Abstract
Purpose
In recent years, a new therapeutic approach, known as immune checkpoint blockade (ICB), has been proposed as approach to improve outcomes in patients with intermediate stage (Barcelona Clinic Liver Cancer, BCLC B) or advanced stage (BCLC C) hepatocellular carcinoma (HCC). Unfortunately, only a select patients can benefit from ICB. Hence, biomarkers that can predict the success and survival of treatment are still necessary.
Patients and Methods
Between 2018 to 2021, 132 patients received ICB treatment for intermediate or advanced stage HCC. Based on the early kinetics of C-reactive protein (CRP), the patients were classified into three groups. The study endpoints were progression-free survival (PFS) and overall survival (OS).
Results
Our findings support the predictive power of early CRP kinetics in determining immunotherapy response for intermediate or advanced HCC. Objective response rates (ORR) were found in 41.2% of CRP flare-responders, 13.3% of CRP responders, and 3.5% of CRP non-responders (p<0.001). Disease control rates (DCR) in the three groups were substantially different (p<0.001). The improved PFS and OS were strongly correlated with the early kinetics of CRP. Compared to CRP non-responders, CRP responders, especially CRP flare-responders, had significantly longer PFS (median PFS: CRP flare-responders: 11.6 months vs CRP responders: 5.2 months vs CRP non-responders: 2.3 months, p<0.001).
Conclusion
The CRP flare response robustly predicts the immunotherapy response and outcomes in patients with HCC. Early CRP kinetics may be an inexpensive, easily implemented and non-invasive biomarker to anticipate response to ICB therapy in intermediate or advanced HCC, with the potential to optimize treatment monitoring.
Keywords: hepatocellular carcinoma, C‐reactive protein kinetics, CRP flare response, immune checkpoint blockade, biomarker, immunotherapy
Introduction
According to 2020 data, primary liver cancer was the sixth most common type of malignancy and the third leading cancer-related death globally.1 Hepatocellular carcinoma (HCC) is the predominant type of primary liver cancer, accounting for 85–90% of cases.1 Due to the asymptomatic nature of the progression of HCC, those identified in intermediate or advanced stages cannot benefit from radical treatments.2 The outlook for treatment with HCC has changed rapidly in recent years. Systematic therapy based on antiangiogenic targeted drugs, immune checkpoint inhibitors, and other drugs has become the preferred treatment mode for patients with advanced HCC and patients with intermediate HCC who are not suitable for local treatment. Atezolizumab-bevacizumab and sindilimab-bevacizumab analogues have been approved in China for the first-line treatment of unresectable or metastatic HCC and in patients who have not previously received systemic antitumor therapy.3–5 Although ICB can be beneficial for some patients, not all experience clinical responses and immune-related adverse events (irAEs) can be serious or even fatal.6,7 For this reason, it is highly beneficial to pinpoint biomarkers that can predict patient response to ICB.
Studies have increasingly demonstrated that a systemic inflammatory response correlates with a worse outcome for individuals with HCC.8–10 C-reactive protein (CRP) is a reliable indicator of systemic inflammation and is an acute phase marker used to measure inflammation in the body.11 Worse clinical prognosis has been associated with higher baseline levels of CRP before the start of oncologic therapy in many malignancies, probably caused by invasive and progressive tumor-induced chronic inflammation.12–16 Unlike adverse prognostic consequences, longitudinal CRP variations after systemic targeted and immune checkpoint therapies have demonstrated therapeutic successes in various cancer entities.17–21 Fukuda et al illustrated the phenomenon of the “flare response”, defined as an initial increase in CRP after the initiation of ICB with a subsequent fall below baseline, which was linked to a considerable decrease in tumor size and a more favorable prognosis.22 The cost-effectiveness and practicality of evaluating early CRP kinetics makes it a suitable biomarker for anticipating response to immunotherapy. However, the effects of early CRP kinetics on ICB treatment in patients with HCC have not yet been fully confirmed. The purpose of this investigation was to determine whether early CRP kinetics, including the appearance of an early spike in CRP after starting therapy, could predict the effectiveness of ICB in patients with intermediate or advanced HCC.
Materials and Methods
Patients and Ethical Statement
The medical records of patients diagnosed with intermediate or advanced HCC and treated with ICB at Nanfang Hospital of Southern Medical University between July 2018 and December 2021 were examined in a retrospective manner. For ICB, pembrolizumab or nivolumab, and sintilimab, tislelizumab, camrelizumab, atezolizumab, or toripalimab were allowed, and 200 mg (atezolizumab: 1200mg, toripalimab: 240 mg), per three weeks, was administered intravenously. The choice of the type of ICB and duration of treatment was a joint decision between physicians and patients in real-world practice. This included information on medical history, lab results, radiological findings, and treatment regimens both prior to and following ICB. All follow-ups occurred between the beginning of ICB and August 20, 2022.
This retrospective study was conducted in accordance with the International Conference on Good Clinical Practice Standards and the ethical guidelines of the Declaration of Helsinki. Documentation of approval was acquired from Nanfang Hospital of Southern Medical University (NFEC-2018-070).
