Abstract
Alzheimer’s disease (AD) is an inflammatory associated disease, in which dysregulated kynurenine pathway (KP) plays a key role. Through KP, L-tryptophan is catabolized into neurotoxic and neuroprotective metabolites. The overactivation of indolamine 2,3-dioxygenase1 (IDO1), the first rate-limiting enzyme of KP, and the abnormal accumulation of KP metabolites have been noted in AD, and blocking IDO1 has been suggested as a therapeutic strategy. However, the expression patterns of KP enzymes in AD, and whether these enzymes are related to AD pathogenesis, have not been fully studied. Herein, we examined the expression patterns of inflammatory cytokines, neurotrophic factors and KP enzymes, and the activity of IDO1 and IDO1 effector pathway AhR (aryl hydrocarbon receptor) in AD mice. We studied the effects of IDO1 inhibitors on Aβ-related neuroinflammation in rat primary neurons, mouse hippocampal neuronal cells, and APP/PS1 mice. The results further demonstrated the importance of IDO1-catalyzed KP in neuroinflammation in Alzheimer’s disease.
Keywords: Indoleamine 2,3-dioxygenase 1; Kynurenine Pathway; Kynureninase; Indoleamine 2,3-dioxygenase 1 Inhibitor; Alzheimer's Disease
Significance Statement
• In AD mice, expressions of inflammatory cytokines, neurotrophic factors, KP enzymes and the activity of IDO1 significantly differ from those in WT mice.
• Aβ induces increase in the expressions of KP enzymes in rat primary neurons and neuronal HT22 cells, while IDO1 inhibitor reverses the effect.
• In APP/PS1 mice, application of IDO1 inhibitor changes the expression patterns of inflammatory cytokines and neurotrophic factors in hippocampus into those similar to WT mice, as well as restores the increased expressions of KP enzymes.
Introduction
Alzheimer’s disease (AD), the most common form of dementia, is characterized by the extracellular amyloid-beta (Aβ) plaques and intraneuronal deposits of neurofibrillary tangles (NFTs) which ultimately lead to the dysfunction and loss of synapses and the eventual death of neuron.1-3 AD is an inflammatory associated disease and dysregulated kynurenine pathway (KP) plays an important role in neuroinflammation cascade in AD pathogenesis.3-5 The KP is a major route of essential amino acid L-tryptophan (L-Trp) catabolism yielding the production of NAD and other neuroactive intermediates both neuroprotective and neurotoxic.5,6
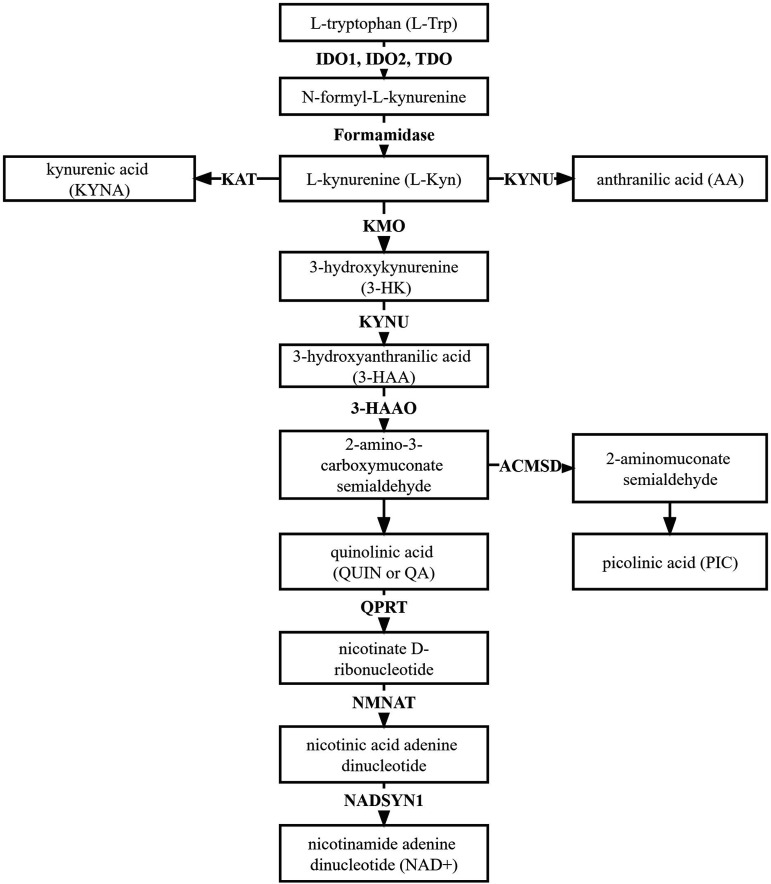
Following the KP, L-Trp is converted to N-formyl-L-kynurenine by indoleamine-2,3-dioxygenase 1 and 2 (IDO1 and 2), and tryptophan 2,3-dioxygenase (TDO), the first rate-limiting enzymes in L-Trp degradation.5-8 Proceeding along the KP, N-formyl-L-kynurenine is metabolized to L-kynurenine (Kyn), the first stable intermediate metabolite, by formamidase 5 (Figure 1). Kyn is the central metabolite of the KP, and can be catabolized through three specific pathways (Figure 1). The main downstream KP enzymes known to catabolize Kyn and its metabolites include kynurenine amino-transferase (KAT), kynureninase (KYNU), kynurenine 3-monooxygenase (KMO), and 3-hydroxyanthrailic acid oxygenase (3-HAAO).5,6 Deamination of Kyn by KAT results in the production of kynurenic acid (KYNA). Kyn is catalyzed by KYNU to form anthranilic acid (AA). Hydroxylation of Kyn by KMO produces 3-hydroxykynurenine (3-HK) which is subsequently converted into 3-hydroxyanthranilic acid (3-HAA) by KYNU.5,9,10 Further catabolism of 3-HAA initially catalyzed by 3-HAAO can lead to the formation of the quinolinic acid (QUIN or QA; the precursor of NAD + synthesis), or picolinic acid (PIC).5-7,9,11 3-HK, 3-HAA and QUIN are neurotoxic metabolites while KYNA and PIC neuroprotective.6,7,9 There is good evidence that these metabolites derived from the KP are causative of AD pathology.6,8-20 However, the expression patterns of KP enzymes in AD, and whether these enzymes are related to AD pathogenesis, have not been fully studied.
Figure 1.
The Kynurenine Pathway (KP). L-tryptophan is catabolized along the KP to form L-kynurenine, which is subsequently metabolized to different neurotoxic or neuroprotective metabolites before converted to NAD+. IDO1, indoleamine 2,3-dioxygenase 1; IDO2, indoleamine 2,3-dioxygenase 2; TDO, tryptophan 2,3-dioxygenase; KAT, kynurenine amino-transferase; KMO, kynurenine 3-monooxygenase; KYNU, kynureninase; 3-HAAO, 3-hydroxyanthrailic acid oxygenase; ACMSD, aminocarboxymuconate semialdehyde decarboxylase; QPRT, nicotinate-nucleotide pyrophosphorylase [carboxylating]; NMNAT, nicotinamide mononucleotide adenylyltransferase; NADSYN1, Glutamine-dependent NAD(+) synthetase.
