Abstract
Aims
Infections resulting from cardiac implantable electronic device (CIED) implantation are severely impacting on patients’ and on health care systems. The use of TYRXTM absorbable antibiotic-eluting envelope has proven to decrease major CIED infections within 12 months of CIED surgery. The aim is to evaluate the impact of the envelope use on infection-related clinical events in a real-world contemporary patient population.
Methods and results
Data on patients undergoing CIED surgery were collected prospectively by participating centers of the One Hospital ClinicalService project. Patients were divided into two groups according to whether TYRXTM absorbable antibiotic-eluting envelope was used or not. Out of 1819 patients, 872 (47.9%) were implanted with an absorbable antibiotic-eluting envelope and included in the Envelope group and 947 (52.1%) patients who did not receive an envelope were included in the Control group. Compared to control, patients in the Envelope group had higher thrombo-embolic or hemorrhagic risk, higher BMI, lower LVEF and more comorbidities. During a mean follow-up of 1.4 years, the incidence of infection-related events was significantly higher in the control compared to the Envelope group (2.4% vs. 0.8%, P = 0.007). The five-year cumulative incidence of infection-related events was 8.1% in the control and 2.1% in the Envelope group (HR: 0.34, 95%CI: 0.14–0.80, P = 0.010).
Conclusion
In our analysis, the use of an absorbable antibiotic-eluting envelope in the general CIED population was associated with a lower risk of systemic and pocket infection.
Keywords: Systemic infection, Pacemaker, Pocket infection, CIED, Antibiotic eluting envelope
Graphical Abstract
Graphical abstract.
What's new?
The percentage of infection-related events in the contemporary population was low, around 2% at 1 year and the use of the envelope in clinical practice seemed to be preferred in case of high-risk infection patients.
The use of the antibacterial envelope was associated with a lower risk of the composite endpoint of systemic or pocket infection by more than 60%. These findings were confirmed on long term follow-up.
When considering a propensity-matched population, the reduction of the risk of the events was higher.
Introduction
Infections resulting from cardiac implantable electronic device (CIED) implantation are rare but serious complications impacting on patients’ outcome and the entire health care system due to hospitalizations, associated complications, increased mortality and costs.1–5 Recently, a randomized clinical trial6 and observational studies have demonstrated the efficacy of an absorbable antibiotic-eluting envelope (TYRX™, Medtronic, Minneapolis, US), in reducing the risk of CIED-related infections in particular in case of CIED replacement procedures, upgrades, revisions, or initial cardiac resynchronization therapy—defibrillator implantation.6–10 Nevertheless, there is still a lack of understanding which patients receive the envelope in the real-world clinical practice, and TYRXTM efficacy in a setting different from a randomized trial. The aim of the present analysis is to describe a large, unselected population undergoing CIED surgery, and observe TYRXTM efficacy in preventing infection- related events along follow up.
Methods
Project design
Consecutive patients undergoing an initial Medtronic CIED implant, or CIED surgery from August 2016 to May 2022 in the 11 Italian centers participating in the One Hospital ClinicalService project were included in the analysis. One Hospital ClinicalService is a clinical data repository and medical care project designed to describe and improve the quality of diagnostic and therapeutic strategies using technologies and therapies in Italian clinical practice. The project consists of a shared environment for the prospective collection, management, analysis, and reporting of data from patients who have received Medtronic therapies. An independent scientific committee of physicians prospectively identifies key clinical questions on an annual basis for purposes of analysis and publication. A charter assigns the ownership of data to the centers and governs the conduct and relationship of the scientific committee and Medtronic. Medtronic did not have any role in identifying research objectives, interpreting results, or drafting the manuscript. In the REINFORCE (REducing INFectiOns thRough Cardiac device Envelope) project, physicians were prospectively aiming to collect patient and Medtronic device data on risk of infection in patients underwent CIED surgery, and to assess the outcomes including systemic or pocket infection in the setting of the daily clinical practice. This project was approved by each site’s Institutional Review Board and Local Ethics Committees and conforms to the principles outlined in the 1975 Declaration of Helsinki as reflected in the a priori approval by the institution's human research committee. Each patient included in the One Hospital ClinicalService project provided informed consent for data collection and analysis.
The objective of this research was to describe the patient population who received the antibacterial envelope during Medtronic CIED implantation or CIED surgery, and to assess the impact of the envelope in preventing infection-related events. The primary efficacy endpoints were defined before the beginning of the analysis and were the incidence of infection-related events (including system infections or pocket infections).
The patient population was divided into two groups. The Envelope group consisted of patients that received an absorbable antibiotic-eluting envelope for the index procedure or a system modification, and the control group included patients who underwent CIED surgery without the use of envelope. Standards of clinical practice at each participating center determined when patients were treated with or without antibiotic eluting envelopes.
