ABSTRACT
Introduction
Negative pressure wound therapy (NPWT) is utilized early after soft tissue injury to promote tissue granulation and wound contraction. Early post-injury transfers via aeromedical evacuation (AE) to definitive care centers may actually induce wound bacterial proliferation. However, the effectiveness of NPWT or instillation NPWT in limiting bacterial proliferation during post-injury AE has not been studied. We hypothesized that instillation NPWT during simulated AE would decrease bacterial colonization within simple and complex soft tissue wounds.
Methods
The porcine models were anesthetized before any experiments. For the simple tissue wound model, two 4-cm dorsal wounds were created in 34.9 ± 0.6 kg pigs and were inoculated with Acinetobacter baumannii (AB) or Staphylococcus aureus 24 hours before a 4-hour simulated AE or ground control. During AE, animals were randomized to one of the five groups: wet-to-dry (WTD) dressing, NPWT, instillation NPWT with normal saline (NS-NPWT), instillation NPWT with Normosol-R® (NM-NPWT), and RX-4-NPWT with the RX-4 system. For the complex musculoskeletal wound, hind-limb wounds in the skin, subcutaneous tissue, peroneus tertius muscle, and tibia were created and inoculated with AB 24 hours before simulated AE with WTD or RX-4-NPWT dressings. Blood samples were collected at baseline, pre-flight, and 72 hours post-flight for inflammatory cytokines interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor alpha. Wound biopsies were obtained at 24 hours and 72 hours post-flight, and the bacteria were quantified. Vital signs were measured continuously during simulated AE and at each wound reassessment.
Results
No significant differences in hemodynamics or serum cytokines were noted between ground or simulated flight groups or over time in either wound model. Simulated AE alone did not affect bacterial proliferation compared to ground controls. The simple tissue wound arm demonstrated a significant decrease in Staphylococcus aureus and AB colony-forming units at 72 hours after simulated AE using RX-4-NPWT. NS-NPWT during AE more effectively prevented bacterial proliferation than the WTD dressing. There was no difference in colony-forming units among the various treatment groups at the ground level.
Conclusion
The hypoxic, hypobaric environment of AE did not independently affect the bacterial growth after simple tissue wound or complex musculoskeletal wound. RX-4-NPWT provided the most effective bacterial reduction following simulated AE, followed by NS-NPWT. Future research will be necessary to determine ideal instillation fluids, negative pressure settings, and dressing change frequency before and during AE.
INTRODUCTION
Complex musculoskeletal wounds (CMWs) comprised up to 50% of all injuries incurred during the Global War on Terror.1 Just over 52,000 veterans were wounded in action during Operation Enduring Freedom and Operation Iraqi Freedom contributing heavily to U.S. health care and personal functional costs.2 Although advances in protective equipment and earlier operative intervention have decreased mortality and limb amputation rates, extremity wounds continue to cause significant morbidity due to the high rate of infection observed in CMW upon arrival at tertiary care centers.3 The most commonly isolated bacteria from CMW during recent military conflicts include Acinetobacter baumannii(AB) and Staphylococcus aureus (SA).3–9 Following complex musculoskeletal injury, military personnel are stabilized by a forward surgical team and treated in the combat theater for the first 24 to 48 hours. Patients then undergo transport via the aeromedical evacuation (AE) system with cabins pressurized to 8,000 ft.10,11 A previous study suggested that exposure to a hypoxic, hypobaric environment during this transport increases bacterial burden within CMW.12
Negative pressure wound therapy (NPWT), or vacuum-assisted wound closure, is the use of subatmospheric pressure over a polyurethane sponge within a wound bed to promote coaptation of wound edges, tissue granulation, angiogenesis, and clearance of edematous fluid and exudate including inflammatory cytokines.13 This type of wound dressing is ideal for use in austere environments with limited resources and personnel as it allows for less frequent dressing changes and protects the wound from additional contamination while preventing wound desiccation. Prior studies have shown that NPWT is effective in reducing bacterial loads within CMW acquired in combat.14–18 However, there have been limited studies evaluating the effectiveness of NPWT during AE. Retrospective reviews during Operation Iraqi Freedom and Operation Enduring Freedom have shown that NPWT is safe during flight with minimal complications.19–21 These reports focused primarily on equipment performance and malfunction but did not address the effectiveness of NPWT in reducing bacterial load and promoting wound healing during and after flight to prevent wound deterioration from the AE process.
Furthermore, early irrigation with large amounts of sterile saline or potable water may reduce bacterial burden within combat wounds.22,23 Negative pressure wound therapy with instillation is a newer method of wound management that dwells a programmed volume of fluid within the wound for a set amount of time at predetermined intervals. This technique can decrease bacterial growth rates within soft tissue wounds over time compared to simple wet-to-dry (WTD) dressing or standard NPWT.24,25 Similar to instillation NPWT, continuous saline irrigation with NPWT aids wound healing and bioburden reduction.26 The purpose of this study was to evaluate whether NPWT strategies are as effective as WTD dressing in reducing bacterial load within a soft tissue wound or CMW during the hypoxic, hypobaric conditions associated with AE. Our hypothesis was that the instillation of normal saline combined with NPWT during simulated flight would result in a decreased bacterial burden within the wound post-flight.
