ABSTRACT
Introduction
Battlefield-related wound infections are a significant source of morbidity among combat casualties. Seasonality of these infections was demonstrated in previous conflicts (e.g., Korea) but has not been described with trauma-related health care–associated infections from the war in Afghanistan.
Methods
The study population included military personnel wounded in Afghanistan (2009-2014) medevac’d to Landstuhl Regional Medical Center and transitioned to participating military hospitals in the United States with clinical suspicion of wound infections and wound cultures collected ≤7 days post-injury. Analysis was limited to the first wound culture from individuals. Infecting isolates were collected from skin and soft-tissue infections, osteomyelitis, and burn soft-tissue infections. Data were analyzed by season (winter [ December 1-February 28/29], spring [March 1-May 31], summer [June 1-August 31], and fall [September 1-November 30]).
Results
Among 316 patients, 297 (94.0%) sustained blast injuries with a median injury severity score and days from injury to initial culture of 33 and 3.5, respectively. Although all patients had a clinical suspicion of a wound infection, a diagnosis was confirmed in 198 (63%) patients. Gram-negative bacilli (59.5% of 316) were more commonly isolated from wound cultures in summer (68.1%) and fall (67.1%) versus winter (43.9%) and spring (45.1%; P < .001). Multidrug-resistant (MDR) Gram-negative bacilli (21.8%) were more common in summer (21.8%) and fall (30.6%) versus winter (7.3%) and spring (19.7%; P = .028). Findings were similar for infecting Gram-negative bacilli (72.7% of 198)—summer (79.5%) and fall (83.6%; P = .001)—and infecting MDR Gram-negative bacilli (27.3% of 198)—summer (25.6%) and fall (41.8%; P = .015). Infecting anaerobes were more common in winter (40%) compared to fall (11%; P = .036). Gram-positive organisms were not significantly different by season.
Conclusion
Gram-negative bacilli, including infecting MDR Gram-negative bacilli, were more commonly recovered in summer/fall months from service members injured in Afghanistan. This may have implications for empiric antibiotic coverage during these months.
INTRODUCTION
Historically, infectious disease complications after battlefield wounds have been a significant cause of both morbidity and mortality among warfighters.1 With improved treatment, mortality rates associated with infected war wounds dropped from 1 in 25 during World War II to 1 in 100 during the Gulf War.1 The dramatic decline in mortality has been attributed to the evolving standard of battlefield war wound care including rapid administration of antibiotics, prompt surgical intervention, and decreased time from point of injury to definitive care. Those surviving previously fatal injuries frequently undergo prolonged hospitalizations with associated infectious complications.2 The vast majority of these infections tend to be health care–associated, with illnesses such as sepsis, pneumonia, and blood stream infections developing a median of 5-7 days post-injury and osteomyelitis occurring 26-28 days post-injury.3 Examination of an outbreak of multidrug-resistant (MDR) Acinetobacter baumannii–calcoaceticus complex infections among personnel wounded in Iraq determined that the source of infection was multifactorial with the primary source being health care–associated transmission of pathogens in field hospitals.4 Despite great strides in improving the treatment of war wounds, infectious complications remain a significant source of morbidity among service members and contribute to poor wound healing after amputation, as well as serve as a source for recurrent infections, osteomyelitis, and sepsis.5,6
To continue to improve outcomes among service members who suffer from war-related wounds, it is critical to understand and be able to predict the microbiology of war wounds and associated infections. One aspect in the development of effective empiric antibiotic regimens is the understanding of seasonality and its role in the microbiologic composition of war wounds.7,8 Since Alexander Fleming began identifying organisms present in war wounds in the pre-antibiotic era, seasonality of war wound microbiology has been of interest in every major conflict to include the World Wars, Korean, Vietnam, and Gulf War conflicts.1,9,10 Additionally, although the seasonality of colonizing and infecting pathogens has previously been described, this has yet to be discussed in relation to battlefield-related infections.11–16 With this in mind, we sought to evaluate the effect that seasonal factors play on the microbiology of wound cultures in service members injured in Afghanistan.