Population and procedural characteristics
During the baseline visit several patients’ clinical characteristics were collected, including age, sex, NYHA class, CHA2DS2-VASc scores, presence of hypertension, diabetes, previous thromboembolic events, presence of structural heart disease with the left ventricular ejection fraction (LVEF) measured by echocardiography, presence of renal insufficiency, immunodeficiency. Moreover, the history of procedures on existing pockets was collected (generator replacement, system revision or upgrade including any lead procedure), device type [pacemaker (PM), implantable cardiac defibrillator (ICD), cardiac resynchronization therapy (CRT)], presence of active or abandoned leads and presence of fever in the 48 h prior to the procedure. Antibiotic prophylaxis was administered in almost all patients according to the clinical practice of each center; cephalosporins being the agent used most frequently. In particular, cefazolin or first generation cephalosporins were given 1 h before the incision. In case of allergy to cefazolin, vancomycin (15 mg/Kg) was administrated 2 h before the incision. All information about any infection-related adverse events occurring during the procedure or during the follow-up was recorded and collected. A pocket infection was defined as superficial cellulitis in the region of the CIED pocket with wound dehiscence, erosion, or purulent drainage or deep incisional (pocket) surgical-site infection or persistent bacteremia according to the definition used in WRAP-IT trial.6 A systemic infection was defined as infection (including positive blood cultures and lead vegetations), persistent bacteraemia or endocarditis involving many different parts of the body or more than one body system at the same time with clinical sign like fever.
Follow-up and event collection
Follow-up visits were made in accordance with the clinical practice of each center, including clinic visits for stiches removal or wound control 10–15 days after the surgery, then every 3–6 months in case of ICD or CRT devices and every 6–12 months in case of PM. The standard visit consisted of an assessment of the patient’s symptoms, an electrocardiogram, device interrogation and device pocket examination, and an assessment of the patient’s medications. If patients missed the scheduled in-hospital follow-up visits, they or their relatives were contacted by phone; after two unsuccessful attempts at phone contact, information on patients’ survival was collected from the National Office of Vital Statistics.
Statistical analysis
Descriptive statistics were used to summarize all results. These include mean and standard deviation and median with interquartile range (IQR) for continuous variables and counts and percentages for categorical variables. Continuous variables were compared between groups using Wilcoxon’s test., and categorical variables were compared between groups using the Chi-square test or Fisher’s exact test, as appropriate. All statistical tests were based on a two-sided significance level of 0.05. Incidence Rates (IRs) were expressed as number of events/100 patient-months, and estimated using Poisson regression models, with deviance scaling to correct for over/under-dispersion. Estimates along with their 95% Confidence Intervals (CIs) were reported. Estimated differences between groups were expressed as Incidence Rate Ratios (IRRs), along with their 95% CIs. For all patients, only clinical events after the implant date (start date) during the study period were considered. The end date was the last contact date. Last contact date was defined as the latest date among in-hospital FU dates, telephone contact dates, clinical event dates system modification date and exit dates. We calculated for each patient the raw PADIT risk scores11 and the relative risk of infection-related events for each score was estimated and reported as Odds Ratio, together with its 95% CI as a sensitivity analysis. To account for differences in baseline characteristics between envelope and control groups, propensity score (PS) method was utilized to estimate an adjusted risk ratio for infection between envelope and control. The PS method was used to adjust the group’s risk ratio in both Poisson and Kaplan–Meier analysis. The PSs for each patient were calculated by using a logistic regression model that included PADIT risk score only. PADIT risk score groups (Low, Medium, and High) were used as the only match. Additionally, a sensitivity analysis of the primary outcome was performed using inverse probability of treatment weighting (IPTW) on PSs. SAS software, version 9.4, (SAS Institute Inc., Cary, NC, USA) was used to perform statistical analyses.
Results
In this analysis, 1819 consecutive subjects underwent an index CIED implantation or system modification in 11 centers. There were 872 (47.9%) subjects in the Envelope group, and 947 (52.1%) in the Control group. The mean percentage of patients treated with envelope per center was 58.6% ± 37. Supplementary material online, table S1.
Baseline and procedural characteristics
Baseline patient characteristics and procedural data are listed in Table 1. There were several differences between the Envelope and Control group, with regard to baseline characteristics. The Envelope group was more likely to be younger, have higher BMI and CHA2D2-VASC score, more likely to have a history of heart failure, ventricular arrhythmic episodes, ischemic heart disease, hypertension, diabetes and chronic kidney disease and have lower (LVEF) and to use anticoagulant drugs. Importantly, the groups of patients differed also for infective risk calculated using PADIT score: in the Envelope group, more than 37% of patients were at high risk according to PADIT score, contrary to 11% in the Control group. (P < 0.001) All other baseline characteristics did not differ, including sex, history of stroke/TIA, and history of atrial fibrillation (AF). (Table 1). Out of 1819 patients, 1817 were in antibiotic prophylaxis during the CIED implant or surgery. In two patients the data was missed.
Table 1.
