The clinical benefits of sodium‐glucose cotransporter‐2 (SGLT2) inhibitors in patients with CKD have been well established in major clinical outcome trials.1,2 Secondary analyses from these landmark trials demonstrated consistent benefits of SGLT2 inhibitors across many subgroups, supporting the widespread use and incorporation in clinical practice guidelines of this new kidney and cardioprotective drug class.3,4 Notwithstanding, there are three important groups of kidney patients in whom SGLT2 inhibitors are not indicated. First are patients with severely impaired kidney function. This seems sensible because the efficacy of SGLT2 inhibitors in these patients could well be diminished. SGLT2 inhibitors block the reabsorption of glucose and sodium in the proximal tubule and enhance glycosuria and natriuresis. In patients with a low number of functioning nephrons, it may be expected that SGLT2 transporters are less effective because the number of SGLT2 transporters containing tubules is reduced. Therefore, patients with an eGFR <20 ml/min per 1.73 m2 and those receiving dialysis were excluded from recent outcome trials. Yet, there are emerging data suggesting that SGLT2 inhibitors may reduce clinically relevant outcomes in these vulnerable high-risk patients.
The DAPA-CKD and EMPA-KIDNEY trials recruited 2906 patients with CKD stage 4 who had an eGFR>25 (DAPA-CKD; N=624) or >20 ml/min per 1.73 m2 (EMPA-Kidney; N=2282).1,2 In both trials, SGLT2 inhibition reduced the risk of the primary kidney outcome by 27% with no evidence that the magnitude of the effect estimate varied by baseline GFR. Moreover, in the DAPA-CKD trial, dapagliflozin reduced the relative risks of heart failure or cardiovascular death or all-cause mortality, both secondary outcomes, in participants with and without CKD stage 4.1 In the EMPA-KIDNEY trial, 245 participants had an eGFR below 20 ml/min per 1.73 m2 at randomization. Although this was a small subgroup, the 27% relative risk reduction in the incidence of the primary kidney outcome was consistent with the effect size in the overall population. These exploratory subgroup data support the hypothesis that SGLT2 inhibitors may exert beneficial effects in patients at advanced stages of CKD. However, the evidence in this specific subgroup is weak, and the benefits observed in exploratory analyses in small subgroups could be the result of chance.
Second, clinical experience with SGLT2 inhibitors in dialysis patients is also limited because of perceived lack of efficacy. However, emerging data from the DAPA-CKD trial, where patients continued study medication when they started dialysis, provide new insights. Safety assessment among participants continuing dapagliflozin or placebo during dialysis did not reveal any between-group differences in adverse events. Moreover, numerically fewer deaths were reported in the dapagliflozin compared with the placebo group.1 These data should, however, be cautiously interpreted because the dapagliflozin and placebo groups were not randomized at dialysis initiation and few participants initiated dialysis (N=68 and N=99, respectively), reducing the reliability of these results.
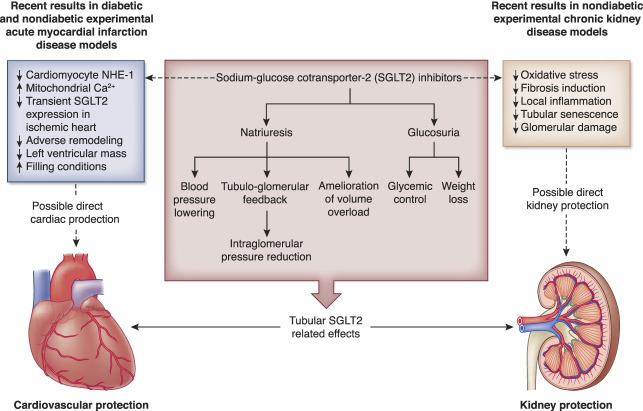
How could it be that the efficacy of SGLT2 inhibitors persists in patients with severely impaired kidney function or on dialysis? Experimental data have suggested direct effects on the kidney as well as the heart that are not mediated via SGLT2 inhibition as presented in Figure 1. For instance, in an experimental study, uremic serum from dialysis patients impaired endothelium-mediated enhancement of relaxation and contraction of cardiomyocytes. Incubation with empagliflozin reversed this process, suggesting direct effects on endothelial dysfunction in coronary microvascular beds.5 In another experimental study, it was shown that isolated endothelial cells of human coronary arteries produce on stimulation with the stressor TNFα more of vasoconstrictor reactive oxygen species and less of the vasodilator nitric oxide. These effects could be blocked by cotreating these isolated cells with the SGLT2 inhibitors empagliflozine and dapagliflozine.6 How exactly SGLT2 inhibitors exert these beneficial effects in the absence of a functioning proximal tubule requires further study. These drugs can also bind directly to sodium–hydrogen exchange 1 transporters in cardiac tissues to promote a cardioprotective effect.7 Moreover, Lee et al. showed that in the case of ischemia, SGLT2 is expressed in the myocardium and that inhibition of this cardiac SGLT2 leads to a smaller infarct size. Finally, binding of SGLT2 inhibitors to SGLT2 expressed in pancreatic α-cells may promote the release of glucagon and reduce insulin, which in turn increases ketone body levels. Increased ketone bodies exert a wide array of beneficial effects in cardiac tissues.8 This notion of SGLT2 transporter–independent cardiac benefits is further supported by a bioinformatic study that used a combined approach of in silico modeling of publicly available RNA sequence datasets coupled with RNA sequencing of cardiac tissues from an experimental model of diabetic rats with heart failure treated with empagliflozin.9 The results also suggested that the inhibition of sodium–hydrogen exchange 1 may explain the heart failure protective effects of SGLT2 inhibitors.9 Such direct cardiac and kidney effects that are independent of proximal tubular SGLT2 could play a role in patients with severe CKD and minimal diuresis, but confirmation in a clinical study is required. Thus, at present, there is no definitive evidence to support a positive benefit-to-risk ratio in patients with severe CKD.
