Abstract
Background
Scoring systems for severe acute pancreatitis (SAP) prediction should be used in conjunction with pre‐test probability to establish post‐test probability of SAP, but data of this kind are lacking.
Objective
To investigate the predictive value of commonly employed scoring systems and their usefulness in modifying the pre‐test probability of SAP.
Methods
Following PRISMA statement and MOOSE checklists after PROSPERO registration, PubMed was searched from inception until September 2022. Retrospective, prospective, cross‐sectional studies or clinical trials on patients with acute pancreatitis defined as Revised Atlanta Criteria, reporting rate of SAP and using at least one score among Bedside Index for Severity in Acute Pancreatitis (BISAP), Acute Physiology and Chronic Health Examination (APACHE)‐II, RANSON, and Systemic Inflammatory Response Syndrome (SIRS) with their sensitivity and specificity were included. Random effects model meta‐analyses were performed. Pre‐test probability and likelihood ratio (LR) were combined to estimate post‐test probability on Fagan nomograms. Pooled severity rate was used as pre‐test probability of SAP and pooled sensitivity and specificity to calculate LR and generate post‐test probability. A priori hypotheses for heterogeneity were developed and sensitivity analyses planned.
Results
43 studies yielding 14,116 acute pancreatitis patients were included: 42 with BISAP, 30 with APACHE‐II, 27 with Ranson, 8 with SIRS. Pooled pre‐test probability of SAP ranged 16.6%–25.3%. The post‐test probability of SAP with positive/negative score was 47%/6% for BISAP, 43%/5% for APACHE‐II, 48%/5% for Ranson, 40%/12% for SIRS. In 18 studies comparing BISAP, APACHE‐II, and Ranson in 6740 patients with pooled pre‐test probability of SAP of 18.7%, post‐test probability when scores were positive was 48% for BISAP, 46% for APACHE‐II, 50% for Ranson. When scores were negative, post‐test probability dropped to 7% for BISAP, 6% for Ranson, 5% for APACHE‐II. Quality, design, and country of origin of the studies did not explain the observed high heterogeneity.
Conclusions
The most commonly used scoring systems to predict SAP perform poorly and do not aid in decision‐making.
Keywords: acute pancreatitis, APACHE‐II, BISAP, meta‐analysis, prediction, RANSON, Revised Atlanta Criteria, scoring system, severe, SIRS
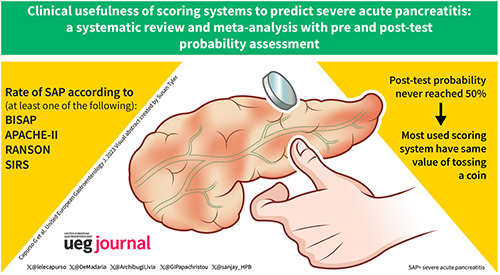
Key summary.
Summarize the established knowledge on this subject
Acute pancreatitis is a common, heterogeneous disease. Most patients experience a mild disease, and predicting a severe course would be of outmost clinical value.
Many scoring systems have been used to this aim, and they all have been shown to have only moderate predictive value. However, as for any test, the sensitivity and specificity of these scores alone cannot be used to accurately estimate the probability of severe disease in individual patients.
What are the significant and/or new findings of this study?
In this systematic review and meta‐analysis, a Bayesian approach was employed for the first time to depict the combination of the likelihood ratios of scoring systems with the pre‐test probability and to estimate the resulting post‐test probabilities.
We included 43 studies yielding 14,116 patients. All scoring systems had limited clinical usefulness as the actual post‐test probability of severe acute pancreatitis never reached 50% when scores were predicting a severe course and ranged between 5% and 12% when they were predicting a non‐severe course.
In real‐life clinical practice, the most used scoring systems to predict severe acute pancreatitis perform poorly and have the same value as tossing a coin. New approaches seem necessary.
BACKGROUND
Acute pancreatitis (AP) is a frequent condition with increasing incidence. 1 AP is a heterogeneous disease, and while most patients experience a mild course, approximately one‐third have local or systemic complications that are associated with increased morbidity, and in cases of persistent (>48 h) organ failure, with high mortality risk. 2 Therefore, classifying AP severity is important to correctly stratify patients with extremely different disease courses. However, the most employed systems to determine AP severity, the Revised Atlanta Classification (RAC) 3 and the Determinant‐Based Classification 4 , take into consideration the presence of local complications and organ failure occurring at any time during the disease course, being “post‐hoc” methods. While such severity classifications are useful for the final categorization of patients, they are not helpful for early management.
Predicting the severity of AP involves detecting, at an early disease stage, those patients most likely to have poor outcomes, which remains a challenge. Accurate early prediction of severity would allow the selection of patients who should be followed more closely, cared for in an intensive care unit, or transferred to tertiary centers. Severity prediction is also essential in the selection of patients to be included in trials.
Many different approaches have been developed to predict AP severity. The most employed scores include some specifically developed for AP, such as the Ranson score 5 and the Bedside Index for Severity in Acute Pancreatitis (BISAP), 6 and others that are not specific for AP, such as the Acute Physiology and Chronic Health Examination (APACHE)‐II 7 and the Systemic Inflammatory Response Syndrome (SIRS). 8 Several other scoring systems and tools employing combinations of physiological, laboratory, and radiographic parameters have been developed, but they all show only moderate positive predictive values. 9
However, as for any test, the sensitivity and specificity of these scores alone cannot be used to accurately estimate the probability of severe disease in individual patients. This depends on their combination into the likelihood ratio (LR), which should then be used in conjunction with pre‐test probability to establish the post‐test probability of severe AP (SAP) in a clinically meaningful manner. 10 In Bayesian statistics, this concept is visually summarized by Fagan's nomogram, a graphical tool that allows the combination of the LR of a test with the pre‐test probability of the outcome of interest to estimate the post‐test probability. 11
As data on the clinical usefulness of predictive scores for SAP are sparse and heterogeneous and given the absence of previous studies of this kind, we designed a systematic review and meta‐analysis to investigate the actual value of the most common predictive scoring systems in modifying the pre‐test probability of developing SAP.
METHODOLOGY
The methodology of the study was developed and reviewed with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 12 and the Meta‐Analyses Of Observational Studies in Epidemiology (MOOSE) 13 checklist. This review was registered in PROSPERO (ID CRD42022368212).
Search strategy
First, a computerized bibliographic search was performed in PubMed and the Cochrane Database of Systematic Reviews to retrieve prior systematic reviews and meta‐analyses on this topic. A PubMed search was then run from inception until 10 September 2022, to identify original studies. The specific search terms are detailed in Supporting Infomation S1. The titles of all identified articles were screened to evaluate eligibility, and the abstracts and/or full texts of potentially relevant papers were further evaluated. We manually searched the reference lists of all the retrieved articles to identify other potentially relevant studies.
Inclusion and exclusion criteria
The selected studies had to meet these criteria: (a) be either retrospective, prospective, cross‐sectional studies, or clinical trials; (b) report on AP patients defined according to RAC; (c) report SAP rate defined according to RAC or rate of organ failure, allowing SAP definition. In this view, the definition of persistent organ failure as renal, cardiovascular, or pulmonary failure lasting >48 h would be considered appropriate; (d) report on at least one of the following scores: BISAP, APACHE‐II, RANSON, and SIRS; (e) report sensitivity and specificity of the score(s) and/or enough data to calculate them; (f) be in English language.
In duplicate publications, the most recent or complete were used. Two independent reviewers (Ruggero Ponz de Leon Pisani and Gaetano Lauri) completed the study identification and selection process, and disagreements were discussed with two other reviewers (Livia Archibugi and Gabriele Capurso). The excluded studies and reasons for exclusion were recorded. Case reports or series, letters, abstracts, reviews, animal, and in vitro studies were excluded, as were studies published before the RAC publication or those that did not allow calculation of severity or employed predictive scores in a non‐standardized manner.
