Abstract
Background:
Anti-mitochondrial antibodies (AMA) and the M2 subtype are considered serological hallmarks in the diagnosis of primary biliary cholangitis (PBC). However, these autoantibodies may be undetectable in some patients. This meta-analysis aimed to evaluate the diagnostic accuracy of serum AMA and M2 for PBC.
Methods:
We systematically searched PubMed, Embase, Web of Science, and the Cochrane Library for relevant studies. Pooled sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), and diagnostic odds ratio (DOR) were calculated using a random-effects model. We also constructed hierarchical summary receiver operating characteristic curves and calculated the area under the curve values.
Results:
Our meta-analysis included 28 studies, of which 24 examined the diagnostic accuracy of AMA for PBC. Pooled sensitivity and specificity of AMA were 84% (95% confidence intervals [CI] 77–90%) and 98% (96–99%), respectively. Pooled LR+, LR−, and DOR were 42.2 (22.1–80.5), 0.16 (0.11–0.24), and 262 (114–601), respectively. Sixteen studies explored the diagnostic value of the M2 subtype, demonstrating pooled sensitivity and specificity of 89% (81–94%) and 96% (93–98%), respectively. Pooled LR+, LR−, and DOR were 20.3 (8.0–51.1), 0.12 (0.05–0.26), and 169 (41–706), respectively. The hierarchical summary receiver operating characteristic curves for both of serum AMA and M2 subtype lie closer to the upper left corner of the plot with area under the curve values of 0.98 (95% CI = 0.96–0.99) and 0.98 (95% CI = 0.96–0.99) respectively.
Conclusion:
This meta-analysis provides evidence affirming the utility of AMA and M2 as sensitive and specific serological hallmarks that can facilitate early screening and diagnosis of PBC.
Keywords: anti-mitochondrial antibody, diagnosis, meta-analysis, performance, primary biliary cholangitis
1. Introduction
Primary biliary cholangitis (PBC) is an autoimmune cholestatic liver disease characterized by destruction of the small intrahepatic bile ducts, resulting in progressive cholestasis, fibrosis, cirrhosis and liver failure if left untreated.[1,2] The global prevalence of PBC is estimated to be between 40 to 400 cases per million population, with a female predominance.[3]
The etiology of PBC remains poorly understood but is believed to involve a combination of genetic susceptibility, environmental triggers, and breakdown of immune tolerance.[4] The hallmark of PBC is the loss of immune tolerance to mitochondrial and nuclear antigens, leading to immune-mediated bile duct damage driven by autoreactive T and B cells.[5] PBC is associated with specific human leukocyte antigen alleles, suggesting a genetic component, as well as viral triggers and molecular mimicry between microbial and self-proteins.[6] Abnormal apoptosis and defective clearance of apoptotic debris may expose intracellular autoantigens to the immune system.[7] B cells are stimulated to produce high-titer autoantibodies such as anti-mitochondrial antibody (AMA) and antinuclear antibody, while autoreactive T cells accumulate in the liver, perpetuating inflammation and bile duct injury.[8]
The presence of AMA was first identified in the 1960s, and their association with PBC was initially characterized during that seminal period.[9,10] AMA are present in up to 95% of PBC patients and target components of the 2-oxoacid dehydrogenase complexes, particularly the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2).[11] AMA recognizes the inner lipoyl domain of PDC-E2 and is highly disease-specific, making it a key serological marker for PBC diagnosis.[12] The predominant AMA subtype is anti-M2, directed against PDC-E2. AMA can be detected years before PBC diagnosis and may predict disease development in asymptomatic individuals.[13,14] Other autoantibodies found in PBC patients include anti-sp100 and anti-gp210, directed against nuclear body and nuclear envelope proteins respectively.[15,16] Anti-gp210 and/or anti-sp100 antibodies were highly specifically in the diagnosis of PBC.[17] Besides, they were reported to be associated with a more rapid progression to cirrhosis and liver failure, which may aid in predicting disease progression, although their exact pathophysiological roles remain unclear.[15]
PBC is characterized by an elevated serum alkaline phosphatase (ALP), reflecting cholestasis and injury to small bile duct epithelial cells (cholangiocytes).[18–20] In healthy individuals, ALP is produced by cells in the liver, bone, intestine, placenta, and kidneys.[21] In cholestatic liver disease, damaged cholangiocytes release increased amounts of ALP into the circulation. ALP levels often correlate with disease severity and can help monitor PBC progression and response to treatment.[22] Other commonly elevated liver enzymes in PBC include aspartate aminotransferase, alanine aminotransferase and gamma-glutamyl transferase.[23,24] However, ALP tends to be disproportionately elevated compared to other enzymes. Serum cholesterol is also frequently increased, while hyperbilirubinemia may occur in later stages due to reduced bile flow.[25,26] The European Association for the Study of the Liver guidelines[27] recommend annual lab monitoring with liver biochemistry including ALP, bilirubin, aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase. Worsening biochemical markers over time, particularly ALP, indicate disease progression and need for therapeutic intervention.[28–30]
Accordingly, diagnosis of PBC is based on a combination of the above-mentioned clinical features, biochemical abnormalities, detectable autoantibodies, and histologic changes. International guidelines such as those from the American Association for the Study of Liver Diseases [31] recommend that 2 of the following criteria are met for diagnosis: Biochemical evidence of cholestasis based on elevated ALP; Presence of AMA or other PBC-specific autoantibodies such as anti-sp100 or anti-gp210; Histologic evidence of nonsuppurative destructive cholangitis and interlobular bile duct destruction. Patients who are AMA-negative pose a greater diagnostic challenge and often require liver biopsy. Early and accurate diagnosis of PBC is important to prevent complications and improve long-term outcomes.
In short, the diagnostic significance of serum markers of AMA remains enigmatic and is not well established. We conducted this meta-analysis to evaluate the performance of serum AMA and M2 subtype in the diagnosis of PBC.
2. Methods
This meta-analysis was accomplished following the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[32] The authors declare that all supporting data are available within the article. Ethics approval and patient consent were not required since all analyses were based on previously published studies. We registered our meta-analysis in PROSPERO (CRD42020189155).