METHODS
Study Population and Data Sources and Definitions
Data were collected from the Department of Defense and Veterans Affairs (DoD-VA) multicenter Trauma Infectious Disease Outcomes Study (TIDOS), an observational study designed to examine infectious complications among wounded service members. Patients were included in TIDOS if they were 18 years or older, were active duty or DoD beneficiaries, and suffered a deployment-related injury requiring medical evacuation to Landstuhl Regional Medical Center (LRMC, Germany) with subsequent transfer to participating military hospitals in the United States (June 1, 2009-December 31, 2014). The participating U.S. military hospitals were Brooke Army Medical Center (BAMC) in San Antonio, TX, and Walter Reed National Military Medical Center in the National Capital Region (National Naval Medical Center and Walter Reed Army Medical Center before September 2011).17,18
To assess the seasonality of wound cultures and wound infections occurring during the early phase of trauma care, inclusion in this analysis required sustaining an injury in Afghanistan with clinical suspicion of infection, and wounds cultured ≤7 days post-injury with corresponding positive microbiologic results (Fig. 1). The population was restricted to early wound infections to focus on the downrange and early medevac wound infection risk, which was thought to most likely be impacted by seasonal variance. The first week of trauma care includes medevac to LRMC (a 2-3 day stay) followed by transition to a U.S. hospital.18 The study was approved by the Institutional Review Board at the Uniformed Services University (Bethesda, MD).
FIGURE 1.
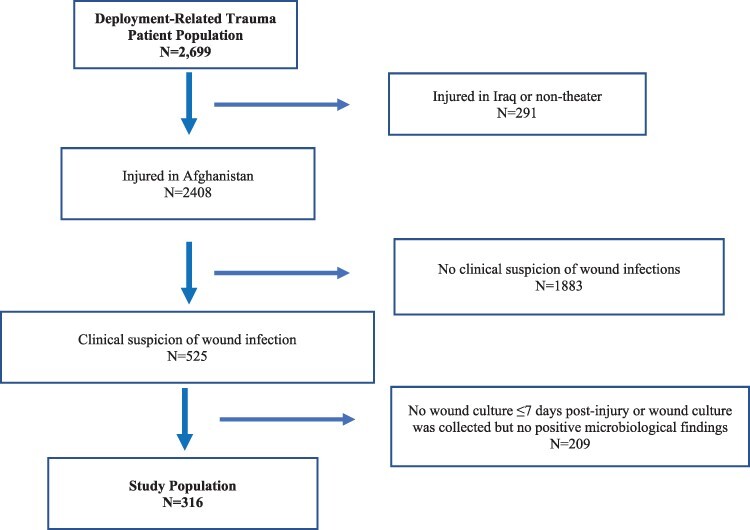
Study population derivation of patients who sustained deployment-related trauma in Afghanistan and clinical signs of wound infection. Study population is restricted to those with clinical suspicion of infection and wound cultures ≤7 days post-injury with corresponding microbiology. Following injury, the first week of trauma care includes medevac and a 2-3 day stay at Landstuhl Regional Medical Center followed by transition to a U.S. hospital.
Data on injury characteristics (e.g., severity and mechanism) were obtained from the DoD Trauma Registry (DoDTR), while information on infections and microbiology were collected from the TIDOS Infectious Disease Module of the DoDTR.19,20 Infections were based on clinical and laboratory findings and classified per standardized definitions of the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network (NHSN).21 Infections not meeting the a priori criteria were included when there was an indicated clinical diagnosis associated with ≥5 days of directed antimicrobial treatment (≥21 days for osteomyelitis). For this analysis, infections were restricted to wound infection (i.e., skin and soft-tissue infections [SSTIs], osteomyelitis, and burn SSTIs).
Infecting isolates were defined as isolates collected from osteomyelitis, superficial or deep wound SSTI, or burn SSTI, as previously defined by TIDOS.18,20 If more than one wound culture was collected, isolates recovered from the first culture were used in the analysis. If service members sustained more than one injury and with multiple cultures from different sites at different time points, only isolates recovered from the first initial culture from each injury site were included in the analysis. Isolates recovered from wound cultures that did not meet clinical diagnostic criteria for infection were classified as colonizing. Multidrug resistance for Gram-negative bacilli was defined as resistance to ≥3 classes of antibiotics (aminoglycosides, beta-lactams, carbapenems, and/or fluoroquinolones) or presence of an extended spectrum β-lactamase or Klebsiella pneumoniae carbapenemase production.22 Vancomycin-resistant Enterococcus spp. and methicillin-resistant Staphylococcus aureus (MRSA) were also considered to be MDR.22
Statistical Analysis
Clinical and microbiologic data were analyzed by season (winter [December 1-February 28/29], spring [March 1-May 31], summer [June 1-August 31], and fall [September 1-November 30]). Categorical variables were compared using chi-square tests. Continuous variables were compared using Mann–Whitney U tests. Statistical analysis was conducted using IBM SPSS Statistics for Mac, version 28.0.1.1 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as P < .05.