Data extraction and quality assessment
From the studies that met the eligibility criteria, the following data were extracted into a Microsoft Excel spreadsheet (Microsoft 2016, Redmond, WA, USA): (a) study—first author, publication year, setting, design, country, accrual period; (b) cases –number, sex, and age, the rate of severity according to RAC; (c) severity score(s)—name, timing at evaluation, employed cut‐off, sensitivity, specificity, +LR and −LR.
The quality of each study included in the quantitative synthesis was assessed by two independent reviewers (Ruggero Ponz de Leon Pisani and Gaetano Laur) using a specific quality appraisal tool developed for prognostic factors. 14 Disagreements were discussed with a third reviewer (Livia Archibugi).
Statistical analysis
A meta‐analysis of all eligible studies was performed using the Comprehensive Meta‐Analysis software package (Biostat, Englewood, N.J., USA). First, the pooled estimate of SAP was calculated to obtain the pre‐test probability. Next, the pooled estimates of the sensitivity and specificity of the different scoring systems were calculated to obtain +LR and −LR. The Der Simonian‐Laird method and a random‐effects model were used. Random‐effects models were chosen, as they consider both sampling variance within the different studies and variation in the underlying effect across studies. The assumption of variation in the underlying effect seems plausible given the different populations, designs, and etiology. Heterogeneity was assessed using the I 2 value and Cochran's Q statistics. An I 2 value ≤ 40% was considered trivial heterogeneity, I 2 > 40 < 75% was considered important heterogeneity, and an I 2 ≥ 75% considerable heterogeneity. Publication bias was assessed using the Begg and Mazumdar test. 15 , 16 Statistical significance was set at p < 0.05. We developed the following a priori hypotheses that would explain heterogeneity and planned sensitivity analyses for (a) area of origin, (b) quality of the study, and (c) study design. An open‐access online calculator (http://araw.mede.uic.edu/cgi‐bin/testcalc.pl) was used to estimate the post‐test probability.
RESULTS
Search results and study selection
There were no previous systematic reviews or meta‐analyses in the Cochrane Database of Systematic Reviews. Out of 64 studies published in PubMed, we retrieved seven prior systematic reviews and meta‐analyses on this topic. However, one only examined the performance of the Ranson score and was published before RAC, 17 three only examined BISAP, 18 , 19 , 20 one reviewed the performance of the Harmless Acute Pancreatitis Score (HAPS), which has the opposite aim of identifying patients who would not develop a severe disease 21 and one focused on the role of the computed tomography index. 22 Notably, the remaining study 23 differed substantially from the present one, as it aimed to investigate the net reclassification improvement using the available scores. Also, its search was terminated in mid‐2016 and mortality was the main outcome.
In our search for original studies, a total of 1894 references were identified (Figure 1). After evaluation of titles, 443 records were removed as not related to the study topic. Thus, the abstracts of the remaining 1446 studies were examined and 911 checked for eligibility. Finally, 43 studies were included. There was absolute agreement among the reviewers for the assessment of eligibility and selection of studies.
FIGURE 1.
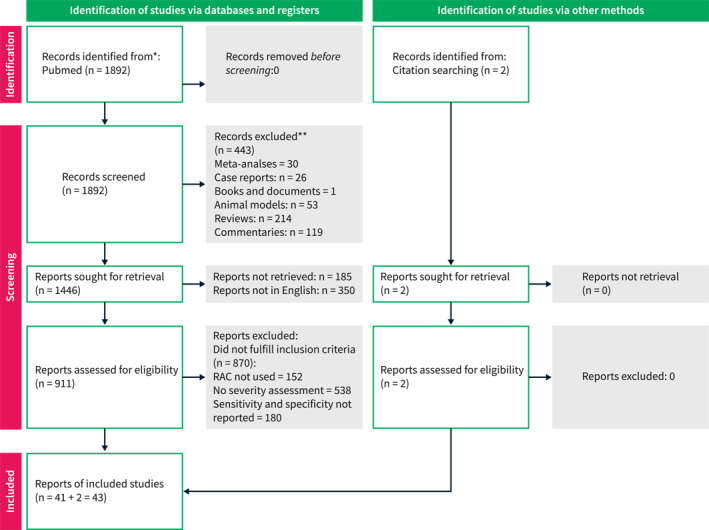
PRISMA 2020 flow diagram with included studies and reasons for exclusion.
Study characteristics
Table 1 presents a summary of relevant studies. The 43 studies 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 included 14,116 AP patients, with mean/median age ranging widely (35–72 years) as the rate of male patients (34%–86%).
TABLE 1.
Main characteristics of the studies eligible for the analyses.
| Study | Reference | Year | Location | Country | Setting a | Design b | Subgroups | Patients | Sex (male %) | Age | Accrual period | Severe AP, N (%) | Score(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mounzer R | 12 | 2012 | Pittsburgh, Boston | USA | M | P | Training | 256 | 52% | 51 (median) | 2003–2010 | 62 (24.2) | BISAP, RANSON, APACHEII, SIRS |
| Mounzer R | 12 | 2012 | Pittsburgh, Boston | USA | M | P | Validation | 397 | 49% | 52 (median) | 2005–2007 | 34 (8.6) | BISAP, RANSON, APACHEII, SIRS |
| Cho YS | 29 | 2013 | Uijeongbu | South Korea | U | R | None | 299 | 69.6% | 52.1 (mean) | 2008–2010 | 22 (7.4) | BISAP, RANSON |
| Khanna AK | 30 | 2013 | Varanasi | India | U | P | None | 72 | 51.4% | 40.5 (mean) | 2010–2012 | 31 (43.1) | BISAP, RANSON, APACHEII, SIRS |
| Park JY | 31 | 2013 | Seoul | South Korea | U | R | None | 303 | 71.2% | 52 (mean) | 2007–2010 | 31 (10.2) | BISAP, APACHEII, RANSON |
| Zhang J | 32 | 2014 | Hefei | China | U | R | None | 155 | 59% | 51.8 (mean) | 2010–2013 | 21 (13.5) | BISAP, APACHEII, RANSON |
| Cho JH | 33 | 2015 | Daegu | South Korea | U | R | None | 161 | 63% | 62.3 (mean) | 2011–2012 | 21 (13) | BISAP, APACHEII, RANSON |
| Mok SRS | 34 | 2015 | Camden | USA | U | P | None | 266 | 59% | 48.8 (mean) | 2011–2014 | 41 (15.4) | BISAP, APACHEII, RANSON |
| Qiu L | 35 | 2015 | Shanghai | China | M | R | None | 129 | 58.9% | 45.2 (mean) | 2008–2014 | 20 (15.5) | BISAP, RANSON, SIRS |
| Sharma V | 36 | 2015 | Chandigarh | India | U | R | None | 105 | 61.9% | 40.6 (mean) | 2013–2014 | 71 (67.6) | BISAP, SIRS |
| Yadav J | 37 | 2016 | Ranchi | India | U | P | None | 119 | 70.6% | 38.9 (mean) | 2012–2014 | 42 (35.2) | BISAP, RANSON |