2.1. Data sources and search strategy
We systematically searched PubMed, Embase, Web of Science, and the Cochrane Library from database inception through May 5, 2023 to identify relevant studies. Two investigators (XQL and ZWJ) independently performed the literature search using a combination of controlled vocabulary terms (e.g., Medical Subject Headings) and free-text keywords. The search strategy focused on the following key concepts: “anti-mitochondrial antibody”, “ama”, “m2”, “autoantibody”, “antibody”, “primary biliary cholangitis”, “primary biliary cirrhosis”, “pbc”, and “autoimmune liver disease”. We presented the specific search strings utilized for each database (Table S1, Supplemental Digital Content, http://links.lww.com/MD/K671). We limited the searches to human studies published in English language. Conference abstracts and other gray literature were excluded. To identify additional relevant articles, we manually searched the reference lists of all included studies, reviews, meta-analyses, and other pertinent publications. The final list of articles to screen was generated by merging citations from both the electronic and manual searches after removing duplicates. Two reviewers independently screened the titles and abstracts of retrieved records for relevance. The full-text version of any article deemed potentially eligible was obtained and further assessed for inclusion based on the predefined criteria. Any discrepancies regarding study selection were resolved by discussion and consensus with a third investigator acting as arbiter.
2.2. Inclusion and exclusion criteria
We included studies that met the following predefined eligibility criteria:
Participants had a diagnosis of PBC established using accepted international criteria, such as the guidelines from European Association for the Study of the Liver[27] or American Association for the Study of Liver Diseases,[31] or through clinical diagnosis based on the combination of histological findings, biochemical liver function tests, imaging, and clinical features.
The diagnostic accuracy of serum AMA and/or M2 subtype was evaluated.
Sensitivity and specificity of AMA or M2 for diagnosing PBC were reported, or sufficient data (such as true positives [TP], false positives [FP], false negatives [FN], and true negatives [TN]) were presented to allow us to indirectly calculate sensitivity and specificity.
Sample size was at least 20 PBC cases. For multiple publications using the same case series, the study with the largest sample or most recent data was selected.
Published in English language.
We excluded studies that met the following criteria:
Did not have adequate clinical data to confirm a PBC diagnosis.
Had overlapping datasets with other included studies.
Did not report sufficient information to allow calculation of diagnostic accuracy.
Had a sample size < 20 PBC patients.
Were publications types such as letters, editorials, reviews, case reports, conference abstracts, or nonhuman studies.
2.3. Data extraction and quality assessment
The reference management software EndNote X8 (Thomson Reuters, New York, NY) was used to manage records retrieved from electronic searches. The full-text versions of all publications that potentially qualified for the meta-analysis were scanned in detail by 2 independent investigators according to the predefined inclusion and exclusion criteria. The decision to include a study was made by consensus, and discrepancies between the 2 investigators at any stage of the study selection process were arbitrated by a third reviewer and resolved upon consensus. The following data were included: the first author, publication year, study location, number of patients, ethnicity, age, diagnostic criteria for PBC, antibody type, detection methods, and quality assessment score. The test results were extracted including TP, FP, FN, and TN. We evaluated critically the risk of bias and applicability based on the revised Quality Assessment of Diagnostic Accuracy Studies tool with 4 key domains including patient selection, index text, reference standard, and flow and timing.[33]
2.4. Statistical analysis
All statistical analyses were performed using StataSE version 17.0 (StataCorp, College Station, TX). The diagnostic accuracy of serum AMA and M2 for PBC was evaluated by generating forest plots of pooled sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), and diagnostic odds ratio (DOR) with corresponding 95% confidence intervals (CIs). We also constructed hierarchical summary receiver operating characteristic (HSROC) curves to assess overall diagnostic performance. HSROC curves and corresponding area under the curve (AUC) demonstrate the inverse relationship between sensitivity and specificity by plotting the trade-off between these measures. Pooled estimates were calculated using a bivariate random-effects model based on the DerSimonian-Laird method. Heterogeneity was evaluated using the I2 statistic, with values ≤ 25%, ≤50% and ≤ 75% indicating low, moderate and high inconsistency between studies, respectively. To explore potential sources of heterogeneity, we performed subgroup analyses and random-effects meta-regression based on study-level covariates including sample size, geographical location, publication year, and test method. Sensitivity analyses were conducted by excluding individual studies to evaluate their influence on overall results. Publication bias was assessed through Deeks’ asymmetry test and Egger regression test.[34] All statistical tests were 2-sided and P < .05 was considered statistically significant.
3. Results
3.1. Characteristics of the included studies
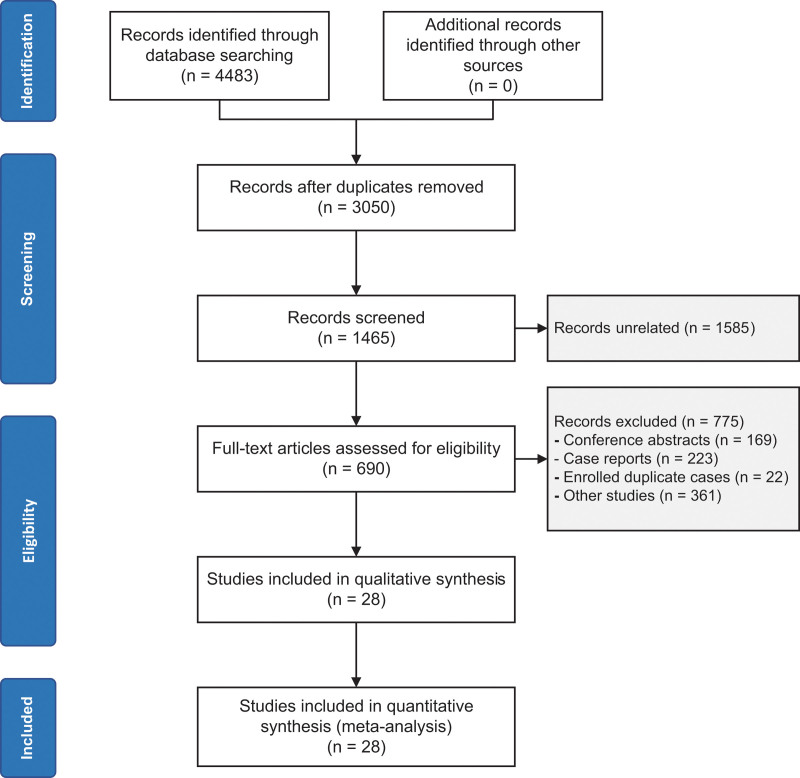
A comprehensive search in the mentioned databases yielded a total of 4483 studies. After removing duplicates, 3050 studies remained. Subsequently, 1585 records were excluded as they were not relevant to our study, leaving us with 1465 articles. Further refinement led to the exclusion of 775 records, categorized as follows: 169 conference abstracts, 223 case reports, 22 duplications with the same samples, and 361 other studies. After thorough evaluation of the full-text, 690 records were considered eligible for our meta-analysis. Eventually, 28 articles were selected to be included in the current study, and there were no disagreements between the 2 investigators. For a visual representation of the study selection process, please refer to Figure 1.