RESULTS
Population Characteristics
Among 2,699 patients included in the TIDOS population, 316 patients met criteria for inclusion in the analysis (Fig. 1). The study population was predominantly male (99.7%) with 297 (94.0%) military personnel injured because of blast-related injury mechanism. The patients generally were severely injured with a median injury severity score of 33 (interquartile range [IQR]: 27-43; Table I). The median days from injury to initial culture was 3.5 (IQR: 3-5 days). Among the 316 patients, 119 (37.6%), 85 (26.9%), 41 (13.0%), and 71 (22.5%) were injured during the summer, fall, winter, and spring seasons, respectively. Although all 316 patients had a clinical suspicion of a wound infection, 198 (62.7%) met the clinical criteria for a wound infection with 164 (83% of 198) having a wound/burn SSTI and 34 (17%) with osteomyelitis. The first wound culture with positive microbiology was collected at LRMC for 232 (73.4%) patients, while 71 (22.5%), 12 (4.0%), and 1 (0.03%) patients had their first positive wound culture collected at a facility in the National Capital Region, BAMC, and during transition between hospitals, respectively.
TABLE I.
Characteristics of Military Personnel Wounded in Afghanistan with Clinical Suspicion of a Wound Infection and Cultures with Positive Microbiologic Findings Collected within 7 Days Post-Injurya
| Characteristics, No. (%) | Patients (N = 316) |
|---|---|
| Male | 315 (99.7) |
| Blast mechanism | 297 (94.0) |
| Injured during | |
| Fall | 85 (26.9) |
| Summer | 119 (37.6) |
| Spring | 71 (22.5) |
| Winter | 41 (13.0) |
| Injury severity scoreb, median (IQR) | 33 (27-43) |
| Days from injury to initial culture, median (IQR) | 3.5 (3-5) |
| Met diagnostic criteria for Wound infections | 198 (62.7) |
| Burn or wound skin and soft-tissue infection | 164 (51.9) |
| Osteomyelitis | 34 (10.8) |
| Culture findingsc | |
| Gram-negative bacilli | 188 (59.5) |
| Gram-positive organisms | 186 (58.9) |
| MDR Gram-negative bacteriad | 69 (21.8) |
| Anaerobes | 41 (12.9) |
| Mixed culture findingse | 156 (49.4) |
IQR: interquartile range; MDR: multidrug-resistant.
Study population is focused on the first week of trauma care following injury, which includes medevac and a 2-3 day stay at Landstuhl Regional Medical Center followed by transition to a U.S. hospital.
See Linn (1995)32 for information on injury severity score.
Includes both colonizing and infection isolates recovered from wound cultures. Colonizing isolates were from wounds with clinical suspicion but did not meet diagnostic criteria for infection.
Multidrug-resistant bacteria primarily included Escherichia coli, Acinetobacter spp., and Enterobacter spp.
Includes a combination of Gram-negative bacilli, Gram-positive organisms, and/or anaerobes.
Microbiology
A total of 188 (59.5%) patients had Gram-negative bacilli isolated on wound cultures, while Gram-positive organisms were recovered from 186 (58.9%) patients and anaerobes were recovered from 41 (12.9%) patients (Table I). Sixty-nine (21.8%) patients had MDR bacteria isolated from their wound cultures, and 156 (49.4%) had mixed culture findings (e.g., combination of Gram-negative bacilli, Gram-positive organisms, and/or anaerobes).
Among the 198 patients who met clinical criteria for a wound infection, Gram-negative bacilli were recovered from wound cultures from 143 (72.7%) patients, Gram-positive organisms from 128 (64.6%) patients, and anaerobes from 30 (15.2%) patients (Table II). In addition, 54 (27.3%) patients had MDR Gram-negative bacteria associated with their wound infection. Ninety-five (48.0%) patients had infections with both Gram-negative bacilli and Gram-positive organisms.
TABLE II.