Kyn has been identified as an endogenous ligand of AhR 21 which is a transcription receptor that responds to extracellular signals to alter cell functions including cell differentiation, apoptosis, immune response and injury repairing.22-24 Increasing evidence shows a deleterious role of AhR in various neural system disease. For instance, Kyn-AhR pathway has been reported to be involved in ischemic brain damage and therefore considered as a potential therapeutic target in stroke. 25 Absence of AhR gene in mice showed protective effect against Huntington’s disease (HD) in HD mimicking transgenic mice. 26 In addition, in Parkinson’s disease mouse model induced by neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrathydropyridine (MPTP), AhR in microglia and astrocytes in the striatum was activated after MPTP treatment. 27 However, the investigation of the association between AhR gene polymorphism and higher risk of AD in Chinese population shows negative result. 28 Up to date, the expression and function of AhR in AD has rarely been explored.
IDO1 is up-regulated by inflammatory molecules and cytokines, such as lipopolysaccharides (LPS), 29 tumor necrosis factor alpha (TNF-α) 30 and most potently by interferon gamma (IFN-γ) which induces both the enzymatic activity and gene expression of IDO1. 31 Activation of IDO1 and KP in microglia and astrocytes is a pathogenic factor of Aβ-associated inflammation in AD.20,32-38 Microglia and astrocytes are the mediators of neuroinflammation in AD and known sites of KP catabolism34,35 which contain all enzymes required for KP progression. 39 In situ data on the KP in neurons are limited. It is known that IFN-γ treated human primary neurons in culture express IDO1 mRNA and protein but do not produce any detectable amount of QUIN.33,34 The induction of IDO1 is related to the down-regulation of brain-derived neurotrophic factor (BNDF) in the prefrontal cortex and hippocampus of inflammation-associated depression mice. 40 Neurotrophic factors are important for neuronal survival 41 and were markedly reduced in the brain of AD patients 42 and AD mouse models. 43 It could be suggested that a neurotrophic deficiency in AD brain could be correlated with IDO1 activation by pro-inflammatory cytokines.
Here, we compared the expressions of inflammatory cytokines, neurotrophic factors, KP enzymes and the activity of IDO1 in AD mice (APOE−/− mice and APP/PS1 mice) and wild type mice at different month age. We also explored the function of IDO1 inhibitors on KP enzymes, inflammatory cytokines and neurotrophic factors expressions in the hippocampus of APP/PS1 mice. Furthermore, using SD rat primary hippocampal neurons and HT22 cells, we first explored the effects of Aβ on KP enzymes and the reversal effects of IDO1 inhibitor on Aβ-treated HT22 cells. Our study suggests that inhibition of IDO1 is a promising therapeutic strategy targeting KP while influencing inflammatory cytokines and neurotrophic factors for the treatment of AD.
Materials and Methods
Cell Culture
SD Rat Primary Hippocampal Neuron Culture
Hippocampal neuron culture was prepared using method previously described by Banker et al 44 with minor modification. The hippocampi from embryonic SD rats were isolated and dissociated with trypsin for 15 min at 37°C. The cell suspension was collected in a cell strainer to obtain the dissociated neurons that were then rapidly transferred into the medium. The neurons were then counted and plated on coverslips coated with poly-D-lysine in 12-well culture plates or six-well culture plates and maintained in Neuro basal medium supplemented with B27 (Invitrogen, CA, USA). Half of the medium was replaced with an identical medium every 3 days. Cultures were kept at 37°C in a humidified incubator with 5% CO2/95% air for 14 days before using.
HT22 Culture
HT22 cells were purchased from the cell bank of the Chinese academy of sciences (Shanghai, China), cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (GIBCO, USA) supplemented with 10% (v/v) fetal bovine serum (FBS), 1% penicillin/streptomycin and NaHCO3 (2 mg/mL), and maintained at 37°C in an atmosphere of 5% CO2 and 95% humidified air. The cells are checked for mycoplasma contamination and cleared for use.
Reagents
1-L-MT was purchased from Sigma-Aldrich. RY103 is a small molecule IDO1 inhibitor designed and developed by our lab. The chemical formulas of RY103 is kept non-disclosed due to confidentiality reasons during the process of patent application.
Aβ 1-42 oligomers solution was prepared by the following protocol: Aβ 1-42 peptide powder (Beyotime, China) was dissolved in hexafluoroisopropanol (HFIP, Sigma-aldrich, USA) and the final concentration of Aβ was 1 mM, and the solution was left in fume hood until HFIP was evaporated, to which DMSO (Sinopharm, China) was added and the final concentration of Aβ was 5 mM. The solution was then stored at 4°C and diluted to the desired concentration with PBS 1 day before treatment.
Western Blot
Tissues or cells were lysed with RIPA lysis buffer (Beyotime, China). After centrifugation, the supernatants were collected as total proteins. Protein concentration was determined with the BCA kit (Beyotime). Lysates (25-40 μg) were separated by 10% SDS-PAGE and transferred onto PVDF membranes. Proteins were probed by western blot assay. The resultant blots were visualized with ECL reagents (Thermo Fisher Scientific, USA) and immunoreactive signals were analyzed by densitometry using Image J software. The antibodies used were as follows: IDO1 (1:1000, Abcam, USA,), AhR (1:1000, HuaBio, China), GAPDH (1:2000, HuaBio).
RNA Isolation and Quantitative Real-Time PCR (qPCR)
Total RNA was isolated from the mice hippocampi or cells using TRIzol reagent (Takara, Japan). Reverse transcription was performed to synthesize cDNA using Premium One-Step RT-PCR kit (Invitrogen, USA). qPCR was performed in triplicate for each sample using a SYBR Green Mastermix kit (Takara), Actb was used as an internal control. The sequences of primers were shown in Table 1.
Table 1.
The Sequences of Primers Used for qPCR analysis of Gene Expression.