| Kumar AH | 38 | 2018 | Rohtak | India | U | P | None | 50 | 34.0% | 48.4 (mean) | 2015–2016 | 14 (28) | BISAP, APACHEII, RANSON |
| He WH | 39 | 2017 | Nanchang | China | U | P | None | 708 | 43.9% | 51.7 (mean) | 2011–2012 | 172 (24.3) | BISAP, APACHEII, SIRS |
| Shi Y | 40 | 2017 | Shenyang | China | U | P | None | 56 | 58% | 54 (median) | 2015–2016 | 10 (13.2) | BISAP, APACHEII |
| Valverde‐Lopez F | 41 | 2017 | Granada | Spain | U | P | None | 269 | 49.9% | 64.5 (mean) | 2010–2012 | 17 (6.3) | BISAP, RANSON |
| Choi HW | 42 | 2018 | Seoul | South Korea | M | R | Training | 115 | 69.5% | 48.5 (mean) | 2013–2016 | 17 (14.8) | BISAP, APACHEII |
| Choi HW | 42 | 2018 | Seoul | South Korea | M | R | Validation | 77 | 66.2% | 46.8 (mean) | 2013–2016 | 11 (14.3) | BISAP, APACHEII |
| de‐Madaria E | 43 | 2018 | Alicante, Barcelona | Spain | M | P | None | 59 | 44.1% | 64 (mean) | NR | 13 (22) | BISAP, SIRS |
| Fei Y | 44 | 2018 | Nanjing | China | M | R | Training | 1073 | 57.3% | 47.3 (mean) | 2013–2016 | 517 (48.1) | BISAP, APACHEII, RANSON |
| Fei Y | 44 | 2018 | Nanjing | China | M | R | Validation | 326 | 51.5% | 56.3 (mean) | 2012–2016 | 126 (38.6) | BISAP, APACHEII, RANSON |
| Gravito‐Soares M | 45 | 2018 | Coimbra | Portugal | U | R | None | 182 | 54.9% | 66.3 (mean) | 2014–2016 | 91 (50) | BISAP, RANSON |
| Hagjer S | 46 | 2018 | Assam | India | U | P | None | 60 | 68.3% | 37.1 (mean) | 2015–2016 | 14 (23.3) | BISAP, APACHEII, RANSON |
| Yang WQ | 47 | 2018 | Shanghai | China | U | R | None | 172 | 61% | 48 (median) | 2012–2017 | 11 (6.4) | APACHEII, RANSON |
| Arif A | 48 | 2019 | Karachi | Pakistan | U | CS | None | 206 | 39.3% | 35.2 (mean) | 2015 | 39 (18.9) | BISAP, RANSON |
| Chen J | 49 | 2019 | Nanchang | China | U | P | None | 113 | 61.1% | 52.9 (mean) | 2016–2018 | 44 (38.9) | BISAP, APACHEII |
| Jain D | 50 | 2019 | Rohtak | India | U | P | None | 50 | 100% | 42 (mean) | NR | 10 (20) | BISAP, APACHEII, RANSON |
| Zhou H | 51 | 2019 | Beijing | China | U | R | None | 406 | 59.6% | 57 (mean) | 2014–2017 | 56 (13.8) | BISAP, APACHEII, RANSON |
| Chatterjee R | 52 | 2020 | Mumbai | India | U | P | None | 87 | 86.2% | 37.7 (mean) | NR | 20 (23) | BISAP, APACHEII |
| Gezer NS | 53 | 2020 | Izmir | Turkey | U | R | None | 80 | 42.5% | 55 (mean) | 2015–2018 | 19 (23.8) | BISAP, RANSON |
| Li M | 54 | 2020 | Hangzhou | China | U | R | None | 238 | 70.2% | 39.7 (median) | 2016–2018 | 60 (25.2) | BISAP, RANSON, APACHEII, SIRS |
| Li Y | 55 | 2020 | Nanjing | China | U | R | Elderly | 368 | 54.6% | 73.8 (mean) | 2015–2018 | 27 (7.3) | BISAP, APACHEII, RANSON |
| Li Y | 55 | 2020 | Nanjing | China | U | R | Young | 550 | 65.2% | 42.1 (mean) | 2015–2018 | 25 (4.5) | BISAP, APACHEII, RANSON |
| Peng R | 56 | 2020 | Panzhihua | China | U | P | None | 309 | 63.4% | 50 (mean) | 2017–2018 | 17 (5.5) | BISAP, APACHEII |
| Satis H | 57 | 2020 | Ankara | Turkey | U | P | Elderly | 113 | 40% | 73.7 (mean) | 2014–2016 | 1 (2.5) | BISAP, APACHEII |
| Silva Vaz P | 58 | 2020 | Castelo Branco | Portugal | U | P | None | 75 | 42.7% | 72 (mean) | 2015–2017 | 13 (17) | BISAP, SIRS |
| Venkatesh NR | 59 | 2020 | Puducherry | India | U | P | None | 164 | NR | 45 (mean) | NR | 104 (63.4) | BISAP, APACHEII, RANSON |
| Zhou T | 60 | 2020 | Nanchong | China | U | R | None | 337 | 55.0% | 50.4 (mean) | 2016–2018 | 17 (5) | BISAP, APACHEII |
| Sun HW | 61 | 2021 | Whenzou | China | U | R | Validation | 568 | 63.5% | 50 (median) | 2017–2019 | 162 (28.5) | BISAP, APACHEII, RANSON |
| Pando E | 62 | 2021 | Barcelona | Spain | U | P | None | 410 | 51.0% | 65.4 (median) | 2015–2020 | 45 (11) | BISAP, APACHEII |
| Wu Q | 63 | 2021 | Nanning | China | U | R | None | 1848 | 68.2% | 48.2 (mean) | 2003–2020 | 684 (37.0) | BISAP, RANSON |
| Shen D | 64 | 2021 | Changsha | China | U | P | None | 143 | 65.7% | 47 (median) | 2019–2020 | 26 (18.1) | BISAP, APACHEII |
| Teng TZJ | 65 | 2021 | Singapore | Singapore | U | R | None | 653 | 58.7% | 58.7 (mean) | 2009–2016 | 81 (12.4) | BISAP, APACHEII, RANSON |
| Wang Y | 66 | 2021 | Shanghai | China | U | P | None | 103 | 58.2% | 46 (median) | 2019–2020 | 31 (30.1) | BISAP, APACHEII |
| Yan G | 67 | 2021 | Chongqing, Suining, Nanchong | China | M | R | None | 465 | 54.4% | 54.6 (mean) | 2018–2020 | 27 (5.8) | BISAP, APACHEII, RANSON |
| Dancu GM | 68 | 2021 | Tmisoara | Romania | U | R | None | 216 | 55.5% | 56.3 (mean) | 2018–2019 | 25 (11.5) | BISAP |
| Bardakcı O | 69 | 2022 | Çanakkale | Turkey | U | R | None | 159 | 39% | 68.6 (mean) | 2017–2019 | 24 (15.1) | BISAP, APACHEII, RANSON |
| Wu B | 70 | 2022 | Chongqing | China | U | P | None | 1046 | 59.4% | 51.6 (mean) | 2020–2021 | 117 (11.2) | BISAP |
U = unicenter, M = multicenter.
R = retrospective, P = prospective, NR = not reported, AP = acute pancreatitis, N = number.
Thirty‐four studies (79%) took place in Institutions in Asia, 24 , 25 , 26 , 27 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 37 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 54 , 55 , 56 , 58 , 59 , 60 , 61 , 62 , 65 7 in Europe, 36 , 38 , 40 , 53 , 57 , 63 , 64 2 in the USA. 9 , 29 Twenty‐one studies were prospective, 9 , 25 , 29 , 32 , 33 , 34 , 35 , 36 , 38 , 41 , 44 , 45 , 47 , 51 , 52 , 53 , 54 , 57 , 59 , 61 , 65 21 retrospective 24 , 26 , 27 , 28 , 30 , 31 , 37 , 39 , 40 , 42 , 46 , 48 , 49 , 50 , 55 , 56 , 58 , 60 , 62 , 63 , 64 and 1 cross‐sectional. 43 Three of the studies included both training and validation sets, 9 , 37 , 39 and one two cohorts of different age groups. 50 The accrual period ranged 2005–2021.
