1. Introduction
Increasing evidence highlights the co-morbidity of physical and psychological illnesses and its significance in disease progression and therapeutic considerations. The bidirectional relationship of the nervous and immune systems is shown to underlie many of such conditions. We conducted a series of studies to investigate inflammation and its neuroendocrine regulatory mechanisms that underlie affective and cognitive outcomes in chronic physical conditions and diseases, including metabolic disorders, cardiovascular disease (CVD) and human immunodeficiency virus (HIV) infection. This paper summarizes our published findings as presented in the symposium titled, Dialing in the Dialogue Between Inflammation and the Brain at the 10th World Congress of Neuroscience in 2019 and is organized in five main sections: 1. Immune dysfunctions with central nervous system (CNS) symptoms; 2. Immune dysregulation underlying depression and depressive symptoms; 3. Neuroendocrine pathways of inflammation regulation in the brain-immune link; 4. Co-morbid conditions and covariates in the interface of physical and mental health outcomes; and 5. Clinical and therapeutic implications. The primary aim of this brief review is to highlight the role of inflammation and immune dysregulation at the interface between physical and neuropsychiatric conditions by summarizing our previous findings rather than it being a comprehensive review, while acknowledging a large body of literature on this topic.
2. Immune dysfunctions with marked CNS symptoms
A large body of literature provides the evidence of the interconnection between physical and CNS outcomes in a wide range of health and disease conditions. The following case report (Verma & Anand, 2014) highlights such interactions in manifestations of severe neuropsychiatric symptoms, as cited in our previous article (Hong & Banks, 2015).
“A 24-year-old unmarried woman presented with 9 months’ history of abnormal behavior and depressed mood, 6 months’ history of tremulousness of both the hands, and history of urinary and bowel incontinence for 3 months. She stopped taking care of self and started showing disinterest and apathy toward day-to-day activities... The patient was observed to be conscious, with impaired sustained attention and recent memory. The patient scored 21 of 30 on the Mini-Mental Status Examination... Magnetic resonance imaging of the brain revealed diffuse cortical atrophy. The CSF analysis showed clear acellular fluid…”
These signs and symptoms of notable neurologic, mood and cognitive disturbances accompanied by brain structural changes clearly indicate CNS pathology, but with unclear etiology at the time of these initial observations. Such cases present diagnostic and, more importantly, therapeutic challenges without careful considerations of other physical signs, symptoms, and history. This particular case accompanies a history of “fever” and infections that suggest an involvement of the immune system and concludes with the confirmed diagnosis of HIV-associated dementia (HAD) and a tragic loss of the patient as described below.
“Eleven months prior to presentation, the patient had fever for a duration of 3 months... in view of the relatively rapid onset of cognitive impairment with prominent motor symptoms, the patient was checked for HIV and was found to be positive... Subsequently, CD4 count was performed and noted to be <30 cells/mm3. She was diagnosed with HAD. However, she died suddenly before we could initiate ART (antiretroviral therapy).”
As a result of early diagnosis and initiation of ART, HAD is no longer common, especially in developed countries where combination ART is readily available. However, HIV-associated neurocognitive disorder (HAND) persists even among adequately treated individuals who are living with HIV (Heaton et al., 2011). This is an example of how prominent CNS outcomes are traced back to a major immunological insult such as a viral infection and subsequent host immune responses. In the literature, the presence of HAND among HIV+ individuals, particularly among those with viral suppression, is attributed to peripheral and neuro-inflammation and trafficking of inflammatory and HIV-infected immune cells into the CNS, further activating pericytes, microglial cells and astrocytes (Hong & Banks, 2015). We have reported that expression levels of a chemokine receptor CXC receptor 3 (CXCR3) is greater on memory T cells found in cerebrospinal fluid (CSF) compared to those in circulating blood which indicates likely migration of activated T cells to the CNS in chronic HIV infection even treated with ART. Percent CXCR3-expressing memory T cells from CSF was significantly higher among 14 HIV+ individuals who were found to be cognitively impaired compared to 16 non-impaired HIV+ individuals, further indicating that CNS infiltration by activated and differentiated T cells likely underlies neurocognitive impairment (Hong et al,. 2013). We also reported greater production of inflammatory cytokines tumor necrosis factor (TNF), interleukin 2 (IL-2), and interferon gamma (IFN-γ) by T cells found in CSF upon stimulation with HIV p24 and significant associations between T cell IFN-γ expression and neurocognitive impairment (Schrier et al., 2015). From the Adolescent Medicine Trials Network for HIV/AIDS interventions study, we reported that the markers of activation of monocytes in circulation underlie HIV-related neurocognitive declines in adolescents and young adults (Kim-Chang et al., 2019). These findings of specific cellular inflammatory activities in the CNS as well as heightened systemic inflammation in the individuals living with HIV collectively indicate that peripheral inflammation and immune activation and subsequent neuroinflammation underlie HAND. It provides the supporting evidence that immune dysregulation and prolonged activation under chronic viral infection in the periphery is detrimental to the CNS outcomes.
