Abstract
Despite a large number of people globally being affected by rare kidney diseases, research support and health care policy programs usually focus on the management of the broad spectrum of CKD without particular attention to rare causes that would require a targeted approach for proper cure. Hence, specific curative approaches for rare kidney diseases are scarce, and these diseases are not treated optimally, with implications on the patients' health and quality of life, on the cost for the health care system, and society. There is therefore a need for rare kidney diseases and their mechanisms to receive the appropriate scientific, political, and policy attention to develop specific corrective approaches. A wide range of policies are required to address the various challenges that target care for rare kidney diseases, including the need to increase awareness, improve and accelerate diagnosis, support and implement therapeutic advances, and inform the management of the diseases. In this article, we provide specific policy recommendations to address the challenges hindering the provision of targeted care for rare kidney diseases, focusing on awareness and prioritization, diagnosis, management, and therapeutic innovation. In combination, the recommendations provide a holistic approach aiming for all aspects of rare kidney disease care to improve health outcomes, reduce the economic effect, and deliver benefits to society. Greater commitment from all the key stakeholders is now needed, and a central role should be assigned to patients with rare kidney disease to partner in the design and implementation of potential solutions.
Keywords: CKD, health policy, policy
Introduction
In 2020, targeted care was available for only 500 of the more than 8000 diagnosable rare diseases.1 Among CKD, rare kidney diseases (affecting <1 in 2000 people2) have up to now received insufficient attention, although an estimated 11.9% of adults and most children with kidney failure suffer from rare kidney diseases.3 This contrasts with the more common causes (e.g., hypertension and diabetes).4 This lack of targeted approaches results in a substantial risk of developing kidney failure, with a need for KRT (dialysis or transplantation),5 and reduced survival and quality of life,6 resulting in significant personal, health care system, and societal costs.7
A broad array of barriers hampers the development of targeted approaches. These barriers include (1) general practitioners having not enough experience; (2) researchers missing access to large databases; (3) industry facing problems conducting controlled trials; and (4) nephrologists lacking diagnostic tools, validated biomarkers, and guidance for targeted therapeutic options. Hence, there is currently a high unmet need for innovative research and a lack of therapeutic solutions that directly target rare kidney diseases.
The aims of this policy paper are to define the obstacles to efficient health care for rare kidney diseases. In addition, it also outlines strategies for targeted policy actions and prioritization that would facilitate slowing down progression. Finally, it addresses the challenges hindering targeted care. With this text, we not only target policy makers, insurers, and funders defining care and research objectives but also providers, researchers, medical societies, and practitioners. In addition, this article should also be of use to patients, especially those in search of inspiration for advocacy.
Methodology
This position statement builds on a literature review and the views of an expert advisory board.
First, we conducted a review of the literature published after 2015 on the diagnosis and management of rare kidney diseases as well as gaps in innovative research, health care approaches, and policy actions to deal with shortcomings in research and clinical care (Figure 1). To find relevant publications, the search terms “progression,” “burden,” and “kidney diseases” or “rare kidney diseases” were used in Google Scholar and PubMed. The retrieved publications were ranked according to their relevance to the search terms and used as a basis for further discussion by the expert group together with additional articles referred to in these articles and included through hand search. Then, a virtual roundtable was organized by the European Kidney Health Alliance (EKHA—for further information about EKHA, see acknowledgments) on February 2–3, 2022. The invited experts have a variety of complementary expertise, including patients' perspectives, rare kidney diseases, patient-reported outcome measures (PROMs), health economics, nephrology guidelines, pediatric nephrology, and links to the European Rare Kidney Disease Reference Network (ERKNet). A total of 11 experts have been involved, all of whom provided their feedback.
Figure 1.
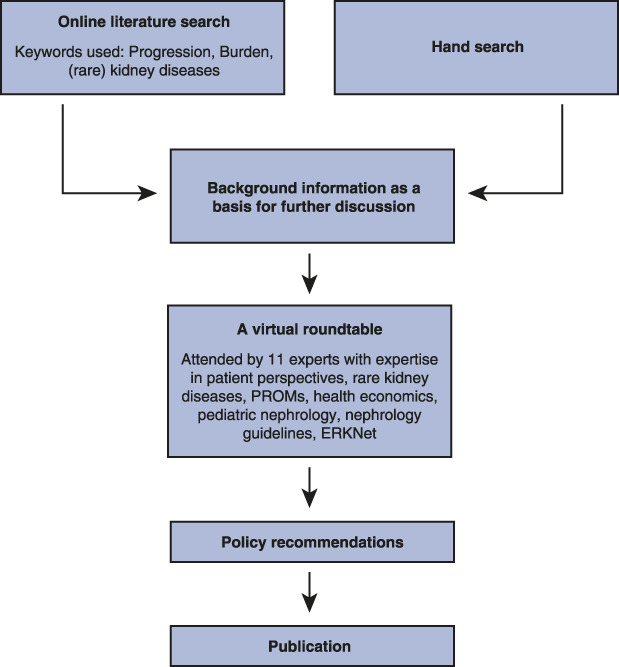
Methodology used to develop current publication. ERKNet, European Rare Kidney Disease Reference Network; PROMs, patient-reported outcome measures.
