Abstract
BACKGROUND:
Brugada syndrome poses significant challenges in terms of risk stratification and management, particularly for asymptomatic patients who comprise the majority of individuals exhibiting Brugada ECG pattern (BrECG). The aim of this study was to evaluate the long-term prognosis of a large cohort of asymptomatic patients with BrECG.
METHODS:
Asymptomatic patients with BrECG (1149) were consecutively collected from 2 Italian centers and followed-up at least annually for 2 to 22 years. For the 539 asymptomatic patients (men, 433 [80%]; mean age, 46±13 years) with spontaneous type 1 documented on baseline ECG (87%) or 12-lead 24-hour Holter monitoring (13%), an electrophysiologic study (EPS) was proposed; for the 610 patients with drug-induced–only type 1 (men, 420 [69%]; mean age, 44±14 years), multiple ECGs and 12-lead Holter were advised in order to detect the occurrence of a spontaneous type-1 BrECG. Arrhythmic events were defined as sudden death or documented ventricular fibrillation or tachycardia.
RESULTS:
Median follow-up was 6 (4–9) years. Seventeen (1.5%) arrhythmic events occurred in the overall asymptomatic population (corresponding to an event-rate of 0.2% per year), including 16 of 539 (0.4% per year) in patients with spontaneous type-1 BrECG and 1 of 610 in those with drug-induced type-1 BrECG (0.03% per year; P<0.001). EPS was performed in 339 (63%) patients with spontaneous type-1 BrECG. Patients with spontaneous type-1 BrECG and positive EPS had significantly higher event rates than patients with negative EPS (7 of 103 [0.7% per year] versus 4 of 236 [0.2% per year]; P=0.025). Among 200 patients who declined EPS, 5 events (0.4% per year) occurred. There was 1 device-related death.
CONCLUSIONS:
The entire population of asymptomatic patients with BrECG exhibits a relatively low event rate per year, which is important in view of the long life expectancy of these young patients. The presence of spontaneous type-1 BrECG associated with positive EPS identifies a subgroup at higher risk. Asymptomatic patients with drug-induced–only BrECG have a minimal arrhythmic risk, but ongoing follow-up with 12-lead Holter monitoring is recommended to detect the appearance of spontaneous type-1 BrECG pattern.
Keywords: arrhythmias, cardiac; Brugada syndrome; death, sudden, cardiac; electrocardiography; electrophysiology
Clinical Perspective.
What Is New?
Asymptomatic patients with Brugada ECG pattern demonstrate a relatively low incidence of arrhythmic events (0.2% per year), and the arrhythmic risk is extremely low in the patients with the true drug-induced–only Brugada ECG (0.03% per year).
The presence of spontaneous type-1 Brugada ECG associated with positive EPS identifies a subgroup of patients with higher arrhythmic risk.
What Are the Clinical Implications?
Patients with true drug-induced–only BrECG pattern confirmed by multiple ECGs and 12-lead 24-hour Holter monitoring (with V1 and V2 leads both on the 4th and 2nd or 3rd intercostal space) exhibit a very low risk, which calls into question the need for therapy, provided that patients adhere to behavioral recommendations and undergo regular follow-up. This observation is important because this subgroup constitutes approximately 50% of asymptomatic patients with BrECG pattern.
Within the cohort of patients displaying spontaneous type-1 Brugada ECG pattern, the electrophysiological study enables the identification of a higher-risk subgroup.
Editorial, see p 1556
Thirty years after the first description of the Brugada syndrome,1 the knowledge of the condition has increased. Currently, consensus exists regarding the treatment of symptomatic patients, including patients with previous history of aborted sudden death or arrhythmic syncope.2,3 For these patients, an implantable cardioverter defibrillator (ICD) is recommended,2,3 based on the consideration that the risk of sudden death outweighs the risk of ICD-related complications. However, symptomatic patients represent only a small percentage of the Brugada patient population. Risk stratification and treatment of the asymptomatic patients are the major challenges at the present time.
Although asymptomatic patients have a low risk of sudden death according to previous studies with relatively short follow-up,4 the total number of arrhythmic events cannot be overlooked. This is because the asymptomatic patient group comprises the majority of patients with Brugada ECG (BrECG) pattern; notably, 80% of patients with sudden death are asymptomatic until the occurrence of the event.5 The current guidelines2,3 do not provide clear indications on how to manage these patients for whom the risk associated with ICD implantation can be equal to or even greater than the intrinsic risk of the disease.6 Because most patients with BrECG pattern are young, asymptomatic, and have a long life expectancy, it becomes very important to define the real incidence of arrhythmic events during long-term follow-up and to identify those patients at higher risk.
The study aimed to evaluate arrhythmic risk in a large population of asymptomatic patients with BrECG pattern, consecutively collected and assessed during long-term (range, 2–22 years) follow-up. The study focused on the event rate in asymptomatic patients with spontaneous type-1 BrECG pattern compared with the event rate of patients with drug-induced–only type-1 BrECG pattern. Moreover, the role of electrophysiologic study (EPS) in the risk stratification of asymptomatic patients with spontaneous type-1 BrECG pattern was investigated further.
METHODS
Study Population
Consecutive patients with spontaneous or drug-induced BrECG pattern were prospectively included in 2 Italian registries (Brugada registry of the Piedmont region and of the Casilino Hospital in Rome), from 2001 to 2022. The 2 registries were linked because one originated from the other after one of the authors (L.C.) moved from Piedmont to Rome. The data from the 2 series were merged because the management of patients was homogeneous. The study conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the medical ethical committee of the referring institutions; all patients gave written informed consent for research purposes. The data that support the findings of this study are available from the corresponding author upon reasonable request.
BrECG pattern was assessed according to the first Consensus Conference criteria7 and subsequent updates.8,9 It was considered diagnostic (ie, type-1 BrECG pattern) when showing a coved-type ST segment elevation ≥2 mm followed by a negative T wave in one or more right precordial leads (V1 and V2),10 including recordings from the second and third intercostal spaces, either spontaneously or after challenge with class-I antiarrhythmic drugs. The presence of structural heart disease was ruled out by clinical examination, laboratory tests, and diagnostic procedures when appropriate. A genetic test, searching for mutations in SCN5A (sodium voltage-gated channel alpha subunit 5) and other Brugada susceptibility genes, was proposed to the patients with spontaneous type-1 BrECG.
From 2001 to 2022, 1669 consecutive patients with BrECG pattern were collected. Patients were classified according to the symptoms reported at the first clinical observation as: patients with sudden death (8 [0.5%]) or aborted sudden death (21 [1.3%]), patients with unexplained syncope (128 [7.6%]) and asymptomatic (1512 [90.6%]). Exclusions criteria included patients with follow-up <2 years and patients on hydroquinidine therapy, due to the potential confounding effect of the drug on the incidence of events. The final study population included 1149 asymptomatic patients (Figure 1A).
Figure 1.