| Species | Genes | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|---|
| Mouse | Ido1 | TGCGTGACTTTGTGGACC | TGGAAGATGCTGCTCTGG |
| Kynu | TAGGCAAACGCCCTTGGATT | TTGTGCCGCTTTGGAGTAGG | |
| Kmo | TGAAGAAGCCCCGCTTTGAT | TCCATGTTTGGAAGGGCGAT | |
| Haao | GAGACACGAACTCTGAGGGAC | CTGCTCCAGGACTCGTAGTATC | |
| Cyp1a1 | CACTAATGGCAAGAGCATGAC | TCACCTTCTGAAGTTTGCTGAC | |
| Ifng | GAGGTCAACAACCCACAGGTC | ACTCCTTTTCCGCTTCCTGA | |
| Tnf | CCCTCACACTCACAAACCAC | ACAAGGTACAACCCATCGGC | |
| Il1b | CTACAGGCTCCGAGATGAACA | CGTTGCTTGGTTCTCCTTGTA | |
| Il10 | AAGGCCATGAATGAATTTGA | TTCGGAGAGAGGTACAAACG | |
| Bdnf | GACGACATCACTGGCTGACA | CAAGTCCGCGTCCTTATGGT | |
| Gdnf | TGGGCTATGAAACCAAGGAGG | CTGCAACATGCCTGGCCTAC | |
| Ngf | CAATAGCTGCCCGAGTGACA | TCCGGTGAGTCCTGTTGAAAG | |
| Actb | CTGTCCCTGTATGCCTCTG | ATGTCACGCACGATTTCC | |
| Rat | Ido1 | TCTGTTCTCGTTTCCTGGTGG | TCCAGTGCTTTCGGGTCTTG |
| Kynu | GGAGATGAGAGCATTGTAACCCT | ATTTTGTGCCGCTTTGGTGT | |
| Kmo | CGTGGAGTCCTACCCCAATG | GAGTAGGCCCCATCACATCC | |
| Haao | GCTGAGGTACTATGTGGGCG | AGGGTTGGGCTTTCCTGTTC | |
| Actb | AGGGTGTAGAGTGTTTGCAGTC | CTCAAGGTGGACAGATGCGG |
Immunofluorescence
The tissues were fixed in 4% paraformaldehyde, embedded in optimum cutting temperature (OCT) compound and cut in thick tissue sections (8 μm). Triton X-100 (.2%) was used to permeabilize tissue or cells and blocking was done using with 10% normal goat serum (Beyotime C0265). The tissue sections were incubated with primary antibodies overnight at 4°C, which is followed by the incubation with secondary antibodies for 1 h at room temperature. The following primary antibodies were used: IDO1 (1:100, Abcam), class III β-tubulin (1:200, HuaBio). The following secondary antibodies were used: Alexa Fluor 488 goat-anti-rabbit, Alexa Fluor 555 goat-anti-mouse. The cell nuclei were then stained with DAPI (1:1000). The brain sections slices were imaged using a laser scanning confocal microscope (Nikon Eclipse A1-Ni, Japan).
Animals
Pregnant SD rats were purchased from Shanghai Jiesijie Experiment Animal Co., Ltd. (Shanghai, China). 3-month-old male APOE−/− (APOE knockout) mice, 3-, 6-, 9-, and 12-month-old male APP/PS1 (APPswe/PSEN1dE9 double-transgenic) mice and the corresponding WT mice (nontransgenic littermates of the APOE−/− and APP/PS1 mice: WTAPOE−/− and WTAPP/PS1) of comparable ages were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). The experimental procedures were approved by the Animal Ethics Committee of Fudan University (No. JS-006) and performed in compliance with ARRIVE guidelines.
Animals Grouping and Administration
Eight-month-old male APP/PS1 mice were randomly divided into four groups: control group, RY103 group, 1-L-MT group and donepezil group (8-15 animals per group). The RY103 group and 1-L-MT group were orally administered 50 mg/kg body weight of 1-L-MT or RY103 per 2 days. Donepezil group and control group were orally administered 1 mg/kg body weight of donepezil or an equal volume of .5% CMC-Na once daily. 1-L-MT, RY103 and donepezil were all dissolved in .5% CMC-Na. Eight-month-old age-matched male C57BL/6 mice were selected randomly as WT group mice that were given .5% CMC-Na. All mice were orally administered for 1 month.
High-Performance Liquid Chromatography (HPLC) Evaluation of IDO Activity
After the mice were sacrificed, blood samples were collected from retro-orbital veins and centrifuged at 3000 g for 15 min to obtain serum. The IDO1 activity in serum was evaluated by measuring the levels of Trp and Kyn with HPLC, as described previously, 45 on an Agilent 1260 series HPLC system (Agilent Technologies, USA) equipped with a quaternary pump as well as a UV detector. Analysis of the samples was performed using an Agilent C18 column (5 μm particle size, L × I.D. 25 cm × 4.6 mm) preceded by a C18 guard column (Dikma, China). The mobile phase was 15 mM acetic acid-sodium acetate buffer (pH 3.6) containing 6% acetonitrile by volume. Column temperature was 25°C. Flow rate was 1 mL/min. The UV detection wavelengths of Trp and Kyn were 280 nm and 360 nm, respectively.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) or standard error of the mean (SEM). One-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test was used to compare several treatment groups with one control group. Student’s t test was used to determine the difference between the two groups. All statistical analyses were performed using GraphPadPrism 6 software. Significance values were set at *P < .05, **P < .01 and ***P < .001.
Results
Change in the Expressions of Inflammatory Cytokines and Neurotrophic Factors and IDO1 Activity in AD Mice
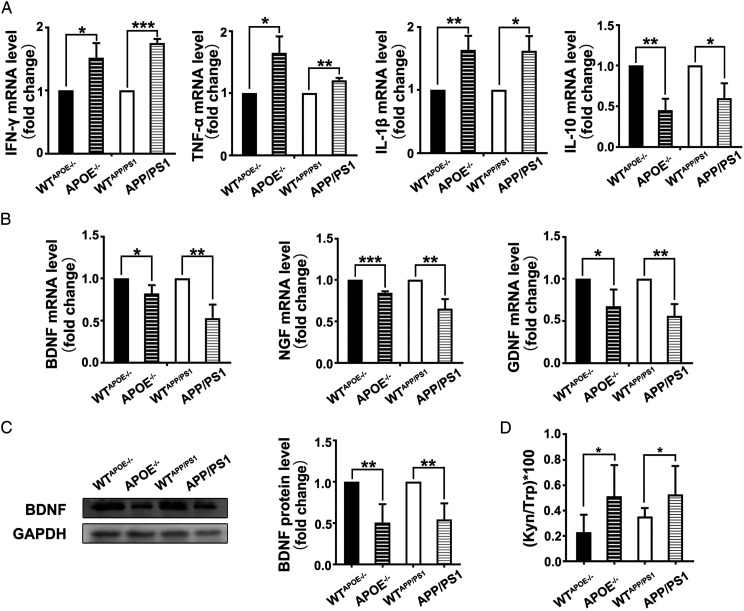
APP/PS1 mice and APOE−/− mice are commonly used animal models for AD studies. 3-month-old APOE−/− mice and 6-month-old APP/PS1 mice bear behavioral and psychological symptoms of dementia.46,47 qPCR analysis was first performed to investigate the potential change in the expressions of inflammatory cytokines in 3-month-old APOE−/− mice and 6-month-old APP/PS1 mice in comparison with wild-type (WT) mice of comparable ages. It was found that the mRNA levels of pro-inflammatory cytokines IFN-γ, TNF-α, interleukin-1β (IL-1β) in the hippocampus of APOE−/− mice and APP/PS1 mice were higher than that of WT mice, while the mRNA expression level of anti-inflammatory cytokine interleukin 10 (IL-10) was lower (Figure 2A).
Figure 2.
Change in the expressions of inflammatory cytokines and neurotrophic factors and IDO1 activity in AD mice. (A) mRNA expressions of IFN-γ, TNF-α, IL-1β and IL-10 quantified by qPCR. (n ≥ 6 mice in each group). (B) mRNA expressions of BDNF, NGF and GDNF quantified by qPCR. (n ≥ 6 mice in each group). (C) Expression of BDNF protein determined by western blot (n ≥ 5 mice in each group). (D) Concentrations of Trp and Kyn in serum determined by HPLC. Kyn/Trp ratio was calculated (n = 9-10 mice in each group).). WTAPOE−/− and APOE−/−: male mice at 3 months of age. WTAPP/PS1 and APP/PS1: male mice at 6 months of age. All data were analyzed by Student’s t test. Data of Fig (A-C) are presented as the mean ± SEM, data of Fig (D) is presented as the mean ± SD, *P < .05, **P < .01, ***P < .01. The mRNA level are normalized to the level of Actb mRNA.
