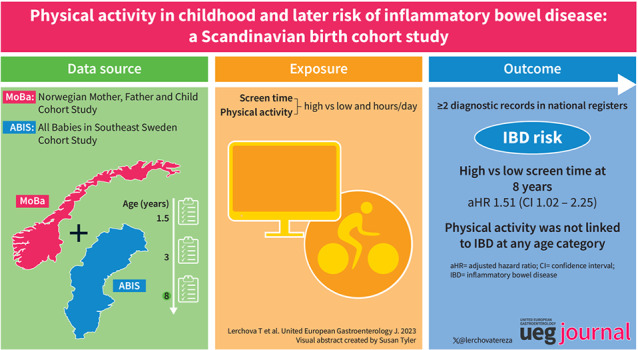
Abstract
Background and Objective
Retrospective data have linked adult physical activity (PA) to reduced risk of inflammatory bowel disease (IBD). We aimed to prospectively examine the association of PA and screen time (ST) in childhood with later risk of IBD, for which data are scarce.
Methods
Using two population‐based birth cohorts (All Babies in Southeast Sweden [ABIS] and Norwegian Mother, Father, and Child Cohort Study [MoBa]), we retrieved parent‐reported data on PA and ST degree at ages 3 and 8 years. Data were modelled as binary (high vs. low) and numerical (hours/day) exposures. Inflammatory bowel disease was defined as ≥2 diagnostic records in national health registers. Cox regression estimated hazard ratios adjusted for potential confounding from parental IBD, country of origin, education, and smoking habits (Adjusted hazard ratio (aHR)). Our 8‐year analyses included a 2‐year lag period to reduce the risk of reverse causation. Cohort‐specific estimates were pooled using random‐effects model.
Result
Among 65,978 participants from ABIS (n = 8810) and MoBa (n = 57,168) with available data, 266 developed IBD. At 3 years, children with high versus low PA had an aHR of 1.12 for IBD (95%CI = 0.87–1.43); high versus low ST showed an aHR of 0.91 (95%CI = 0.71–1.17). Conversely, at 8 years, high versus low ST was associated with increased risk of later IBD (aHR = 1.51; 95%CI = 1.02–2.25), but PA at 8 years, was not linked to IBD (aHR = 1.19; 95%CI = 0.80–1.76). Subtype‐specific analyses for Crohn's disease and ulcerative colitis did not differ appreciably.
Conclusion
Acknowledging possible confounding variables, children with high versus low ST at 8 years were at increased risk of IBD. In contrast, PA degree was not linked to IBD at any age category.
Keywords: children, Crohn's disease, inflammatory bowel disease, physical activity, screen time, ulcerative colitis
Key summary.
The established knowledge on this subject
Multiple environmental factors, including sedentary lifestyle, have been linked to the rising incidence of inflammatory bowel disease (IBD).
In adults, low physical activity (PA) has been positively associated with IBD risk.
Childhood PA has shown short‐ and long‐term health benefits.
The significant and/or new findings of this study
This is the first study to analyse a prospective cohort to assess relationship between IBD and childhood PA.
High compared to low 8‐years screen‐time was positively associated with the risk of IBD.
We neither observed association between IBD and PA at 3 years nor at 8 years of age.
INTRODUCTION
The degree of physical activity (PA) affects short‐ and long‐term health outcomes in children and adults, including gastrointestinal diseases. 1 , 2 There is also evidence of the direct impact of PA on the immune system, including a decline in lymphocyte counts, reduced number of secreted immunoglobulins, increased number of neutrophils, and alterations in their or macrophage functions. 3
Ecological evidence has linked the rising incidence of inflammatory bowel disease (IBD) to multiple environmental factors, including an increasingly sedentary lifestyle. 4 Low PA has been positively associated with later IBD development in adults, but data were mostly retrospective and subject to recall bias and even reverse causation. 5 , 6 , 7 , 8 To our knowledge, there has been no previous prospective examination of the degree of PA and screen time (ST) in childhood with the risk of later IBD.
This study used prospectively collected data from two Scandinavian birth cohorts to determine the relationship between PA and ST in childhood and the risk of IBD development.
METHODS
Study population
In this study, we leveraged prospectively collected questionnaire data obtained from two population‐based birth cohorts: All Babies in Southeast Sweden (ABIS) 9 and the Norwegian Mother, Father, and Child Cohort Study (MoBa) 10 and linked data from national health registers 11 , 12 (Figure 1).
FIGURE 1.
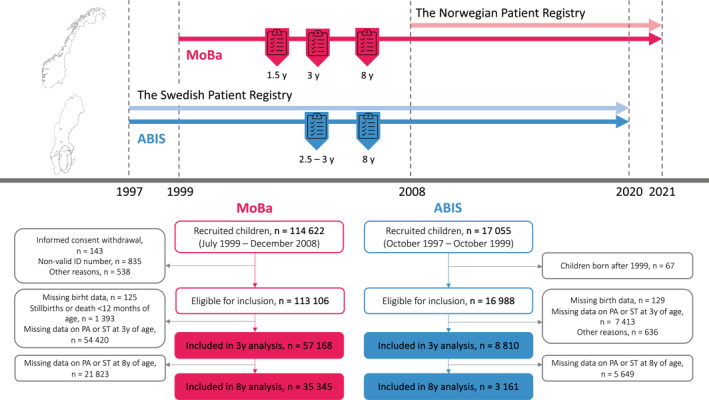
Flowchart and study design. Analyses were performed using ABIS data retrieved at ages 2.5–3 and 8 years (degree of physical activity and screen time). The MoBa data were retrieved at 1.5, 3 and 8 years of age (degree of physical activity and screen time). Line “2020” represents the end of the data capture in ABIS and line “2021” characterises the end of the data capture in MoBa. All children in ABIS and MoBa included in this study were linked at the individual level using a personal identification number (PIN) to data from the national health registers. ABIS, All Babies in Southeast Sweden; MoBa, The Norwegian Mother, Father and Child Cohort Study; PA, physical activity; ST, screen time; y, years.