Regarding the studies' quality, the analysis of study participation and attrition was not relevant as dealing with all patients hospitalized for the disease of interest with the availability of data on the outcome. The QUIPS tool takes into consideration other 4 items: (a) prognostic factor measurement, (b) outcome measurement, (c) study confounding and statistical analysis, and (d) reporting. These were scored as low, moderate, or high risk of bias according to the tool. We considered 13 studies that had a low risk of bias for all items as of “high quality” and the remaining 30 as of “moderate quality” (Supplementary Table S1).
BISAP score
There were 42 studies investigating BISAP 9 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 in a total of 13,944 patients. In these studies, the pooled prevalence of severity (pre‐test probability) was 17.8% (14.3%–22.2%) with considerable heterogeneity (I 2 = 96.9%) (Supplementary Figure S1a). There was no publication bias (Kendall's tau with continuity correction =–0.094; p = 0.35).
The pooled sensitivity and specificity of BISAP in these studies were 74.4% and 81.5%, both with considerable heterogeneity (I 2 = 97.3 and 95.5%) (Supplementary Figure S1b and S1c).
Figure 2a summarizes the performance of the BISAP score according to such data: with a pre‐test probability of 17.8% and +LR and −LR of 4.09 and 0.31, respectively, the post‐test probability of SAP was 47% when BISAP was positive and 6% when negative.
FIGURE 2.
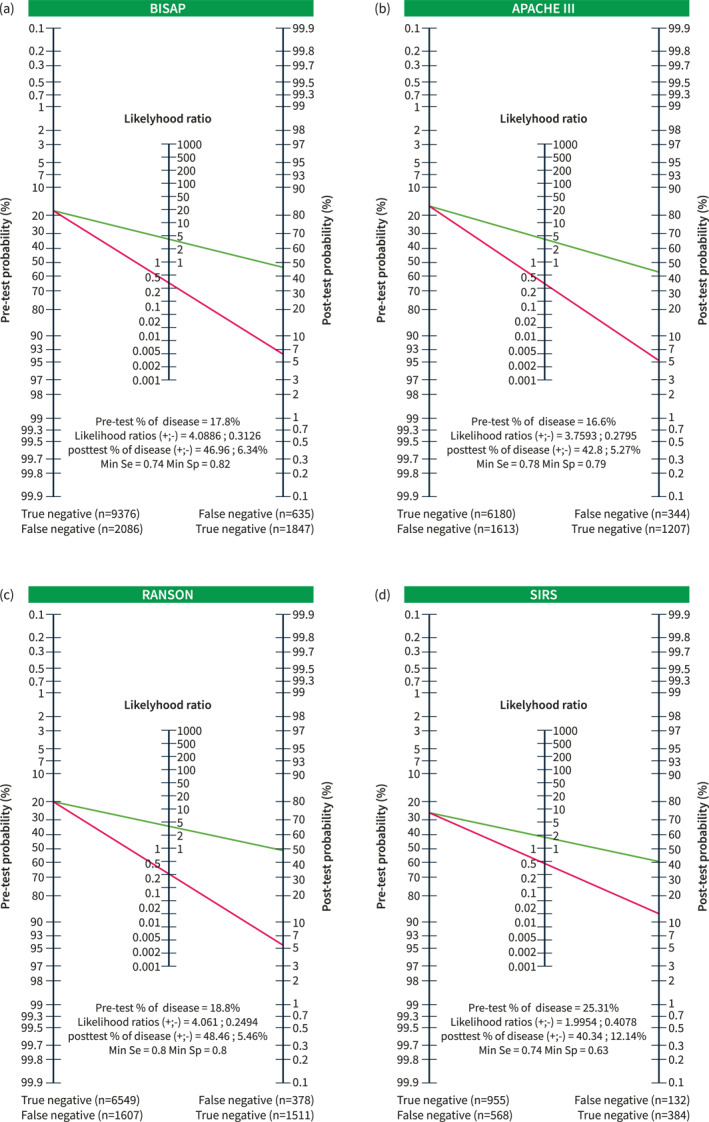
Panel (a) performance of the Bedside Index for Severity in Acute Pancreatitis (BISAP) score in 42 studies with a pre‐test probability of 17.8% and positive and negative likelihood ratios of 4.09 and 0.31, respectively, and the post‐test probability of severe acute pancreatitis (SAP) is 47% when BISAP is positive and 6% when it is negative. With this performance, only 1847 of the 2482 patients who eventually developed SAP would have been correctly identified as true positives, with 635 false negatives; only 9376 of the 11,462 patients experiencing non‐severe AP would have been correctly classified, with 2086 having a false positive prediction of SAP. Panel (b) performance of the Acute Physiology and Chronic Health Examination (APACHE)‐II score in 30 studies with a pre‐test probability of 16.6% and positive and negative likelihood ratios of 3.76 and 0.28, respectively, and the post‐test probability of SAP is 43% when APACHE is positive and 5% when it is negative. With this performance, only 1207 of the 1551 patients who eventually developed SAP would have been correctly identified as true positives, with 344 false negatives, and only 6180 of the 7793 patients experiencing non‐severe AP would have been correctly classified, with 1613 having a false positive prediction of SAP. Panel (c) performance of the Ranson score in 27 studies with a pre‐test probability of 18.8% and positive and negative likelihood ratios of 4.06 and 0.25, respectively. The post‐test probability of SAP is 48% when Ranson is positive and 5% when it is negative. With this performance, only 1511 of the 1889 patients who eventually developed SAP would have been correctly identified as true positives, with 378 false negatives; only 6549 of the 8156 patients experiencing non‐severe AP would have been correctly classified, with 1607 having a false positive prediction of SAP. Panel (d) performance of the Systemic Inflammatory Response Syndrome (SIRS) score in eight studies with a pre‐test probability of 25.3% and positive and negative likelihood ratios of 1.99 and 0.41, respectively, with a post‐test probability of SAP of 40% when SISR was positive and 12% when it was negative. With this performance, only 384 of the 516 patients who eventually developed SAP would have been correctly identified as true positives, with 132 false negatives, and only 955 of the 1523 patients with non‐severe AP would have been correctly classified, with 568 having a false positive prediction of SAP.
APACHE‐II score
Thirty studies 9 , 25 , 26 , 27 , 28 , 29 , 33 , 34 , 35 , 37 , 39 , 41 , 42 , 44 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 54 , 55 , 56 , 57 , 59 , 60 , 61 , 62 , 64 investigated APACHE‐II in 9344 patients. In these studies, the pooled prevalence of severity (pre‐test probability) was 16.6% (12.6%–21.5%), with considerable heterogeneity (I 2 = 96.8%) (Supplementary Figure S2a). There was no publication bias (Kendall tau with continuity correction = –0.10; p = 0.37).
The pooled sensitivity and specificity of APACHE II in these studies were 77.8% and 79.3%, both with considerable heterogeneity (I 2 = 96.1 and 96.3%, respectively) (Supplementary Figure S2b and S2c).
Figure 2b summarizes the performance of the APACHE II score according to such data: with a pre‐test probability of 16.6% and +LR and −LR of 3.76 and 0.28, respectively, the post‐test probability of SAP was 43% when APACHE was positive and 5% when negative.
Ranson score
There were 27 studies 9 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 32 , 33 , 36 , 39 , 40 , 41 , 42 , 43 , 45 , 46 , 48 , 49 , 50 , 54 , 56 , 58 , 60 , 62 , 64 investigating Ranson in 10,044 patients. In these studies, the pooled prevalence of severity (pre‐test probability) was 18.8% (14.3%–24.2%) with considerable heterogeneity (I 2 = 97.4%) (Supplementary Figure S3a). There was no publication bias (Kendall's tau with continuity correction = –0.16; p = 0.19).
