Abstract
Purpose:
The aim of this study was to investigate the effects of exercise therapy on physical function and quality of life (QOL) in older patients with non-Hodgkin lymphoma undergoing inpatient chemotherapy, including differences between patients with and without sarcopenia.
Methods:
Thirty-one inpatients aged 70 years or older participated in this study. Grip and knee extensor strength, 6-minute walking test, body composition, nutritional status, fatigue and health-related QOL at admission and discharge were compared. In addition, the patients were classified into sarcopenic and non-sarcopenic groups, and a comparison between admission and discharge and 2-way ANOVA were performed.
Results:
Overall, grip strength and skeletal muscle mass were significantly lower at discharge than at admission (P < .05); however, QOL significantly improved (P < .05). In the non-sarcopenia group, grip strength, right knee extension muscle strength, and skeletal muscle mass were all significantly lower at discharge than at admission (P < .05); however, this was not the case in the sarcopenia group. In terms of QOL, improvements were observed in different items in the non-sarcopenia and sarcopenia groups. There was a significant interaction between admission to discharge time period and sarcopenia regarding left grip strength, right knee extensor strength, and QOL.
Conclusion:
Exercise therapy is effective in improving QOL in older non-Hodgkin lymphoma patients undergoing inpatient chemotherapy. However, the effect of exercise therapy and optimal exercise load may differ between non-sarcopenia and sarcopenia patients. Therefore, it is necessary to consider exercise therapy in the future, taking into account the presence or absence of sarcopenia.
Keywords: older non-Hodgkin lymphoma, older adults, sarcopenia, exercise therapy, physical function, quality of life
Introduction
With the current aging of the population worldwide, 1 the number of elderly patients with cancer is expected to increase. Although the definition of the term “elderly” in hematological malignancies cancer patients has not been defined, Buske et al recommended at The European Society for Medical Oncology consensus conference that patients aged 70 years or older be considered elderly. 2 Patients with non-Hodgkin lymphoma (NHL), in particular, have a mean age at diagnosis of 67 years 3 ; these are the most common hematopoietic tumors in an elderly population. Because chemotherapy in the elderly is associated with many comorbidities and a high risk of treatment-related mortality, chemotherapy doses and intervals should be carefully determined for elderly patients with NHL.4,5 Since aggressive chemotherapy may be difficult for patients with significantly impaired mental and physical function, as well as independence in activities of daily living (ADL), it is important for elderly patients with NHL to maintain mental and physical function and ADL, not only from the perspective of health-related quality of life (QOL), but also to facilitate treatment.
Exercise therapy has been reported to be effective in improving muscle strength,6,7 ADL, 8 walking ability, 8 and QOL7,9 in patients with malignant lymphoma. 10 Therefore, exercise therapy may play an important role in maintaining the physical and mental function and lifestyle of elderly patients with NHL and hence in administering chemotherapy. However, the effectiveness of exercise therapy for elderly patients with NHL has not been fully investigated, and the optimal intensity and frequency of exercise are unknown.
In recent years, it has also become increasingly clear that sarcopenia affects functional outcome. For example, the presence of sarcopenia has been reported to adversely affect ADL independence at hospital discharge in stroke patients, 11 as well as functional recovery after exercise therapy in patients with hip fracture and knee osteoarthritis.12,13 Therefore, the possibility that the presence of sarcopenia may similarly affect the effectiveness of exercise therapy in patients with NHL cannot be denied.
In light of the above background, examining the effectiveness of exercise therapy for patients with malignant lymphoma aged 70 years or older, including the effect of sarcopenia, will provide important information that will contribute to the development of effective intervention programs in the future. Thus, the purpose of this study was to investigate the effects of exercise therapy on physical function and QOL in patients with NHL aged 70 years or older undergoing inpatient chemotherapy, including differences between patients with and without sarcopenia.
Methods
Study Design
The present study was a prospective observational study. The inclusion criteria were follows: patients 1) with NHL who received inpatient chemotherapy and exercise between April 2018 and January 2022 at Kita-Fukushima Medical Center; and 2) who were aged ≥70 years.
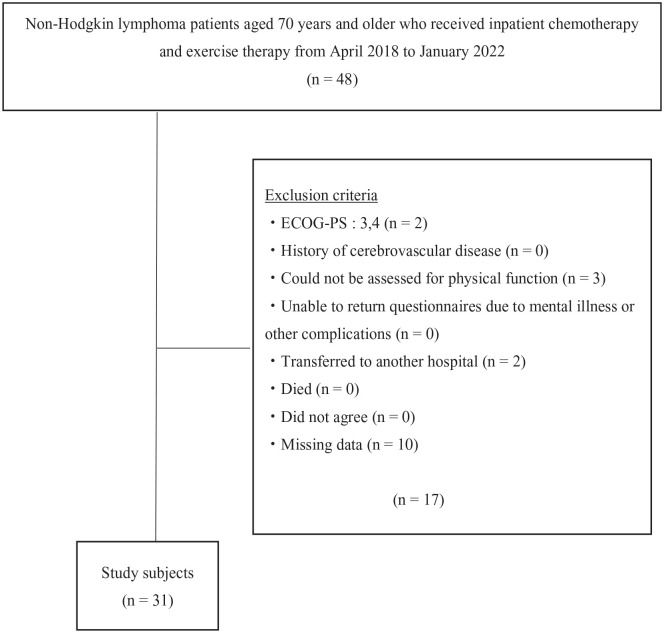
The exclusion criteria were as follows: patients with an Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 3 or more at admission, those who had a history of cerebrovascular disease, those who could not be assessed for physical function, those who were unable to return questionnaires due to mental illness or other complications, those who had transferred to another hospital, those who had died, and those who had not agreed to participate in the study (Figure 1).