All Babies in Southeast Sweden is a prospective birth cohort study inviting all 21,700 children born between 1 October 1997 and 1 October 1999 in the southeast region of Sweden to participate. 9 Parents of 17,055 children consented to participate (79% participation rate). We used ABIS questionnaires administered at birth and 2.5–3 and 8 years of age.
MoBa is a prospective birth cohort study conducted by the Norwegian Institute of Public Health. Between 1999 and 2009, pregnant women throughout Norway were invited to participate. A total of 114,500 children (41% response rate) were included in the cohort. 10 Questionnaire data from early pregnancy, 0.5 years, 1.5, 3, and 8 years of age were included in this work.
Exposure
Our primary exposure variables were the degree of PA and ST as reported in ABIS and MoBa at ages 3 and 8 years. A secondary exposure concerned the degree of PA and ST at age 1.5 years (these data were only available in MoBa). Given the structure of the questionnaires, PA exposure at 1.5 and 3 years was generally related to the amount of outdoor activity. In contrast, at 8 years, the data focused more on time spent in organised sports and active movement (e.g., jumping, running). The exposure of ST included sedentary activity defined as time spent in front of any screen (e.g., computer, television) at all time points (1.5, 3, and 8 years of age). For ST, information was reported in multiple questions (e.g., a specific question on time spent in front of the television, computer, or video games), we used the sum of the individual's total reported ST as the final exposure. The average amount of PA and ST per day was calculated for items reflecting PA and ST by weekdays (e.g., school days and weekends) and seasons (e.g., summer and winter). Harmonisation between ABIS and MoBa questionnaires is detailed in Supplementary Table S1.
We modelled PA and ST separately as binary (high vs. low) and numerical variables (per hour/day). Motivated by previous studies 13 , 14 and reflecting the structure of the questionnaire, we set the closest possible option of ≥2 h/day as the limit for high PA at 3 years in both cohorts. However, considering differences in questionnaires and PA distribution in MoBa and ABIS, cohort‐specific cut‐offs were used for the 8‐year data (ABIS: high ≥2 h/day, low <2 h/day; MoBa: high ≥1 h/day, low <1 h/day). All cut‐offs for ST were similar in ABIS and MoBa. Given the distribution of ST and reflecting approximate median levels at 3 years, high ST was defined as ≥1 h/day and low as <1 h/day. At 8 years, high ST was defined as ≥2 h/day and low as <2 h/day (Supplementary Table S1). To ensure their appropriateness, cut‐off values were compared against the New Canadian PA Guidelines. 15 Because children with high PA may be more likely to have low ST, and vice versa, we also compared children with reports of “High PA and Low ST” versus children with “Low PA and High ST” at 1.5 (only MoBa) 3, and 8 years using the PA and ST categorisations as described above.
Outcome
All Babies in Southeast Sweden and MoBa participants with an IBD diagnosis were identified using the country‐specific personal identification number using linked individual‐level data from the Swedish National Patient Register 12 and the Norwegian Patient Registry, respectively. 11 These registers, which share a similar data structure, contain nationwide diagnostic data on all inpatient and hospital‐based outpatient care in Sweden and Norway. Similar to previous research, 16 IBD was defined as a minimum of two International Classification of Diseases (ICD‐10) codes for the disease (Supplementary Table S2). This register‐based diagnostic algorithm has been shown to have a positive predictive value of 93% for IBD in adults and children on medical record review. 16 , 17 , 18 The date of the first record of IBD was set as the time of the diagnosis. Subtype‐specific ICD‐10 codes were used to distinguish UC from CD. 19 Events of IBD‐U were included in the outcome of IBD overall, but we did not examine it as a different outcome because of the higher misclassification risk of this subtype diagnosis. 16
Other data
Based on a critical evaluation of the current literature and available data, 20 , 21 we pre‐selected covariates associated with PA measures or IBD. Using the at‐birth questionnaire (ABIS), questionnaires in pregnancy, and at 6 months (MoBa), we retrieved information on parental IBD, parental/maternal origin, maternal immune‐mediated comorbidities, smoking during pregnancy, and educational level. Immune‐mediated diseases concern type 1 or insulin‐treated diabetes before/during pregnancy, rheumatoid arthritis, and autoimmune thyroid disease. In ABIS origin was defined by parental country of birth (Sweden or other [if Sweden has not been reported as the country of birth of the mother or father]) and in MoBa by the native language of the mother (Norwegian or any other language). The highest attained educational level was divided into three categories: low (9–11 years), medium (12 years), and high (≥13 years of education). Other covariates were classified as shown in Table 1. In MoBa, parental IBD diagnoses were defined using data from the Norwegian Patient Registry 11 and in ABIS as reported at birth.
TABLE 1.
