Abstract
BACKGROUND AND OBJECTIVES:
Spreading depolarizations (SDs) are a pathological mechanism that mediates lesion development in cerebral gray matter. They occur in ∼60% of patients with severe traumatic brain injury (TBI), often in recurring and progressive patterns from days 0 to 10 after injury, and are associated with worse outcomes. However, there are no protocols or trials suggesting how SD monitoring might be incorporated into clinical management. The objective of this protocol is to determine the feasibility and efficacy of implementing a treatment protocol for intensive care of patients with severe TBI that is guided by electrocorticographic monitoring of SDs.
METHODS:
Patients who undergo surgery for severe TBI with placement of a subdural electrode strip will be eligible for enrollment. Those who exhibit SDs on electrocorticography during intensive care will be randomized 1:1 to either (1) standard care that is blinded to the further course of SDs or (2) a tiered intervention protocol based on efficacy to suppress further SDs. Interventions aim to block the triggering and propagation of SDs and include adjusted targets for management of blood pressure, CO2, temperature, and glucose, as well as ketamine pharmacotherapy up to 4 mg/kg/ hour. Interventions will be escalated and de-escalated depending on the course of SD pathology.
EXPECTED OUTCOMES:
We expect to demonstrate that electrocorticographic monitoring of SDs can be used as a real- time diagnostic in intensive care that leads to meaningful changes in patient management and a reduction in secondary injury, as compared with standard care, without increasing medical complications or adverse events.
DISCUSSION:
This trial holds potential for personalization of intensive care management by tailoring therapies based on monitoring and confirmation of the targeted neuronal mechanism of SD. Results are expected to validate the concept of this approach, inform refinement of the treatment protocol, and lead to larger-scale trials.
KEY WORDS: Decompressive craniectomy, Electrocorticography, Intensive care, Ketamine, Spreading depolarization, Spreading depression, Traumatic brain injury
ABBREVIATIONS:
- BTACT
Brief Test of Adult Cognition by Telephone
- CDE
common data elements
- CMRO2
cerebral metabolic rate of oxygen
- CPP
cerebral perfusion pressure
- CRF
case report form
- DC
direct current
- ECG
electrocardiogram
- ECoG
electrocorticography
- ETCO2
end-tidal carbon dioxide
- GOS-E
Glasgow Outcome Score-Extended
- INDICT
Inhibition With Combination Therapy
- MAP
mean arterial pressure
- N.A.
not applicable
- PbtO2
brain tissue oxygen
- QOLIBRI
Quality of Life After Brain Injury
- SBP
systolic blood pressure
- SD
spreading depolarization
- TBI
traumatic brain injury.
Traumatic brain injury (TBI) is a heterogeneous disease, and precision medicine principles are needed to advance and personalize treatment options. Although identification of mechanistic targets to guide delivery of the right therapy to the right patients has proven to be challenging,1,2 one candidate that has emerged is spreading depolarizations (SDs). SDs are pathological waves that propagate through gray matter at 2 to 5 mm/min and have been characterized in experimental studies as a proven, requisite mechanism of necrotic lesion development, mediating excitotoxic Ca2+ loading and neurotransmitter release, microvascular constriction, and metabolic depletion.3,4
In patients who require surgical treatment for TBI or stroke, SDs can be monitored by electrocorticography during intensive care (Figure 1).5 Using this method, it is found that SDs occur in ∼60% of TBI cases, beginning within hours of injury and continuing through 7 to 10 days. Some patients have relatively sparse SD occurrence, whereas approximately one third have clusters of repetitive SDs, often leading to persistent isoelectricity of brain activity. Such patterns are independently associated with worse 6-month outcomes.6-9 Other clinical TBI studies have found that SDs are associated with excitotoxicity and metabolic crisis,10 seizures,11 and spreading ischemia.12 In patients with aneurysmal subarachnoid hemorrhage, SDs are similarly an independent mechanism of early and delayed infarction and worse patient outcomes.13,14
FIGURE 1.
