Abstract
Two methanotrophic bacteria, Methylobacter albus BG8 and Methylosinus trichosporium OB3b, oxidized atmospheric methane during batch growth on methanol. Methane consumption was rapidly and substantially diminished (95% over 9 days) when washed cell suspensions were incubated without methanol in the presence of atmospheric methane (1.7 ppm). Methanotrophic activity was stimulated after methanol (10 mM) but not methane (1,000 ppm) addition. M. albus BG8 grown in continuous culture for 80 days with methanol retained the ability to oxidize atmospheric methane and oxidized methane in a chemostat air supply. Methane oxidation during growth on methanol was not affected by methane deprivation. Differences in the kinetics of methane uptake (apparent Km and Vmax) were observed between batch- and chemostat-grown cultures. The Vmax and apparent Km values (means ± standard errors) for methanol-limited chemostat cultures were 133 ± 46 nmol of methane 108 cells−1 h−1 and 916 ± 235 ppm of methane (1.2 μM), respectively. These values were significantly lower than those determined with batch-grown cultures (Vmax of 648 ± 195 nmol of methane 108 cells−1 h−1 and apparent Km of 5,025 ± 1,234 ppm of methane [6.3 μM]). Methane consumption by soils was stimulated by the addition of methanol. These results suggest that methanol or other nonmethane substrates may promote atmospheric methane oxidation in situ.
Despite the contribution of soils to global methane budgets (8, 12, 25, 29), the methane oxidizers active in situ remain unidentified and are considered novel (11, 22, 28). In some respects, soil methanotrophy appears physiologically distinct from methanotrophy in cultures. For example, soil methanotrophs appear capable of oxidizing atmospheric methane indefinitely given suitable temperatures and soil water contents. In contrast, methanotrophs in culture consume atmospheric methane only transiently after growth at high methane concentrations (30). Differences in the kinetics of methane uptake (Vmax, apparent Km, and threshold) also distinguish soil methanotrophs from known isolates (2, 3). For example, soils typically exhibit a high affinity for methane (approximately 10 nM), whereas pure cultures have a relatively low affinity (>1 μM) (18).
Many of the factors that affect soil methane oxidation, including agriculture and disturbances in soil water content and soil nitrogen, have been examined extensively (1, 10, 13, 24, 29, 31). However, relatively little is known about the substrate(s) supporting growth and activity. For example, the extent to which soil methanotrophs depend on atmospheric methane (about 1.7 ppm, equivalent to a dissolved methane concentration in soil water of about 2.5 nM at 22°C) as a sole source of carbon and energy is unknown. Bender and Conrad (4) demonstrated that numbers of soil methanotrophs increase only at methane concentrations of ≥7,000 ppm, an observation that raises doubts about the role of methane as a sole carbon and energy source. More recently, methanotrophic activity in forest soil was found to be unaffected by methane deprivation, which suggests that nonmethane substrates may support soil methanotrophs (5). However, obligate methanotrophs are restricted to a limited range of C1 compounds for growth and energy (9), and use of multicarbon compounds has been reported for only a few presumed facultative methanotrophs (34).
Despite the ability of methanotrophs to grow on some nonmethane substrates, little is known about the role of such compounds as supplementary carbon and energy sources. Methanotrophs have been reported to grow on methanol, methylamine, or trimethylamine (e.g., see references 9 and 14), but the significance of these substrates appears limited at best. Results presented here demonstrate that pure cultures of methanotrophs oxidize atmospheric methane during growth on methanol and that they retain this activity during methanol-limited growth. Soils treated with methanol also showed enhanced methane consumption. On the basis of these collective results, we suggest that methanotrophic activity in soils depends at least partially on nonmethane substrates for maintenance; we also suggest that methanol is the most important nonmethane substrate.
MATERIALS AND METHODS
Growth and maintenance of organisms.
Methylobacter albus BG8 (formerly Methylomonas albus BG8) and Methylosinus trichosporium OB3b were obtained from R. S. Hanson. Cultures were maintained on agar slants of nitrate mineral salts medium (NMS) at 30°C (20), under an initial atmosphere of approximately 30 to 70% methane-air; subcultures were transferred every 4 to 5 weeks. To prepare an inoculum for batch and continuous cultivation studies, 4 ml of NMS medium was added to a 3- to 4-day-old slant; the slant was vortexed briefly to resuspend the cells, and the liquid was transferred to a 1-liter flask containing 160 ml of NMS medium. Both cultures were grown on methane (approximately 30 to 70% methane-air) at 30°C with shaking (125 rpm).
Atmospheric methane consumption during batch growth on methanol.
A volume (100 μl) of M. albus BG8 or M. trichosporium OB3b cultures from mid-exponential growth phase was used to inoculate NMS medium (20 ml) containing methanol (25 mM) in 160-ml serum bottles sealed with butyl rubber stoppers. The cultures were maintained at 30°C with shaking (125 rpm). Atmospheric methane consumption by cultures that had been equilibrated with ambient air at 30°C was determined by measuring the decrease in headspace methane over time by gas chromatography (20). The optical density of cultures at a wavelength of 600 nm during incubation was also determined for a parallel set of bottles. All experiments were performed in triplicate.
Substrate utilization by M. albus BG8.