Study characteristics of participants in All Babies in Southeast Sweden (ABIS) and MoBa birth cohorts at 3 years of age.
| ABIS | MoBa | |||||||
|---|---|---|---|---|---|---|---|---|
| All n = 8810 | IBD a n = 65 | CD n = 20 | UC n = 34 | All n = 57,168 | IBD a n = 201 | CD n = 92 | UC n = 52 | |
| Sex (%) | ||||||||
| Female | 4239 (48.1) | 33 (50.8) | 7 (35.0) | 18 (52.9) | 27,896 (48.8) | 89 (44.3) | 40 (43.5) | 30 (57.7) |
| Male | 4571 (51.9) | 32 (49.2) | 13 (65.0) | 16 (47.1) | 29,272 (51.2) | 112 (55.7) | 52 (56.5) | 22 (42.3) |
| Time of follow‐up b (years) | ||||||||
| Mean (SD) | 19.3 (0.9) | 14.2 (3.8) | 13.2 (4.2) | 14.6 (3.6) | 13.3 (2.0) | 9.6 (3.6) | 9.8 (3.3) | 10.5 (3.9) |
| Median (interquartile range) | 19.3 (18.8; 19.8) | 15.3 (12.6; 16.7) | 13.1 (11.3; 16.1) | 15.6 (13.1; 16.8) | 13.2 (11.7; 14.8) | 10.1 (7.6; 12.4) | 9.8 (8.0; 12.0) | 11.7 (8.9; 13.5) |
| Calendar year of study entry (birth) (%) | ||||||||
| 1997 | 901 (10.2) | 8 (12.3) | 2 (10.0) | 4 (11.8) | ‐ | ‐ | ‐ | ‐ |
| 1998 | 4680 (53.1) | 35 (53.8) | 12 (60.0) | 16 (47.1) | ‐ | ‐ | ‐ | ‐ |
| 1999 | 3229 (36.7) | 22 (33.8) | 6 (30.0) | 14 (41.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2000 | ‐ | ‐ | ‐ | ‐ | 3 (0.0) | 0 (0.0) | 0 (0.00) | 0 (0.0) |
| 2001 | ‐ | ‐ | ‐ | ‐ | 1063 (1.9) | 6 (3.0) | 1 (0.5) | 3 (5.8) |
| 2002 | ‐ | ‐ | ‐ | ‐ | 5067 (8.9) | 27 (13.4) | 13 (14.1) | 6 (11.5) |
| 2003 | ‐ | ‐ | ‐ | ‐ | 6917 (12.1) | 34 (16.9) | 12 (13.0) | 13 (25.0) |
| 2004 | ‐ | ‐ | ‐ | ‐ | 7852 (13.7) | 30 (14.9) | 13 (14.1) | 10 (19.2) |
| 2005 | ‐ | ‐ | ‐ | ‐ | 8943 (15.6) | 33 (16.4) | 13 (14.1) | 9 (17.3) |
| 2006 | ‐ | ‐ | ‐ | ‐ | 9592 (16.8) | 26 (12.9) | 15 (16.3) | 4 (7.7) |
| 2007 | ‐ | ‐ | ‐ | ‐ | 8625 (15.1) | 18 (9.0) | 9 (9.8) | 4 (7.7) |
| 2008 | ‐ | ‐ | ‐ | ‐ | 7232 (12.7) | 24 (11.9) | 14 (15.2) | 3 (5.8) |
| 2009 | ‐ | ‐ | ‐ | ‐ | 1874 (3.3) | 3 (1.5) | 2 (2.2) | 0 (0.0) |
| PA level at 3 years c (%) | ||||||||
| Low | 4180 (47.4) | 32 (49.2) | 8 (40.0) | 18 (52.9) | 37,142 (65.0) | 122 (60.7) | 61 (66.3) | 28 (53.9) |
| High | 4535 (51.5) | 32 (49.2) | 11 (55.0) | 16 (47.1) | 19,605 (34.2) | 78 (38.8) | 31 (33.7) | 24 (46.1) |
| Missing | 95 (1.1) | 1 (1.5) | 1 (5.0) | 0 (0.0) | 421 (0.7) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| ST level at 3 years d (%) | ||||||||
| Low | 3978 (45.2) | 30 (46.2) | 11 (55.0) | 14 (41.2) | 33,543 (58.7) | 124 (61.7) | 52 (56.5) | 39 (75.0) |
| High | 4423 (50.2) | 33 (50.8) | 8 (40.0) | 19 (55.9) | 23,401 (40.9) | 77 (38.3) | 40 (43.5) | 13 (25.0) |
| Missing | 409 (4.6) | 2 (3.1) | 1 (5.0) | 1 (2.9) | 224 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Parental IBD (%) | ||||||||
| No | 8702 (98.8) | 63 (96.9) | 19 (95.0) | 33 (97.1) | 55,809 (97.6) | 196 (97.5) | 90 (97.8) | 50 (96.2) |
| Yes | 108 (1.2) | 2 (3.1) | 1 (5.0) | 1 (2.9) | 1359 (2.4) | 5 (2.5) | 2 (2.2) | 2 (3.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | (0.0) |
| Parental origin e (%) | ||||||||
| Norwegian/Swedish | 7841 (89.0) | 55 (84.6) | 18 (90.0) | 27 (79.4) | 53,895 (94.3) | 193 (96.0) | 89 (96.7) | 51 (98.1) |
| Other country | 773 (8.8) | 9 (13.8) | 2 (10.0) | 6 (17.6) | 2805 (4.9) | 8 (4.0) | 3 (3.3) | 1 (1.9) |
| Missing | 196 (2.2) | 1 (1.5) | 0 (0.0) | 1 (2.9) | 468 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Maternal comorbidity f (%) | ||||||||
| No | 8506 (96.5) | 63 (96.9) | 20 (100.0) | 32 (94.1) | 54,862 (96.0) | 192 (95.5) | 88 (95.7) | 49 (94.2) |
| Yes | 304 (3.5) | 2 (9.1) | 0 (0.0) | 2 (5.9) | 2306 (4.0) | 9 (4.5) | 4 (4.3) | 3 (5.8) |
| Missing | 0 (0.0) | (0.0) | (0.0) | (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Maternal educational level g (%) | ||||||||
| Compulsory school | 584 (6.6) | 7 (10.8) | 2 (10.0) | 5 (14.7) | 3164 (5.5) | 15 (7.5) | 7 (7.6) | 4 (7.7) |
| High school | 4747 (53.9) | 35 (53.8) | 10 (50.0) | 18 (52.9) | 15,166 (26.5) | 58 (28.9) | 23 (25.0) | 17 (32.7) |
| University/College | 3277 (37.2) | 22 (33.8) | 8 (40.0) | 10 (29.4) | 38,163 (66.8) | 127 (63.2) | 62 (67.4) | 31 (59.6) |
| Missing | 20 (0.2) | 1 (1.5) | 0 (0.0) | 1 (2.9) | 675 (1.2) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Maternal smoking during pregnancy (%) | ||||||||
| No | 7857 (89.2) | 57 (87.7) | 18 (90.0) | 29 (85.3) | 52,104 (91.1) | 176 (87.6) | 85 (92.4) | 41 (78.8) |