On the basis of this search and the roundtable, a comprehensive text was distilled,8 which is summarized in the current publication.
Literature Analysis
Awareness and Prioritization
A fundamental obstacle to an efficient approach to rare kidney diseases is a lack of awareness, resulting in their nonprioritization by policy makers. CKDs at large (which include rare kidney diseases) are not listed among the health research priorities of the European Commission.9 In addition, at the population level, awareness is limited. Less than 10% of patients with stage 3 CKD are aware of their disease.10,11 At the physician level, awareness of rare kidney diseases is low, resulting in patients being referred to a nephrologist too late.11
Diagnosis
Diagnosis of rare kidney diseases is problematic and has historically relied on kidney biopsies, which have shortcomings (invasive, risk of bleeding, insufficient sample size for diagnosis, limitations in access to histologic techniques and expertise for interpretation, and limited prognostic value) and are not systematically used.12 Patients with rare kidney diseases often spend years visiting multiple health care providers before receiving an accurate diagnosis. Next-generation sequencing techniques are emerging as the new diagnostic standard for genetic rare kidney diseases as they noninvasively amplify diagnostic accuracy, help decipher molecular mechanisms, allow genetic counseling, and offer possibilities for carrier testing.10 Unfortunately and despite rapidly decreasing costs, access to genetic testing is still limited in many health care systems. In addition, variants of unknown significance often make interpretation challenging. Silico models and expert consensus may help in solving part of these issues.13
Additional diagnostic methods also have a limited effect. Although population-wide urine screening programs are cost-effective,14 only 24% of countries globally deploy a CKD detection program on the basis of national guidelines or policies.15 Especially, for nongenetic rare kidney disorders, the identification of specific urinary biomarkers (liquid biopsy) is urgently needed.16
The need for adequate and timely screening is not specific to rare kidney diseases but may necessitate more than the usual determination of estimated GFR and albuminuria (hematuria, urinary sediment morphologic analysis, genetic screening, and specific biomarkers). With today's limited therapeutic resources, some may question the usefulness of an early diagnosis, but it will still allow a number of appropriate lifestyle measures and BP control. Early action will gain importance because the number of specific therapies will increase, as has already been demonstrated in selected diseases (e.g., cystinosis17 and primary hyperoxaluria18).
Lack of Innovation
There have been therapeutic advances for some conditions, such as atypical hemolytic uremic syndrome,19 lupus nephritis,20 cystinosis,21 membranous nephropathy,22 primary hyperoxaluria,23 and autosomal dominant polycystic kidney disease,24 while other approaches are under consideration.25–27 However, much more rare kidney diseases face therapeutic challenges, while currently accepted therapies may show differences in efficacy per individual patient, highlighting the need for more funding and investments in innovative therapies.8 Even if effective therapies are available, the lack of accurate biomarkers makes it difficult to predict or monitor progression, identify clinical phenotypes, and conduct clinical studies.16
Management
In addition, the limited usage of clinical guidelines by clinicians,28 the scarcity of specific recommendations for rare kidney diseases, limited access to nephrology care and multidisciplinary teams,29 poor therapeutic adherence, health illiteracy,30 and limited interaction between patients and primary care physicians31 all jeopardize the proper management of rare kidney diseases.
The lack of targeted approaches places a considerable clinical and economic burden on society: As these diseases evolve to an advanced stage, they increase the risk of a large range of complications, each with an effect on health economy and outcomes, even well before KRT is needed.32 As CKD acts as a comorbidity accelerator,33 it reduces life expectancy at all stages and ages.34 In addition, health-related quality of life is severely affected and decreases as kidney insufficiency progresses.35 Children born with severe congenital and rare nephropathies face a high likelihood of altered physical, cognitive, and psychosocial development.10
Although transplantation is the ideal KRT,36 patients with rare kidney diseases may relapse after transplantation or be noneligible.37 For these patients, the disease burden is extremely high because dialysis with worse outcomes and quality of life is the only possible remaining option.
The potential economic effect is considerable, despite the small population size, as indicated by our estimates on the basis of the prevalence of some important rare kidney diseases (Table 1).38–55 This analysis essentially focuses on the cost of patients receiving dialysis therapy. It is difficult to find reliable data on the cost of these earlier CKD stages, especially regarding rare kidney diseases, but we can extrapolate from overall CKD data that the costs for CKD patients not on KRT will exceed costs for patients on KRT6,56 due to the much larger number of patients with early-stage CKD. Rare kidney diseases may impose higher relative costs than other causes of CKD at similar stages, either because of associated conditions (e.g., other affected organs in inherited diseases) or more rapid progression, as reported for autosomal dominant polycystic kidney disease.57,58 To put costs of KRT into perspective compared with other health care costs, it is generally accepted that KRT consumes at least 2% of overall health care budgets for 0.1%–0.2% of the general population.31 Lower-income countries in general consume proportionally more of their health care budgets on KRT.55,59 Only if affordable new treatments emerge with the potential of preventing progression, can a reduction of disease burden and the annual cost per patient be expected.
Table 1.