3. Immune dysregulation underlying depression and depressive symptoms
Development of depression from cytokine infusion.
Numerous studies have also examined underlying immune processes of a range of neuropsychiatric outcomes, and a large body of literature indicate a link between inflammation and depression. Beyond numerous associations shown between depression and elevated inflammatory mediators, a causal relationship of inflammatory cytokines and development of depressive symptoms is demonstrated in clinical studies of disease conditions for which a high-dose (e.g., 20 million U/square meter of body surface area, 5 days per week for 12 weeks) interferon-α (IFN- α) treatment is applied, such as melanoma (Capuron et al., 2002) (Musselman et al., 2001) and hepatitis-C (Raison et al., 2009). They further demonstrated that 12-weeks of IFN- α treatment resulted in the development of clinically diagnosed major depression in up to about 50% of patients in the placebo but only about 10% in the antidepressant paroxetine treatment group. These findings offer the evidence of a causal link between peripheral inflammatory mediators and major depression and a therapeutic promise that a standard antidepressant treatment is effective in its prevention. An earlier observation by Renault and colleagues (1987) also reported that about 20% of patients with chronic viral hepatitis that were treated with courses of IFN- α infusion had developed side effects after 1 – 3 months of the therapy. These side effects were labeled as, “personality syndrome” such as irritability and short temper; “affective syndrome” such as emotional lability and depression; and “delirium” such as confusion, paranoia and suicidal potential. These neuropsychiatric sequelae of the IFN-α treatment improved within a few days following dose reduction, and were resolved upon therapy termination (Renault et al., 1987). It is important to note that depression and other psychiatric symptoms were found in a subset of the patients undergoing the IFN-α treatment in these studies, but not all, which raises a salient question of which individual factors may contribute to this “selective vulnerability” to cytokine-induced depression. There is no definitive answer available currently.
Greater prevalence of depression and levels of depressive mood in inflammatory diseases.
The association between elevated basal levels of inflammation and affective dysfunction is also observed among individuals with chronic physical diseases. Although such data are often correlational in nature and present challenges in deciphering causal relationships, they offer the evidence of inflammation as a likely underlying factor of co-morbid physical and affective disorders. Those individuals with chronic inflammatory conditions such as CVD often report subclinical yet markedly elevated levels of depressive symptoms. We have been investigating the role of inflammation and its dysregulation in CVD and chronic metabolic disorders. We have observed that the plasma levels of inflammatory markers C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor (TNF), and soluble intercellular molecule-1 (sICAM-1) were greater among 39 patients with New York Heart Association class II – IV heart failure (HF) compared to 40 healthy control participants after adjusting for age (Hong and Pruitt et al., 2008). In parallel, levels of depressive mood in those HF patients, assessed using the Beck Depression Inventory (BDI-1A), were significantly higher than control participants (BDI total scores of 12 vs. 5) and, furthermore, significantly associated with the levels of CRP, IL-6 and sICAM-1. In particular, vascular inflammatory marker sICAM-1 was predictive of BDI scores 114 after controlling for age, gender, body mass index (BMI), and B-type natriuretic peptide. Indeed, HF patients are at greater risk for major depression at about 20% prevalence (Rustad, Stern, Hebert, & Musselman, 2013) and high depressive mood, both of which are associated with higher mortality (Jiang et al., 2001). These data indicate that the underlying inflammatory state of CVD may be a mechanism of greater prevalence of depression and levels of subclinical depressive mood found in these individuals.