METHODS
Animal Model
This study was reviewed and approved by the University of Cincinnati Institutional Animal Care and Use Committee and by the U.S. Air Force Medical Support Agency Office of Research Oversight and Compliance. Animals were cared for by a program approved by the Association for Assessment and Accreditation of Laboratory Animal Care International and in compliance with the National Research Council’s 2011 Guide for the Care and Use of Laboratory Animals as well as the DoD Instruction 3216.01. Forty-seven female Yorkshire pigs weighing 34.9 ± 0.6 kg were obtained from Isler Genetics (Prospect, OH) and acclimated for 48 to 72 hours before experimentation. Animals were housed alone or in pairs and provided with food and water without restriction, except for the night before study initiation to prevent aspiration during induction of anesthesia. Pigs were sedated with tiletamine hydrochloride (Telazol) and xylazine hydrochloride (each 5 mg/kg administered intramuscularly; Henry Schein Animal Health, Dublin, OH). Sedated pigs were placed in a supine position and orotracheally intubated, then maintained on a ventilator (Ohmeda, Madison, WI) in pressure control mode during non-altitude portions of the experiment, and then transferred to an Impact 731 Series ventilator (IMPACT Instrumentation, West Caldwell, NJ) for simulated AE due to approved performance at altitude for ventilation.
Bacterial Inoculation
Clinical strains of AB and SA were acquired from collaborators at Shriners Children’s Hospital (Cincinnati, OH). These samples were aliquoted into 200 μL portions and stored at −80 °C. Four days before inoculation, the AB samples were thawed and mixed with 5 mL of tryptic soy agar broth (Thermo Fisher Scientific, Waltham, MA). Staphylococcus aureus samples were thawed and mixed with 5 mL of Luria-Bertani broth (Thermo Fisher Scientific, Waltham, MA). Stock cultures were grown for approximately 92 hours in a 37 °C incubator at which time a dilution of 108 colony-forming units (CFUs)/mL was obtained and confirmed via spectrometry.
Porcine Simple Tissue Wound Model
Swine were anesthetized before inducing wound models. Swine were assigned into one of the two wound model arms: simple tissue wound (n = 37) or CMW (n = 10). These two arms were included to determine if the depth and severity of injury would affect the bacterial clearance ability of each dressing type. Animals in the simple tissue wound arm had a ground (n = 12) and flight (n = 25) component, whereas those in the CMW arm only underwent flight. Ground controls were compared to respective flight groups to determine the effect of altitude on bacterial clearance as a secondary objective. Pigs within the simple tissue wound group underwent creation of a dorsal soft tissue wound on the cephalad portion of the torso as depicted in Figure 1A.12 Sharp dissection was utilized to create two 4 × 4-cm soft tissue defects to the level of the fascia with a connecting bridge between to facilitate NPWT placement. After hemostasis was achieved, 200 μL of 108 CFUs/mL AB was inoculated into one side of the wound and 200 μL of 108 CFUs/mL SA was inoculated into the other side of the wound (Fig. 1A). The wound was then dressed in a WTD dressing for the first 24 hours post-surgery to simulate a point-of-injury battlefield dressing.
FIGURE 1.
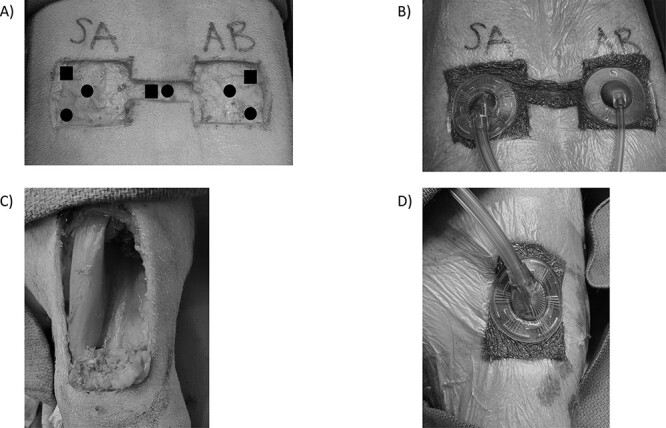
(A) Soft tissue wounds with overlying diagram showing biopsy locations at 24 and 72 hours after simulated AE. (B) Soft tissue wounds with negative pressure wound therapy in place. (C) A complex musculoskeletal wound on the left anterior hind leg demonstrating removal of a portion of the peroneus tertius muscle. (D) NPWT is applied to the complex musculoskeletal wound with black sponge.