Figure 1.
Flow chart of the search.
Regarding the baseline age in the selected studies, participants ages ranged from 22 to 88 years. The presence of AMA was detected in 24 studies using various methods, including indirect immunofluorescence (IIF) in 20 studies, enzyme-linked immunosorbent assay (ELISA) in 3 studies, and in 1 study, the method was not reported. On the other hand, the sensitivity and specificity of the M2 subtype were reported in 16 articles, using ELISA in 12 studies, ELISA and Western blot in 1 study each, and the methods were not reported in 2 studies. These studies were conducted across different countries, with representation from America (2 studies), China (8 studies), France (2 studies), Greece (1 study), Italy (4 studies), Japan (4 studies), and 1 multicenter study. Additionally, there was 1 study each from Canada, Korea, Spain, Tunisia, Colombia, and the UK.
To assess the quality of the included studies, Quality Assessment of Diagnostic Accuracy Studies scores were employed, with the studies ranging from 5 to 8, indicating a moderately high quality overall. The main characteristics of the selected studies can be found in Table 1.
Table 1.
Main characteristics of included studies.
| Author | Year | Age | Study location | Criteria | Antibody | Methods | QUADAS-2 |
|---|---|---|---|---|---|---|---|
| Assassi[1] | 2009 | 62.7 ± 7.81 | America | Clinical features* | AMA/M2 | IIF/ELISA | 6 |
| Bandin[2] | 1996 | NR | France | NR | AMA | IIF | 8 |
| Bargou[3] | 2008 | 58.9 ± 13.4 | Tunisia | NR | AMA/M2 | IIF/ELISA | 7 |
| Cavazzana[4] | 2011 | 48 ± 11.7 | Italy | EASL | AMA | IIF | 8 |
| Chung[5] | 2016 | 53 (32–72) | UK | NR | AMA | ELISA | 6 |
| Dighiero[6] | 1987 | NR | France | NR | AMA/M2 | IIF/ELISA | 5 |
| Gabeta[7] | 2007 | 62 (19–87) | Greece | Clinical features* | AMA/M2 | IIF/ELISA | 8 |
| Guatibonza-García[8] | 2021 | 56.9 ± 10.2 | Colombia | Biopsy | AMA | IIF | 5 |
| Han[9] | 2017 | 56.9 (28–85) | Korea | AASLD 2009 | AMA/M2 | IIF/ELISA | 8 |
| Hu[10] | 2011 | 54.0 (46–60.3) | China | AASLD 2000 | AMA/M2 | IIF/ELISA | 6 |
| Imura-Kumada[11] | 2012 | 56.7 ± 8.4 | Japan | NR | M2 | ELISA | 7 |
| Jong-Hon[12] | 2001 | NR | Japan | NR | AMA | IIF | 6 |
| Lee[13] | 1983 | NR | America | Clinical features* | AMA | IIF | 5 |
| Liu and Liu[14] | 2010 | 55 ± 15 | China | EASL | AMA | IIF | 7 |
| Liu and Norman[15] | 2010 | NR | Multicenter† | EASL | AMA | IIF | 6 |
| Lu[16] | 2017 | 52.94 ± 1.49 | China | AASLD 2009 | M2 | NR | 7 |
| Milkiewicz[17] | 2009 | 48 ± 2 | Canada | Clinical features* | M2 | ELISA | 6 |
| Muratori[18] | 2004 | 58.1 ± 14 | Italy | Clinical features* | AMA/M2 | IIF/ELISA/WB | 7 |
| Nagai[19] | 1983 | NR | Japan | Clinical features* | AMA/M2 | IIF/ELISA | 5 |
| Oertelt[20] | 2007 | 67 (35–82) | Italy | Clinical features* | AMA | ELISA | 7 |
| Romero-Gómez[21] | 2004 | 55.4 ± 12.5 | Spain | Clinical features* | AMA | IIF | 8 |
| Sakugawa[22] | 2003 | 22–88 | Japan | Clinical features* | AMA | IIF | 8 |
| Sun[23] | 2019 | 32–76 | China | AASLD 2009 | AMA/M2 | IIF/WB | 7 |
| Tang[24] | 2017 | 45 (19–61) | China | AASLD 2009 | M2 | ELISA | 8 |
| Villalta[25] | 2015 | 41.1 (2–87) | Italy | AASLD 2009 | AMA/M2 | IIF/Line blot | 7 |
| Wang[26] | 2015 | 52 (44–77) | China | AASLD 2009 | AMA/M2 | ELISA/ELISA | 7 |
| Yang J[27] | 2016 | 56.89 ± 9.87 | China | EASL | AMA/M2 | NR/NR | 8 |
| Yang Z[28] | 2012 | 56 ± 12 | China | AASLD 2009 | AMA | IIF | 7 |
AASLD = the American Association for the Study of Liver Diseases, AMA/M2 = anti-mitochondrial antibody/M2 subtype, AU = arbitrary units, EASL = European Association for the Study of the Liver, ELISA = enzyme-linked immunosorbent assay, IIF, indirect immunofluorescence, NR = not reported, WB = Western blot.