Study population. A, Inclusion criteria. B, Incidence of arrhythmic events during median follow-up (FU) and event rate per year in the entire asymptomatic population with Brugada ECG pattern and in the subgroups with spontaneous and drug-induced–only type 1 ECG pattern. EPS indicates electrophysiologic study.
Ninety-three of these patients were included in the FINGER (France, Italy, Netherlands, Germany) study but had a shorter follow-up at that time (median 1.7 [0.7–3.5] years).4
Management of Asymptomatic Patients
Upon initial clinical observation, patients diagnosed with BrECG pattern were provided with comprehensive recommendations, including avoidance of specific drugs, large meals, excessive alcohol intake, and prompt treatment of fever. These recommendations constitute the first therapeutic step in managing patients with BrECG and are in accordance with the guidelines outlined on the website www.brugadadrugs.org.11
Our treatment approach differed for patients with drug-induced type-1 BrECG and spontaneous type-1 BrECG. For patients with drug-induced type-1 BrECG pattern, multiple ECGs (at least one per year) and, if available, a 12-lead 24-hour Holter monitoring were proposed in order to search for the occurrence of the spontaneous type-1 BrECG. As long as no spontaneous type-1 BrECG pattern was documented, these patients continued clinical follow-up, including 12-lead 24-hour ECG Holter, with no therapy except behavioral recommendations. For patients with spontaneous type-1 BrECG documented on 12-lead ECG or 12-lead 24-hour Holter monitoring at baseline or during follow-up, EPS was proposed.
The EPS protocol consisted of a maximum of 2 ventricular extrastimuli, from 2 ventricular sites (apex first and then right ventricular outflow tract), at 2 different pacing cycle lengths (600 and 400 ms); extrastimuli were delivered with 10-ms decrements up to the shortest coupling interval which resulted in ventricular capture, but without going below 160 ms. EPS was considered positive if sustained ventricular arrhythmias (eg, ventricular fibrillation [VF], polymorphic ventricular tachycardia [VT], or monomorphic VT lasting >30 s or requiring emergency intervention) were induced. ICD implantation was proposed based on EPS results or according to the clinical judgment of the referring physician, taking into consideration the patient’s preference. ICDs were programmed with a long detection time and a single VF zone ≥210 bpm, with antitachycardia pacing during capacitor charging.
Risk-Scoring Models
During the past 5 years, different risk scores have been proposed for diagnosis and risk stratification of patients with BrECG pattern: Sieira,12 Shanghai,13 and Honarbakhsh score models.14 When applied to specific segments of the population with BrECG pattern, such as the asymptomatic patients considered in the current study, these models require customization based on the appropriate variables, which can potentially impact their performance. The Sieira score model12 is based on 6 variables, of which only 3 were appropriate for our population: spontaneous type-1 BrECG pattern (1 point), early familial sudden death (1 point), and inducible EPS (2 points), with a possible maximum score value of 4.
The Shanghai score system13 is based on 10 variables, of which only 5 were appropriate for the population of the current study: spontaneous type-1 ECG (3.5 points) or drug-induced type-1 ECG (2 points), atrial fibrillation at a young age (0.5 points), family history of Brugada syndrome (2 points), family history of early sudden death (1 point), and probable pathogenic mutation in Brugada syndrome susceptibility genes (0.5 points), with a possible maximum score of 7.5 points.
The score model developed by Honarbakhsh et al14 was based on 4 variables, of which 3 were appropriate for our population: spontaneous type-1 BrECG pattern (14 points), early repolarization in the peripheral leads (12 points), and type-1 BrECG pattern in the peripheral leads (9 points), with a possible maximum score of 35 points. We retrospectively investigated the predictive ability and accuracy of these 3 risk scores in our population of asymptomatic patients.
Follow-Up
All patients underwent regular outpatient re-evaluation at the referring center, at least once per year. Evaluation included a review of symptoms, clinical examination, 12-lead ECG and, when available, 12-lead 24-hour Holter monitoring with V1 and V2 leads both recorded on the fourth and on the second or third intercostal spaces. ICD carriers were followed in the pacemaker clinic of the referring center every 6 months. Patients were considered to have an arrhythmic event at follow-up in the event of occurrence of sudden death in the absence of other plausible explanations, if VF or sustained VT were documented, or if appropriate ICD intervention was delivered for VF or VT. ICD-related complications were recorded during the follow-up. Inappropriate shocks were defined as those delivered in the absence of ventricular arrhythmias.
Statistical Analysis
Continuous variables satisfying Shapiro-Wilk for normality (age) were reported as mean±SD and compared with t test; otherwise follow-up years were reported as median with first and third interquartile range and compared with Mann-Whitney nonparametric test. Arrhythmic events were reported as counts and timing of occurrence.
After univariable analysis, potential predictors of events were assessed with the multivariable logistic regression and expressed as P value and odds ratio (OR). The event rate per year (ie, the count of the event during risk time [cumulative length of exposure of all persons in a group]) was used to compare the rate of events between groups with different exposure lengths. The statistical significance of the difference between 2 rates was expressed by rate ratio, and P value calculated by the z-test.
The impact of a predictor on the timing of arrhythmic events was assessed using 2 approaches: (1) Kaplan-Meier freedom-from-event curves and associated cumulative hazard for events as a function of time, wherein comparison between 2 groups with different values of the predictor was performed with log-rank test and expressed as P value and hazard ratio (HR) with 95% CI; and (2) Cox proportional hazard regression, in which the P value and estimated risk ratio (RR), along with 95% CI, were determined for each covariate.
The performance of a variable as event predictor according to a model was estimated using the receiver operating characteristics curve with area under the curve. For all possible thresholds, sensitivity (SE) and specificity (SP), as well as positive and negative predictive values, were computed using the number of true and false positives and true and false negatives. The most opportune threshold for the risk region was determined by maximization of the harmonic mean between the parameters of sensitivity and specificity and Youden index (ie, SE+SP−1).
Statistical significance corresponded to P values <0.05 and 95% CI for OR, RR, and HR, not including 1. All analyses were performed using StatPlus for Macintosh (build 8.0.1.0/Core v.7.7.11; AnalystSoft, USA).