Figure 1.
Study flow chart.
This study was conducted with the approval of the Ethical Review Committee of the Kita-Fukushima Medical Center (Approval No. 74). Written informed consent was obtained from each participant.
Definition of Sarcopenia
Sarcopenia was diagnosed according to Asian Working Group for Sarcopenia 201914 (AWGS 2019) consensuses. AWGS 2019 retains the previous definition of sarcopenia, but revises the diagnostic algorithm, protocols and some criteria: handgrip strength <28 kg for men and <18 kg for women, and 6 -meter walk <1.0 m/s, and Short Physical Performance Battery ≤ 9 or five-time chair stand test ≥12 seconds, and Skeletal muscle mass index (SMI) of bioimpedance <7.0kg/m2 in men and <5.7 kg/m2 in women is defined as sarcopenia 14 . In this study, sarcopenia was defined to be a combination of low SMI and low muscle strength.
Exercise Therapy Intervention
All subjects underwent physical therapy with a focus on resistance and aerobic exercises once a day, 6 days a week. performed on the Borg Scale 13 (somewhat hard),15 -17 which is commonly used in cancer patients. Lower-extremity resistance training consisted of squats and calf raises with body weight, and manual resistance exercise for hip flexors, hip abductors, knee extensors, and ankle dorsiflexors. Each exercise was performed 10 to 20 times for 2 to 3 sets, depending on the subject’s muscle strength and endurance. Upper-extremity strength training consisted of shoulder flexion, shoulder abduction, and elbow joint flexion/extension using weights appropriate for the patient’s muscle strength, and each exercise was performed 10 to 20 times for 2 to 3 sets.
During the cytopenia phase after chemotherapy, there were times when the blood test results were lower than the “criteria for discontinuing rehabilitation in cancer patients 18 ”; however, exercise therapy was continued after further evaluation of the patient’s physical condition. As a countermeasure against infection, if the patient’s white blood cell count was ≤2,000/μl, the physical therapy was performed not in the rehabilitation room, but in the ward or hospital room, and the same exercises were performed in the ward.
When the platelet count was <50,000/μl, resistance equipment such as weights were used. In addition, these patients were allowed to walk more briskly and go up and down stairs. When the platelet count was <20,000/μl, the patients were instructed to engage in light exercise such as sitting or standing exercises and walking without resistance. 19 When the hemoglobin level was ≤8 g/dl and the physician judged that blood transfusion was necessary, physical therapy was performed after the transfusion was completed.
Measurements
Admission evaluations were performed prior to initiation of first chemotherapy (either on the day of admission or the next day), and discharge evaluations were performed just prior to discharge after completion of all the chemotherapy courses.
Attributes of the Participants
Data on the following participant attributes were collected: age, gender, weight, body mass index (BMI), ECOG-PS at admission, type of NHL, type of chemotherapy, number of courses of chemotherapy, exercise therapy adherence and total number of days of hospitalization.
Physical Function Assessment
Physical function assessment included muscle strength (grip strength and knee extensor strength), 6-minute walk tests (6 MWT), and body composition. Handgrip strength was measured using a standard adjustable-handle dynamometer (GRIP D; TAKEI Scientific Instruments Co. Ltd, Niigata, Japan). The measurement was performed in the standing position with the upper limbs drooping. The participants performed 2 trials for each hand alternatively. Knee extensor strength was measured using hand-held dynamometers (μ-tas F1; ANIMA Co., Tokyo, Japan), and was tested with the patient sitting with the knee flexed at approximately 90°. The dynamometer was applied proximal to the malleoli. The participants performed 3 trials for each side. For both measurements, the highest value was selected for analysis.
The 6MWT was performed according to American Thoracic Society standards. 20 If patients experienced symptoms such as dyspnea, back pain, or leg pain, they were allowed to rest as needed, resume if they were able to, or terminate the session if they were unable to continue.
Body composition was measured by bioelectrical impedance analysis (BIA) using In Body S10 (In Body S10; In Body Japan Co. Ltd, Tokyo, Japan), and skeletal muscle mass (SMM) and SMI were extracted. SMM refers to the total mass of body skeletal muscle, whereas SMI is a measure of muscle mass in relation to the subject’s height. SMI is used to assess sarcopenia, and is calculated by dividing the limb skeletal muscle mass (kg) by the square of the height (m2). Measurements were taken in the supine position with arms and legs extended, and the patient was asked to remain at rest during the measurement.