Porcine CMW Model
Swine in the complex wound arm (n = 10) underwent an extremity injury model adapted from the U.S. Army Institute of Surgical Research.22,27 Briefly, the left hind leg was shaved and prepped. A 5 × 2-cm area of skin and fascia were removed using sharp dissection exposing the peroneus tertius muscle. Crush injury was induced by clamping the exposed peroneus tertius muscle with two Kelly clamps for 1-minute duration. A 4 × 2-cm portion of the peroneus tertius muscle was then removed using electrocautery (Fig. 1C). Kerrison rongeurs were utilized to create a superficial 1 × 1-cm defect in the periosteum and anterior cortex of the mid-tibia to allow for muscle and bone injury without inhibiting independent post-injury ambulation. After hemostasis was achieved, 200 μL of 108 CFUs/mL AB was distributed within the wound and a WTD dressing was applied. Acinetobacter baumannii was selected based on its higher prevalence in recent combat wounds and based on the initial data from the simple tissue wound arm of the study.6–8
Wound Treatment Groups
Three different vacuum-assisted closure (VAC) systems were utilized within this study: VAC ULTA, VAC VERAFLO, and VAC RX-4 (each from Kinetic Concepts Inc., St. Paul, MN). Swine within the simple tissue wound arm (n = 37) were separated into five flight groups (n = 5) and four ground groups (n = 3): WTD dressing, VAC ULTA (NPWT), VAC RX-4 NPWT (RX-4-NPWT) (flight only), VAC VERAFLO instillation NPWT with either normal saline (NS-NPWT) or Normosol-R® (NM-NPWT) (Fig. 2A). The RX-4-NPWT group was added later in the study due to the observation that standard wound vacuum machines had trouble maintaining −125 mmHg during flight. This group was not included in the ground controls based on preliminary data lacking significant differences in bacterial levels between respective flight and ground dressing types and based on the principle of minimization of animal utilization when possible (Figs. 3, 4). Only control WTD (n = 5) and RX-4-NPWT (n = 5) were tested in complex wounds based on analysis of the simple wound model, revealing RX-4-NPWT to be the most effective dressing type for bacterial reduction compared to WTD dressing (72 hours post-flight AB: WTD 9.0 × 106 vs. RX-4-NPWT 4.93 × 1010, P = .0004; SA: WTD 3 × 106 vs. RX-4-NPWT 4.86 × 109, P = .0002). No ground controls were completed for CMW based on the lack of bacterial differences between flight and ground groups in the simple tissue wound arm and to minimize overall animal utilization (Figs. 3, 4). Pigs within the NPWT, NS-NPWT, NM-NPWT, and RX-4-NPWT groups each had pre-cut black sponge pieces placed within the wound and covered with sterile plastic drapes (Fig. 1B). Negative pressure was set to −125 mmHg as this is the most commonly used setting in military applications. NPWT was initiated just before simulated flight to ensure adequate seal to the device across each wound. Pigs within the NS-NPWT and NM-NPWT groups underwent instillation of 36 mL of the designated fluid with 10 minutes of dwell time for each hour of vacuum therapy. Following simulated AE, swine were recovered and monitored for 72 hours before euthanasia. During this time, pigs within the WTD dressing groups underwent dressing change at 24 hours. Pigs in the NPWT, NS-NPWT, NM-NPWT, and RX-4-NPWT groups were maintained in a NPWT system using a black sponge with a VAC PREVENA PLUS (Kinetic Concepts Inc., St. Paul, MN) suction device that was secured to the dorsum with a mesh vest, tape, and staples.
FIGURE 2.
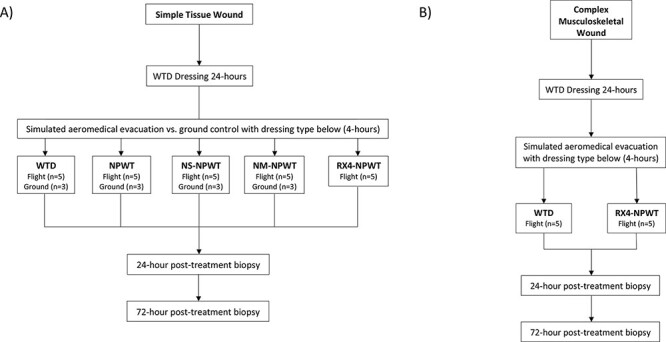
(A) A flowchart demonstrating treatment groups for the simple tissue wound arm. (B) A flowchart demonstrating treatment groups for the complex musculoskeletal wound arm.
FIGURE 3.
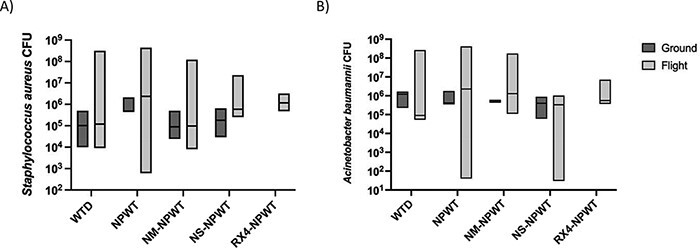
(A) A box-and-whisker plot demonstrating no significant differences in Staphylococcus aureus CFUs between treatment groups in flight at 24 hours post–simulated aeromedical evacuation/ground control in simple tissue wounds. (B) A box-and-whisker plot demonstrating no significant differences in Acinetobacter baumannii CFUs between treatment groups in flight at 24 hours post–simulated aeromedical evacuation/ground control in simple tissue wounds (median ± interquartile range). There were no differences noted between flight and ground groups, or ground groups compared to one another, although ground groups were not powered to detect statistical differences.
FIGURE 4.