Subsequently, the expressions of neurotrophic factors in these AD mice were investigated. It was shown that the mRNA levels of brain-derived neurotrophic factor (BDNF), neurotrophin nerve growth factor (NGF) and glial cell-derived neurotrophic factor (GDNF) and the protein expression of BDNF in the hippocampus of two kinds of AD mice were lower than that of WT mice (Figure 2B and C).
In addition, the concentrations of Trp and Kyn in the serum were analyzed using HPLC and the ratio of Kyn to Trp was calculated (Table 2). It was found that the serum IDO1 activity, represented by Kyn/Trp ratio, of AD mice was significantly higher than that of WT mice (Figure 2D).
Table 2.
Concentrations (Mean ± SD) of Trp and Kyn and the Kyn/Trp Ratio in the Serum of AD mice.
| WTAPOE−/−(n = 9) | APOE−/−(n = 9) | WTAPP/PS1 (n = 10) | APP/PS1 (n = 10) | |
|---|---|---|---|---|
| Trp (μmol/L) | 79.6 ± 16.9 | 66.5 ± 10.7 | 81.8 ± 11.6 | 79.8 ± 12.3 |
| Kyn (μmol/L) | .16 ± .06 | .33 ± .12 | .29 ± .07 | .41 ± .16 |
| (Kyn/Trp) × 100 | .20 ± .14 | .50 ± .25* | .35 ± .07 | .51 ± .22* |
WTAPOE−/− and APOE−/−: Male mice at 3 months of age. WTAPP/PS1 and APP/PS1: Male mice at 6 months of age. n = 9-10 Mice in Each Group.
Together, we observed that the expressions of inflammatory cytokines and neurotrophic factors, and IDO1 activity in AD mice are significantly different to that of WT mice. Similarly, it has previously been reported that the i.c.v. injection of Aβ oligomers, the chief cause of neurotoxicity in AD, induced an increase in IDO1 activity while caused a down-regulation in BDNF levels in the prefrontal cortex and hippocampus. 41
Change in the Expressions of KP Enzymes and AhR Activation in AD Mice
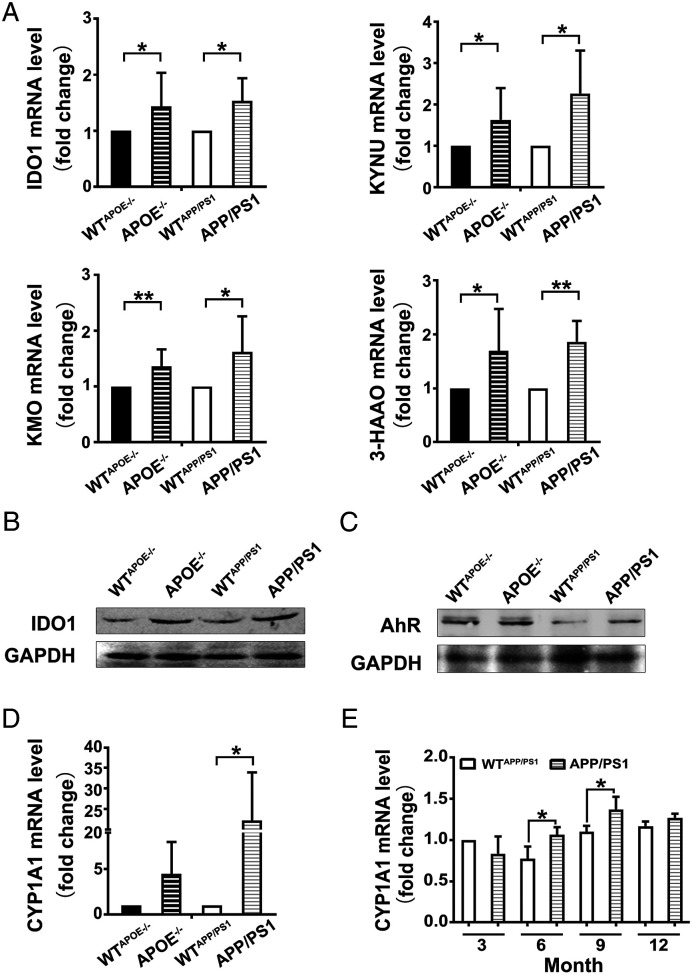
The mRNA expressions of KP enzymes including IDO1, KYNU, KMO and 3-HAAO in 3-month-old APOE−/− mice and 6-month-old APP/PS1 mice were analyzed using qPCR. The mRNA levels of IDO1, KYNU, KMO and 3-HAAO in the hippocampus of AD mice were found to be higher compared to that of WT mice, which suggested that IDO1-mediated KP was up-regulated in the hippocampus of AD mice (Figure 3A). Subsequently, with western blot analysis, the up-regulated protein expressions of IDO1 and AhR in the hippocampus of AD mice were observed (Figure 3B and C).
Figure 3.
Increased expression of the KP enzymes and AhR activation in the hippocampus of AD mice. A. mRNA expressions of KP enzymes quantified by qPCR. (n ≥ 6 mice in each group). (B and C) Expressions of IDO1 and AhR proteins determined by western blot. (n ≥ 5 mice in each group). WTAPOE−/− and APOE−/−: male mice at 3 months of age. WTAPP/PS1 and APP/PS1: male mice at 6 months of age. (D and E) mRNA expression of CYP1A1 quantified by qPCR. (n ≥ 3 mice in each group). All data were analyzed by Student’s t test and expressed as the mean ± SEM, *P < .05, **P < .01. The mRNA expression levels are normalized to the level of Actb mRNA.
Then the expressions of main cytochrome P450 enzyme, one of the target genes of AhR, in 3-month-old APOE−/− mice and 6-month-old APP/PS1 mice were analyzed. It was found that the mRNA levels of CYP1A1 in the hippocampus of two kinds of AD mice were higher compared to that of WT mice, and the data of APP/PS1 had statistical significance (Figure 3D). Therefore, the CYP1A1 mRNA level in the whole brain of 3-, 6-, 9- and 12-month-old APP/PS1 mice were further analyzed by qPCR. CYP1A1 mRNA level in APP/PS1 mice was found to be higher than that in WT mice in most age groups except 3-month-old group, especially in 6- and 9-month-old groups (Figure 3E). Taken together, our results suggest that AhR was activated in the brain especially in the hippocampus of AD mice, and it was also correlated to month age and the intensification of dementia of the mice.
