Abstract
Background
Heart rate is the main determinant factor of the child’s cardiac output in the first year of life. Thus, bradycardia decreases cardiac output leading to fatal cardiac arrhythmias, cardiac arrest, and even death. The objective of this study is to determine the prevalence of bradycardia and its associated factors after induction of general anesthesia among pediatric patients operated at Hawassa University Comprehensive Specialized Hospital (HUCSH).
Methods
Prospective observational study was employed at HUCSH by using a systematic random sampling technique. Pediatric surgical patients less than 6 years old were included in the study. Data were entered into Epi data statistical software (version 4.6.0.) and exported to SPSS (version 25.0). Categorical data were analyzed using chi-square statistics, and continuous data were analyzed using Student’s t-test. Bivariable logistic regression was used to select candidate variables for multivariable logistic regression.
Results
The prevalence of bradycardia among 205 pediatric patients included in this study was 19.5%. Preoperative risk of hypoxia, opioids premedication, inhalational induction (halothane), difficult intubation, intraoperative complications, and significant surgical blood loss were independently associated with bradycardia.
Conclusion
The prevalence of bradycardia after induction of general anesthesia was 19.5%. Preoperative risk of hypoxia, opioids premedication, inhalational induction (particularly with halothane), difficult intubation, intraoperative complications such as hypoxia, and significant blood loss were significantly associated with bradycardia.
Keywords: anesthesia, bradycardia, general anesthesia, induction of anesthesia, pediatrics bradycardia, pediatrics surgery
Introduction
Bradycardia is defined as a clinical state of decreased heart rate from the normal range for specific age groups. It is described in terms of age groups as a heart rate less than 100 BPM, <80 BPM, <70 BPM, <60 BPM that lasts for longer than 30 seconds for infants, toddlers, younger children, school-age children, and adolescents, respectively.1–3
It is the most common complication after induction of general anesthesia in infants as compared to older children and is associated with an increased risk of mortality and morbidity. Heart rate is the determinant factor of cardiac output in the pediatric population. Hence, bradycardia may compromise the child’s cardiac output leading to insufficient oxygen delivery to the vital organs. This may lead to serious cardiac arrhythmias such as cardiac arrest, and even death.4–6
It is the most common problem we encountered during pediatric general anesthesia in a variety of clinical situations. It may occur secondary to exaggerated vagal response in association with airway or ventilator complications, rapid sequence induction, and regional anesthesia. Hypoxia due to hypoventilation may lead to bradycardia, which is the leading cause of cardiopulmonary arrests in pediatric patients. Administration of certain medications such as inhalation induction with halothane and high dose of intravenous opioids may precipitate bradycardia in pediatric patients during anesthesia.4,7–9
Pediatric patients, especially infants and younger children, had experienced a more pronounced vagal response as compared to the adult population to certain reflex stimuli, which may be induced by a variety of physiological factors such as repeated suctioning, laryngoscopy stimulation, tracheal intubation, and repeated doses of succinylcholine.1,10,11 It is also associated with certain electrolyte disturbances like increased serum potassium levels (hyperkalemia), the presence of infectious conditions, and hypothermia.10,12,13
This clinical syndrome occurred at any time during anesthesia, predominantly during the maintenance phase of anesthesia, with airway complications remaining the most common cause of bradycardia in pediatric patients under anesthesia.4
The incidence of bradycardia is decreased among pediatric patients premedicated with atropine and dexmedetomidine, airway protection with an endotracheal tube, and high body weight.14,15
There was a limited study conducted on bradycardia after induction of anesthesia among pediatric patients. This may increase the failure rate in the proper diagnosis and management, as well as limit adherence to perioperative standards to handle pediatric surgical patients. Thus, assessment of the prevalence of bradycardia and identifying the associated factors after induction of general anesthesia helps to have appropriate perioperative anesthesia standards, good communication skills, and a preventive plan. This is also important to identify the associated factors observed, with a view to making recommendations for early recognition and management. To the best of our knowledge, there is no study conducted on the prevalence of bradycardia and its associated factors after induction of general anesthesia among pediatric surgical patients. Therefore, we aimed to determine the prevalence of bradycardia and its associated factors after induction of general anesthesia among pediatric patients operated at HUCSH, Ethiopia.
Methods and Materials
Study Area, Study Period, Study Design, and Study Population
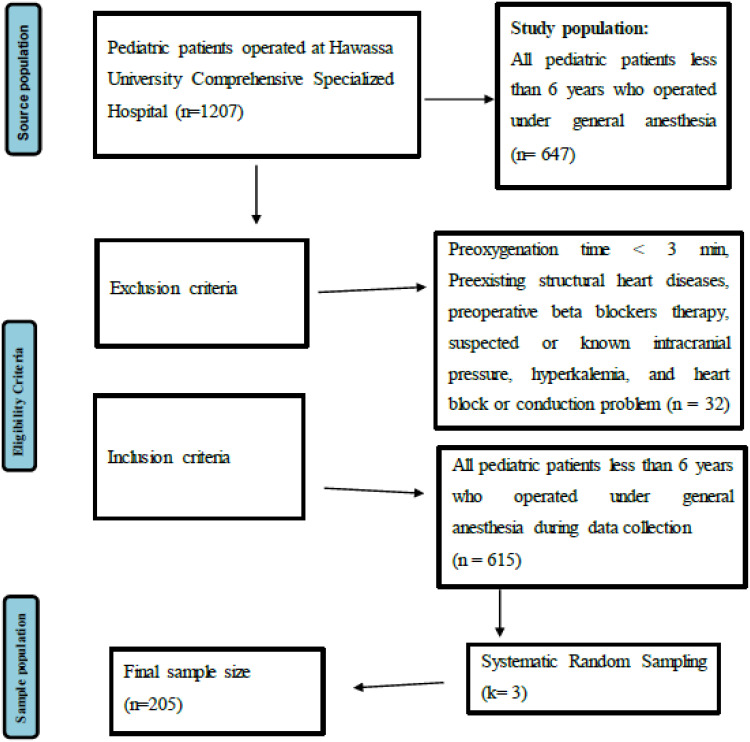
A prospective observational study was conducted in the Hawassa University Comprehensive Specialized Hospital from January 01 to April 30, 2022 (Figure 1).
Figure 1.
Flow chart summarizing the study design and procedures of the study conducted on the prevalence of bradycardia and its associated factors after induction of general anesthesia among pediatric patients operated at Hawassa University Comprehensive Specialized Hospital.