We also observed that impaired regulation of monocytic inflammatory activities mediated by the sympathoadrenal system are associated with greater somatic depressive symptoms assessed by BDI among individuals with stage I hypertension or advanced prehypertension compared to normotensive individuals (Hong et al., 2015). These findings indicate that the elevated levels of inflammation in CVD likely mediate major depression or elevated depressive mood observed in affected individuals, suggesting the need of monitoring depressive symptoms in patients with inflammatory diseases. We also postulate that chronic sympathetic activation and elevated circulating catecholamine levels that are often observed in hypertension and HF contribute to desensitization/downregulation of adrenergic receptors (ARs) on immune cells, which then leads to unregulated or elevated cellular inflammatory activities and underlies depression-CVD co-morbidity. The following section includes expanded discussions of sympathoadrenal system-mediated inflammatory activities.
4. Neuroendocrine pathways of inflammation regulation in the brain-immune link
The communication between the brain and immune system is bi-directional. Understanding the mechanisms by which the brain communicates to the peripheral immune system and the immune system communicates back to the brain is critical in interrogating the pathophysiology of health and disease conditions with immune-dysregulation. We have conducted a series of studies to investigate the neuroendocrine regulatory mechanisms of inflammatory activities of immune cells with a particular focus on innate immunity.
4.1. Sympathoadrenergic pathway.
Monocytes express ARs through which adrenergic agonists play regulatory roles, and monocytic inflammatory responses are shown to be suppressed through activation of beta-adrenergic receptor (β-ARs) (Padro & Sanders, 2014) (Scanzano & Cosentino, 2015). Using an ex vivo model of lipopolysaccharide (LPS) stimulated circulating monocytes with and without a β-AR agonist isoproterenol and intracellular TNF detection by flow cytometry, we have examined β-AR mediated regulation of inflammatory responses (“BARIC”) in investigations of the interface between physical and mental health. We have reported that monocytic demargination responses during acute physical exercise, which is typically marked with mobilization of “proinflammatory” CD16+ monocytes under sympathoadrenergic activation, is blunted among individuals with stage I hypertension or advanced prehypertension (Dimitrov et al., 2013). This finding suggests that individuals with hypertension exhibit diminished responsivity of monocytes to sympathoadrenal activation, as pathophysiology of hypertension includes sympathetic overdrive and thus, desensitization/downregulation of ARs. We followed these findings with an investigation in which we observed that BARIC of circulating monocytes is, indeed, significantly diminished in individuals with prehypertension compared to normotensive participants (Hong et al., 2015), as aforementioned. Impaired BARIC was also associated with the following CVD risk factors: older age and higher levels of blood pressure (BP), BMI, fasting glucose, triglycerides, total and low-density cholesterol levels, and somatic depressive symptoms.
Although leukocytes, including monocytes, express both β1 and β2-ARs as well as α-ARs, under naturalistic stress (e.g., an exercise test) and physiological levels of sympathoadrenal activation, monocyte trafficking and inflammatory responses are mediated via β2-ARs, which is about 100 times more sensitive than β1-AR-mediated inflammatory regulation (Dimitrov, Hulteng, & Hong, 2017). Our recent work also highlights the importance of BARIC in its sexually dimorphic associations with depressive symptoms, such that impaired BARIC was predictive of somatic depressive symptoms (e.g., fatigue, pain) in women but not men (Kohn et al., 2019). Given the major role that monocytes and macrophages play in CVD and vascular inflammation, the implications of BARIC in investigations of chronic stress or depression in individuals at high risk for or with CVD are of high clinical significance and therapeutic value.