Substrate utilization by M. albus BG8 was determined by using washed cell suspensions of both batch (methane-grown) and chemostat (methanol-limited) cultures. Cultures were serially diluted (approximately 500 and 1,000 cells ml−1 for batch and chemostat cultures, respectively), and samples (100 μl) were plated on NMS agar plates containing various substrates (methane, methanol [vapor], formate, glucose, glucose plus casein hydrolysate, fructose, acetate, succinate, malate, pyruvate, citrate, glutamate, alanine, and serine). Substrates were added at 10 mM unless otherwise indicated. The plates were incubated at 30°C under ambient air, except for the methane treatment, for which an atmosphere of 30% methane in air was used. Colonies were counted after 19 days of incubation. The plates were incubated further to confirm that all colonies had been counted. Control treatments without added substrates were incubated under ambient air as the sole source of carbon and energy.
Stability of atmospheric methane consumption.
The stability of atmospheric methane consumption by washed cultures of M. albus BG8 was determined with methane (1.7 ppm) in the presence or absence of methanol. Cultures of M. albus BG8 (160 ml) in mid-exponential growth phase were harvested by centrifugation, and cell pellets were washed and resuspended in a phosphate buffer to an optical density of 4 (equivalent to approximately 1.4 × 109 cells ml−1). Phosphate buffer was prepared as for NMS medium, but Higgins salts were excluded. Washed cell suspensions (2 ml) were transferred to 120-ml glass jars that were sealed with neoprene rubber stoppers and incubated at 30°C with shaking (125 rpm). Cell suspensions were also sprayed on 10 g of autoclaved sand in 120-ml jars that were sealed with neoprene rubber stoppers and incubated statically. The effect of methanol on the stability of atmospheric methane consumption was examined by injecting methanol (1 μl) with a microsyringe onto the walls of the sealed glass jars at various times. At intervals, atmospheric methane consumption rates were determined by equilibrating the cultures under ambient air and measuring headspace methane. Methane uptake rate constants (k) were calculated by regression analysis of logarithmically transformed concentration data. The effects of methane and methanol additions on atmospheric methane uptake by M. albus BG8 incubated for 17 days with ambient air as the sole source of carbon and energy were examined by exposing cultures for 1 h to methane (1,000 ppm) or methanol (10 mM), respectively; atmospheric methane consumption rates were determined subsequently.
Continuous-culture studies.
For continuous cultivation, M. albus BG8 was grown under methanol limitation as follows. A 650-ml volume of NMS medium containing methanol (25 mM) in a 1-liter glass vessel was inoculated with mid-exponential-phase cultures. Temperature and pH were maintained at 30°C and 7.3, respectively; pH control was unnecessary. Air continuously sparged the culture at a flow rate of 73 ml min−1. Methane concentrations in the inlet and outlet gas streams were measured periodically. After 24-h growth, NMS medium containing methanol (25 mM) was supplied at a flow rate of 10 ml h−1, which corresponded to a dilution rate of 0.015 h−1, and the culture was stirred with a magnetic stirrer. The purity of the culture was checked regularly by microscopic examination.
Atmospheric methane uptake was monitored frequently by removing samples from the glass vessel via an aseptic sampling port. Cultures (2 ml) were transferred to 120-ml glass jars and maintained at 30°C with shaking (250 rpm). Atmospheric methane consumption by cultures equilibrated with ambient air was determined by measuring the decrease in headspace methane over time. Samples were also removed from the glass vessel for absorbance measurements at 600 nm, cell enumeration, and pH and microscopic analyses. The total number of culturable cells in the chemostat was determined by spread plate counts. The chemostat cultures were serially diluted, and samples were plated on NMS agar plates and incubated at 30°C under an initial atmosphere of approximately 30% methane. Colonies were counted after 7 to 10 days. The plates were further incubated up to 30 days to confirm that all colonies had been counted.
To determine whether methanol or atmospheric methane was responsible for maintaining methane monooxygenase (MMO) activity, the air sparging the culture was replaced with hydrocarbon-free air (<0.03 ppm of methane; Scott Specialty Gases, Inc.) at a flow rate of 73 ml min−1 between 55 and 85 days. Measurements of the outlet gas streams confirmed removal of traces of methane. The rate of atmospheric methane consumption, absorbance at 600 nm, and cell number were monitored during methane starvation. After 85 days, the hydrocarbon-free air was replaced with ambient air (1.7 ppm of methane).
Kinetic studies.
The kinetic parameters of methane oxidation (apparent Km and Vmax) for M. albus BG8 were determined by using washed cell suspensions of both batch (methane-grown) and chemostat (methanol-limited) cultures. Cultures of M. albus BG8 were harvested by centrifugation; cell pellets were washed and resuspended in phosphate buffer to an optical density of 0.6 (equivalent to approximately 2 × 108 cells ml−1). Washed cell suspensions (5 ml) were transferred to 160-ml serum bottles that were sealed with butyl rubber stoppers and incubated at 30°C with shaking (250 rpm). Methane consumption rates (V) were measured at various methane concentrations [S] ranging between 500 and 30,000 ppm in the headspace. Apparent Km and Vmax values were obtained from nonlinear fits of the data to the Michaelis-Menten equation V = Vmax [S]/(Km + [S]). Concentrations of dissolved methane were calculated from the gas-phase concentrations by using mole fractions (33).
14CH4 utilization studies.