Definition of Early CRP Kinetics
Patients were divided into groups based on how Fukuda et al defined CRP rates. Patients had to have their CRP levels taken at least once during the initial six weeks of ICB treatment as a baseline (based on the most recent CRP level from ICB therapy). Those classified as “CRP flare-responders” had CRP levels that increased to at least double the baseline within the first month of ICB, and then dropped back below the baseline within three months. Those whose baseline CRP levels decreased by 30% or more in the initial three months of treatment and did not double from the baseline in the first month were categorized as “CRP responders”, while the rest were labeled as “CRP non-responders”.
Eligibility Criteria and Data Collection
The inclusion criteria were [1] age ≥ 18 years; [2] unresectable HCC that was categorized as Barcelona Clinic Liver Cancer (BCLC) stage B or C; [3] well-maintained liver function of Child-Pugh class A or B; [4] Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; [5] had at least one measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; and [6] had an overall survival of more than three months. The exclusion criteria were [1] secondary liver cancer; [2] had a history of other malignant tumors; [3] had contraindications for immunotherapy; [4] Combined present infection that causes acute increase in CRP in patients; [5] had a history of physical organ transplantation or bone marrow suppression; and [6] patients who were lost to follow-up.
Gender, age, BCLC stage, liver function class, ECOG PS score, tumor size, CRP level (mg/L), a-fetoprotein (AFP) level (ng/L), neutrophil-lymphocyte ratio (NLR), prior treatment and regimen in conjunction with ICB were among the demographic and clinical characteristics.
Baseline CRP levels (up to six weeks prior to ICB therapy), at least one additional CRP measurement at first staging or clinical progression, and at least one additional CRP measurement during the first and third months of treatment are recommended. RECIST v1.1 recommends using enhanced computed tomography (CT) and/or multiparametric magnetic resonance imaging (MRI) scans to evaluate radiation response at baseline, four to six weeks after the start of treatment, and roughly two to three months at each subsequent interval.
Statistical Analysis
Response and progression to ICB treatment, as well as death from any reason, were considered according to RECIST v1.1 criteria. The time from the commencement of therapy to the first disease progression or death from any cause is known as progression-free survival (PFS). Overall survival (OS) is the period of time from the start of treatment until mortality, the follow-up cut-off date, or the date of loss of follow-up. Using multivariate Kaplan-Meier survival analysis with Log rank test, the PFS and OS after the start of the ICB were calculated. The predictive significance of early CRP kinetics was assessed using univariate and multivariate Cox regression analyses of PFS and OS. Variables were incorporated into multivariate Cox regression models only when the survival impact in univariate analysis was significant. SPSS25.0 and STATA12.0 were used to conduct the statistical studies. Categorical variables are reported as median, frequency, and range. The Kruskal–Wallis test, Pearson’s chi-squared test, and Fisher’s exact test were used to compare groups. P values < 0.05 were regarded as significant in all two-sided analyses.
Results
Patient Characteristics
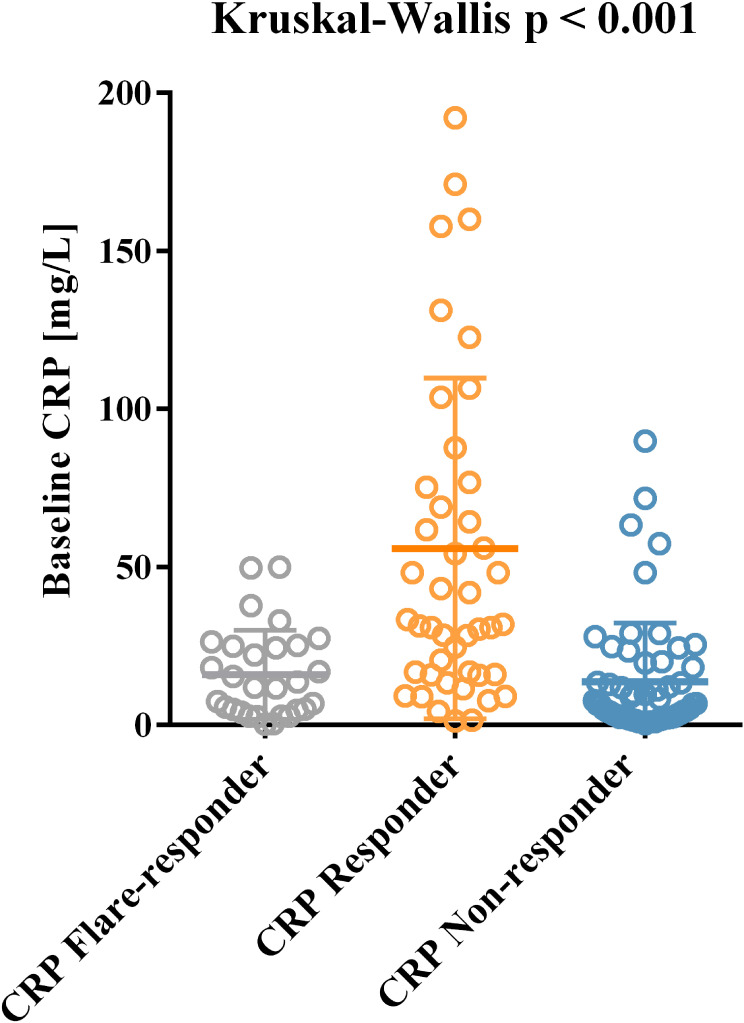
A total of 132 patients with intermediate to advanced HCC received ICB treatment at the Liver Oncology ward of the Southern Hospital during the study period. Of these, 108 patients aged <60 years were enrolled, with 120 (90.9%) males and 12 (9.1%) females. Before starting ICB, 87 patients (65.9%) had undergone various antitumor treatments, including radical treatment (surgical excision and ablation), local treatment (transhepatic artery chemoembolization [TACE] and liver artery infusion chemotherapy [HAIC]), and targeted therapy. A total of 123 (93.2%) patients began other treatment regimens in combination with ICB, of which 4 (3.0%) patients received ICB in combination with TACE/HAIC, 67 (50.8%) patients received ICB in combination with targeted therapy, and 52 (39.4%) patients received ICB in combination with TACE/HAIC and targeted therapy. The baseline clinical parameters did not show significant differences (Table 1), with the exception of baseline CRP, which was higher in CRP responders than in CRP flare-responders or CRP non-responders (Figure 1; p<0.001).