The pooled sensitivity and specificity of Ranson in these studies were 80% and 80.3%, both with considerable heterogeneity (I 2 = 97.1 and 96.6%, respectively) (Supplementary Figure S3b and S3c).
Figure 2c summarizes the performance of the Ranson score according to such data: with a pre‐test probability of 18.8% and +LR and −LR of 4.06 and 0.25, respectively, the post‐test SAP probability was 48% when Ranson was positive and 5% when negative.
SIRS score
Eight studies 9 , 25 , 30 , 31 , 34 , 38 , 49 , 53 investigated SIRS in 2039 patients. The pooled prevalence of severity (pre‐test probability) was 25.3% (17%–35.8%), with considerable heterogeneity (I 2 = 94.6%) (Supplementary Figure S4a). There was no publication bias (Kendall's tau with continuity correction = −0.02; p = 0.91). The pooled sensitivity and specificity of SIRS in these studies were 74.4% and 62.7%, both with considerable heterogeneity (I 2 = 97.1 and 90.3%, respectively) (Supplementary Figure S4b,c).
Figure 2d summarizes the performance of the SIRS score according to such data, with a pre‐test probability of 25.3% and +LR and −LR ratios of 1.99 and 0.41, respectively, and a post‐test probability of SAP of 40% when SISR is positive and 12% when negative.
Comparison of the performance of the different scores and sensitivity analysis
Only 3 studies 9 , 25 , 54 compared all four examined scores; four compared the BISAP, APACHE‐II, and SIRS. To obtain the most comprehensive comparison of the performance of the investigated scores, we selected 18 studies 9 , 25 , 26 , 27 , 28 , 29 , 33 , 39 , 41 , 45 , 46 , 49 , 50 , 54 , 56 , 60 , 62 , 64 that compared the accuracy of the BISAP, APACHE‐II, and Ranson scores for a total of 6740 patients. In this cohort, the pre‐test SAP probability was 18.7% (13.1%–26.1%) (Supplementary Figure S5) with considerable heterogeneity (I 2 = 97.6%).
Figure 3 summarizes the performances of the three scoring systems in this subgroup. Notably, with a pooled pre‐test probability of 18.7%, the performance of the three Scoring Systems was very similar. The post‐test probability when the scores were positive was 46% for APACHE II, 48% for BISAP, and 50% for Ranson. On the other hand, when the scores were negative, the post‐test probability of a severe course was as low as 5% for APACHE‐II, 6% for Ranson, and 7% for BISAP.
FIGURE 3.
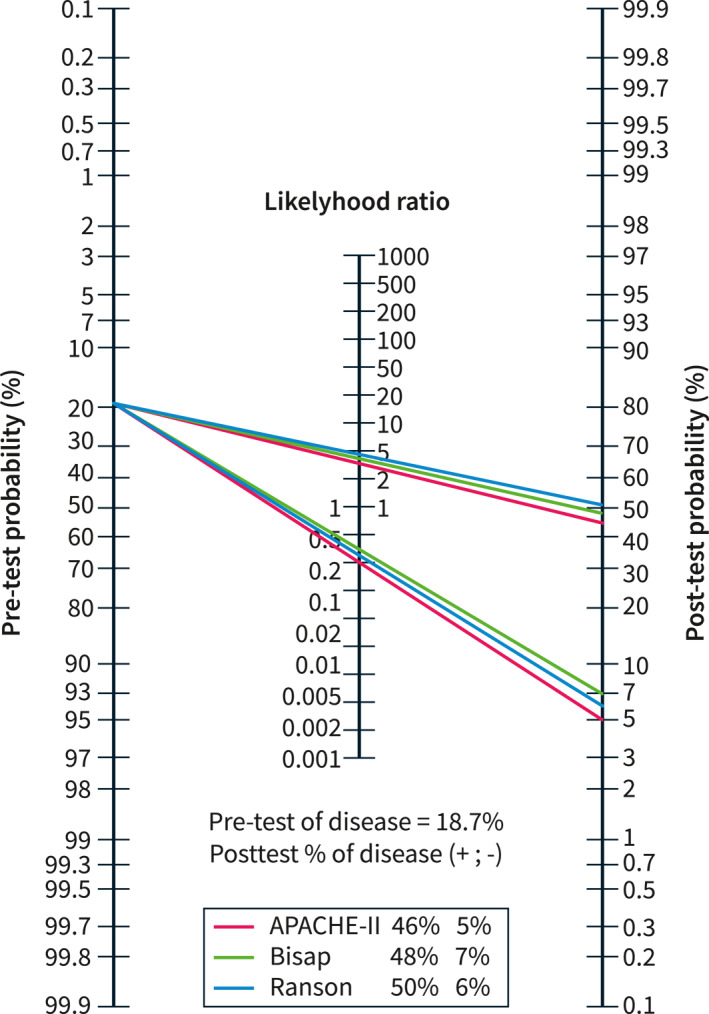
Performance of the Bedside Index for Severity in Acute Pancreatitis (BISAP), Acute Physiology and Chronic Health Examination (APACHE)‐II, and Ranson scores in 18 studies with a pooled pre‐test probability of 18.7% for severe acute pancreatitis. The post‐test probabilities when the scores were positive were similar: 48% for BISAP, 46% for APACHE‐II, and 50% for Ranson. However, when the scores were negative, the post‐test probability dropped to 7% for BISAP, 6% for Ranson, and 5% for APACHE‐II.
As for the sensitivity analyses (Supplementary Table S2), the quality, design and country of origin did not account for the observed heterogeneity. As most of the examined studies were conducted in Asia, where etiology, comorbidities and lifestyle are very different from those of Western countries (Europe and USA), we further investigated the performance of the scoring systems separately in such subgroups (Supplementary Figure S6). The performance was generally worse in studies conducted in Western Countries with post‐test probabilities of a severe course when a score was positive being as low as 38% for BISAP, 19% for APACHE‐II, 40% for Ranson and 27% for SIRS, compared to ,respectively, 50%, 48%, 49% and 51% in Asia.
DISCUSSION
In an individual patient, the pre‐test probability of SAP is usually not higher than 20%. The purpose of the prediction scores is to generate a post‐test probability of SAP that is as high as possible. Many different approaches have been developed, and many scoring systems that combine laboratory and clinical features are commonly employed for this purpose. 66
One of the limitations of scoring systems is that generalization may not be possible as they were developed and validated in certain groups of patients, but the clinicians need to make a prediction about the individual patient they are caring for. There are several ways the sensitivity and specificity can be combined into a single score. The most used method is the receiver operator characteristic curve, which plots sensitivity and specificity presenting the performance as “area under the curve” While this may be of help in comparing the accuracy of different systems, it has limited clinical relevance for individual patients. A better approach is to derive the post‐test probability by combining the expected pre‐test probability for the patient population with the positive and negative LR and using a nomogram to read the post‐test probability. 11
In the present study, we systematically retrieved literature on predictive scores of SAP defined according to RAC and calculated their performance using a Bayesian approach for the first time. We retrieved data from 43 studies conducted on >14,000 AP patients to investigate the accuracy of BISAP, APACHE‐II, Ranson, and SIRS in predicting SAP. We first calculated the pre‐test probability. Thereafter, the sensitivity and specificity of each score were calculated. These data were employed to generate positive and negative LR and post‐test probabilities of SAP for each score. There was no publication bias.