Figure 1.
Potential cardiorenal protective mechanisms for SGLT inhibition mediated via tubular SGLT2 and via mechanisms working direct on the heart and the kidney. NHE-1, sodium–hydrogen exchange 1; SGLT2, sodium‐glucose cotransporter‐2. Adapted from Udell JA, Jones WS, Petrie MC, et al. Sodium glucose cotransporter-2 inhibition for acute myocardial infarction: JACC review topic of the week. J Am Coll Cardiol. 2022;79(20): 2058–2068.
The third group of patients who could benefit from SGLT2 inhibitors but were excluded from recent large-scale outcome trials are patients living with a kidney transplant. Besides the fact that many of these patients have (severely) decreased kidney function, there is another issue that deserves attention in this specific group. In general, SGLT2 inhibitors are well tolerated and safe. Main side effects are, however, an increased incidence of urinary tract and genital infections. Patients living with a kidney transplant have a higher chance of (severe) urinary tract infections, which may lead to kidney function loss. The benefit-to-risk ratio in kidney transplant recipients deserves therefore special attention. Another specific clinical dilemma in patients with kidney transplantation is post-transplant diabetes mellitus, which occurs in 10%–20% of kidney transplant recipients due to prednisolone and other immunosuppressive therapy. SGLT2 inhibitors may be salutary not only because of their kidney and cardiac benefits but also because they reduce the risk of new-onset diabetes.1 A number of studies have explored the effects of SGLT2 inhibition in kidney transplant recipients. Most of these studies were case reports or small retrospective case series, limiting the quality and reliability of the data. A small randomized controlled clinical trial in 44 kidney transplant recipients showed that 24-week treatment with empagliflozin reduced HbA1c and body weight compared with placebo. During the relatively short follow-up period, empagliflozin was well tolerated with no imbalance in adverse events or immunosuppressive drug levels, including calcineurin inhibitors.10 Kidney function over time or the incidence of clinical outcomes was not assessed. A more recent observational clinical practice study aimed to address this gap. In this study, 226 kidney transplant recipients with type 2 diabetes who received SGLT2 inhibitors >90 days were pair-matched with kidney transplant recipients with type 2 diabetes not using SGLT2 inhibitors. During a mean follow-up of 5.2 years, SGLT2 inhibition was associated with a reduction in the risk of all-cause mortality, death-censored graft survival, or doubling of serum creatinine.11 SGLT2 inhibitors were well tolerated with no difference in the incidence of bacterial or fungal urinary tract infections. Diabetic ketoacidosis did not occur during follow-up in both groups. Although these findings are reassuring, the retrospective character of this study, without randomization and without blinded end point collection, precludes a definitive assessment of benefits and risks.
In conclusion, although there are some preliminary promising data, it is at present unclear whether SGLT2 inhibition will be effective in preventing clinically relevant outcomes and be sufficiently safe in patients with severe CKD that are not included in the present label. The RENAL LIFECYCLE trial (NCT05374291) was recently started to investigate the potential benefit of SGLT2 inhibitors in these patients. The trial aims to address the efficacy and safety of dapagliflozin 10 mg versus placebo in at least 1500 patients with (1) an eGFR <25 ml/min per 1.73 m2; (2) dialysis patients with residual diuresis >500 ml/24-hour; or (3) kidney transplant recipients with an eGFR ≤45 ml/min per 1.73 m2. These patients are recruited at clinical practice sites in Europe and Australia. The primary study end point is a composite end point of kidney failure, heart failure hospitalization, or all-cause mortality. The results are awaited in 4 years. In the absence of reliable data from randomized controlled clinical trials, we recommend being restrictive with the use of SGLT2 inhibitors in patients with severe CKD, on dialysis, or living with a kidney transplant.