Nutritional Measurements
Nutritional status was measured using the Mini Nutritional Assessment (MNA®) and Geriatric Nutritional Risk Index (GNRI). The MNA® is a comprehensive 18-item nutritional assessment tool for the elderly, 21 but it has also been used in cancer patients and has been reported to be associated with survival rate, cancer progression, and health-related QOL.22,23
Fatigue Measurement
Fatigue was measured using Brief Fatigue Inventory 24 (BFI). The BFI is a simple questionnaire for assessing fatigue in patients with cancer and hematopoietic tumors. 25 It consists of 9 questions related to the intensity of fatigue and daily life obstacles that are each rated on a scale of 0 to 10, with the average score calculated; a higher average score indicates a severe case.
Health-Related QOL
Health-related QOL was measured using the Medical Outcome Study 36-item Short-Form Health Survey (SF-36), which assesses physical and mental health components across the following 8 domains: physical functioning (PF), physical role function (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), emotional role functioning (RE), and mental health (MH). We also calculated 3 factor summary scores from the scores of these 8 subscales: physical component summary (PCS), mental component summary (MCS), and role/social component summary (RCS). 26 The SF-36 measures of health-related QOL were on a 0 to 100 scale, with higher scores indicating better QOL. This self-administered questionnaire is widely used to examine patients undergoing chemotherapy for various types of cancer and those who have undergone hematopoietic stem cell transplantation.27 -29
Statistical Analysis
Clinical and demographic characteristics were compared between sarcopenia and non-sarcopenia groups. Physical function, body composition, nutritional status, fatigue, and QOL at admission and discharge were compared in each overall subjects, sarcopenia group, and non-sarcopenia groups. Parametric and nonparametric tests were used, depending on the normality of each variable. In addition, a 2-way analysis of variance was performed to examine differences in each assessment of the 2 groups. Statistical analysis was performed using SPSS 27 (SPSS Japan Inc, Tokyo, Japan) at a significance level of ≤5%.
Results
The attributes of all subjects are summarized in Table 1. The overall mean age was 76.0 ± 5.6 years, height was 156.1 ± 4.5 cm, and weight was 54.0 ± 15.9 kg. Fourteen were male (17 female). The most common type of NHL was DLBCL, and the most common form of chemotherapy was R-CHOP. All subjects received chemotherapy as planned, with a complete response rate of 74.2% and a partial response rate of 25.8%. In the non-sarcopenia group 65.0% had a complete response, and 35% had a partial response, whereas in the sarcopenia group, 90.9% had a complete response and 9.1% had a partial response. There was no significant difference in response rate between the 2 groups.
Table 1.
Clinical and Demographic Characteristics of Patients With Malignant Lymphoma.
| Characteristics | Total n = 31 |
Non-Sarcopenia n = 20 (65%) |
Sarcopenia n = 11 (35%) |
P-value |
|---|---|---|---|---|
| Age, years | 76.0 ± 5.6 | 75.9 ± 4.5 | 76.1 ± 7.4 | .90 |
| Height, cm | 156.1 ± 8.9 | 159.9 ± 8.5 | 151.2 ± 7.7 | <.05 |
| Body weight, kg | 54.0 ± 15.9 | 60.6 ± 9.6 | 44.4 ± 6.2 | <.05 |
| BMI, kg/m2 | 22.1 ± 5.6 | 23.5 ± 6.4 | 19.4 ± 2.2 | <.05 |
| Sex | ||||
| Male | 14 (45.0) | 12 (60.0%) | 2 (18.0%) | <.05 |
| Female | 17 (55.0) | 8 (40.0%) | 9 (82.0%) | |
| Performance status | ||||
| 0 | 22 (71.0) | 17 (85.0%) | 5 (45.4%) | .06 |
| 1 | 5 (16.0) | 2 (10.0%) | 3 (27.3%) | |
| 2 | 4 (13.0) | 1 (5.0%) | 3 (27.3%) | |
| Non-Hodgkin Lymphoma Type | ||||
| DLBCL | 29 (93.6) | 19 (95.0%) | 10 (91.0%) | |
| FL | 1 (3.2) | 1 (5.0%) | 0 (0%) | |
| MALT | 1 (3.2) | 0 (0%) | 1 (9.0%) | |
| Chemotherapy | ||||
| R-CHOP | 26 (83.9) | 18 (90.0%) | 8 (72.7%) | |
| Number of courses | 6.5 ± 0.9 | 6.7 ± 1.0 | 6.3 ± 0.7 | |
| Other than R-CHOP | 5 (16.1) | 2 (10.0%) | 3 (27.3%) | |
| Number of courses | 5.8 ± 0.4 | 6.0 ± 0.0 | 5.7 ± 0.6 | |
| Treatment effect | .20 | |||
| Complete response | 23 (74.2%) | 13 (65.0%) | 10 (90.9%) | |
| Partial response | 8 (25.8%) | 7 (35.0%) | 1 (9.1%) | |
| Adherence to Exercise Therapy, % | 95.5 ± 4.3 | 95.6 ± 5.1 | 95.3 ± 2.6 | .89 |
| Length of hospitalization, days | 143.5 ± 28.7 | 143.9 ± 27.2 | 142.8 ± 33.8 | .93 |
Values are presented as means ± standard deviations (SD) or number. Statistical testing at baseline was performed using independent Student’s t-tests or Pearson’s χ2 tests. P < .05 compared with the non-sarcopenia and sarcopenia groups.