Including histological findings, biochemical liver function tests, imaging, or clinical features.
Including United States, Canada, United Kingdom, Greece, Italy, Japan.
3.2. Overall diagnostic accuracy
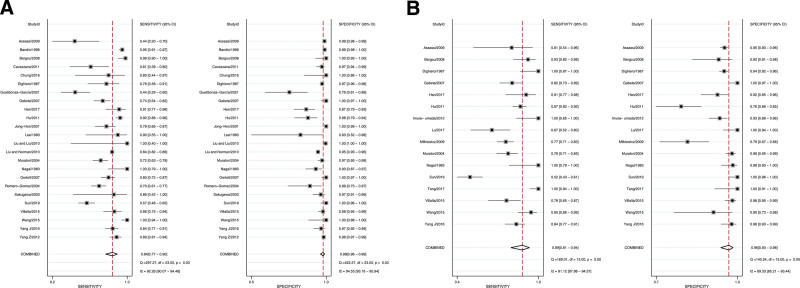
Out of the 28 studies that met the inclusion criteria, 24 of them identified the presence of AMA in both PBC patients and controls. The pooled sensitivity and specificity were calculated to be 84% (95% CI = 77%–90%) and 98% (95% CI = 96%–99%), respectively. However, the Q tests for pooled sensitivity and specificity indicated significant heterogeneity among the studies, with I2 values of 92.26 (95% CI = 90.07–94.46) for sensitivity and 94.55 (95% CI = 93.16–95.94) for specificity (P = .00) (Fig. 2A). The pooled LR+, LR-, and DOR were 42.2 (95% CI = 22.1–80.5), 0.16 (95% CI = 0.11–0.24), and 262 (95% CI = 114–601), respectively. We displayed a detailed overview of the raw data, including TP, FP, FN, and TN, as well as the diagnostic accuracy of serum AMA for each individual study (Table S2, Supplemental Digital Content, http://links.lww.com/MD/K672).
Figure 2.
Forest plots of pooled sensitivity and specificity. (A) Pooled sensitivity (left) and specificity (right) of AMA with 95% confidence intervals (95% CIs), (B) pooled sensitivity (left) and specificity (right) of M2 subtype with 95% CIs. AMA = anti-mitochondrial antibody.
Regarding the detection of M2, a total of 16 articles reported on this subtype. The meta-analysis demonstrated a pooled sensitivity and specificity of 89% (95% CI = 76%–95%) and 96% (95% CI = 90%–98%), respectively. Similar to the AMA analysis, significant heterogeneity was found among the studies, as evidenced by Q tests with I2 values of 98.29 (95% CI = 97.92–98.67) for sensitivity and 94.39 (95% CI = 92.60–96.17) for specificity (P = .00) (Fig. 2B). The pooled LR+, LR-, and DOR were calculated to be 20.3 (95% CI = 8.0–51.1), 0.12 (95% CI = 0.05–0.26), and 169 (95% CI = 41–706), respectively. The detailed data and diagnostic performance of serum M2 subtype for each study were also demonstrated (Table S3, Supplemental Digital Content, http://links.lww.com/MD/K673).
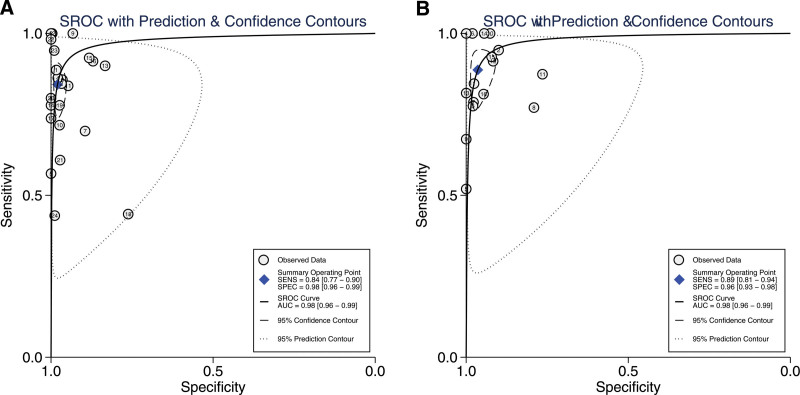
Furthermore, the diagnostic accuracy of individual studies was visually represented in a HSROC plot. In the HSROC curve, the pooled sensitivity and specificity were indicated by the summary operating point, while the 95% CI of both pooled and individual sensitivity and specificity were circled by the 95% Confidence Contour and the Prediction Contour. The HSROC curve for quantitative AMA and M2 demonstrated high accuracy, with the AUC being 0.98 (95% CI = 0.96–0.99) for both AMA and M2, suggesting excellent diagnostic performance for PBC (Fig. 3).
Figure 3.
The hierarchical summary receiver operating characteristic (HSROC) curve in PBC diagnosis. (A) AMA, (B) M2 subtype. AMA = anti-mitochondrial antibody, PBC = primary biliary cholangitis.