| Yes | 752 (8.5) | 7 (10.8) | 2 (10.0) | 4 (11.8) | 4346 (7.6) | 24 (11.9) | 7 (7.6) | 10 (19.2) |
| Missing | 201 (2.3) | 1 (1.5) | 0 (0.0) | 1 (2.9) | 718 (1.3) | 1 (0.5) | 0 (0.0) | 1 (1.9) |
Abbreviations: ABIS, All Babies in Southeast Sweden; CD, Crohn's disease; IBD, inflammatory bowel disease; MoBa, The Norwegian Mother, Father and Child Cohort Study; PA, physical activity; SD, standard deviation; ST, screen time; UC, ulcerative colitis.
IBD also includes individuals with a mix of codes for UC, CD, or IBD unclassified (Supplementary Table S2).
Follow‐up time starting at the time of questionnaire administration and ending at the time of IBD, CD, or UC diagnosis or for comparators, at the end of data capture 2020 (ABIS)/2021 (MoBa).
Low PA equals <2 h/day.
Low ST equals <1 h/day.
Other country if any parent was born in another country than Sweden (ABIS) or any parent having another native language than Norwegian (MoBa).
Any type 1/insulin‐treated diabetes, autoimmune thyroid disease, or rheumatoid arthritis.
University/College equals at least 13 years of education, high school equals 12 years, and compulsory school equals 9–11 years.
Statistical analysis
Cox proportional‐hazard regression was used to separately estimate cohort‐specific hazard ratios and 95% confidence intervals (CIs) for IBD overall, CD and UC. Analyses for the outcome CD excluded participants who developed UC and IBD‐U; likewise, UC analyses excluded participants who developed CD or IBD‐U. For 1.5‐year (MoBa only) and 3‐year exposure analyses, follow‐up started at the time of questionnaire administration (i.e., at 1.5 years for MoBa and 3 years of age for ABIS). However, to reduce the risk of reverse causation (e.g., low PA being a prodromal manifestation of IBD rather than a risk factor for the disease) our 8‐year analyses applied a 2‐year‐lag period before the start of follow‐up (i.e., in 8‐year analyses only events at 10 years of age or later were considered).
The proportional‐hazard assumption was tested using Schoenfeld residuals, 22 interaction with time analyses, and visual assessment of the data. The proportional‐hazard assumption was found valid for the overall risk of IBD. Cohort‐specific estimates were pooled using a random‐effects model. 23 Apart from crude risk estimates, we adjusted for the child's sex, parental/maternal origin, parental IBD, maternal comorbidities, educational level, and smoking during pregnancy. We also performed analyses stratified by sex and in sensitivity analyses the amount of PA was also adjusted for ST at the corresponding age, and vice versa. Follow‐up ended at the time of IBD diagnosis (i.e., first out of minimum two records of IBD) or by censoring at the end of data capture: 31 December 2020 (ABIS) and 31 December 2021 (MoBa).
The correlation between PA and ST degree at the same age was assessed by Spearman's rank correlation coefficient.
Statistical analyses were done using R Statistics Software (version 4.2.2 and 4.1.3, R Foundation, R packages: Survival, survminer, meta, metafor) and SPSS Statistics (version 29.0).
Ethics
Informed consent was retrieved from the guardians of all ABIS and MoBa participants after written and oral information.
RESULTS
Pooled analyses included 65,978 participants (ABIS: n = 8,810, 48% girls; Table 1] and MoBa: n = 57,168, 49% girls) with data on PA at age 3 (Figure 1, Flowchart). During 928,981 person‐years of follow‐up, 266 participants were diagnosed with IBD (ABIS, n = 65; MoBa, n = 201), corresponding to an incidence rate of 38.33 per 100,000 person‐years in ABIS and 26.47 per 100,000 person‐years in MoBa. Starting at the age of 3 years, the median overall follow‐up time was 19.3 years in ABIS (interquartile range [IQR] 18.8–19.8) and 13.2 years in MoBa (IQR 11.7–14.8]); accordingly, 16.9% of IBD diagnosis was first recorded in adulthood (≥18 years). At 3 years, children with high versus low PA had broadly similar baseline characteristics, including maternal education level and smoking habits (Supplementary Table S3). Degree of PA and ST were only weakly negatively correlated in both cohorts (e.g., 8‐year PA and ST in MoBa yielded a Spearman rank correlation coefficient of −0.054, p < 0.001; Supplementary Tables S4, S5).