Results of Clinical Cultures Collected from Patients with Wound Infections within 7 Days Post-Injury Stratified by Season
| Patients with infecting isolates by Season | ||||||
|---|---|---|---|---|---|---|
| Organism, No. (%) | Patients with infecting isolatesa (n = 198) | Winter (n = 20) | Spring (n = 45) | Summer (n = 78) | Fall (n = 55) | P-value |
| Gram-negative | 143 (72.7) | 12 (60.0) | 23 (51.1) | 62 (79.5) | 46 (83.6) | 0.001 |
| MDR | 54 (27.3) | 2 (10.0) | 9 (20.0) | 20 (25.6) | 23 (41.8) | 0.015 |
| Escherichia coli | 52 (26.3) | 4 (20.0) | 10 (22.2) | 18 (23.1) | 20 (36.4) | 0.267 |
| MDR | 37 (18.7) | 2 (10.0) | 7 (15.6) | 13 (15.7) | 15 (27.3) | 0.245 |
| Pseudomonas spp. | 44 (22.2) | 4 (20.0) | 5 (11.1) | 19 (24.4) | 16 (29.1) | 0.144 |
| MDR | 11 (5.6) | 0 | 1 (2.2) | 4 (5.1) | 6 (10.9) | 0.160 |
| Acinetobacter baumannii | 29 (14.6) | 0 | 3 (6.7) | 16 (20.5) | 10 (18.2) | 0.009 |
| MDR | 16 (8.1) | 0 | 2 (4.4) | 7 (9.0) | 7 (12.7) | 0.237 |
| Enterobacter spp. | 52 (26.3) | 6 (30.0) | 8 (17.8) | 21 (26.9) | 17 (30.9) | 0.463 |
| MDR | 12 (6.1) | 0 | 3 (6.7) | 4 (5.1) | 5 (9.1) | 0.508 |
| Aeromonas spp. | 16 (8.1) | 0 | 1 (2.2) | 11 (14.1) | 4 (7.3) | 0.024 |
| MDR | 1 (0.5) | 0 | 0 | 0 | 1 (1.8) | – |
| Mixed Gram-negative | 12 (6.1) | 0 | 0 | 8 (10.3) | 4 (7.3) | 0.001 |
| Gram-positive | 128 (64.6) | 16 (80.0) | 32 (71.1) | 43 (55.1) | 37 (67.3) | 0.099 |
| Enterococcus spp. | 96 (48.5) | 13 (65.0) | 26 (57.8) | 32 (41.0) | 25 (45.5) | 0.126 |
| VRE | 9 (4.5) | 1 (5.0) | 3 (6.7) | 2 (2.6) | 3 (6.7) | 0.727 |
| Coagulase-negative Staphylococcus | 23 (11.6) | 2 (10.0) | 2 (4.4) | 10 (12.8) | 9 (16.4) | 0.249 |
| Bacillus spp. | 54 (27.3) | 6 (30.0) | 15 (33.3) | 20 (25.6) | 13 (23.6) | 0.716 |
| Mixed Gram-positive | 11 (5.6) | 3 (15.0) | 2 (4.4) | 3 (3.8) | 3 (6.7) | 0.903 |
| Both Gram-negative and Gram-positive | 95 (48.0) | 11 (55.0) | 19 (42.2) | 35 (44.9) | 30 (54.5) | 0.001 |
| Anaerobes | 30 (15.2) | 8 (40.0) | 6 (13.3) | 10 (12.8) | 6 (10.9) | 0.036 |
MDR: multidrug-resistant; VRE: vancomycin-resistant Enterococcus spp.
As patients may have more than one isolate recovered, the numbers will sum to more than the total number of patients.
Seasonality
Among the 316 patients with clinical suspicion of wound infections (Table I), there was a greater frequency of Gram-negatives being recovered from patients injured in the summer season (n = 81, 68.1% of 119 patients injured in summer) and fall (n = 57, 67.1% of 85) versus winter (n = 18, 43.9% of 41) and spring (n = 32, 45.1% of 71; P < .001). For the 69 patients with MDR Gram-negative bacteria isolated from their wound cultures, there was a similar distribution: summer (n = 26, 21.8% of 119) and fall (n = 26, 30.6% of 85) versus winter (n = 3, 7.3% of 41) and spring (n = 14, 19.7% of 71; P = .028). Gram-positive organisms were recovered from 60 (50.4% of 119) patients injured in the summer, 54 (63.5% of 85) patients injured in the fall, 26 (63.4% of 41) patients injured in the winter, and 46 patients injured in the spring (64.8% of 71) with no significant differences.
Among the 198 patients who met clinical criteria for a wound infection, 20 (10.1%) were injured during the winter season, 45 (22.7%) in the spring, 78 (39.4%) in the summer, and 55 (27.8%) in the fall (Table II). Approximately 73% of the patients with infections had Gram-negative bacilli isolated from their wound cultures with the cultures primarily identifying Escherichia coli (26% of 198), Enterobacter spp. (26%), and Pseudomonas spp. (22%). Other Gram-negative pathogens included A. baumannii and Aeromonas spp., which were isolated from 14.6% and 8.1% of patients, respectively. A seasonal variation was seen for the recovery of Gram-negative bacilli with 60.0% of patients injured in the winter season isolating Gram-negatives compared to 51.1% of those during spring, 79.5% during summer, and 83.6% during the fall (P < .001). Regarding specific pathogens, there was a seasonal variation with A. baumannii with greater recovery during the summer (20.5%) and fall (18.2%) compared to the winter (zero) and spring (6.7%; P = .009). No significant variation was seen for E. coli, Pseudomonas spp., Enterobacter spp., or Aeromonas spp. (Table II). Fifty-four (27%) patients had cultures identifying MDR Gram-negative bacilli and a similar seasonal variation was noted with a greater proportion in the summer (25.6%) and fall (41.8%) compared to the winter (10%) and spring (20%; P = .015). The majority of MDR Gram-negatives recovered were E. coli and A. baumannii with a trend toward greater recovery in the summer and fall; however, there was no significant seasonal variation for any of the individual MDR Gram-negatives. Specifically, among the 52 patients with cultures that grew E. coli, 37 (71%) had MDR E. coli. For the 29 patients who had cultures with A. baumannii, 16 (55%) were MDR. Twelve (6.1%) and 95 (48.0%) patients with infections had wound cultures that recovered multiple Gram-negative bacilli and both Gram-negative bacilli and Gram-positive organisms, respectively, with greater recovery in the summer and fall seasons (P = .001).