Aβ Induced Increase in the Expressions of KP Enzymes in Rat Primary Neurons and Neuronal HT22 Cells, While IDO1 Inhibitor Reversed the Effect
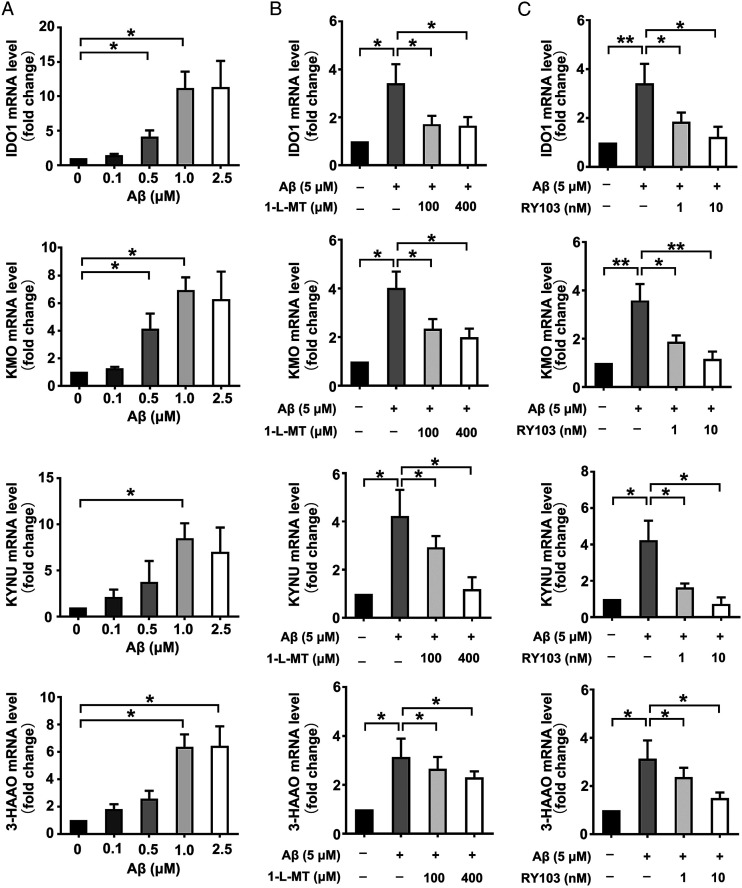
The KP up-regulation and the metabolite-related neurotoxicity resulted from IDO1 overactivation are downstream of amyloid plaques neurotoxicity, but the effect of Aβ oligomers on IDO1 and KP has been less studied. Using THP-1 cells and human primary PBMC as models for microglia, O. Takikawa et al found that both monocytic cell types pretreated with Aβ (1-42), one of the main components of amyloid plaques, become highly responsive to a secondary stimulation with the proinflammatory cytokine IFN-γ and markedly activate the KP through induction of IDO. 33 Recently, using SD rat primary hippocampal neurons and mouse hippocampal neuronal HT22 cells, we explored the effect of Aβ on IDO1-Kyn-AhR pathway in neurons, which has shown that different concentrations of Aβ led to a dose-dependent increase in the protein expression of IDO1 and AhR while IDO1 inhibitors decreased the enhanced expressions of IDO1, AhR protein and CYP1A1 mRNA caused by Aβ treatment. 48 However, the alterations on gene expressions of IDO1 and other KP enzymes and the reversal effects of IDO1 inhibitors in Aβ effected neurons have not been studied previously. Herein, it was shown that Aβ treatment led to an increase in the mRNA levels of IDO1 and other KP enzymes including KYNU, KMO, and 3-HAAO in SD rat primary hippocampal neurons (Figure 4A). The impact of Aβ on IDO1 and KP in mouse hippocampal neuronal HT22 cells has rarely been reported. Similar to the observation in SD rat primary hippocampal neurons, it was also found that the expressions of KP enzymes were up-regulated. The variation tendency of IDO1 mRNA level in Aβ-treated rat primary neurons and HT22 cells is consistent with that of data from Aβ-treated human microglia models, also reported by Takikawa et al. 33 Further, IDO1 inhibitor 1-L-MT and RY103 decreased the enhanced gene expression of KP enzymes in HT22 cells (Figure 4B and C). These findings together with our previously reported data provide further support for the opinion that neurotoxic Aβ also activates KP in neurons, KP is activated under AD condition, IDO1 and KP play important roles in AD pathogenesis.
Figure 4.
Aβ induced increase in the expressions of KP enzymes in rat neurons and HT22 cells, while IDO1 inhibitor reversed the effect. (A-C) mRNA expressions of KP enzymes quantified by qPCR. (A) SD rat primary hippocampal neurons were treated with Aβ of different concentrations (.1, .5, 1, 2.5 μM) for 24 h. (B and C) HT22 cells were incubated with Aβ (5 μM), Aβ (5 μM) supplemented with 1-L-MT (100, 400 μM) or RY103 (1, 10 nM) for 24 h. All data were analyzed by one-way ANOVA followed by Dunnett’s post hoc test and expressed as the mean ± SEM. *P < .05, **P < .01. The mRNA Values are normalized to the level of Actb mRNA.
Taken together, we demonstrated that in neurons, Aβ up-regulated the gene expressions of KP enzymes, which could be reversed by IDO1 inhibitor.
Application of IDO1 Inhibitor Changed the Expression Pattern of Inflammatory Cytokines and Neurotrophic Factors in the Hippocampus of APP/PS1 Mice
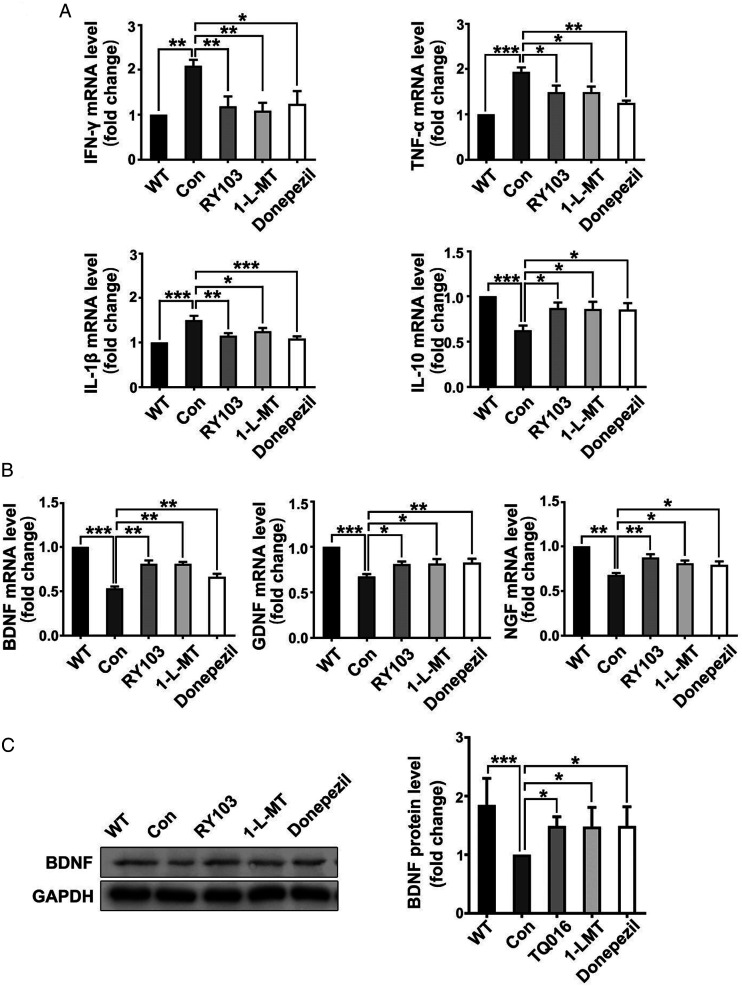
We recently have reported the effects of IDO1 inhibitor on cognitive performance of APP/PS1 mice. 48 Herein, we explored the effects of IDO1 inhibitor (RY103 and 1-L-MT) on the expressions of inflammatory cytokines and neurotrophic factors in the hippocampus of APP/PS1 mice. Commercialized AD drug Donepezil, an acetylcholinesterase (AChE) inhibitor, was used as a reference compound, as reports showed that Donepezil had neuroprotective effect on AD mice by increasing expressions of neurotrophic factors 49 and decreasing inflammatory cytokines production. 50 We discovered in the hippocampus, the mRNA levels of IFN-γ, TNF-α, IL-1β in the IDO1 inhibitor and Donepezil groups were lower than the control group, while the mRNA level of IL-10 was significantly increased in the IDO1 inhibitor group (Figure 5A). Furthermore, application of IDO1 inhibitors significantly increased the mRNA levels of BDNF, NGF, GDNF and the protein level of BDNF in the hippocampus (Figure 5B and C), which is consistent with previous observation that Donepezil could suppress pro-inflammatory cytokines and neurotrophic factors in AD mice. These results indicated that RY103 abrogated neuroinflammatory response and neurotrophic deficiency in the hippocampus of AD mice, suggesting its neuroprotective effect.