Estimated annual economic burden of dialysis in patients with rare kidney diseases
| Disease | Worldwide Prevalence (×103) | % with Kidney Failure | Cost LIC (×106) | Cost MIC (×106) | Cost HIC (×106) | Global Cost (×106) |
|---|---|---|---|---|---|---|
| Alport syndrome | 157.2 | 51 | $46.9 | $808.5 | $458.8 | $1314.2 |
| Atypical hemolytic uremic syndrome | 39.3 | 65 | $14.9 | $257.7 | $146.1 | $418.7 |
| Autosomal dominant polycystic kidney disease | 12,500.0 | 28 | $2040.9 | $35,213.9 | $19,981.3 | $57,236.1 |
| Focal and segmental glomerulosclerosis | 552.0 | 50 | $16.1 | $277.4 | $157.4 | $450.9 |
| C3 glomerulopathy | 78.6 | 50 | $23.0 | $396.3 | $224.9 | $644.2 |
| Goodpasture syndrome | 7.9 | 30 | $1.3 | $23.8 | $13.5 | $38.6 |
| IgA nephropathy | 1988.6 | 20 | $232.5 | $4010.9 | $2275.9 | $6519.3 |
| Membranous nephropathy | 4345.0 | 10 | $253.9 | $4381.7 | $2486.4 | $7122.0 |
The estimated worldwide prevalence of each rare disease was extrapolated from refs 38–46. We applied the prevalence statistic to the populations living in low-, middle-, and high-income country regions as provided by the World Bank: https://data.worldbank.org/indicator/SP.POP.TOTL. Similarly, the percentage of patients with rare kidney diseases with kidney failure is based on refs. 45,47–54. The global annual economic burden of kidney failure patients who progressed to dialysis was calculated based on the data provided in ref. 55. These figures do not account for indirect costs (hospitalization, drugs, physiotherapy, and transport) and productivity losses, which would add considerably to the global cost. Cost calculations assume that every valid candidate would be treated by dialysis. Amounts mentioned are not actual costs but are estimated values, based in part on extrapolations. LIC, low-income countries; MIC, middle-income countries; HIC, high-income countries.
Summary
There are still large gaps in policy and treatment of rare kidney diseases regarding awareness, prioritization, diagnosis, innovation, and management (Table 2). Health care systems have traditionally focused on managing rare kidney diseases only after their progression to advanced stages rather than investing resources in early detection and delay of progression. Despite the worrying consequences, health care plans rarely focus on determining early enough whether kidney disease is caused by a rare condition.
Table 2.
Challenges hindering the provision of targeted care for rare kidney diseases
| Challenges | Description |
|---|---|
| Awareness and prioritization challenges | • Limited prioritization by policy makers and absence of guidelines • Not enough focus on avoiding disease progression and too much focus on managing kidney failure • Limited physician and patient awareness of rare kidney diseases • Limited availability of data on rare kidney diseases • Few patient advocacy groups focused on rare kidney diseasesa |
| Diagnostic challenges | • Limited implementation of urine screening programs to diagnose kidney disease • Limitations of current diagnostic methods • Lack of accurate biomarkers • Limited knowledge of underlying defects of those rare kidney diseases that are genetically determined • Delay in receiving an accurate diagnosis • Limited access to genetic testing |
| Research and development challenges | • Limited availability of targeted treatments • Clinical study design challenges • Poor translation of research developments into clinical care |
| Management challenges | • Lack of specific clinical guidelines • Limited use of existing clinical guidelines by clinicians • Limited access to nephrologist care • Poor management of kidney function decline and progression of the disease • Inequities in the level of access to minimal preventive care, hemodialysis, and transplantation, among countries and patient subgroups • Limited contact time between physicians and patients • Limited access to multidisciplinary teams |
Some patient organizations focusing on rare kidney diseases and kidney diseases at large are presented in Table 5.
Discussion
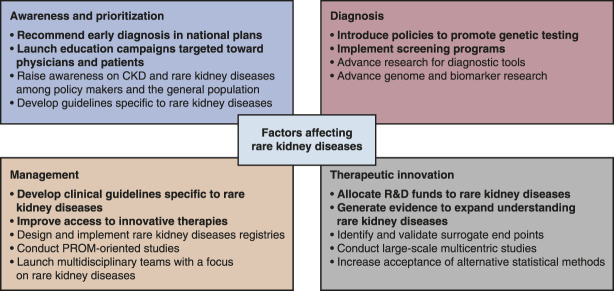
Policy actions should be considered to increase awareness, improve diagnosis, support therapeutic advances, and inform the management of rare kidney diseases (Figure 2 and Table 3). On the basis of the obtained information and the discussion during the roundtable, the expert panel ranked the most important focus points for policy action in the order of importance (Table 4).
Figure 2.
Four categories that need policy action regarding rare kidney diseases and potential policy solutions for each. The items in bold were prioritized by the expert panel responsible for this publication. PROM, patient-reported outcome measure; R&D, research and development.
Table 3.