RESULTS
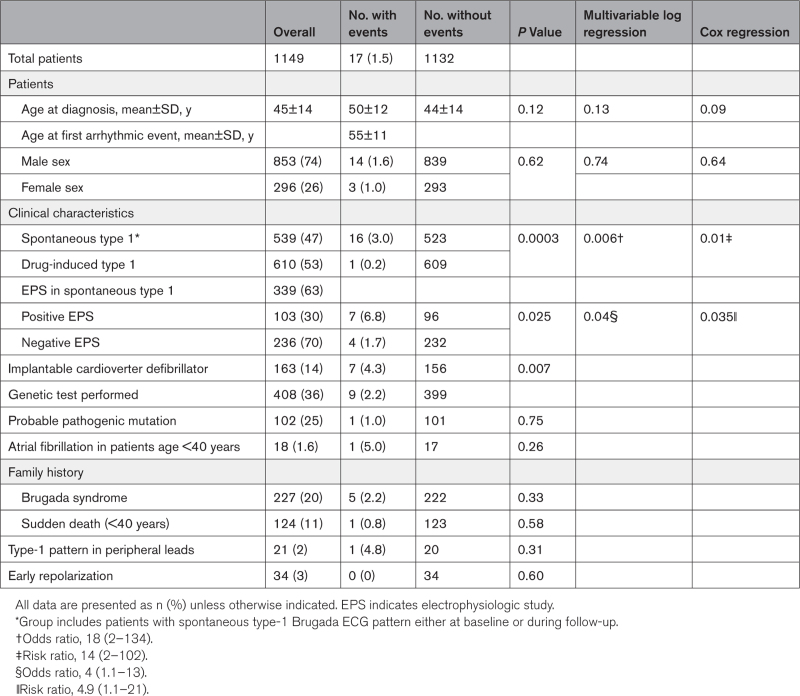
The study included 1149 asymptomatic patients with BrECG pattern and with a mean age of 45±14 years at the time of diagnosis. Of those, 853 (74%) were men with a mean age of 44±14 years, and 296 (26%) were women with a mean age of 47±15 years (Table 1). There were 539 (47%) patients with spontaneous type-1 BrECG pattern documented on 12-lead ECG or 12-lead 24-hour Holter monitoring (men, 433 [80%]; mean age, 46±13 years). The other 610 (53%) patients had drug-induced–only type-1 BrECG pattern (men, 420 [69%]; mean age, 44±14 years). An average of 5 (4–8) ECGs per patient were recorded and 668 (58%) patients underwent at least one 12-lead 24-hour Holter monitoring.
Table 1.
Clinical Characteristics of Asymptomatic Patients With Brugada ECG Pattern
Additionally, 72 patients (11.8%) originally classified as drug-induced were reclassified as spontaneous type-1 during the follow-up, after 12-lead 24-hour Holter documenting a spontaneous type 1 BrECG pattern; thus, 13% of patients ultimately diagnosed with spontaneous type-1 BrECG were initially diagnosed as drug-induced BrECG. The mean time interval for documenting spontaneous type-1 BrECG pattern on 12-lead Holter was 17±14 months from the first visit.
EPS was proposed to all 539 patients with spontaneous type 1 and performed in 339 (63%). Of these 339 patients, 103 (30%) had positive EPS, with 90 (87%) receiving an ICD; and 236 (70%) had negative EPS, with 27 (11%) receiving an ICD. A total of 200 patients (37%) refused EPS; ICD was implanted in 11 (5.5%) of them. In asymptomatic patients with spontaneous type-1 BrECG, a total of 128 ICDs were implanted, with 12 (9%) being subcutaneous devices. Another 35 ICDs (4 subcutaneous) were implanted in the drug-induced–only group based on the clinical judgment of the referring physician and taking into consideration patient preference.
Arrhythmic Events in Overall Asymptomatic Population at Follow-Up
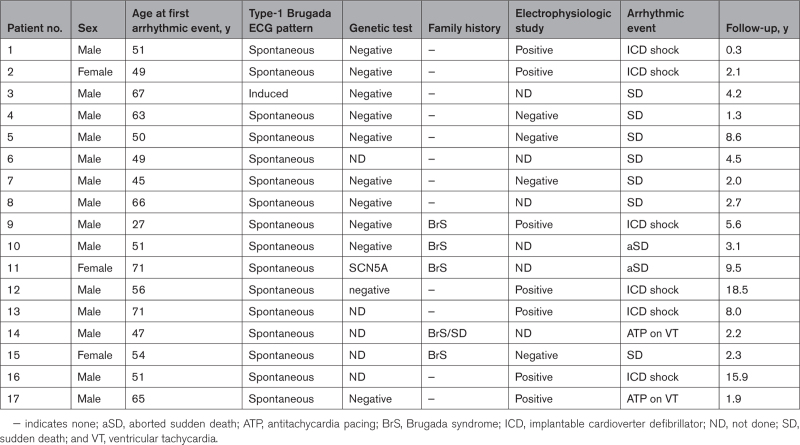
During a median follow-up of 6 (4–9) years (cumulative observation time, 8094 person-years), 17 arrhythmic events occurred in the 1149 asymptomatic patients with BrECG pattern (0.2% per year; Figure 1B). Five of 17 events occurred between the 8th and 18th year of follow-up. The mean age at the occurrence of the arrhythmic event was 55±11 years for men (n=14) and 58±12 years for women (n=3). Table 2 details the features of the 17 patients with arrhythmic events during follow-up. Five events occurred after a meal, 4 during sleep, and 2 during sport activity. One patient died suddenly during preparation for colonoscopy and one had a sustained VT with a concomitant finding of anemia. The other 4 arrhythmic events occurred during ordinary daily activities. None of the 17 arrhythmic events occurred during fever or while taking contraindicated drugs. The 7 patients with appropriate ICD interventions started hydroquinidine therapy and remained free from arrhythmic events thereafter. The 2 patients with aborted sudden death had no arrhythmic recurrences after ICD implantation.
Table 2.
Clinical Characteristics of Patients with Arrhythmic Events
Arrhythmic Events at Follow-Up in Patients With Spontaneous Versus Drug-Induced Type-1 BrECG
A total of 16 arrhythmic events occurred in patients with spontaneous type-1 BrECG during a median observation time of 7 (4–11) years (cumulative follow-up, 4287 person-years). In contrast, only a single event occurred during the median observation time of 5 (4–8) years (cumulative follow-up, 3804 person-years) in patients with drug-induced–only BrECG. The rate of events was 0.4% per year among patients with spontaneous type 1 and 0.03% per year among patients with drug-induced–only type-1 BrECG (P<0.0001; rate ratio, 15 [2–110]).
Figure 2 shows the Kaplan-Meier freedom-from-event functions for the 2 cohorts (spontaneous versus drug-induced). The difference between the 2 functions was significant, with P<0.0001 and an HR of 14 (5–35).
Figure 2.
Asymptomatic Brugada patients: spontaneous vs induced type-1 ECG. Kaplan-Meier curves indicate probability of freedom from arrhythmic events in asymptomatic patients with spontaneous vs drug-induced type-1 Brugada ECG pattern. P value refers to log-rank test. Spont. type 1 indicates spontaneous type 1.
Among patients classified as spontaneous type 1 based on 12-lead ECG, 14 of 467 (3.2%) had events, whereas among those reclassified as spontaneous type 1 after 12-lead 24-hour Holter monitoring, 2 of 72 (2.8%) had events (P=0.99).
The only patient with drug-induced type 1 who died suddenly during follow-up never had 12-lead 24-hour Holter performed during follow-up. No events occurred in the 35 drug-induced–only patients in which an ICD was implanted, according to patient or referring physician choice.