Figure 5.
Application of IDO1 inhibitor changed the expression pattern of inflammatory cytokines and neurotrophic factors in the hippocampus of APP/PS1 mice (8-month-old, male). (A) mRNA expressions of IFN-γ, TNF-α, IL-1β and IL-10 in the hippocampus quantified by qPCR. (B) mRNA expressions of BDNF, NGF and GDNF in the hippocampus quantified by qPCR. The mRNA values are normalized to the level of Actb mRNA. (n = 6-7 mice in each group). (C) Expression of BDNF protein in the hippocampus determined by western blot. (n = 6-7 mice in each group). All data were analyzed by one-way ANOVA followed by Dunnett’s post hoc test and expressed as the mean ± SEM. *P < .05, **P < .01.
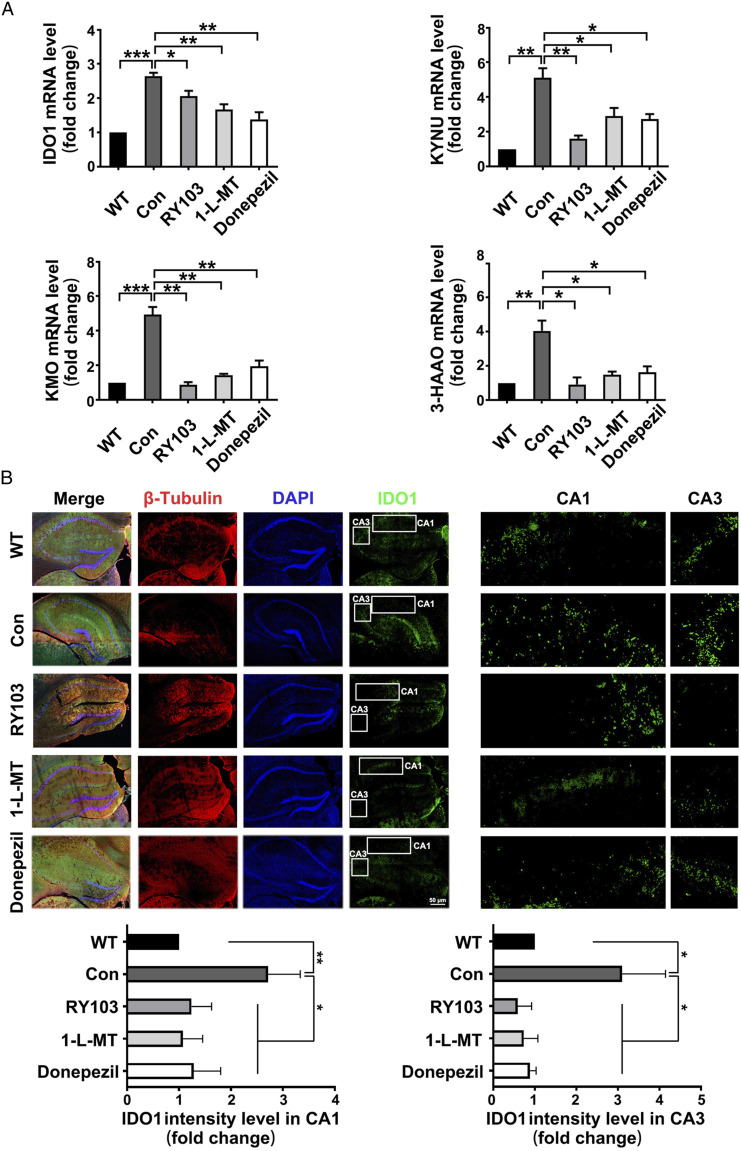
IDO1 Inhibitor Treatment Restored the Increased Expressions of KP Enzymes in Hippocampus of APP/PS1 Mice
The mRNA expressions of KP enzymes in APP/PS1 mice with or without IDO1 inhibitor treatment were tested. For the first time, we found that IDO1 inhibitor decreased the mRNA levels of IDO1 and other KP enzymes including KYNU, KMO and 3-HAAO (Figure 6A). Furthermore, immunostaining scanning showed reduced IDO1 expression in the hippocampus of RY103 and 1-L-MT groups (Figure 6B). It is interesting to find that Donepezil exhibited similar effect as that of IDO1 inhibitors on both IDO1 and KP. This result is consistent with our previous observation that Donepezil could suppress IDO1 activity in AD mice. 29 These results indicated that IDO1 inhibitors attenuated the aberrant KP in APP/PS1 mice.
Figure 6.
IDO1 inhibitor treatment restored the increased expressions of KP enzymes in hippocampus of APP/PS1 mice (8-month-old, male). (A) mRNA expressions of KP enzymes in the hippocampus quantified by qPCR. The mRNA values are normalized to the level of Actb mRNA. (n = 3-5 mice in each group). (B) Immunostaining of IDO1 (green), neuronal nuclei (DAPI, blue) and β-tubulin (Red) in hippocampus (×400). Bar = 50 μm. The right panel exhibited an enlarged view of the representative region (CA1 and CA3 areas of hippocampus) in the white box. (n = 3-5 mice in each group). Data were analyzed by one-way ANOVA followed by Dunnett’s post hoc test and expressed as the mean ± SEM, *P < .05, **P < .01, ***P < .001.
