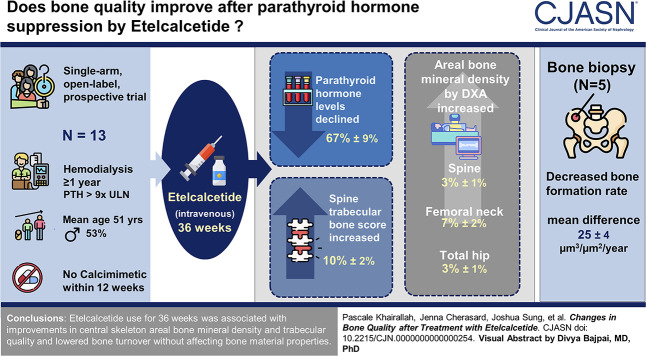
Visual Abstract
Keywords: hyperparathyroidism; mineral metabolism; bones, stones, and mineral metabolism
Abstract
Introduction
Secondary hyperparathyroidism is associated with osteoporosis and fractures. Etelcalcetide is an intravenous calcimimetic for the control of hyperparathyroidism in patients on hemodialysis. Effects of etelcalcetide on the skeleton are unknown.
Methods
In a single-arm, open-label, 36-week prospective trial, we hypothesized that etelcalcetide improves bone quality and strength without damaging bone–tissue quality. Participants were 18 years or older, on hemodialysis ≥1 year, without calcimimetic exposure within 12 weeks of enrollment. We measured pretreatment and post-treatment areal bone mineral density by dual-energy X-ray absorptiometry, central skeleton trabecular microarchitecture by trabecular bone score, and peripheral skeleton volumetric bone density, geometry, microarchitecture, and estimated strength by high-resolution peripheral quantitative computed tomography. Bone–tissue quality was assessed using quadruple-label bone biopsy in a subset of patients. Paired t tests were used in our analysis.
Results
Twenty-two participants were enrolled; 13 completed follow-up (mean±SD age 51±14 years, 53% male, and 15% White). Five underwent bone biopsy (mean±SD age 52±16 years and 80% female). Over 36 weeks, parathyroid hormone levels declined 67%±9% (P < 0.001); areal bone mineral density at the spine, femoral neck, and total hip increased 3%±1%, 7%±2%, and 3%±1%, respectively (P < 0.05); spine trabecular bone score increased 10%±2% (P < 0.001); and radius stiffness and failure load trended to a 7%±4% (P = 0.05) and 6%±4% increase (P = 0.06), respectively. Bone biopsy demonstrated a decreased bone formation rate (mean difference −25±4 µm3/µm2 per year; P < 0.01).
Conclusions
Treatment with etelcalcetide for 36 weeks was associated with improvements in central skeleton areal bone mineral density and trabecular quality and lowered bone turnover without affecting bone material properties.
Clinical Trial registry name and registration number:
The Effect of Etelcalcetide on CKD-MBD (Parsabiv-MBD), NCT03960437
Introduction
Chronic Kidney Disease–Mineral Bone Disorder (CKD-MBD) is a complication of kidney failure.1 CKD-MBD causes impairments in calcium, phosphate, vitamin D metabolism, and fibroblast growth factor 23 (FGF23), leading to secondary hyperparathyroidism. Elevated levels of parathyroid hormone (PTH) result in higher risk of fractures, cardiovascular events, and death.2,3 The high PTH level increases bone turnover rates, decreases cortical bone mass, destroys cortical and trabecular microarchitecture, and alters bone mineral and collagen content and structure. The resulting global impairment in bone strength is associated with a more than four-fold higher fracture risk in patients with kidney failure treated by hemodialysis compared with age-matched individuals.4,5 PTH suppression has been the mainstay of fracture prevention therapeutics in patients treated by hemodialysis. According to the Kidney Disease Improving Global Outcomes workgroup, patients with kidney failure and concurrent hyperparathyroidism should have PTH levels targeted toward two-to-nine times the upper limit of the reference PTH range.6 PTH suppression to this range is believed to minimize the risk of adverse skeletal effects and is associated with better patient survival.7
Calcimimetics are Food and Drug Administration–approved agents that suppress PTH production for the treatment of hyperparathyroidism in patients with kidney failure.6 Cinacalcet and etelcalcetide are administered orally and intravenously, respectively.8,9 Despite known relationships between hyperparathyroidism, bone loss, fractures, and cardiovascular events in patients with CKD,10–13 clinical trials have not shown that cinacalcet-induced PTH suppression reduces fracture or cardiovascular events in patients on hemodialysis.2,14 Etelcalcetide is associated with greater adherence and remains at therapeutic concentrations for a longer period, thus resulting in superior PTH suppression.6,15–17 We hypothesized that etelcalcetide would result in sustained PTH suppression and have favorable effects on bone density and quality in patients with kidney failure on hemodialysis. Thus, this pilot trial investigated relationships between 36 weeks of PTH suppression by etelcalcetide and skeletal changes using a multimodality approach to provide in-depth skeletal phenotyping both before and after etelcalcetide use.
Methods
Trial Site and Population
This pilot trial was conducted at the Rogosin Institute Dialysis Clinics of NewYork-Presbyterian Hospital, New York, NY, between May 23, 2019, and July 13, 2021 (NCT NCT03960437; registry date May 23, 2019). The trial was approved by the Columbia University Irving Medical Center Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Owing to the coronavirus disease 2019 (COVID-19) pandemic, this trial was terminated early before full enrollment (Figure 1). Eligibility criteria are discussed in detail in the Supplemental Methods.
Figure 1.
