoAT activities in at least some strains of E. coli will make the need for expressing either or both of the corresponding clostridial genes unnecessary.
To our knowledge, this work is the first attempt to explore the potential of the heterologous expression of C. acetobutylicum metabolic pathways in an industrially relevant host such as E. coli. Many further improvements are possible beyond this initial study. Further optimization of the synthetic acetone formation operon as well as of fermentation conditions may lead to much higher rates of acetone formation. Wild-type and recombinant C. acetobutylicum strains form acetone at ca. 0.5 to 1 and 1 to 1.7 mM/h/A600, respectively (16). One would expect that E. coli strains with double or triple such productivities can be eventually developed. This, combined with the inexpensive acetone separation process and the development of improved technologies for obtaining inexpensive sugars from lignocellulosics, may lead to an economically attractive acetone fermentation. Specifically, acetone’s high volatility (boiling point of 56.5°C) allows its in situ removal from the fermentation broth by gas stripping (by air or O2 sparging already used in the process), especially under the higher fermentation temperatures (37 to 40°C or higher) that can be employed. A continuous or semicontinuous high-density E. coli fermentation will further reduce capital and operating costs to a fraction of the corresponding costs of the traditional C. acetobutylicum AB fermentation. Finally, industrial E. coli strains that can readily ferment the sugars in biomass hydrolysates (for ethanol production) have been reported (13).
An effective synthetic acetone operon may also prove useful in the in situ removal of acetate from high-density E. coli fermentations for the production of recombinant proteins (1, 2). Relatively complicated schemes have been developed to enable a tight control of glucose uptake and cell growth rates in high-cell-density fermentations in order to prevent the accumulation of high acetic acid levels (1, 2, 9, 10, 22, 25, 26, 32), which limit biomass formation. Acetone’s high volatility allows its removal from the fermentation broth by gas stripping (see above), thus diminishing any potential inhibitory effects due to acetone accumulation. This strategy may thus eliminate the need for complex fermentation control schemes to prevent acetate accumulation.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Nancy Ekdawi.
This research was supported by National Science Foundation (USA) grant BES 96332217.
REFERENCES
- 1.Aristidou A A, San K-Y, Bennett G N. Modification of central metabolic pathway in Escherichia coli to reduce acetate accumulation by heterologous expression of the Bacillus subtilis acetolactate synthase gene. Biotechnol Bioeng. 1994;44:944–951. doi: 10.1002/bit.260440810. [DOI] [PubMed] [Google Scholar]
- 2.Aristidou A A, San K-Y, Bennett G N. Metabolic engineering of Escherichia coli to enhance recombinant protein production through acetate reduction. Biotechnol Prog. 1995;11:475–478. doi: 10.1021/bp00034a019. [DOI] [PubMed] [Google Scholar]
- 3.Bennett G N, Rudolph F B. The central metabolic pathway from acetyl-CoA to butyryl-CoA in Clostridium acetobutylicum. FEMS Microbiol Rev. 1995;17:241–249. [Google Scholar]
- 4.Bermejo Martinez L L. Heterologous expression of Clostridium acetobutylicum genes in Escherichia coli for acetone production. M.S. thesis. Evanston, Ill: Northwestern University; 1996. [Google Scholar]
- 5.Clark D P. The fermentation pathways of Escherichia coli. FEMS Microbiol Rev. 1989;63:223–234. doi: 10.1016/0168-6445(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 6.Dadgar A M, Foutch G L. Improving the acetone-butanol fermentation process with liquid-liquid extraction. Biotechnol Prog. 1988;4:36–39. [Google Scholar]
- 7.Ishii S, Taya M, Kobayashi T. Production of butanol by Clostridium acetobutylicum in extractive fermentation system. J Chem Eng Jpn. 1985;18:125–130. [Google Scholar]
- 8.Jones D T, Woods D R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986;50:484–525. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleman G L, Chalmers J J, Luli G W, Strohl W R. A predictive and feedback control algorithm maintains a constant glucose concentration in fed-batch fermentations. Appl Biochem Biotechnol. 1991;57:910–917. doi: 10.1128/aem.57.4.910-917.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleman G L, Chalmers J J, Luli G W, Strohl W R. Glucose-stat, a glucose-controlled continuous culture. Appl Biochem Biotechnol. 1991;57:918–923. doi: 10.1128/aem.57.4.918-923.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin E C, Luchi S. Regulation of gene expression in fermentative and respiratory systems in Escherichia coli and related bacteria. Annu Rev Genet. 1991;25:361–387. doi: 10.1146/annurev.ge.25.120191.002045. [DOI] [PubMed] [Google Scholar]