Gram-positives were isolated from 128 (65%) patients who met criteria for a wound infection (Table II), with Enterococcus spp. (48.5% of 198), Bacillus spp. (27.3%), and coagulase-negative Staphylococcus (11.6%) being the most common pathogens isolated. Nine patients had cultures that grew Enterococcus spp. isolates that were vancomycin-resistant, and there was no significant seasonal variation. Staphylococcus aureus was not isolated from the clinical cultures. Recovery of Gram-positives was comparable across the seasons with 80.0%, 71.1.%, 55.1%, and 67.3% of patients having Gram-positives identified from their wound cultures during the winter, spring, summer, and fall seasons, respectively. No statistically significant seasonal variation for Enterococcus spp., Bacillus spp., or coagulase-negative Staphylococcus was observed (Table II). Eleven (5.6%) patients had mixed Gram-positive organisms recovered from their cultures with no seasonal variation. Thirty (15.2%) patients with infections also isolated anaerobes in their wound cultures with a greater frequency of recovery during the winter (40.0%) compared to spring (13.3%), summer (12.8%), and fall (10.9%; P = .036).
DISCUSSION
Among service members admitted to military hospitals in the United States with wound cultures collected during the first week following deployment-related trauma, Gram-negatives, including MDR Gram-negative infecting organisms, mixed Gram-negative infections (multiple Gram-negative bacilli and combination of Gram-negative bacilli and Gram-positive organisms), were more common in the summer and fall months in service members injured in Afghanistan. Interestingly, although there were not any seasonal differences noted for most of the commonly isolated Gram-negative organisms, such as E. coli and Enterobacter spp., A. baumannii was recovered in a higher proportion of patients in the summer when compared to the fall, winter, and spring months.
Our data build on the earlier work of Weintrob et al.,23 which examined the seasonality of MDR Gram-negative bacilli colonization recovered from admission surveillance swabs among wounded military personnel included in TIDOS over a 3-year period. A significantly higher proportion of MDR Gram-negative colonization was identified among patients following admission to a hospital in the United States compared to at LRMC (12.4% versus 6.6%; P < .001), indicating the occurrence of health care–associated acquisition of MDR Gram-negative bacilli.23 The highest proportion of MDR Gram-negative bacilli colonization occurred in summer months (i.e., August), while the lowest proportions were in February and March. This earlier assessment provided important data on seasonal trends in MDR Gram-negative colonization; however, it did not examine seasonal trends for individual pathogens or trends related to wound infections. Thus, our current study fills an important gap in our understanding of the microbiology of war wounds from Afghanistan and could have implications for empiric antibiotic coverage during the various seasons of the year.
To continue to improve outcomes among service members who suffer from wound infections, it is critical to understand and predict the microbiology of war wounds, as well as the seasonality of health care–associated infections occurring among combat casualties transitioning through the combat casualty health care system. This process and the inherent challenges of understanding the answer to these questions date back to Alexander Fleming in the pre-antibiotic era. Analyses of wound infections in the First World War identified Gram-positive organisms Clostridium perfringens and Streptococcus pyogenes to be the primarily isolated pathogenic organisms.9,10 Similarly in the colder climate of the Korean War, wounds primarily were infected with Gram-positive organisms.10 These experiences led to early post-traumatic antibiotic prophylaxis recommendations covering commonly implicated Gram-positive organisms. However, during the Vietnam conflict, Heggers and colleagues hypothesized increasing rates of enteric Gram-negative wound infections due to antimicrobial pressure and the warm and wet climate.9 Unlike previous studies, the retrospective cohort analysis performed by Heggers and colleagues identified that the vast majority of extremity wound infections grew enteric Gram-negative pathogens. Additional work revealed that although S. aureus was the predominant microorganism found in initial wound cultures throughout the year in Vietnam, there was seasonal variation—with Staphylococcus epidermidis the most common organism in January, transitioning to Gram-negatives, E. coli and Pseudomonas aeruginosa in June and July, respectively.24 Nonetheless, the timing between injury and culture, prior antimicrobial exposures, mechanisms and severity of associated injuries, surgical debridement, and association with later infection were not detailed within this report. Our findings reported herein resolve some of those important gaps for the Afghanistan conflict by examining wound cultures collected during the first week of care post-injury rather than at the point of injury and, as such, provide information on infections that were likely health care–associated.