4.2. Hypothalamic-pituitary-adrenal pathway.
Elevated glucocorticoid (GC) levels and altered glucocorticoid receptor (GR) function have been shown in depression. A continued interests in the field include whether GC levels and GR function contribute to elevated inflammation underlying depression (Perrin, Horowitz, Roelofs, Zunszain, & Pariante, 2019). Given the immunosuppressive function of glucocorticoids, the greater levels of both cortisol and inflammatory cytokines in depression presents a seemingly paradoxical phenomenon that leads to a potential role of GR dysregulation. Notably, in a recent meta-analysis, Perrin and colleagues (2019) reported “only modest evidence” for the association between inflammation and GC resistance assessed by a mix of in vivo and ex vivo tests (i.e., dexamethasone stimulation test, GR function and expression assays) in the context of depression. This highlights the need of more studies directly investigating GC-GR dynamics, GR sensitivity, and downstream inflammatory responses. Ex vivo and in vitro studies should also carefully consider the dose and type of GR agonists to more reflect the physiological milieu in which GC-GR dysregulation-induced inflammation underlies depression. Using the ex vivo monocyte model mentioned above and the stimulation of GRs with exogenous cortisol of physiological levels (0, 3.6, 7.2 and 36 μg/dL, representing low to high levels), we found that poor GR-mediated inflammation regulation is associated with higher somatic depressive symptoms measured by BDI among healthy individuals in spite of the subclinical symptom levels (Cheng, Dimitrov, Pruitt, & Hong, 2016). We have also shown that the inflammation-suppressing effects of cortisol of the examined concentrations for blood monocytes is mostly mediated through GR rather than mineralocorticoid receptors. Together, our data indicate that inflammatory activities of monocytes are mediated through the two major neuroendocrine pathways that are the sympathoadrenal and hypothalamic pituitary adrenal (HPA) axes and underlie the link between physical pathology such as CVD and depressive symptoms. Our initial data also show that regulation of cellular inflammatory activities of monocytes are mediated by α7 nicotinic acetylcholine receptors (α7 nAChRs), and this cellular index of the cholinergic anti-inflammatory pathway (CAP) is associated with vascular inflammatory markers and elevated depressive mood and fatigue in older (60–90 years old) individuals with hypertension 197 (unpublished data).
5. Co-morbid conditions and covariates at the interface of physical and mental health outcomes and therapeutic implications
In our studies investigating inflammation as an intersecting factor of physical and psychological conditions, we have examined key co-morbid conditions that are closely related to both inflammation and CVD, such as obesity. Furthermore, obesity and depression are often comorbid risk factors for each other (Luppino et al., 2010), and inflammation likely underlie both conditions (Capuron, Lasselin, & Castanon, 2017). We have observed the inter-relations among obesity, depressive symptoms, and GR-mediated inflammation, and found that the obesity-inflammation association was mediated by elevated somatic depressive symptoms in a small group of young to middle-aged adults ranging from lean (23%) to overweight (23%) to obese (54%) (Cheng et al., 2016). In addition, we have reported sex as a potentially significant factor in the obesity-inflammation-depressive symptoms associations such that the obesity-depression comorbidity tends to be stronger and BARIC is associated with somatic depressive symptoms in women but not in men as mentioned above. Among men, metabolic syndrome is a stronger factor in depressive symptoms rather than BARIC (Kohn et al., 2019). A more pronounced relationship between inflammation and depression in women compared to men is in agreement with the literature (Derry, Padin, Kuo, Hughes, & Kiecolt-Glaser, 2015). These data in key co-varying factors highlight the need of careful consideration of such individual characteristics in therapeutic approaches in the era of promoting personalized medicine and precision health.
6. Clinical and therapeutic implications
Various therapeutic approaches are informed by available clinical and mechanistic evidence of the link between inflammation and neuropsychiatric outcomes. For example, circulating levels of high sensitivity CRP greater than 5 mg/L was found to be a promising tool with which patients with treatment-resistant depression can be identified as likely responders to a TNF antagonist infliximab treatment in reducing depressive symptoms (Raison et al., 2013). There exist a few completed and ongoing NIH-funded clinical trials to investigate the safety and efficacy of infliximab in major and bipolar depression (https://clinicaltrials.gov).