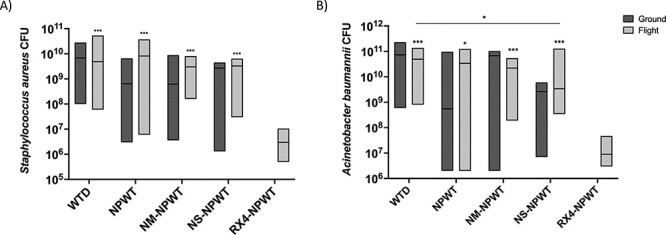
A box-and-whisker plot demonstrating significant differences in (A) Staphylococcus aureus and (B) Acinetobacter baumannii CFUs between RX-4-NPWT–treated simple tissue wounds and all other wound management groups at 72 hours post–simulated flight (* above each group denotes significant differences between that group and RX-4-NPWT). There is also a significant difference in A. baumannii CFUs between WTD dressing and NS-NPWT at 72 hours post-flight in simple tissue wounds (median ± interquartile range). There were no differences noted between flight and ground groups, or ground groups compared to one another, although ground groups were not powered to detect statistical differences. * P < .05; ** P < .01; and *** P < .001.
An altitude chamber (Abbess Instruments, Ashland, MA) was utilized to simulate AE to an altitude of 8,000 ft for 4 hours.28 Ground control animals were placed within the altitude chamber without a change in altitude. Heart rate, respiratory rate, systolic arterial pressure, and oxygen saturation (SpO2) were monitored continuously and noninvasively throughout simulated AE. Swine in simulated AE were maintained at an SpO2 of 82-85% to simulate a hypoxic environment consistent with an altitude of 8,000 feet.10 The study conformed with the ARRIVE guidelines (SDC-1). No pigs were excluded from the study or the analyses. Swine were randomized to either ground or flight treatment arms. The sample size was determined using the primary outcome of measure being bacterial counts in the wound based on our previous investigation of wounds after altitude exposure. The primary outcome of this study was wound bacteria quantity at 72 hours after simulated flight.
Sample Size and Power
This study was designed for 80% power and an alpha of 0.05. Using previously published data,12 we estimated the ratio of counts between WTD dressing–treated and NPWT-treated wounds would be no more than 50%, with a 33.3% coefficient of variation, and assumed that bacterial counts were lognormally distributed. Thus, for a one-sided test, a minimum sample size of eight animals per comparison (both groups) was established. We included five pigs in each of the flight treatment groups and three pigs in each of the ground treated groups as the intent of the study was primarily to compare wound-dressing groups with simulated altitude exposure. Study personnel were not blinded to treatment groups during the study or during the analysis period.
Bacterial Quantification
Bacteria were quantified via previously described methods.14,29 Briefly, tissue samples were collected from each wound at 24 hours and 72 hours post-flight. Biopsies were obtained using a 6 × 5-mm punch biopsy (Integra LifeSciences, Princeton, NJ). Samples were obtained from the lateral inferior aspect of the simple tissue wound at 24 hours and subsequently from the lateral superior aspect and center of the wound at 72 hours (Fig. 1A). Samples were placed in 2 mL of Dulbecco’s phosphate-buffered saline (Fisher Scientific, Hampton, NH), homogenized, and then serially diluted before being plated on trypticase soy agar plates (Fisher Scientific, Hampton, NH). Plates from 24-hour samples were incubated at 37 °C for 24 hours before the quantification of CFUs. Plates from 72-hour samples were incubated at 37 °C for 72 hours before the quantification of CFUs. Bacterial counts from the side and center biopsy locations at 72 hours were averaged. Swine were euthanized on post-injury day 4.
Serum Analysis
Blood samples were collected before surgery, before simulated AE, and at 72 hours post-AE. Whole blood was placed in serum separator tubes (BD Bioscience, San Diego, CA) and centrifuged at 1,000 g for 10 min. Serum was collected and subsequently analyzed for pro-inflammatory cytokines interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) using a Qplex Porcine Chemokine High Sensitivity enzyme-linked immunosorbent assay according to the manufacturer protocol (Quansys Biosciences, Logan, UT).
Statistical Analysis
JMP Pro 16 (JMP, Cary, NC) was used for all statistical analyses except the power analysis, which used SAS (Cary, NC.) Prism 6 (GraphPad Software, La Jolla, California) was utilized to produce graphical figures. Data are presented as the median and interquartile range. Two-sided pairwise Wilcoxon rank sum tests were used to compare group culture results, and two-way analysis of variance was utilized to compare serum cytokines; the results of a Shapiro–Wilk normality test demonstrated that culture data were not normally distributed and serum cytokine results were normally distributed. A P value ≤ .05 was considered significant. The statistical power was set at 0.80.
RESULTS
Systemic Inflammatory Response
There were no significant differences in the heart rate or temperature among any of the simple or complex wound groups. No differences in serum IL-1β, IL-6, IL-8, or TNF-α were noted between any of the groups whether at ground or during simulated AE (SDC-2). There were also no differences within each treatment group over time during the study, suggesting a lack of systemic response to the bacterial colonization of the wounds.