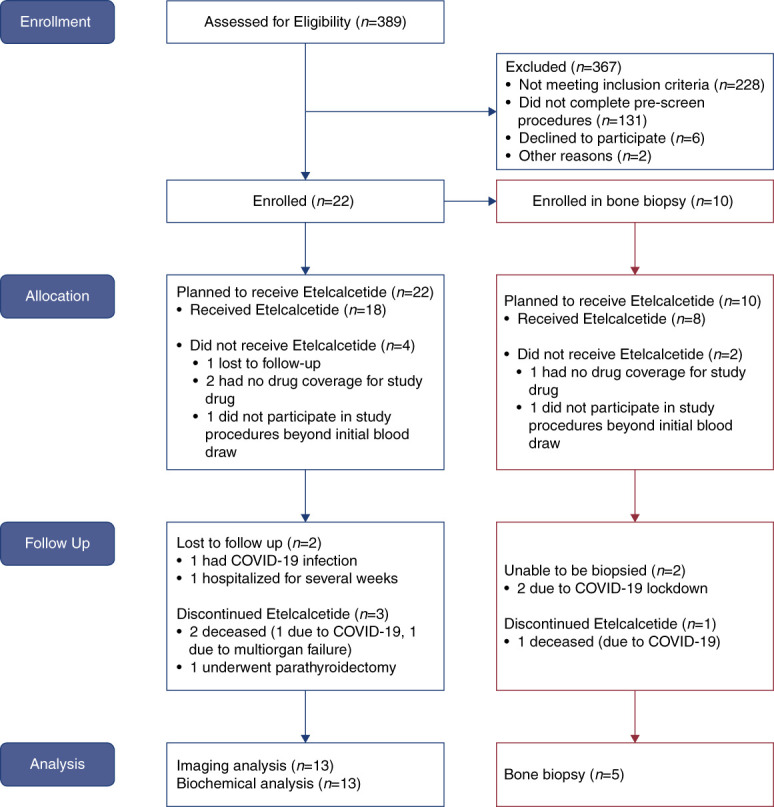
Consort diagram. COVID-19, coronavirus disease 2019. Figure 1 can be viewed in color online at www.cjasn.org.
Trial Design
This was a trial of adult patients with kidney failure treated by hemodialysis (Supplemental Figure 1). The trial was originally designed for three primary outcomes: (1) change in hardness on bone biopsy by nanoindentation, (2) change in areal bone mineral density at the femoral neck by dual-energy X-ray absorptiometry (DXA), and (3) change in calcification propensity by T50. Secondary outcomes included change in bone mineral density of the spine and total hip by DXA and change in bone formation rate (BFR) and mineralization density by biopsy. Owing to the COVID-19 pandemic and early termination, the trial did not meet its recruitment goal. Reanalysis of the power calculations using the final sample size of 13 participants who completed the investigation at the time of termination demonstrated adequate power to assess the primary outcome of percent change in femoral neck areal bone mineral density and the secondary outcomes of change in areal bone mineral density of the spine and total hip. We also determined that other imaging measures with high relevance to bone quality were also powered with 13 participants (i.e., trabecular bone score and high-resolution peripheral quantitative computed tomography [HR-pQCT] outcomes); these were included post hoc as secondary outcomes. A subset that qualified and consented for bone biopsy underwent a quadruple-label transiliac crest bone biopsy procedure. Details on etelcalcetide administration, blood collection, and skeletal imaging are discussed in the Supplemental Methods.
Laboratory Measurements
The analysis of the laboratory studies is discussed in detail in the Supplemental Methods.
DXA, Trabecular Bone Score, and HR-pQCT Measurements
Areal bone mineral density by DXA was measured using a Hologic Horizon densitometer (Hologic, Inc., Waltham, MA) in the array (fan beam) mode. Volumetric bone mineral density and microarchitecture were measured at the distal radius and tibia using the second-generation HR-pQCT scanner (XCT2; Scanco Medical AG, Brüttisellen, Switzerland).18–20 Estimated bone strength (stiffness and failure load) was estimated from the HR-pQCT images using microfinite element analysis on the basis of a voxel conversion approach. Details on measurements are included in the Supplemental Methods.
Bone Biopsy and Histomorphometry and Analysis of Bone Tissue Properties
This trial used a quadruple-label transiliac crest biopsy method. Biopsies underwent colocalized Raman spectroscopy and nanoindentation using a custom hybrid system to assess composition and nanomechanical properties between the two different labels within trabecular bone. Details on bone biopsy performance and analysis are included in the Supplemental Methods.
Statistical Analyses
Statistical analyses were conducted using SAS (version 9.4, SAS Institute, Cary, NC). Categorical data were compared using the chi-squared test. Continuous data were evaluated for normality and outliers before statistical testing. Data were log-transformed when appropriate. Outliers were kept in the dataset if their outcomes were consistent with known relationships between PTH and bone outcomes. A before–after treatment comparison using a complete case analytic approach was conducted using paired t tests for all primary and secondary outcomes. Relationships between biochemical measures and skeletal outcomes were assessed using Spearman correlations. Mixed model linear regression was used to explore relationships between changes in PTH and bone turnover markers (BTMs) and skeletal measures pretreatment and post-treatment (see Supplemental Methods for sample size calculation).
Results
Demographics
Twenty-two participants enrolled in this trial, 13 participants completed skeletal imaging studies, and five completed the bone biopsy substudy (Figure 1). At baseline, the mean age±SD (years), weight±SD (kg), and height±SD (cm) were 52±14 years, 79±18 kg, and 163±7 cm, respectively. Most participants self-identified as male (54%) and non-Hispanic Black (54%). The most prevalent comorbidities among the participants were hypertension (93%), coronary heart disease (46%), and type II diabetes (31%) (Table 1).
Table 1.