Our group has focused on non-pharmacological behavioral therapeutics such as physical exercise to mitigate inflammation-related physical and mental symptoms. As mentioned above, acute physical exercise leads to the re-distribution of immune cells marked by transient but significant leukocytosis followed by leukocyte re-margination that is mediated in part by sympathoadrenal and HPA activation, respectively. Furthermore, we have reported that an acute bout of moderate exercise (20 minutes at 65–70% VO2 peak, 12–13 on Borg’s perceived exertion scale) resulted in reduced LPS-stimulated TNF production by circulating monocytes, indicating potential anti-inflammatory effects of acute exercise on a per-cell basis (Dimitrov et al., 2017) as well as a contribution of monocyte re-distribution. At the same time, we also, note that our data show a higher relative increase of CD16+ monocytes (“non-classical” monocytes) compared to non-CD16-expressing monocytes (“classical” monocytes) as a result of acute exercise while the classical monocytes are the majority in numbers among the monocyte subsets before and after exercise. Thus, whether reduced average TNF production by monocytes post exercise truly indicates anti-inflammatory effects of acute exercise warrants additional investigations. The remaining contradiction is the findings of greater plasma levels of some inflammatory cytokines post-exercise in the literature and the resulting notion of acute exercise being a pro-inflammatory stimulus that is contrary to the consensus of regular exercise being anti-inflammatory. Although the current literature does not provide a clear explanation for plasma levels vs. cellular activity of inflammatory responses in relation to acute exercise, we postulate that sub-population specific leukocyte redistribution during exercise likely contributes to this potentially complex phenomenon. In addition, cardiorespiratory fitness was a significant predictor of BARIC beyond the effect of obesity such that the association of impaired BARIC with increasing BMI disappeared after adjusting for fitness (Hong, Dimitrov, Pruitt, Shaikh, & Beg, 2014).
Meanwhile, the negative association between fitness and plasma levels of TNF and IL-1β was mediated by BARIC. Taken together, our findings indicate that moderate bouts of exercise may promote downregulation of cellular inflammatory activities and lead to improved BARIC through the sympathoadrenal pathway. Thus, we postulate that regular moderate physical activity is a promising behavioral therapeutic approach to mitigate inflammation that underlies comorbid physical and mental disorders.
Summary
We, as well as other investigators, have shown the evidence of the immunological underpinnings at the center of comorbid neuropsychiatric and physical illnesses. In this article, the summarized findings from our studies highlighting the bidirectional communications between the brain and inflammatory processes via sympathoadrenal and HPA pathways, as well as cholinergic anti-inflammatory pathway (Figure 1). Our findings of the associations of immune activation and inflammation with cognitive dysfunction in chronic HIV infection and with depressive symptoms in CVD provide the evidence of inflammatory dysregulation as an underlying pathophysiology of these comorbid conditions. The focus on inflammation at the interface of co-existing physical and mental pathology such as CVD and depression offers a mechanistic insight with a therapeutic promise as shown in TNF blocker treatment effects for patients suffering from treatment-resistant depression in which treatment responders can be identified based on the elevated inflammatory state using a CRP-based threshold. Next, our findings show that inflammation also underlies subclinical levels of depressive symptoms in other chronic inflammatory conditions such as obesity. Third, our data of β-AR, GR, and α7 nAChR-mediated regulation of monocytic inflammatory responses highlight the significant role of neuroendocrine mechanisms, which informs promising behavioral intervention strategies such as exercise that act upon such neuroendocrine pathways. Lastly, considerations of individual factors such as obesity or metabolic state and sex are of clinical significance, given their observed associations with inflammation as well as depression.
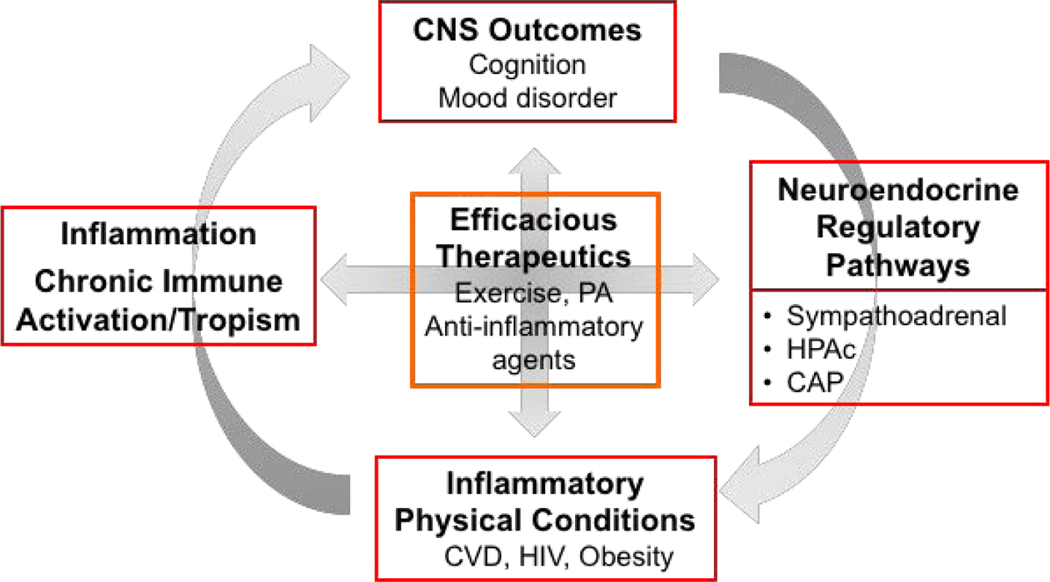
Figure 1.