Ground Controls
There were no significant differences in SA or AB quantification at 24 hours among the various wound management techniques at ground (Fig. 3A, B). There were also no differences in SA or AB bacterial load at 24 hours between flight animals and ground controls for each specific treatment modality (Fig. 3A, B).
There were no differences in SA or AB colonization in the simple tissue wound model at 72 hours among ground treated groups (Fig. 4A, B). When comparing each wound management strategy at the ground level vs. simulated AE, there were no differences in SA or AB bacterial counts at 72 hours post-flight (Fig. 4A, B).
Simulated AE
There were no significant differences in SA or AB quantification at 24 hours between the various wound management techniques during simulated flight (Fig. 3A, B). The simple tissue wound arm demonstrated a decrease in both AB and SA CFUs at 72 hours after simulated AE for flight RX-4-NPWT–treated pigs compared to each of the following groups: WTD, NPWT, NS-NPWT, and NM-NPWT (Fig. 4A, B). There was also a decrease in AB bacterial counts at 72 hours post-flight in NS-NPWT–treated animals compared to WTD dressing–treated animals (Fig. 4B). By contrast, there were no significant differences in AB bacterial load at 24 or 72 hours after simulated flight between WTD dressing–treated and RX-4-NPWT–treated complex wounds. Animals treated with WTD dressing following CMW had 1.71 × 106 CFUs (7.25 x 105, 6.20 × 106), whereas those treated with RX-4-NPWT had 1.08 × 106 CFUs (2.92 x 105, 3.57 × 106) at 24 hours after simulated flight. At 72 hours post-AE, animals in the CMW arm treated with WTD dressing had 4.00 × 106 CFUs (2.15 x 106, 1.61 × 1010), whereas those treated with RX-4-NPWT had 4.30 × 106 CFUs (4.88 x 105, 6.00 × 106).
DISCUSSION
In the present study, we examined the effect of simulated AE and NPWT on bacterial colonization within simple and complex soft tissue wounds. We found that there were no significant differences in SA or AB clearance between ground and flight wounds at 24 hours following simulated AE regardless of wound management treatment strategies. Both SA and AB were significantly reduced during flight when utilizing the RX-4-NPWT system compared to other wound management strategies. These data demonstrated that use of the RX-4-NPWT system is more effective than other treatment modalities at reducing bacterial load during simulated AE. Furthermore, NPWT with NS instillation was more effective than WTD dressings at reducing AB bacterial load by 72 hours after simulated AE.
Previous work from our group has demonstrated an increase in bacterial growth during the hypoxic, hypobaric environment innate to AE.12 By contrast, the current study failed to demonstrate similar bacterial growth during AE compared to ground controls. This may be due to the difference in bacteria utilized in the study, as the current study utilized AB and SA, whereas the previous study inoculated Pseudomonas aeruginosa in a complex wound. Staphylococcus aureus is a facultative anaerobe, whereas AB and Pseudomonas spp. are obligate aerobes. Therefore, the hypoxic environment during simulated AE may be better tolerated by SA than the other two types of bacteria. The present study demonstrates reduced bacterial colonization in both SA and AB when using RX-4-NPWT during flight. Other wound management techniques failed to reduce SA or AB CFUs at ground or post-flight. Lalliss et al. have previously demonstrated no change in SA bacterial load with NPWT, whereas Pseudomonas aeruginosa bacterial counts decreased.30 Mouës et al. performed a randomized clinical trial evaluating bacterial clearance with WTD dressings vs. NPWT, which actually revealed an increase in gram-positive bacteria when using NPWT31; this may suggest that anaerobic bacteria retain the ability to proliferate even in an NPWT dressing better than aerobic bacteria. Our previous study also utilized a caprine model instead of a porcine model, which may have contributed to differences in the bacterial growth within the wound.
Since the initial publications in 1997, NPWT has been considered to reduce bioburden within wounds.32 However, multiple studies since that time have shown no change or an increase in bacterial counts within wounds treated with NPWT.24,31,33–36 Our results showed that although NPWT limits gram-negative proliferation better than WTD dressings on the ground and in flight, bacterial CFUs still increase from the time of inoculation to 72 hours post-AE. This may be related to the fact that some previous studies have included wounds that were copiously irrigated before NPWT. We chose not to irrigate wounds after inoculation in our study to allow for bacterial growth before simulated AE as would occur from the battlefield before definitive care.
Instillation NPWT is a relatively new approach to the NPWT technology, which allows fluid to dwell within a wound bed based on a preset duration and frequency. This technique has been shown to decrease bacterial growth rates within soft tissue wounds over time compared to WTD dressings or standard NPWT.24,25 Instillation NPWT demonstrated less bacterial growth than NPWT alone or WTD dressings during flight, but this was only significant for instillation with normal saline. This study utilized Normosol to compare to normal saline to determine if the inherent composition and pH of the instillation fluid would influence the ability to inhibit bacterial proliferation. The results suggest that instillation NPWT with normal saline may reduce bacterial proliferation, which could be due to the pH of 5.0 of normal saline. Davis et al. previously demonstrated less Pseudomonas growth within a soft tissue wound at 21 days using NPWT with NS compared to WTD dressing or standard NPWT.24 Our results support this finding as NS-NPWT limited bacterial proliferation during flight compared to WTD dressing. Giri et al. also demonstrated increased bacterial clearance within human wounds at 10 days using NPWT with NS instillation.25 Although our study only utilized NPWT with instillation during the 4-hour simulated AE period, a larger difference in bioburden may have been observed if the wounds had been treated with instillation NPWT for a longer period of time. However, continuous instillation was not possible in the current ambulatory porcine model, so instillation was only utilized during the simulated flight period with anesthesia used.