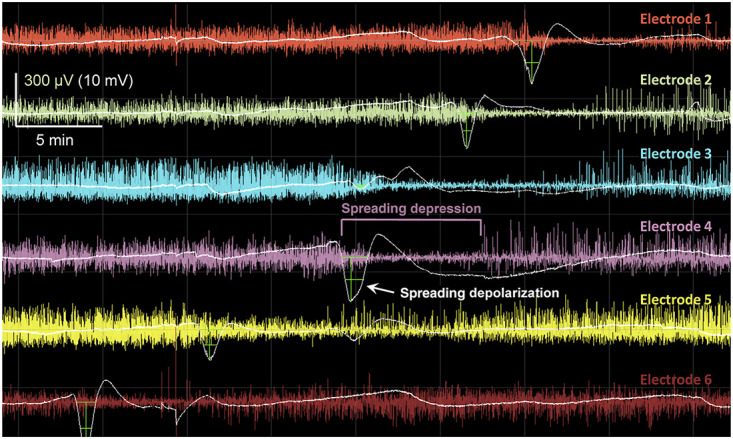
Spreading depolarizations in electrocorticographic recordings of severe brain trauma. Illustration of a spreading depolarization propagating along a 6-contact subdural electrode strip, sequentially from electrodes 6 to 1. For each electrode, the raw recording is filtered for the typical electroencephalography band of 0.5 to 50 Hz (color traces) and the slow potential recording (<0.1 Hz) is overlaid (white traces; negative is down). The slow potentials are filtered by subtraction of the 30-minute moving median to correct for baseline drift. Green crosshairs mark the negative direct current shifts that are characteristic of spreading depolarizations. The sustained (typically 1-3 minutes) mass depolarization of cortex precludes electrical signaling, resulting in a spreading depression of the amplitude of electroencephalography band activity.
Based on this evidence, several neurosurgical centers have incorporated electrocorticographic monitoring of SD in standard care of acute brain injury. This is justified in part by the known efficacy of ketamine as a treatment to block SDs15-20 because diagnosis can lead to treatment. More broadly, the increasing use of ketamine in intensive care is partly attributable to its effect on SDs.21,22 However, the use of this diagnosis-treatment combination raises several important questions that have not yet been answered with adequate evidence.23 For instance, how accurately can SD data be read in real time for use in patient management? How aggressively should SDs be treated based on their severity or patterning? Is ketamine the optimal or only treatment? Is there evidence of benefit?
To begin addressing these questions, we developed a trial that tests the use of SD monitoring to guide intensive care management of patients with surgical TBI. Our approach was guided by several primary considerations. First, we aimed to use selective inclusion and mechanistic targeting to the maximal extent possible. We therefore focused the study on only those patients who have SDs and adopted a therapeutic approach that is guided by continuous SD monitoring. Second, we aimed to minimize the use of therapy, in both duration and intensity, to reduce patient risk. Third, we aimed to conform with the concept of neurointensive care as a systems process that involves interacting causal pathways and iterations of diagnosis-treatment.5 Finally, a fourth consideration was that SD is not only a mechanism but also a marker of injury.24 Because SD is triggered in cortical hotspots that cross a threshold of critical physiological dysfunction, its occurrence may indicate the need for interventions to stabilize perilesional tissue. Therefore, the therapeutic approach is based on both adjustment of physiological targets to prevent SD ignition and direct pharmacological blockade of SDs.
The approach to preventing SDs is based on the finding that SDs are triggered by focal mismatches of energy supply and demand,25 ie, conditions of relative ischemia. Clinical and experimental studies have identified several factors that can trigger SD in tissue that is near the threshold of functional failure. These include low plasma glucose,26-28 low mean arterial pressure (MAP) or cerebral perfusion pressure (CPP),6,25,29-31 tissue hypoxia,25 and fever.29,32 Although current intensive care guidelines offer general and sometimes broad target ranges for physiological values, there is evidence that patients may benefit from more personalized management to narrower targets. By incorporating adjustment of these systemic physiology risk factors in the treatment protocol, here, we further test the idea that SDs can be used as a sensitive measure to guide intensive care management toward systemic values that are optimized for the individual patient.
STUDY GOALS AND OBJECTIVES
Objective 1 is to determine the feasibility of implementing a treatment protocol for intensive care of patients with severe TBI guided by real-time SD monitoring. Feasibility will be assessed by compliance with a tier-based treatment algorithm, including (1) ability to identify SDs in real time and adjust treatment tiers as prescribed, (2) the degree to which therapeutic goals prescribed at each tier are pursued and achieved, and (3) the difference in therapeutic intensity with SD-guided care vs standard care.
Objective 2 is to determine the effects of an SD-guided intensive care protocol to reduce secondary injury and improve cerebral physiology. End points to be compared between the treatment and control arms will be (1) continuous and categorical measures of SD occurrence/burden;5,6 (2) secondary measures of cerebral physiology, including intracranial pressure (ICP), CPP, and brain tissue oxygen (PbtO2); and (3) medical complications and adverse events.