Approaches to modify health care systems to allow efficient approaches to rare kidney diseases
| Potential Policy Approaches to Address | Action Points |
|---|---|
| Awareness and prioritization | • Increase awareness of the clinical and economic burden of rare kidney diseases • Generate evidence to guide the development of national CKD plans and guidelines for rare kidney diseases • Introduce targeted measures to increase physicians' awareness of available screening programs • Support the organization of patient registries and initiatives to extend or combine databases • Introduce educational campaigns targeted to the general public • Increase focus on early detection and preventive measures and recommend inclusion of preventive measures in health care action plans |
| Diagnostic | • Collaborate to advance research on rare kidney disease diagnosis • Expand genetic testing offerings • Implement or expand urine screening programs |
| Research and development | • Allocate funds for research and development of novel diagnostic tools and treatments for rare kidney diseases • Increase efforts to translate research developments into clinical care • Establish national and international initiatives to generate evidence on rare kidney diseases, if needed based on surrogate end points (e.g., in too small populations for controlled hard end point studies) • Encourage stakeholders to invest in and collaborate on the inclusion of PROMs as a research target |
| Management | • Develop clinical guidelines specific for the management of rare kidney diseases • Introduce initiatives to homogenize access among countries and social classes to preventive care, hemodialysis, and transplantation • Incentivize medical graduates to specialize in nephrology • Establish multidisciplinary teams to better manage rare kidney disease patients |
PROMs, patient-reported outcome measures.
Table 4.
Most important elements to be considered for policy action as defined by the expert panel participating in this project, with the corresponding barriers and involved stakeholders
| Policy Action | Barriers (Lack of Solutions) | Involved Stakeholders |
|---|---|---|
| Recommend early diagnosis in national plans | Structured action plans, willingness to implement early screening, inefficient screening and interpretation of results | Governments, regulators, medical professionals |
| Increase physicians' awareness of urine screening programs and their implementation | Education, organization | Medical associations, medical professionals |
| Develop and expand existing clinical guidelines specific to rare kidney diseases | Evidence, training in evidence-based medicine and guideline development, funding | Medical associations, guidance bodies |
| Introduce policies to promote clinically relevant genetic testing | Funding; appropriate analytic technology, technical training in genetic analysis, trained personnel, targeted requests by clinicians | Governments, funders, research institutes, researchers, medical professionals |
| Allocate more research and development funds to rare kidney diseases | Funding, regulation | Governments, funders |
| Provide early optimal access to innovative treatments | Funding, evidence base, education of medical professionals, affordable price setting | Governments, pharmaceutical industry |
| Generate evidence to expand the understanding of rare kidney diseases | Funding, organization, privacy regulations | Pharmaceutical industry, medical professionals, research institutions, governments, funders |
| Set up specific rare kidney disease registries | Funding, infrastructure for big data analysis, privacy regulations, transparency in data communication and sharing | International, national, and regional medical associations; pharmaceutical industry |
This ranking was the final result of the 2-day European Kidney Health Alliance Roundtable on the topic. The first ranking round was spread over five topics: (1) lack of awareness; (2) the diagnostic challenge; (3) the research and development challenges; (4) the health care system challenges; and (5) management challenges. The top scores for each topic were then submitted to a final vote with the intention to define which topics were considered most important for the panel.
The order from top to bottom indicates the order of importance according to the panel of experts, but this order may be adapted to the local needs of countries or regions. The ranking should also be considered as specific for this panel in the situation of this specific meeting. A different panel of experts might have come to different conclusions.
Increase Awareness
The general perception that kidney diseases at large (including rare kidney diseases) receive less attention than other diseases cannot be denied, although it is difficult to support this statement by objective figures. One reason may be that many other rare diseases are perceived to be fatal. By contrast, many people not directly confronted with kidney diseases think that the problem of kidney failure is solved by dialysis and transplantation, without knowing that CKD is a silent killer leading to premature death in all those affected, with dialysis and even transplantation never restoring outcomes to normal. One additional problem is that people with kidney diseases are often too discreet vis-à-vis others about their condition and not always inclined to become vocal, which may be due to their multimorbid condition, combined with cognitive dysfunction, often making active advocacy discussions demanding.
To increase awareness and prioritization among policy makers, evidence to guide optimal approaches is needed. Transnational registries and other initiatives, such as public–private partnerships, can help build large databases to provide this evidence. Policy makers should be made aware of the clinical, mental, and economic burden of rare kidney diseases and the benefits of targeted approaches. Awareness among physicians and patients can be improved through comprehensive guidelines and educational campaigns.
All those involved, including especially the implicated health care professionals, but, if feasible, also the patients with CKD, should do as much effort as possible to inform the public about the risks and burden of rare kidney diseases. Patients themselves and patient organizations (Table 5) may be the most credible spokespersons to explain the burden of rare kidney diseases and should also be involved as much as possible in defining targets for innovation and optimal care. The role of patients is quintessential in awareness campaigns and in meetings with all those involved in policy decisions. More availability of information about their situation should help to create the desired level of awareness.
Table 5.