Baseline characteristics of participants who completed a prospective, open-label, single-arm clinical trial of etelcalcetide conducted in New York
| Characteristic | Completed Trial (n=13) | Biopsy (n=5) | No Biopsy (n=8) |
|---|---|---|---|
| Baseline age, median (IQR) | 51 (44–61) | 55 (40–61) | 50 (45–55) |
| Sex, n (%) | |||
| Female | 6 (46) | 4 (80) | 2 (25) |
| Race, n (%) | |||
| White | 2 (15) | 0 (0) | 2 (25) |
| Black | 7 (54) | 4 (80) | 3 (38) |
| Native Hawaiian | 3 (23) | 0 (0) | 3 (37) |
| Unknown | 1 (8) | 1 (20) | 0 (0) |
| Ethnicity, n (%) | |||
| Hispanic/Latinx | 2 (15) | 1 (20) | 1 (13) |
| Non-Hispanic | 11 (85) | 4 (80) | 7 (88) |
| Weight, kg, median (IQR) | 79 (66–86) | 86 (79–91) | 67 (58–82) |
| Height, cm, median (IQR) | 163 (155–168) | 155 (155–161) | 164 (163–169) |
| Tobacco usage, n (%) | |||
| Never | 6 (46) | 1 (20) | 5 (62.5) |
| Current | 5 (39) | 2 (40) | 3 (37.5) |
| Past | 2 (15) | 2 (40) | 0 (0) |
| Alcohol use, n (%) | |||
| Never | 3 (23) | 0 (0) | 3 (38) |
| Former | 7 (54) | 4 (80) | 3 (38) |
| Current | 3 (23) | 1 (20) | 2 (25) |
| Comorbidities, n (%) | |||
| Hypertension | 12 (92) | 4 (80) | 8 (100) |
| Congestive heart failure | 4 (31) | 2 (40) | 2 (25) |
| Coronary heart disease | 6 (46) | 3 (60) | 3 (38) |
| MI | 1 (8) | 0 (0) | 1 (13) |
| Hyperlipidemia | 3 (23) | 1 (20) | 2 (25) |
| Stroke | 1 (8) | 0 (0) | 1 (13) |
| Type II diabetes | 4 (31) | 2 (40) | 2 (25) |
| Liver disease | 1 (8) | 0 (0) | 1 (13) |
| Known osteoporosis at any skeletal site, n (%) | |||
| Yes | 1 (8) | 0 (0) | 1 (13) |
| No | 12 (92) | 5 (100) | 7 (88) |
| Menopausea, n (%) | |||
| Premenopausal | 1 (1.7) | 1 (25) | 0 (0) |
| Menopausal | 5 (833) | 3 (75) | 2 (100) |
| Medications, n (%) | |||
| Prednisone | 3 (23) | 2 (40) | 1 (13) |
| Vitamin D2/D3 supplement | 5 (39) | 2 (40) | 3 (38) |
| Vitamin D receptor analog | 11 (85) | 5 (100) | 6 (75) |
| Phosphate binder | |||
| Calcium acetate | 1 (7.7) | 0 (0) | 1 (13) |
| Sevelamer | 7 (53.9) | 2 (40) | 5 (63) |
| Calcium carbonate | 1 (7.7) | 0 (0) | 1 (13) |
| Atraumatic fractures, n (%) | |||
| Yes | 4 (31) | 1 (25) | 3 (38) |
| No | 9 (69) | 4 (75) | 5 (63) |
| Biochemical screenings mean (min, max) | |||
| PTH time 1 (KDIGO reference range: 160–721 pg/ml) | 1288 (815, 2570) | 1122 (836, 1553) | 1393 (815, 2570) |
| PTH time 2 (KDIGO reference range: 160–721 pg/ml) | 1434 (957, 2632) | 1429 (957, 2632) | 1437 (962, 1998) |
| Calcium (reference range 8.6–10.0 mg/dl) | 9.3 (8.7, 9.7) | 9.4 (9, 9.7) | 9.2 (8.7, 9.7) |
| Total alkaline phosphatase (reference range 46–116 IU/L) | 116 (51, 182) | 116 (85, 163) | 116 (51, 182) |
| Phosphorus (reference range 2.8–4.5 mg/dl) | 6.2 (5.2, 6.8) | 6.0 (5.0, 6.9) | 6.3 (5.4, 6.8) |
IQR, interquartile range; MI, myocardial infarction; PTH, parathyroid hormone; KDIGO, Kidney Disease Improving Global Outcomes.
Six female participants were included in the trial, four of them were in the biopsy arm and two were not in the biopsy arm.
Baseline Levels of Biochemical Studies and Areal Bone Mineral Density
Before the dose titration period began, the mean first and second screening PTH was 1288±488 and 1434±522 pg/ml, respectively. Baseline blood and areal bone mineral density by DXA were measured before etelcalcetide administration (Table 2 and Supplemental Table 3). Baseline median levels of bone-specific alkaline phosphatase and C-telopeptide were approximately two-fold and ten-fold greater than the premenopausal reference ranges, respectively. Levels of intact and C-terminal FGF23 and sclerostin were markedly elevated above their respective reference ranges.
Table 2.
Biochemical and bone imaging baseline, follow-up, and change data throughout the trial
| Biochemicals | Baseline Visit | 36-wk Visit | Change (%) | P Value |
|---|---|---|---|---|
| Whole PTH 1–84, pg/ml,a | 692 (561–732) | 156 (82–239) | −67±9 | <0.001 |
| Calcium, mg/dl | 9.3 (9.0–9.6) | 9.3 (8.4–10.1) | −2±3 | 0.5 |
| Phosphate, mg/dl | 6.2 (5.2–6.8) | 5.9 (4.8–6.8) | −1±11 | 0.7 |
| Bone-specific alkaline phosphatase, U/L,b | 59.9 (40.0–71.3) | 26.0 (20.5–36.7) | −45±4 | <0.001 |
| C-telopeptide, ng/ml,b | 6.7 (3.8–10.6) | 2.2 (0.8–5.4) | −63±6 | <0.001 |
| FGF23 C-terminal, RU/mL,c | 9148 (6678–13,176) | 8856 (1694–19,465) | −15±13 | 0.2 |
| FGF23 intact, pg/ml,c | 10,868 (5544–13,834) | 8569 (1755–22,530) | −18±13 | 0.2 |
| Sclerostin, ng/ml,b | 1.98 (1.11–2.46) | 2.18 (1.38–3.30) | 34±11 | 0.01 |
| Areal bone mineral density (mg HA/cm 2 ) | mean±SEM | |||
| Lumbar spine | 0.99±0.17 | 1.01±0.17 | 3±1 | 0.04 |
| Femoral neck | 0.72±0.13 | 0.77±0.15 | 7±2 | 0.002 |
| Total hip | 0.83±0.14 | 0.85±0.15 | 3±1 | 0.04 |
| ⅓ radius | 0.66±0.12 | 0.65±0.13 | −0.6±1 | 0.6 |
| Ultra-distal radius | 0.39±0.09 | 0.39±0.09 | −1±1 | 0.4 |
| Spine trabecular bone score | ||||
| Trabecular bone score | 1.2±0.2 | 1.3±0.1 | 10±2 | <0.001 |
| HR-pQCT at the radius | ||||
| Stiffness | 49,253±17,257 | 53,341±19,979 | 7±4 | 0.05 |
| Failure load | 2686±948 | 2903±1107 | 6±4 | 0.06 |
| Cortical area | 51±15 | 50±14 | −3±1 | <0.001 |
| Cortical thickness | 0.8±0.2 | 0.8±0.2 | −2±1 | <0.005 |
| Cortical pore diameter | 0.2±0.02 | 0.18±0.03 | 5±3 | 0.07 |
| Trabecular endocortical area | 258±55 | 262±57 | 0.7±0.3 | 0.02 |
| Trabecular number | 1.36±0.30 | 1.34±0.28 | −3±1 | 0.01 |
| HR-pQCT at the tibia | ||||
| Stiffness | 160,724±44,270 | 159,507±41,150 | −0.1±2 | 0.6 |
| Failure load | 8778±2317 | 8726±2157 | 0.02±2 | 0.6 |
| Cortical area | 241±47 | 234±45 | −2±1 | 0.04 |
| Cortical thickness | 4.8±1.0 | 4.7±1.0 | −2±1 | 0.05 |
| Cortical pore diameter | 0.20±0.04 | 0.21±0.05 | 4±2 | 0.03 |
| Trabecular endocortical area | 606±104 | 599±107 | 0.2±0.1 | 0.1 |
| Trabecular number | 1.2±0.2 | 1.1±0.2 | −1±1 | 0.2 |
N=13 for all cells except N=12 for HR-pQCT at the radius at the 36-week visit and N=11 for HR-pQCT at the tibia at the 36-week visit. PTH, parathyroid hormone; FGF23, fibroblast growth factor 23; HR-pQCT, high-resolution peripheral quantitative computed tomography.