M. albus BG8, M. trichosporium OB3b, and Methylocystis parvus OBBP were grown in batch cultures of NMS medium as described above. Late-exponential-phase cultures were harvested by centrifugation (10,000 × g, 10 min at 4°C), washed in phosphate buffer twice, and resuspended in fresh NMS medium. The cultures were then incubated with ambient air at 30°C with shaking for 4 days. Two-milliliter aliquots with an A600 of about 0.8 were transferred to 30-ml serum bottles containing ambient air immediately after the cultures were resuspended and at intervals of 1, 2, and 4 days. The serum bottles were sealed with neoprene stoppers, after which 50 μl of a [14C]methane stock (about 74 kBq cm−3; 2.07 GBq mmol−1) were injected to create a headspace methane concentration of about 2 ppm. At intervals over a 6- to 8-h period, 1 cm3 of headspace was sampled by needle and syringe and transferred to a sealed scintillation vial containing 2 ml of 0.1 N KOH to trap 14CO2, which was assayed after addition of Scintiverse BD counting fluid. At the final time point, 2 ml of 1 N HCl was injected to acidify the medium; after degassing of 14CO2 and unreacted [14C]methane, 0.5 ml of the medium was transferred to scintillation vials to determine the amount of radioactivity incorporated into cells or cell metabolites. M. albus BG8 batch culture grown with methanol and continuous culture grown as described above were treated similarly. The continuous culture had been growing for 99 days prior to sampling; the batch culture was harvested from exponential and stationary phases. All assays were conducted in triplicate.
Effect of methanol additions on soil methane consumption.
The effect of methanol additions on the methane-consuming capacity of forest soil was examined by injecting methanol (1 μl) at various intervals onto the walls of sealed jars containing 10 g (fresh weight) of sieved soil as described previously (5). The soils were incubated at 25°C. At various intervals, atmospheric methane consumption rates were determined.
RESULTS
Atmospheric methane oxidation during batch growth on methanol.
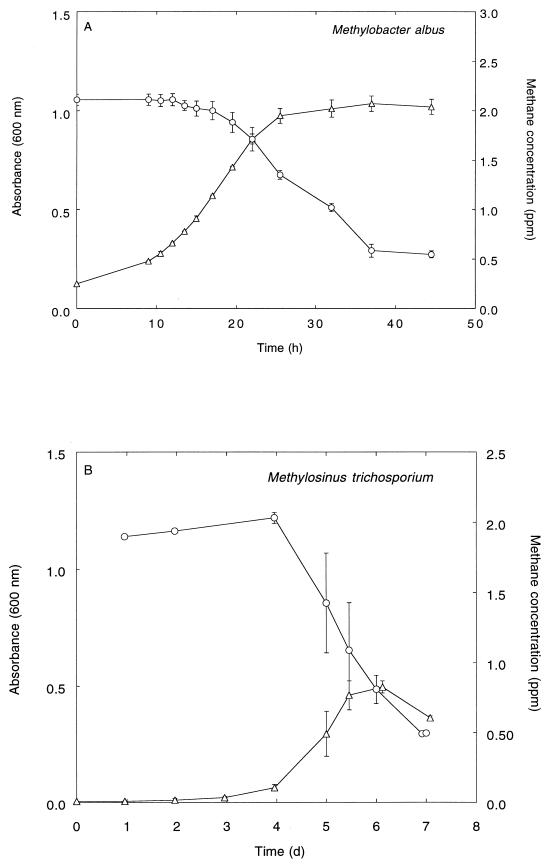
Pure cultures of M. albus BG8 (group I) and M. trichosporium OB3b (group II) were capable of growth on methanol (25 mM). During batch growth on methanol, traces of methane in the bottle headspaces were utilized. For M. albus BG8, methane concentrations decreased from an initial concentration of 2.1 ppm to 0.55 ppm over 2 days (Fig. 1A). The specific growth rate of M. albus on methanol was 0.11 h−1, equivalent to a doubling time of 6.5 h. During batch growth on methanol by M. trichosporium OB3b, methane concentrations decreased from an initial concentration of 2 ppm to 0.5 ppm over 7 days (Fig. 1B).
FIG. 1.
Atmospheric methane oxidation by pure cultures of methanotrophic bacteria during batch growth on methanol (25 mM). (A) M. albus BG8; (B) M. trichosporium OB3b. ▵, absorbance; ○, methane concentration. Each point represents the mean of triplicate determinations ± 1 standard error. d, days.
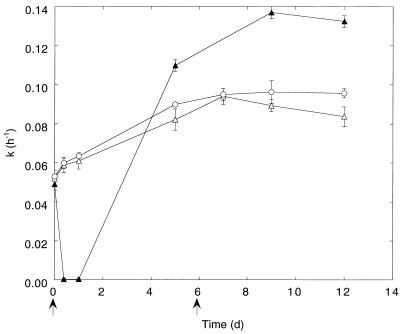
Stability of atmospheric methane consumption.