Worldwide organizations focusing on rare kidney diseases
| Organization | Acronym | Location | Main Focus |
|---|---|---|---|
| European Patient Advocacy Group within European Rare Kidney Disease Reference Network | ePAG in ERKNet | Europe | All rare kidney diseases |
| Federation of European Patient Groups with rare/genetic kidney diseases | FEDERG | Belgium | All rare/genetic kidney diseases |
| Alport Syndrome Foundation | USA | Alport syndrome | |
| Atypical hemolytic uremic syndrome alliance | aHUS alliance | International | Atypical hemolytic uremic syndrome |
| Ciliopathy Alliance | Europe | Ciliopathies including polycystic kidney diseases | |
| NephCure Kidney International | USA | Rare nephrotic syndromes | |
| NephcEurope | Europe | Rare nephrotic syndromes | |
| Cystinosis Network Europe | Europe | Nephropathic cystinosis | |
| Cystinosis Research Foundation | USA | Nephropathic cystinosis | |
| Polycystic Kidney Disease Foundation | PKD Foundation | USA | Polycystic kidney disease |
| Rare Kidney Disease Foundation | USA | Adult tubulointerstitial kidney disease | |
| Association pour l’Information et la Recherche sur les Maladies rénales Génétiques France, España | AIRG. AIRG-E | France, Spain | Genetic rare kidney diseases |
Regarding CKDs at large, various initiatives have been launched to increase awareness and to facilitate research and innovation such as “The Decade of the Kidney” (2020–2030),60 which is led by the American Association of Kidney Patients and supported by EKHA. This initiative is aimed at kidney diseases at large, which by definition also include rare kidney diseases, but it does not address them specifically. Thus, targeted initiatives are needed to specifically support rare kidney diseases.
Improve Diagnosis
Diagnostic challenges could be addressed by greater scientific collaboration, implementation of first-line genetic testing,61 and identification of alarm signs through the implementation of especially urinary screening programs. Collaborative research should be encouraged to detect genetic causes of rare kidney diseases and reliable diagnostic biomarkers and predictors of progression.62 Further research efforts should help identify the phenotypes for whom genetic testing and targeted treatment would have added clinical value. A tentative list of rare kidney diseases where genetic disease identification is likely to change clinical practice has recently been published61 and needs to be validated and refined.
Support Therapeutic Approaches
More funds for basic and translational research should be allocated, incorporating shared priorities from patients, caregivers, and health care providers.63 Large-scale multicentric initiatives should underpin the evidence base for effective therapies, combined with the search for validation and implementation of surrogate end points (such as improved levels of proteinuria or GFR slope) that are technically feasible on a large scale, reproducible, and acceptable for reimbursement bodies. Those would enable more controlled clinical studies in rare kidney diseases with the possibility to obviate the need for hard outcome studies, for which a large number to treat and a long follow-up time are needed before valid conclusions can be drawn. In addition, research for alternative statistical trial designs for small populations and efforts to efficiently identify candidates for clinical trials might be helpful. With more than 16,000 patients enrolled at 50 centers since 2019, the European Rare Kidney Disease Registry exemplifies how to facilitate the identification of suitable patients for trials.64
As PROMs ensure that interventions effectively improve the conditions that matter most to patients, there should be investment and collaboration from all stakeholders in the development of objective parameters for the assessment of PROMs, as well as PROM-oriented studies. The European Rare Disease Coordination and Support Action has recently collated an online repository of PROMs for rare diseases. Only eight of 811 PROMs addressed rare kidney diseases, demonstrating the eminent need to invest in research on PROMs for this disease domain.65
Management
To inform management, the scientific community should develop clinical guidelines specific for rare kidney diseases and establish protocols targeted toward primary care and other non-nephrology physicians, providing a basis for standardized diagnoses and therapies.10 The units in ERKNet which adopted 40 existing guideline documents for management started a systematic effort to fill in areas where guidance was lacking. Ten guideline documents have been published to date by ERKNet specialists, usually in collaboration with other experts in adult and pediatric nephrology.66 Centralized multidisciplinary teams—including nephrologists and other specialties—can facilitate the uptake of these guidelines and optimize management. Referral time could be reduced by incentives encouraging medical graduates to specialize in nephrology and advance innovation by enthusing them to research rare kidney diseases.
This text contains a number of recommendations on how more attention for rare kidney diseases could be obtained. Stakeholders can make their choice among the proposed options, in function of their situation. Hopefully, this may help to generate better approaches and outcomes for this forgotten population.
By adopting a holistic approach targeting all aspects of rare kidney disease care, it should be possible to improve patient quality of life and health outcomes and reduce the economic effect of the later-stage disease. A central role should be assigned to patients with rare kidney disease patients as partners in the definition and implementation of solutions. Greater commitment from all key stakeholders is now needed to consider and eventually adopt the policy solutions identified in this study.
Acknowledgments
The authors are immensely grateful to Professor Panos Kanavos (PhD, Associate Professor in International Health Policy—Department of Health Policy; Program Director—Medical Technology Research Group; and Deputy Director—LSE Health. London School of Economics and Political Science, London, UK) for his comments on an earlier version of the manuscript.
The authors thank Dr. Chris Henshall for providing insightful comments and suggestions to prepare the Policy Roundtable and for masterfully moderating the discussion at the Roundtable. Dr. Henshall's contribution was fundamental to eliciting and collecting the experts' views that informed the content of this publication.