Table 1.
Comparison of Baseline Parameters Between CRP Flare-Responders, CRP Responders and CRP Non-Responders
| Characteristic | Overall N=132 |
Flare-Responder N=29 |
Responder N=45 |
Non-Responder N=58 |
p value |
|---|---|---|---|---|---|
| Gender | 0.816 | ||||
| male | 120 (90.9%) | 27 (93.1%) | 40 (88.9%) | 53 (91.4%) | |
| female | 12 (9.1%) | 2 (6.9%) | 5 (11.1%) | 5 (8.6%) | |
| Age (year) | 0.158 | ||||
| ≤60 | 108 (81.8%) | 27 (93.1%) | 34 (75.6%) | 47 (81.0%) | |
| >60 | 24 (18.2%) | 2 (6.9%) | 11 (24.4%) | 11 (19.0%) | |
| BCLC stage | 0.485 | ||||
| B | 35 (26.5%) | 10 (34.5%) | 12 (26.7%) | 13 (22.4%) | |
| C | 97 (73.5%) | 19 (65.5%) | 33 (73.3%) | 45 (77.6%) | |
| Child-Pugh grade | 0.833 | ||||
| A | 112 (84.9%) | 25 (86.2%) | 37 (82.2%) | 50 (86.2%) | |
| B | 20 (15.1%) | 4 (13.8%) | 8 (17.8%) | 8 (13.8%) | |
| ECOG PS | 0.472 | ||||
| 0 | 113 (85.6%) | 24 (82.8%) | 33 (73.3%) | 41 (70.7%) | |
| ≥1 | 19 (14.4%) | 5 (17.2%) | 12 (26.7%) | 17 (29.3%) | |
| Tumor size (cm) | 0.167 | ||||
| ≤5 | 39 (29.5%) | 5 (17.2%) | 17 (37.8%) | 17 (29.3%) | |
| >5 | 93 (70.5%) | 24 (82.8%) | 28 (62.2%) | 41 (70.7%) | |
| Prior treatment, Ablation | 0.183 | ||||
| YES | 33 (25.0%) | 5 (17.2%) | 9 (20.0%) | 19 (32.8%) | |
| NO | 99 (75.0%) | 24 (82.8%) | 36 (80.0%) | 39 (67.2%) | |
| Prior treatment, Surgery | 0.536 | ||||
| YES | 15 (11.4%) | 3 (10.3%) | 7 (15.6%) | 5 (8.6%) | |
| NO | 117 (88.6%) | 26 (89.7%) | 38 (84.4%) | 53 (91.4%) | |
| Prior treatment, TACE/HAIC | 0.819 | ||||
| YES | 75 (56.8%) | 15 (51.7%) | 26 (57.8%) | 34 (58.6%) | |
| NO | 57 (43.2%) | 14 (48.3%) | 19 (42.2%) | 24 (41.4%) | |
| Prior treatment, Target therapy | 0.612 | ||||
| YES | 49 (37.1%) | 9 (31.0%) | 19 (42.2%) | 21 (36.2%) | |
| NO | 83 (62.9%) | 20 (69.0%) | 26 (57.8%) | 37 (63.8%) | |
| Combined treatment, TACE/HAIC | 0.100 | ||||
| YES | 56 (42.4%) | 17 (89.5%) | 19 (42.2%) | 20 (34.5%) | |
| NO | 76 (57.6%) | 12 (10.5%) | 26 (57.8%) | 38 (65.5%) | |
| Combined treatment, Target therapy | 0.721 | ||||
| YES | 119 (90.2%) | 25 (86.2%) | 41 (91.1%) | 53 (91.4%) | |
| NO | 13 (9.8%) | 4 (13.8%) | 4 (8.9%) | 5 (8.6%) | |
| AFP level (ng/L) | 0.916 | ||||
| ≤400 | 62 (47.0%) | 14 (48.3%) | 20 (44.4%) | 28 (48.3%) | |
| >400 | 70 (53.0%) | 15 (51.7%) | 25 (65.6%) | 30 (51.7%) | |
| NLR | 0.160 | ||||
| <3 | 62 (47.0%) | 16 (55.2%) | 16 (35.6%) | 30 (51.7%) | |
| ≥3 | 70 (53.0%) | 13 (44.8%) | 29 (64.4%) | 28 (48.3%) | |
| CRP level (mg/dL) | <0.001 | ||||
| ≤5 | 38 (28.8%) | 9 (31.0%) | 3 (6.7%) | 26 (44.8%) | |
| >5 | 94 (71.2%) | 20 (69.0%) | 42 (93.3%) | 32 (55.2%) |
Abbreviations: BCLC stage, Barcelona Clinic Liver Cancer stage; ECOG PS, Eastern Cooperative Oncology Group performance status; TACE, transcatheter arterial chemoembolization; HAIC, hepatic arterial infusion of chemotherapy; AFP, a-fetoprotein; NLR, Neutrophil-lymphocyte ratio; CRP, C-reactive protein.