Operational Definition
Anemia is a clinical condition of low hemoglobin levels. It is described in age groups as: hemoglobin levels <10.3 g/dL (3–5 months),16 <11.0 g/dL (6–59 months), and <11.5 g/dL (5–11 years).17,18
Bradycardia after induction of general anesthesia is described as a 20% or greater decrease in heart rate as compared to the baseline value before induction of general anesthesia or during stable anesthesia.19 It is assessed after induction of general anesthesia until recovery from anesthesia.
Induction of general anesthesia is defined as the time at which the patient has reversible loss of consciousness with the administration of intravenous or inhalational anesthetic agents.
Difficult intubation is defined as a clinical situation in which tracheal intubation requires greater than 10 minutes.20
Hypothermia is the decrease in the basal body temperature to less than or equal to 35 °C.
Significant (massive) blood loss is loss of greater than 50% of blood volume within three hours or loss of 1.5 mL/kg/minute for 20 minutes, or blood loss requiring 40 mL/kg of red blood cells.21
Sample Size Determination and Sampling Techniques
Sample Size Determination
The sample size was calculated by the single population proportion formula using a 50% proportion of the pediatric population with bradycardia, a 95% confidence interval, and a margin of error of 5%. Data were obtained on the size of the pediatric population operated at HUCSH over the past three months of last year (similar season). Likewise, the number of pediatric patients who underwent surgery in the previous three months was 360; an average of 88–92 pediatric patients per month underwent surgery. Therefore, by substituting within the formula: sample size n: n = N*n / (n + N – 1) = 186. Where: n = number of sample size, p = proportion of bradycardia following induction of general anesthesia, 50%. Finally, after adding a 10% non-response rate, a total sample size of 205 was applied for the study.
Sampling Techniques
All pediatric patients who met the inclusion criteria were incorporated into the study by using a systematic random sampling technique.
Study Variables
Dependent Variable
Bradycardia after induction of general anesthesia
Independent Variables
Socio-demographic characteristics: age, sex, weight
Preoperative factors:
ASA class, preoperative risk of hypoxia (decreased levels of arterial oxygen saturation), preoperative hemoglobin levels, premedication with opioids, anticholinergics, steroids, etc., and the nature of the surgery.
Intraoperative factors:
Vital signs (non-invasive blood pressure, heart rate, peripheral oxygen saturation, and temperature), induction of anesthesia, induction agent, maintenance of anesthesia, intraoperative complications such as hypoxia and hypercarbia, hypothermia, significant blood loss, hypoglycemia, and others (fluid overload, hypotension, drug adverse events, etc.)
Airway-related (techniques of airway management: face mask, LMA, ETT)
Types of surgery (orthopedics, general surgery, airway procedures like foreign body removal, plastic and reconstructive surgery, gynecologic procedures)
Data Collection Instruments and Techniques
Data were collected by a using structured questionnaire. The training was given to data collectors (four MSc students), and one MSc anesthesia professional as supervisor. Covid-19 prevention strategy like social distancing, hand sanitizers, face mask, and disposable gloves was provided for each data collector, and they would apply these strategies with the individual study participant. Immediately at admission to the operation room the patient's socio-demographic factors like age, gender, and weight were recorded. The preoperative factors including ASA (American Society of Anesthesiologists physical status), elective/emergency, premedication, and any pre-existing risk of hypoxia were reviewed from the patient chart and recorded.
The intraoperative factors such as vital signs (non-invasive blood pressure, heart rate, peripheral oxygen saturation, and temperature), type of anesthesia, type of induction and maintenance agent, usage of analgesics, episodes of desaturation during induction and intubation, techniques of airway management (face mask, laryngeal mask, endotracheal tube), types of surgery (orthopedics, general surgery, airway procedures like foreign body, vascular procedures, plastic and reconstructive surgery, gynecologic procedures), site of surgery (EENT, head, abdomen, chest, extremity), metabolic impairment (hypoglycemia, fluid over- or under-load), and other factors like significant blood loss and hypothermia were reviewed from the anesthesia chart, operation note, surgical and anesthesia log book, and recorded.
Bradycardia was assessed with a pulse-oximeter, ECG, peripheral pulse, and/or pericardial stethoscope and was recorded. Immediately the patient was given the necessary treatments including removing the precipitating factors or reflex stimuli (if any), atropine, oxygen, adrenaline, and chest compression.
Data Management, Processing, and Analysis
Data were collected using a pretested structured questionnaire with multiple choices and open-ended questions on respondents’ socio-demographic characteristics, and on preoperative and intraoperative variables. Pretest was done for 5% of the sample population. Then, possible amendments were made. Regular supervision and follow-up were made during data collection. After the questionnaire was checked manually for completeness and coded, data were entered into Epi data statistical software (version 4.6.0.) and exported to SPSS windows statistical software (version 25.0) for data processing and performing other statistical analyses. Descriptive data were summarized using descriptive statistics. Categorical data were analyzed using chi-square statistics, and continuous data were analyzed using Student’s t-test. Binary logistic regression was used to identify factors associated with bradycardia after induction of general anesthesia. Bivariable logistic regression was used to select candidate variables for multivariable logistic regression. Variables with a p-value less than 25% in the bivariable logistic regression were candidates for the multivariable logistic regression. Before fitting the final model, multicollinearity was checked using the variable inflation factor. The value of variable inflation factor was determined and its value of 1 was obtained, which indicates absence of collinearity. Multivariable logistic regression was fitted to identify factors independently associated with bradycardia after induction of general anesthesia, and to control confounders. In the final model, adjusted odds ratio and 95% confidence interval were used, respectively, to measure the strength of association and statistical significance. Hosmer and Lemeshow’s goodness-of-fit test was used to test model fitness.
Ethical Consideration
An ethical clearance letter was obtained from Hawassa University Institutional Review Board. Written informed consent was obtained from each study participant's parents/legal guardian and approved by the ethics committee in line with the Declaration of Helsinki. Names or any other personal identifiers of study participants were not recorded. The WHO Covid-19 prevention strategy was applied during data collection.
Results
Socio-demographic Characteristics of Pediatrics Patients Operated at HUCSH
A total of 205 pediatric patients were included in the study making a 100% response rate. Greater than half (57.1%) of the study participants were males, and the rest, 88 (42.9%) were females. The mean age and weight of pediatric patients operated at HUCSH were 1.20 ± 4.72 years and 15.30 ± 7.47 kg, respectively (Table 1).
Table 1.