The univariable analysis of the variables reported in Table 1 indicated that the occurrence of arrhythmic events in the overall population had the strongest significant association with spontaneous type-1 BrECG pattern. In fact, patients with spontaneous type-1 BrECG had 16 events (3%), whereas patients with drug-induced–only type 1 had one event (0.16%; P=0.0003).
The multivariable logistic regression (performed for spontaneous type 1, sex, atrial fibrillation, family history of Brugada syndrome, and type-1 pattern in peripheral leads) confirmed the significant association between the occurrence of arrhythmic events and the presence of spontaneous type 1 (P=0.006; OR, 18 [2–134]). The Cox proportional hazard regression determined a significant association not only with the occurrence but also with the timing of the arrhythmic event by spontaneous type 1 (P=0.01; RR, 14 [2–102]; Table 1).
Role of EPS in Arrhythmic Events at Follow-Up for Spontaneous Type-1 BrECG Patients
In 539 asymptomatic patients with spontaneous type-1 BrECG, 339 (63%) underwent EPS—of those, 103 patients (30%) had positive EPS. In this group, 7 patients (6.8%) received an appropriate ICD intervention. In 50 of 103 patients (49%), the site of VF induction at EPS was the apex and 3 (6%) had an arrhythmic event at follow-up. In 53 of 103 patients (51%), the site of VF induction was the right ventricular outflow tract and 4 patients (8%) had an arrhythmic event (P=0.99). In 84 of 103 patients (82%), VF was induced with a coupling interval ≥200 ms and 5 patients (6%) had an arrhythmic event at follow-up; in 19 of 103 patients (18%), VF was induced with a coupling interval <200 ms and 2 (of 19) patients (10%; P=0.50) had an arrhythmic event at follow-up.
A total of 236 had a negative EPS. In this group, there were 4 (1.6%) sudden deaths during follow-up: 2 events occurred in the second year of follow-up, 1 in the third year, and 1 after 8 years.
The univariable analysis indicated a positive EPS as a significant risk factor for arrhythmic events during follow-up among patients with spontaneous type-1 BrECG pattern (positive EPS versus negative EPS P=0.025). The multivariable logistic regression (run over positive EPS, sex, atrial fibrillation, family history of Brugada syndrome, and type-1 pattern in peripheral leads) confirmed positive EPS to be the only characteristic associated with the occurrence of arrhythmic events (P=0.02; OR, 4.8 [1.3–18]). The Cox proportional-hazard regression determined a significant impact of positive EPS not only on the occurrence of the event but also on the timing (P=0.037; RR, 4.1 [1.1–15]; Table S1).
The 7 events in patients with positive EPS occurred during a median observation time of 9 (6–12.5) years (cumulative follow-up, 973 person-years), whereas 4 events in the patients with negative EPS occurred during a median observation time of 8 (5–11) years (cumulative follow-up, 2013 person-years). The rate of events in the 2 groups were respectively 0.7% per year for positive EPS and 0.2% per year for negative EPS (rate ratio, 3.6 [1.1–12]; P=0.03).
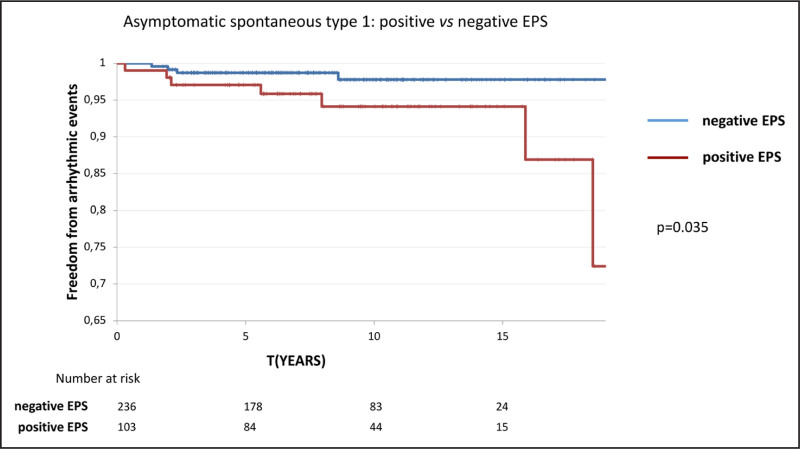
Figure 3 displays the Kaplan-Meier functions for the 2 cohorts of positive and negative EPS (P=0.035; HR, 3.4 [1.1–12]). The risk of arrhythmic events in patients with spontaneous type-1 BrECG and positive EPS was 2% at 5 years and 5% at 10 years.
Figure 3.
Asymptomatic spontaneous type 1: positive vs negative EPS. Kaplan-Meier curves for the probability of freedom from arrhythmic events for asymptomatic patients with spontaneous type-1 Brugada ECG pattern and positive EPS vs negative EPS. P value refers to log-rank test. EPS indicates electrophysiologic study.
Two hundred (37%) asymptomatic patients with spontaneous type-1 BrECG declined EPS. In this group, 5 (2.5%) events (2 sudden death, 2 aborted sudden death, and 1 VT) occurred during a median follow-up of 6 (4–9) years (cumulative risk time, 1301 person-years), yielding a 0.4%-per-year rate of arrhythmic events.
Figure 4A compares event rate in the 4 classes of patients with BrECG pattern: spontaneous type 1 and positive EPS (7 of 103 [0.7% per year]), spontaneous type 1 and no EPS (5 of 200 [0.4% per year]), spontaneous type 1 and negative EPS (4 of 236 [0.2% per year]) and drug-induced–only type 1 (1 of 619 [0.03% per year]). Figure 4B compares the cumulative hazard curves derived from the Kaplan-Meier survival functions of the 4 groups.
Figure 4.
Event rate and cumulative hazard for subgroups of asymptomatic patients with Brugada ECG pattern. A, Plots representing the event rate per year in the 4 subgroups of asymptomatic patients with Brugada ECG pattern: (left to right) patients with spontaneous type-1 BrECG pattern and positive electrophysiologic study (EPS); spontaneous type-1 BrECG pattern and EPS not done; spontaneous type-1 BrECG pattern and negative EPS; and drug-induced–only type-1 BrECG pattern. Spontaneous type 1 and positive EPS vs negative EPS: rate ratio (RR), 3.6 (1.1–14); P=0.03. Spontaneous type 1 and positive EPS vs no EPS: RR, 1.9 (0.6–6.4); P=0.27. Spontaneous type 1 and positive EPS vs drug-induced: RR, 27 (4–622); P<0.0001. B, Cumulative hazard ratio (HR) derived from Kaplan-Meier survival functions for the same 4 subgroups. Spontaneous type 1 and positive EPS vs negative EPS: HR, 3.4 (1.1–12); P= 0.036. Spontaneous type 1 and positive EPS vs no EPS: HR, 1.6 (0.5–5); P=0.36. Spontaneous type 1 and positive EPS vs drug-induced: HR, 24 (5.5–107); P<0.0001.