Figure 1.
Distribution of baseline CRP across different CRP kinetics groups.
Response and Outcomes by Early CRP Kinetics
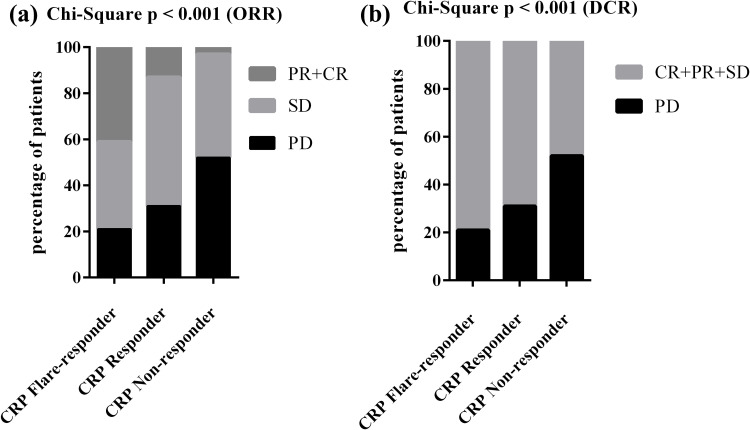
Early CRP kinetics were associated with better radiographic outcomes (Table 2). Of the total 132 patients, 29 (22.0%) were CRP flare-responders, 45 (34.1%) were CRP responders, and 58 (43.9%) were non-responders to CRP. Those who experienced a CRP flare had an objective response (OR; partial remission (PR)/complete remission (CR)) rate of 41.4%, while 13.3% of CRP responders and 3.5% of CRP non-responders achieved an OR (Figure 2a) (p<0.001). None of the patients in the CRP non-responder group achieved CR, and CR rates were 1/45 (2.2%) vs 3/29 (10.3%) for the CRP responder and CRP flare-responders, respectively. Disease control rates (DCR) were 79.3%, 68.9%, and 48.3% for CRP flare-responders, CRP responders, and CRP non-responders, respectively (Figure 2b; p<0.001).
Table 2.
Efficacy According to Different Early CRP Kinetics
| Flare-Responder N=29 |
Responder N=45 |
Non-Responder N=58 |
p value | |
|---|---|---|---|---|
| Overall survival | ||||
| Median (95% CI), months | – | 20.6 (15.5–25.7) | 10.0 (3.1–16.9) | <0.001 |
| Progression-free survival | ||||
| Median (95% CI), months | 11.6 (7.5–15.7) | 5.2 (2.3–8.1) | 2.3 (1.1–3.6) | <0.001 |
| Best radiological response | ||||
| CR | 3 (10.3%) | 1(2.2%) | 0 (0.0%) | |
| PR | 9 (31.1%) | 5(11.1%) | 2 (3.5%) | |
| PD | 6 (20.7%) | 14 (31.1%) | 30 (51.7%) | |
| SD | 11 (37.9%) | 25 (55.6%) | 26 (44.8%) | |
| ORR (CR+PR) | 12 (41.4%) | 6(13.3%) | 2 (3.5%) | <0.001 |
| DCR (CR+PR+SD) | 23 (79.3%) | 31 (68.9%) | 28 (48.3%) | <0.001 |
Abbreviations: CI, confidence interval; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease; ORR, objective response rate; DCR, disease control rate.
Figure 2.
Distribution of response at first staging according to RECIST v1.1 among the different CRP kinetics groups. P value based on Chi-square test for ORR (a) and DCR (b).