3.3. Heterogeneity analysis and publication bias
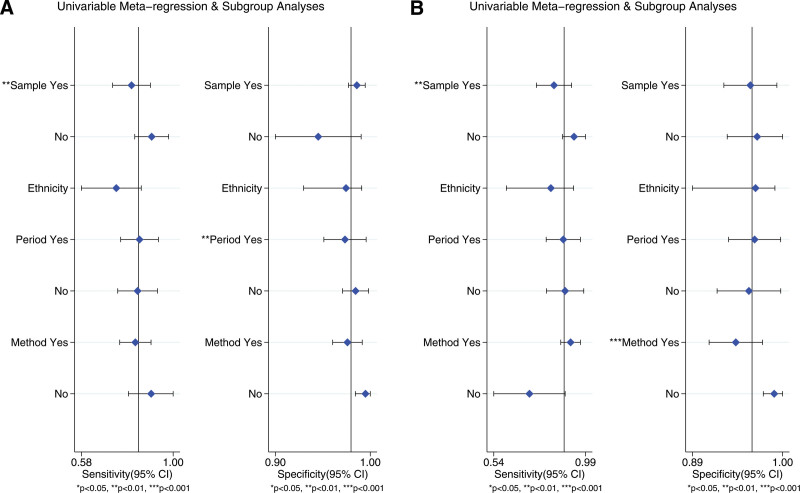
To investigate the potential sources of heterogeneity in our study, we conducted meta-regression analysis since the I2 values of the pooled sensitivity and specificity exceeded 50%. Various subgroups were defined based on sample size (< or more than 200), ethnicity (African, Asian, Caucasian, or multicenter), study period (published before or after 2010), and the detection assay used for AMA (IIF or non-IIF) and M2 (ELISA or non-ELISA). The results of the meta-regression revealed that sample size (P < .001) and study period (P < .001) were potential contributors to heterogeneity for the pooled sensitivity and specificity in AMA, as depicted in Figure 4A. As for M2, sample size (P < .001) and detection assay (P < .001) were identified as potential sources of heterogeneity for the pooled sensitivity, as shown in Figure 4B. However, it is important to interpret these findings with caution.
Figure 4.
The meta-regression analysis. (A) AMA, (B) M2 subtype. AMA = anti-mitochondrial antibody.
To further assess the impact of individual studies on the heterogeneity in our meta-analysis, we conducted a sensitivity analysis by iteratively removing each study. The results indicated that the omission of single-study estimates had minimal influence on the overall diagnostic accuracy (Figure S1, Supplemental Digital Content, http://links.lww.com/MD/K674). Additionally, we examined publication bias using Deeks’ funnel plot asymmetry test. The results indicated no apparent publication bias (Figure S2, Supplemental Digital Content, http://links.lww.com/MD/K675). Furthermore, the Egger test suggested that our meta-analysis for AMA demonstrated stability and consistency (β = 1.07, 95% CI = −0.584 to 2.723, P = .193). However, for M2, there was significant publication bias (β = 3.342, 95% CI = 1.998–4.686, P < .05).
4. Discussion
This meta-analysis of 28 studies assessed the sensitivity and specificity of AMA and M2 for the diagnosis of PBC. The pooled results demonstrated that these antibodies have a favorable accuracy for PBC diagnosis, with pooled sensitivity and specificity of 85%, 98%, and 89%, 96%, respectively. The corresponding AUCs were 0.98 for both AMA and M2, suggesting excellent diagnostic performance for PBC. The pooled LR + and LR- for AMA were 46.2 and 0.15, indicating that a positive AMA result was nearly 46 times more likely in a person with PBC compared to a person without the disease, and a negative AMA result reduced the probability of diagnosing PBC to 15%. Similarly, there was slightly inferior diagnostic performance for M2 subtype, with pooled LR + and LR− of 20.3 and 0.12, respectively. These results, combined with the pooled sensitivity and specificity, can be used to distinguish PBC from other liver diseases or healthy controls. These findings were consistent with the previous studies that there were significant higher positivity of serum AMA and M2 identified in cases with PBC compared with the controls.[35]
Despite the encouraging results, there was considerable variation in sensitivity (43.8%–100% for AMA detection, 52.0%–100% for M2 determination) and specificity (83.3%–100% for AMA detection, 76.5%–100% for M2 detection) across individual studies.[36–59] Heterogeneity is a potential problem in meta-analysis that can affect the validity of results in a systematic review and needs to be quantified.[60] Thus, we performed meta-regression based on the classification of sample size, ethnicity, study period, and detection assay for AMA and M2. The results showed that for the detection of both AMA and M2 subtype, there was significantly lower sensitivity in studies with small sample sizes compared with studies with large sample sizes. Similarly, specificity for diagnosing PBC was influenced by publication year in the detection of AMA and detection method in M2 examination. We suspect that this is likely due to variable sensitivity and specificity in different detection methods and the instability of results from small sample size studies. Additionally, we conducted sensitivity analysis by sequentially excluding individual studies. We also performed Deeks funnel plot asymmetry test and Egger test to explore outliers and publication bias, with the results confirming the relative robustness of our meta-analysis.
The pathogenesis of PBC is complex and involves a combination of genetic, environmental, and immunological factors.[1] The hallmark of the disease is the loss of immune tolerance to mitochondrial and nuclear antigens, leading to immune-mediated damage to the bile ducts.[61] While the exact mechanisms underlying the production of AMA and M2 subtype are not fully understood, it is believed that abnormal apoptosis and defective clearance of apoptotic debris may expose intracellular autoantigens to the immune system, leading to the production of autoantibodies.[62] AMA targets components of the 2-oxoacid dehydrogenase complexes, particularly PDC-E2, which is highly specific to PBC.[63] Similarly, the M2 subtype is directed against PDC-E2 and is the most PBC-specific AMA subtype.[63] The diagnostic value of AMA and M2 lies in their high sensitivity and specificity for PBC, making them valuable tools for screening and ruling out other liver diseases. On the other hand, despite these advancements, the diagnosis of PBC can still be challenging due to the overlap of these markers in other liver diseases.[64] For example, the presence of these autoantibodies is not pathognomonic for PBC, they have been reported in various other liver conditions, including chronic active hepatitis and cryptogenic cirrhosis, which may lead to false positives in PBC diagnosis.[65,66] To ensure accurate diagnosis, international guidelines recommend the use of specific diagnostic criteria, including biochemical evidence of cholestasis based on elevated ALP levels and the presence of AMA or other PBC-specific autoantibodies.[27,31] Additionally, histologic evidence of nonsuppurative destructive cholangitis and interlobular bile duct destruction is often required to confirm the diagnosis, especially in AMA-negative cases.[67] However, these guidelines also acknowledge the limitations of AMA and M2 subtype, and the need for careful interpretation in certain clinical contexts.[27,31]
Several knowledge gaps remain regarding the optimal use of AMA and M2 testing for PBC diagnosis and disease monitoring, especially the clinical features and optimal management of these patients require further elucidation.[68] Long-term studies are needed to determine whether changing autoantibody levels predict treatment response and prognosis.[69] Future guidelines would benefit from head-to-head comparisons of testing modalities to standardize protocols and reference ranges. Additional research should also clarify the mechanisms of autoantibody production in PBC and their potential utility as biomarkers for early diagnosis or screening asymptomatic individuals.[70] Regarding the detection methods for AMA and M2, the variability across studies highlights the need for standardized, evidence-based guidelines to enable accurate and consistent PBC diagnosis worldwide.