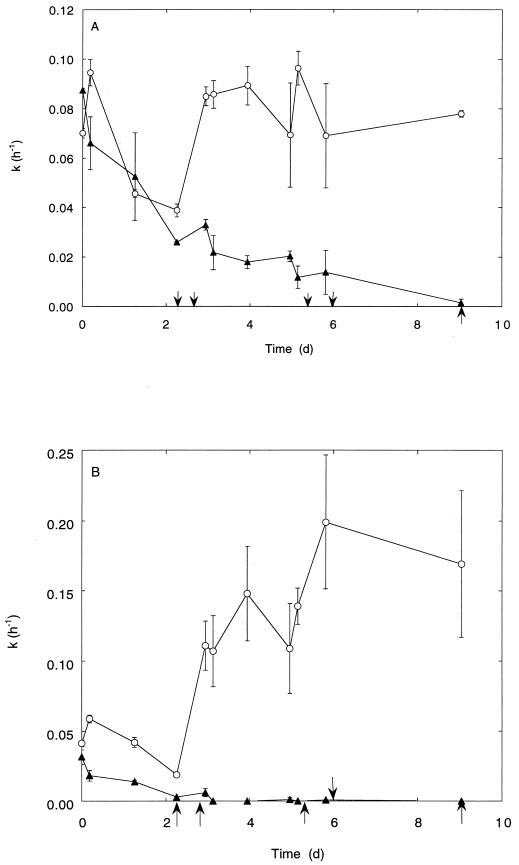
M. albus BG8 consumed atmospheric methane transiently following growth at high methane concentrations. However, the uptake rate constants for atmospheric methane consumption decreased during subsequent incubations with atmospheric methane as the sole source of carbon and energy; after 9 days, 95% of the original activity was lost. In contrast, cell suspensions supplemented with methanol at intervals retained the ability to oxidize atmospheric methane (Fig. 2A). In addition, an immediate stimulation of atmospheric methane consumption was observed after addition of methanol (10 mM) but not methane (1,000 ppm) to cultures maintained for 17 days on atmospheric methane (data not shown).
FIG. 2.
Stability of atmospheric methane consumption by pure cultures of M. albus BG8 incubated without a solid support (A) and added to a solid support (B). ▴, cultures incubated with CH4 (1.7 ppm); ○, cultures incubated with CH4 (1.7 ppm) plus methanol. Methanol (25 μmol) was added at the times indicated (arrows). Data are means of triplicate determinations ± 1 standard error. d, days.
The capacity of cell suspensions added to autoclaved sand to consume atmospheric methane was initially lower than that of liquid cultures (Fig. 2B). However, after 6 days of incubation in the presence of methanol, rates of methane oxidation increased twofold compared to those of liquid incubations at the same initial cell density.
Continuous-culture studies.
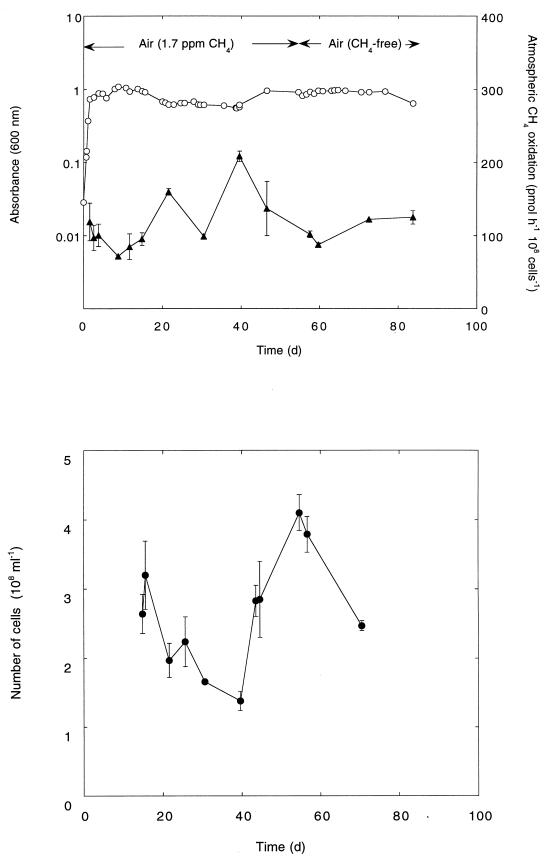
M. albus BG8 grown in continuous culture (dilution rate, 0.015 h−1) for 80 days with methanol retained the ability to oxidize atmospheric methane (Fig. 3). The rate of atmospheric methane consumption and biomass of samples taken from steady-state continuous culture (means ± standard errors) were 126 ± 13 pmol of methane 108 cells−1 h−1 and 2.65 × 108 ± 0.3 × 108 cells ml−1, respectively. The methane-oxidizing activity of M. albus BG8 was relatively unaffected by methane starvation during growth on methanol. There were also no significant differences in A600 or consistent trends in the cell number of samples taken from steady-state continuous culture in the presence or absence of atmospheric methane (Fig. 3). However, the ability of M. albus BG8 to grow on methane changed during continuous cultivation. Colonies enumerated on plates were visible within 10 days of incubation with methane (30% in air) for samples obtained during the first 30 days of chemostat operation. After this time, decreased colony growth was evident, fewer colonies were visible within 10 days, and plates required further incubation (about 20 to 30 days) to count all colonies.
FIG. 3.
Atmospheric methane oxidation during continuous cultivation of M. albus BG8 under methanol limitation. ○, absorbance; ▴, methane oxidation; •, cell number. Data are means of triplicate determinations ± 1 standard error. d, days.
Kinetics of methane uptake.