Risk Scores Predictions for Asymptomatic Patients
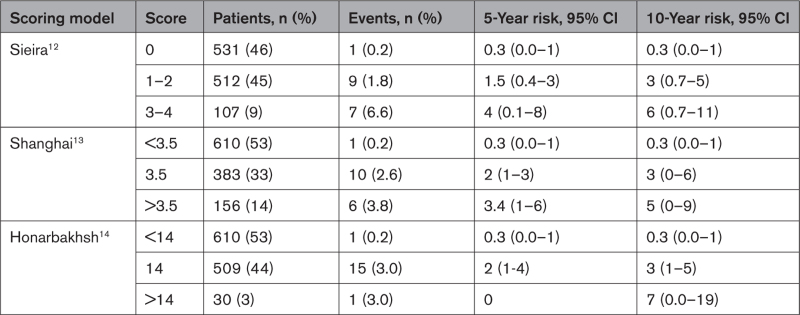
We retrospectively evaluated the performance of the Sieira,12 Shanghai,13 and Honarbakhsh14 scores in predicting the risk of events in the study population by receiver operating characteristics curve procedure (Figure 5): the area under the curve was 0.75, 0.76, and 0.74 for Sieira, Shanghai, and Honarbakhsh scores, respectively (P>0.5). The event-free survival probability curves according to the 3 scores are shown in Figures S1 through S3. The 5- and 10-year risks of arrhythmic events for each score model applied to the study population are shown in Table 3.
Figure 5.
Receiver operating characteristics curve for 3 risk scores applied to the study population. AUC indicates area under the curve; NPV, negative predictive value; and PPV, positive predictive value.
Table 3.
Arrhythmic Risk of Study Population Across Scoring Models
ICD Complications
Of the 163 patients with ICD, 38 (23%) experienced at least 1 device-related complication (20 [(12%] inappropriate shocks; 17 [10%] lead malfunctions; 10 [6%] infections requiring ICD removal and reimplantation) during a cumulative time of 1247 person-years (median follow-up, 9 [6–13] years), yielding a complication rate of 3% per year. One patient died during the lead extraction procedure performed for device infection. Among the 16 patients with subcutaneous ICD, 3 (19%) had complications: 2 patients experienced inappropriate shocks (1 for myopotential oversensing; 1 for atrial fibrillation) and 1 had a localized pocket infection.
DISCUSSION
We report a series of 1149 asymptomatic patients with BrECG pattern during a median follow-up of 6 (4–9) years (range, 2–22 years). There were several main findings of the study. First, among asymptomatic patients with true drug-induced–only type-1 ECG (ie, patients for whom the spontaneous type-1 BrECG pattern was searched and excluded with repeat ECG and 12-lead 24-hour Holter with V1 and V2 leads both on the fourth and second intercostal spaces), the event rate is extremely low (0.03% per year). Second, patients with spontaneous type-1 BrECG pattern had significantly greater risks for arrhythmia than drug-induced–only type-1 patients (0.4% per year; P=0.0005). While this risk is numerically low, it is clinically non-negligible considering the young age and long life-expectancy of these patients. Third, for patients with asymptomatic spontaneous type-1 BrECG pattern, those with positive EPS had a yearly risk of arrhythmic events that was greater than that of patients with negative EPS (0.7% per year versus 0.2% per year; P=0.04); a negative EPS identifies a low, but not zero-risk group. Fourth, arrhythmic events often occurred several years after initial presentation, which indicates the need to plan for indefinite follow-up in patients with BrECG.
Data from the literature have shown a gradual decrease in incidence of arrhythmic events in the asymptomatic patients with BrECG pattern since Brugada syndrome was first described: in 199815, the reported event rate was 10% per year, and subsequent studies have reported a lower incidence of events: between 0.3% and 1.4% per year.4,16–18 One of the reasons for the progressive reduction of arrhythmic risk in the whole asymptomatic population could be the fact that the number of asymptomatic patients with drug-induced–only BrECG pattern has increased over time. Moreover, improved patient management by cardiologists as well as increased patient awareness regarding adherence to behavioral recommendations may contribute to this decrease.11 In this study, the risk of spontaneous arrhythmias in asymptomatic patients with BrECG pattern appears to be even less than those reported in previous publications.4,15–18 Our lower event rate could reflect selection bias: we excluded from analysis all patients with spontaneous type-1 BrECG pattern and positive EPS (therefore, belonging to a subgroup with a higher arrhythmic risk) who were treated with hydroquinidine. It is likely that some of these patients could have had an arrhythmic event if left untreated.
Considering the asymptomatic patients with drug-induced–only type-1 BrECG pattern, the present study showed an event rate about 10× less than in the FINGER study (0.03% versus 0.35%).4 In the FINGER study,4 which included a large number of patients from 5 different centers in different countries, mean follow-up was 3 years, and patients classified as drug-induced type 1 had a single ECG recorded.4 In the current study, patients with drug-induced–only Brugada pattern underwent multiple ECGs (at least one per year), as well as yearly 12-lead 24-hour Holter when available. In this process, it was possible to uncover several true spontaneous type-1 patients who were initially classified as drug-induced (≈20% of those who underwent 12-lead Holter, in line with the data reported in the literature),19–21 which allowed for both a more accurate composition of the drug-induced–only population in the study and a more precise attribution of the arrhythmic events. In fact, due to the well-known intradaily and interdaily fluctuations of the Brugada pattern, spontaneous type-1 BrECG may have been missed with less accurate and frequent monitoring, leading to an underestimation of the arrhythmic risk for these patients.
Although this study confirms that the asymptomatic patients with drug-induced–only type-1 BrECG pattern are at very low risk, a pharmacological challenge with ajmaline or flecainide remains useful to unmask a type-1 BrECG pattern in the case of dubious ECG.7,22 Indeed, patients with a negative drug test can be reassured, and for them, no further follow-up is needed. On the other side, patients with a positive drug test should be provided with lifesaving behavioral recommendations and made aware of the need for regular follow-up visits, each with repeat ECGs and 12-lead 24-hour Holter monitoring (both with V1 and V2 electrodes in standard and higher position) to detect the appearance of a spontaneous type-1 BrECG pattern.
Spontaneous type-1 BrECG pattern represents itself as an important risk factor for arrhythmic events, as reported in the literature,4,23–25 but in the current study, the risk difference is particularly high: it is 14× greater, mainly due to the extremely low incidence of arrhythmic events among true drug-induced–only type-1 patients (0.4% per year versus 0.03% per year). Regardless, 0.4% per year is a numerically low value, but not clinically negligible. Indeed, of the 539 patients with spontaneous type-1 BrECG pattern (documented at 12-lead ECG or at 12-lead Holter), 16 (3%) experienced at least one arrhythmic event during the follow-up. This percentage is far from low, considering that patients with BrECG pattern are often very young and their arrhythmic risk increases substantially when considering life expectancy. For this reason, risk stratification appears particularly important within the subgroup of asymptomatic patients with spontaneous type-1 BrECG pattern.