Abbreviations: BMI, body mass index; ECOG-PS, eastern cooperative oncology group performance status; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MALT, mucosa-associated lymphoid tissue. R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone.
The adherence to exercise therapy was 95.5 ± 4.3%, and the total length of hospitalization was 143.5 ± 28.7 days. The percentage of sarcopenia among all subjects was 35%. Compared to the non-sarcopenia group, the sarcopenia group had significantly lower height, weight, and BMI (P < .05), as well as significantly more women (P < .05). There were no significant differences in age, ECOG-PS at admission, adherence, or total length of hospitalization between the sarcopenia and non-sarcopenia groups.
First, the results of physical function and nutritional status, fatigue, and QOL from admission to discharge for all subjects are shown in Table 2. Regarding physical function, grip strength and SMM were significantly lower at discharge than at admission (P < .05); regarding QOL, GH, MH, PCS, and MCS were significantly better at discharge than at admission (P < .05).
Table 2.
Physical Function, Muscle Mass, Body Weight, GNRI, MNA®, BFI, and QOL at Admission and Discharge of the Study Participants.
| Variables | Admission | Discharge | P-value |
|---|---|---|---|
| Rt Handgrip (kgf) | 23.5 ± 9.1 | 21.2 ± 7.3 | <.01 |
| Lt Handgrip (kgf) | 21.6 ± 9.6 | 19.7 ± 7.1 | <.05 |
| Rt Knee ext (kgf) | 22.2 ± 9.0 | 21.2 ± 7.4 | .18 |
| Lt Knee ext (kgf) | 21.8 ± 8.8 | 21.4 ± 7.6 | .52 |
| 6MWT (m) | 414.4 ± 81.5 | 424.9 ± 81.5 | .37 |
| SMM (kg) | 20.7 ± 4.9 | 19.7 ± 4.5 | <.01 |
| SMI (kg/m2) | 6.2 (5.6-7.0) | 5.7 (5.2-6.8) | .08 |
| Body weight (kg) | 55.0 ± 11.5 | 53.8 ± 11.3 | .13 |
| GNRI (points) | 100.1 ± 12.6 | 101.9 ± 9.0 | .33 |
| MNA® | 21.5 (19.0-23.5) | 24.0 (22.0-26.0) | <.05 |
| BFI | 2.6 (0.8-3.4) | 1.2 (0.3-2.8) | .50 |
| Physical functioning | 66.0 ± 23.5 | 73.8 ± 19.6 | .10 |
| Role physical | 63.5 ± 31.2 | 65.3 ± 31.1 | .82 |
| Bodily pain | 64.6 ± 26.9 | 76.2 ± 23.9 | .09 |
| General health | 43.4 ± 14.6 | 55.2 ± 15.7 | <.01 |
| Vitality | 51.3 ± 20.6 | 57.5 ± 20.7 | .33 |
| Social functioning | 74.0 ± 27.0 | 72.5 ± 26.8 | .82 |
| Role emotional | 67.7 ± 27.5 | 77.0 ± 29.1 | .16 |
| Mental health | 61.4 ± 20.2 | 72.0 ± 19.1 | <.05 |
| PCS | 36.6 ± 11.5 | 42.0 ± 12.3 | <.05 |
| MCS | 49.2 ± 8.7 | 54.1 ± 9.7 | <.05 |
| RCS | 43.1 ± 17.6 | 41.8 ± 13.5 | .74 |
Mean ±SD or Median (25–75%tile).
Abbreviations: Rt, right; Lt, left; Knee ext, knee extension; 6MWT, 6-minute walk test; SMM, skeletal muscle mass; SMI, skeletal muscle mass index; GNRI, geriatric nutritional risk index; MNA®, mini nutritional assessment; BFI, brief fatigue inventory; SF-36, MOS 36-item short-form health survey; PCS, physical component summary; MCS, mental component summary; RCS, role/social. Component summary.
Second, the results of physical function and nutritional status, fatigue, and QOL from admission to discharge for the sarcopenia and non-sarcopenia groups are shown in Table 3. The non-sarcopenia group had significantly lower bilateral grip strength and right knee extension muscle strength (P < .01) and SMM (P < .05) at discharge than at admission and significantly increased MNA (P < .01). On the one hand, in the sarcopenia group, there were no significant differences in any of the variables. As a result of 2-way ANOVA, there were significant interaction effects between time period (admission and discharge) and groups in left grip strength and right knee extensor strength. (P < .05).
Table 3.