The main result is that all scoring systems have a similar, limited, and clinical usefulness, as the post‐test probability of SAP never reached 50% (Figure 2) when the score was positive and ranged between 5% and 12% when negative. Therefore, if these scoring systems are used to predict a non‐severe course of AP, the HAPS score should be preferentially employed. 21
To obtain a more reliable figure of the scoring systems' performance, we further focused on a subset of 18 studies that compared the accuracy of the BISAP, APACHE II, and Ranson scores in predicting SAP. In this cohort, the pre‐test probability of SAP was 18.7%, and the performances of the three scoring systems were very similar (Figure 3), with a post‐test probability ≤50% when the scores were positive. This means that in real‐life clinical practice, the use of these scores to predict SAP has the same value as tossing a coin.
The present study has strengths. This is the first systematic review with rigorous methodology to calculate the actual performance of scoring systems that have been employed for decades to predict SAP probability. However, there are limitations, mainly related to the heterogeneity of the studies. Despite pre‐planned sensitivity analyses that included an evaluation of the quality, design and country of origin, no reasons for the observed high heterogeneity were found. However, there are many factors that are intrinsic to single patients, such as AP etiology, 67 age and comorbidities, 68 triglyceride and glucose levels, 69 and the setting where the patient is treated (hospital volume and resources), 70 which have an influence on AP course and may account for heterogeneity. Individual data analysis would be necessary to further investigate these aspects. Also, we separately investigated the performance of the four scoring systems in studies conducted in Asia versus Western countries, with findings of a much worse performance in the latter group. Whether this is due to the lower number of enrolled patients or to actual differences in the applicability of the systems must be established.
Our results reinforce the need for novel alternative approaches to predict an AP course. One would be to monitor the dynamic AP evolution during its course instead of focusing on a rather rare outcome, such as persistent organ failure. The Pancreatitis Activity Scoring System (PASS), which includes organ failure, SIRS, abdominal pain, the need for opiates, and the ability to tolerate oral diet as variables, was developed for this aim 71 and found to be able to track the clinical trajectories of an AP episode, anticipating deterioration and complications. However, its accuracy for predicting SAP at a single time point is limited.
Several novel tools are based on computed tomography (CT) imaging. The most obvious limitation of radiological approaches is that a CT scan is not required on admission in most patients.
Another novel approach uses information theory and machine learning to select the best‐performing panel of circulating cytokines, which reflects the magnitude of inflammatory response. Angiopoietin‐2, hepatocyte growth factor, interleukin‐8, resistin, and tumor necrosis factor receptor‐1 were the highest‐ranking cytokines in the derivation cohort. A Random Forest classifier trained the 5‐cytokine panel in the verification cohort and achieved a 10‐fold cross‐validated accuracy of 0.89, which significantly outperformed the prognostic accuracy of existing laboratory tests and clinical scores. 72 As multiple factors interact in a nonlinear, complex, and unpredictable manner to determine the actual risk of developing SAP, artificial intelligence algorithms might be an appropriate tool to improve prediction ability. In a recent large cohort study, machine learning models were employed to examine simple variables such as respiratory rate, body temperature, abdominal rebound tenderness, sex, age, and glucose levels. The accuracy of the model was as high as 89% and a user‐friendly web application (“EASY”) was developed for wider applications. 73
In conclusion, we have systematically reviewed the performance of the most commonly employed scoring systems to predict AP severity, with findings that underline their poor performance in everyday clinical practice.
It is likely that artificial intelligence will become a more common approach for rapid, early, and accurate prediction of AP severity and outcomes, which will outperform existing scoring systems.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest or funding to report regarding the study.
Supporting information
Supporting Information S1
Supporting Information S2
Supporting Information S3
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Figure S11
Figure S12
Figure S13
Table S1
Table S2
ACKNOWLEDGEMENT
Open access funding provided by BIBLIOSAN.
Capurso G, Ponz de Leon Pisani R, Lauri G, Archibugi L, Hegyi P, Papachristou GI, et al. Clinical usefulness of scoring systems to predict severe acute pancreatitis: a systematic review and meta‐analysis with pre and post‐test probability assessment. United European Gastroenterol J. 2023;11(9):825–836. 10.1002/ueg2.12464
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta‐analysis. Gastroenterology. 2022;162(1):122–134. 10.1053/j.gastro.2021.09.043 [DOI] [PubMed] [Google Scholar]
- 2. Sternby H, Bolado F, Canaval‐Zuleta HJ, Marra‐Lopez C, Hernando‐Alonso A, Del‐Val‐Antonana A, et al. Determinants of severity in acute pancreatitis: a nation‐wide multicenter prospective cohort study. Ann Surg. 2019;270(2):348–355. 10.1097/SLA.0000000000002766 [DOI] [PubMed] [Google Scholar]
- 3. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis ‐ 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 4. Dellinger EP, Forsmark CE, Layer P, Lèvy P, Maravì‐Poma E, Petrov MS, et al. Determinant‐based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256(6):875–880. 10.1097/SLA.0b013e318256f778 [DOI] [PubMed] [Google Scholar]
- 5. Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Localio SA. Objective early identification of severe acute pancreatitis. Am J Gastroenterol. 1974;61(6):443–451. [PubMed] [Google Scholar]
- 6. Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population‐based study. Gut. 2008;57(12):1698–1703. 10.1136/gut.2008.152702 [DOI] [PubMed] [Google Scholar]
- 7. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE‐II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 8. Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg. 2006;93(6):738–744. 10.1002/bjs.5290 [DOI] [PubMed] [Google Scholar]
- 9. Mounzer R, Langmead CJ, Wu BU, Evans AC, Bishehsari F, Muddana V, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142(7):1476–1482. 10.1053/j.gastro.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 10. Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;15;16(9):981–991. [DOI] [PubMed] [Google Scholar]
- 11. Fagan TJ. Letter: nomogram for Bayes theorem. N Engl J Med. 1975;293(5):257–258. 10.1056/NEJM197507312930513 [DOI] [PubMed] [Google Scholar]
- 12. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Pettycrew M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (prisma‐p) 2015: elaboration and explanation. BMJ. 2015;350(jan02 1):g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14. Hayden JA, Côtè P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–437. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 16. Egger M, Smith GD, Schneider M, Minder C. Papers bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Bernardinis M, Violi V, Roncoroni L, Boselli AS, Giunta A, Peracchia A. Discriminant power and information content of Ranson’s prognostic signs in acute pancreatitis: a meta‐analytic study. Crit Care Med. 1999;27(10):2272–2283. 10.1097/00003246-199910000-00035 [DOI] [PubMed] [Google Scholar]