Socio-demographic Characteristics of Pediatric Patients Operated at Hawassa University Comprehensive Specialized Hospital, Ethiopia (n=205)
| Variables | Categories | Frequency (%) | Bradycardia After General Anesthesia, Frequency (%) | |
|---|---|---|---|---|
| Yes (n=40) | No (n=165) | |||
| 40 (19.5) | 165 (80.5) | |||
| Sex | Male | 117 (57.1) | 26 (22.2) | 91 (77.8) |
| Female | 88 (42.9) | 14 (15.9) | 74 (84.1) | |
| Age | 1.20±4.72 | 1.91±1.78 | 2.92±5.29 | |
| Weight (kg) | 15.30±7.47 | 11.12±7.26 | 16.3±7.18 | |
Prevalence of Bradycardia After Induction of General Anesthesia Among Pediatric Patients Operated at HUCSH
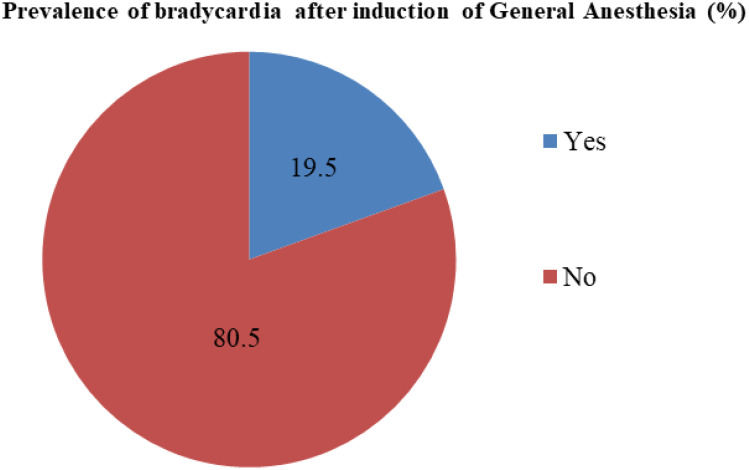
The prevalence of bradycardia after induction of general anesthesia among pediatric patients operated at Hawassa University Comprehensive Specialized Hospital was 40 (19.5%); CI 16-22. (Figure 2). More than half 24 (60%), of pediatric bradycardia cases were quickly resolved with oxygen administration and controlling reflex activity and aggressive management of hypoxia, 9 (22.5%) needed atropine administration, 5 (12.5%) were self-limited, and 2 (5%) were managed with adrenaline administration and chest compression.
Figure 2.
Prevalence of bradycardia after induction of general anesthesia among pediatric patients operated at Hawassa University Comprehensive Specialized Hospital.
The prevalence of bradycardia after general anesthesia among pediatric patients who were given opioids premedication was 69.2%. The prevalence of bradycardia after induction of general anesthesia among pediatric patients who had preoperative risk of hypoxia (decreased levels of arterial oxygen saturation) was 35.4%. The prevalence of bradycardia after induction of general anesthesia among pediatric patients who had intraoperative complications (hypoxia, hypoglycemia, hypothermia, hypotension, significant blood loss, etc.) was 28.1%. The prevalence of bradycardia after induction of general anesthesia among pediatric patients who had a difficult intubation and who had significant blood loss were 79.4% and 22.2%, respectively.
Preoperative Factors
As the distribution of preoperative factors showed, regarding the ASA status of the pediatric patients, nearly two-thirds (65.4%) were ASA I, while 71 (34.6%) were ASA II. Anticholinergics were the most frequently given premedication, 60 (29.3%); followed by opioids, 39 (19.0%); and other premedication like diazepam and steroids, 27 (13.2%). A total of 63 (30.7%) pediatric patients had undergone emergency surgery. The mean preoperative hemoglobin level of pediatric patients operated at Hawassa University Comprehensive Specialized Hospital was 12.24 ± 5.23 g/dL (Table 2).
Table 2.
A Cross-Tabulation of the Preoperative Factors Among Pediatric Patients Operated at Hawassa University Comprehensive Specialized Hospital, Ethiopia (n=205)
| Variables | Categories | Frequency (%) | Bradycardia After General Anesthesia, Frequency (%) | |||
|---|---|---|---|---|---|---|
| Yes (n=40) | No (n=165) | |||||
| 40 (19.5) | 165 (80.5) | |||||
| ASA class | ASA-1 | 134 (65.4) | 16 (11.9) | 118 (88.1) | ||
| ASA-2 | 71 (34.6) | 24 (33.8) | 47 (66.2) | |||
| Premedication | Anticholinergics | Yes | 60 (29.3) | 16 (26.7) | 44 (73.3) | |
| No | 145 (70.7) | 24 (16.6) | 121 (83.4) | |||
| Opioids | Yes | 39 (19) | 27 (69.2) | 12 (30.8) | ||
| No | 166 (81) | 23 (13.9) | 143 (86.1) | |||
| Others (steroids, diazepam) | Yes | 27 (13.2) | 8 (29.6) | 19 (70.4) | ||
| No | 178 (86.8) | 32 (18) | 146 (82) | |||
| Preoperative hemoglobin levels (g/dL) | 12.24±5.23 | 7.05±1.40 | 12.64±1.74 | |||
| Preoperative risk of hypoxia (decreased levels of arterial oxygen saturation) | Yes | 79 (38.5) | 28 (35.4) | 51 (64.6) | ||
| No | 126 (61.5) | 12 (9.5) | 114 (90.5) | |||
| Nature surgery | Emergency | 63 (30.7) | 20 (31.7) | 43 (68.5) | ||
| Elective | 142 (69.3) | 20 (14.1) | 122 (85.9) | |||
Intraoperative Factors
The mean intraoperative blood pressure, heart rate, peripheral oxygen saturation, and body temperature levels of pediatric patients operated at Hawassa University Comprehensive Specialized Hospital were 84.12 ± 22.88 mmHg/ 45.13 ± 12.72 mmHg, 113 ± 43 beats per minute, 96.1% ± 3.90%, 36.22 ± 1.31 °C, respectively. More than three-fourths (76.6%) of the pediatric patients had general anesthesia induced with intravenous anesthetics, and inhalational induction was used in 35 (17.1%). Among these, 62 (30.2%) of the pediatric patients were induced with ketamine, and the rest were induced with: propofol, 73 (35.6%); ketofol, 54 (26.3%); and halothane, 10 (4.9%). Regarding the maintenance of general anesthesia, 88 (42.9%) of the pediatric patients were maintained with halothane. Greater than half (62.9%) of pediatric patients had their airway managed with an endotracheal tube. Greater than three-fourths (80%) of the pediatric patients were given vecuronium for intraoperative muscle relaxation (Table 3).
Table 3.