Some limitations should be considered when interpreting the findings. First, restricting our search to only 4 databases and English articles may have excluded relevant studies and introduced language bias. Second, variability in the diagnostic criteria used as reference standards could have led to misclassification, especially for borderline cases relative to current guidelines. Third, the use of different detection methods for AMA and M2 across studies likely contributed to heterogeneity in results. Head-to-head comparisons of testing modalities are needed to identify optimal approaches. Fourth, most included studies comprised Asian and Caucasian populations, limiting generalizability to other ethnicities that should be evaluated in future research. Finally, evidence of publication bias suggests selective reporting may have inflated accuracy estimates in some studies. However, we conducted a comprehensive literature search and applied predefined strict inclusion criteria to ensure all participants had definitively diagnosed PBC per accepted guidelines or typical presentation. We also used multiple independent reviewers for study selection, data extraction, and quality assessment with validated tools to minimize risk of bias. Thus, the reliability and objectivity of the results are reasonably assured.
In summary, this comprehensive meta-analysis found that AMA and M2 antibodies demonstrate high diagnostic accuracy for PBC. However, clinicians should interpret serological results in the full clinical context, considering clinical features, other antibodies, and histological findings. Further research is required to establish standardized testing guidelines, elucidate the variability of autoantibody levels over disease course, and identify potential novel biomarkers.
Author contributions
Conceptualization: Qingling Xu, Weijia Zhu, Yufeng Yin.
Data curation: Qingling Xu, Weijia Zhu, Yufeng Yin.
Formal analysis: Yufeng Yin.
Methodology: Weijia Zhu.
Software: Weijia Zhu.
Supervision: Yufeng Yin.
Writing – original draft: Qingling Xu, Weijia Zhu.
Writing – review & editing: Yufeng Yin.
Supplementary Material
Abbreviations:
- AMA
- anti-mitochondrial antibody
- ALP
- alkaline phosphatase
- AUC
- area under the curve
- CI
- confidence intervals
- DOR
- diagnostic odds ratios
- ELISA
- enzyme-linked immunosorbent assay
- FN
- false negatives
- FP
- false positives
- HSROC
- hierarchical summary receiver operating characteristic
- IIF
- indirect immunofluorescence
- LR−
- negative likelihood ratios
- LR+
- positive likelihood ratios
- PBC
- primary biliary cholangitis
- PDC-E2
- E2 subunit of the pyruvate dehydrogenase complex
- TN
- true negatives
- TP
- true positives
The PRISMA checklist has been adopted.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
How to cite this article: Xu Q, Zhu W, Yin Y. Diagnostic value of anti-mitochondrial antibody in patients with primary biliary cholangitis: A systemic review and meta-analysis. Medicine 2023;102:45(e36039).
Contributor Information
Qingling Xu, Email: xuqingling0926@163.com.
Weijia Zhu, Email: weijiazhu01@163.com.
References
- [1].Zhao Y, Wei S, Chen L, et al. Primary biliary cholangitis: molecular pathogenesis perspectives and therapeutic potential of natural products. Front Immunol. 2023;14:1164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bosch A, Dumortier J, Maucort-Boulch D, et al. Preventive administration of UDCA after liver transplantation for primary biliary cirrhosis is associated with a lower risk of disease recurrence. J Hepatol. 2015;63:1449–58. [DOI] [PubMed] [Google Scholar]
- [3].Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D, et al. The challenges of primary biliary cholangitis: what is new and what needs to be done. J Autoimmun. 2019;105:102328. [DOI] [PubMed] [Google Scholar]
- [4].Yang Y, He X, Rojas M, et al. Mechanism-based target therapy in primary biliary cholangitis: opportunities before liver cirrhosis? Front Immunol. 2023;14:1184252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Taylor SA, Assis DN, Mack CL. The contribution of B cells in autoimmune liver diseases. Semin Liver Dis. 2019;39:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mulinacci G, Palermo A, Gerussi A, et al. New insights on the role of human leukocyte antigen complex in primary biliary cholangitis. Front Immunol. 2022;13:975115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang YW, Lin CI, Chen HW, et al. Apoptotic biliary epithelial cells and gut dysbiosis in the induction of murine primary biliary cholangitis. J Transl Autoimmun. 2023;6:100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Webb GJ, Siminovitch KA, Hirschfield GM. The immunogenetics of primary biliary cirrhosis: a comprehensive review. J Autoimmun. 2015;64:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Walker JG, Doniach D, Roitt IM, et al. Serological tests in diagnosis of primary biliary cirrhosis. Lancet. 1965;1:827–31. [DOI] [PubMed] [Google Scholar]
- [10].Doniach D, Roitt IM, Walker JG, et al. Tissue antibodies in primary biliary cirrhosis, active chronic (lupoid) hepatitis, cryptogenic cirrhosis and other liver diseases and their clinical implications. Clin Exp Immunol. 1966;1:237–62. [PMC free article] [PubMed] [Google Scholar]
- [11].Invernizzi P, Crosignani A, Battezzati PM, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–5. [DOI] [PubMed] [Google Scholar]
- [12].Harada K, Sudo Y, Kono N, et al. In situ nucleic acid detection of PDC-E2, BCOADC-E2, OGDC-E2, PDC-E1alpha, BCOADC-E1alpha, OGDC-E1, and the E3 binding protein (protein X) in primary biliary cirrhosis. Hepatology. 1999;30:36–45. [DOI] [PubMed] [Google Scholar]