Abbreviations: PR, partial remission; CR, complete remission; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
Survival Analysis by Early CRP Kinetics
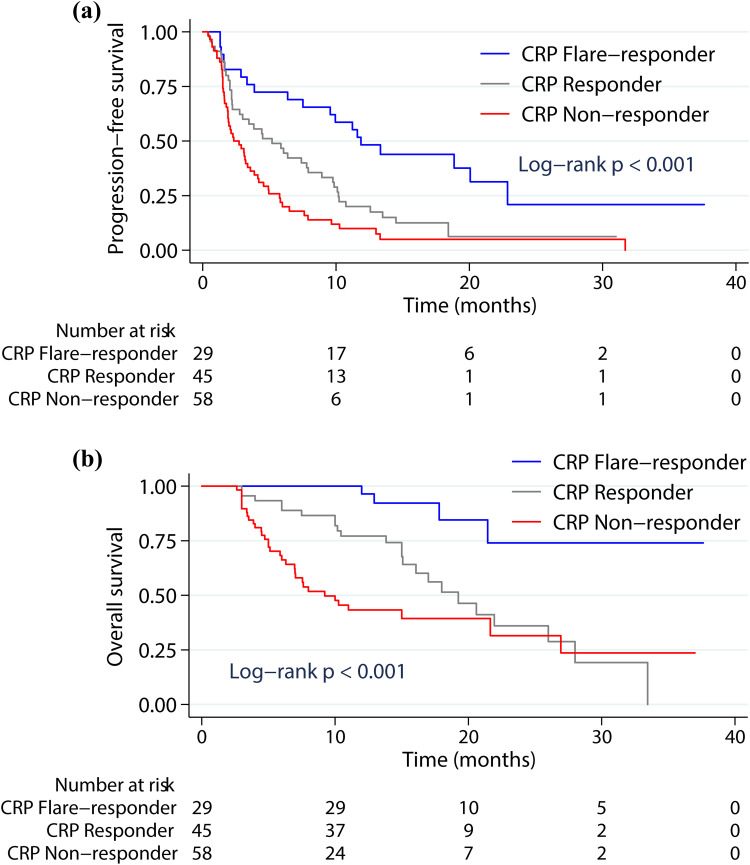
After the initiation of ICB, the median PFS was 11.6 months (95% confidence interval (CI) 7.5–15.7 months) for CRP flare-responders, 5.2 months (95% CI 2.3–8.1 months) for CRP responders and 2.3 months (95% CI 1.1–3.6 months) for CRP non-responders, with significant differences between groups (Figure 3a; p<0.001; Table 2). The Kaplan-Meier curve analysis of OS was comparable to that of PFS. The median OS for the three groups of CRP flare-responders, CRP responders, and CRP non-responders was not reached vs 20.6 months (95% CI: 15.5–25.7 months) vs 10.0 months (95% CI: 3.1–16.9 months) (Figure 3b; p<0.001; Table 2). Thus, early CRP kinetics appeared to be a significant and independent indicator of the tumor reaction to ICB, leading to a remarkable improvement in PFS and OS after the initiation of immunotherapy.
Figure 3.
Kaplan-Meier survival curves showing the progression-free survival (PFS) (a) and overall survival (OS) (b) after ICB initiation stratified according to CRP kinetics groups.
Cox Regression Analysis
The results of the univariate and multivariate Cox regression analysis are presented in Table 3 and Table 4. Early CRP kinetics and baseline AFP levels were identified as prognostic variables for PFS in patients with HCC based the univariate Cox proportional model. In the multivariate Cox regression model, the effects of early CRP kinetics and baseline AFP levels were still significant, indicating these as significant prognostic factors for PFS. CRP responders had a 37% lower multivariate corrected risk of tumor progression than CRP non-responders (hazard ratio (HR)=0.63, 95% CI 0.41–0.95, p=0.03), and CRP flare-responders had a 74% reduced risk compared with CRP non-responders (HR=0.26, 95% CI 0.15–0.46, p<0.001) (Table 3). None of the other clinically relevant factors under investigation had any predictive value for PFS. Baseline AFP levels and early CRP kinetics were linked to OS in the univariate analysis, and in the multifactorial analysis, these remained independent prognostic factors for OS, with CRP non-responders having significantly lower OS than CRP responders (p=0.03) and CRP flare-responders (p<0.001) (Table 4).
Table 3.
Univariable and Multivariable Cox Regression Analyses of Risk Factors for Progression-Free Survival
| Univariable HR (95% CI) |
p value | Multivariable HR (95% CI) |
p value | |
|---|---|---|---|---|
| Gender | ||||
| Male | – |
0.22 |
||
| Female | 1.46 (0.80–2.67) | |||
| Age, y | ||||
| ≤60 | – |
0.65 |
||
| >60 | 1.12 (0.69–1.80) | |||
| Child-Pugh grade | ||||
| A | – |
0.87 |
||
| B | 1.05 (0.62–1.78) | |||
| BCLC stage | ||||
| B | – |
0.46 |
||
| C | 1.17 (0.77–1.78) | |||
| ECOG PS | ||||
| 0 | – |
0.80 |
||
| ≥1 | 1.06 (0.68–1.64) | |||
| Tumor size (cm) | ||||
| ≤5 | – |
0.64 |
||
| >5 | 1.10 (0.74–1.64) | |||
| CRP level | ||||
| ≤5 mg/dl | – |
0.21 |
||
| >5 mg/dl | 1.30 (0.86–1.94) | |||
| AFP level | ||||
| ≤400 ng/mL | – |
0.03 |
– |
0.01 |
| >400 ng/mL | 1.51 (1.04–2.20) | 1.64 (1.12–2.39) | ||
| NLR | ||||
| <3 | – |
0.72 |
||
| ≥3 | 1.07 (0.74–1.55) | |||
| Early CRP kinetics | ||||
| Non-responder | – | – | ||
| Responder | 0.62 (0.41–0.94) | 0.02 | 0.63 (0.41–0.95) | 0.03 |
| Flare-responder | 0.28 (0.16–0.48) | <0.001 | 0.26 (0.15–0.46) | <0.001 |
Abbreviations: BCLC stage, Barcelona Clinic Liver Cancer stage; ECOG PS, Eastern Cooperative Oncology Group performance status; CRP, C-reactive protein; AFP, a-fetoprotein; NLR, Neutrophil-lymphocyte ratio; HR, hazard ratio; CI, confidence interval.