Professor Franz Schaefer is a member of the European Reference Network for Rare Kidney Diseases (ERKNet).
The European Kidney Health Alliance (EKHA—https://ekha.eu) is a not-for-profit organization defending the case of kidney patients and the nephrological community at the level of the European Commission. The EKHA network has five full members (the European Renal Association, the International Society of Nephrology, the European Kidney Patients Federation, the European Dialysis and Transplant Nurses Association-European Renal Care Association, and the Dutch Kidney Foundation) next to 30 National or Regional Societies as affiliated members.
EKHA is the recipient of support from the European Union in the context of the Annual Work Program 2022 on the prevention of non-communicable diseases of EU4Health, topic ID EU4H-2022-PJ02, project #101101220 PREVENTCKD.
Next to this grant, EKHA is funded by membership fees and unrestricted grants.
Novartis Pharma AG has provided an unrestricted grant, allowing the literature review and the organization of the Roundtable to inform this publication.
Disclosures
W.J.W. Bos reports research funding from Dutch Kidney Foundation, Leading the Change, Zilveren Kruis Insurance, and ZonMW and advisory or leadership roles for ICHOM working group CKD (chair), various committees on Value Based Healthcare of the Association of Dutch University Hospitals (NFU), and various committees of the Dutch Nephrology Quality Initiative “Nefrovisie.” R. Coppo reports consultancy for Amgen, Anylam, Argenx, Bayer, Calliditas, Chinook, Menarini, Novartis, Otsuka-Visterra, Purespring, Reata, and Stadapharm; honoraria from Amgen, Bayer, Chinook, Menarini, Novartis, Purespring, Stadapharm, and Travere; royalties from UpToDate; advisory or leadership roles for Nephrology Dialysis Transplantation Editorial Board and as an Associate Editor for Pediatric Nephrology; and speakers bureau for Stadpharm and Travere. E. Damato reports employment with and consultancy for Charles River Associates. F. Fakhouri reports consultancy for, honoraria from, and advisory or leadership roles for Alexion, Alnylam, Apellis, Novartis, and Roche. I. Nistor reports speaker fee for AstraZeneca, Boeringer Ingelheim, and Zentiva. A. Ortiz reports consultancy for Fresenius Medical Care, Genzyme Sanofi, and Travere; research funding from AstraZeneca, Mundipharma, and Sanofi Genzyme; honoraria from Advicciene, Alexion, Amgen, Amicus, Astellas, AstraZeneca, Bayer, Chiesi, Freeline, Fresenius Medical Care, Idorsia, Kyowa Kirin, Menarini, Otsuka, Sanofi Genzyme, and Vifor Fresenius Medical Care Renal Pharma; advisory or leadership roles for Board of Directors IIS-Fundacion Jimenez Diaz UAM, Dutch Kidney Foundation scientific advisory board, SOMANE and ERA Councils, and Spanish Society of Nephrology (member); roles on the Editorial Boards of Journal of Nephrology, JASN and Peritoneal Dialysis International; and honoraria for speaker engagements for Advicciene, Alexion, Amgen, Amicus, Astellas, AstraZeneca, Bayer, Chiesi, Fresenius Medical Care, Idorsia, Kyowa Kirin, Menarini, Otsuka, Sanofi-Genzyme, and Vifor Fresenius Medical Care Renal Pharma. M. Pistollato reports employment with, consultancy for, and research funding from Charles River Associates. F. Schaefer reports consultancy for Alexion, Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Fresenius Medical Care, GSK, Otsuka, Purespring, and Roche; research funding from Fresenius Medical Care, Novartis, and Roche; honoraria from Amgen, Otsuka, and Roche; textbook royalties from Springer; and Scientific Advisory Board activities for Alexion and Otsuka. F. Schaefer is a member of the European Reference Network for Rare Kidney Diseases (ERKNet). R. Vanholder reports consultancy for AstraZeneca, Baxter, Fresenius Kabi, Fresenius Medical Care, GlaxoSmithKline, Kibow, Nipro, Nextkidney, and Novartis; honoraria from Baxter Healthcare and Fresenius Medical Care; royalties from UpToDate; and speakers bureau for Fresenius Medical Care, Baxter Healthcare, and Nipro. All remaining authors have nothing to disclose.
Funding
This work was supported from Novartis.
Author Contributions
Conceptualization: Willem J.W. Bos, Rosanna Coppo, Fadi Fakhouri, Alister Humphreys, Ionut Nistor, Alberto Ortiz, Michele Pistollato, Franz Schaefer, Eveline Scheres, Raymond Vanholder.
Formal analysis: Elaine Damato, Michele Pistollato.
Methodology: Elaine Damato, Michele Pistollato, Raymond Vanholder.
Project administration: Eveline Scheres, Raymond Vanholder.
Supervision: Raymond Vanholder.
Writing – original draft: Elaine Damato, Michele Pistollato, Raymond Vanholder.