A Cross-Tabulation of the Intraoperative Factors Among Pediatric Patients Operated at Hawassa University Comprehensive Specialized Hospital, Ethiopia (n=205)
| Variables | Categories | Frequency (%) | Bradycardia After General Anesthesia, Frequency (%) | ||
|---|---|---|---|---|---|
| Yes (n=40) | No (n=165) | ||||
| 40 (19.5) | 165 (80.5) | ||||
| Vital signs | Blood pressure (mmHg) | 84.12±22.88 / 45.13±12.72 | 65±33 / 29.50±17.11 | 91±23 / 51.02±9.91 | |
| Heart rate (beats per minute) | 113±43 | 113±43 | 109±41 | ||
| Oxygen saturation (%) | 96.1±3.90 | 89.23±9.08 | 97.03±2.51 | ||
| Temperature (°C) | 36.22±1.31 | 35.43±1.01 | 36.05±0.09 | ||
| Induction of general anesthesia | IV induction | Yes | 157 (76.6) | 14 (8.9) | 143 (91.1) |
| No | 48 (23.4) | 26 (54.2) | 22 (45.8) | ||
| Inhalational induction | Yes | 35 (17.1) | 14 (40) | 21 (60) | |
| No | 170 (82.9) | 26 (15.3) | 144 (84.7) | ||
| Induction agent | Ketamine | Yes | 62 (30.2) | 12 (19.4) | 50 (80.6) |
| No | 143 (69.8) | 28 (19.6) | 115 (80.4) | ||
| Propofol | Yes | 73 (35.6) | 16 (21.9) | 57 (78.1) | |
| No | 132 (64.4) | 24 (18.2) | 108 (81.8) | ||
| Ketofol | Yes | 54 (26.3) | 6 (11.1) | 48 (88.9) | |
| No | 151 (73.7) | 34 (22.5) | 117 (77.5) | ||
| Halothane | Yes | 10 (4.9) | 6 (60) | 4 (40) | |
| No | 195 (95.1) | 34 (17.4) | 161 (82.6) | ||
| Airway management | Face mask | Yes | 46 (22.4) | 6 (13) | 40 (87) |
| No | 159 (77.6) | 34 (21.4) | 125 (78.6) | ||
| LMA | Yes | 22 (10.7) | 4 (18.2) | 18 (81.8) | |
| No | 183 (89.3) | 36 (19.7) | 147 (80.3) | ||
| ETT | Yes | 129 (62.9) | 11 (8.5) | 118 (91.5) | |
| No | 76 (37.1) | 29 (38.2) | 47 (61.8) | ||
| Difficult intubation | Yes | 34 (16.6) | 27 (79.4) | 7 (20.6) | |
| No | 171 (83.4) | 13 (7.6) | 158 (92.4) | ||
| Maintenance of anesthesia | Isoflurane | 65 (31.7) | 11 (16.9) | 54 (83.1) | |
| Halothane | 88 (42.9) | 19 (21.6) | 69 (78.4) | ||
| Propofol | 831 (15.1) | 8 (38.1) | 13 (61.9) | ||
| Others (ketofol, ketamine) | 21 (10) | 2 (6.5) | 29 (93.5) | ||
| Types of surgery | Ophthalmological | 73 (35.6) | 9 (12.3) | 64 (87.7) | |
| General surgery | 59 (28.8) | 13 (22) | 46 (78) | ||
| Neurosurgery | 31 (15.1) | 7 (22.6) | 24 (77.4) | ||
| Urology | 24 (11.7) | 5 (20.8) | 19 (79.2) | ||
| ENT | 11 (5.4) | 4 (36.4) | 7 (63.6) | ||
| Others (ortho, plastic, OMFS) | 7 (3.4) | 2 (28.6) | 5 (71.4) | ||
| Muscle relaxant used during maintenance | Succinylcholine | 21 (20) | 14 (66.7) | 7 (33.3) | |
| Vecuronium | 84 (80) | 28 (33.3) | 56 (66.7) | ||
| Intraoperative complications | Yes | 57 (27.8) | 16 (28.1) | 4 1 (71.9) | |
| No | 148 (72.2) | 24 (16.2) | 124 (83.8) | ||
| Complications | No | 148 (72.2) | 24 (16.2) | 124 (83.8) | |
| Hypoxia | 21 (10.2) | 5 (23.8) | 16 (76.2) | ||
| Significant blood loss | 9 (4.4) | 2 (22.2) | 7 (77.8) | ||
| Hypothermia | 11 (5.4) | 2 (18.2) | 9 (81.8) | ||
| Hypoglycemia | 8 (3.9) | 3 (37.5) | 5 (62.5) | ||
| Others (fluid load) | 8 (3.9) | 4 (50) | 4 (50) | ||
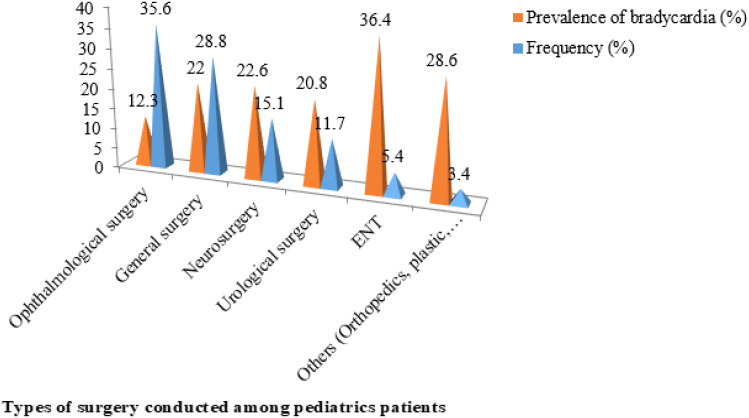
Among the types of surgery pediatric patients underwent, ophthalmological surgery was the most frequently performed type of surgical procedure relative to the other specialties, 73 (35.6%), as described by the bar chart (Figure 3).
Figure 3.
The frequency of types of surgery conducted and its respective prevalence of bradycardia after induction of general anesthesia among pediatric patients operated at Hawassa University Comprehensive Specialized Hospital.
Factors Associated with Bradycardia After Induction of General Anesthesia in Pediatric Patients Operated at HUCSH
Results of Bivariable Binary Logistic Regression of Factors Associated with Bradycardia After Induction of General Anesthesia in Pediatric Patients Operated at HUCSH
Bivariable logistic regression was fitted to identify candidate variables for the multivariable logistic regression. Accordingly, ASA class, preoperative risk of hypoxia (decreased levels of arterial oxygen saturation), premedication with opioids and anticholinergics, induction of general anesthesia with both intravenous and inhalational agents (halothane), airway management with face mask or ETT, difficult airway, maintenance of anesthesia, types of surgery performed, and intraoperative complications, such as hypoxia, significant blood loss, and hypoglycemia, were associated with bradycardia after induction of general anesthesia at a p-value of less than 25% (Table 4)
Table 4.