Comparison of Participants’ Functions Between the Sarcopenia and Non-Sarcopenia Groups.
| Non-Sarcopenia n = 20 (65%) | Sarcopenia n = 11 (35%) | Non-Sarcopenia vs Sarcopenia Interaction | |||||
|---|---|---|---|---|---|---|---|
| Variables | Admission | Discharge | p-value | Admission | Discharge | p-value | p-value |
| Rt Handgrip (kgf) | 27.5 ± 8.7 | 24.4 ± 6.7 | <.01 | 16.1 ± 3.9 | 15.4 ± 4.5 | .13 | .06 |
| Lt Handgrip (kgf) | 25.7 ± 8.8 | 22.8 ± 6.3 | <.01 | 14.1 ± 5.7 | 13.9 ± 4.5 | .80 | <.05 |
| Rt Knee ext (kgf) | 26.1 ± 8.4 | 23.9 ± 7.4 | <.05 | 15.3 ± 5.0 | 16.3 ± 4.3 | .31 | <.05 |
| Lt Knee ext (kgf) | 25.6 ± 8.2 | 24.9 ± 6.4 | .46 | 15.0 ± 4.8 | 15.0 ± 5.1 | .96 | .60 |
| 6MWT (m) | 428.7 ± 74.9 | 435.4 ± 79.3 | .65 | 388.3 ± 89.9 | 405.7 ± 85.5 | .41 | .66 |
| SMM (kg) | 22.7 ± 4.6 | 21.5 ± 4.4 | <.01 | 17.0 ± 2.9 | 16.5 ± 2.7 | .23 | .24 |
| SMI (kg/m2) | 6.7(6.1-7.3) | 6.6(5.5-7.2) | .23 | 5.4 (4.6-5.6) | 4.9 (4.6-5.7) | .22 | .68 |
| Body weight (kg) | 60.6 ± 9.6 | 59.3 ± 9.3 | .27 | 44.6 ± 6.2 | 43.6 ± 7.0 | .23 | .85 |
| GNRI (points) | 104.5 ± 11.8 | 105.2 ± 8.1 | .68 | 91.7 ± 9.7 | 95.6 ± 7.5 | .39 | .40 |
| MNA® | 22.5(20.5-23.5) | 25.0(23.0-26.5) | <.05 | 18.3 (17.0-20.5) | 21.8 (20.3-23.5) | .21 | .89 |
| BFI | 1.3(0.6-2.8) | 1.3 (0.6-2.6) | .76 | 3.7 (1.8-5.9) | 0.6 (0.2-3.2) | .16 | .13 |
| Physical functioning | 71.5 ± 22.5 | 77.4 ± 13.1 | .21 | 54.4 ± 22.4 | 66.3 ± 28.8 | .31 | .55 |
| Role physical | 69.9 ± 31.3 | 62.9 ± 30.1 | .30 | 50.0 ± 27.9 | 70.0 ± 32.9 | .32 | .10 |
| Bodily pain | 64.8 ± 25.9 | 76.6 ± 22.0 | .08 | 64.3 ± 30.8 | 75.3 ± 29.3 | .53 | .96 |
| General health | 42.5 ± 13.7 | 56.2 ± 15.3 | <.01 | 45.5 ± 17.1 | 52.9 ± 17.4 | .16 | .25 |
| Vitality | 56.3 ± 20.0 | 56.3 ± 22.8 | .96 | 39.9 ± 17.6 | 60.2 ± 16.7 | .07 | .12 |
| Social functioning | 74.3 ± 28.8 | 69.9 ± 29.7 | .60 | 73.4 ± 24.5 | 78.1 ± 19.8 | .69 | .53 |
| Role emotional | 72.1 ± 29.6 | 71.6 ± 31.7 | .95 | 58.3 ± 20.9 | 88.5 ± 19.4 | <.05 | <.05 |
| Mental health | 65.0 ± 18.8 | 72.7 ± 10.2 | .21 | 53.8 ± 22.1 | 70.6 ± 17.6 | .08 | .39 |
| PCS | 40.2 ± 12.8 | 45.5 ± 11.5 | <.05 | 32.4 ± 9.7 | 36.4 ± 13.2 | .47 | .07 |
| MCS | 49.2 ± 8.6 | 54.4 ± 10.6 | .05 | 49.2 ± 9.6 | 53.4 ± 7.8 | .24 | .82 |
| RCS | 45.6 ± 18.6 | 38.1 ± 14.0 | .09 | 38.2 ± 15.0 | 48.6 ± 10.5 | .22 | <.05 |
Mean ±SD or Median (25%–75%tile). Mean ±SD, all values are points.
Abbreviations: Rt, right; Lt, left; Knee ext, knee extension; 6MWT, 6-minute walk test; SMM, skeletal muscle mass; SMI, skeletal muscle mass index; GNRI, geriatric nutritional risk index; MNA®, mini nutritional assessment; BFI, brief fatigue inventory; SF-36, MOS 36-item short-form health survey; PCS, physical component summary; MCS, mental component summary; RCS, role/social. Component summary.