Writing – review & editing: Willem J.W. Bos, Rosanna Coppo, Elaine Damato, Fadi Fakhouri, Alister Humphreys, Ionut Nistor, Alberto Ortiz, Michele Pistollato, Franz Schaefer, Eveline Scheres, Raymond Vanholder.
References
- 1.Lamoreaux K, Sebastien Lefebvre MS, Levine DS, Erler W, Hume T. Rare X: The Power of Being Counted. 2020. Accessed August 8, 2022. https://rare-x.org/wp-content/uploads/2022/05/be-counted-052722-WEB.pdf [Google Scholar]
- 2.EURODIS. About Rare Diseases. Accessed August 8, 2022, Accessed April 14, 2023. https://www.eurordis.org/about-rare-diseases [Google Scholar]
- 3.Wühl E van Stralen KJ Wanner C, et al. Renal replacement therapy for rare diseases affecting the kidney: an analysis of the ERA–EDTA Registry. Nephrol Dial Transplant. 2014;29(suppl 4):iv1–iv8. doi: 10.1093/ndt/gfu030 [DOI] [PubMed] [Google Scholar]
- 4.Foreman KJ Marquez N Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/s0140-6736(18)31694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurlow JS Joshi M Yan G, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. 2021;52(2):98–107. doi: 10.1159/000514550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanholder R Annemans L Bello AK, et al. Fighting the unbearable lightness of neglecting kidney health: the decade of the kidney. Clin Kidney J. 2021;14(7):1719–1730. doi: 10.1093/ckj/sfab070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S, Amedia CA. Economic burden of chronic kidney disease. J Eval Clin Pract. 2008;14(3):422–434. doi: 10.1111/j.1365-2753.2007.00883.x [DOI] [PubMed] [Google Scholar]
- 8.EKHA. Addressing the human and societal burden of chronic kidney disease and rare kidney diseases in national plans: a call for policy intervention. Working Paper. 2022. [Google Scholar]
- 9.European Commission. Why the EU Supports Health Research and Innovation. Accessed April 16, 2023. https://research-and-innovation.ec.europa.eu/research-area/health_en accessed 18/06/2023 [Google Scholar]
- 10.Aymé S Bockenhauer D Day S, et al. Common elements in rare kidney diseases: conclusions from a kidney disease: improving global outcomes (KDIGO) Controversies Conference. Kidney Int. 2017;92(4):796–808. doi: 10.1016/j.kint.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsiao LL. Raising awareness, screening and prevention of chronic kidney disease: it takes more than a village. Nephrology. 2018;23(suppl 4):107–111. doi: 10.1111/nep.13459 [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran R Sulaiman S Chauhan P, et al. Challenges in diagnosis and management of glomerular disease in resource-limited settings. Kidney Int Rep. 2022;7(10):2141–2149. doi: 10.1016/j.ekir.2022.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical Genome Resource. Kidney Disease Clinical Domain Working Group. Accessed April 14, 2023. https://clinicalgenome.org/working-groups/clinical-domain/clingen-kidney-disease-clinical-domain-working-group/ [Google Scholar]
- 14.Thomas B Matsushita K Abate KH, et al. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28(7):2167–2179. doi: 10.1681/ASN.2016050562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuen BL, Chadban SJ, Demaio AR, Johnson DW, Perkovic V. Chronic kidney disease and the global NCDs agenda. BMJ Glob Health. 2017;2(2):e000380. doi: 10.1136/bmjgh-2017-000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mischak H. Pro: urine proteomics as a liquid kidney biopsy: no more kidney punctures. Nephrol Dial Transplant. 2015;30(4):532–537. doi: 10.1093/ndt/gfv046 [DOI] [PubMed] [Google Scholar]
- 17.Van Stralen KJ Emma F Jager KJ, et al. Improvement in the renal prognosis in nephropathic cystinosis. Clin J Am Soc Nephrol. 2011;6(10):2485–2491. doi: 10.2215/CJN.02000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groothoff JW Metry E Deesker L, et al. Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope. Nat Rev Nephrol. 2023;19(3):194–211. doi: 10.1038/s41581-022-00661-1 [DOI] [PubMed] [Google Scholar]
- 19.Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681–696. doi: 10.1016/s0140-6736(17)30062-4 [DOI] [PubMed] [Google Scholar]
- 20.Rovin BH Teng YKO Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397(10289):2070–2080. doi: 10.1016/s0140-6736(21)00578-x [DOI] [PubMed] [Google Scholar]
- 21.Brodin-Sartorius A Tête MJ Niaudet P, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81(2):179–189. doi: 10.1038/ki.2011.277 [DOI] [PubMed] [Google Scholar]
- 22.Fervenza FC Appel GB Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46. doi: 10.1056/nejmoa1814427 [DOI] [PubMed] [Google Scholar]