Baseline characteristics of the total population and statistical comparisons between the two groups of patients: subjects in the envelope group vs. the control group
| Baseline Characteristic | Total (n = 1819) |
Envelope (N = 872) |
Control (N = 947) |
P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (yrs/old) | 72.8 ± 14.0 | 72.1 ± 14.1 | 73.4 ± 13.9 | 0.036 |
| Gender (Male) | 69.5% (1263) | 69.2% (603) | 69.7% (660) | 0.830 |
| BMI (kg/m²) | 26.7 ± 4.6 | 27.1 ± 4.7 | 26.5 ± 4.4 | 0.049 |
| Medical history | ||||
| History of HF | 52.1% (947) | 64.8% (565) | 39.9% (382) | <0.001 |
| History of VT/VF | 16.2% (294) | 18.1% (157) | 14.5% (137) | 0.038 |
| History of AT/AF | 38.4% (698) | 40.5% (353) | 36.5% (345) | 0.084 |
| Paroxysmal AF | 18.2% (331) | 17.4% (151) | 18.9% (180) | 0.402 |
| Persistent AF | 5.4% (98) | 6.2% (54) | 4.7% (44) | 0.190 |
| Permanent AF | 14.8% (269) | 16.9% (147) | 12.8% (122) | 0.014 |
| Ischemic Heart Disease | 34.1% (620) | 39.5% (341) | 29.0% (279) | <0.001 |
| Valvular Disease | 32.6% (593) | 41.0% (349) | 24.7% (244) | <0.001 |
| History of Stroke/TIA | 4.4% (80) | 4.9% (43) | 3.9% (37) | 0.307 |
| Hypertension | 76.6% (1393) | 79.4% (691) | 74.1% (702) | 0.008 |
| Diabetes | 27.6% (502) | 33.8% (294) | 22.0% (208) | <0.001 |
| Chronic Kidney Disease | 25.2% (458) | 36.8% (320) | 14.0% (138) | <0.001 |
| CHADS₂≥2 | 73.2% (1331) | 80.4% (701) | 66.5% (530) | <0.001 |
| CHA₂DS₂-VASc ≥ 4 | 50.1% (911) | 58.9% (513) | 42.3% (398) | <0.001 |
| COPD | 11.5% (209) | 12.5% (109) | 10.6% (100) | 0.209 |
| LVEF (%) | 43.4 ± 14.3 | 40.7 ± 13.2 | 46.2 ± 14.9 | <0.001 |
| Risk Score | ||||
| PADIT Risk Score | 4.4 ± 3.2 | 5.6 ± 3.1 | 3.3 ± 2.8 | <0.001* |
| Low (Score: 0–4) | 49.6% (903) | 31.1% (271) | 66.7% (632) | |
| Medium (Score: 5–6) | 26.6% (483) | 31.7% (276) | 21.9% (207) | |
| High (Score: ≥7) | 23.8% (433) | 37.3% (325) | 11.4% (108) | |
| Implantable device Type | ||||
| CRT-D | 32.9% (599) | 40.6% (352) | 26.7% (247) | <0.001* |
| CRT-P | 8.4% (152) | 8.5% (74) | 8.3% (78) | |
| ICD-DC | 11.1% (201) | 10.3% (90) | 11.7% (111) | |
| ICD-SC | 7.9% (143) | 6.6% (57) | 8.9% (86) | |
| PM | 39.7% (724) | 34.0% (299) | 44.4% (425) | |
| De Novo CIED | 64.7% (1178) | 45% (393) | 82.8% (785) | <0.001 |
| De Novo PM | 52.9% (623) | 51.2% (201) | 53.8% (422) | 0.457 |
| De Novo ICD | 47.1% (555) | 48.8% (192) | 46.2% (363) | 0.457 |
| Previous CIED implantation | 35.2% (641) | 54.9% (479) | 17.1% (162) | <0.001 |
| To PM | 61.2% (392) | 65.0% (308) | 52.1% (84) | 0.008 |
| To ICD | 38.8% (249) | 35.0% (171) | 47.9% (78) | 0.008 |
| Antiplatelets and Anticoagulant use | ||||
| Antiplatelets | 33.5% (609) | 32.1% (279) | 34.7% (330) | 0.251 |
| Anticoagulant | 35.9% (653) | 39.4% (343) | 32.6% (310) | 0.004 |
Significative P-values are in bold.
Abbreviations: BMI, Body mass index; HF, heart failure; AF, atrial fibrillation; VT, ventricular tachyarrhythmias; VF, Ventricular fibrillation; TIA, transient ischemic attack; LVEF, left ventricular ejection fraction; CRT-D, Cardiac Resynchronization Therapy defibrillator; CRT-P, Cardiac Resynchronization Therapy Pacemaker; ICD-DC, dual chamber implantable defibrillator; ICD-SC, single chamber defibrillator; PM, Pacemaker.
*Statistical test conducted between entire Envelope cohort vs. Control group on device distribution.
Out of 1819, 39.7% of patients were implanted with a PM, with a significant difference between the two groups (34.0% and 44.4% in the Envelope and control group, respectively, P < 0.001). In the Envelope group, 40.6% of subjects were implanted with a CRT-D, while 26.7% in the Control group received a CRT-D (P < 0.001). In the whole population, 1178 (65%) were de-novo patients, while 641 (35%) had a previous CIED implantation.