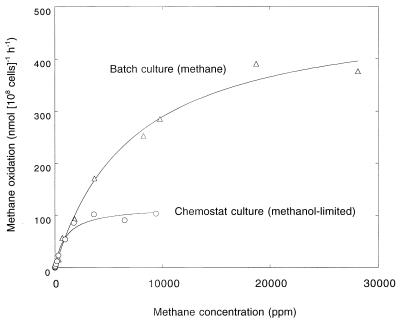
Differences in methane oxidation kinetics were observed for washed cell suspensions of batch- and chemostat-grown cultures of M. albus BG8 (Fig. 4). The Vmax and apparent Km values (means ± standard errors) of methanol-limited chemostat cultures were 133 ± 46 nmol of methane 108 cells−1 h−1 and 916 ± 235 ppm of methane (equivalent to a dissolved concentration of 1.2 μM), respectively. These values were significantly lower than those determined for batch-grown cultures (Vmax of 648 ± 195 nmol of methane 108 cells−1 h−1 and Km of 5,025 ± 1,234 ppm of methane [6.3 μM]). There were no significant differences in Vmax and apparent Km for washed cell suspensions taken from steady-state continuous culture in the presence or absence of atmospheric methane (Table 1).
FIG. 4.
Rates of methane consumption by M. albus BG8 at concentrations between 500 and 30,000 ppm.
TABLE 1.
Kinetics of methane uptake by M. albus BG8a
| Treatment | Methane concn | Km (ppm) | Vmaxb |
|---|---|---|---|
| Batch (methane) | 20% | 5,025 ± 1,234 | 648 ± 195 |
| Chemostat (methanol limited) | 1.7 ppm | 916 ± 235 | 133 ± 46 |
| <0.03 ppm | 1,119 ± 42 | 133 ± 45 |
Results are the means for triplicate cultures ± standard errors of the means.
Nanomoles of CH4 108 cells−1 h−1.
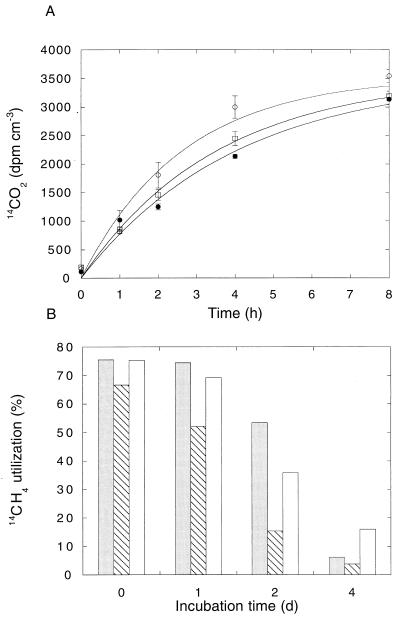
14CH4 utilization studies.
Production of 14CO2 in all cultures fit an exponential relationship of the form y = 1 − e−xt (Fig. 5A), consistent with exponentially decreasing methane as typically observed for cultures at near-ambient methane concentrations. Of the three cultures examined, activity was least for M. trichosporium OB3b, though the differences among cultures were not substantial. Activity also decreased with increasing incubation time in all cultures, especially after 2 days, and most dramatically in M. trichosporium OB3b (Fig. 5B; note that these assays involved short-term incubations of replicate treatments rather than continuous incubations with [14C]methane). Total radiomethane uptake during 6- to 8-h incubations as measured by 14CO2 production and incorporation was initially about 65 to 75% and declined with time to <10% in all cases (Fig. 5B). The percentage of total uptake defined as incorporation (cells and cell metabolites) was generally <10% for M. parvus OBBP and M. trichosporium OB3b; incorporation was initially low for M. albus BG8 but increased with time to about 45% (Table 2). Incorporation was relatively high for both methanol-limited continuous culture of M. albus BG8 and M. albus BG8 grown in batch on methanol; higher values were obtained from the latter during exponential growth (Table 2).
FIG. 5.
14CH4 utilization by pure cultures of methanotrophic bacteria incubated with ambient air. (A) 14CO2 production. ○, M. parvus; □, M. albus BG8; •, M. trichosporium OB3b. (B) 14CH4 utilization. , M. parvus; ▧, M. albus BG8; □, M. trichosporium OB3b. Data are means of triplicate determinations ± 1 standard error. d, days.
TABLE 2.
Percent incorporation of radiolabelled 14CH4 into biomass and cell metabolites by various cultures of methanotrophsa
| Time (days) | % Incorporation of
14CH4
|
||||
|---|---|---|---|---|---|
| M. parvus | M. trichosporium | M. albus | M. albus Ch | M. albus BM | |
| 0 | 4.5 ± 0.8 | 6.1 ± 0.4 | 2.0 ± 0.0 | 38.8 ± 2.2 | 29.4 ± 1.1 |
| 1 | 5.4 ± 0.2 | 5.1 ± 0.1 | 6.9 ± 1.6 | ||
| 2 | 5.0 ± 0.7 | 27.1 ± 4.9 | 23.8 ± 1.6 | ||
| 4 | 8.5 ± 0.3 | 10.1 ± 0.6 | 45.3 ± 0.8 | ||
M. parvus, M. trichosporium, and M. albus were grown under batch conditions with about 30% methane and then subjected to an incubation with atmospheric methane concentrations (see text for details). M. albus Ch was grown in a methanol-limited chemostat, and M. albus BM was grown in a batch culture with methanol; samples of the latter were obtained from stationary-phase growth. Values are means of triplicates ± 1 standard error.
Effect of methanol additions on soil methane consumption.
The addition of methanol (2.5 μmol g [fresh weight] of soil−1) resulted in a rapid inhibition of soil methane consumption (Fig. 6). However, 5 days after treatment, the rate constants for atmospheric methane consumption were significantly higher than those for the control treatments (Student’s t test; P < 0.05). No stimulatory effect on methane consumption was observed after the addition of 0.05 μmol of methanol g (fresh weight) of soil−1 (Fig. 6).