Table 4.
Univariable and Multivariable Cox Regression Analyses of Risk Factors for Overall Survival
| Univariable HR (95% CI) |
p value | Multivariable HR (95% CI) |
p value | |
|---|---|---|---|---|
| Gender | ||||
| Male | – |
0.32 |
||
| Female | 1.54 (0.66–3.62) | |||
| Age, y | ||||
| ≤60 | – |
0.50 |
||
| >60 | 1.27 (0.64–2.51) | |||
| Child-Pugh grade | ||||
| A | – |
0.56 |
||
| B | 1.23 (0.61–2.51) | |||
| BCLC stage | ||||
| B | – |
0.65 |
||
| C | 1.16 (0.62–2.15) | |||
| ECOG PS | ||||
| 0 | – |
0.46 |
||
| ≥1 | 1.24 (0.70–2.21) | |||
| Tumor size (cm) | ||||
| ≤5 | – |
0.78 |
||
| >5 | 1.08 (0.62–1.90) | |||
| CRP level | ||||
| ≤5 mg/dl | – |
0.23 |
||
| >5 mg/dl | 1.40 (0.80–2.44) | |||
| AFP level | ||||
| ≤400 ng/mL | – |
0.04 |
– |
0.04 |
| >400 ng/mL | 1.71 (1.00–2.91) | 1.71 (1.00–2.91) | ||
| NLR | ||||
| <3 | – |
0.32 |
||
| ≥3 | 1.30 (0.77–2.17) | |||
| Early CRP kinetics | ||||
| Non-responder | – | – | ||
| Responder | 0.57 (0.33–0.97) | 0.04 | 0.55 (0.32–0.94) | 0.03 |
| Flare-responder | 0.12 (0.04–0.34) | <0.001 | 0.12 (0.04–0.33) | <0.001 |
Abbreviations: BCLC stage, Barcelona Clinic Liver Cancer stage; ECOG PS, Eastern Cooperative Oncology Group performance status; CRP, C-reactive protein; AFP, a-fetoprotein; NLR, Neutrophil-lymphocyte ratio; HR, hazard ratio; CI, confidence interval.
Discussion
The introduction of ICB has caused a dramatic change in the treatment environment for patients with advanced HCC, yet the survival outcomes of those with the same subtype can vary greatly due to the molecular heterogeneity of advanced HCC.23,24 It is crucial to identify predictors of response to ICB among patients with advanced HCC.
To date, AFP levels have been proposed for the regular screening, diagnosis, and classification of the prognosis of liver cancer.25,26 Ma et al proposed that serum high-sensitivity CRP (hs-CRP) may have the potential to be used as an effective diagnostic tool to supplement AFP for the diagnosis of HCC and to improve the identification of AFP negative HCC in patients with HBV-associated cirrhosis.27 Previous practice has suggested that the CRP level was a powerful indicator of the prognostic the outcome of ICB treatment in a variety of types of cancer. Higher levels of CRP are positively associated with worse OS and PFS,28–31 but the shift and early variation in systematic inflammation following therapeutic intervention has largely gone unnoticed. Recently, Fukuda et al proposed the use of early CRP kinetics to predict response to therapy and survival in patients with metastatic renal cell carcinoma (MRCC) receiving nivolumab immunotherapy.22 However, its predictive importance in patients with HCC who received ICB therapy is yet unknown. In the present study, we demonstrated that early CRP dynamics profoundly affects the ICB response in patients with HCC. A better tumor response and a longer PFS were substantially correlated with the CRP flare response. Most (80%) of the CRP flare-responders had disease control during the follow-up period. Both baseline AFP levels and early CRP kinetics have a prognostic value in the multivariate Cox regression analysis in this study. Therefore, early CRP kinetics may be a useful and efficient approach to classifying patients with HCC and improving the response rate to ICB. Early CRP kinetics have the potential to act a biomarker that guides treatment decisions for HCC similar to that of AFP.
In our cohort, CRP flare-responders had lower baseline CRP levels. One possible explanation for this phenomenon is that baseline CRP levels, which differ significantly between CRP-responsive groups, may represent the initial immunogenicity or the tumor microenvironment of patients with HCC. The liver, as an immune-exempt organ, is the home of numerous macrophages.32 Macrophages, triggered by cytokines from the tumor microenvironment, can transform into different types of tumor-associated macrophages (TAMs), primarily M1 and M2. The former generates anti-tumor substances such as tumor necrosis factor alpha (TNF-α) and triggers anti-tumor immune responses. In contrast, M2 macrophages are associated with pro-tumorigenic and anti-inflammatory effects.33–35 During the initial stages of tumor growth, M1 TAMs are the primary types present, while M2 TAMs become more prevalent in the middle and later stages. As tumor progresses, M1 TAMs switch to M2 TAMs, and an increase in the number of M2 TAMs is associated with a worse prognosis and unsuccessful treatment outcomes in patients with cancer.36–38 It has been observed that several effector chemokines and cytokines are involved in the tumor microenvironment, which is known to promote tumor development and invasiveness.39–41 When inflammation or tissue damage occurs, cytokines such as interleukin, TNF, and interferon are released, leading to the production of CRP by the liver. Nakayama et al demonstrated that an increase in CRP levels is associated with an immunosuppressive microenvironment populated by regulatory T cells and M2 macrophages.42 Nonetheless, the immunological basis of HCC that could explain the observed early CRP kinetics remains to be clarified. To better understand the heterogeneity of the ICB response and to improve patient categorization and prediction of treatment responses, additional research is needed to explore potential distinctions between initial CRP, different kinetics of CRP after immunotherapy, and the composition of the tumor microenvironment.