Inflammation and neuroendocrine pathways at the interface of central nervous system (CNS) and the immune system in health and disease. The reciprocal interactions between the brain and physical health outcomes are governed through the neuroendocrine pathways (sympathoadrenal and HPAc axes, CAP) and inflammatory processes which also interact. In this article, we presented data that highlight such associations in CVD, HIV infection, and obesity in association with depression or subclinical depressive symptoms and neurocognition. Non-pharmacological interventions such as exercise or PA which affects both neuroendocrine and inflammatory processes present a promising therapeutic opportunity for co-morbid physical and mental conditions.
CNS, central nervous system; HPAc, hypothalamic-pituitary-adrenal-cortical; CAP, cholinergic anti-inflammatory pathway; CVD, cardiovascular disease; HIV, human immunodeficiency virus; PA, physical activity
Highlights.
Inflammation dysregulation underlies CVD and depressive symptoms
β-adrenergic and glucocorticoid receptors mediate monocytic TNF responses
Both acute and chronic exercise offer anti-inflammatory strategies
Acknowledgement
The author would like to express gratitude to the collaborators and coauthors of cited work within and outside of UC San Diego who made this work possible. This work was in part supported by the research grants HL126056 and AG063328 from the NIH of which SH is the principal investigator.
Abbreviations
- α7 nAChRs alpha
7 nicotinic acetylcholine receptors
- ART
antiretoroviral therapy
- β-AR
beta-adrenergic receptor
- BARIC
beta-adrenergic receptor (β-AR) mediated inflammation control
- BDI
Beck depression inventory
- BP
blood pressure
- CNS
central nervous system
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- CVD
cardiovascular disease
- GC
glucocorticoid
- GR
glucocorticoid receptor
- HAD
HIV-associated dementia
- HAND
HIV-associated neurocognitive disorder
- HF
heart failure
- HPA
hypothalamic pituitary adrenal
- HIV
human immunodeficiency virus
- IFN-α
interferon alpha
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- sICAM-1
soluble intercellular molecule-1
- TNF
tumor necrosis factor
Footnotes
Disclosure Statement: The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, & Miller AH (2002). Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology, 26(5), 643–652. doi: 10.1016/s0893-133x(01)00407-9 [DOI] [PubMed] [Google Scholar]
- Capuron L, Lasselin J, & Castanon N (2017). Role of Adiposity-Driven Inflammation in Depressive Morbidity. Neuropsychopharmacology, 42(1), 115–128. doi: 10.1038/npp.2016.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Dimitrov S, Pruitt C, & Hong S (2016). Glucocorticoid mediated regulation of inflammation in human monocytes is associated with depressive mood and obesity. Psychoneuroendocrinology, 66, 195–204. doi: 10.1016/j.psyneuen.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry HM, Padin AC, Kuo JL, Hughes S, & Kiecolt-Glaser JK (2015). Sex Differences in Depression: Does Inflammation Play a Role? Curr Psychiatry Rep, 17(10), 78. doi: 10.1007/s11920-015-0618-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Hulteng E, & Hong S (2017). Inflammation and exercise: Inhibition of monocytic intracellular TNF production by acute exercise via beta2-adrenergic activation. Brain Behav Immun, 61, 60–68. doi: 10.1016/j.bbi.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Shaikh F, Pruitt C, Green M, Wilson K, Beg N, & Hong S (2013). Differential TNF production by monocyte subsets under physical stress: blunted mobilization of proinflammatory monocytes in prehypertensive individuals. Brain Behav Immun, 27(1), 101–108. doi: 10.1016/j.bbi.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, . . . Grant I (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol, 17(1), 3–16. doi: 10.1007/s13365-010-0006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, & Banks WA (2015). Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun, 45, 1–12. doi: 10.1016/j.bbi.