Physical activity and risk of later inflammatory bowel disease development
Of the 65,462 children for whom 3‐year data were available, approximately one third (n = 24,140, 37%) were categorised into high PA group. In pooled analyses, high versus low PA at 3 years was not associated with the risk of IBD, CD, and UC (IBD, adjusted [a]HR = 1.12 [95%CI = 0.87–1.43]; CD, Adjusted hazard ratio (aHR) = 1.00 [95%CI = 0.67–1.48]; UC, aHR = 1.23 [95%CI = 0.66–2.32]; Figure 2, Supplementary Figures S1, S2). Similarly, when modelled as a numerical exposure, PA at 3 years was not associated with IBD (pooled aHR = 1.15 [95%CI = 0.95–1.39]; Figure 2) or its subtypes (Supplementary Figures S1, S2). In line with the 3‐year analysis, pooled analyses for PA at 8 years were not associated with the risk of IBD (high vs. low, aHR = 1.19 [95%CI = 0.80–1.76]; per hour/day, aHR = 0.99 [95%CI = 0.88–1.11]; Figure 3) or its subtypes (Supplementary Figures S3, S4).
FIGURE 2.
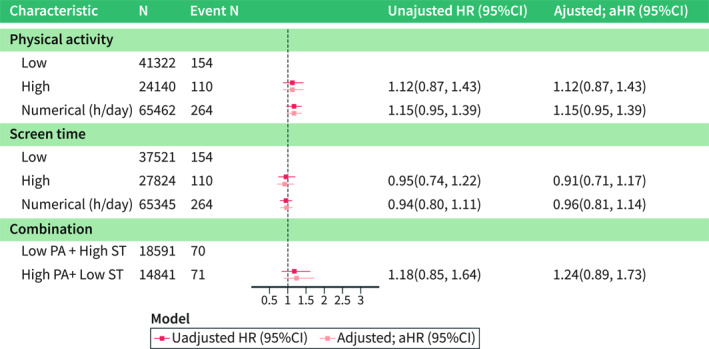
Pooled analyses of physical activity at 3 years and later risk for inflammatory bowel disease. Adjusted hazard ratio (aHR) accounted for child's sex, parental/maternal origin, parental IBD, maternal immune‐mediated comorbidities (any insulin‐treated diabetes: type 1 or gestational, rheumatoid arthritis or autoimmune thyroid disease), educational level, and smoking during pregnancy. Combination exposure reflects only individuals who had “High PA + Low ST” versus “Low PA + High ST”; the number of participants is lower due to ignoring children who had low PA + low ST level and high PA + high ST level. Numerical exposure of PA/ST examines the level of PA/ST using the original data distribution in hours per day. CI, confidence interval; h, hours; HR, hazard ratio; PA, physical activity; ST, screen time.
FIGURE 3.
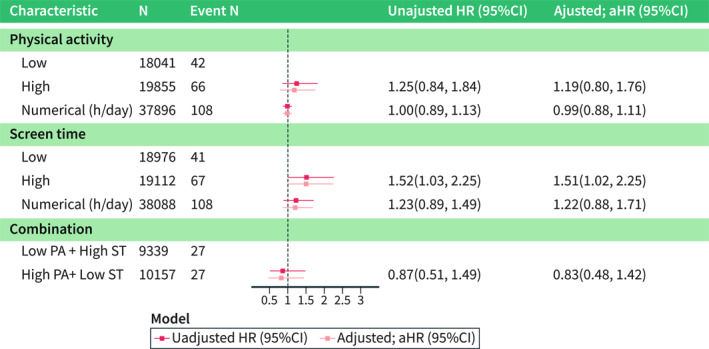
Pooled analyses of physical activity at 8 years and later risk for inflammatory bowel disease. Adjusted hazard ratio (aHR) accounted for child's sex, parental/maternal origin, parental IBD, maternal immune‐mediated comorbidities (any insulin‐treated diabetes: type 1 or gestational, rheumatoid arthritis or autoimmune thyroid disease), educational level, and smoking during pregnancy. Combination exposure reflects only individuals who had “High PA + Low ST” versus “Low PA + High ST”; the number of participants is lower due to ignoring children who had low PA + low ST level and high PA + high ST level. Numerical exposure of PA/ST examines the level of PA/ST using the original data distribution in hours per day. CI, confidence interval; h, hours; HR, hazard ratio; PA, physical activity; ST, screen time.
Screen time and risk of later inflammatory bowel disease development
Among the 65,345 participants with available ST information at 3 years, nearly half of the children (n = 27,824, 43%) sat in front of any screen for ≥1 h per day (i.e., high ST degree). In pooled analyses, participants with high versus low ST at age 3 years did not have a significantly increased risk of IBD or its subtypes (IBD, aHR = 0.91 [95%CI = 0.71–1.17]; CD, aHR = 0.98 [95% = CI 0.58–1.66]; UC, aHR = 0.75 [95%CI = 0.28–1.98]; Figure 2, Supplementary Figures S1, S2). Similar results emerged when assessing ST as a numerical exposure (IBD, pooled aHR = 0.96 [95%CI = 0.81–1.14]; Figure 2, Supplementary Figures S1, S2). A total of 38,088 children entered the pooled 8‐year ST analysis. Here, in contrast to the previous analysis, high versus low ST was positively associated with a later risk of IBD (aHR = 1.51 [95%CI = 1.02–2.25]). An additional sensitivity analysis, in which ST was mutually adjusted to the degree of PA at the age 8, showed a nearly identical result (IBD, aHR = 1.51 [95%CI = 1.01–2.25]). The aHRs for CD and UC yielded a pooled aHR of 1.36 [95%CI 0.51–3.62] and 1.36 [95%CI 0.67–2.74], respectively. When ST at 8 years was modelled as a numerical variable, it was not significantly associated with IBD or its subtypes (Figure 3, Supplementary Figures S3, S4).