CRP is an acute-phase protein that rapidly activated and is a nonspecific indicator of inflammation caused by both acute and chronic inflammation, as well as by unknown trauma. Correct discrimination between ICB-mediated antitumor immune responses, infections, and autoimmune adverse effects that cause CRP elevations is important. To avoid interference from external variables, CRP levels should be tested when the disease is stable. Furthermore, it is essential to examine whether a combination of CRP and other markers, such as serum amyloid A and procalcitonin, can differentiate between the response to ICB-associated treatment and systemic infection.
Our study had some major limitations. First, we recognize that this was a retrospective analysis and had a relatively short follow‐up period, particularly for the meaningful endpoint of OS. Additionally, this was a single-center study, and its conclusion may not be as convincing as that of a multi-center study. Third, due to the retrospective nature of our analysis, there were unavoidable selection biases and confounders.
In conclusion, we conducted an in-depth analysis of early CRP kinetics in a cohort of patients with HCC who received ICB. The ICB reaction is predicted by the CRP flare response, which translates into favorable outcomes (PFS, OS, DCR, ORR). Evaluation of CRP kinetics is a promising prospective therapeutic biomarker that is inexpensive, simple to use, and may be more feasible than the programmed death ligand PD-1 (PD-L1) expression assay. Thus, additional prospective studies are needed to confirm the application of early CRP kinetics as a prognostic biomarker and to further explore its potential for dynamic therapeutic stratification of patients with HCC receiving ICB therapy.
Conclusion
The CRP flare response robustly predicts the immunotherapy response and outcomes in patients with HCC. Early CRP kinetics may be an inexpensive, easily implemented and non-invasive biomarker to anticipate response to ICB therapy in intermediate or advanced HCC, with the potential to optimize treatment monitoring.
Funding Statement
This work was partly supported by grants from the National Natural Science Foundation of China (81970539, 82172257) and the Health Care Major Project of Guangzhou (202206080001). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data Sharing Statement
The datasets used and/or analyzed during the current study available from the corresponding author Xiaoyong Zhang (xiaoyzhang@smu.edu.cn) on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of Nanfang Hospital (NFEC-2018-070). All patients provided their written informed consent to enter the study.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi: 10.1016/S0140-6736(22)01200-4 [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150:atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. [DOI] [PubMed] [Google Scholar]
- 5.Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi: 10.1080/13543784.2022.2009455 [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi: 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 8.Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. doi: 10.1038/s41698-018-0048-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pocino K, Stefanile A, Basile V, et al. Cytokines and hepatocellular carcinoma: biomarkers of a deadly embrace. J Pers Med. 2022;13(1):5. doi: 10.3390/jpm13010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathak A, Agrawal A. Evolution of C-reactive protein. Front Immunol. 2019;10:943. doi: 10.3389/fimmu.2019.00943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu M, Ma Z, Zhang X, et al. C-reactive protein and cancer risk: a pan-cancer study of prospective cohort and Mendelian randomization analysis. BMC Med. 2022;20(1):301. doi: 10.1186/s12916-022-02506-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Socha MW, Malinowski B, Puk O, et al. C-reactive protein as a diagnostic and prognostic factor of endometrial cancer. Crit Rev Oncol Hematol. 2021;164(103419):103419. doi: 10.1016/j.critrevonc.2021.103419 [DOI] [PubMed] [Google Scholar]
- 14.Machado D, Marques C, Dias M, Campainha S, Barroso A. Inflammatory prognostic biomarkers in advanced non-small cell lung cancer. Pulmonology. 2019;25(3):181–183. doi: 10.1016/j.pulmoe.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Katagiri R, Yamaji T, et al. Association between C-reactive protein and risk of overall and 18 site-specific cancers in a Japanese case-cohort. Br J Cancer. 2022;126(10):1481–1489. doi: 10.1038/s41416-022-01715-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatanaka T, Kakizaki S, Hiraoka A, et al. Prognostic impact of C-reactive protein and alpha-fetoprotein in immunotherapy score in hepatocellular carcinoma patients treated with atezolizumab plus bevacizumab: a multicenter retrospective study. Hepatol Int. 2022;16(5):1150–1160. doi: 10.1007/s12072-022-10358-z [DOI] [PubMed] [Google Scholar]
- 17.Ozawa Y, Amano Y, Kanata K, et al. Impact of early inflammatory cytokine elevation after commencement of PD-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol. 2019;36(4):33. doi: 10.1007/s12032-019-1255-3 [DOI] [PubMed] [Google Scholar]