STUDY DESIGN
Inclusion/Exclusion
Inclusion criteria are (1) emergency craniotomy to treat acute TBI within 72 hours post-trauma, (2) placement of subdural electrode strip for neuromonitoring during the emergency craniotomy procedure, (3) enrollment within 24 hour after electrode strip placement, and (4) age 18 to 80 years. Exclusion criteria are (1) persistent bilateral nonreactive pupils or other evidence of nonsurvivable injury, (2) decompressive craniectomy to treat refractory ICP subsequent to diffuse injury, (3) coenrollment in another therapeutic TBI trial, and (4) pregnancy.
Protocol Overview
Electrode strips are used as a standard care procedure for electrocorticography monitoring during intensive care. If research consent is obtained, subjects will be randomized 1:1 to either standard care (control) or SD-guided care (treatment) as soon as an SD is observed. There is no time limit for when randomization may occur. In the control arm, treatment will be blinded to electrocorticography-based recognition of SDs and will adhere to national guidelines for intensive care of patients with TBI. In the treatment arm, management will follow a tiered intervention protocol based on efficacy to suppress further SD pathology. Patients who are enrolled but do not exhibit SDs will not be randomized, but will be followed for the study duration. Figure 2 shows the study flow chart.
FIGURE 2.
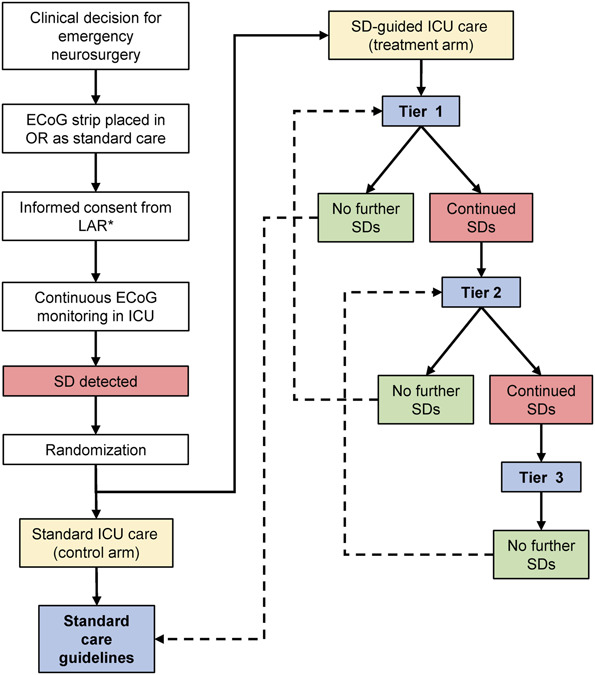
Trial flowchart. Patients with traumatic brain injury who require emergency surgery and receive an electrocorticography strip for monitoring will be eligible for enrollment. After consent, patients will be randomized only if and when spreading depolarizations are first observed on electrocorticography. Patients randomized to the control arm will receive standard care with blinding of the care team to the further course of depolarizations. Those randomized to the treatment arm will initially be treated at Tier 1 of the SD-guided ICU care protocol. Tier 1 treatments are directed at correcting conditions of systemic and cerebral physiology that may contribute to triggering of depolarizations. Tiers 2-3 escalate the Tier 1 management goals and also add pharmacological treatment with ketamine. Patients are escalated and de-escalated between tiers based on success or failure of therapy to suppress further depolarizations, as specified in Table 1. ECoG, electrocorticography; ICU, intensive care unit; SD, spreading depolarization.
Control Arm
In the standard care arm, electrocorticography will inform patient management based only on identification of seizures and ictal-interictal continuum patterns. Electrocorticography displays that allow recognition of SDs will be disabled, and intensive care will be blinded to the further course of SD pathology. Patient management will follow published national guidelines with common intensive care unit (ICU)–based targets for physiological intervention that are believed to mitigate the development of secondary brain injuries.33-36 Invasive monitoring devices will be used at the treating physicians' discretion. Sedation will be maintained with fentanyl or its analog and either propofol or midazolam, as needed,37 and patients in both study arms will receive a minimum of 500 mg levetiracetam twice daily as standard prophylaxis to prevent early seizures. Use of ketamine will not be allowed in the control arm except in the case of status epilepticus that is not controlled with standard antiseizure medications, including intravenous midazolam. Ketamine is Food and Drug Administration-approved as an anesthetic agent, but does not have approved indications for the treatment of seizures or SDs.