Infection-related events
During a mean follow up time of 1.4 ± 1.7 years (1.5 ± 1.7 in the Envelope and 1.4 ± 1.6 in the control group, P = 0.534), 27/1819 (1.5%) patients experienced a pocket infection, 3 (0.2%) a systemic infection. Table 2 The Control group had significantly higher overall pocket infection or systemic infection rates as compared with the Envelope group (2.4% (23/947) vs. 0.8% (7/872), P = 0.007).
Table 2.
Infective related events of the total population and comparison between the two groups of patients: subjects in the envelope group vs. the control group
| Clinical Event | Total (N = 1819) |
Envelope (N = 872) |
Control (N = 947) |
P-Value |
|---|---|---|---|---|
| At least one infection-related clinical event (Systemic or Pocket infection) | 1.6% (30/1819) | 0.8% (7/872) | 2.4% (23/947) | 0.007 |
| Pocket infection | 1.5% (27/1819) | 0.6% (5/872) | 2.3% (22/947) | 0.002 |
| Systemic infection | 0.2% (3/1819) | 0.2% (2/872) | 0.1% (1/947) | 0.516 |
Significative P-values are in bold.
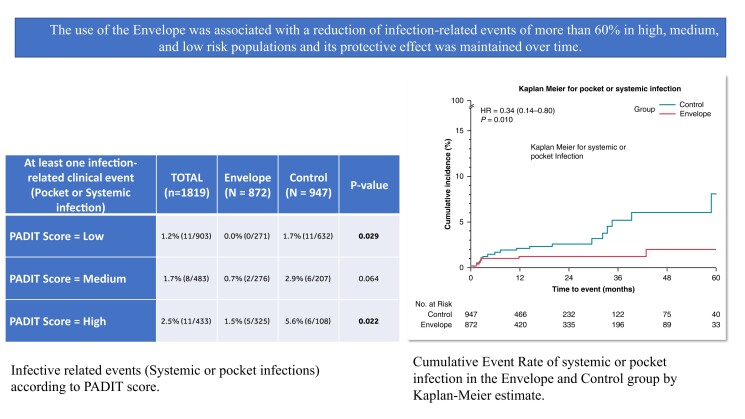
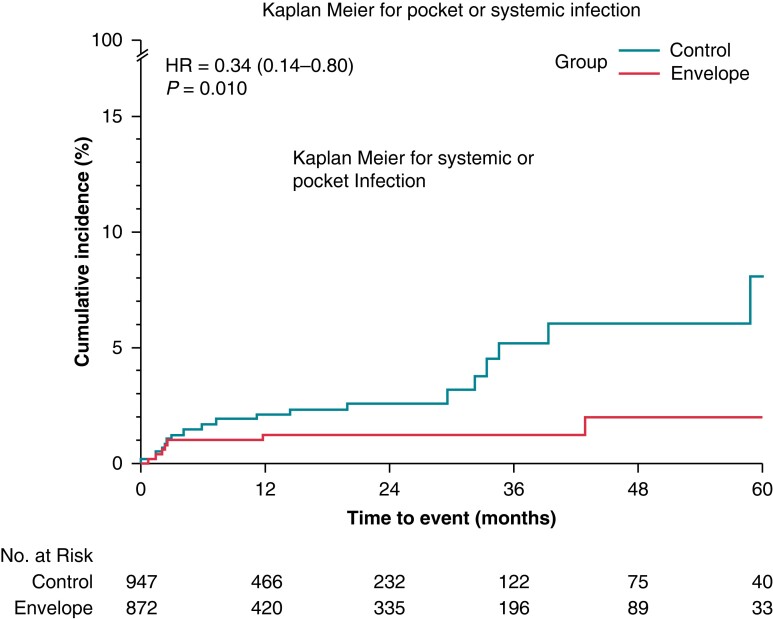
All pocket infections resulted in CIED system removal (device and leads). Pocket infection occurred in five subjects (0.6%) in the Envelope group and in 22 (2.3%) patients in the Control group, P = 0.002, as shown in Table 2. Systemic infection occurred in three subjects, two in the Envelope group, and one in the control group. Out of those three subjects, two died as a consequence of the systemic infection. The monthly rate per 100 patients of the composite endpoint of pocket infection and systemic events was 0.04 (95% CI 0.06–0.06) and 0.16 (95% CI 0.14–0.19) in the Envelope and Control group, respectively (P < 0.001). The IRs confirm the protective effect of the envelope in the Envelope group with respect to infection-related events, with a risk reduction of 62% (IRR: 0.38, 95% CI 0.19–0.38, P < 0.001). We adjusted the IRs taking into account each center in order to consider the differences in the usage of envelope amongst participating centers between Envelope and Control groups. The findings confirmed the main analysis: The adjusted monthly rate per 100 patients was 0.03 (95% CI 0.02–0.04) and 0.15 (95% CI 0.12–0.18) in the Envelope and Control group, respectively (adjusted IRR: 0.20, 95% CI 0.14–0.28, P < 0.001). The unadjusted survival analysis for risk showed the 5-year event rate of 2.1% (95% CI 0.8–5.0%) in the Envelope group vs. 8.1% (95% CI 4.3–15.0%) in the Control group (HR: 0.34, 95%CI: 0.14–0.80, P = 0.010), as shown in Figure 1 panel A. Table 3 shows the incidence of infection related events according to PADIT risk scores. Out of 903 patients with a low PADIT score (Score: 0–4), 11 (1.2%) had at least infection-related event: no events occurred in the 271 patients in the envelope cohort, while 11 (1.7%) occurred in the 632 patients in the Control group (P = 0.029). In contrast, out of 433 patients with high PADIT scores (score ≥7), there were 11 events (2.5%): 5 of 325 (1.5%) in the Envelope group, and 6 of 108 (5.6%) in the control group, P = 0.022. Out of 1178 patients with de-novo CIED implantation, in 18 (1.5%) occurred a systemic or pocket infection: 3 (0.8%) in the Envelope group vs. 15 (1.9%) in the Control group (P = 0.130). The monthly rate per 100 patients was 0.04 (95%CI 0.03–0.07) in the Envelope and 0.13 (95% CI 0.11–0.16) in the Control group, P < 0.001. Supplementary material online, table S2A showed the event rates per group. In the group of patients with previous CIED surgery, systemic or pocket infection occurred in 12 patients: 4(0.8%) in the Envelope group and 8 (4.9%) in the control group, P < 0.001. The monthly rate per 100 patients was 0.04 (95% CI: 0.03–0.06) in the Envelope and 0.25 (95% CI 0.19–0.32) in the Control group, P < 0.001. Supplementary material online, table S2B.