FIG. 6.
Effect of methanol addition on soil methane consumption. ▴, 25 μmol; ○, 0.5 μmol; ▵, control treatments. Methanol additions were made at the times shown (arrows). Experiments were carried out with sieved soils. Each point represents the mean of triplicate determinations ± 1 standard error. d, days.
DISCUSSION
The ability to maintain atmospheric methane consumption for extended periods is one of the primary traits distinguishing soil methanotrophs from known methanotrophic cultures. The latter can consume atmospheric methane only transiently after growth at high methane concentrations and are unable to grow with atmospheric methane as a sole source of carbon and energy (30). However, results presented here demonstrate that M. albus BG8 (a group I methanotroph) retained the ability to oxidize atmospheric methane when grown in continuous culture with methanol. Such cultures may serve as models for soil methane consumption and provide predictive insights regarding the behavior of soil methanotrophy with respect to controls and responses to methane deprivation. Although physiologically novel methanotrophs growing solely on atmospheric methane may yet be proven to exist in soils, long-term atmospheric methane consumption by M. albus BG8 when using methanol suggests as an alternative that methanotrophic activity in soils could be supported by substrates other than methane.
Of the various nonmethane substrates, methanol is perhaps the most important as a supplement for methanotrophs. It can be derived from methoxylated sugars (e.g., pectins) and various aromatics in lignin (19). These sources likely dwarf terrestrial sources for other C1 substrates, such as methylamines. The significance of other substrates, especially multicarbon substrates, appears minimal (30). A few reports have indicated that select multicarbon substrates (e.g., glucose) support growth of facultative methanotrophs (e.g., Methylobacterium spp. [26, 34]). However, these organisms are not considered dominant in soils, although this may be a reflection of the conditions used for enrichments and isolation procedures (14). In addition, M. albus BG8 grown on methanol does not subsequently grow on common multicarbon substrates, suggesting that the inability to use multicarbon substrates is not a function of repression by methane.
Methanol can act as a sole source of carbon and energy for methanotrophs (9). Group I methanotrophs (e.g., Methylobacter and Methylomonas spp.) appear to grow readily on it. Although methanol can be toxic to group II methanotrophs (e.g., Methylosinus and Methylocystis spp.), they have been preadapted to growth on high methanol concentrations (15). The ability of group II taxa to use methanol is significant because they have been suggested as the dominant populations in soils (14).
Though methanotrophs can use methanol, the expression and activity of MMO during growth on methanol are uncertain. For example, Hou et al. (16) have concluded that MMO was induced by methane and that growth of M. trichosporium OB3b, Methylococcus capsulatus, and Methylobacterium organophilum on methanol resulted in a loss of MMO activity. Other reports have shown that MMO activity was retained during growth of methanotrophs on methanol (7, 17, 23, 27). In agreement with the latter studies, M. albus BG8 has continuously expressed MMO during growth in a methanol-limited chemostat in the presence of atmospheric methane, in spite of the fact that methane was at most a secondary carbon source. In addition, MMO expression was observed for a 30-day period during which the chemostat was sparged with hydrocarbon-free air. Contrary to the findings of Hou et al. (16), this indicates that MMO expression is constitutive and not methane dependent.
In contrast to expression, maximum levels of M. albus BG8 MMO activity varied during continuous cultivation on methanol. This was evident from a decrease in Vmax per cell compared to that of batch-grown cultures (Table 1). A decrease in Vmax can be attributed to regulation of MMO by methane concentration (or flux). A decrease can also be explained in part by the diminution in size (about 25%) over time of methanol-grown cells relative to that of the initial inoculum. The effect of size is illustrated by an expression for diffusion-limited substrate uptake for a spherical cell, in which Vmax = 4πr2kDC0, where r is cell radius, k is a transport coefficient related to the number of uptake sites and time the sites are occupied by substrate, D is the diffusion coefficient, and C0 is the concentration of substrate in the bulk medium (6). Regardless of the mechanism, changes in Vmax observed for M. albus BG8 suggest that cell-specific activities for soil methanotrophs are probably lower than for methanotrophs in culture; further isolates obtained by enrichment with high methane concentrations may exhibit characteristics that differ from those expressed in situ.
The affinity for methane (apparent Km) of pure cultures of methanotrophic bacteria can also vary as a function of growth conditions: copper concentration affects apparent Km (22), and apparent Km for M. albus BG8 in batch culture exceeds that for continuous methanol-limited culture fivefold (Table 1). The explanation for this difference is unclear. Although Km can be related to cell radius (Km = krD−1 [6]), changes in cell size account for only a fraction of the change in Km. This suggests the possibility of either posttranscriptional modifications of a single MMO or expression of a higher-affinity enzyme.
Alternatively, a model recently proposed by Koch (21) suggests that apparent Km may change in response to the dynamics of substrate utilization as determined by the coupling between transport, growth, and internal substrate pools. In Koch’s model, apparent Km can change even if the amounts of transport proteins and their reaction rate constants are unaltered. The general model may be particularly useful when applied to methanotrophs because the first product of methane oxidation, methanol, is sometimes excreted and affects the kinetics of MMO. These properties indicate that the coupling of methanol to growth and regulation of intracellular methanol concentrations may be key components of a control system for methane uptake kinetics.