Results of Bivariable Logistic Regression of Factors Associated with Bradycardia After Induction of General Anesthesia Among Pediatric Patients Operated at Hawassa University Comprehensive Specialized Hospital, Ethiopia (n=205)
| Variables | Categories | Bradycardia After General Anesthesia, Frequency (%) | COR (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|
| Yes (n=40) | No (n=165) | ||||||
| 40 (19.5) | 165 (80.5) | ||||||
| ASA class | ASA-I | 16 (11.9) | 118 (88.1) | 1 | |||
| ASA-II | 24 (33.8) | 47 (66.2) | 0.27 (0.13–0.54) | 0.14 | |||
| Preoperative risk of hypoxia (desaturation) | No | 12 (9.5) | 114 (90.5) | 1 | |||
| Yes | 28 (35.4) | 51 (64.6) | 5.22 (2.45–11.0) | 0.12 | |||
| Premedications | Anticholinergics | Yes | 16 (26.7) | 44 (73.3) | 1 | ||
| No | 24 (16.6) | 121 (83.4) | 1.83 (0.89–3.77) | 0.21 | |||
| Opioids | No | 13 (13.9) | 143 (86.1) | 1 | |||
| Yes | 27 (69.2) | 12 (30.8) | 26.5 (10.9–64.2) | 0.24 | |||
| Others (steroids, diazepam) | No | 32 (18) | 146 (82) | 1 | |||
| Yes | 8 (29.6) | 19 (70.4) | 1.92 (0.21–1.29) | 0.26 | |||
| Nature of surgery | Elective | 20 (14.1) | 122 (85.9) | 1 | |||
| Emergency | 20 (31.7) | 43 (68.5) | 0.35 (0.17–0.72) | 0.28 | |||
| Induction of general anesthesia | IV induction | Yes | 14 (8.9) | 143 (91.1) | 1 | ||
| No | 26 (54.2) | 22 (45.8) | 0.08 (0.04–0.18) | 0.22 | |||
| Inhalational induction | No | 26 (15.3) | 144 (84.7) | 1 | |||
| Yes | 14 (40) | 21 (60) | 3.69 (1.67–8.17) | 0.10 | |||
| Induction agent | Ketamine | Yes | 12 (19.4) | 50 (80.6) | 1 | ||
| No | 28 (19.6) | 115 (80.4) | 0.98 (0.46–2.10) | 0.36 | |||
| Propofol | No | 24 (18.2) | 108 (81.8) | 1 | |||
| Yes | 16 (21.9) | 57 (78.1) | 1.26 (0.39–1.61) | 0.52 | |||
| Ketofol | Yes | 6 (11.1) | 48 (88.9) | 1 | |||
| No | 34 (22.5) | 117 (77.5) | 0.43 (0.17–1.09) | 0.30 | |||
| Halothane | No | 34 (17.4) | 161 (82.6) | 1 | |||
| Yes | 6 (60) | 4 (40) | 7.10 (1.90–26.5) | 0.08 | |||
| Airway management | Face mask | No | 34 (21.4) | 125 (78.6) | 1 | ||
| Yes | 6 (13) | 40 (87) | 0.55 (0.22–1.41) | 0.21 | |||
| LMA | Yes | 4 (18.2) | 18 (81.8) | 1 | |||
| No | 36 (19.7) | 147 (80.3) | 0.91 (0.29–2.85) | 0.86 | |||
| ETT | No | 29 (38.2) | 47 (61.8) | 1 | |||
| Yes | 11 (8.5) | 118 (91.5) | 0.15 (0.07–0.34) | 0.18 | |||
| Difficult intubation | No | 13 (7.6) | 158 (92.4) | 1 | |||
| Yes | 27 (79.4) | 7 (20.6) | 46.8 (17.2–51.4) | 0.02 | |||
| Maintenance of anesthesia | Isoflurane | 11 (16.9) | 54 (83.1) | 1 | |||
| Halothane | 19 (21.6) | 69 (78.4) | 1.35 (0.59–3.08) | 0.06 | |||
| Propofol | 8 (38.1) | 13 (61.9) | 3.02 (0.87–18.3) | 0.07 | |||
| Others (ketofol, ketamine) | 2 (6.5) | 29 (93.5) | 0.34 (0.16–1.24) | 0.12 | |||
| Muscle relaxants used | Succinylcholine | 14 (66.7) | 7 (33.3) | 0.25 (0.36–1.15) | 0.28 | ||
| Vecuronium | 28 (33.3) | 56 (66.7) | 1 | ||||
| Types of surgery | Others (ortho, plastic, OMFS) | 2 (28.6) | 5 (71.4) | 1 | |||
| ENT | 4 (36.4) | 7 (63.6) | 1.43 (0.25–8.16) | 0.19 | |||
| Urology | 5 (20.8) | 19 (79.2) | 0.66 (0.48–16.9) | 0.25 | |||
| Neurosurgery | 7 (22.6) | 24 (77.4) | 0.73 (0.22–10.3) | 0.17 | |||
| General surgery | 13 (22) | 46 (78) | 0.71 (0.09–5.43) | 0.23 | |||
| Ophthalmological | 9 (12.3) | 64 (87.7) | 0.35 (0.22–8.66) | 0.34 | |||
| Intraoperative complications | No | 24 (16.2) | 124 (83.8) | 1 | |||
| Yes | 16 (28.1) | 41 (71.9) | 2.02 (0.24–1.02) | 0.16 | |||
| Complications | No | 24 (16.2) | 124 (83.8) | 1 | |||
| Hypoxia | 5 (23.8) | 16 (76.2) | 1.61 (0.17–7.06) | 0.15 | |||
| Blood loss | 2 (22.2) | 7 (77.8) | 1.47 (0.23–8.78) | 0.16 | |||
| Hypothermia | 2 (18.2) | 9 (81.8) | 1.15 (1.9–2.90) | 0.46 | |||
| Hypoglycemia | 3 (37.5) | 5 (62.5) | 3.10 (0.60–1.73) | 0.22 | |||
| Others (fluid load) | 4 (50) | 4 (50) | 5.17 (1.54–4.83) | 0.39 | |||
Notes: Bold p-value = Significant association on bivariable logistic regression at p-value <0.25. 1- Reference group.
Factors Independently Associated with Bradycardia After Induction of General Anesthesia Among Pediatric Patients Operated at HUCSH
Multivariable logistic regression was fitted to identify independent factors associated with bradycardia after induction of general anesthesia. Accordingly, preoperative risk of hypoxia (decreased level of arterial oxygen saturation), opioids premedication, inhalational induction (particularly with halothane), difficult intubation, intraoperative complications such as hypoxia, and significant surgical blood loss were significantly associated with bradycardia after induction of general anesthesia in a multivariable logistic regression at a p-value of less than 5%.