Figure 1.
Panel A cumulative event rate of systemic or pocket infection in the envelope and control group by Kaplan–Meier estimate.
Table 3.
Infective related events according to PADIT score
| At least one infection-related clinical event (Pocket or Systemic infection) | ||||
|---|---|---|---|---|
| PADIT Score = Low | 1.2% (11/903) | 0.0% (0/271) | 1.7% (11/632) | 0.029 |
| PADIT Score = Medium | 1.7% (8/483) | 0.7% (2/276) | 2.9% (6/207) | 0.064 |
| PADIT Score = High | 2.5% (11/433) | 1.5% (5/325) | 5.6% (6/108) | 0.022 |
| At least one Pocket infection | ||||
| PADIT Score = Low | 1.1% (10/903) | 0.0% (0/271) | 1.6% (10/632) | 0.037 |
| PADIT Score = Medium | 1.7% (8/483) | 0.7% (2/276) | 2.9% (6/207) | 0.064 |
| PADIT Score = High | 2.1% (9/433) | 0.9% (3/325) | 5.6% (6/108) | 0.003 |
Significative P-values are in bold.
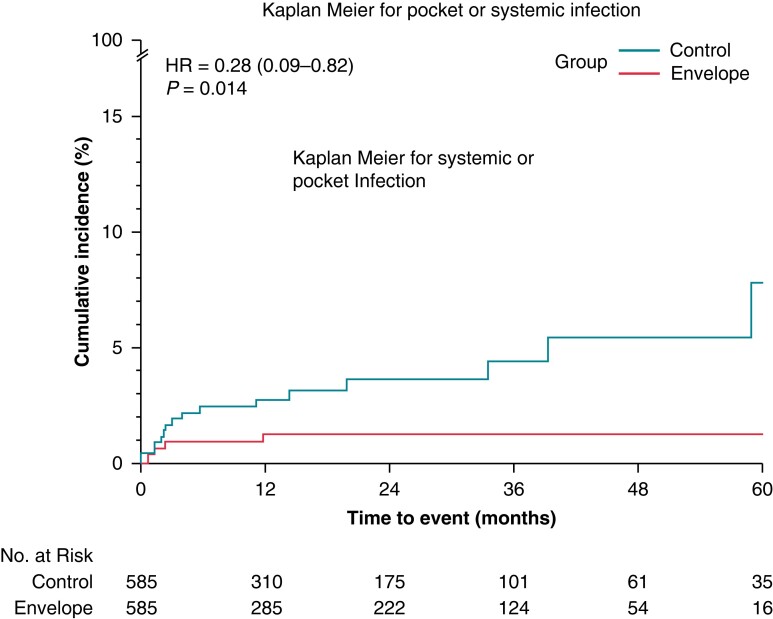
A matched cohort sub-analysis
PS matching, using PADIT score as variable, identified 585 pairs of patients with balanced baseline characteristics with respect to PADIT Score.11 Baseline characteristics are shown in Supplementary material online, Table S3. The mean follow up was 1.5 years with no significant differences between the two groups of patients. The risk of systemic or pocket infection at 60 months post implant was 7.7% (95% CI: 3.7%—15.4%) in the control group and 1.2% (95% CI: 0.5%–3.3%) in the Envelope group (HR: 0.28, 95% CI: 0.09–0.82, P = 0.014) as shown in Figure 2 panel A. The incidence of infection related events is shown in Supplementary material online, table S4.
Figure 2.
Cumulative event rate of systemic or pocket infection in the propensity matched cohort: envelope and control group by Kaplan–Meier estimate.