- 18. Gao W, Yang HX, Ma CE. The value of BISAP score for predicting mortality and severity in acute pancreatitis: a systematic review and meta‐analysis. PLoS One. 2015;10(6):10. 10.1371/journal.pone.0130412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang YX, Li L. Evaluating the ability of the bedside index for severity of acute pancreatitis score to predict severe acute pancreatitis: a meta‐analysis. Med Princ Pract. 2016;25(2):137–142. 10.1159/000441003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chandra S, Murali A, Bansal R, Agarwal D, Holm A. The Bedside Index for Severity in Acute Pancreatitis: a systematic review of prospective studies to determine predictive performance. J Community Hosp Intern Med Perspect. 2017;7(4):208–213. 10.1080/20009666.2017.1361292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maisonneuve P, Lowenfels AB, Lankisch PG. The harmless acute pancreatitis score (HAPS) identifies non‐severe patients: a systematic review and meta‐analysis. Pancreatology. 2021;21(8):1419–1427. 10.1016/j.pan.2021.09.017 [DOI] [PubMed] [Google Scholar]
- 22. Mikó A, Vigh É, Mátrai P, Soòs A, Garami A, Balaskò M, et al. Computed tomography severity index vs. Other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta‐analysis. Front Physiol. 2019;10:1002. 10.3389/fphys.2019.01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di MY, Liu H, Yang ZY, Bonis PAL, Tang JL, Lau J. Prediction models of mortality in acute pancreatitis in adults: a systematic review. Ann Intern Med. 2016;165(7):482–490. 10.7326/M16-0650 [DOI] [PubMed] [Google Scholar]
- 24. Cho YS, Kim HK, Jang EC, Yeom JO, Kim SY, Yu JY, et al. Usefulness of the bedside index for severity in acute pancreatitis in the early prediction of severity and mortality in acute pancreatitis. Pancreas. 2013;42(3):483–487. 10.1097/MPA.0b013e318267c879 [DOI] [PubMed] [Google Scholar]
- 25. Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE‐II, CTSI Scores, IL‐6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surg. 2013;2013:367581–367610. 10.1155/2013/367581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park JY, Jeon TJ, Ha TH, Hwang JT, Sinn DH, Oh TH, et al. Bedside index for severity in acute pancreatitis: comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int. 2013;12(6):645–650. 10.1016/s1499-3872(13)60101-0 [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Shahbaz M, Fang R, Liang B, Gao C, Gao H, et al. Comparison of the BISAP scores for predicting the severity of acute pancreatitis in Chinese patients according to the latest Atlanta classification. J Hepatobiliary Pancreat Sci. 2014;21(9):689–694. 10.1002/jhbp.118 [DOI] [PubMed] [Google Scholar]
- 28. Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21(9):2387–2394. 10.3748/wjg.v21.i8.2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mok SRS, Mohan S, Elfant AB, Judge TA. The acute physiology and chronic health evaluation IV, a new scoring system for predicting mortality and complications of severe acute pancreatitis. Pancreas. 2015;44(8):1314–1319. 10.1097/MPA.0000000000000432 [DOI] [PubMed] [Google Scholar]
- 30. Qiu L, Sun RQ, Jia RR, Ma XY, Cheng L, Tang MC, et al. Comparison of existing clinical scoring systems in predicting severity and prognoses of hyperlipidemic acute pancreatitis in Chinese patients: a retrospective study. Med (United States). 2015;94(23):e957. 10.1097/MD.0000000000000957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma V, Rana SS, Sharma RK, Kang M, Gupta R, Bhasin DK. A study of radiological scoring system evaluating extrapancreatic inflammation with conventional radiological and clinical scores in predicting outcomes in acute pancreatitis. Ann Gastroenterol. 2015;28(3):399–404. [PMC free article] [PubMed] [Google Scholar]
- 32. Yadav J, Yadav SK, Kumar S, Baxla RG, Sinha DK, Bodra P, et al. Predicting morbidity and mortality in acute pancreatitis in an Indian population: a comparative study of the BISAP score, Ranson’s score and CT severity index. Gastroenterol Rep. 2016;4(3):216–220. 10.1093/gastro/gov009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar AH, Griwan MS. A comparison of APACHE‐II, BISAP, Ranson’s score and modified CTSI in predicting the severity of acute pancreatitis based on the 2012 revised Atlanta Classification. Gastroenterol Rep. 2018;6(2):127–131. 10.1093/gastro/gox029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He WH, Zhu Y, Zhu Y, Jin Q, Xu HR, Xion ZJ, et al. Comparison of multifactor scoring systems and single serum markers for the early prediction of the severity of acute pancreatitis. J Gastroenterol Hepatol. 2017;32(11):1895–1901. 10.1111/jgh.13803 [DOI] [PubMed] [Google Scholar]
- 35. Shi Y, Liu Y, Liu YQ, Gao F, Li JH, Li QJ, et al. Early diagnosis and severity assessment of acute pancreatitis (AP) using MR elastography (MRE) with spin‐echo echo‐planar imaging. J Magn Reson Imag. 2017;46(5):1311–1319. 10.1002/jmri.25679 [DOI] [PubMed] [Google Scholar]
- 36. Valverde‐López F, Matas‐Cobos AM, Alegría‐Motte C, Jiménez‐Rosales R, Úbeda‐Muñoz M, Redondo‐Cerezo E. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. 2017;32(9):1649–1656. 10.1111/jgh.13763 [DOI] [PubMed] [Google Scholar]
- 37. Choi HW, Park HJ, Choi SY, Do JH, Yoon NY, Ko A, et al. Early prediction of the severity of acute pancreatitis using radiologic and clinical scoring systems with classification tree analysis. Am J Roentgenol. 2018;211(5):1035–1043. 10.2214/AJR.18.19545 [DOI] [PubMed] [Google Scholar]
- 38. de‐Madaria E, Molero X, Bonjoch L, Casas J, Cardenas‐Jaen K, Montenegro A, et al. Oleic acid chlorohydrin, a new early biomarker for the prediction of acute pancreatitis severity in humans. Ann Intensive Care. 2018;8(1):1. 10.1186/s13613-017-0346-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fei Y, Gao K, Tu J, Wang W, Zong GQ, Li WQ. Predicting and evaluation the severity in acute pancreatitis using a new modeling built on body mass index and intra‐abdominal pressure. Am J Surg. 2018;216(2):304–309. 10.1016/j.amjsurg.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 40. Gravito‐Soares M, Gravito‐Soares E, Gomes D, Almeida N, Tomé L. Red cell distribution width and red cell distribution width to total serum calcium ratio as major predictors of severity and mortality in acute pancreatitis. BMC Gastroenterol. 2018;18(1):108. 10.1186/s12876-018-0834-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis – a prospective observational study. Int J Surg. 2018;54(Pt A):76–81. 10.1016/j.ijsu.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 42. Yang WQ, Yang Q, Chen WJ, Zhang XB, Xu QQ, Qiao Y, et al. Low FT3 is a valuable predictor of severe acute pancreatitis in the emergency department. J Dig Dis. 2018;19(7):431–438. 10.1111/1751-2980.12609 [DOI] [PubMed] [Google Scholar]
- 43. Arif A, Jaleel F, Rashid K. Accuracy of BISAP score in prediction of severe acute pancreatitis. Pak J Med Sci. 2019;35(4):1008–1012. 10.12669/pjms.35.4.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen J, Wan J, Shu W, Yang X, Xia L. Association of serum levels of silent information regulator 1 with persistent organ failure in acute pancreatitis. Dig Dis Sci. 2019;64(11):3173–3181. 10.1007/s10620-019-05647-x [DOI] [PubMed] [Google Scholar]
- 45. Jain D, Bhaduri G, Jain P. Different scoring systems in acute alcoholic pancreatitis: which one to follow? an ongoing dilemma. Arq Gastroenterol. 2019;56(3):280–285. 10.1590/S0004-2803.201900000-53 [DOI] [PubMed] [Google Scholar]
- 46. Zhou H, Mei X, He X, Lan T, Guo S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase. Med (United States). 2019;98(16):e15275. 10.1097/MD.0000000000015275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chatterjee R, Parab N, Sajjan B, Nagar VS. Comparison of acute physiology and chronic health evaluation ii, modified computed tomography severity index, and bedside index for severity in acute pancreatitis score in predicting the severity of acute pancreatitis. Indian J Crit Care Med. 2020;24(2):99–103. 10.5005/jp-journals-10071-23343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gezer NS, Bengi G, Baran A, Erkmen PE, Topalak OS, Altay C, et al. Comparison of radiological scoring systems, clinical scores, neutrophil‐lymphocyte ratio and serum C‐reactive protein level for severity and mortality in acute pancreatitis. Rev Assoc Med Bras. 2020;66(6):762–770. 10.1590/1806-9282.66.6.762 [DOI] [PubMed] [Google Scholar]