Cell contents are median (interquartile range), mean (SD), or percent change (±SEM).
Healthy population reference range 5–39 pg/ml.
Premenopausal reference range bone-specific alkaline phosphatase 11.6–29.6 U/L, C-telopeptide 0.112–0.738 ng/ml, sclerostin range 0.026–0.273 ng/ml.
No reference ranges reported.
Mean T-scores were within the osteopenic range for all sites except the lumbar spine. The first quartile of the T-score for both the one-third (−2.69) and ultra-distal radius (−2.69) were within the osteoporotic range.
Serum Studies at Each Visit
On the basis of the levels of intact PTH obtained in the hemodialysis unit for clinical management, median levels of intact PTH required 24 weeks to reach the trial-specific target range of 2–5 times upper limit of normal (Supplemental Figure 2). Fifty-three percent and 69% of participants met the PTH target by 24 and 36 weeks, respectively. The levels of whole PTH 1–84 and the bone formation and resorption markers, bone-specific alkaline phosphatase, and C-telopeptide, respectively, at baseline and 36 weeks are presented in Table 2. At 12 and 36 weeks compared with the baselined levels of PTH 1–84 levels (P < 0.05 for both), bone-specific alkaline phosphatase (P < 0.001 for 36 weeks), C-telopeptide (P < 0.05 for both), and sclerostin (P < 0.01 for 36 weeks) decreased. The levels of C-terminal FGF23 did not change significantly over the course of the trial.
Skeletal Imaging Pretreatment versus Post-Treatment
DXA, trabecular bone score, and HR-pQCT were used to compare differences in areal and volumetric bone density, microarchitecture, geometry, and biomechanical estimates of bone mechanical properties at the central and peripheral skeleton before and after 36 weeks of etelcalcetide-induced PTH suppression (Table 2). By DXA, there were increases in areal bone mineral density at the spine, femoral neck, and total hip, respectively. These corresponded to increases in age-adjusted and sex-adjusted Z-scores of 0.3±0.1, 0.4±0.1, and 0.2±0.1 at the lumbar spine, femoral neck, and total hip, respectively. By trabecular bone score, spine trabecular microarchitecture improved by 10%±2%. By HR-pQCT at the radius, finite element modeling estimates of stiffness and failure load trended to a 7%±4% (P = 0.05) and 6%±4% increase (P = 0.06), respectively. In contrast to the increases in the estimates of mechanical properties, there were decreases in cortical area and thickness and trabecular number and increases in trabecular endocortical area. By HR-pQCT at the tibia, there were no changes in finite element modeling estimates of stiffness and failure load. There were decreases in cortical area and thickness and increases in average cortical pore diameter.
Relationships between PTH Suppression and Changes in Skeletal Imaging
We used Spearman correlations and linear mixed regression models to evaluate relationships between baseline and post-treatment changes in PTH, bone-specific alkaline phosphatase, C-telopeptide, and post-treatment changes in skeletal imaging outcomes. By Spearman correlations, there were no relationships between biochemical measures and areal bone mineral density at any skeletal site. At the radius by HR-pQCT, decreases in bone-specific alkaline phosphatase and C-telopeptide were related to increases in cortical area (rho −0.63 and −0.65; P < 0.05 for both), and decreases in C-telopeptide were associated with increases in cortical thickness (rho −0.64; P < 0.05). At the tibia by HR-pQCT, decreases in C-telopeptide were associated with decreases in periosteal circumference (rho 0.62; P < 0.05). Mixed linear models were adjusted for baseline bone imaging and biochemical measures (Supplemental Table 1). By DXA, decreases in PTH, bone-specific alkaline phosphatase, and C-telopeptide were associated with decreased areal bone mineral density at the spine. By contrast, decreases in PTH and bone-specific alkaline phosphatase predicted increased areal bone mineral density at the cortical one-third radius. By HR-pQCT at the radius, decreases in PTH and bone remodeling markers predicted cortical thickening and an expansion of cortical area. However, total bone and trabecular density and trabecular microarchitecture improved in response to either less robust decreases or even an increase in PTH and remodeling markers. By HR-pQCT at the tibia, decreases in PTH and bone remodeling markers predicted increases in cortical density and decreases in cortical porosity. Similar to the radius, less robust decreases or even an increase in PTH and bone remodeling markers predicted increases in total and trabecular density, improvements in trabecular microarchitecture, and increases in computational measures of bone mechanical properties—stiffness and failure load.