Comparing children with reports of “High PA and Low ST” versus children with “Low PA and High ST”, we found no association with the risk of IBD at 3 years (pooled aHR = 1.24 [95%CI = 0.89–1.73]; Figures 2 and 3). The 8‐year pooled analysis showed similar findings (IBD, aHR = 0.83 [95%CI = 0.48–1.42]; Supplementary Figures S1, S4).
Cohort‐specific aHRs for IBD, CD, and UC by PA and ST were largely consistent (Supplementary Tables S6–S11). Also, sex‐stratified analyses showed similar results to our main analyses (data not shown).
We did not observe any significant effects of the adjusting covariates on IBD risk (data not shown).
Sub‐analyses
In MoBa 1.5‐year‐olds with high versus low PA or high versus low ST were not at increased risk of later IBD (aHR = 1.07 [95%CI = 0.78–1.45] and aHR = 0.91 [95%CI = 0.70–1.16], respectively (Supplementary Figures S5–S7).
DISCUSSION
Based on two Scandinavian birth cohorts, this prospective study found high versus low ST at 8 years of age associated with an increased risk for later development of IBD. However, measures of ST at age 3 years and PA at 8 and 3 years were not related to subsequent risk of IBD.
Previous literature
In adults, several studies have found an inverse relationship between PA and IBD development. In 1990, Sonnenberg et al. reported that occupations involving physical exercise had a protective association with IBD development. 8 Two other adult retrospective studies have linked low PA with an increased risk of IBD. 6 , 7 In the only prior paediatric study in this field, children who had less than two sports activities per week were at increased risk of CD and UC. 24 Still, causal inference of these results may have been hampered by the retrospective design of the studies, making them vulnerable to recall bias or even reverse causation. Generally, retrospective data collection may also reduce the precision of PA exposure assessment and have limited possibilities of adjusting for potential confounders. Previous studies have also suffered from generalisability issues, as they have only included hospitalised IBD patients who typically have more severe IBD diagnoses or comorbidities.
Prospective data on PA and IBD risk are scarce. Based on 194,711 women in the Nurses' Health Study Cohorts, high versus low PA was significantly associated with lower risk of subsequent CD (aHR = 0.56 [95%CI = 0.37–0.84]), but not UC (aHR = 0.91 [95%CI = 0.65–1.26]). 25 Within the same adult population cohort, a recent prospective study proposed that a healthy lifestyle, including adequate PA, might have prevented 61% of CD and 42% of UC cases. 26 Contrary, a Danish prospective cohort study showed no relationship between IBD risk and the degree of PA amongst elderly individuals. 27
We did not identify any previous work that specifically addressed ST and the risk of IBD.
In our prospective birth cohort study, high versus low ST at 8 years of age was linked to the risk of later IBD (aHR = 1.51 [95%CI = 1.02–2.25]). However, 8‐year ST modelled as a numerical variable was not significantly associated with IBD, suggesting a possible threshold effect of ST. Also, ST at age 3 years was not linked to IBD. There are several possible explanations for our findings. First, it is possible that ST is a marker of other health behaviours (e.g., diet) that influences the risk of IBD. Following this explanation, it could be conjectured that the amount of ST at age 8 years, as opposed to 3 years, might indicate this health behaviour more precisely. Second, while we adjusted for several potential confounders, including maternal education level, we cannot rule out that residual confounding from other socioeconomic characteristics or the living environment (i.e., rural vs. urban area) may still have affected our results.
We did not find an association between an 8‐year PA and later IBD risk. While PA and ST are likely to be correlated (Supplementary Tables S4, S5), existing evidence suggests that PA and ST have distinct effects on physiological markers and health parameters. 28 A higher level of PA is associated with lower inflammatory markers, while prolonged ST or sedentary behaviour is generally linked to increased inflammation. 29 However, the variation in PA at 3 and 8 years in our cohorts was relatively small, precluded a more granular exposure categorisation, and may have increased the risk of a type 2 error. This lower variance aligns with the observation of a decline in PA during early adolescence, usually at 12–14 years. 30 The reduced sample size at the 8‐year follow‐up may also have increased the risk of a type 2 error. It should be noted that some questions (mainly at age 3 years) did not allow a detailed evaluation of PA. Thus, the nature of the data may reduce the accuracy of our exposure assessment and bias the results towards the null.
Strengths and limitations
This study has several strengths. First, the large population‐based data set minimises the risk of selection bias and should ensure external validation of the results to similar populations. Second, the prospective exposure assessment eliminates the risk of recall bias. Third, we used high‐quality data from national patient registries 11 , 12 and a validated IBD algorithm that has previously shown a 93% consistency with a clinical IBD diagnosis. Fourth, using two independent Scandinavian cohorts improved the statistical power, allowing us to test the consistency of the results across cohorts. In addition, comprehensive questionnaire data allowed us to adjust for several potential confounders, such as parental IBD and smoking habits.