In any case, the ability of a methanotroph to display different kinetic properties as a function of growth conditions raises questions about the meaning of previous observations showing distinct high- and low-affinity methane uptake systems in soils (2). Do the former represent novel methanotrophs (for example, see references 2, 11, and 28), or “typical” methanotrophs adapted to growth regimens differing from those that promote significant cell proliferation? Might the low-affinity populations also express a high-affinity MMO under suitable growth conditions? If expression of a high-affinity MMO requires prolonged exposure to low (e.g., atmospheric) methane concentrations and a cosubstrate (e.g., methanol), comparisons of the properties of batch-grown cultures and soils may be misleading.
14CH4 metabolism illustrates further the need for examining cultures prior to drawing conclusions about either atmospheric methane consumption capacities or differences relative to soils. 14CH4 incorporation is generally low (<10% [Table 2]) for pure cultures incubated with near-ambient methane. Although 14CH4 incorporation increases somewhat over time for M. albus BG8, activity overall decreases and the capacity for atmospheric methane consumption is greatly diminished (Fig. 5B). In contrast, the level of 14CH4 incorporation by M. albus BG8 grown in a methanol-limited chemostat and incubated with ambient methane is significantly higher than that of either M. trichosporium OB3b or M. parvus OBBP incubated with ambient methane only (Table 2). Although 14CH4 incorporation by batch-grown M. albus BG8 after incubation for 4 days with atmospheric methane is comparable to that of cells from a methanol-limited chemostat, total methane consumption by the former is less than one-third that of the latter, and the uptake capacity for chemostat-grown cells appears to be stable indefinitely, while that of the batch culture is not. 14CH4 incorporation by M. albus BG8 from a methanol-limited chemostat is comparable to 14CH4 incorporation by soils, which also have a relatively stable capacity for atmospheric methane consumption (28, 30, 32). This similarity may indicate that soil methanotrophs are supported at least in part by methanol or other nonmethane substrates.
In a recent analysis, forest soil methanotrophy was unaffected by methane deprivation (5). This suggests a possible supporting role for nonmethane substrates, e.g., methanol. However, despite the ability of methanotrophs to grow on methanol, little is known about its utilization in soils. Previous attempts to stimulate soil methanotrophic activity with a variety of C1 substrates (methanol and formate) and various organics (glucose, acetate, starch, malate, and β-hydroxybutyrate) have proven unsuccessful (5, 30). In the present study, methane consumption was stimulated in soils for which no enhancement had been observed before (5, 30). The difference between this study and earlier studies may reflect differences in the mass and timing of methanol additions or differences in the physiological status of the methanotrophs. With respect to the latter point, rate constants for methane consumption in the present study were about fourfold lower than in earlier studies. A role for methanol in soil methane consumption is particularly interesting since the potential for growth of soil methanotrophs on atmospheric methane alone is limited (28). Methanol may help support the growth and activity of methanotrophs in soils by two mechanisms: (i) as a substrate for growth and energy production and (ii) as a source of reducing equivalents that are required for the continued oxidation of atmospheric methane.
In conclusion, results presented here demonstrate that methanol facilitates continued atmospheric methane oxidation by methanotrophic cultures. Consequently, isolates obtained by traditional enrichment may be representative of populations active in situ. Involvement of nonmethane substrates in soil may also explain why enrichments using high concentrations of methane as a sole carbon and energy source have apparently failed to produce high-affinity methanotrophs (30). This hypothesis should be tested by evaluating methanotrophic activity of cultures under conditions which reflect the soil environment (e.g., nutrient limitation and mixed-substrate utilization).
ACKNOWLEDGMENTS
We acknowledge financial support from the U.S. Department of Agriculture (CSRS-CRP 94-37107-0488).
We also acknowledge K. R. Hardy for technical support.
Footnotes
Contribution 312 from the Darling Marine Center.
REFERENCES
- 1.Adamsen A P S, King G M. Methane consumption in temperate and subarctic forest soils: rates, vertical zonation, and responses to water and nitrogen. Appl Environ Microbiol. 1993;59:485–490. doi: 10.1128/aem.59.2.485-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender M, Conrad R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4mixing ratios. FEMS Microbiol Ecol. 1992;101:261–270. [Google Scholar]
- 3.Bender M, Conrad R. Kinetics of methane oxidation in oxic soils. Chemosphere. 1993;26:687–696. [Google Scholar]
- 4.Bender M, Conrad R. Effect of CH4 conditions on the induction of CH4oxidation activity. Soil Biol Biochem. 1995;27:1517–1527. [Google Scholar]
- 5.Benstead J, King G M. Response of methanotrophic activity in forest soil to methane availability. FEMS Microbiol Ecol. 1997;23:333–340. [Google Scholar]
- 6.Berg H C. Random walks in biology. Princeton, N.J: Princeton University Press; 1991. [Google Scholar]