- [13].Ergenc I, Gozaydinoglu B, Keklikkiran C, et al. The risk of development of primary biliary cholangitis among incidental antimitochondrial M2 antibody-positive patients. Hepatol Forum. 2023;4:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen BH, Wang QQ, Zhang W, et al. Screening of anti-mitochondrial antibody subtype M2 in residents at least 18 years of age in an urban district of Shanghai, China. Eur Rev Med Pharmacol Sci. 2016;20:2052–60. [PubMed] [Google Scholar]
- [15].Jaskowski TD, Nandakumar V, Novis CL, et al. Presence of anti-gp210 or anti-sp100 antibodies in AMA-positive patients may help support a diagnosis of primary biliary cholangitis. Clinica Chimica Acta. 2023;540:117219. [DOI] [PubMed] [Google Scholar]
- [16].Luettig B, Boeker KHW, Schoessler W, et al. The antinuclear autoantibodies Sp100 and gp210 persist after orthotopic liver transplantation in patients with primary biliary cirrhosis. J Hepatol. 1998;28:824–8. [DOI] [PubMed] [Google Scholar]
- [17].Abenavoli L, Procopio AC, Cinaglia P, et al. Clinical patterns of primary biliary cholangitis: comparison between two European case series. Rev Recent Clin Trials. 2022;17:136–42. [DOI] [PubMed] [Google Scholar]
- [18].Parés A. Primary biliary cholangitis. Med Clin. 2018;151:242–9. [DOI] [PubMed] [Google Scholar]
- [19].Bossen L, Rebora P, Bernuzzi F, et al. Soluble CD163 and mannose receptor as markers of liver disease severity and prognosis in patients with primary biliary cholangitis. Liver Int. 2020;40:1408–14. [DOI] [PubMed] [Google Scholar]
- [20].Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871–7. [DOI] [PubMed] [Google Scholar]
- [21].Warnes TW. Alkaline phosphatase. Gut. 1972;13:926–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Berdichevski T, Cohen-Ezra O, Pappo O, et al. Positive antimitochondrial antibody but normal serum alkaline phosphatase levels: could it be primary biliary cholangitis? Hepatol Res. 2017;47:742–6. [DOI] [PubMed] [Google Scholar]
- [23].Zein CO, Angulo P, Lindor KD. When is liver biopsy needed in the diagnosis of primary biliary cirrhosis? Clin Gastroenterol Hepatol. 2003;1:89–95. [DOI] [PubMed] [Google Scholar]
- [24].Gerussi A, Bernasconi DP, O’Donnell SE, et al. Measurement of gamma glutamyl transferase to determine risk of liver transplantation or death in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2021;19:1688–1697.e14. [DOI] [PubMed] [Google Scholar]
- [25].Schaffner F. Paradoxical elevation of serum cholesterol by clofibrate in patients with primary biliary cirrhosis. Gastroenterology. 1969;57:253–5. [PubMed] [Google Scholar]
- [26].Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut. 1979;20:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].EASL Clinical Practice Guidelines. The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145–72. [DOI] [PubMed] [Google Scholar]
- [28].Trivella J, John BV, Levy C. Primary biliary cholangitis: epidemiology, prognosis, and treatment. Hepatol Commun. 2023;7:e0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].De Vincentis A, D’Amato D, Cristoferi L, et al. Predictors of serious adverse events and non-response in cirrhotic patients with primary biliary cholangitis treated with obeticholic acid. Liver Int. 2022;42:2453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abenavoli L, Procopio AC, Fagoonee S, et al. Primary biliary cholangitis and bile acid Farnesoid X receptor agonists. Diseases. 2020;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the study of liver diseases. Hepatology. 2019;69:394–419. [DOI] [PubMed] [Google Scholar]
- [32].Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313:1657–65. [DOI] [PubMed] [Google Scholar]
- [33].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [34].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hu S, Zhao F, Wang Q, et al. The accuracy of the anti-mitochondrial antibody and the M2 subtype test for diagnosis of primary biliary cirrhosis: a meta-analysis. Clin Chem Lab Med. 2014;52:1533–42. [DOI] [PubMed] [Google Scholar]
- [36].Assassi S, Fritzler MJ, Arnett FC, et al. Primary biliary cirrhosis (PBC), PBC autoantibodies, and hepatic parameter abnormalities in a large population of systemic sclerosis patients. J Rheumatol. 2009;36:2250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bandin O, Courvalin JC, Poupon R, et al. Specificity and sensitivity of gp210 autoantibodies detected using an enzyme-linked immunosorbent assay and a synthetic polypeptide in the diagnosis of primary biliary cirrhosis. Hepatology. 1996;23:1020–4. [DOI] [PubMed] [Google Scholar]
- [38].Bargou I, Mankai A, Jamaa A, et al. Detection of M2 antimitochondrial antibodies by dot blot assay is more specific than by enzyme linked immunosorbent assay. Pathol Biol (Paris). 2008;56:10–4. [DOI] [PubMed] [Google Scholar]
- [39].Cavazzana I, Ceribelli A, Taraborelli M, et al. Primary biliary cirrhosis-related autoantibodies in a large cohort of Italian patients with systemic sclerosis. J Rheumatol. 2011;38:2180–5. [DOI] [PubMed] [Google Scholar]
- [40].Chung BK, Guevel BT, Reynolds GM, et al. Phenotyping and auto-antibody production by liver-infiltrating B cells in primary sclerosing cholangitis and primary biliary cholangitis. J Autoimmun. 2017;77:45–54. [DOI] [PubMed] [Google Scholar]
- [41].Dighiero G, Magnac C, de Saint Martin J, et al. Detection of anti-mitochondrial antibodies by ELISA and Western-blot techniques and identification by one and two-dimensional gel electrophoresis of M2 target antigens. Clin Exp Immunol. 1987;70:640–8. [PMC free article] [PubMed] [Google Scholar]
- [42].Gabeta S, Norman GL, Liaskos C, et al. Diagnostic relevance and clinical significance of the new enhanced performance M2 (MIT3) ELISA for the detection of IgA and IgG antimitochondrial antibodies in primary biliary cirrhosis. J Clin Immunol. 2007;27:378–87. [DOI] [PubMed] [Google Scholar]