Since the earliest studies,25 EPS has been proposed as a useful tool to better identify the patients at risk, but this finding was not confirmed by other studies, especially in the asymptomatic patients.23,26 More recently, the metanalysis from Sroubek et al27 demonstrated that induction of VT or VF at EPS was a marker of arrhythmic risk in the overall Brugada population; however, a consensus has not yet been reached. This explains why the current guidelines have a class-IIb indication for EPS in this group of patients.2,3
Data from the current study may represent a significant advancement in this debate, attesting to the usefulness of EPS in identifying asymptomatic patients with spontaneous type-1 BrECG who may be at greater risk for arrhythmia. In fact, in this subgroup, patients with positive EPS showed an event rate of 0.7% per year, greater than that of patients with negative EPS (0.2% per year). Therefore, it seems that EPS improves the ability to stratify arrhythmic risk for asymptomatic patients with spontaneous type-1 BrECG. Among patients with positive EPS, there were no sudden deaths because the 7 patients (6.8%) with ventricular arrhythmias during the follow-up were protected by an ICD. Unfortunately, there is still a non-negligible number of false negative results from EPS. In that group, 4 sudden deaths occurred in patients who were not protected by an ICD.
In case of positive EPS, the most common treatment currently offered is an ICD. Unfortunately, ICD implantation carries a significant risk of complications in which incidence increases over time, especially in a young population with a long life-expectancy.28,29
In this study, the risk of arrhythmic events in patients with spontaneous type-1 BrECG and positive EPS was 2% at 5 years and 5% at 10 years. Assuming that ICD implantation can effectively prevent sudden death, the number of patients needed to treat with an ICD to save 1 life should be respectively 50 at 5 years and 20 at 10 years. Although these values make ICD implantation a reasonable therapy in this subgroup of patients, it is difficult to establish a definite risk cut-off for prophylactic ICD implant in patients with BrECG pattern. Currently, that choice depends on various factors including the patient’s will and the risk–benefit ratio of the treatment.
In retrospectively evaluating the study population on the basis of the 3 risk scores proposed in the past 5 years,12–14 it emerges that all of 3 have a moderate predictive capacity when applied to the subgroup of asymptomatic patients with BrECG pattern and a high number of false-positive results. The scores allow the identification of patients at very low risk (ie, those with drug-induced–only type-1 BrECG), whereas the considered variables do not discriminate, within the asymptomatic patients with spont-type-1 BrECG pattern, those at higher risk and who therefore need a treatment, similarly to what observed by Probst et al.30
The primary concern today is that there are not many treatment alternatives to ICD. In the current guidelines, quinidine therapy and epicardial transcatheter ablation are indicated only for patients who refuse or have contraindications to ICD implantation.2,3 ICD therapy is certainly effective in preventing sudden death, but is not free of complications. In this study, 23% of implanted patients had complications, including one death during lead extraction (1 of 163 [0.6%]). For this reason, ICD cannot be the only treatment for asymptomatic patients with BrECG pattern. Subcutaneous ICD, recently introduced in clinical practice, reduces the risks related to catheter extraction and systemic infections, but it is not suitable for everyone due to failure of preimplantation screening in some cases (as much as 18% and even greater if screening is done during a pharmacological challenge or exercise test), and it does not overtake the psychological problems and risk of inappropriate shocks.31–34 There was a 19% rate of complications in this subgroup of our study.
Considering other therapeutic approaches available, quinidine seems effective in preventing spontaneous and induced ventricular arrhythmias.35–40 However, adverse effects, leading to drug discontinuation, can occur in 14% to 36% of patients and the drug is not available in all countries.35,39,40
In the past years, epicardial transcatheter ablation of the Brugada substrate showed efficacy in preventing VF recurrence,41,42 and, more recently, the efficacy of substrate ablation was confirmed in a long-term follow-up in high-risk symptomatic Brugada patients with few side effects.43 If the data on both the efficacy and safety of the ablation procedure (especially the absence of proarrhythmic effects) are eventually confirmed by future prospective studies based on international collaboration with long-term follow-up, asymptomatic patients with a spontaneous type-1 BrECG pattern and positive EPS might benefit also from this treatment.
Limitations
In this study, the population with drug-induced BrECG experienced a single arrhythmic event over an observation time of 3806 person-years. While from a clinical point of view a single event is good since it means that the accurate patient follow-up, with multiple ECGs, 12-lead Holter monitoring and behavioral recommendations, was effectively shielding adverse events, from a statistical point of view, it lowers the power of statistical tests and the robustness of our conclusions.
The number of arrhythmic events, in patients with spontaneous type-1 BrECG and ICD, might have been overestimated, because some appropriate ICD interventions might have occurred on self-terminating ventricular arrhythmias.
It cannot be excluded that some of the arrhythmic events occurred in the patients with negative EPS and may have been identified using a stimulation protocol with up to 3 extra stimuli, but this would have also increased the number of false-positive results.
Conclusions
The entire asymptomatic population with BrECG pattern exhibits a relatively low annual event rate. However, considering the young age and long life-expectancy of such patients, this risk remains noteworthy.
Asymptomatic patients with true drug-induced–only BrECG pattern, confirmed through multiple ECGs and 12-lead 24-hour Holter monitoring (with V1 and V2 electrodes placed in standard and higher position), have an extremely low arrhythmic risk, provided they follow the first therapeutic step (avoid specific medications and adhere to behavioral recommendations) and undergo regular follow-up. Conversely, asymptomatic patients with spontaneous type-1 BrECG represent a higher-risk subgroup and their arrhythmic risk can be further stratified through EPS.
To avoid the challenging decision between lifelong risk of disease-related events and complications associated with ICD implantation, the research of alternative therapeutic strategies should be encouraged.
ARTICLE INFORMATION
Sources of Funding
None.
Disclosures
None.
Supplemental Material
Table S1
Figures S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BrECG
- Brugada ECG
- EPS
- electrophysiologic study
- ICD
- implantable cardioverter defibrillator
- VF
- ventricular fibrillation
- VT
- ventricular tachycardia
F. Gaita, N. Cerrato, and C. Giustetto contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.064689.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 1553.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Fiorenzo Gaita, Email: fiorenzo.gaita@gmail.com.
Carla Giustetto, Email: carla.giustetto@unito.it.
Annamaria Martino, Email: martinoannamaria@yahoo.it.
Laura Bergamasco, Email: laura.bergamasco@gmail.com.
Michele Millesimo, Email: michele.millesimo@gmail.com.
Lorella Barbonaglia, Email: lorybarbonaglia@gmail.com.
Paula Carvalho, Email: paula.carvalho@tiscali.it.
Domenico Caponi, Email: drdomenicocaponi@gmail.com.
Andrea Saglietto, Email: andrea.saglietto@live.com.
Giacomo Bonacchi, Email: giacomobonacchi1@gmail.com.
Francesca Bianchi, Email: fbianchi@mauriziano.it.