Pediatric patients who had a preoperative risk of hypoxia (decreased levels of arterial oxygen saturation) were 1.33 times (AOR: 1.33, 95% CI: 0.12–1.46) more likely to develop bradycardia as compared to those who had no risk of preoperative desaturation (hypoxia). Pediatric patients who were given opioids premedication were 2.4 times (AOR: 2.4, 95% CI: 0.03–0.22) more likely to experience bradycardia following induction of general anesthesia as compared to those who were not given opioids premedication.
On the other hand, pediatric patients who had general anesthesia with inhalational induction were 10.5 times (AOR: 10.5, 95% CI: 2.80–39.7), and in particular those given halothane induction were 1.5 times (AOR: 1.5, 95% CI: 1.22–14.5), more likely to develop bradycardia following induction of general anesthesia as compared to those pediatric patients who had general anesthesia induction with intravenous anesthetics. Those pediatric patients who had difficult intubation were 2.35 times (AOR: 2.35, 95% CI: 0.46–0.88) more likely to experience bradycardia following induction of general anesthesia as compared to those who were easily intubated (first pass success).
Pediatric patients who had experienced intraoperative complications were 1.65 times (AOR: 1.65, 95% CI: 0.04–0.77) more likely to develop bradycardia after induction of general anesthesia as compared to those who had a smooth intraoperative course (no complication). Those pediatric patients who had intraoperative hypoxia were 6.5 times (AOR: 6.5, 95% CI: 5.22–45.12) more likely to develop bradycardia after induction of general anesthesia as compared to those who had normal arterial oxygen saturation. Pediatric patients who had significant surgical blood loss were 14.6 times (AOR: 14.6, 95% CI: 1.75–5.76) more likely to develop bradycardia after induction of general anesthesia as compared to those with minimal blood loss (Table 5).
Table 5.
Results of Multivariable Logistic Regression of Factors Independently Associated with Bradycardia After Induction of General Anesthesia Among Pediatric Patients Operated at Hawassa University Comprehensive Specialized Hospital, Ethiopia (n=205)
| Variables | Categories | AOR (95% CI) | P-value | |
|---|---|---|---|---|
| ASA class | ASA-I | 1 | ||
| ASA-II | 1.95 (8.20–24.74) | 0.08 | ||
| Risk of hypoxia (desaturation) | No | 1 | ||
| Yes | 1.33 (0.12–1.46) | 0.04 | ||
| Premedications | Anticholinergics | Yes | 1 | |
| No | 0.59 (0.04–8.44) | 0.20 | ||
| Opioids | No | 1 | ||
| Yes | 2.40 (0.03–0.22) | 0.01 | ||
| Induction of general anesthesia | IV induction | Yes | 1 | |
| No | 1.03 (3.15–13.9) | 0.21 | ||
| Inhalational induction | No | 1 | ||
| Yes | 10.5 (2.80–39.7) | 0.02 | ||
| Induction agent | Halothane | No | 1 | |
| Yes | 1.50 (1.22–14.5) | 0.04 | ||
| Airway management | Face mask | No | 1 | |
| Yes | 19.5 (0.49–7.75) | 0.11 | ||
| ETT | No | 1 | ||
| Yes | 1.38 (3.56–5.41) | 0.12 | ||
| Difficult intubation | No | 1 | ||
| Yes | 2.35 (0.46–0.88) | 0.001 | ||
| Maintenance of anesthesia | Isoflurane | 1 | ||
| Halothane | 0.13 (0.04–8.44) | 0.43 | ||
| Propofol | 0.71 (0.08–24.83) | 0.89 | ||
| Others (ketofol, ketamine) | 0.70 (0.00–2.0) | 0.38 | ||
| Types of surgery | Others (ortho, plastic, OMFS) | 1 | 0.53 | |
| ENT | 0.23 (0.01–0.27) | 0.43 | ||
| Urology | 0.61 (1.11–80) | 0.37 | ||
| Neurosurgery | 0.30 (10.0–22.2) | 0.26 | ||
| General surgery | 0.40 (1.02–5.22) | 0.25 | ||
| Intraoperative complications | No | 1 | ||
| Yes | 1.65 (0.04–0.77) | 0.02 | ||
| Complications | No | 1 | 0.09 | |
| Hypoxia | 6.5 (5.22–45.12) | 0.03 | ||
| Significant blood loss | 14.6 (1.75–5.76) | 0.04 | ||
| Hypoglycemia | 0.24 (4.14–11.9) | 0.73 | ||
Notes: Bold: Significant association on multivariable logistic regression.
Discussion
In the present study, we reported the prevalence of bradycardia after induction of general anesthesia among pediatric patients operated at Hawassa University Comprehensive Specialized Referral Hospital was 40 (19.5%); CI 16-22.
The finding of our study is in corroboration with the studies conducted in the Netherlands and Iran, which showed that the prevalence of bradycardia during induction of general anesthesia among the pediatric population was 16.2% and 16.46%, respectively.5,22
The finding of our study is high as compared to the study conducted in Philadelphia, which revealed that the prevalence of bradycardia after induction of general anesthesia among pediatric patients was 8.9%. The possible explanation for the discrepancy of this difference might be due to the exclusion of pediatric patients who underwent emergency surgery and had a difficult airway, which increases the perils to develop bradycardia during general anesthesia in the previous study.2
In the current study, pediatric patients who had a preoperative risk of hypoxia (decreased levels of arterial oxygen saturation) were 1.33 times (AOR: 1.33, 95% CI: 0.12–1.46), and intraoperative hypoxia were 6.5 times (AOR: 6.5, 95% CI: 5.22–45.12), more likely to develop bradycardia as compared to those who had no risk of preoperative desaturation (hypoxia). The finding of our study agreed with the result of previous studies which found that bradycardia during or after induction of general anesthesia in pediatric patients was strongly associated with hypoxia. The possible explanation for this might be due to the exaggerated vagal tone in response to hypoxia, resulting in a slow heart rate (bradycardia) in neonates, infants, and small children.1,2,6,10,22–24
Pediatric patients who were given opioids premedication were 2.4 times (AOR: 2.4, 95% CI: 0.03–0.22) more likely to experience bradycardia following induction of general anesthesia as compared to those who were not given opioids premedication. This finding agreed with another study that reported that the episodes of bradycardia in the pediatric population were highly associated with high doses of intravenous opioids during anesthesia. The possible explanation for opioid-induced bradycardia might be due to depression of sympathetic nervous system tone leading to unopposed vagal tone, and decreased cardiac output from peripheral vasodilatation.25–27