Bone Biopsy
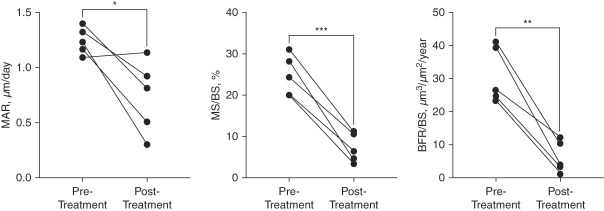
Quadruple-label bone biopsy with quantitative histomorphometry was used to measure pretreatment and post-treatment dynamic measures of histomorphometry (Figure 2 and Supplemental Figure 3) and material properties.21 There were decreases in the cancellous BFR (mean difference −25±4 µm3/µm2 per year; P < 0.01), mineralizing surface to bone surface ratio (mean difference −18%±2%; P < 0.001) and mineral apposition rate (mean difference 0.5±0.2 µm/d; P < 0.05). Changes in dynamic parameters at the cortical compartment were not significant (Supplemental Figure 4). Nanoindentation and Raman spectroscopy revealed no difference in modulus, hardness, or collagen–mineral properties (Supplemental Figures 5 and 6).
Figure 2.
Dynamic measures of histomorphometry from quadruple-label bone biopsy after 36 weeks of etelcalcetide treatment. Data presented as individual values for each of the five patients. *P < 0.05; **P < 0.01; ***P < 0.001. Reference ranges (mean+SD, on the basis of postmenopausal women aged 45–74 years)21 are as follows: MAR (0.53+0.08), MS/BS (7.0+4.1), and BFR/BS (8+6). BFR/BS, bone formation rate/bone surface; MAR, mineral apposition rate; MS/BS, mineralizing surface/bone surface.
Adverse Events
Five participants experienced adverse events during the duration of the trial, none of which were believed to be related to the trial intervention. The three serious adverse events included a cardiac event during anesthesia for arteriovenous fistula revision (N=1), a 2-week hospitalization for vertigo (N=1), and COVID-19 infection (N=1). Two participants reported nonserious adverse events, including a gastrointestinal disorder (N=1) and cold sores (N=1). There were two deaths, both unrelated to the trial drug. One participant died secondary to COVID-19 infection, and one participant died from multiorgan failure.
Discussion
In this single-arm pilot trial of participants on hemodialysis with severe secondary hyperparathyroidism, we found that after administration of etelcalcetide, PTH levels were lowered and maintained to the lower half of the Kidney Disease Improving Global Outcomes PTH range in most patients by 24 weeks of drug administration and that mean levels of circulating markers of bone formation and resorption were suppressed compared with pretreatment. By DXA and trabecular bone score at the central skeleton, there were increases in areal bone mineral density at the spine and hip and in the trabecular bone quality score at the spine, respectively. In contrast, by HR-pQCT at the peripheral skeleton, we found changes that are associated with worsening bone quality—increases in cortical pore diameter and decreases in cortical area and thickness. We report that PTH and bone remodeling had differential effects on cortical and trabecular bone, which were associated with paradoxical findings on bone mechanical property estimates. By quadruple-label bone biopsy, there were improvements (via suppression) in bone remodeling without effects on tissue material properties. These findings suggest that PTH reduction and control by etelcalcetide in patients on hemodialysis with severe hyperparathyroidism improves areal bone mineral density and trabecular microarchitecture at the central skeleton and suppresses bone turnover rates.
Studies in patients with CKD stages 3–4,22 on dialysis,4,5 and after transplantation23 demonstrated that patients with CKD have a 2- to 100-fold higher fracture risk compared with age-matched and sex-matched individuals without CKD. Although antifracture strategies are needed to mitigate the effects of CKD and osteoporosis on morbidity and mortality,24,25 there are no therapies with proven efficacy for the prevention of bone loss and fracture in CKD.26,27 We reported increases in areal bone mineral density at the spine, femoral neck, and total hip. Not only are these increases in areal bone mineral density over 36 weeks of treatment in the range of yearly gains reported for osteoporotic agents in the general population, but the Study to Advance Bone Mineral Density as a Regulatory End Point reported that increases in total hip bone mineral density of 2.7% over 24 months was associated with at least a 10% reduction in all-type fractures.28 Therefore, these findings suggest that PTH suppression with etelcalcetide may have efficacy as a bone-protective agent in patients with hyperparathyroidism on hemodialysis.
Owing to direct relationships between impairments in bone density and quality and fracture risk, an optimal antifracture agent in patients with kidney failure should improve both these aspects of bone mechanical properties. Hyperparathyroidism impairs bone mechanics, making it an important cause of bone loss and fractures in patients with CKD. However, despite treatments targeting biochemical abnormalities of CKD-MBD, fracture incidence of the peripheral skeleton more than doubled from 1992 to 2009 in patients with CKD grade 5 requiring dialysis.29 Cinacalcet failed to significantly reduce fracture incidence in the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial in the primary intention-to-treat analysis but did demonstrate an antifracture signal in post hoc analyses of older patients with more severe hyperparathyroidism.30 Although cinacalcet has been shown to improve bone mineral density at the proximal femur, it did not affect spine bone mineral density.31,32 Therefore, there is significant interest in the ability of more potent PTH suppression to improve both bone density at all skeletal sites and bone quality and to lower fracture risk. Etelcalcetide is a Food and Drug Administration–approved calcimimetic for the treatment of hyperparathyroidism in patients on hemodialysis.6,17 In a head-to-head clinical trial against cinacalcet, etelcalcetide was shown to provide superior control of PTH.6 We found that treatment with etelcalcetide reduced the levels of PTH over time. This reduction in PTH is likely responsible for our findings including (1) maintained suppression of circulating markers of bone turnover, suggesting decreased global skeletal bone remodeling rates; (2) increased age-adjusted and sex-adjusted Z-scores at the spine and femoral neck; (3) improved spine trabecular microarchitecture; and (4) lowered BFRs measured by bone biopsy. These findings strongly support the need for large-scale investigations on the utility of PTH reduction by etelcalcetide to protect the skeleton in patients with hyperparathyroidism on hemodialysis.
Although we reported improvements in areal bone mineral density and trabecular microarchitecture at the central skeleton and in bone remodeling, we found paradoxical changes in bone density and quality at the peripheral skeleton by HR-pQCT. PTH anabolic effects on trabecular bone may have offset catabolic effects on cortical bone, resulting in increases in stiffness and failure load. Although we did not detect relationships between changes in bone turnover markers and areal bone mineral density by DXA, we attribute this to DXA's two-dimensional and low-resolution image. Overall, these findings are difficult to interpret in the absence of a control group, with our small sample size, and PTH levels not meeting the target range for most trial participants until week 24 of the investigation. The small sample size did not enable assessments of relationships between variability in PTH levels, a proxy for PTH pulsatility, and anabolic effects on the skeleton. However, numerous studies have shown that bone loss in the setting of hyperparathyroidism negatively affects the cortical skeleton.10,33–35 Cortical bone comprised more than 85% of the skeleton and is essential to supporting axial loads.36–38 In simulated models of bone loss, reductions in cortical thickness had greater negative effect on whole bone strength than reductions in trabecular number or thickness.39 Thus, small increases in cortical porosity, from high PTH, may disproportionately affect bone mechanics and contribute substantially to the risk of fractures.33 Our data suggest that more successful PTH suppression might have mitigated cortical losses.