Elisa Silvetti, Email: elisa.silvetti@gmail.com.
Cinzia Crescenzi, Email: crescenzi.cinzia@gmail.com.
Stefano Canestrelli, Email: stefano.canestrelli@yahoo.it.
Melissa De Maio, Email: demaiomelissa@libero.it.
Gaetano Maria De Ferrari, Email: gaetanomaria.deferrari@unito.it.
Giuseppe Musumeci, Email: giuseppe.musumeci@gmail.com.
Francesco Rametta, Email: ramettafra@gmail.com.
Marco Scaglione, Email: marco.scaglione.at@gmail.com.
Leonardo Calò, Email: leonardocalo.doc@gmail.com.
REFERENCES
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j [DOI] [PubMed] [Google Scholar]
- 2.Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, Charron P, Corrado D, Dagres N, de Chillou C, et al. ; ESC Scientific Document Group. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262 [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e190–e252. doi: 10.1016/j.hrthm.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 4.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, Babuty D, Sacher F, Giustetto C, Schulze-Bahr E, et al. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada syndrome registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026 [DOI] [PubMed] [Google Scholar]
- 5.Raju H, Papadakis M, Govindan M, Bastiaenen R, Chandra N, O’Sullivan A, Baines G, Sharma S, Behr ER. Low prevalence of risk markers in cases of sudden death due to Brugada syndrome relevance to risk stratification in Brugada syndrome. J Am Coll Cardiol. 2011;57:2340–2345. doi: 10.1016/j.jacc.2010.11.067 [DOI] [PubMed] [Google Scholar]
- 6.Olde Nordkamp LR, Wilde AA, Tijssen JG, Knops RE, van Dessel PF, de Groot JR. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013;6:91–100. doi: 10.1161/CIRCEP.112.975268 [DOI] [PubMed] [Google Scholar]
- 7.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Perez Riera AR, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005;111:659–670. doi: 10.1161/01.CIR.0000152479.54298.51 [DOI] [PubMed] [Google Scholar]
- 8.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 9.Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, et al. J-Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Heart Rhythm. 2016;13:e295–e324. doi: 10.1016/j.hrthm.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter S, Sarkozy A, Paparella G, Henkens S, Boussy T, Chierchia GB, Brugada R, Brugada J, Brugada P. Number of electrocardiogram leads displaying the diagnostic coved-type pattern in Brugada syndrome: a diagnostic consensus criterion to be revised. Eur Heart J. 2010;31:1357–1364. doi: 10.1093/eurheartj/ehq049 [DOI] [PubMed] [Google Scholar]
- 11.Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DM, Priori SG, Tan HL, Hiraoka M, Brugada J, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (wwwbrugadadrugsorg). Heart Rhythm. 2009;6:1335–1341. doi: 10.1016/j.hrthm.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieira J, Conte G, Ciconte G, Chierchia GB, Casado-Arroyo R, Baltogiannis G, Di Giovanni G, Saitoh Y, Juliá J, Mugnai G, et al. A score model to predict risk of events in patients with Brugada syndrome. Eur Heart J. 2017;38:1756–1763. doi: 10.1093/eurheartj/ehx119 [DOI] [PubMed] [Google Scholar]
- 13.Kawada S, Morita H, Antzelevitch C, Morimoto Y, Nakagawa K, Watanabe A, Nishii N, Nakamura K, Ito H. Shanghai score system for diagnosis of Brugada syndrome: validation of the score system and system and reclassification of the patients. JACC Clin Electrophysiol. 2018;4:724–730. doi: 10.1016/j.jacep.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 14.Honarbakhsh S, Providencia R, Garcia-Hernandez J, Martin CA, Hunter RJ, Lim WY, Kirkby C, Graham AJ, Sharifzadehgan A, Waldmann V, et al. ; Brugada Syndrome Risk Investigators. A primary prevention clinical risk score model for patients with brugada syndrome (BRUGADA-RISK). JACC Clin Electrophysiol. 2021;7:210–222. doi: 10.1016/j.jacep.2020.08.032 [DOI] [PubMed] [Google Scholar]
- 15.Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: a marker for sudden death in patients without demonstrable structural heart disease. Circulation. 1998;97:457–460. doi: 10.1161/01.CIR.97.5.457 [DOI] [PubMed] [Google Scholar]
- 16.Priori SG, Napolitano C, Gasparini M, Pappone C, Della Bella P, Giordano U, Bloise R, Giustetto C, De Nardis R, Grillo M, et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–1347. doi: 10.1161/hc1102.105288 [DOI] [PubMed] [Google Scholar]
- 17.Eckardt L, Probst V, Smits JPP, Bahr ES, Wolpert C, Schimpf R, Wichter T, Boisseau P, Heinecke A, Breithardt G, et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation. 2005;111:257–263. doi: 10.1161/01.CIR.0000153267.21278.8D [DOI] [PubMed] [Google Scholar]
- 18.Giustetto C, Drago S, Demarchi PG, Dalmasso P, Bianchi F, Masi AS, Carvalho P, Occhetta E, Rossetti G, Riccardi R, et al. ; Italian Association of Arrhythmology and Cardiostimulation (AIAC)-Piedmont Section. Risk stratification of the patients with Brugada type electrocardiogram: a community-based prospective study. Europace. 2009;11:507–513. doi: 10.1093/europace/eup006 [DOI] [PubMed] [Google Scholar]
- 19.Cerrato N, Giustetto C, Gribaudo E, Richiardi E, Barbonaglia L, Scrocco C, Zema D, Gaita F. Prevalence of type 1 Brugada electrocardiographic pattern evaluated by twelve-lead twenty-four-hour Holter monitoring. Am J Cardiol. 2015;115:52–56. doi: 10.1016/j.amjcard.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 20.Extramiana F, Maison-Blanche P, Badilini F, Messali A, Denjoy I, Leenhardt A. Type 1 electrocardiographic burden is increased in symptomatic patients with Brugada syndrome. J Electrocardiol. 2010;43:408–414. doi: 10.1016/j.jelectrocard.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 21.Gray B, Kirby A, Kabunga P, Freedman SB, Yeates L, Kanthan A, Medi C, Keech A, Semsarian C, Sy RW. Twelve-lead ambulatory electrocardiographic monitoring in Brugada syndrome: potential diagnostic and prognostic implications. Heart Rhythm. 2017;14:866–874. doi: 10.1016/j.hrthm.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 22.Wolpert C, Echternach C, Veltmann C, Antzelevitch C, Thomas GP, Spehl S, Streitner F, Kuschyk J, Schimpf R, Haase KK, et al. Intravenous drug challenge using flecainide and ajmaline in patients with Brugada syndrome. Heart Rhythm. 2005;2:254–260. doi: 10.1016/j.hrthm.2004.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priori SG, Gasparini M, Napolitano C, Della Bella P, Ottonelli AG, Sassone B, Giordano U, Pappone C, Mascioli G, Rossetti G, et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064 [DOI] [PubMed] [Google Scholar]