Although our work was done on a large prospective dataset, it has some limitations. First, the follow‐up time was mostly limited to childhood and hence did not enable examination of adult‐onset IBD, when IBD diagnosis becomes more prevalent; therefore, we cannot rule out that some children may later develop IBD after study follow‐up. Second, participation rates differed between ABIS (79%) and MoBa (41%), which may be related to these cohorts' regional versus national approach. Third, compared to the general Norwegian population, mothers smoking during pregnancy 31 and women with low educational levels are underrepresented in MoBa. However, these differences have been shown not to affect the choice of exposure‐outcome associations, 31 reducing the impact of self‐selection bias. Lastly, data in this study were restricted to exposures between 3 and 8 years, and hence could not examine the influence on IBD from PA occurring during adolescence, at which PA tends to be more structured compared to younger ages.
CONCLUSION
This is the first paper to analyse two prospective cohorts for assessing the relationship between PA in a paediatric population and the later risk of IBD. Acknowledging the presence of possible confounding variables, we found high compared to low ST at age 8 years to be associated with an increased risk of subsequent IBD, while PA degree showed no such association, which motivates further prospective research in this area.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICAL STATEMENT
At the time of recruitment into ABIS and MoBa, broad, informed consent was obtained from all participants after written and oral consent. The establishment of MoBa and the initial data collection were based on a license from the Norwegian Data Protection Agency and approval from the Regional Committees for Medical and Health Research Ethics. The Norwegian Health Registry Act currently regulates the MoBa cohort. The Regional Committees for Medical and Health Research Ethics approved the present study in 2020 (REK id 153,328). The ABIS study was approved at the Research Ethics Committees of the Faculty of Health Sciences at Linköping University, Sweden, 1997/96,287 and 2003/03–092, and the Medical Faculty of Lund University, Sweden, and connection to national registers Dnr 03–513 and 2013/253‐32 Research Ethics Committee of the Faculty of Health Sciences at Linköping University, Sweden. All Babies in Southeast Sweden data storage at the University of Gothenburg has been approved by the Ethical Review Authority (Dnr 2020–06581). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institution's human research committee.
Supporting information
Supplementary Information S1
ACKNOWLEDGEMENTS
We thank all the families of ABIS and MoBa for their participation. MoBa is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research and is conducted by the Norwegian Institute of Public Health. KM: Birgitta och Göran Karlssons Stiftelse: Swedish Society for Medical Research, The Swedish Research Council, and ALF funding. All Babies in Southeast Sweden was supported by Barndiabetesfonden (Swedish Child Diabetes Foundation); Swedish Council for Working Life and Social Research, Grant/Award Numbers: FAS2004‐1775, FAS2004–1775; Swedish Research Council, Grant/Award Numbers: K2005‐72X‐11242‐11A and K2008‐69X‐20826‐01‐4, K2008‐69X‐20826‐01‐4; Östgöta Brandstodsbolag; Medical Research Council of Southeast Sweden (FORSS); JDRF Wallenberg Foundation, Grant/Award Number: K 98‐99D‐12813‐01A; ALF‐and LFoU grants from Region Östergötland and Linköping university, Sweden; Joanna Cocozza Foundation. The funders had no role in study design, data collection, analysis, drafting the manuscript, or publication process.
Lerchova T, Östensson M, Sigvardsson I, Størdal K, Guo A, Mårild K, et al. Physical activity in childhood and later risk of inflammatory bowel disease: a Scandinavian birth cohort study. United European Gastroenterol J. 2023;11(9):874–883. 10.1002/ueg2.12469
Karl Mårild and Johnny Ludvigsson contributed equally as co‐senior authors.
DATA AVAILABILITY STATEMENT
Data are available on a reasonable request.
REFERENCES
- 1. Peters HP, De Vries WR, Vanberge‐Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48(3):435–439. 10.1136/gut.48.3.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saakslahti A, Numminen P, Varstala V, Helenius H, Tammi A, Viikari J, et al. Physical activity as a preventive measure for coronary heart disease risk factors in early childhood. Scand J Med Sci Sports. 2004;14(3):143–149. 10.1111/j.1600-0838.2004.00347.x [DOI] [PubMed] [Google Scholar]
- 3. Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev. 2018;17(1):53–72. 10.1016/j.autrev.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 4. Piovani D, Danese S, Peyrin‐Biroulet L, Bonovas S. Environmental, nutritional, and socioeconomic determinants of IBD incidence: a global ecological study. J Crohns Colitis. 2020;14(3):323–331. 10.1093/ecco-jcc/jjz150 [DOI] [PubMed] [Google Scholar]
- 5. Cucino C, Sonnenberg A. Occupational mortality from inflammatory bowel disease in the United States 1991‐1996. Am J Gastroenterol. 2001;96(4):1101–1105. 10.1111/j.1572-0241.2001.03747.x [DOI] [PubMed] [Google Scholar]
- 6. Klein I, Reif S, Farbstein H, Halak A, Gilat T. Preillness non dietary factors and habits in inflammatory bowel disease. Ital J Gastroenterol Hepatol. 1998;30(3):247–251. [PubMed] [Google Scholar]
- 7. Persson PG, Leijonmarck CE, Bernell O, Hellers G, Ahlbom A. Risk indicators for inflammatory bowel disease. Int J Epidemiol. 1993;22(2):268–272. 10.1093/ije/22.2.268 [DOI] [PubMed] [Google Scholar]