Discussion
Research on the relationship between the KP and AD has mainly focused on the neuroactive metabolites of KP13,17,51 and the overactivation of IDO1 in microglia and astrocytes,20,32,37 while change in KP enzymes other than IDO1 in AD brain has rarely been explored. Herein, using AD mice models and HT22 cells, it is first observed that in IDO1 active AD environment, whether in mouse model or induced by Aβ treatment, expressions of KP enzymes (Figures 3 and 4) and levels of inflammatory cytokines are higher, while expressions of neurotrophic factors are lower (Figure 2). Application of IDO inhibitor attenuates the effect (Figures 5 and 6). These results are consistent with our previous study in which we demonstrated that Aβ up-regulated IDO1-Kyn-AhR signal pathway as a pathogenic mechanism, 48 and thus provide further evidence on the role of KP in AD, suggesting that targeting IDO1 may represent a novel therapeutic approach to combat the neuronal dysfunction associated with AD.
IDO1 has been known as an important immune checkpoint in the past two decades. KP metabolites like Kyn and KYNA are also important regulators of the innate and adaptive immune system, including suppression of T cell responses, proliferation and survival. 52 IDO1 exerts immunosuppressive effects via three downstream effector pathways, two mediated by the general control non-depressible 2 (GCN2) and the mammalian target of rapamycin (mTOR) kinases which respond to Trp deprivation and one mediated by AhR which can be activated by Kyn. 53 The significance of GCN2 mediated mechanism has been doubted in recent studies 54 while the function of IDO1-Kyn-AhR signaling pathway has been highly valued, both in cancer immunotherapy 55 and in AD studies. 49 Our present study reported the connection between IDO1-mediated KP and AhR in the hippocampus of APOE−/− and APP/PS1 mice, which provided another evidence on the modulatory function of IDO1 on downstream pathway in neural system.
Higher levels of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β and lower levels of anti-inflammatory cytokines such as IL-10 in the AD patients have been previously observed. 50 Beyond that, lower levels of BDNF, NGF and GDNF have been detected in AD patients or AD mice. 56 In the present study, upon treatment of IDO1 inhibitors, the elevated mRNA expressions of pro-inflammatory cytokines and the decreased mRNA expressions of anti-inflammatory cytokines and neurotrophin were attenuated in the hippocampus of APP/PS1 mice (Figure 6).
KP-targeting strategy represents a promising new approach in the treatment of AD, several published studies showed therapeutic efficacy in inhibition of KP enzymes IDO1 or KMO in AD animals.32,35,57,58 Inhibition of KMO decreases the levels of its product 3-HK, as well as the downstream metabolite QUIN, while causing an increase in production of neuroprotective KYNA. KMO inhibitor JM6 can shunt KP metabolism toward enhanced neuroprotective KYNA production, prevent spatial memory deficits, anxiety-related behavior, and synaptic loss in APPtg mouse model of AD. 58 IDO1, the first rate-limiting enzyme of KP, has shown great potential as a therapeutic target for the treatment of neurodegeneration. 59 Coptisine, a natural product identified as uncompetitive inhibitor of IDO1, can inhibit IDO1 activity in the blood and ameliorate AD phenotypes in APP/PS1 mice. 32 The 3xTg-AD mice treated with DWG-1036, a novel synthesized IDO1 inhibitor, showed better memory in the trace fear conditioning task and significant improvements in learning. 35 However, the effects of DWG-1036 treatment on the behavioral tasks were variable, and sex differences were apparent. High doses of DWG-1036 resulted in reduced body weight, particularly in females. 35 Our present findings provide further evidence demonstrating the associations between IDO1 and AD, showing great therapeutic potential of IDO1 inhibition strategy.
To summarize, our study demonstrates that KP is up-regulated in Aβ-treated rat primary neurons and neuronal HT22 cells, and the hippocampus of APOE−/− and APP/PS1 mice. IDO1 inhibitors decreased the expressions of KP enzymes including IDO1, KYNU, KMO and 3-HAAO, attenuated the up-regulated KP in Aβ-treated HT22 cells and in the hippocampus of APP/PS1 mice. By using IDO1 inhibitors, abrogated neuroinflammatory response and neurotrophic deficiency in the hippocampus of APP/PS1 mice was alleviated. These data present that targeting IDO1 may represent a novel therapeutic approach to combat the neuronal dysfunction associated with AD.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Open Research Fund of State Key Laboratory of Genetic Engineering, Fudan University (No. SKLGE-2119), and sponsored by Shanghai Sailing Program (21YF1436900).
ORCID iD
Zhen Ning Tony He https://orcid.org/0000-0003-4143-3219
References
- 1.Ranasinghe KG, Cha J, Iaccarino L, et al. Neurophysiological signatures in Alzheimer’s disease are distinctly associated with TAU, amyloid-β accumulation, and cognitive decline. Sci Transl Med. 2020;12(534):eaaz4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heppner FL, Ransohoff RM, Becher B. Immune attack: The role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358-372. [DOI] [PubMed] [Google Scholar]
- 4.Webers A, Heneka MT, Gleeson PA. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol Cell Biol. 2020;98(1):28-41. [DOI] [PubMed] [Google Scholar]
- 5.Tan L, Yu JT, Tan L. The kynurenine pathway in neurodegenerative diseases: Mechanistic and therapeutic considerations. J Neurol Sci. 2012;323(1-2):1-8. [DOI] [PubMed] [Google Scholar]
- 6.Lovelace MD, Varney B, Sundaram G. et al. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology. 2017;112:373-388. [DOI] [PubMed] [Google Scholar]
- 7.Savitz J. The kynurenine pathway: A finger in every pie. Mol Psychiatry. 2020;25(1):131-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: Potential role in AIDS dementia complex. J Neuroinflammation. 2005;2(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009;4(7):e6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz MJ, Guillemin GJ, Teipel SJ, Buerger K, Hampel H. Increased 3-hydroxykynurenine serum concentrations differentiate Alzheimer’s disease patients from controls. Eur Arch Psychiatry Clin Neurosci. 2013;263:345-352. [DOI] [PubMed] [Google Scholar]
- 11.Guillemin GJ, Cullen KM, Lim CK. et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27(47):12884-12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Nicolazzo JA, Wen L. et al. Expression of tryptophan 2, 3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One. 2013;8(4):e59749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2, 3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31(4):395-404. [DOI] [PubMed] [Google Scholar]
- 14.Duleu S, Mangas A, Sevin F, Veyret B, Bessede A, Geffard M. (2010) Circulating antibodies to IDO/THO pathway metabolites in Alzheimer’s disease. Int J Alzheimer’s Dis 2010, 501541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer’s disease. J Neural Transm. 1999;106:165-181. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs KR, Lim CK, Blennow K. et al. Correlation between plasma and CSF concentrations of kynurenine pathway metabolites in Alzheimer’s disease and relationship to amyloid-β and tau. Neurobiol Aging. 2019;80:11-20. [DOI] [PubMed] [Google Scholar]
- 17.Hartai Z, Juhász A, Rimanóczy Á. et al. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochem Int. 2007;50(2):308-313. [DOI] [PubMed] [Google Scholar]