Treatment Arm
In this arm, patterns of SD in electrocorticography will be used to guide patient management according to a tier-based therapeutic escalation and de-escalation protocol (Table 1). The protocol intent is to suppress SDs using the most conservative treatment possible, using more aggressive treatments only when necessary, and only for the duration that is necessary. Tier changes are determined by the success or failure of SD suppression at the given tier, according to the specified criteria. De-escalation from higher tiers occurs after shorter durations of SD suppression to minimize the use of pharmacotherapy at higher doses. Escalation and de-escalation may occur as many times as necessary. Treatments should be initiated within 1 hour of tier assignment, and all treatments for the tier should be pursued unless contraindicated according to clinical judgment. All treatments and management practices other than those specified will be the same as those for the control arm. Ketamine will not be used for ICU analgosedation except in accordance with this protocol.
TABLE 1.
SD-Guided ICU Care Protocol
| Tier | Target thresholds and treatments | Escalation | De-escalation |
|---|---|---|---|
| 1 | SBP >120 mm Hg, or CPP >70 mm Hga | To Tier 2 as soon as 3 SDs occur in a 24-h period | Return to standard care targets if no SDs occur for 48 h: |
| ETCO2 to goal PaCO2 ≥35 mm Hg as tolerated by ICPa,b | • SBP >110 or CPP >60 | ||
| Core temperature <38.5°C | • ETCO2 ≥35 mm Hg | ||
| ICP < 22 mm Hga | • Core temperature <38.5°C | ||
| PbtO2 >20 mm Hga | • Serum glucose >80 mg/dL | ||
| Serum glucose concentrations 140-180 mg/dL | • ICP, PbtO2: no change | ||
| 2 | Continue Tier 1 goals for SBP/CPP, ICP, glucose, and PbtO2 | To Tier 3 as soon as 3 SDs occur in a 24-h period | Return to Tier 1 if no SDs occur for 24 h |
| ETCO2 to goal PaCO2 ≥ 40 mm Hg as tolerated by ICPa,b | |||
| Core temperature < 37.0°C | |||
| Initiate ketamine at 1 mg/kg/h (16.67 mcg/kg/min) | |||
| • Lower dosing may be used as per clinical judgment | |||
| • Fentanyl and either midazolam or propofol should be used concurrently for analgosedation | |||
| 3 | Continue Tier 2 goals | N.A. | Return to Tier 2 if no SDs occur for 12 h |
| Increase ketamine to 2-4 mg/kg/h (33.33-66.67 mcg/kg/min) | |||
| • Start at 2 mg/kg/h (33.33 mcg/kg/min). Increase dose by 0.5 mg/kg/h (8.33 mcg/kg/min) if SD occurs >15 min after previous dose start. Titrate up to 4 mg/kg/h maximum dose, as needed | |||
| • Ensure protected airway before initiation of Tier 3 intervention | |||
| • Fentanyl and either midazolam or propofol should be used concurrently for analgosedation |
CPP, cerebral perfusion pressure; ETCO2, end-tidal concentration of expired carbon dioxide; ICP, intracranial pressure; ICU, intensive care unit; PaCO2, partial pressure of arterial carbon dioxide; PbtO2, Brain tissue oxygen; N.A., not applicable; SD, spreading depolarization; SBP, systolic blood pressure.
Only applicable to patients undergoing intracranial neuromonitoring as part of clinical care.
Arterial blood gases will be drawn every 6 h and correlated with ETCO2.
Note: Tier 3 includes ketamine treatment at 2 to 4 mg/kg/h, a dose that requires ventilatory assistance. Thus, patients at Tier 1 can be extubated when clinically indicated, and Tier 2 will be the maximum for patients who are breathing spontaneously. For intubated patients at Tiers 2-3, mechanical ventilation will be continued until criteria are met for de-escalation to Tier 1. Extubation and de-escalation of such patients who have not met these criteria are allowed per clinical judgment but will be recorded as a protocol violation. Mechanical ventilation will not be extended because of SD considerations for longer than 7 days total.
Bold indicates the difference compared with standard care.
Duration
Electrocorticography will be monitored for a minimum of 5 days and, in the treatment arm, until a period of 24 hours with no SDs has been achieved. It may be terminated earlier if no longer clinically feasible.