- 8. Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. 1990;31(9):1037–1040. 10.1136/gut.31.9.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ludvigsson JF, Abis Study G. Epidemiological study of constipation and other gastrointestinal symptoms in 8000 children. Acta Paediatr. 2006;95(5):573–580. 10.1111/j.1651-2227.2006.tb02286.x [DOI] [PubMed] [Google Scholar]
- 10. Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol. 2016;45(2):382–388. 10.1093/ije/dyw029 [DOI] [PubMed] [Google Scholar]
- 11. Bakken IJ, Ariansen AMS, Knudsen GP, Johansen KI, Vollset SE. The Norwegian patient Registry and the Norwegian Registry for primary health care: research potential of two nationwide health‐care registries. Scand J Pub Health. 2020;48(1):49–55. 10.1177/1403494819859737 [DOI] [PubMed] [Google Scholar]
- 12. Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136. 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 13. Bernhardsen GP, Stensrud T, Nystad W, Ekelund U. Pre‐ and post‐natal factors and physical activity in childhood: the Norwegian Mother, Father and Child Cohort study. Scand J Med Sci Sports. 2020;30(11):2264–2274. 10.1111/sms.13781 [DOI] [PubMed] [Google Scholar]
- 14. Carlsson E, Ludvigsson J, Huus K, Faresjo M. High physical activity in young children suggests positive effects by altering autoantigen‐induced immune activity. Scand J Med Sci Sports. 2016;26(4):441–450. 10.1111/sms.12450 [DOI] [PubMed] [Google Scholar]
- 15. Tremblay MS, Warburton DE, Janssen I, Paterson DH, Latimer AE, Rhodes RE, et al. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011;36(1):36–46. 7‐58. 10.1139/h11-009 [DOI] [PubMed] [Google Scholar]
- 16. Jakobsson GL, Sternegard E, Olen O, Myrelid P, Ljung R, Strid H, et al. Validating inflammatory bowel disease (IBD) in the Swedish national patient register and the Swedish quality register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–221. 10.1080/00365521.2016.1246605 [DOI] [PubMed] [Google Scholar]
- 17. Mouratidou N, Malmborg P, Jaras J, Sigurdsson V, Sandstrom O, Fagerberg UL, et al. Identification of childhood‐onset inflammatory bowel disease in Swedish healthcare registers: a validation study. Clin Epidemiol. 2022;14:591–600. 10.2147/clep.s358031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostensson M, Bjorkqvist O, Guo A, Stordal K, Halfvarson J, Marild K, et al. Epidemiology, validation, and clinical characteristics of inflammatory bowel disease: the ABIS birth cohort study. BMC Gastroenterol. 2023;23(1):199. 10.1186/s12876-023-02840-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Everhov AH, Halfvarson J, Myrelid P, Sachs MC, Nordenvall C, Soderling J, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 Years or older in Sweden. Gastroenterology. 2018;154(3):518–528.e15. 10.1053/j.gastro.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 20. Agrawal M, Sabino J, Frias‐Gomes C, Hillenbrand CM, Soudant C, Axelrad JE, et al. Early life exposures and the risk of inflammatory bowel disease: systematic review and meta‐analyses. EClinicalMedicine. 2021;36:100884. 10.1016/j.eclinm.2021.100884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Med Sci Sports Exerc. 2000;32(5):963–975. 10.1097/00005768-200005000-00014 [DOI] [PubMed] [Google Scholar]
- 22. Schoenfeld D. Partial residuals for the proportional hazards regression‐model. Biometrika. 1982;69(1):239–241. 10.1093/biomet/69.1.239 [DOI] [Google Scholar]
- 23. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 24. Hlavaty T, Toth J, Koller T, Krajcovicova A, Oravcova S, Zelinkova Z, et al. Smoking, breastfeeding, physical inactivity, contact with animals, and size of the family influence the risk of inflammatory bowel disease: a Slovak case‐control study. United Eur Gastroenterol J. 2013;1(2):109–119. 10.1177/2050640613478011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalili H, Ananthakrishnan AN, Konijeti GG, Liao X, Higuchi LM, Fuchs CS, et al. Physical activity and risk of inflammatory bowel disease: prospective study from the Nurses' Health Study cohorts. BMJ. 2013;347(nov14 4):f6633. 10.1136/bmj.f6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopes EW, Chan SSM, Song M, Ludvigsson JF, Hakansson N, Lochhead P, et al. Lifestyle factors for the prevention of inflammatory bowel disease. Gut. 2022;72(6):1093–1100. 10.1136/gutjnl-2022-328174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rasmussen NF, Bech BH, Rubin KH, Andersen V. Associations between participation in, intensity of, and time spent on leisure time physical activity and risk of inflammatory bowel disease among older adults (PA‐IBD): a prospective cohort study. BMC Publ Health. 2021;21(1):634. 10.1186/s12889-021-10492-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliveira RG, Guedes DP. Physical activity, sedentary behavior, cardiorespiratory fitness and metabolic syndrome in adolescents: systematic review and meta‐analysis of observational evidence. PLoS One. 2016;11(12):e0168503. 10.1371/journal.pone.0168503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parsons TJ, Sartini C, Welsh P, Sattar N, Ash S, Lennon LT, et al. Physical activity, sedentary behavior, and inflammatory and hemostatic markers in men. Med Sci Sports Exerc. 2017;49(3):459–465. 10.1249/mss.0000000000001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dumith SC, Gigante DP, Domingues MR, Kohl HW, 3rd . Physical activity change during adolescence: a systematic review and a pooled analysis. Int J Epidemiol. 2011;40(3):685–698. 10.1093/ije/dyq272 [DOI] [PubMed] [Google Scholar]
- 31. Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, et al. Self‐selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23(6):597–608. 10.1111/j.1365-3016.2009.01062.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information S1
Data Availability Statement
Data are available on a reasonable request.