- 12.Linden J C, Moreira A R, Lenz T G. Acetone and butanol. In: Moo-Young M, Blanch H W, Drew S, Wang D I C, editors. Comprehensive biotechnology: the principles, applications, and regulations of biotechnology in industry, agriculture, and medicine. New York, N.Y: Pergamon Press; 1985. pp. 915–930. [Google Scholar]
- 13.Lindsay S E, Bothast R J, Ingram L O. Improved strains of recombinant Escherichia coli for ethanol production from sugar mixtures. Appl Microbiol Biotechnol. 1995;43:70–75. doi: 10.1007/BF00170625. [DOI] [PubMed] [Google Scholar]
- 14.Luli G W, Strohl W R. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol. 1990;56:1004–1011. doi: 10.1128/aem.56.4.1004-1011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mermelstein L D, Papoutsakis E T, Petersen D J, Bennett G N. Metabolic engineering of Clostridium acetobutylicum for increased solvent production by enhancement of acetone formation enzyme activities using a synthetic acetone operon. Biotechnol Bioeng. 1993;42:1053–1060. doi: 10.1002/bit.260420906. [DOI] [PubMed] [Google Scholar]
- 16.Mermelstein L D, Welker N E, Petersen D J, Bennett G N, Papoutsakis E T. Genetic and metabolic engineering of Clostridium acetobutylicum ATCC824. Ann N Y Acad Sci. 1994;721:54–68. doi: 10.1111/j.1749-6632.1994.tb47376.x. [DOI] [PubMed] [Google Scholar]
- 17.Papoutsakis E T, Bennett G N. Cloning, structure, and expression of acid and solvent pathway genes of Clostridium acetobutylicum. In: Woods D R, editor. The clostridia and biotechnology. Stoneham, Mass: Butterworth-Heinemann; 1993. pp. 157–199. [PubMed] [Google Scholar]
- 18.Petersen D, Jeffrey W C, Vanderleyden J, Bennett G N. Sequence and arrangement of genes encoding enzymes of the acetone-production pathway of Clostridium acetobutylicum ATCC824. Gene. 1993;123:93–97. doi: 10.1016/0378-1119(93)90545-e. [DOI] [PubMed] [Google Scholar]
- 19.Petersen D J, Bennett G N. Purification of acetoacetate decarboxylase from Clostridium acetobutylicum ATCC 824 and cloning of the acetoacetate decarboxylase gene in Escherichia coli. Appl Environ Microbiol. 1990;56:3491–3498. doi: 10.1128/aem.56.11.3491-3498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen D J, Bennett G N. Cloning of the Clostridium acetobutylicum ATCC 824 acetyl coenzyme A acetyltransferase (thiolase; EC 2. 3. 1.9) gene. Appl Environ Microbiol. 1991;57:2735–2741. doi: 10.1128/aem.57.9.2735-2741.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi N, Maddox I S, Friedl A. Application of continuous substrate feeding to the ABE fermentation: relief of product inhibition using extraction, perstraction, stripping, and pervaporation. Biotechnol Prog. 1992;8:382–390. [Google Scholar]
- 22.Riesenberg D, Schulz V, Knorre W A, Polh H-D, Korz D, Sanders E A, Rob A, Deckwer W-D. High cell density cultivation of Escherichia coli at controlled specific growth rate. J Biotechnol. 1991;20:17–28. doi: 10.1016/0168-1656(91)90032-q. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 24.Shiloach J, Kaufman J, Guillard A S, Fass R. Effect of glucose supply strategy on acetate accumulation, growth and recombinant protein production by Escherichia coli BL21(1DE3) and Escherichia coli JM109. Biotechnol Bioeng. 1996;49:421–428. doi: 10.1002/(SICI)1097-0290(19960220)49:4<421::AID-BIT9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24a.Soucaille, P., and E. T. Papoutsakis. Unpublished data.
- 25.Strohl W R, Schlasner S M, Lorensen P L. Microcomputer-control of fermentation processes. BioTechniques. 1986;4:336–345. [Google Scholar]
- 26.Tartakovsky B, Ulitzur S, Sheintuch M. Optimal control of fed-batch fermentation with autoinduction of metabolite production. Biotechnol Prog. 1995;11:80–87. [Google Scholar]
- 27.Wiesenborn D P, Rudolph F B, Papoutsakis E T. Thiolase from Clostridium acetobutylicum ATCC 824 and its role in the synthesis of acids and solvents. Appl Environ Microbiol. 1988;54:2717–2722. doi: 10.1128/aem.54.11.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesenborn D P, Rudolph F B, Papoutsakis E T. Coenzyme A transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol. 1989;55:323–329. doi: 10.1128/aem.55.2.323-329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods D. The genetic engineering of microbial solvent production. Trends Biotechnol. 1995;13:259–264. doi: 10.1016/S0167-7799(00)88960-X. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Tsao G T. Enhanced acetone-butanol fermentation using repeated fed-batch operation coupled with cell recycle by membrane and simultaneous removal of inhibitory products by adsorption. Biotechnol Bioeng. 1995;47:444–450. doi: 10.1002/bit.260470405. [DOI] [PubMed] [Google Scholar]
- 31.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 32.Yee L, Blanch H W. Recombinant protein expression in high cell density fed-batch culture of Escherichia coli. Bio/Technology. 1992;10:1550–1555. doi: 10.1038/nbt1292-1550. [DOI] [PubMed] [Google Scholar]