- [43].Guatibonza-García V, Gaete PV, Pérez-Londoño A, et al. Poor performance of anti-mitochondrial antibodies for the diagnosis of primary biliary cholangitis in female Colombian patients: a single-center study. World J Gastroenterol. 2021;27:4890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Han E, Jo SJ, Lee H, et al. Clinical relevance of combined anti-mitochondrial M2 detection assays for primary biliary cirrhosis. Clin Chim Acta. 2017;464:113–7. [DOI] [PubMed] [Google Scholar]
- [45].Hu C, Deng C, Song G, et al. Prevalence of autoimmune liver disease related autoantibodies in Chinese patients with primary biliary cirrhosis. Dig Dis Sci. 2011;56:3357–63. [DOI] [PubMed] [Google Scholar]
- [46].Jong-Hon K, Yajima R, Karino Y, et al. Development of a new enzyme-linked immunosorbent assay for the detection of anti-M2 in primary biliary cirrhosis. Hepatol Res. 2001;21:1–7. [DOI] [PubMed] [Google Scholar]
- [47].Lee WM, Shelton LL, Galbraith RM. Antibodies to hepatocyte membrane antigens in chronic liver disease: detection by immunofluorescence after Bouin’s fixation. J Histochem Cytochem. 1983;31:1246–9. [DOI] [PubMed] [Google Scholar]
- [48].Liu H, Liu Y, Wang L, et al. Prevalence of primary biliary cirrhosis in adults referring hospital for annual health check-up in Southern China. BMC Gastroenterol. 2010;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liu H, Norman GL, Shums Z, et al. PBC screen: an IgG/IgA dual isotype ELISA detecting multiple mitochondrial and nuclear autoantibodies specific for primary biliary cirrhosis. J Autoimmun. 2010;35:436–42. [DOI] [PubMed] [Google Scholar]
- [50].Muratori P, Muratori L, Gershwin ME, et al. “True” antimitochondrial antibody-negative primary biliary cirrhosis, low sensitivity of the routine assays, or both? Clin Exp Immunol. 2004;135:154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nagai S, Manns M, Meyer zum Büschenfelde KH, et al. Detection of mitochondrial antibodies directed against the primary biliary cirrhosis (M2) antigen by an enzyme-linked immunosorbent assay (ELISA). J Immunol Methods. 1983;60:77–88. [DOI] [PubMed] [Google Scholar]
- [52].Oertelt S, Rieger R, Selmi C, et al. A sensitive bead assay for antimitochondrial antibodies: chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659–65. [DOI] [PubMed] [Google Scholar]
- [53].Romero-Gomez M, Wichmann I, Crespo J, et al. Serum immunological profile in patients with chronic autoimmune cholestasis. Am J Gastroenterol. 2004;99:2150–7. [DOI] [PubMed] [Google Scholar]
- [54].Sakugawa H, Nakasone H, Nakayoshi T, et al. Epidemiology of primary biliary cirrhosis among women with elevated gamma-glutamyl transpeptidase levels in Okinawa, Japan. Hepatol Res. 2003;26:330–6. [DOI] [PubMed] [Google Scholar]
- [55].Sun Q, Wang Q, Feng N, et al. The expression and clinical significance of serum IL-17 in patients with primary biliary cirrhosis. Ann Transl Med. 2019;7:389–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Villalta D, Sorrentino MC, Girolami E, et al. Autoantibody profiling of patients with primary biliary cirrhosis using a multiplexed line-blot assay. Clin Chim Acta. 2015;438:135–8. [DOI] [PubMed] [Google Scholar]
- [57].Wang L, Sun X, Qiu J, et al. Increased numbers of circulating ICOS (+) follicular helper T and CD38 (+) plasma cells in patients with newly diagnosed primary biliary cirrhosis. Dig Dis Sci. 2015;60:405–13. [DOI] [PubMed] [Google Scholar]
- [58].Yang J, Yu YL, Jin Y, et al. Clinical characteristics of drug-induced liver injury and primary biliary cirrhosis. World J Gastroenterol. 2016;22:7579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yang Z, Liang Y, Qin B, et al. Clinical significance of conventional serum autoantibodies for various liver diseases in a Chinese population. Clin Biochem. 2012;45:203–6. [DOI] [PubMed] [Google Scholar]
- [60].Kulinskaya E, Hoaglin DC. Estimation of heterogeneity variance based on a generalized Q statistic in meta-analysis of log-odds-ratio. Res Synth Methods. 2023;14:671–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Colapietro F, Lleo A, Generali E. Antimitochondrial antibodies: from bench to bedside. Clin Rev Allergy Immunol. 2022;63:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lleo A, Bowlus CL, Yang GX, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lleo A, Invernizzi P, Mackay IR, et al. Etiopathogenesis of primary biliary cirrhosis. World J Gastroenterol. 2008;14:3328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Duan W, Chen S, Li S, et al. The future risk of primary biliary cholangitis (PBC) is low among patients with incidental anti-mitochondrial antibodies but without baseline PBC. Hepatol Commun. 2022;6:3112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kaplowitz N, Finlayson NDC, Walmsley P, et al. Hepatobiliary diseases associated with serum antimitochondrial antibody (AMA). Am J Med. 1973;54:725–30. [DOI] [PubMed] [Google Scholar]
- [66].Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. 2021;123:102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Michieletti P, Wanless IR, Katz A, et al. Antimitochondrial antibody negative primary biliary cirrhosis: a distinct syndrome of autoimmune cholangitis. Gut. 1994;35:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].John BV, Dahman B, Deng Y, et al. Rates of decompensation, hepatocellular carcinoma and mortality in AMA-negative primary biliary cholangitis cirrhosis. Liver Int. 2022;42:384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gideon MH, Jessica KD, Graeme JMA, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut. 2018;67:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chew M, Bowlus CL. Primary biliary cholangitis: diagnosis and treatment. Liver Res. 2018;2:81–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.