Additionally, a sensitivity analysis of the primary outcome using IPTW on PSs was performed, as shown in Supplementary material online, table S5 and S6. The results confirmed the main analysis.
Discussion
Main results
The main findings of the present study are as follows: (i) in contemporary clinical practice, the absorbable antibiotic eluting envelope was more frequently used to prevent infection-related complications in the cohort of patients with higher infective risk scores; (ii) use of the antibacterial envelope was associated with a lower risk of the composite endpoint of systemic or pocket infection by more than 60% and when considering a propensity-matched population, the reduction of the risk of the events was higher, (iii) these findings were confirmed on long term follow-up.
Patient population
Infections resulting from CIED implantation are rare but are associated with significant morbidity, mortality and increased cost.1–4 The majority of infections involve the device pocket, but they can lead to infective endocarditis and progress into systemic infections.1–4 In some patients with worse prognoses, systemic infection may lead to lead- related endocarditis progressing to pocket infection.12,13 The rates of infections in the CIED populations ranges from 1% to 19.9%1,2 and depend on several factors including clinical characteristics and presence of comorbidities, procedural complexity and numbers and times to re-interventions. Recently, a large, randomized study demonstrated the incremental benefit in using an antibacterial envelope in reducing the rate of overall CIED infections by approximately 40%.6 Moreover, the envelope has been showed to prevent hematoma from transitioning into an infection.14 In our study, we prospectively collected data on baseline characteristics, envelope usage and infection-related events in consecutive patients during routine clinical practice. The antibacterial envelope was used in 48% of observed patients, and these patients were at higher infective risk (mean PADIT score 5.6 ± 3.1 vs. 3.3 ± 2.8 in the Envelope and control group, respectively), with more comorbidities, and more often implanted with CRT-D, compared to the cohort of patients without envelopes. In contrast, the RI-AIAC study showed that in a real-life cohort of patients receiving a CIED, the envelope was used in a few selected cases (2% of enrolled patients). These differences in the use of envelope could be explained by the older cohort of patients, and the higher percentage of PM implants in the study by Boriani et al. (RI-AIAC), two factors that are related to a lesser infection risk (PADIT).15
Our findings showed that among the participating centers the median value of percentage of patients with envelope was 68.3% (I-III IQR: 24–86%). This heterogeneous situation depended on the choice and clinical practice of each center.
Efficacy/effectiveness in preventing infections
The WRAP-IT study6 showed the TYRXTM was significantly more effective at preventing infection than standard infection-control strategies alone with an event rate at 1 year of 0.7% and 1.2%, respectively (hazard ratio, 0.60; 95% CI, 0.36 to 0.98; P = 0.04). In our analysis, at 1 year the event rate of systemic or pocket infection was 1.3% (95% CI 0.6%–2.8%) in the Envelope group and 2.1% (95% CI: 1.2%–3.6%) in the Control group, increasing at 2.1% (95% CI 0.8%–5.0%) and 8.1% (95% CI: 3.5%–10.3%) at the 5th year. This raises the hypotheses that preventing bacterial seeding at any index procedure may prevent pocket infection at a later stage.16 Interestingly, a long-term analysis of the WRAP-IT trial data17 showed that infections continued to rise at 12 months post-procedure and that device-related infections are time-dependent and not confined to the 12 months after the index procedure. Although data on repeated procedures (lead repositioning, pocket revision) that might represent further opportunities for pocket infection are missing in both these studies the hypothesis of a sustained benefit of TYRXTM at long term should not be neglected. In our study, when propensity matched populations are considered, the rate of infection-related events increased only in the control group, while in the envelope group remained stable in the first-year post-procedure. These data further support that the use of the antibiotic envelope, on the top of antibiotic prophylaxis, should be included in any peri-operative plan targeted to minimize the infection risk in appropriately selected patients, on the basis of their clinical profile and predicted risk of CIED infections in combination with a series of clinical measures and logistical-organizational features.11–21
In this study, the overall incidence of systemic or pocket infection was around 1.6%. In particular, 0.2% was system infection, while 1.5% pocket infection. The large use of antibiotic prophylaxis, standard protocols to prevent infections including chlorhexidine skin preparation and preventive strategies in case of increased risk, may influence these occurrences. The diagnosis of pocket or systemic infection is very challenging. However, in this study, systemic infections are only diagnosticated in case of presence of clinical signs with positive blood cultures and the presence of lead vegetations. In the general CIED population, current data have reported that CIED infection ranged from 0.1–0.7% to 4% depending on the type of device, procedure, and centers.6,22 When high risk population was assessed, we found that the incidence of infection rose to 5.6% during a mean follow up of 1.4 years.
Our results were in line with the results of previous studies, showing a risk reduction of 62% in the incidence of CIED infection. The WRAP-IT demonstrated a 40% reduction in CIED infections and a 60% reduction in major pocket infections6 in high-risk patients with a positive cost-effective analysis. Moreover, we also reported a reduction in the incidence in low-risk patients according to PADIT risk score. A reduction in the prize of the envelope might broaden the use of the device after further evidence. More larger and randomized studies are needed to corroborate these early findings.