In the current study, pediatric patients who had general anesthesia with inhalational induction were 10.5 times (AOR: 10.5, 95% CI: 2.80–39.7) (in particular halothane induction were 1.5 times (AOR: 1.5, 95% CI: 1.22–14.5)) more likely to develop bradycardia after induction of general anesthesia as compared to those pediatric patients who had general anesthesia induction with intravenous anesthetics. Our finding is in agreement with the study conducted by Kraemer et al that showed that greater than half (57%) of the pediatric patients develop bradycardia during inhaled induction of anesthesia. The finding of the current study is supported by another study which concluded that bradycardia is highly associated with infants who had been induced with a high concentration of inhalational anesthetics. Similarly, our finding is consistent with the study that reported an increased risk of bradycardia among children following inhalational induction, particularly after sevoflurane induction. The possible explanation for this might be due to the direct depressant effect of inhalational anesthetics on the myocardium, and might be from the resulting decrease in the mean arterial pressure as a consequence of reduced systemic vascular resistance thereby decreasing cardiac out in a dose-dependent manner.1,23,28
Those pediatric patients who had difficult intubation were 2.35 times (AOR: 2.35, 95% CI: 0.46–0.88), more likely to experience bradycardia following induction of general anesthesia as compared to those who had been easily intubated (first pass success). The finding of the present study is corroborated by the studies conducted in Iran, the United Kingdom, and Philadelphia, which found that bradycardia after induction of general anesthesia in pediatric patients was associated with multiple attempts of direct laryngoscopy during tracheal intubation of the infant. The finding of our study is consistent with another study which described that difficult intubation (airway) increases the risk of respiratory adverse events leading to cardiovascular complications like bradycardia, ventricular arrest, and asystole. The possible explanation for this might be that prolonged apnea increases the risk of hypoxic stimulation of the carotid bodies resulting in decreased heart rate (bradycardia).2,10,20,22,29
A pediatric patient who had experienced intraoperative complications such as hypoxia and significant bleeding was 1.65 times (AOR: 1.65, 95% CI: 0.04–0.77) more likely to develop bradycardia after induction of general anesthesia as compared to those who had a smooth intraoperative course (no complication). A pediatric patient who had significant surgical blood loss was 14.6 times (AOR: 14.6, 95% CI: 1.75–5.76) more likely to experience bradycardia after induction of general anesthesia as compared to those who had minimal surgical blood loss. The finding of our study is consistent with the results of different studies that reported that progressive or significant bleeding is strongly associated with sinus bradycardia. The possible explanation for this might be due to significant blood loss stimulating the parasympathetic nervous systems and slowing the heart rate (bradycardia).30–32
Strength and Limitation of the Study
The present study was focused on the prevalence and associated factors of bradycardia in pediatric surgical patients by using all available assessment tools in combination (precordial stethoscope, ECG, pulse-oximetry); this increases the validity and reliability of our study finding. We have not studied the postoperative outcome of bradycardia among pediatric surgical patients; both short-term and long-term consequences need longitudinal studies with a large sample size.
Conclusion and Recommendation
The prevalence of bradycardia after induction of general anesthesia among pediatric patients operated at Hawassa University Comprehensive Specialized Hospital was 19.5%.
Preoperative risk of hypoxia (decreased level of arterial oxygen saturation), opioids premedication, inhalational induction (particularly with halothane), difficult intubation, intraoperative complications such as hypoxia, and significant surgical blood loss were significantly associated with bradycardia on multivariable logistic regression at p-value less than 5%.
Anesthesia Teams
Early identification, minimizing, or avoiding pediatric patients at risk of hypoxia, maintaining normal ventilation avoiding hypoxia, and hypercarbia, and considering multimodal analgesia to minimize the requirement of opioids may reduce the prevalence of bradycardia during general anesthesia. The use of intravenous anesthetics might be preferred for induction of general anesthesia.
Experienced anesthesia staff should handle the pediatric patients at risk of difficult intubation, in face of prepared necessary airway equipment’s facilitating easy intubation.
Surgical Teams
Surgical teams should apply the proper measures to minimize surgical blood loss such as the use of cautery, allowing the experienced surgeon to do the procedure, and minimizing the use of systemic vasoconstrictors (timely and adequate volume replacement in the case of significant bleeding).
Acknowledgments
We would like to acknowledge Hawassa University, College of Medicine and Health Science. Our sincere appreciation goes to our study participants for their time.
Abbreviations
ASA, American Society of Anesthesiologists. BPM, beats per minute. ECG, electrocardiography. EENT, eye, ear, nose, throat. HUCSH, Hawassa University Comprehensive Specialized Hospital. LMA, laryngeal mask airway. OMFS, oral and maxillofacial surgery. RSI, rapid sequence induction. SPSS, Statistical Packages for Social Sciences. WHO, World Health Organization.
Data Sharing Statement
Data are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The ethical letter was taken from Hawassa University, College of Medicine and Health Science Institutional Review Board with Ref. no. HUCMHS/1719/22. Written informed consent was obtained from each study participant's parents/legal guardian and approved by the ethics committee.
Publication Consent
We received proper patient written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kraemer FW, Stricker PA, Gurnaney HG, et al. Bradycardia during induction of anesthesia with sevoflurane in children with down syndrome. Anesth Analg. 2010;111(5):1259–1263. doi: 10.1213/ANE.0b013e3181f2eacf [DOI] [PubMed] [Google Scholar]
- 2.Gálvez JA, Acquah S, Ahumada L, et al. Hypoxemia, bradycardia, and multiple laryngoscopy attempts during anesthetic induction in infants: a single-center, retrospective study. Anesthesiology. 2019;131(4):830–839. doi: 10.1097/ALN.0000000000002847 [DOI] [PubMed] [Google Scholar]
- 3.Keenan RL, Shapiro JH, Kane F, Simpson P. Bradycardia during anesthesia in infants: an epidemiologic study. Anesthesiology. 1999;80(5):976–987. doi: 10.1097/00000542-199405000-00005 [DOI] [PubMed] [Google Scholar]
- 4.Watterson LM, Morris RW, Westhorpe RN, Williamson JA. Crisis management during anaesthesia: bradycardia. Qual Saf Health Care. 2005;14(3):7–14. doi: 10.1136/qshc.2002.004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradycardia in children during general anaesthesia. card arrhythm - new considerations; 2012.