In the setting of increasing rates of Gram-negative and MDR wound infections noted in the recent conflicts in Iraq and Afghanistan, Murray et al. evaluated the microbiology of wounds at the time of injury during Operation Iraqi Freedom and noted that the majority of organisms were susceptible Gram-positives (although MRSA was noted rarely).25 Further studies related to MDR colonization26 and infections27 did show variation of MDR Gram-negative organism and MRSA recovery, which was not clearly seasonal but thought to potentially correlate with increased casualty rates and the strain on the medical evacuation chain, especially as related to infection control measures. However, the study evaluating MDR colonization was confined to a single center over a 3-year period when >95% of casualty admissions were injured in Iraq.26
It is important to note that combat casualty care has substantially advanced in recent conflicts. Compared to the Vietnam and Korean, conflict-injured personnel are now rapidly transitioned out of theater to LRMC (a 2-3 day stay) followed by transition to tertiary care centers in the United States for definitive care. This transition process was vastly expedited compared to that in the Vietnam and Korean conflicts.1,2,18 Nevertheless, when comparing our study’s results to those seen in historical conflicts, our data enhance lessons learned from previous conflicts. Unlike the predominant Gram-positive infecting isolates seen during the World Wars and in the Korean War, our results show higher rates of Gram-negative infecting isolates in summer months, similar to that seen in the Vietnam War.9,10 In addition, because of increasing rates of combat-related extremity injuries occurring secondary to improvised explosive devices, military personnel may be increasingly exposed to pelvic floor injuries, resulting in early enteric Gram-negative colonization and subsequent infections.28 These factors seem to be reflected in our data, given the majority (72.7%) of patients had cultures that yielded Gram-negative organisms. Similarly, in the Vietnam conflict, Gram-positive organisms, such as S. epidermidis, were found to be more frequently isolated in the winter months.24 Although our data did not reach statistical significance, they did suggest proportionally higher numbers of Gram-positive organisms during winter months than in summer.
Our study has limitations inherent to retrospective analyses. In addition, while assessing for seasonal variation of culture findings, potential confounding factors were not examined. This includes combat fighting seasons (i.e., high casualty numbers occurring April through September), ability for service members to bathe effectively, proximity to combat support hospitals, prior prophylactic antibiotic therapy, and specific type of blast-related injury mechanism, which have been proposed as potential factors for variance in microbiology of war wounds.27,29–31 Health care environmental factors related to the transition of pathogens through the combat casualty care pipeline may also impact the microbiologic makeup of infecting isolates but are yet to be studied. Although prospective studies would be ideal to assess this question, our results suggest that there may be a role to consider seasonal variation in the empiric treatment of war wounds, as well as methods to reduce risk for service member colonization.
The identification of environmental, injury mechanism, and antimicrobial resistance patterns is vitally important in ensuring appropriate empiric regimens for the treatment of wound infections. Our results show that there is seasonal variance of microbiologic makeup of wound infections during the Afghanistan conflicts. With seasonal variation of Gram-negative, anaerobic, and MDR organisms, empiric antibiotic regimens may miss more commonly isolated infecting organisms during various seasons throughout the year.
CONCLUSION
Gram-negative bacilli, including MDR Gram-negatives linked with infections, were more commonly recovered in summer/fall months from service members injured in Afghanistan. This may have implications for empiric antibiotic coverage during these months. In addition, these results show that environmental and climate factors can influence the microbiologic makeup of infecting wounds. Further studies exploring various aspects of environmental and climate impacts on wound microbiology are warranted.
ACKNOWLEDGMENTS
We are indebted to the TIDOS team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their contributions to the success of this project.
Contributor Information
Capt Matthew A Soderstrom, Infectious Disease Service, Brooke Army Medical Center, Joint Base San Antonio, Fort Sam Houston, TX 78234, USA.
Lt Col Dana M Blyth, Infectious Disease Service, Walter Reed National Military Medical Center, Bethesda, MD 20889, USA.
M Leigh Carson, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817, USA.
CDR Wesley R Campbell, Infectious Disease Service, Walter Reed National Military Medical Center, Bethesda, MD 20889, USA.
Maj Joseph M Yabes, Infectious Disease Service, Brooke Army Medical Center, Joint Base San Antonio, Fort Sam Houston, TX 78234, USA.
Faraz Shaikh, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817, USA.
Laveta Stewart, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817, USA.