- 18.Gulaj E, Pawlak K, Bien B, Pawlak D. Kynurenine and its metabolites in Alzheimer’s disease patients. Adv Med Sci. 2010;55(2):204-211. [DOI] [PubMed] [Google Scholar]
- 19.Okuda S, Nishiyama N, Saito H, Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70(1):299-307. [DOI] [PubMed] [Google Scholar]
- 20.Bonda DJ, Mailankot M, Stone JG. et al. Indoleamine 2, 3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer’s disease. Redox Rep. 2010;15(4):161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Opitz CA, Litzenburger UM, Sahm F. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197-203. [DOI] [PubMed] [Google Scholar]
- 22.Xu K, Yang Z, Shi R, Luo C, Zhang Z. Expression of aryl hydrocarbon receptor in rat brain lesions following traumatic brain injury. Diagn Pathol. 2016;11:72-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akahoshi E, Yoshimura S, Ishihara-Sugano M. Over-expression of AhR (aryl hydrocarbon receptor) induces neural differentiation of Neuro2a cells: Neurotoxicology study. Environ Health. 2006;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30(9):447-454. [DOI] [PubMed] [Google Scholar]
- 25.Cuartero MI, Ballesteros I, de la Parra J. et al. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130(23):2040-2051. [DOI] [PubMed] [Google Scholar]
- 26.Angeles-López QD, García-Lara L, Aguirre-Pineda N. et al. The absence of the aryl hydrocarbon receptor in the R6/1 transgenic mouse model of Huntington’s disease improves the neurological phenotype. Behav Brain Res. 2021;408:113230. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Zhao WJ, Quan W. et al. Dynamic changes of activated AHR in microglia and astrocytes in the substantia nigra-striatum system in an MPTP-induced Parkinson’s disease mouse model. Brain Res Bull. 2021;176:174-183. [DOI] [PubMed] [Google Scholar]
- 28.Lin GF, Ma QW, Zhang DS, Zha YL, Lou KJ, Shen JH. Polymorphism of alpha-estrogen receptor and aryl hydrocarbon receptor genes in dementia patients in Shanghai suburb. Acta Pharmacol Sin. 2003;24(7):651-656. [PubMed] [Google Scholar]
- 29.Fujigaki S, Saito K, Sekikawa K. et al. Lipopolysaccharide induction of indoleamine 2, 3-dioxygenase is mediated dominantly by an IFN-γ-independent mechanism. Eur J Immunol. 2001;31(8):2313-2318. [DOI] [PubMed] [Google Scholar]
- 30.Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine. 2000;12(6):588-594. [DOI] [PubMed] [Google Scholar]
- 31.Munn DH, Sharma MD, Lee JR. et al. Potential regulatory function of human dendritic cells expressing indoleamine 2, 3-dioxygenase. Science. 2002;297(5588):1867-1870. [DOI] [PubMed] [Google Scholar]
- 32.Yu D, Tao BB, Yang YY. et al. The IDO inhibitor coptisine ameliorates cognitive impairment in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2015;43(1):291-302. [DOI] [PubMed] [Google Scholar]
- 33.Yamada A, Akimoto H, Kagawa S, Guillemin GJ, Takikawa O. Proinflammatory cytokine interferon-γ increases induction of indoleamine 2, 3-dioxygenase in monocytic cells primed with amyloid β peptide 1–42: Implications for the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;110(3):791-800. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee P, Zetterberg H, Goozee K. et al. Plasma neurofilament light chain and amyloid-β are associated with the kynurenine pathway metabolites in preclinical Alzheimer’s disease. J Neuroinflammation. 2019;16(1):186-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fertan E, Stover KR, Brant MG. et al. Effects of the novel IDO inhibitor DWG-1036 on the behavior of male and female 3xTg-AD mice. Front Pharmacol. 2019;10:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillemin GJ, Smythe GA, Veas LA, Takikawa O, Brew BJ. Aβ1-42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport. 2003;14(18):2311-2315. [DOI] [PubMed] [Google Scholar]
- 37.Kincses ZT, Toldi J, Vécsei L. Kynurenines, neurodegeneration and Alzheimer’s disease. J Cell Mol Med. 2010;14(8):2045-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souza LC, Jesse CR, Antunes MS. et al. Indoleamine-2, 3-dioxygenase mediates neurobehavioral alterations induced by an intracerebroventricular injection of amyloid-β1-42 peptide in mice. Brain Behav Immun. 2016;56:363-377. [DOI] [PubMed] [Google Scholar]
- 39.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105-112. [DOI] [PubMed] [Google Scholar]
- 40.Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. 2013;8(4):900-920. [DOI] [PubMed] [Google Scholar]
- 41.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7-23. [DOI] [PubMed] [Google Scholar]
- 42.Holsinger RM, Schnarr J, Henry P, Castelo VT, Fahnestock M. Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer’s disease. Brain Res Mol Brain Res. 2000;76(2):347-354. [DOI] [PubMed] [Google Scholar]
- 43.Fukumoto K, Mizoguchi H, Takeuchi H. et al. Fingolimod increases brain-derived neurotrophic factor levels and ameliorates amyloid β-induced memory impairment. Behav Brain Res. 2014;268:88-93. [DOI] [PubMed] [Google Scholar]
- 44.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126(3):342-397. [DOI] [PubMed] [Google Scholar]
- 45.Liang H, Chen M, Qi F. et al. The proatherosclerotic function of indoleamine 2, 3-dioxygenase 1 in the developmental stage of atherosclerosis. Signal Transduct Target Ther. 2019;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neurodegeneration in the central nervous system of apoE-deficient mice. Exp Neurol. 1995;136(2):107-122. [DOI] [PubMed] [Google Scholar]
- 47.Yan P, Bero AW, Cirrito JR. et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci. 2009;29(34):10706-10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duan Z, Zhang S, Liang H. et al. Amyloid β neurotoxicity is IDO1–Kyn–AhR dependent and blocked by IDO1 inhibitor. Signal Transduct Target Ther. 2020;5(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salminen A. Activation of aryl hydrocarbon receptor (AhR) in Alzheimer’s disease: Role of tryptophan metabolites generated by gut host-microbiota. J Mol Med. 2023;101:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gezen-Ak D, Dursun E, Hanağası H. et al. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis. 2013;37(1):185-195. [DOI] [PubMed] [Google Scholar]
- 51.Schwarcz R, Stone TW. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology. 2017;112:237-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197-209. [DOI] [PubMed] [Google Scholar]
- 53.Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41:41-48. [DOI] [PubMed] [Google Scholar]
- 54.Sonner JK, Deumelandt K, Ott M. et al. The stress kinase GCN2 does not mediate suppression of antitumor T cell responses by tryptophan catabolism in experimental melanomas. OncoImmunology. 2016;5(12):e1240858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon Axis. Clin Cancer Res. 2019;25(5):1462-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015;6(5):331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu X, He SY, Li Q. et al. Investigation of multi-target-directed ligands (MTDLs) with butyrylcholinesterase (BuChE) and indoleamine 2, 3-dioxygenase 1 (IDO1) inhibition: The design, synthesis of miconazole analogues targeting Alzheimer’s disease. Bioorg Med Chem. 2018;26(8):1665-1674. [DOI] [PubMed] [Google Scholar]
- 58.Zwilling D, Huang SY, Sathyasaikumar KV. et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Platten M, Nollen EA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18(5):379-401. [DOI] [PubMed] [Google Scholar]