METHODOLOGY
Electrocorticography
Electrocorticography is used in standard care of patients with surgical TBI as a sensitive measure of seizures and epileptiform activity38 and is the gold standard for SD monitoring.5,39 A 6-contact subdural electrode strip is placed typically on pericontusional frontal or temporal lobe gyri and exteriorized with generous tunneling. In the ICU, electrode leads are connected to a direct current (DC)–coupled amplifier (Advanced ICU Amplifier, Micromed S.p.A) and Moberg Component Neuromonitoring System-350 monitor (Micromed S.p.A).5,39 At the end of monitoring, strips are removed at the bedside by gentle traction. There have been no hemorrhagic or infectious complications using this procedure.5,6,9,40
Protocol Implementation and Compliance
Component Neuromonitoring System CarePath (Micromed S.p.A) is customizable bedside software that has been used in other tier-based intensive care management studies.41 It will be used to aid protocol compliance and documentation of study-related interventions and decisions. It will display, prompt, and record tier changes, treatment targets, interventions, and decision criteria.
Case Report Forms
Clinical data regarding injury characteristics and hospital course will be collected in accordance with National Institute of Neurological Disorders and Stroke common data elements (CDEs) for TBI42 and will be entered in a secure, online database maintained in compliance with Health Insurance Portability and Accountability Act and 21CFRpart11 (QuesGen Systems, Inc). Data fields include established prognostic variables,43 demographics, injury presentation, medications, vital signs, therapeutic intensity level, neurological examinations, arterial blood gases, complete blood count, biochemistry, surgeries, adverse events, complications, and outcomes.
CONCLUSION
The monitoring and recognition of SDs is an emerging specialization in neurointensive care, and causes, interpretations, and treatments remain active topics in translational neuroscience. As such, our approach to address emerging clinical applications was first to examine and establish the feasibility of incorporating SD monitoring in neurocritical care. We attempt to do so by developing a protocol based on best available evidence and consensus opinion of the authors. The protocol here provides one answer to the question, “what should a clinician do when spreading depolarizations are observed in a patient,”23 and thus provides a first point of comparison for alternative or complementary approaches. It may also serve as a recommendation for those centers currently using electrocorticography in standard care.
Alternative trials designs were discussed and have been advocated by others. For instance, the protocol could be simplified by escalating treatments more aggressively or initiating treatments only when SDs occur repetitively (ie, clusters). Furthermore, treatments could be reduced to 1 or 2 tiers or even to a single modality of ketamine pharmacotherapy. Another approach might forego the requirement of electrocorticography and test the benefits of ketamine as a preferred agent for analgosedation,22,44 allowing a larger trial and inclusion of nonsurgical patients. We consider these rational options, but favored an approach based on the potential of SD monitoring for personalized medicine. Specifically, the trial design provides for individualized clinical management by (1) targeting therapy only to patients in whom the targeted mechanism is diagnostically confirmed (with a comparable control arm) and (2) adjusting treatment dose and duration based on individual pathology and response to treatment.
Importantly, this protocol is a trial of a system of care, and not of a single treatment modality. The system involves the iterative interaction between a novel diagnostic and a multimodal treatment algorithm, guided by continuing assessment of the impact of treatment on the target mechanism. As a feasibility trial, it will assess (1) the capability to diagnose SDs in a timely and continuing manner, (2) the capability to implement and adjust treatments accordingly, and (3) the impact of an SD-guided protocol on the course and intensity of care delivered. As a treatment trial, it will assess the efficacy of interventions to suppress SDs as a potentially modifiable secondary injury process. Trial results could lead to refinement of the treatment algorithm in further Phase 2 trials or possibly to larger-scaler trials that address the long-term goal of improving patient outcomes.
Trial Status
The study opened for enrollment at the University of Cincinnati on December 14, 2022.
Safety Considerations
Risk Mitigation
Most study interventions are within accepted standards of care, and risks are therefore considered minimal. Furthermore, consistent with the study's feasibility aims, all treatments prescribed by protocol are recommendations. Decisions to implement individual treatments will be at the discretion of the treating physician, who may decline particular treatments based on clinical judgment. There is potential risk, however, in Tier 3 treatment with ketamine prescribed at 2 to 4 mg/kg/hour, a dosing that requires mechanical ventilation. Although little is known about optimal ventilator weaning and tracheal extubation in patients with TBI,45 there are risks associated with prolongation of ventilation, including prolonged length of stay, ventilator-associated pneumonia, and tracheolaryngeal injury. To mitigate risk, patients who are not already on mechanical ventilation will not be escalated to Tier 3. For patients already at Tier 3 treatment, mechanical ventilation will be maintained for study purposes until criteria for de-escalation to Tier 2 are met, depending on other clinical contexts. However, prolongation for study purposes will not exceed 7 days.