- 7.Best D J, Higgins I J. Methane-oxidizing activity and membrane morphology in a methanol-grown obligate methanotroph, Methylosinus trichosporiumOB3b. J Gen Microbiol. 1981;125:73–84. [Google Scholar]
- 8.Born M, Dörr H, Levin I. Methane consumption in aerated soils of the temperate zone. Tellus. 1990;42B:2–8. [Google Scholar]
- 9.Bowman J P, Sly L I, Nichols P D, Hayward A C. Revised taxonomy of the methanotrophs: description of Methylobacter gen. nov., emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and proposal that the family Methylococcaceaeincludes only the group I methanotrophs. Int J Syst Bacteriol. 1993;43:735–753. [Google Scholar]
- 10.Castro M S, Steudler P A, Melillo J M, Aber J D, Bowden R D. Factors controlling atmospheric methane consumption by temperate forest soils. Global Biogeochem Cycles. 1995;9:1–10. [Google Scholar]
- 11.Conrad R. Soil microorganisms as controllers of atmospheric trace gases. Microbiol Rev. 1996;60:609–640. doi: 10.1128/mr.60.4.609-640.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crutzen P J. Methane’s sinks and sources. Nature. 1991;350:380–381. [Google Scholar]
- 13.Goulding K W T, Hütsch B W, Webster C P, Willison T W, Powlson D S. The effect of agriculture on methane oxidation in soil. Phil Trans R Soc Lond A. 1995;351:313–325. [Google Scholar]
- 14.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou C T, Laskin A I, Patel R N. Growth and polysaccharide production by Methylocystis parvusOBBP on methanol. Appl Environ Microbiol. 1978;37:800–804. doi: 10.1128/aem.37.5.800-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou C T, Patel R, Laskin A I, Barnabe N. Microbial oxidation of gaseous hydrocarbons: epoxidation of C2 to C4n-alkenes by methanotrophic bacteria. Appl Environ Microbiol. 1979;38:127–134. doi: 10.1128/aem.38.1.127-134.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyder S L, Meyers A, Cayer M L. Membrane modulation in a methylotrophic bacterium Methylococcus capsulatus(Texas) as a function of growth substrate. Tissue Cell. 1979;11:597–610. doi: 10.1016/0040-8166(79)90017-x. [DOI] [PubMed] [Google Scholar]
- 18.Joergensen L, Degn H. Mass spectrometric measurements of methane and oxygen utilization by methanotrophic bacteria. FEMS Microbiol Lett. 1983;20:331–335. [Google Scholar]
- 19.King G M. Ecophysiological characteristics of obligate methanotrophic bacteria and methane oxidation in situ. In: Murrell J C, Kelly D P, editors. Microbial growth on C1 compounds. Andover, United Kingdom: Intercept Ltd.; 1993. pp. 303–313. [Google Scholar]
- 20.King G M, Adamsen P S. Effects of temperature on methane consumption in a forest soil and in pure cultures of the methanotroph Methylomonas rubra. Appl Environ Microbiol. 1992;58:2758–2763. doi: 10.1128/aem.58.9.2758-2763.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch A L. Microbial physiology and ecology of slow growth. Microbiol Mol Biol Rev. 1997;61:305–318. doi: 10.1128/mmbr.61.3.305-318.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lidstrom M E. Environmental molecular biology approaches: promises and pitfalls. In: Murrell J C, Kelly D P, editors. Microbiology of atmospheric trace gases. Sources, sinks and global change processes. Berlin, Germany: Springer Verlag; 1996. pp. 121–134. [Google Scholar]
- 23.Linton J D, Vokes J. Growth of the methane utilizing bacterium MethylococcusNCIB 11083 in mineral salts medium with methanol as the sole source of carbon. FEMS Microbiol Lett. 1978;4:125–128. [Google Scholar]
- 24.Mosier A, Schimel D, Valentine D, Bronson K, Parton W. Methane and nitrous oxide fluxes in native, fertilized and cultivated grasslands. Nature. 1991;350:330–332. [Google Scholar]
- 25.Oremland R S, Culbertson C W. Importance of methane-oxidizing bacteria in the methane budget as revealed by the use of a specific inhibitor. Nature. 1992;356:421–423. [Google Scholar]
- 26.Patt T E, Cole G C, Bland J, Hanson R S. Isolation and characterisation of bacteria that grow on methane and organic compounds as sole sources of carbon and energy. J Bacteriol. 1974;120:955–964. doi: 10.1128/jb.120.2.955-964.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prior S D, Dalton H. The effect of copper ions on membrane content and methane monooxygenase activity in methanol-grown cells of Methylococcus capsulatus(Bath) J Gen Microbiol. 1985;131:155–163. [Google Scholar]
- 28.Roslev P, Iversen N, Henriksen K. Oxidation and assimilation of atmospheric methane by soil methane oxidizers. Appl Environ Microbiol. 1997;63:874–880. doi: 10.1128/aem.63.3.874-880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnell S, King G M. Mechanistic analysis of ammonium inhibition of atmospheric methane consumption in forest soils. Appl Environ Microbiol. 1994;60:3514–3521. doi: 10.1128/aem.60.10.3514-3521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnell S, King G M. Stability of methane oxidation capacity to variations in methane and nutrient concentrations. FEMS Microbiol Ecol. 1995;17:285–294. [Google Scholar]
- 31.Steudler P A, Bowden R D, Melillo J M, Aber J D. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature. 1989;341:314–315. [Google Scholar]
- 32.Whalen S C, Reeburgh W S. Consumption of atmospheric methane by tundra soils. Nature. 1990;346:160–162. [Google Scholar]
- 33.Wilhelm E, Battino R, Wilcock J. Low-pressure solubility of gases in liquid water. Chem Rev. 1977;77:219–262. [Google Scholar]
- 34.Zhao S-J, Hanson R S. Variants of the obligate methanotroph isolate 761M capable of growth on glucose in the absence of methane. Appl Environ Microbiol. 1984;48:807–812. doi: 10.1128/aem.48.4.807-812.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]