- 6.Richard L, Keenan MD, Jay H, et al. Keenan-1994-Bradycardia during anesthesia in infants. An epidemiologic study. Anesthesiology. 1994;80(5):976–982. [DOI] [PubMed] [Google Scholar]
- 7.Brandom BW, Colligan J, Brandom BW. Frequency of anesthesia-related complications in children with Down syndrome under general anesthesia for noncardiac procedures. Pediatr Anesth. 2004;14(1):733–738. doi: 10.1111/j.1460-9592.2004.01329.x [DOI] [PubMed] [Google Scholar]
- 8.Litman RS, Fields RG, Litman RS. Complications during rapid sequence induction of general anesthesia in children: a benchmark study. Pediatr Anesth. 2010;20(5):421–424. doi: 10.1111/j.1460-9592.2010.03287.x [DOI] [PubMed] [Google Scholar]
- 9.Hovgaard HL, Juhl-olsen P, Edelstein S. Suxamethonium-Induced Hyperkalemia: a Short Review of causes and recommendations for clinical applications. Crit Care Res Pract. 2021;2021:25. doi: 10.1155/2021/6613118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon CH, Kim S. Intraoperative management of critical arrhythmia. Korean J Anesthesiol. 2017;70(2):120–126. doi: 10.4097/kjae.2017.70.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fastle RK, Roback MG. Pediatric rapid sequence intubation: incidence of reflex bradycardia and effects of pretreatment with atropine. Pediatr Emerg Care. 2004;20(10):651–655. doi: 10.1097/01.pec.0000142947.35394.81 [DOI] [PubMed] [Google Scholar]
- 12.Associate JCF, Quezado ZMN. Hypothermia-induced bradycardia in a neonate receiving dexmedetomidine B. J Clin Anesth. 2007;02:290–292. [DOI] [PubMed] [Google Scholar]
- 13.Piotrowski AJ, Fendler WM. Hyperkalemia and cardiac arrest following succinylcholine administration in a 16-year-old boy with acute nonlymphoblastic leukemia and sepsis. Pediatr Crit Care Med. 2007;8(2):183–185. doi: 10.1097/01.PCC.0000257103.96579.B2 [DOI] [PubMed] [Google Scholar]
- 14.Jiang B, Yao L, Zhao H, Liang J, Feng Y. Low body weight predicted bradycardia and desaturation in retinopathy of prematurity surgeries: a retrospective cohort study. Front Pediatr. 2020;8(May):1–7. doi: 10.3389/fped.2020.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong M, Man Y, Fu Q. Incidence of bradycardia in pediatric patients receiving dexmedetomidine anesthesia: a meta-analysis. Int J Clin Pharm. 2017;39(1):139–147. doi: 10.1007/s11096-016-0411-5 [DOI] [PubMed] [Google Scholar]
- 16.Torres MAA, Braga JAP, Taddei JAAC, Nóbrega FJ. Anemia em lactentes de baixa renda em aleitamento materno exclusivo. J Pediatr. 2006;82(4):284–288. [Google Scholar]
- 17.Hemachitra J, Monisha A. Risk of infant anemia in 3–6 months old babies and its association with maternal anemia. Int J Contemp Pediatr. 2018;5(3):938. doi: 10.18203/2349-3291.ijcp20181517 [DOI] [Google Scholar]
- 18.Li H, Xiao J, Liao M, et al. Anemia prevalence, severity and associated factors among children aged 6–71 months in rural Hunan Province, China: a community-based cross-sectional study. BMC Public Health. 2020;20(1):1–13. doi: 10.1186/s12889-020-09129-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsson S, Asceirsson B, Magnusson J. Propofol-fentanyl anesthesia compared to thiopental-halothane with special reference to recovery and vomiting after pediatric strabismus surgery *. Acta Anaesthesiol Scand. 1992;36(2):182–186. doi: 10.1111/j.1399-6576.1992.tb03448.x [DOI] [PubMed] [Google Scholar]
- 20.Aida J, Oda Y, Kasagi Y, Ueda M, Nakada K, Okutani R. The management of difficult intubation in infants: a retrospective review of anesthesia record database. JA Clin Rep. 2015;1(1):10–13. doi: 10.1186/s40981-015-0020-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuluaga Giraldo M. Pediatric perioperative bleeding – basic considerations. Colomb J Anesthesiol. 2013;41(1):44–49. [Google Scholar]
- 22.Ebadi A, Saki N, Nikakhlagh S, Rahim F. Adenoidectomy-induced bradycardia in anesthetized children. Eur J Dent Med. 2009;1:8–15. [Google Scholar]
- 23.Green DH, Townsend P, Bagshaw O, Stokes MA. Nodal rhythm and bradycardia during inhalation induction with sevoflurane in infants: a comparison of incremental and high-concentration techniques. BJA. 2000;85(3):368–370. doi: 10.1093/bja/85.3.368 [DOI] [PubMed] [Google Scholar]
- 24.Kjeld T, Isbrand AB, Linnet K, et al. Extreme hypoxia causing brady-arrythmias during apnea in elite breath-hold divers. Front Physiol. 2021;12:1–12. doi: 10.3389/fphys.2021.712573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krantz MJ, Palmer RB, Haigney MCP. Cardiovascular complications of opioid use: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77(2):205–223. doi: 10.1016/j.jacc.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Davis JM, Shenberger J, Terrin N, et al. Comparison of safety and efficacy of methadone vs morphine for treatment of neonatal abstinence syndrome a randomized clinical trial. JAMA Pediatr. 2018;172(8):741–748. doi: 10.1001/jamapediatrics.2018.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davala S, Hansbury A, Miller M, Boateng J, Shrestha H, Wachman EM. Pilot study comparing adverse cardiorespiratory events among pharmacologically and nonpharmacologically treated infants undergoing monitoring for neonatal abstinence syndrome. J Pediatr X. 2020;3:100027. doi: 10.1016/j.ympdx.2020.100027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecturer WB, Msn TV, Specialist A. Hemodynamic changes in children with Down syndrome during and following inhalation induction of anesthesia with sevoflurane. J Clin Anesth. 2010;22(8):592–597. doi: 10.1016/j.jclinane.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 29.Disma N, Virag K, Riva T, et al. Difficult tracheal intubation in neonates and infants. NEonate and Children audiT of Anaesthesia pRactice IN Europe (NECTARINE): a prospective European multicentre observational study. Br J Anaesth. 2021;126(6):1173–1181. doi: 10.1016/j.bja.2021.02.021 [DOI] [PubMed] [Google Scholar]
- 30.Thomas I, Dixon J. Bradycardia in acute haemorrhage Case reports. BMJ. 2004;328(7437):451–453. doi: 10.1136/bmj.328.7437.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkman E, Watts S. Haemodynamic changes in trauma. Br J Anaesth. 2014;113(2):266–275. doi: 10.1093/bja/aeu232 [DOI] [PubMed] [Google Scholar]
- 32.Bell K, Elmograbi A, Smith A, Kaur J. Paradoxical bradycardia and hemorrhagic shock. Baylor Univ Med Cent Proc. 2019;32(2):240–241. doi: 10.1080/08998280.2018.1559386 [DOI] [PMC free article] [PubMed] [Google Scholar]