Adverse Events and Oversight
Adverse events that could be caused by study procedures will be monitored and documented. A physician medical monitor will review all adverse events and unanticipated problems involving risk to subjects. The monitor will have oversight responsibilities, with power to halt the study or mandate protocol changes. Adverse events, unanticipated problems involving risk to subjects or others, protocol deviations, complaints, and other reportable events will be disclosed to the University of Cincinnati Institutional Review Board and Human Research Protection Office, Army Medical Research and Materiel Command, in accordance with respective policies.
Follow-up
Tests administered at 6-month postinjury are the Glasgow Outcome Score-Extended (GOS-E),46,47 the Brief Test of Adult Cognition by Telephone (BTACT),48 and the Quality of Life After Brain Injury (QOLIBRI).49,50 Tests will be given preferably at a follow-up visit or alternatively by telephone.
Data Management and Statistical Analysis
Power and Sample Size
Using data from a previous cohort6 and an unpaired two-sample t-test with variance heterogeneity, the study was powered to detect a 50% reduction in SD count at 80% power and α = 0.05, 2-sided significance level. To determine total sample size (n = 71), it was assumed that 40% of enrolled patients would not have SD and would not be randomized (n = 29) and that the remaining 60% of patients with SDs would be randomized to the experimental arms (n = 21 each).
Data Management
Access to the case report form (CRF) database will be user-specific and password-protected. The database has automated checks for accuracy of data entry and will prompt the user when inaccurate or inconsistent entries are made. To ensure data integrity, an audit trail will log all entries and changes.
Quality Assurance
Blinding
In the control arm, the clinical team will be blinded to SD data by disabling electrocorticography displays required for SD recognition (ie, long time scales and frequencies <0.1 Hz). Such displays will remain available to research staff for quality assurance purposes only and will be password-protected. Formal scoring of SDs5 and 6-month outcome assessments will be conducted with blinding to the treatment arm.
Randomization
Assignment to the study arm will follow computer-generated randomization grids prepared for each site with random block sizes (2, 4, and 6) and will be managed in the QuesGen database.
EXPECTED OUTCOMES OF THE STUDY
We expect to demonstrate that electrocorticographic monitoring of SDs can be used as a real-time diagnostic in intensive care that leads to meaningful changes in patient management and a reduction in secondary injury, as compared with standard care, without increasing medical complications or adverse events.
Duration of the Project
We expect that 12 subjects can be enrolled annually at each study site. The expected duration based on 3 sites is 2 years of active enrollment.
Project Management
The study is led by author JAH and will be managed through the Clinical Trials Division, Department of Neurosurgery, University of Cincinnati.
Ethics
Informed Consent
Consent procedures will be conducted in accordance with Good Clinical Practices and Declaration of Helsinki ethical principles. Because all eligible patients will be obtunded or comatose, surrogate consent will be sought from a legally authorized representative.
Risk-Benefit
Benefits of study participation considerably outweigh risks. Subjects randomized to the treatment arm are likely to receive the most benefit because SD monitoring to guide intensive care is hypothesized to reduce secondary brain injury and improve cerebral physiology. In addition, there is intention for all subjects to benefit from study participation. Benefit may be derived from increased surveillance related to study procedures, including attention to complications and adverse events, and completion of 6-month outcome questionnaires.
Contributor Information
Jens P. Dreier, Email: jens.dreier@charite.de.
Laura B. Ngwenya, Email: ngwenyla@ucmail.uc.edu.
Ramani Balu, Email: ramanibalu1@gmail.com.
Andrew P. Carlson, Email: andrewcarlson@salud.unm.edu.
Brandon Foreman, Email: foremabo@ucmail.uc.edu.
Funding
This material is based on the work supported by the Congressionally Directed Medical Research Programs (CDMRP) under Contract No. W81XWH-21-C-0075. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the CDMRP. Jens P. Dreier acknowledges support from DFG DR 323/10-1 and Era-Net Neuron EBio2, with funds from BMBF (01EW2004). Andrew P. Carlson received funding from NIH P20GM109089.
Disclosures
The authors have no conflicts to disclose. Laura P. Ngwenya has financial relationships with Abbott and Biogen. Andrew P. Carlson has a financial relationship with Cerebroscope. Brandon Foreman has financial relationships with UCH Pharma, Marinus Pharmaceuticals, and Sage Therapeutics.