This is the first trial to assess bone tissue–level effects of PTH reduction by etelcalcetide and is the first bone biopsy trial of a calcimimetic to assess tissue effects with nanoscale measures of bone quality. Several studies have used paired bone biopsy methods to assess effects of the oral calcimimetic, cinacalcet, on dynamic measures of remodeling.40–42 Yajima et al.42 reported that 52 weeks of cinacalcet lowered activation frequency and fibrosis in four patients with hyperparathyroidism on hemodialysis. Malluche et al.41 evaluated the effects of 12-month treatment with cinacalcet versus a standard-of-care comparator group on bone histology in 32 patients (19 cinacalcet, 13 standard of care). Cinacalcet lowered activation frequency, BFR, and fibrosis surface. The Bone Biopsy Study for Dialysis Patients with Secondary Hyperparathyroidism of End Stage Renal Disease (BONAFIDE) study was a multicenter, open-label, single-arm study to characterize the skeletal response of 6–12 months of cinacalcet treatment in adult patients with hyperparathyroidism treated by hemodialysis.40 Cinacalcet lowered BFRs, and in 25% of study participants, bone histology was normalized. The current trial used a novel quadruple-label approach to evaluate pretreatment and post-treatment effects of PTH suppression by etelcalcetide on bone–tissue. We found that PTH suppression led to reduced BFRs. All patients had high turnover before etelcalcetide, and with 36 weeks of treatment, bone turnover decreased to normal or near normal (on the basis of laboratory historical controls). Raman spectroscopy and nanoindentation, which measure collagen and mineral quality and bone hardness/modulus, respectively, did not change with treatment. Although our small sample size affected our ability to test for these outcomes, we did not detect trends toward worsening bone tissue–level quality due to PTH suppression.
Our trial has limitations. The small sample size is attributed to the enrollment period coinciding with the COVID-19 pandemic and the need for early termination. Although the sample size affected generalizability, the effect of etelcalcetide on suppressing PTH production permitted adequate power to assess a primary and some secondary trial outcomes. This was a single-arm trial and uncontrolled; therefore, it is not possible to determine whether the observed changes were due to temporal trends in PTH decline rather than treatment with etelcalcetide. Our study design permitted assessment of the effects of etelcalcetide on skeletal outcomes rather than identifying independent effects of PTH reduction per se. Patients selected for this trial had high PTH despite standard treatment for secondary hyperparathyroidism; thus, regression to the mean, unrelated to treatment, may have affected some of the longitudinal PTH trends. Furthermore, there was substantial loss to follow-up, which could have led to selection bias.
Bone imaging assesses important aspects of bone strength, such as bone mass (i.e., density) and microstructural aspects of bone quality, and can identify how bone-targeted strategies affect the skeleton. However, fracture studies are needed to determine efficacy of bone-targeted therapies. The quadruple-label approach used in our trial has not been validated against the turnover, mineralization, and volume system in CKD; thus, the results may not exactly mirror those from that approach. The quadruple-label approach quantifies changes in dynamic but not static measures of histomorphometry. Only post-treatment and not pretreatment bone volume is assessed by the method. We also note that the biopsy subset of the trial is not representative of the general CKD population because it comprised 80% female and 80% Black participants. This investigation used imaging methods to quantify bone volume at the peripheral skeleton where associations between imaging-based bone volume and fracture risk have been established. Despite these limitations, this pilot trial was strengthened by its prospective design and use of state-of-the-art multimodality methods to evaluate skeletal responses to PTH suppression by etelcalcetide.
In conclusion, this pilot single-arm trial found that the use of etelcalcetide in patients with kidney failure treated by hemodialysis was associated with improved areal bone mineral density and trabecular bone properties at the central skeleton. At the same time, at the peripheral skeleton, persistent elevations in PTH were associated with cortical bone losses along with anabolic effects on trabecular bone. Bone biopsy demonstrated significant suppression of remodeling. These data suggest that etelcalcetide improves bone properties and may be a potent therapeutic agent to prevent bone loss and fractures in kidney failure patients on hemodialysis. Double-blinded active comparator-controlled trials are needed to determine whether etelcalcetide has antifracture efficacy in patients with kidney failure.
Supplementary Material
Acknowledgments
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant No. UL1TR001873. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
P.K. and J.C. contributed equally to this work.
See related editorial, “New Insights into the Effects of Etelcalcetide on Bone Health,” on pages 1388–1390.
Disclosures
S. Agarwal, M.A. Aponte, and M. Bucovsky report the employment with Columbia University. M.R. Allen reports employment with Roudebush Veterans Administration; research funding from MBX Biosciences; and role on the Editorial Boards for Bone, Calcified Tissue International, and Osteoporosis International. M. Fusaro reports consultancy for Vifor-Pharma and honoraria from Abiogen and Amgen. P. Khairallah reports role on the Editorial Board of American Journal of Kidney Diseases. D. McMahon provides statistical consulting services to Hospital for Special Surgery and New York University, both located in New York City. T.L. Nickolas reports employment with Columbia University; consultancy for Alnylam and Pharmacosmos; research funding from Amgen; honoraria from Alnylam and Pharmacosmos; and advisory or leadership roles for Amgen and Pharmacosmos. Columbia University has licensed patents on NGAL to Abbott Diagnostics and Alere. J. Silberzweig reports consultancy work for Honeywell, Kaneka, and St. Gobain; stock in A&T, American Express, IBM, and Wells Fargo; research funding as the national principal investigator for two clinical trials sponsored by Kaneka: one is using their Lixelle device for treatment of Beta-2-microglobulin amyloidosis in patients with ESKD and the other is using lipid apheresis for the treatment of focal glomerulosclerosis; and advisory or leadership roles for American Society of Nephrology: Nephrologists Transforming Dialysis Safety COVID-19 and Other Current and Emerging Threats workgroup, COVID-19 Response team, and Emergency Partnership Initiative. J. Silberzweig's wife is an employee of Anthem and holds stock in the company. J. Sung reports ownership interest in Alphabet, Amazon, Bank of America, Citigroup, Intel, and Meta. J. Wallace reports employment with Boardable and roles on the Editorial Boards for Advances in Computed Tomography, Bone, Bone Reports, Current Osteoporosis Reports, Journal of Biomechanical Engineering, PLOS One, and Scientific Reports. All remaining authors have nothing to disclose.