The VAC RX-4 system was designed for the military with the purpose of being able to simultaneously manage up to four wounds with one device. It is the first NPWT system approved by the U.S. Air Force for in-flight use since the VAC Freedom therapy unit during the early 2000s.19 A significant limitation in AB bacterial proliferation in the simple tissue wound was noted in RX-4-NPWT–treated animals undergoing simulated flight compared to all other flight treatment groups. Interestingly, we noted a difference in SA and AB CFUs between wounds treated with VAC ULTA NPWT and VAC RX-4-NPWT. Per technical specifications, the VAC RX-4 and the VAC ULTA devices are rated for pressures between 700 hPa and 1,060 hPa, which are equivalent to atmospheric pressure between −389.1 m (1,253 ft) and 3,010 m (9,878 ft). Initially, we attributed the decrease in bacterial count in the RX-4-NPWT groups to the RX-4’s improved ability to maintain full negative pressure at altitude; however, this may not be the case as both systems are rated for the same altitude. There may be other proprietary engineering differences that separate the VAC ULTA and the VAC RX-4 systems; however, these are not published. The RX-4 system was designed specifically for Critical Care Transport teams in the U.S. Air Force. Based on our results, the RX-4-NPWT system outperformed the VAC ULTA NPWT system during simulated AE for increased clearance of both gram-positive and gram-negative bacteria. Future studies should evaluate whether the same proprietary technology within the RX-4 can be used for a more compact, single-wound device that would need to be tested at the ground level and at altitude.
There are limitations to our study that must be considered. This study only included three animals per ground group as part of the secondary objective to determine if altitude affected bacterial clearance. The determination of sample size analysis revealed that a minimum of four animals per group were needed to demonstrate statistical differences. Although our primary flight groups had five animals each, ground groups had only three animals, which could have resulted in a type 2 error in our ground vs. flight analysis. We chose to study SA and AB based on these being two of the most common isolated bacterial species within contaminated combat wounds.3–9 Other bacteria such as Escherichia coli, Enterococcus faecium, and Pseudomonas aeruginosa are common within military wounds and may require dedicated investigation. This study implemented various types of wound management techniques only during the 4-hour simulated AE period. During the other time points of the survival period, wounds were either dressed in WTD dressing or standard NPWT using a PREVENA PLUS VAC system (Kinetic Concepts Inc., St. Paul, MN). Although this mirrors clinical practice, it does not completely isolate the portion of AE for bacterial analysis. Some of the differences between WTD dressing and variations of NPWT may be due to NPWT being applied during the entirety of the post-flight survival period. There were also occasional equipment malfunctions with the PREVENA PLUS VAC systems, which led to periods of time during which there was a lack of effective negative pressure on the wound. These periods, although infrequent and relatively short, are not uncommon clinically as well but could contribute to bacterial growth in an anaerobic environment under the dressing. Lastly, there are little data on ideal instillation volume, duration, and frequency during NPWT. Further investigation will be needed to determine how instillation settings affect bacterial growth within the wound.
Based on our study, utilization of NPWT and instillation NPWT during AE are safe and effective. The hypoxic, hypobaric environment of AE did not independently affect bacterial growth after simple or complex wounds. RX-4-NPWT during flight demonstrates increased bacterial clearance compared to WTD dressing–treated animals for both SA and AB. The RX-4-NPWT system provided the most effective bacterial reduction following simulated AE. Although we demonstrate improvement in bioburden, further research will be necessary to establish the effectiveness of RX-4-NPWT at altitude with various injury patterns and types of bacterial contamination. Future studies will focus on the determination of ideal instillation fluids, negative pressure settings, and dressing change frequency before and during AE.
Supplementary Material
ACKNOWLEDGMENTS
Judy Heyl and Karla Sloan assisted with animal procedures and data collection. We would like to thank Maia Smith, PhD, for assistance with statistical analysis.
Contributor Information
Matthew R Baucom, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Taylor E Wallen, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Jaclyn Youngs, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Kathleen E Singer, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Aaron M Delman, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Rebecca M Schuster, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Thomas C Blakeman, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Richard Strilka, United States Air Force School of Aerospace Medicine, En Route Care Training Department, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Timothy A Pritts, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
Michael D Goodman, Department of Surgery, University of Cincinnati, Cincinnati, OH 45267-0558, USA.
SUPPLEMENT SPONSORSHIP
This article appears as part of the supplement “Proceedings of the 2022 Military Health System Research Symposium,” sponsored by the Assistant Secretary of Defense for Health Affairs.
SUPPLEMENTARY MATERIAL
SUPPLEMENTARY MATERIAL is available at Military Medicine online.
FUNDING
This study was supported by the DoD grant (No. USAF FA8650-20-2-6G36) and NIH-Ruth Kirschstein T32 training grant (No. 5T32GM008478-29).