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Dimitrov S, Cheng T, Redwine L, Pruitt C, Mills PJ, . . . Wilson K (2015). Beta-adrenergic receptor mediated inflammation control by monocytes is associated with blood pressure and risk factors for cardiovascular disease. Brain Behav Immun, 50, 31–38. doi: 10.1016/j.bbi.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Dimitrov S, Pruitt C, Shaikh F, & Beg N (2014). Benefit of physical fitness against inflammation in obesity: role of beta adrenergic receptors. Brain Behav Immun, 39, 113–120. doi: 10.1016/j.bbi.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Pruitt C, Woods BG, Zapanta C, Linke S, Redwine L, … Mills PJ (2008). Depressive symptoms & inflammation in congestive heart failure vs. healthy individuals. Psychosom Med, 70(3), A-49. [meeting abstract] [Google Scholar]
- Hong S, Schrier RD, Kao Y, Vaida F, Cherner M, Crescini M,…Letendre S (2013). Does migration of immune cells into the CNS play a role in HIV-associated neurocognitive impairment during antiretroviral therapy? Brain Behav Immun, 32, suppl. 1, p e40–1. [meeting abstract] [Google Scholar]
- Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, . . . O’Connor CM (2001). Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med, 161(15), 1849–1856. doi: 10.1001/archinte.161.15.1849 [DOI] [PubMed] [Google Scholar]
- Kim-Chang JJ, Donovan K, Loop MS, Hong S, Fischer B, Venturi G, . . . Sleasman JW (2019). Higher soluble CD14 levels are associated with lower visuospatial memory performance in youth with HIV. Aids, 33(15), 2363–2374. doi: 10.1097/qad.0000000000002371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn JN, Cabrera Y, Dimitrov S, Guay-Ross N, Pruitt C, Shaikh FD, & Hong S (2019). Sex-specific roles of cellular inflammation and cardiometabolism in obesity-associated depressive symptomatology. Int J Obes (Lond), 43(10), 2045–2056. doi: 10.1038/s41366-019-0375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, & Zitman FG (2010). Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry, 67(3), 220–229. doi: 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS,…Miller AH (2001). Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Eng J Med, 344, 961–966. DOI: 10.1056/NEJM200103293441303 [DOI] [PubMed] [Google Scholar]
- Padro CJ, & Sanders VM (2014). Neuroendocrine regulation of inflammation. Semin Immunol, 26(5), 357–368. doi: 10.1016/j.smim.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin AJ, Horowitz MA, Roelofs J, Zunszain PA, & Pariante CM (2019). Glucocorticoid Resistance: Is It a Requisite for Increased Cytokine Production in Depression? A Systematic Review and Meta-Analysis. Front Psychiatry, 10, 423. doi: 10.3389/fpsyt.2019.00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, . . . Miller AH (2009). Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry, 65(4), 296–303. doi: 10.1016/j.biopsych.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, . . . Miller AH (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry, 70(1), 31–41. doi: 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault PF, Hoofnagle JH, Park Y, Mullen KD, Peters M, Jones DB, . . . Jones EA (1987). Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med, 147(9), 1577–1580. [PubMed] [Google Scholar]
- Rustad JK, Stern TA, Hebert KA, & Musselman DL (2013). Diagnosis and treatment of depression in patients with congestive heart failure: a review of the literature. Prim Care Companion CNS Disord, 15(4). doi: 10.4088/PCC.13r01511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzano A, & Cosentino M (2015). Adrenergic regulation of innate immunity: a review. Front Pharmacol, 6, 171. doi: 10.3389/fphar.2015.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, & Letendre S (2015). Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One, 10(2), e0116526. doi: 10.1371/journal.pone.0116526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, & Anand KS (2014). HIV presenting as young-onset dementia. J Int Assoc Provid AIDS Care, 13(2), 110–112. doi: 10.1177/2325957413488173 [DOI] [PubMed] [Google Scholar]