Regarding QOL, the non-sarcopenia group showed significant improvement in GH (P < .01) and PCS (P < .01) at discharge compared to admission, with no significant differences in the other variables. The sarcopenia group showed a significant improvement in RE at discharge compared to admission (P < .05), with no significant differences in the other items. The interaction in the sarcopenia and non-sarcopenia groups was significantly different in regards to RE and RCS (P < .05).
Discussion
Exercise therapy is known to be effective in improving physical function and QOL in NHL patients; however, its effectiveness in elderly NHL patients remains unclear. In addition, it has become clear that sarcopenia, which is common in the elderly, affects the functional outcomes of many diseases. Since NHL patients are often elderly, the present study investigated the progress of rehabilitation outcomes in elderly patients with NHL who had undergone exercise using Borg 13 as an index, including the presence or absence of sarcopenia.
In the present study, we found that in elderly NHL patients who had undergone chemotherapy and exercise therapy during hospitalization, both grip strength and SMM had significantly decreased, whereas MNA and some items of QOL had significantly improved at discharge compared to admission. The reason for the decrease in grip strength may be related to the content of the patients’ training. The upper-extremity strength training performed in this study was shoulder and elbow exercises, and did not include grip-specific training. These results suggest that elderly patients with NHL may also require grip strength training. On the one hand, our results suggest that knee extension strength and QOL were maintained or improved in elderly patients with NHL who received exercise therapy. It should be noted that due to design issues in this study, it was not possible to state whether exercise therapy affects the maintenance of knee muscle strength and improvement of QOL. However, considering that previous studies7,9 have reported that exercise therapy improves QOL in cancer patients undergoing chemotherapy, exercise therapy may have contributed to the improvement of QOL in elderly patients with NHL in in the present study.
Regarding the presence of sarcopenia, the sarcopenia rate for all subjects was 35%. The prevalence of sarcopenia has varied from a minimum of 24.6% 30 to a maximum of 54.9% 31 in studies using L3-SMI, and the only previous study 32 using BIA approximated the present results at 36%. In the present study, the sarcopenia group had significantly lower height, weight, and BMI than the non-sarcopenia group, possibly because there were more women in the sarcopenia group. Although previous studies have reported no sex difference in the prevalence of sarcopenia,33,34 when looking at age and sex differences, sarcopenia tends to be more common in women under the age of 80 and in men over the age of 80. 35 Considering that the average age of the subjects in the present study was 76.0 years, the results are consistent with previous studies. It has also been reported that the prevalence of sarcopenia is higher in women than in men with NHL, 35 and the results of the present study results are similar to those of the reports.
One of the main findings of this study was that elderly NHL patients with and without sarcopenia experienced different outcomes, despite having undergone similar exercise regimens based on Borg scale 13. In a comparison of the sarcopenia and non-sarcopenia groups, the non-sarcopenia group showed a trend toward decreased muscle strength and SMM, while the sarcopenia group showed a trend toward maintained or increased muscle strength. An interaction between the time of assessment (admission and discharge) and the presence or absence of sarcopenia was also observed for some muscle strengths, indicating that the progress of patient’s functional status differed between the groups. There were no differences in weight or nutritional status between the groups, making it unlikely that nutritional status influenced the differences in progress of patient’s functional status. The reason for the difference in progress of patient’s functional status between the groups is a matter of speculation, but one possibility is that the exercise intensity required to maintain and improve muscle strength differs between sarcopenic and non-sarcopenic groups. A systematic review by Beckwee et al 36 reported that resistance training for sarcopenia is sufficient for muscle strengthening even at low intensities (≤50% 1RM) and the recommended frequency is 7 to 9 times per set, 3 times per week, for 2 to 3 sets. The exercise regimen in this study, indexed to Borg 13 of exercise therapy, was performed once a day, 6 days a week, which may have been a sufficient intensity for those with sarcopenia. On the other hand, for the non-sarcopenia group, which had higher physical function at admission, the intensity was not high enough, and a stronger load may have been necessary.
Regarding QOL, the only items that significantly improved were GH and PCS in the non-sarcopenia group and RE in the sarcopenia group. The current results showed no significant improvement in either group, but there were no significant decreases in any of the items, indicating an overall trend toward maintenance and improvement. Although this study did not investigate causality, exercise therapy may be associated with the maintenance and improvement of QOL regardless of the presence or absence of sarcopenia. However, there is an interaction between RE and RCS in the non-sarcopenia and sarcopenia groups, and exercise therapy for the sarcopenia group may be associated with improved social aspects of QOL.
A limitation of this study is the small sample size. The required sample size, calculated before the study began, was set at a = 0.05, power = 0.8, and effect size (d) =0.5, which would require 34 patients. However, due to the influences of the COVID-19 pandemic, it was difficult to recruit enough patients to meet these requirements within the study enrollment period. In addition, this was a single-arm and pre-post comparison study. Since various factors are involved in rehabilitation outcomes, the various changes in the subjects in the present study were not due to exercise therapy alone. In the future, in addition to securing a larger sample, it will be necessary to review the nutritional assessment and evaluate activity levels.