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY
The data that support the findings of this study are available on request from the corresponding author. All data are freely accessible.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC)
This study was reviewed and approved by the University of Cincinnati Institutional Animal Care and Use Committee (19-06-06-01) and by the U.S. Air Force Medical Support Agency Office of Research Oversight and Compliance.
AUTHOR CONTRIBUTIONS
K.E.S., T.E.W., T.C.B., T.A.P., and M.D.G. contributed to study conception and design. M.R.B., T.E.W., J.Y., K.E.S., R.S., and T.C.B. contributed to acquisition of data. M.R.B., T.E.W., J.Y., A.M.D., and M.D.G. analyzed and interpreted the data. M.R.B., T.E.W., and M.D.G. drafted the manuscript. M.R.B., T.E.W., K.E.S., J.Y., R.S., T.A.P. and M.D.G. made the critical revision of the article. Each author has made final approval of the article. Furthermore, each author certifies that this material has not been and will not be published or submitted to any other publication before its appearance in the Military Supplement of the Journal of Trauma and Acute Care Surgery.
INSTITUTIONAL CLEARANCE
Cleared for release AFRL 2022-3014.
REFERENCES
- 1. Owens BD, Kragh JF Jr., Wenke JC, Macaitis J, Wade CE, Holcomb JB: Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 2008; 64(2): 295–9.doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Department of Defense : Casualty status. Available at https://www.defense.gov/casualty.pdf; accessed December 27, 2021.
- 3. Hospenthal DR, Crouch HK, English JF, et al. : Multidrug-resistant bacterial colonization of combat-injured personnel at admission to medical centers after evacuation from Afghanistan and Iraq. J Trauma 2011; 71(1 Suppl): S52–7.doi: 10.1097/TA.0b013e31822118fb. [DOI] [PubMed] [Google Scholar]
- 4. Fischer D, Veldman A, Schafer V, Diefenbach M: Bacterial colonization of patients undergoing international air transport: a prospective epidemiologic study. J Travel Med 2004; 11(1): 44–8.doi: 10.2310/7060.2004.13647. [DOI] [PubMed] [Google Scholar]
- 5. Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK: Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis 2007; 45(4): 409–15.doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 6. Weintrob AC, Murray CK, Xu J, et al. : Early infections complicating the care of combat casualties from Iraq and Afghanistan. Surg Infect (Larchmt) 2018; 19(3): 286–97.doi: 10.1089/sur.2017.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petersen K, Riddle MS, Danko JR, et al. : Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007; 245(5): 803–11.doi: 10.1097/01.sla.0000251707.32332.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scott P, Deye G, Srinivasan A, et al. : An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US Military Health Care System associated with military operations in Iraq. Clin Infect Dis 2007; 44(12): 1577–84.doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 9. Sheppard FR, Keiser P, Craft DW, et al. : The majority of US combat casualty soft-tissue wounds are not infected or colonized upon arrival or during treatment at a continental US military medical facility. Am J Surg 2010; 200(4): 489–95.doi: 10.1016/j.amjsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 10. Hurd WW, Beninati W, SpringerLink : Aeromedical Evacuation: Management of Acute and Stabilized Patients, 2nd ed. Springer International Publishing; 2019. [Google Scholar]
- 11. Araiza A, Duran M, Surani S, Varon J: Aeromedical transport of critically ill patients: a literature review. Cureus 2021; 13(5): e14889.doi: 10.7759/cureus.14889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Earnest RE, Sonnier DI, Makley AT, et al. : Supplemental oxygen attenuates the increase in wound bacterial growth during simulated aeromedical evacuation in goats. J Trauma Acute Care Surg 2012; 73(1): 80–6.doi: 10.1097/TA.0b013e31824cf215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Putnis S, Khan WS, Wong JM: Negative pressure wound therapy - a review of its uses in orthopaedic trauma. Open Orthop J 2014; 8: 142–7.doi: 10.2174/1874325001408010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Topaz M, Tan H, et al. : Treatment of infected soft tissue blast injury in swine by regulated negative pressure wound therapy. Ann Surg 2013; 257(2): 335–44.doi: 10.1097/SLA.0b013e318269d1ca. [DOI] [PubMed] [Google Scholar]
- 15. Hinck D, Franke A, Gatzka F: Use of vacuum-assisted closure negative pressure wound therapy in combat-related injuries—literature review. Mil Med 2010; 175(3): 173–81.doi: 10.7205/milmed-d-09-00075. [DOI] [PubMed] [Google Scholar]
- 16. Leininger BE, Rasmussen TE, Smith DL, Jenkins DH, Coppola C: Experience with wound VAC and delayed primary closure of contaminated soft tissue injuries in Iraq. J Trauma 2006; 61(5): 1207–11.doi: 10.1097/01.ta.0000241150.15342.da. [DOI] [PubMed] [Google Scholar]