REFERENCES
- 1.Maas AIR, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics. 2010;7(1):115-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maas AI, Menon DK, Lingsma HF, Pineda JA, Sandel ME, Manley GT. Re-orientation of clinical research in traumatic brain injury: report of an international workshop on comparative effectiveness research. J Neurotrauma. 2012;29(1):32-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartings JA, Shuttleworth CW, Kirov SA, et al. The continuum of spreading depolarizations in acute cortical lesion development: examining Leao's legacy. J Cereb Blood Flow Metab. 2017;37(5):1571-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439-447. [DOI] [PubMed] [Google Scholar]
- 5.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab. 2017;37(5):1595-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartings JA, Andaluz N, Bullock MR, et al. Prognostic value of spreading depolarizations in patients with severe traumatic brain injury. JAMA Neurol. 2020;77(4):489-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartings JA, Watanabe T, Bullock MR, et al. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain 2011;134(5):1529-1540. [DOI] [PubMed] [Google Scholar]
- 8.Hartings JA, Vidgeon S, Strong AJ, et al. Surgical management of traumatic brain injury: a comparative-effectiveness study of 2 centers. J Neurosurg. 2014;120(2):434-446. [DOI] [PubMed] [Google Scholar]
- 9.Hartings JA, Bullock MR, Okonkwo DO, et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011;10(12):1058-1064. [DOI] [PubMed] [Google Scholar]
- 10.Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J Neurotrauma. 2016;33(19):1775-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabricius M, Fuhr S, Willumsen L, et al. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin Neurophysiol. 2008;119(9):1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinzman JM, Andaluz N, Shutter LA, et al. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain. 2014;137(11):2960-2972. [DOI] [PubMed] [Google Scholar]
- 13.Dreier JP, Winkler MKL, Major S, et al. Spreading depolarizations in ischaemia after subarachnoid haemorrhage, a diagnostic phase III study. Brain. 2022;145(4):1264-1284. [DOI] [PubMed] [Google Scholar]
- 14.Dreier JP, Woitzik J, Fabricius M, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129(12):3224-3237. [DOI] [PubMed] [Google Scholar]
- 15.Sakowitz OW, Kiening KL, Krajewski KL, et al. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40(8):e519-e522. [DOI] [PubMed] [Google Scholar]
- 16.Hertle DN, Dreier JP, Woitzik J, et al. Effect of analgesics and sedatives on the occurrence of spreading depolarizations accompanying acute brain injury. Brain 2012;135(8):2390-2398. [DOI] [PubMed] [Google Scholar]
- 17.Hartings JA, Ngwenya LB, Carroll CP, Foreman B. Letter to the Editor. Ketamine sedation for the suppression of spreading depolarizations. J Neurosurg. 2019;130(6):2095-2097. [DOI] [PubMed] [Google Scholar]
- 18.Carlson AP, Abbas M, Alunday RL, Qeadan F, Shuttleworth CW. Spreading depolarization in acute brain injury inhibited by ketamine: a prospective, randomized, multiple crossover trial. J Neurosurg. 2019;130(5):1513-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos E, Olivares-Rivera A, Major S, et al. Lasting s-ketamine block of spreading depolarizations in subarachnoid hemorrhage: a retrospective cohort study. Crit Care. 2019;23(1):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Porras R, Santos E, Scholl M, et al. The effect of ketamine on optical and electrical characteristics of spreading depolarizations in gyrencephalic swine cortex. Neuropharmacology. 2014;84:52-61. [DOI] [PubMed] [Google Scholar]
- 21.Herzer G, Mirth C, Illievich UM, Voelckel WG, Trimmel H. Analgosedation of adult patients with elevated intracranial pressure: survey of current clinical practice in Austria. Wiener klinische Wochenschrift. 2018;130(1-2):45-53. [DOI] [PubMed] [Google Scholar]
- 22.Madsen FA, Andreasen TH, Lindschou J, Gluud C, Moller K. Ketamine for critically ill patients with severe acute brain injury: protocol for a systematic review with meta-analysis and Trial Sequential Analysis of randomised clinical trials. PLoS One. 2021;16(11):e0259899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helbok R, Hartings JA, Schiefecker A, et al. What should a clinician do when spreading depolarizations are observed in a patient? Neurocrit Care. 2019;32(1):306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartings JA. Spreading depolarization monitoring in neurocritical care of acute brain injury. Curr Opin Crit Care. 2017;23(2):94-102. [DOI] [PubMed] [Google Scholar]
- 25.von Bornstadt D, Houben T, Seidel JL, et al. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron. 2015;85(5):1117-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens AR, Ng IHX, Helmy A, Hutchinson PJA, Menon DK, Ercole A. Glucose dynamics of cortical spreading depolarization in acute brain injury: a systematic review. J Neurotrauma. 2019;36(14):2153-2166. [DOI] [PubMed] [Google Scholar]