- 18.Riedl JM, Barth DA, Brueckl WM, et al. C-Reactive Protein (CRP) levels in immune checkpoint inhibitor response and progression in advanced non-small cell lung cancer: a bi-center study. Cancers. 2020;12(8):2319. doi: 10.3390/cancers12082319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Lu L, He Z, et al. C-reactive protein levels predict responses to PD-1 inhibitors in hepatocellular carcinoma patients. Front Immunol. 2022;13:808101. doi: 10.3389/fimmu.2022.808101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kijima T, Yamamoto H, Saito K, et al. Early C-reactive protein kinetics predict survival of patients with advanced urothelial cancer treated with pembrolizumab. Cancer Immunol Immunother. 2021;70(3):657–665. doi: 10.1007/s00262-020-02709-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasuda Y, Saito K, Yuasa T, et al. Early response of C-reactive protein as a predictor of survival in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Int J Clin Oncol. 2017;22(6):1081–1086. doi: 10.1007/s10147-017-1166-2 [DOI] [PubMed] [Google Scholar]
- 22.Fukuda S, Saito K, Yasuda Y, et al. Impact of C-reactive protein flare-response on oncological outcomes in patients with metastatic renal cell carcinoma treated with nivolumab. J Immunother Cancer. 2021;9(2):e001564. doi: 10.1136/jitc-2020-001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoni M, Rizzo A, Kucharz J, et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365–1379. doi: 10.1007/s00262-022-03349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol. 2021;7(1):113–123. doi: 10.1001/jamaoncol.2020.3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley RK, Meyer T, Rimassa L, et al. Serum alpha-fetoprotein levels and clinical outcomes in the Phase III CELESTIAL study of cabozantinib versus placebo in patients with advanced Hepatocellular carcinoma. Clin Cancer Res. 2020;26(18):4795–4804. doi: 10.1158/1078-0432.CCR-19-3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian S, Chen Y, Zhang Y, et al. Clinical value of serum AFP and PIVKA-II for diagnosis, treatment and prognosis of hepatocellular carcinoma. J Clin Lab Anal. 2023;37(1):e24823. doi: 10.1002/jcla.24823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma LN, Liu XY, Lu ZH, et al. Assessment of high-sensitivity C-reactive protein tests for the diagnosis of hepatocellular carcinoma in patients with hepatitis B-associated liver cirrhosis. Oncol Lett. 2017;13(5):3457–3464. doi: 10.3892/ol.2017.5890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toide M, Saito K, Yasuda Y, et al. Prognostic significance of c-reactive protein in patients with non-metastatic papillary renal cell carcinoma: results from the INternational Marker Consortium for Renal Cancer (INMARC) cohort. Clin Genitourin Cancer. 2022;20(4):e276–e282. doi: 10.1016/j.clgc.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Minichsdorfer C, Gleiss A, Aretin MB, Schmidinger M, Fuereder T. Serum parameters as prognostic biomarkers in a real world cancer patient population treated with anti PD-1/PD-L1 therapy. Ann Med. 2022;54(1):1339–1349. doi: 10.1080/07853890.2022.2070660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Zhang D, Zeng H, et al. Inflammation-based scores predict responses to PD-1 inhibitor treatment in intrahepatic cholangiocarcinoma. J Inflamm Res. 2022;15::5721–5731. doi: 10.2147/JIR.S385921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Baum Y, Alemozaffar M, et al. C-reactive protein in urologic cancers. Mol Aspects Med. 2015;45:28–36. doi: 10.1016/j.mam.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 32.Gao B, Jeong W-I, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2007;47(2):729–736. doi: 10.1002/hep.22034 [DOI] [PubMed] [Google Scholar]
- 33.Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: from mechanisms to therapy. Cancer Commun. 2022;42(11):1112–1140. doi: 10.1002/cac2.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suthen S, Lim CJ, Nguyen PHD, et al. Hypoxia-driven immunosuppression by Treg and type-2 conventional dendritic cells in HCC. Hepatology. 2022;76(5):1329–1344. doi: 10.1002/hep.32419 [DOI] [PubMed] [Google Scholar]
- 35.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6(3):1670–1690. doi: 10.3390/cancers6031670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oura K, Morishita A, Tani J, Masaki T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci. 2021;22(11):5801. doi: 10.3390/ijms22115801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung PS. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin Mol Hepatol. 2022;28(3):333–350. doi: 10.3350/cmh.2021.0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H, Peng X, Yang S, et al. Targeting tumor-associated macrophages in hepatocellular carcinoma: biology, strategy, and immunotherapy. Cell Death Discov. 2023;9(1):65. doi: 10.1038/s41420-023-01356-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144(3):512–527. doi: 10.1053/j.gastro.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 41.Sanghera C, Teh JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39(11):2008–2023. doi: 10.1111/liv.14220 [DOI] [PubMed] [Google Scholar]
- 42.Nakayama T, Saito K, Kumagai J, et al. Higher serum C-reactive protein level represents the immunosuppressive tumor microenvironment in patients with clear cell renal cell carcinoma. Clin Genitourin Cancer. 2018;16(6):e1151–e1158. doi: 10.1016/j.clgc.2018.07.027 [DOI] [PubMed] [Google Scholar]