- 17. Machen S: Management of traumatic war wounds using vacuum-assisted closure dressings in an austere environment. US Army Med Dep J 2007; 17–23. [PubMed] [Google Scholar]
- 18. Pirela-Cruz MA, Machen MS, Esquivel D: Management of large soft-tissue wounds with negative pressure therapy-lessons learned from the war zone. J Hand Ther 2008; 21(2): 196–202. quiz 203.doi: 10.1197/j.jht.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 19. Couch KS, Stojadinovic A: Negative-pressure wound therapy in the military: lessons learned. Plast Reconstr Surg 2011; 127(Suppl 1): 117S–130S.doi: 10.1097/PRS.0b013e3181fd344e. [DOI] [PubMed] [Google Scholar]
- 20. Fang R, Dorlac WC, Flaherty SF, et al. : Feasibility of negative pressure wound therapy during intercontinental aeromedical evacuation of combat casualties. J Trauma 2010; 69(Suppl 1): S140–5.doi: 10.1097/TA.0b013e3181e452a2. [DOI] [PubMed] [Google Scholar]
- 21. Pollak AN, Powell ET, Fang R, Cooper EO, Ficke JR, Flaherty SF: Use of negative pressure wound therapy during aeromedical evacuation of patients with combat-related blast injuries. J Surg Orthop Adv 2010; 19(1): 44–8. [PubMed] [Google Scholar]
- 22. Svoboda SJ, Owens BD, Gooden HA, Melvin ML, Baer DG, Wenke JC: Irrigation with potable water versus normal saline in a contaminated musculoskeletal wound model. J Trauma 2008; 64(5): 1357–9.doi: 10.1097/TA.0b013e31816e3476. [DOI] [PubMed] [Google Scholar]
- 23. Bassetto F, de Antoni E, Rizzato S, Scarpa C: Management of acute and chronic wounds using negative pressure wound therapy with instillation and dwell time: a retrospective review of a 100-patient cohort in Padova, Italy. Wounds 2021.doi: 10.25270/wnds/081421.01. [DOI] [PubMed] [Google Scholar]
- 24. Davis K, Bills J, Barker J, Kim P, Lavery L: Simultaneous irrigation and negative pressure wound therapy enhances wound healing and reduces wound bioburden in a porcine model. Wound Repair Regen 2013; 21(6): 869–75.doi: 10.1111/wrr.12104. [DOI] [PubMed] [Google Scholar]
- 25. Giri P, Krishnaraj B, Chandra Sistla S, et al. : Does negative pressure wound therapy with saline instillation improve wound healing compared to conventional negative pressure wound therapy? - A randomized controlled trial in patients with extremity ulcers. Ann Med Surg (Lond) 2021; 61: 73–80.doi: 10.1016/j.amsu.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiyokawa K, Takahashi N, Rikimaru H, Yamauchi T, Inoue Y: New continuous negative-pressure and irrigation treatment for infected wounds and intractable ulcers. Plast Reconstr Surg 2007; 120(5): 1257–65.doi: 10.1097/01.prs.0000279332.27374.69. [DOI] [PubMed] [Google Scholar]
- 27. Svoboda SJ, Bice TG, Gooden HA, Brooks DE, Thomas DB, Wenke JC: Comparison of bulb syringe and pulsed lavage irrigation with use of a bioluminescent musculoskeletal wound model. J Bone Joint Surg Am 2006; 88(10): 2167–74.doi: 10.2106/JBJS.E.00248. [DOI] [PubMed] [Google Scholar]
- 28. Aerospace Medical Association; Aviation Safety Committee; Civil Aviation Subcommittee : Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med 2008; 79(4): 433–9.doi: 10.3357/asem.2272.2008. [DOI] [PubMed] [Google Scholar]
- 29. Xu L, McLennan SV, Lo L, et al. : Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care 2007; 30(2): 378–80.doi: 10.2337/dc06-1383. [DOI] [PubMed] [Google Scholar]
- 30. Lalliss SJ, Stinner DJ, Waterman SM, Branstetter JG, Masini BD, Wenke JC: Negative pressure wound therapy reduces pseudomonas wound contamination more than Staphylococcus aureus. J Orthop Trauma 2010; 24(9): 598–602.doi: 10.1097/BOT.0b013e3181ec45ba. [DOI] [PubMed] [Google Scholar]
- 31. Moues CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE: Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004; 12(1): 11–7.doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 32. Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W: Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997; 38(6): 553–62.doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 33. Weed T, Ratliff C, Drake DB: Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg 2004; 52(3): 276–9. discussion 279-80.doi: 10.1097/01.sap.0000111861.75927.4d. [DOI] [PubMed] [Google Scholar]
- 34. Moues CM, van den Bemd GJ, Heule F, Hovius SE: Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg 2007; 60(6): 672–81.doi: 10.1016/j.bjps.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 35. Khashram M, Huggan P, Ikram R, Chambers S, Roake JA, Lewis DR: Effect of TNP on the microbiology of venous leg ulcers: a pilot study. J Wound Care 2009; 18(4): 164–7.doi: 10.12968/jowc.2009.18.4.41608. [DOI] [PubMed] [Google Scholar]
- 36. Birke-Sorensen H, Malmsjo M, Rome P, et al. : Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer)—steps towards an international consensus. J Plast Reconstr Aesthet Surg 2011; 64: S1–16.doi: 10.1016/j.bjps.2011.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. All data are freely accessible.