Conclusion
Exercise therapy on the Borg scale 13 is feasible and safe enough for NHL patients over 70 years of age during inpatient chemotherapy. Regarding the effects of the subject’s exercise therapy on physical function, both knee extensor strength and endurance were maintained between admission and discharge; however, there was a decrease in grip strength in both hands in our participants. Furthermore, the same exercise therapy was administered in the non-sarcopenia and sarcopenia groups from the time of admission, with differences in changes in physical function and QOL. Therefore, it is necessary to consider the content of exercise therapy in the future, taking into account the presence or absence of sarcopenia.
Acknowledgments
Not applicable.
Footnotes
Author Contributions: Ryuichi Kasahara, Ryohei Jinbo, Shinichiro Morishita, Yutaka Shiga, Hideo Kimura, Miki Furukawa, Mai Owari and Tatsuyuki Kai made substantial contributions to the conception and design. Material preparation, data collection and analysis were performed by Ryuichi Kasahara, Ryohei Jinbo, Junko Kubota, Aya Takano, Shoko Takahashi, Yuka Ohashi, Kazumi Jinbo, Yuichi Yamamoto, Takaaki Fujita and Shinichiro Morishita. Ryuichi Kasahara, Takaaki Fujita, Tatsuyuki Kai and Shinichiro Morishita were major contributors in drafting and writing the manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials: The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a Grant-in-Aid for scientific research (C) (grant no. 21K11176) from the Japan Society for the Promotion of Science.
Consent to Participate: Informed consent was obtained from all individual participants included in the study.
Consent to Publish: Written informed consent was obtained from the patients regarding publishing their data.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Kita-Fukushima Medical Center (Approval No. 74).
ORCID iD: Shinichiro Morishita  https://orcid.org/0000-0002-4841-948X
https://orcid.org/0000-0002-4841-948X
References
- 1. World Health Organization (WHO). Ageing and health. Accessed May 14, 2022. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 2. Buske C, Hutchings M, Ladetto M, et al. ESMO Lymphoma Consensus Conference Panel Members. ESMO Consensus Conference on malignant lymphoma: general perspectives and recommendations for the clinical management of the elderly patient with malignant lymphoma. Ann Oncol. 2018;29(3):544-562. doi: 10.1093/annonc/mdx413 [DOI] [PubMed] [Google Scholar]
- 3. Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of Non-Hodgkin’s lymphoma. Med Sci. 2021;9(1):5. doi: 10.3390/medsci9010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jung YH, Woo IS, Han CW. Clinical characteristics and outcomes in diffuse large B cell lymphoma patients aged 70 years and older: a single-center experience with a literature review. Korean J Intern Med. 2015;30(5):684-693. doi: 10.3904/kjim.2015.30.5.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Storti S, Spina M, Pesce EA, et al. Rituximab plus bendamustine as front-line treatment in frail elderly (>70 years) patients with diffuse large B-cell non-Hodgkin lymphoma: a phase II multicenter study of the fondazione Italiana Linfomi. Haematologica. 2018;103(8):1345-1350. doi: 10.3324/haematol.2017.186569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sahin U, Dundar I, Celebi MM, et al. The effects of exercise prescription on aerobic performance and quality of life during the course of lymphoma chemotherapy: results of a prospective controlled study. Clin Lymphoma Myeloma Leuk. 2022;22(1):e15-e25. doi: 10.1016/j.clml.2021.07.018 [DOI] [PubMed] [Google Scholar]
- 7. Cox MC, Nusca SM, Di Landro F, et al. Exercise training (ET) in adult and elderly patients receiving anti-lymphoma treatments is feasible and may improve the provision of care. Leuk Lymphoma. 2021;62(3):560-570. doi: 10.1080/10428194.2020.1842396 [DOI] [PubMed] [Google Scholar]
- 8. Fukushima T, Nakano J, Ishii S, et al. Low-intensity exercise therapy with high frequency improves physical function and mental and physical symptoms in patients with haematological malignancies undergoing chemotherapy. Eur J Cancer Care. 2018;27(6):e12922. doi: 10.1111/ecc.12922 [DOI] [PubMed] [Google Scholar]
- 9. Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25(2):493-499. doi: 10.1093/annonc/mdt568 [DOI] [PubMed] [Google Scholar]
- 10. Courneya KS, Sellar CM, Stevinson C, et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol. 2009;27(27):4605-4612. doi: 10.1200/JCO.2008.20.0634 [DOI] [PubMed] [Google Scholar]
- 11. Matsushita T, Nishioka S, Taguchi S, Yamanouchi A. Sarcopenia as a predictor of activities of daily living capability in stroke patients undergoing rehabilitation. Geriatr Gerontol Int. 2019;19(11):1124-1128. doi: 10.1111/ggi.13780 [DOI] [PubMed] [Google Scholar]
- 12. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28:1569-1576. doi: 10.1007/s00198-017-3929-z [DOI] [PubMed] [Google Scholar]