Funding
T.L. Nickolas: Amgen (2018736).
Author Contributions
Conceptualization: Matthew R. Allen, Maria Fusaro, Donald McMahon, Thomas L. Nickolas, Joseph Wallace.
Data curation: Sanchita Agarwal, Matthew R. Allen, Jenna Cherasard, Pascale Khairallah, Thomas L. Nickolas, Joshua Sung.
Formal analysis: Sanchita Agarwal, Matthew R. Allen, Jenna Cherasard, Donald McMahon, Corinne E. Metzger, Thomas L. Nickolas, Rachel K. Surowiec, Joseph Wallace.
Funding acquisition: Matthew R. Allen, Thomas L. Nickolas.
Investigation: Matthew R. Allen, Maria Alejandra Aponte, Thomas L. Nickolas, Joseph Wallace.
Methodology: Matthew R. Allen, Jenna Cherasard, Pascale Khairallah, Donald McMahon, Corinne E. Metzger, Thomas L. Nickolas, Joshua Sung, Joseph Wallace.
Project administration: Matthew R. Allen, Maria Alejandra Aponte, Mariana Bucovsky, Pascale Khairallah, Thomas L. Nickolas, Joshua Sung, Joseph Wallace.
Resources: Thomas L. Nickolas.
Software: Donald McMahon, Thomas L. Nickolas.
Supervision: Matthew R. Allen, Pascale Khairallah, Thomas L. Nickolas, Joseph Wallace.
Validation: Donald McMahon, Thomas L. Nickolas.
Visualization: Thomas L. Nickolas.
Writing – original draft: Matthew R. Allen, Maria Fusaro, Pascale Khairallah, Donald McMahon, Corinne E. Metzger, Thomas L. Nickolas, Rachel K. Surowiec, Joseph Wallace.
Writing – review & editing: Matthew R. Allen, Karim El Hachem, Gail N. Frumkin, Maria Fusaro, Pascale Khairallah, Donald McMahon, Corinne E. Metzger, Thomas L. Nickolas, Linda Schulman, Jeffrey Silberzweig, Rachel K. Surowiec, Joseph Wallace.
Data Sharing Statement
Anonymized data created for the study are or will be available in a persistent repository upon publication.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B799.
Supplemental Table 1. Relationships between post-treatment changes in levels of PTH, bone-specific alkaline phosphatase, and C-telopeptide and post-treatment changes in bone imaging using mixed linear models adjusted for baseline bone imaging and biochemical measures.
Supplemental Table 2. Phosphate binders, vitamin D supplement, and vitamin D receptor analog use at baseline and at study completion.
Supplemental Table 3. Doses of phosphate binders and vitamin D receptor analogs at baseline and at study completion.
Supplemental Table 4. Biochemical and bone imaging baseline, follow-up, and change data throughout the trial for the participants who underwent a bone biopsy.
Supplemental Figure 1. Study outline for the assessments of measures collected over 36 weeks of study participation.
Supplemental Figure 2. Median levels of serum intact parathyroid hormone (PTH) obtained by the hemodialysis unit for clinical management.
Supplemental Figure 3. Quadruple-label method to assess dynamic indices of histomorphometry. Tetracycline double label was used at baseline before initiation of etelcalcetide (white arrow, green fluorescence), and declomycin double label was used 36 weeks after treatment (red arrow, blue–green fluorescence). Dynamic indices of histomorphometry were assessed between the individual labels.
Supplemental Figure 4. Dynamic measures of histomorphometry in cortical bone from quadruple-label bone biopsy before and after 36 weeks of etelcalcetide treatment.
Supplemental Figure 5. Bone material strength measured by nanoindentation in trabecular bone before and after 36 weeks of etelcalcetide treatment.
Supplemental Figure 6. Bone crystal and collagen properties measured by Raman spectroscopy before and after 36 weeks of etelcalcetide treatment.