Cost-efficacy consideration
The use of the envelope is associated with additional costs which need to be compared to the benefits it provides.23A common method to compare costs and benefits is cost-effectiveness analysis. In cost-effectiveness analysis, the incremental costs of an intervention are compared to the willingness to pay threshold for a better health outcome. The antibacterial envelope has been reported to be cost-effective in selected patients at increased risk of infection in Italy based on the WRAP-IT study. The number needed to treat ranged from 35–185 in the different patient groups. The sensitivity analysis showed the risk of infection required for the envelope to be cost-effective was around 2.7% for Italy.24 In this study, the risk of systemic or pocket infection 5 years post-implant were 8.1% in the control group, 6.0% percentage points higher than in treatment group. This results in a number needed to treat (NNT) per infection avoided of 17. It is highly suggestive that use of the antibacterial envelope was cost-effective in this real-world cohort of patients with higher infective risk scores than in the above-mentioned cost-effectiveness analysis for Italy.
Limitations
This analysis presents some limitations, including: (i) its non-randomized observational nature, so bias could be present in patient selection and treatment; in particular, selection bias and possible presence of the imbalanced distribution in baseline characteristics may have affected the data set as it is possible that there were factors (e.g. risk of infection, drug therapy, attitude of physicians, economic issues) which influenced envelope usage. To overcome this issue, sensitivity analyses based on the PS matching and IPTW on PSs was additionally conducted to control for this imbalance and potential bias; (ii) data of infection-related events were collected during in hospital follow-up visits but some events treated in other hospitals may have been missed; (iii) Pocket and systemic infection were judged to be adverse event based on the description provided by the physician; (iv) the absence of a standardized protocol for the follow up; (v) the envelope group had more comorbidities compared to the control group, though paired-group analyses based on similar PADIT score risk were conducted, and found similar findings; and (vi) the data were based on the clinical practice of several participating centers with different standard-of-care procedures. No recommendations were provided to the participating centers in terms of pharmacological and antibiotic treatment prior to and following the procedure. However, these limitations are balanced by the accurate picture of real-world clinical patient treatment that these data provide.
Conclusions
In our real-world experience, the use of an absorbable antibiotic-eluting envelope in the general CIED population appeared of clinical value, being associated with a lower risk of the composite endpoint of systemic and pocket infection. The percentage of infection-related events in the contemporary population was low, around 2% at 1 year and the use of the envelope in clinical practice seemed to be preferred in case of high-risk infection patients. The use of the Envelope was associated with a reduction of infection-related events of more than 60% in high, medium, and low risk populations and its protective effect was maintained over time.
Supplementary Material
Acknowledgements
We would like to thank all nurses and technicians that helped during the data collection.
Contributor Information
Matteo Ziacchi, Institute of Cardiology, IRCCS Azienda Ospedaliero Universitaria di Bologna, Via Massarenti 9, 40125 Bologna, Italy.
Mauro Biffi, Institute of Cardiology, IRCCS Azienda Ospedaliero Universitaria di Bologna, Via Massarenti 9, 40125 Bologna, Italy.
Saverio Iacopino, GVM, Maria Cecilia Hospital, Cotignola.
Michele di Silvestro, Umberto I, Enna.
Procolo Marchese, Presidio ospedaliero G. Mazzoni, Ascoli Piceno.
Francesca Miscio, Ospedale ‘Teresa Masselli Mascia’, San Severo, Foggia.
Vincenzo Paolo Caccavo, Ospedale F. Miulli, Acqua Viva delle Fonti.
Gabriele Zanotto, Ospedale Mater Salutis di Legnago.
Luca Tomasi, Azienda Ospedaliero-Universitaria di Verona.
Antonio Dello Russo, Azienda Ospedaliero Universitaria delle Marche, Ancona.
Luca Donazzan, Cardiology Department, Ospedale San Maurizio, Bolzano, Italy.
Giuseppe Boriani, Cardiology Division, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Policlinico di Modena, Modena, Italy.
Supplementary material
Supplementary material is available at Europace online.
Compliance with ethical standards
All procedures performed in this project involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical approval
This project was approved by each site’s Institutional Review Board and Local Ethics Committees.
Informed Consent.
Each patient included in the ClinicalService project provided informed consent for data collection and analysis.
Funding
Open access fee supported by TRXITALY.
Data availability
The datasets generated during and/or analysed during the current study are available on reasonable request.