Acknowledgments
The RENAL LIFECYCLE Trial is an investigator-initiated trial funded by grants from the Dutch Kidney Foundation and the Australian NHMRC, with minor co-funding (study medication) from AstraZeneca. AstraZeneca does not have a role in the conception, design, collection of data, analysis, and interpretation of the study results.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Renal Life Cycle Trial Investigators: Aiko de Vries, Leiden UMC; Alferso Abrahams, UMC Utrecht; Jeroen van der Net, Albert Schweitzer Ziekenhuis; Marc Hemmelder, Maastricht UMC; Marc Vervloet, Amsterdam UMC; Rob van Kruijsdijk, Radboud UMC; Dirk Kuyper, UZ Leuven; Clare Arnott, TGI; Sunil Badve, TGI; Christoph Wanner, University hospital Würzburg.
Contributor Information
Collaborators: Aiko de Vries, Alferso Abrahams, Jeroen van der Net, Marc Hemmelder, Marc Vervloet, Rob van Kruijsdijk, Dirk Kuyper, Clare Arnott, Sunil Badve, and Christoph Wanner
Disclosures
S. Berger reports consultancy for Chiesi and Novartis; research funding from Chiesi and Novartis; honoraria from Astellas and Novartis; and advisory or leadership roles for Advisory Board for Novartis and Supervisory Board for Dutch Transplant Foundation. R.T. Gansevoort reports consultancy for AstraZeneca, Bayer, Galapagos, Mironid, and Sanofi-Genzyme; research funding from Abbvie, AstraZeneca, Bayer, Galapagos, Otsuka Pharmaceuticals, Roche, and Sanofi-Genzyme; role on the Editorial Boards of American Journal of Kidney Diseases, CJASN, Journal of Nephrology, Kidney360, and Nephron Clinical Practice; and role as Editor of Nephrology Dialysis Transplantation and member of the Council of the European Renal Association. H.J.L. Heerspink reports ongoing consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli-Lilly, Gilead, Janssen, Merck, Novartis, Novo Nordisk, and Travere Pharmaceuticals; research funding from AstraZeneca, Boehringer Ingelheim, Janssen research support (grant funding directed to employer), and Novo Nordisk; lecture fees from AstraZeneca and Novo Nordisk; and speakers bureau for AstraZeneca.
Funding
None.
Author Contributions
Conceptualization: Stefan Berger, Ron T. Gansevoort, Hiddo J.L. Heerspink.
Funding acquisition: Stefan Berger.
Writing – original draft: Ron T. Gansevoort, Hiddo J.L. Heerspink.
Writing – review & editing: Stefan Berger, Ron T. Gansevoort.
References
- 1.Heerspink HJL Stefánsson BV Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/nejmoa2024816 [DOI] [PubMed] [Google Scholar]
- 2.Herrington WG Staplin N Wanner C, et al.; The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/nejmoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossing P Caramori ML Chan JCN, et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5):S1–S127. doi: 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 4.UK Kidney Association Clinical Practice Guideline Working Group. Sodium-Glucose Co-transpOrter-2 (SGLT-2) Inhibition in Adults with Kidney Disease. Accessed February 2, 2023. https://ukkidney.org/sites/renal.org/files. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juni RP Al-Shama R Kuster DWD, et al. Empagliflozin restores chronic kidney disease–induced impairment of endothelial regulation of cardiomyocyte relaxation and contraction. Kidney Int. 2021;99(5):1088–1101. doi: 10.1016/j.kint.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 6.Uthman L Homayr A Juni RP, et al. Empagliflozin and dapagliflozin reduce ROS generation and restore NO bioavailability in tumor necrosis factor α-stimulated human coronary arterial endothelial cells. Cell Physiol Biochem. 2019;53(5):865–886. doi: 10.33594/000000178 [DOI] [PubMed] [Google Scholar]
- 7.Uthman L Baartscheer A Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia. 2018;61(3):722–726. doi: 10.1007/s00125-017-4509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodson DJ, Rorsman P. A variation on the theme: SGLT2 inhibition and glucagon secretion in human islets. Diabetes. 2020;69(5):864–866. doi: 10.2337/dbi19-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iborra-Egea O Santiago-Vacas E Yurista SR, et al. Unraveling the molecular mechanism of action of empagliflozin in heart failure with reduced ejection fraction with or without diabetes. JACC Basic Transl Sci. 2019;4(7):831–840. doi: 10.1016/j.jacbts.2019.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halden TAS Kvitne KE Midtvedt K, et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care. 2019;42(6):1067–1074. doi: 10.2337/dc19-0093 [DOI] [PubMed] [Google Scholar]
- 11.Lim J Kwon S Jeon Y, et al. The efficacy and safety of SGLT2 inhibitor in diabetic kidney transplant recipients. Transplantation. 2022;106(9):e404–e412. doi: 10.1097/tp.0000000000004228 [DOI] [PubMed] [Google Scholar]