References
- 1.Levin A Bakris GL Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–38. doi: 10.1038/sj.ki.5002009 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM Block GA Correa-Rotter R, et al. The EVOLVE Trial Investigators. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/nejmoa1205624 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/nejmoa041031 [DOI] [PubMed] [Google Scholar]
- 4.Alem AM Sherrard DJ Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–399. doi: 10.1046/j.1523-1755.2000.00178.x [DOI] [PubMed] [Google Scholar]
- 5.Maravic M, Ostertag A, Torres PU, Cohen-Solal M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int. 2014;25(1):159–165. doi: 10.1007/s00198-013-2435-1 [DOI] [PubMed] [Google Scholar]
- 6.Block GA Bushinsky DA Cheng S, et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317(2):156–164. doi: 10.1001/jama.2016.19468 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Martin JL Martinez-Camblor P Dionisi MP, et al. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30(9):1542–1551. doi: 10.1093/ndt/gfv099 [DOI] [PubMed] [Google Scholar]
- 8.Alexander ST Hunter T Walter S, et al. Critical cysteine residues in both the calcium-sensing receptor and the allosteric activator AMG 416 underlie the mechanism of action. Mol Pharmacol. 2015;88(5):853–865. doi: 10.1124/mol.115.098392 [DOI] [PubMed] [Google Scholar]
- 9.Walter S Baruch A Dong J, et al. Pharmacology of AMG 416 (Velcalcetide), a novel peptide agonist of the calcium-sensing receptor, for the treatment of secondary hyperparathyroidism in hemodialysis patients. J Pharmacol Exp Ther. 2013;346(2):229–240. doi: 10.1124/jpet.113.204834 [DOI] [PubMed] [Google Scholar]
- 10.Nickolas TL Stein EM Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013;28(8):1811–1820. doi: 10.1002/jbmr.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.a2 [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez OM Mannstadt M Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. doi: 10.1056/nejmoa0706130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floege J Kim J Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–1955. doi: 10.1093/ndt/gfq219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moe SM Abdalla S Chertow GM, et al. Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events EVOLVE Trial Investigators. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE trial. J Am Soc Nephrol. 2015;26(6):1466–1475. doi: 10.1681/ASN.2014040414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell G, Huang S, Martin KJ, Block GA. A randomized, double-blind, phase 2 study evaluating the safety and efficacy of AMG 416 for the treatment of secondary hyperparathyroidism in hemodialysis patients. Curr Med Res Opin. 2015;31(5):943–952. doi: 10.1185/03007995.2015.1031731 [DOI] [PubMed] [Google Scholar]
- 16.Martin KJ Pickthorn K Huang S, et al. AMG 416 (velcalcetide) is a novel peptide for the treatment of secondary hyperparathyroidism in a single-dose study in hemodialysis patients. Kidney Int. 2014;85(1):191–197. doi: 10.1038/ki.2013.289 [DOI] [PubMed] [Google Scholar]
- 17.Block GA Bushinsky DA Cunningham J, et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317(2):146–155. doi: 10.1001/jama.2016.19456 [DOI] [PubMed] [Google Scholar]
- 18.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41(4):505–515. doi: 10.1016/j.bone.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 19.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. doi: 10.1016/j.bone.2010.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacNeil JA, Boyd SK. Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2008;30(6):792–799. doi: 10.1016/j.medengphy.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 21.Recker RR, Kimmel DB, Parfitt MA, Davies MK, Keshawarz N, Hinders S. Static and tetracycline-based bone histomorphometric data from 34 normal postmenopausal females. J Bone Mineral Res. 2009;3(2):133–144. doi: 10.1002/jbmr.5650030203 [DOI] [PubMed] [Google Scholar]
- 22.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17(11):3223–3232. doi: 10.1681/ASN.2005111194 [DOI] [PubMed] [Google Scholar]
- 23.Ball AM Gillen DL Sherrard D, et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA. 2002;288(23):3014–3018. doi: 10.1001/jama.288.23.3014 [DOI] [PubMed] [Google Scholar]
- 24.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol. 2013;24(9):1461–1469. doi: 10.1681/ASN.2012090916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SM, Long J, Montez-Rath M, Leonard M, Chertow GM. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res. 2016;31(10):1803–1809. doi: 10.1002/jbmr.2862 [DOI] [PubMed] [Google Scholar]
- 26.Khairallah P, Nickolas TL. Updates in CKD-associated osteoporosis. Curr Osteoporos Rep. 2018;16(6):712–723. doi: 10.1007/s11914-018-0491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962–969. doi: 10.2215/CJN.11031017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eastell R Vittinghoff E Lui LY, et al. Validation of the surrogate threshold effect for change in bone mineral density as a surrogate endpoint for fracture outcomes: the FNIH-ASBMR SABRE project. J Bone Mineral Res. 2022;37(1):29–35. doi: 10.1002/jbmr.4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denburg M, Nickolas TL. Declining hip fracture rates in dialysis patients: is this winning the war? Am J Kidney Dis. 2018;71(2):154–156. doi: 10.1053/j.ajkd.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 30.Moe SM Abdalla S Chertow GM, et al. Effects of cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE trial. J Am Soc Nephrol. 2015;26(6):1466–1475. doi: 10.1681/ASN.2014040414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lien YH, Silva AL, Whittman D. Effects of cinacalcet on bone mineral density in patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2005;20(6):1232–1237. doi: 10.1093/ndt/gfh829 [DOI] [PubMed] [Google Scholar]
- 32.Tsuruta Y, Okano K, Kikuchi K, Tsuruta Y, Akiba T, Nitta K. Effects of cinacalcet on bone mineral density and bone markers in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol. 2013;17(1):120–126. doi: 10.1007/s10157-012-0665-8 [DOI] [PubMed] [Google Scholar]
- 33.Cooper DM, Kawalilak CE, Harrison K, Johnston BD, Johnston JD. Cortical bone porosity: what is it, why is it important, and how can we detect it? Curr Osteoporos Rep. 2016;14(5):187–198. doi: 10.1007/s11914-016-0319-y [DOI] [PubMed] [Google Scholar]
- 34.Parfitt AM. A structural approach to renal bone disease. J Bone Miner Res. 1998;13(8):1213–1220. doi: 10.1359/jbmr.1998.13.8.1213 [DOI] [PubMed] [Google Scholar]
- 35.Sharma AK Toussaint ND Masterson R, et al. Deterioration of cortical bone microarchitecture: critical component of renal osteodystrophy evaluation. Am J Nephrol. 2018;47(6):376–384. doi: 10.1159/000489671 [DOI] [PubMed] [Google Scholar]
- 36.Nickolas TL Stein E Cohen A, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol. 2010;21(8):1371–1380. doi: 10.1681/ASN.2009121208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickolas TL Cremers S Zhang A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011;22(8):1560–1572. doi: 10.1681/ASN.2010121275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Augat P, Reeb H, Claes LE. Prediction of fracture load at different skeletal sites by geometric properties of the cortical shell. J Bone Miner Res. 2009;11(9):1356–1363. doi: 10.1002/jbmr.5650110921 [DOI] [PubMed] [Google Scholar]
- 39.Pistoia W, van Rietbergen B, Ruegsegger P. Mechanical consequences of different scenarios for simulated bone atrophy and recovery in the distal radius. Bone. 2003;33(6):937–945. doi: 10.1016/j.bone.2003.06.003 [DOI] [PubMed] [Google Scholar]
- 40.Behets GJ Spasovski G Sterling LR, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;87(4):846–856. doi: 10.1038/ki.2014.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malluche HH Monier-Faugere MC Wang G, et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008;69(04):269–278. doi: 10.5414/cnp69269 [DOI] [PubMed] [Google Scholar]
- 42.Yajima A, Akizawa T, Tsukamoto Y, Kurihara S, Ito A, Group KS. Impact of cinacalcet hydrochloride on bone histology in patients with secondary hyperparathyroidism. Ther Apher Dial. 2008;12(suppl 1):S38–S43. doi: 10.1111/j.1744-9987.2008.00630.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data created for the study are or will be available in a persistent repository upon publication.