- 13. Liao CD, Chen HC, Huang SW, Liou TH. Impact of sarcopenia on rehabilitation outcomes after total knee replacement in older adults with knee osteoarthritis. Ther Adv Musculoskelet Dis. 2021;13. doi: 10.1177/1759720x21998508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300-307.e2. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 15. van Waart H, Stuiver MM, van Harten WH, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918-1927. doi: 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 16. Hacker ED, Larson JL, Peace D. Exercise in patients receiving hematopoietic stem cell transplantation: lessons learned and results from a feasibility study. Oncol Nurs Forum. 2011;38(2):216-223. doi: 10.1188/11.ONF.216-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined Resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340-347. doi: 10.1200/JCO.2009.23.2488 [DOI] [PubMed] [Google Scholar]
- 18. Gerber LH, Valgo M. Rehabilitation for patients with cancer diagnoses. In: DeLisa JA, Gans BM (eds) Rehabilitation Medicine: principles and Practice, 3rd ed. Lippincott-Raven Publishing; 1998;1293-1317. [Google Scholar]
- 19. Morishita S, Nakano J, Fu JB, Tsuji T. Physical exercise is safe and feasible in thrombocytopenic patients with hematologic malignancies: A narrative review. Hematology. 2020;25(1):95-100. doi: 10.1080/16078454.2020.1730556 [DOI] [PubMed] [Google Scholar]
- 20. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 21. Vellas B, Guigoz Y, Garry PJ, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15(2):116-122. doi: 10.1016/S0899-9007(98)00171-3 [DOI] [PubMed] [Google Scholar]
- 22. Torbahn G, Strauss T, Sieber CC, Kiesswetter E, Volkert D. Nutritional status according to the mini nutritional assessment (MNA)® as potential prognostic factor for health and treatment outcomes in patients with cancer – a systematic review. BMC Cancer. 2020;20(1):594. doi: 10.1186/s12885-020-07052-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang X, Edwards BJ. Malnutrition in older adults with cancer. Curr Oncol Rep. 2019;21(9):80. doi: 10.1007/s11912-019-0829-8 [DOI] [PubMed] [Google Scholar]
- 24. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186-1196. doi: [DOI] [PubMed] [Google Scholar]
- 25. Jafari H, Janati Y, Yazdani J, Bali N, Hassanpour S. The effect of relaxation technique on fatigue levels after stem cell transplant. Iran J Nurs Midwifery Res. 2018;23(5):388-394. doi: 10.4103/ijnmr.IJNMR_26_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suzukamo Y, Fukuhara S, Green J, et al. Validation testing of a three-component model of Short Form-36 scores. J Clin Epidemiol. 2011;64(3):301-308. doi: 10.1016/j.jclinepi.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 27. Syrjala KL, Stover AC, Yi JC, Artherholt SB, Abrams JR. Measuring social activities and social function in long-term cancer survivors who received hematopoietic stem cell transplantation. Psychooncology. 2010;19(5):462-471. doi: 10.1002/pon.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samuel SR, Maiya GA, Babu AS, Vidyasagar MS. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013;137(3):515-520. [PMC free article] [PubMed] [Google Scholar]
- 29. Morishita S, Kaida K, Yamauchi S, et al. Relationship of physical activity with physical function and health-related quality of life in patients having undergone allogeneic haematopoietic stem-cell transplantation. Eur J Cancer Care. 2017;26(4). doi: 10.1111/ecc.12669 [DOI] [PubMed] [Google Scholar]
- 30. Go SI, Park MJ, Song HN, et al. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle. 2016;7(5):567-576. doi: 10.1002/jcsm.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lanic H, Kraut-Tauzia J, Modzelewski R, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. 2014;55(4):817-823. doi: 10.3109/10428194.2013.816421 [DOI] [PubMed] [Google Scholar]
- 32. Kamiya T, Mizuno K, Ogura S, et al. A prospective observational study evaluating sarcopenia by using the bioelectrical impedance analysis in elderly patients with hematologic malignancies. Blood. 2018;132(Supplement 1):4851-4851. doi: 10.1182/blood-2018-99-114545 [DOI] [Google Scholar]
- 33. Yoshimura N, Muraki S, Oka H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017;28(1):189-199. doi: 10.1007/s00198-016-3823-0 [DOI] [PubMed] [Google Scholar]
- 34. Shafiee G, Keshtkar A, Soltani A, et al. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16(1):21. doi: 10.1186/s40200-017-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirchengast S, Huber J. Gender and age differences in lean soft tissue mass and sarcopenia among healthy elderly. Anthropol Anz. 2009;67(2):139-151. doi: 10.1127/0003-5548/2009/0018 [DOI] [PubMed] [Google Scholar]
- 36. Beckwée D, Delaere A, Aelbrecht S, et al. Exercise interventions for the prevention and treatment of sarcopenia. A systematic umbrella review. J Nutr Health Aging. 2019;23(6):494-502. doi: 10.1007/s12603-019-1196-8 [DOI] [PubMed] [Google Scholar]