- 49. Li M, Xing XK, Lu ZH, Guo F, Su W, Lin YJ, et al. Comparison of scoring systems in predicting severity and prognosis of hypertriglyceridemia‐induced acute pancreatitis. Dig Dis Sci. 2020;65(4):1206–1211. 10.1007/s10620-019-05827-9 [DOI] [PubMed] [Google Scholar]
- 50. Li Y, Zhang J, Zou J. Evaluation of four scoring systems in prognostication of acute pancreatitis for elderly patients. BMC Gastroenterol. 2020;20(1):165. 10.1186/s12876-020-01318-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peng R, Zhang L, Zhang ZM, Wang ZQ, Liu GY, Zhang XM. Chest computed tomography semi‐quantitative pleural effusion and pulmonary consolidation are early predictors of acute pancreatitis severity. Quant Imaging Med Surg. 2020;10(2):451–463. 10.21037/qims.2019.12.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Satiş H, Kayahan N, Sargin ZG, Karataş A, Çeliker D. Evaluation of the clinical course and prognostic indices of acute pancreatitis in elderly patients: a prospective study. Acta Gastroenterol Bel. 2020;83(3):413–417. [PubMed] [Google Scholar]
- 53. Silva‐Vaz P, Abrantes AM, Morgado‐Nunes S, Castelo‐Branco M, Gouveia A, Botelho MF, et al. Evaluation of prognostic factors of severity in acute biliary pancreatitis. Int J Mol Sci. 2020;21(11):1–18. 10.3390/ijms21124300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Venkatesh NR, Vijayakumar C, Balasubramaniyan G, Kandhasami SC, Sundaramurthi S, Sreenath SS, et al. Comparison of different scoring systems in predicting the severity of acute pancreatitis: a prospective observational study. Cureus. 2020;12(2):e6943. 10.7759/cureus.6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou T, Chen Y, Wu JL, Deng Y, Zhang J, Sun H, et al. Extrapancreatic inflammation on magnetic resonance imaging for the early prediction of acute pancreatitis severity. Pancreas. 2020;49(1):46–52. 10.1097/MPA.0000000000001425 [DOI] [PubMed] [Google Scholar]
- 56. Sun HW, Lu JY, Weng YX, Chen H, He QY, Liu R, et al. Accurate prediction of acute pancreatitis severity with integrative blood molecular measurements. Aging. 2021;13(6):8817–8834. 10.18632/aging.202689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pando E, Alberti P, Mata R, Gomez MJ, Vidal L, Cirera A, et al. Early changes in Blood Urea Nitrogen (BUN) can predict mortality in acute pancreatitis: comparative study between BISAP score, APACHE‐II, and other laboratory markers‐A prospective observational study. Can J Gastroenterol Hepatol. 2021;2021:6643595–6643598. 10.1155/2021/6643595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu Q, Wang J, Qin M, Yang H, Liang Z, Tang G. Accuracy of conventional and novel scoring systems in predicting severity and outcomes of acute pancreatitis: a retrospective study. Lipids Health Dis. 2021;20(1):41. 10.1186/s12944-021-01470-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shen D, Tang C, Zhu S, Huang G. Macrophage migration inhibitory factor is an early marker of severe acute pancreatitis based on the revised Atlanta classification. BMC Gastroenterol. 2021;21(1):34. 10.1186/s12876-020-01598-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Teng TZJ, Tan JKT, Baey S, Gunasekaran SK, Junnarkar SP, Low JK, et al. Sequential organ failure assessment score is superior to other prognostic indices in acute pancreatitis. World J Crit Care Med. 2021;10(6):355–368. 10.5492/wjccm.v10.i6.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y, Xu Z, Zhou Y, Xie M, Qi X, Xu Z, et al. Leukocyte cell population data from the blood cell analyzer as a predictive marker for severity of acute pancreatitis. J Clin Lab Anal. 2021;35(7):e23863. 10.1002/jcla.23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yan G, Li H, Bhetuwal A, McClure MA, Li Y, Yang G, et al. Pleural effusion volume in patients with acute pancreatitis: a retrospective study from three acute pancreatitis centers. Ann Med. 2021;53(1):2003–2018. 10.1080/07853890.2021.1998594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dancu GM, Popescu A, Sirli R, Danila M, Bende F, Tarta C, et al. The BISAP score, NLR, CRP, or BUN: which marker best predicts the outcome of acute pancreatitis? Med (United States). 2021;100(51):E28121. 10.1097/MD.0000000000028121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bardakcı O, Akdur G, Das M, Sıddıkoğlu D, Akdur O, Beyazit Y. Comparison of different risk stratification systems for prediction of acute pancreatitis severity in patients referred to the emergency department of a tertiary care hospital. Ulusal Travma ve Acil Cerrahi Dergisi. 2022;28(7):967–973. 10.14744/tjtes.2021.51892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu B, Yang J, Dai Y, Xiong L. Combination of the BISAP score and miR‐155 is applied in predicting the severity of acute pancreatitis. Int J Gen Med. 2022;15:7467–7474. 10.2147/IJGM.S384068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Windsor JA. Assessment of the severity of acute pancreatitis: No room for complacency. Pancreatology. 2008;8(2):105–109. 10.1159/000123604 [DOI] [PubMed] [Google Scholar]
- 67. Kamal A, Akshintala VS, Kamal MM, El Zein M, Besharati S, Kumbhari V, et al. Does etiology of pancreatitis matter? Differences in outcomes among patients with post‐endoscopic retrograde cholangiopancreatography, acute biliary, and alcoholic pancreatitis. Pancreas. 2019;48(4):574–578. 10.1097/MPA.0000000000001283 [DOI] [PubMed] [Google Scholar]
- 68. Szakács Z, Gede N, Pécsi D, Izbèki F, Papp M, Kovàcs G, et al. Aging and comorbidities in acute pancreatitis II.: a cohort‐analysis of 1203 prospectively collected cases. Front Physiol. 2019;9:1776. 10.3389/fphys.2018.01776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nagy A, Juhàsz M, Gorbe A, Vàradi A, Izbèki F, Vincze A, et al. Glucose levels show independent and dose‐dependent association with worsening acute pancreatitis outcomes: post‐hoc analysis of a prospective, international cohort of 2250 acute pancreatitis cases. Pancreatology. 2021;21(7):1237–1246. 10.1016/j.pan.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 70. Murata A, Matsuda S, Mayumi T, Yokoe M, Kuwabara K, Ichimiya Y, et al. Effect of hospital volume on clinical outcome in patients with acute pancreatitis, based on a National Administrative Database. Pancreas. 2011;40(7):1018–1023. 10.1097/MPA.0b013e31821bd233 [DOI] [PubMed] [Google Scholar]
- 71. Buxbaum J, Quezada M, Chong B, Gupta N, Yao YC, Lane C, et al. The Pancreatitis Activity Scoring System predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am J Gastroenterol. 2018;113(5):755–764. 10.1038/s41395-018-0048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Langmead C, Lee P, Paragomi P, Greer P, Stello K, Hart PA, et al. A novel 5‐cytokine panel outperforms conventional predictive markers of persistent organ failure in acute pancreatitis. Clin Transl Gastroenterol. 2021;12(5):e00351. 10.14309/ctg.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kui B, Pintér J, Molontay R, Nagy M, Farkas N, Gede N, et al. EASY‐APP: an artificial intelligence model and application for early and easy prediction of severity in acute pancreatitis. Clin Transl Med. 2022;12(6):e842. 10.1002/ctm2.842 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Supporting Information S3
Figure S1
Figure S2
Figure S3
Figure S4
Figure S5
Figure S6
Figure S7
Figure S8
Figure S9
Figure S10
Figure S11
Figure S12
Figure S13
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