References
- 1. Blomström-Lundqvist C, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MGet al. . European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020;22:515–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Korantzopoulos P, Sideris S, Dilaveris P, Gatzoulis K, Goudevenos JA. Infection control in implantation of cardiac implantable electronic devices: current evidence, controversial points, and unresolved issues. Europace 2016;18:473–8. [DOI] [PubMed] [Google Scholar]
- 3. Sławiński G, Lewicka E, Kempa M, Budrejko S, Racza G. Infections of cardiac implantable electronic devices: epidemiology, classification, treatment, and prognosis. Adv Clin Exp Med 2019;28:263–70. [DOI] [PubMed] [Google Scholar]
- 4. Roder C, Gunjaca V, Otome O, Gwini SM, Athan E. Cost and outcomes of implantable cardiac electronic device infections in Victoria, Australia. Heart Lung Circ 2020;29:e140–6. [DOI] [PubMed] [Google Scholar]
- 5. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–9. [DOI] [PubMed] [Google Scholar]
- 6. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss Eet al. . Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- 7. Chaudhry U, Borgquist R, Smith JG, Mörtsell D. Efficacy of the antibacterial envelope to prevent cardiac implantable electronic device infection in a high-risk population. Europace 2022;24:1973–80. [DOI] [PubMed] [Google Scholar]
- 8. Pranata R, Tondas AE, Vania R, Yuniadi Y. Antibiotic envelope is associated with reduction in cardiac implantable electronic devices infections especially for high-power device-systematic review and meta-analysis. J Arrhythm 2019;36:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GRet al. . Cost-effectiveness of an antibacterial envelope for cardiac implantable electronic device infection prevention in the US healthcare system from the WRAP-IT trial. Circ Arrhythm Electrophysiol 2020;13:e008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henrikson CA, Sohail MR, Acosta H, Johnson EE, Rosenthal L, Pachulski Ret al. . Antibacterial envelope is associated with low infection rates after implantable cardioverter-defibrillator and cardiac resynchronization therapy device replacement results of the citadel and centurion studies. JACC Clin Electrophysiol 2017;3:1158–67. [DOI] [PubMed] [Google Scholar]
- 11. Krahn AD, Longtin Y, Philippon F, Birnie DH, Manlucu J, Angaran Pet al. . Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol 2018;72:3098–109. [DOI] [PubMed] [Google Scholar]
- 12. Birnie DH, Wang J, Alings M, Philippon F, Parkash R, Manlucu Jet al. . Risk factors for infections involving cardiac implanted electronic devices. J Am Coll Cardiol 2019;74:2845–54. [DOI] [PubMed] [Google Scholar]
- 13. Diemberger I, Biffi M, Lorenzetti S, Martignani C, Raffaelli E, Ziacchi Met al. . Predictors of long-term survival free from relapses after extraction of infected CIED. Europace 2018;20:1018–27. [DOI] [PubMed] [Google Scholar]
- 14. Tarakji KG, Korantzopoulos P, Philippon F, Biffi M, Mittal S, Poole JEet al. . Infectious consequences of hematoma from cardiac implantable electronic device procedures and the role of the antibiotic envelope: A WRAP-IT trial analysis. Heart Rhythm 2021;18:2080–6. [DOI] [PubMed] [Google Scholar]
- 15. Boriani G, Proietti M, Bertini M, Diemberger I, Palmisano P, Baccarini Set al. . Incidence and predictors of infections and all cause death in patients with cardiac implantable electronic devices: the Italian nationwide RI-AIAC registry. J Pers Med 2022;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleemann T, Becker T, Strauss M, Dyck N, Weisse U, Saggau Wet al. . Prevalence of bacterial colonization of generator pockets in implantable cardioverter defibrillator patients without signs of infection undergoing generator replacement or lead revision. Europace 2010;12:58–63. [DOI] [PubMed] [Google Scholar]
- 17. Mittal S, Wilkoff BL, Kennergren C, Poole JE, Corey R, Bracke FAet al. . The World-wide Randomized Antibiotic Envelope Infection Prevention (WRAP-IT) trial: long-term follow-up. Heart Rhythm 2020;17:1115–22. [DOI] [PubMed] [Google Scholar]
- 18. Tarakji KG, Korantzopoulos P, Philippon F, Biffi M, Mittal S, Poole JEet al. . Risk factors for hematoma in patients undergoing cardiac device procedures: A WRAP-IT trial analysis. Heart Rhythm 2022;3:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu Bet al. . Clinically significant pocket hematoma in- creases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol 2016;67:1300–8. [DOI] [PubMed] [Google Scholar]
- 20. Ziacchi M, Massaro G, Angeletti A, Statuto G, Diemberger I, Martignani Cet al. . Preoperative checklist to reduce the risk of cardiac implantable electronic device infections. Pacing Clin Electrophysiol 2022;45:262–9. [DOI] [PubMed] [Google Scholar]
- 21. Callahan TD, Tarakji KG, Wilkoff BL. Antibiotic eluting envelopes: evidence, technology, and defining high-risk populations. Europace 2021;23:iv28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RTet al. . 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. [DOI] [PubMed] [Google Scholar]
- 23. Boriani G, Vitolo M, Wright DJ, Biffi M, Brown B, Tarakji KGet al. . Infections associated with cardiac electronic implantable devices: economic perspectives and impact of the TYRX™ antibacterial envelope. Europace 2021;23:iv33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boriani G, Kennergren C, Tarakji KG, Wright DJ, Ahmed FZ, McComb JMet al. . Cost-effectiveness analyses of an absorbable antibacterial envelope for use in patients at increased risk of cardiac implantable electronic device infection in Germany, Italy, and England. Value Health 2021;24:930–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available on reasonable request.