- 27.Strong AJ, Smith SE, Whittington DJ, et al. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia. Stroke. 2000;31(1):214-222. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann U, Sukhotinsky I, Eikermann-Haerter K, Ayata C. Glucose modulation of spreading depression susceptibility. J Cereb Blood Flow Metab. 2013;33(2):191-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartings JA, Strong AJ, Fabricius M, et al. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 2009;26(11):1857-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sukhotinsky I, Yaseen MA, Sakadzic S, et al. Perfusion pressure-dependent recovery of cortical spreading depression is independent of tissue oxygenation over a wide physiologic range. J Cereb Blood Flow Metab. 2010;30(6):1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helbok R, Schiefecker AJ, Friberg C, et al. Spreading depolarizations in patients with spontaneous intracerebral hemorrhage: association with perihematomal edema progression. J Cereb Blood Flow Metab. 2017;37(5):1871-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiefecker AJ, Kofler M, Gaasch M, et al. Brain temperature but not core temperature increases during spreading depolarizations in patients with spontaneous intracerebral hemorrhage. J Cereb Blood Flow Metab. 2018;38(3):549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle international severe traumatic brain injury consensus Conference (SIBICC). Intensive Care Med. 2019;45(12):1783-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carney N, Totten AM, O'Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6-15. [DOI] [PubMed] [Google Scholar]
- 35.Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46(5):919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okonkwo DO, Shutter LA, Moore C, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med. 2017;45(11):1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghori KA, Harmon DC, Elashaal A, et al. Effect of midazolam versus propofol sedation on markers of neurological injury and outcome after isolated severe head injury: a pilot study. Crit Care Resusc. 2007;9(2):166-171. [PubMed] [Google Scholar]
- 38.Foreman B, Lee H, Okonkwo DO, et al. The relationship between seizures and spreading depolarizations in patients with severe traumatic brain injury. Neurocrit Care. 2022;37(S1):31-48. [DOI] [PubMed] [Google Scholar]
- 39.Hartings JA, Li C, Hinzman JM, et al. Direct current electrocorticography for clinical neuromonitoring of spreading depolarizations. J Cereb Blood Flow Metab. 2017;37(5):1857-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drenckhahn C, Windler C, Major S, et al. Complications in aneurysmal subarachnoid hemorrhage patients with and without subdural electrode strip for electrocorticography. J Clin Neurophysiol. 2016;33(3):250-259. [DOI] [PubMed] [Google Scholar]
- 41.Bernard F, Barsan W, Diaz-Arrastia R, Merck LH, Yeatts S, Shutter LA. Brain Oxygen Optimization in Severe Traumatic Brain Injury (BOOST-3): a multicentre, randomised, blinded-endpoint, comparative effectiveness study of brain tissue oxygen and intracranial pressure monitoring versus intracranial pressure alone. BMJ Open. 2022;12(3):e060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maas AI, Harrison-Felix CL, Menon D, et al. Standardizing data collection in traumatic brain injury. J Neurotrauma. 2011;28(2):177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hukkelhoven CW, Steyerberg EW, Habbema JDF, et al. Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma. 2005;22(10):1025-1039. [DOI] [PubMed] [Google Scholar]
- 44.Klass A, Sanchez-Porras R, Santos E. Systematic review of the pharmacological agents that have been tested against spreading depolarizations. J Cereb Blood Flow Metab. 2018;38(7):1149-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robba C, Poole D, McNett M, et al. Mechanical ventilation in patients with acute brain injury: recommendations of the European Society of Intensive Care Medicine consensus. Intensive Care Med. 2020;46(12):2397-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teasdale GM, Pettigrew LE, Wilson JL, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the glasgow outcome scale. J Neurotrauma. 1998;15(8):587-597. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JL, Pettigrew LE, Teasdale GM. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573-585. [DOI] [PubMed] [Google Scholar]
- 48.Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the brief test of adult cognition by telephone. Age Ageing. 2006;35(6):629-632. [DOI] [PubMed] [Google Scholar]
- 49.Truelle JL, Koskinen S, Hawthorne G, et al. Quality of life after traumatic brain injury: the clinical use of the QOLIBRI, a novel disease-specific instrument. Brain Inj. 2010;24(11):1272-1291. [DOI] [PubMed] [Google Scholar]
- 50.Harfmann EJ, deRoon-Cassini TA, McCrea MA, Nader AM, Nelson LD. Comparison of four quality of life inventories for patients with traumatic brain injuries and orthopedic injuries. J Neurotrauma. 2020;37(12):1408-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]