David R Tribble, Infectious Disease Clinical Research Program, Department of Preventive Medicine and Biostatistics, Uniformed Services University of the Health Sciences, Bethesda, MD 20814, USA.
BG Clinton K Murray, Infectious Disease Service, Brooke Army Medical Center, Joint Base San Antonio, Fort Sam Houston, TX 78234, USA.
MAJ John L Kiley, Infectious Disease Service, Brooke Army Medical Center, Joint Base San Antonio, Fort Sam Houston, TX 78234, USA.
SUPPLEMENT SPONSORSHIP
This article appears as part of the supplement “Proceedings of the 2022 Military Health System Research Symposium,” sponsored by the Assistant Secretary of Defense for Health Affairs.
FUNDING
This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072; the Defense Health Program, U.S. DoD, under award HU0001190002; and the Department of the Navy under the Wounded, Ill, and Injured Program and the Military Infectious Disease Research Program.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author.
Institutional Review Board (HUMAN SUBJECTS)
Approved by Uniformed Services University (Bethesda, MD).
INSTITUTIONAL CLEARANCE
Approved by Brooke Army Medical Center, Walter Reed National Military Medical Center, the Uniformed Services University of the Health Sciences, and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc.
INDIVIDUAL AUTHOR CONTRIBUTIONS
Drs Soderstrom, Blyth, Campbell, Yabes, Stewart, Tribble, Murray, and Kiley, Ms Carson, and Mr Shaikh contributed to concept development, study design, and data analysis/interpretation. Drs Soderstrom and Kiley prepared the manuscript draft, and all authors reviewed and approved the manuscript.
REFERENCES
- 1. Murray CK, Hinkle MK, Yun HC: History of infections associated with combat-related injuries. J Trauma 2008; 64(3 Suppl): S221–3.doi: 10.1097/TA.0b013e318163c40. [DOI] [PubMed] [Google Scholar]
- 2. Blyth DM, Yun HC, Tribble DR, Murray CK: Lessons of war: Combat-related injury infections during the Vietnam War and Operation Iraqi and Enduring Freedom. J Trauma Acute Care Surg 2015; 79(4 Suppl 2 Proceedings of 2014 Military Health System Research Symposium): S227–35.doi: 10.1097/TA.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tribble DR, Li P, Warkentien TE, et al. : Impact of operational theater on combat and noncombat trauma-related infections. Mil Med 2016; 181(10): 1258–68.doi: 10.7205/MILMED-D-15-00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott P, Deye G, Srinivasan A, et al. : An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis 2007; 44(12): 1577–84.doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 5. Murray CK: Infectious disease complications of combat-related injuries. Crit Care Med 2008; 36(7 Suppl): S358–64.doi: 10.1097/CCM.0b013e31817e2ffc. [DOI] [PubMed] [Google Scholar]
- 6. McDonald JR, Liang SY, Li P, et al. : Infectious complications after deployment trauma: following wounded United States military personnel into Veterans Affairs care. Clin Infect Dis 2018; 67(8): 1205–12.doi: 10.1093/cid/ciy280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eardley WG, Brown KV, Bonner TJ, Green AD, Clasper JC: Infection in conflict wounded. Philos Trans R Soc Lond B Biol Sci 2011; 366(1562): 204–18.doi: 10.1098/rstb.2010.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hutley EJ, Green AD: Infection in wounds of conflict—old lessons and new challenges. J R Army Med Corps 2009; 155(4): 315–9.doi: 10.1136/jramc-155-04-14. [DOI] [PubMed] [Google Scholar]
- 9. Heggers JP, Barnes ST, Robson MC, Ristroph JD, Omer GE Jr.: Microbial flora of orthopaedic war wounds. Mil Med 1969; 134(8): 602–3.doi: 10.1093/milmed/134.8.602. [DOI] [PubMed] [Google Scholar]
- 10. Altemeier WA: Treatment of wounds of violence. Postgrad Med 1954; 16(2): 111–6.doi: 10.1080/00325481.1954.11712291. [DOI] [PubMed] [Google Scholar]
- 11. Kritsotakis EI, Groves-Kozhageldiyeva A: A systematic review of the global seasonality of infections caused by Acinetobacter species in hospitalized patients. Clin Microbiol Infect 2020; 26(5): 553–62.doi: 10.1016/j.cmi.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 12. Blot K, Hammami N, Blot S, Vogelaers D, Lambert ML: Seasonal variation of hospital-acquired bloodstream infections: A national cohort study. Infect Control Hosp Epidemiol 2022; 43(2): 205–11.doi: 10.1017/ice.2021.85. [DOI] [PubMed] [Google Scholar]