- 24.Sieira J, Ciconte G, Conte G, Chierchia GB, DE Asmundis C, Baltogiannis G, Di Giovanni G, Saitoh Y, Irfan G, Casado-Arroyo R, et al. Asymptomatic Brugada syndrome: clinical characterization and long-term prognosis. Circ Arrhythm Electrophysiol. 2015;8:1144–1150. doi: 10.1161/CIRCEP.114.003044 [DOI] [PubMed] [Google Scholar]
- 25.Brugada J, Brugada R, Brugada P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation. 2003;108:3092–3096. doi: 10.1161/01.CIR.0000104568.13957.4F [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Gerss J, Schulze-Bahr E, Wichter T, Vahlhaus C, Wilde AAM, Breithardt G, Eckardt L. Role of programmed ventricular stimulation in patients with Brugada syndrome: a meta-analysis of worldwide published data. Eur Heart J. 2007;28:2126–2133. doi: 10.1093/eurheartj/ehm116 [DOI] [PubMed] [Google Scholar]
- 27.Sroubek J, Probst V, Mazzanti A, Delise P, Hevia JC, Ohkubo K, Zorzi A, Champagne J, Kostopoulou A, Yin X, et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome: a pooled analysis. Circulation. 2016;133:622–630. doi: 10.1161/CIRCULATIONAHA.115.017885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olde Nordkamp LRA, Postema PG, Knops RE, Van Dijk N, Limpens J, Wilde AAM, De Groot JR. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–454. doi: 10.1016/j.hrthm.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 29.Migliore F, Martini N, Calo’ L, Martino A, Winnicki G, Vio R, Condello C, Rizzo A, Zorzi A, Pannone L, et al. Predictors of late arrhythmic events after generator replacement in Brugada syndrome treated with prophylactic ICD. Front Cardiovasc Med. 2022;9:964694. doi: 10.3389/fcvm.2022.964694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Probst V, Goronflot T, Anys S, Tixier R, Briand J, Berthome P, Geoffroy O, Clementy N, Mansourati J, Jesel L, et al. Robustness and relevance of predictive score in sudden cardiac death for patients with Brugada syndrome. Eur Heart J. 2021;42:1687–1695. doi: 10.1093/eurheartj/ehaa763 [DOI] [PubMed] [Google Scholar]
- 31.Jespersen CHB, Krøll J, Bhardwaj P, Winkel BG, Jacobsen PK, Jøns C, Haarbo J, Kristensen J, Johansen JB, Philbert BT, et al. Severity of Brugada syndrome disease manifestation and risk of new-onset depression or anxiety: a Danish nationwide study. Europace. 2023;25:euad112. doi: 10.1093/europace/euad112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olde Nordkamp LRA, Conte G, Rosenmöller BRAM, Warnaars JLF, Tan HL, Caputo ML, Regoli F, Moccetti T, Auricchio A, Knops RE, et al. Brugada syndrome and the subcutaneous implantable cardioverter-defibrillator. J Am Coll Cardiol. 2016;68:665–666. doi: 10.1016/j.jacc.2016.05.058 [DOI] [PubMed] [Google Scholar]
- 33.Conte G, Kawabata M, De Asmundis C, Taravelli E, Petracca F, Ruggiero D, Caputo ML, Regoli F, Chierchia GB, Chiodini A, et al. High rate of subcutaneous implantable cardioverter-defibrillator sensing screening failure in patients with Brugada syndrome: a comparison with other inherited primary arrhythmia syndromes. Europace. 2018;20:1188–1193. doi: 10.1093/europace/eux009 [DOI] [PubMed] [Google Scholar]
- 34.Tachibana M, Nishii N, Morita H, Nakagawa K, Watanabe A, Nakamura K, Ito H. Exercise stress test reveals ineligibility for subcutaneous implantable cardioverter defibrillator in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2017;28:1454–1459. doi: 10.1111/jce.13315 [DOI] [PubMed] [Google Scholar]
- 35.Andorin A, Gourraud JB, Mansourati J, Fouchard S, le Marec H, Maury P, Mabo P, Hermida JS, Deharo JC, Delasalle B, et al. The QUIDAM study: hydroquinidine therapy for the management of Brugada syndrome patients at high arrhythmic risk. Heart Rhythm. 2017;14:1147–1154. doi: 10.1016/j.hrthm.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 36.Bouzeman A, Traulle S, Messali A, Extramiana F, Denjoy I, Narayanan K, Marijon E, Hermida JS, Leenhardt A. Long-term follow-up of asymptomatic Brugada patients with inducible ventricular fibrillation under hydroquinidine. Europace. 2014;16:572–577. doi: 10.1093/europace/eut279 [DOI] [PubMed] [Google Scholar]
- 37.Belhassen B, Rahkovich M, Michowitz Y, Glick A, Viskin S. Management of Brugada syndrome: thirty-three-year experience using electrophysiologically guided therapy with class 1A antiarrhythmic drugs. Circ Arrhythm Electrophysiol. 2015;8:1393–1402. doi: 10.1161/CIRCEP.115.003109 [DOI] [PubMed] [Google Scholar]
- 38.Malhi N, Cheung CC, Deif B, Roberts JD, Gula LJ, Green MS, Pang B, Sultan O, Konieczny KM, Angaran P, et al. Challenge and impact of quinidine access in sudden death syndromes: a national experience. JACC Clin Electrophysiol. 2019;5:376–382. doi: 10.1016/j.jacep.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 39.Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation. 2004;110:1731–1737. doi: 10.1161/01.CIR.0000143159.30585.90 [DOI] [PubMed] [Google Scholar]
- 40.Hermida JS, Denjoy I, Clerc J, Extramiana F, Jarry G, Milliez P, Guicheney P, Di Fusco S, Rey JL, Cauchemez B, et al. Hydroquinidine therapy in Brugada syndrome. J Am Coll Cardiol. 2004;43:1853–1860. doi: 10.1016/j.jacc.2003.12.046 [DOI] [PubMed] [Google Scholar]
- 41.Nademanee K, Hocini M, Haïssaguerre M. Epicardial substrate ablation for Brugada syndrome. Heart Rhythm. 2017;14:457–461. doi: 10.1016/j.hrthm.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 42.Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano M, Vitale R, Cuko A, Giannelli L, Calovic Z, et al. Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol. 2017;10:e005053. doi: 10.1161/CIRCEP.117.005053 [DOI] [PubMed] [Google Scholar]
- 43.Nademanee K, Chung FP, Sacher F, Nogami A, Nakagawa H, Jiang C, Hocini M, Behr E, Veerakul G, Jan Smit J, et al. Long-term outcomes of Brugada substrate ablation: a report from BRAVO (Brugada Ablation of VF Substrate Ongoing Multicenter Registry). Circulation. 2023;147:1568–1578. doi: 10.1161/circulationaha.122.063367 [DOI] [PubMed] [Google Scholar]