- 13. Freeman J, Anderson D, Sexton DJ: Emerging evidence for seasonality of gram-negative bacterial infections. Infect Control Hosp Epidemiol 2009; 30(8): 813–4.doi: 10.1086/597506. [DOI] [PubMed] [Google Scholar]
- 14. Leekha S, Diekema DJ, Perencevich EN: Seasonality of staphylococcal infections. Clin Microbiol Infect 2012; 18(10): 927–33.doi: 10.1111/j.1469-0691.2012.03955.x. [DOI] [PubMed] [Google Scholar]
- 15. Richet H: Seasonality in Gram-negative and healthcare-associated infections. Clin Microbiol Infect 2012; 18(10): 934–40.doi: 10.1111/j.1469-0691.2012.03954.x. [DOI] [PubMed] [Google Scholar]
- 16. Sagi HC, Cooper S, Donahue D, Marberry S, Steverson B: Seasonal variations in posttraumatic wound infections after open extremity fractures. J Trauma Acute Care Surg 2015; 79(6): 1073–8.doi: 10.1097/TA.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 17. Tribble DR, Conger NG, Fraser S, et al. : Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 2011; 71(1 Suppl): S33–42.doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tribble DR, Murray CK, Lloyd BA, et al. : After the battlefield: Infectious complications among wounded warriors in the Trauma Infectious Disease Outcomes Study. Mil Med 2019; 184(Suppl 2): 18–25.doi: 10.1093/milmed/usz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB: Trauma system development in a theater of war: Experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2006; 61(6): 1366–72.doi: 10.1097/01.ta.0000245894.78941.90. [DOI] [PubMed] [Google Scholar]
- 20. Tribble DR, Spott MA, Shackleford SA, Gurney JM, Murray CK: Department of Defense Trauma Registry Infectious Disease Module impact on clinical practice. Mil Med 2022; 187(Supplement_2): 7–16.doi: 10.1093/milmed/usac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention : CDC/NHSN surveillance definitions for specific types of infections. 2022. Available at http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf; accessed November 21, 2022.
- 22. Division of Healthcare Quality Promotion : The National Healthcare Safety Network (NHSN) Manual (Patient Safety Component)-Protocol: multidrug-resistant organism (MDRO) and Clostridioides difficile infection (MDRO/CDI) module. 2023. Available at https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf; accessed February 27, 2023.
- 23. Weintrob AC, Murray CK, Lloyd B, et al. : Active surveillance for asymptomatic colonization with multidrug-resistant Gram negative bacilli among injured service members - a three year evaluation. Msmr 2013; 20(8): 17–22. [PMC free article] [PubMed] [Google Scholar]
- 24. Matsumoto T, Wyte SR, Moseley RV, Hawley RJ, Lackey GR: Combat surgery in communication zone. I. War wound and bacteriology (preliminary report). Mil Med 1969; 134(9): 655–65.doi: 10.1093/milmed/134.9.655. [DOI] [PubMed] [Google Scholar]
- 25. Murray CK, Roop SA, Hospenthal DR, et al. : Bacteriology of war wounds at the time of injury. Mil Med 2006; 171(9): 826–9.doi: 10.7205/MILMED.171.9.826. [DOI] [PubMed] [Google Scholar]
- 26. Murray CK, Yun HC, Griffith ME, et al. : Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Mil Med 2009; 174(6): 598–604.doi: 10.7205/MILMED-D-03-8008. [DOI] [PubMed] [Google Scholar]
- 27. Campbell WR, Li P, Whitman TJ, et al. : Multi-drug–resistant Gram-negative infections in deployment-related trauma patients. Surg Infect (Larchmt) 2017; 18(3): 357–67.doi: 10.1089/sur.2017.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart L, Shaikh F, Bradley W, et al. : Combat-related extremity wounds: injury factors predicting early onset infections. Mil Med 2019; 184(Suppl 1): 83–91.doi: 10.1093/milmed/usy336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE: The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg 2011; 253(6): 1184–9.doi: 10.1097/SLA.0b013e31820752e3. [DOI] [PubMed] [Google Scholar]
- 30. Tribble DR, Rodriguez CJ, Weintrob AC, et al. : Environmental factors related to fungal wound contamination after combat trauma in Afghanistan, 2009-2011. Emerg Infect Dis 2015; 21(10): 1759–69.doi: 10.3201/eid2110.141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilbert LJ, Li P, Murray CK, et al. : Multidrug-resistant gram-negative bacilli colonization risk factors among trauma patients. Diagn Microbiol Infect Dis 2016; 84(4): 358–60.doi: 10.1016/j.diagmicrobio.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linn S: The injury severity score: Importance and uses. Ann Epidemiol 1995; 5(6): 440–6.doi: 10.1016/1047-2797(95)00059-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