- 23.Liu A Zhao J Shah M, et al. Nedosiran, a candidate siRNA drug for the treatment of primary hyperoxaluria: design, development, and clinical studies. ACS Pharmacol Transl Sci. 2022;5(11):1007–1016. doi: 10.1021/acsptsci.2c00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller RU Messchendorp AL Birn H, et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA working group on inherited kidney disorders, the European rare kidney disease reference network and polycystic kidney disease international. Nephrol Dial Transplant. 2022;37(5):825–839. doi: 10.1093/ndt/gfab312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang X, Xu G. An update on targeted treatment of IgA nephropathy: an autoimmune perspective. Front Pharmacol. 2021;12:715253. doi: 10.3389/fphar.2021.715253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas-Rivera J, Fervenza FC, Ortiz A. Recent clinical trials insights into the treatment of primary membranous nephropathy. Drugs. 2022;82(2):109–132. doi: 10.1007/s40265-021-01656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teisseyre M Cremoni M Boyer-Suavet S, et al. Advances in the management of primary membranous nephropathy and rituximab-refractory membranous nephropathy. Front Immunol. 2022;13:859419. doi: 10.3389/fimmu.2022.859419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperati CJ Soman S Agrawal V, et al. Primary care physicians’ perceptions of barriers and facilitators to management of chronic kidney disease: a mixed methods study. PloS One. 2019;14(8):e0221325. doi: 10.1371/journal.pone.0221325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torreggiani M Chatrenet A Fois A, et al. Unmet needs for CKD care: from the general population to the CKD clinics. How many patients are we missing? Clin Kidney J. 2021;14(10):2246–2254. doi: 10.1093/ckj/sfab055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor DM Fraser S Dudley C, et al. Health literacy and patient outcomes in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2018;33(9):1545–1558. doi: 10.1093/ndt/gfx293 [DOI] [PubMed] [Google Scholar]
- 31.Vanholder R Annemans L Brown E, et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13(7):393–409. doi: 10.1038/nrneph.2017.63 [DOI] [PubMed] [Google Scholar]
- 32.Braun L, Sood V, Hogue S, Lieberman B, Copley-Merriman C. High burden and unmet patient needs in chronic kidney disease. Int J Nephrol Renovasc Dis. 2012;5:151–163. doi: 10.2147/ijnrd.s37766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan EYF Yu EYT Chin WY, et al. Burden of CKD and cardiovascular disease on life expectancy and health service utilization: a cohort study of Hong Kong Chinese hypertensive patients. J Am Soc Nephrol. 2019;30(10):1991–1999. doi: 10.1681/ASN.2018101037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Society of Nephrology. ISN Global Kidney Health Atlas. 2019. Accessed May 19, 2023. https://www.theisn.org/wp-content/uploads/2021/05/GKHAtlas_2019_WebFIle-1.pdf. [Google Scholar]
- 35.Mujais SK Story K Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–1301. doi: 10.2215/CJN.05541008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanholder R Domínguez-Gil B Busic M, et al. Organ donation and transplantation: a multi-stakeholder call to action. Nat Rev Nephrol. 2021;17(8):554–568. doi: 10.1038/s41581-021-00425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uffing A Pérez-Sáez MJ Mazzali M, et al. Recurrence of FSGS after kidney transplantation in adults. Clin J Am Soc Nephrol. 2020;15(2):247–256. doi: 10.2215/CJN.08970719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alport Syndrome News. Prevalence of Alport Syndrome. 2023. Accessed April 14, 2023. https://alportsyndromenews.com/prevalence-of-alport-syndrome [Google Scholar]
- 39.Yan K, Desai K, Gullapalli L, Druyts E, Balijepalli C. Epidemiology of atypical hemolytic uremic syndrome: a systematic literature review. Clin Epidemiol. 2020;12:295–305. doi: 10.2147/clep.s245642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.PKD International. What is ADPKD. 2023. Accessed April 14, 2023. https://pkdcure.org/what-is-adpkd/ [Google Scholar]
- 41.NORD. Focal Segmental Glomerulosclerosis. 2018. Accessed April 14, 2023. https://rarediseases.org/rare-diseases/focal-segmental-glomerulosclerosis/ [Google Scholar]
- 42.Novartis Data on File. Investigator’s Brochure. Edition 7. Release date: 24-Mar 2020.
- 43.Greco A Rizzo MI De Virgilio A, et al. Goodpasture’s syndrome: a clinical update. Autoimmun Rev. 2015;14(3):246–253. doi: 10.1016/j.autrev.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 44.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430. doi: 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 45.Fervenza FC, Sethi S, Glassock RJ. Idiopathic membranoproliferative glomerulonephritis: does it exist? Nephrol Dial Transplant. 2012;27(12):4288–4294. doi: 10.1093/ndt/gfs288 [DOI] [PubMed] [Google Scholar]
- 46.Willey CJ, Coppo R, Schaefer F, Mizerska-Wasiak M, Mathur M, Schultz MJ. The incidence and prevalence of IgA nephropathy in Europe. Nephrol Dial Transplant. 2023:gfad082. doi: 10.1093/ndt/gfad082 (Preprint posted May 8, 2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson S, Padala SA, Bush JS. Alport syndrome. In: StatPearls. StatPearls Publishing; 2022. [Google Scholar]
- 48.Nguyen MH, Mathew JJ, Denunzio TM, Carmichael MG. Diagnosis of atypical hemolytic uremic syndrome and response to eculizumab therapy. Hawaii J Med Public Health. 2014;73(9 suppl 1):22–24. [PMC free article] [PubMed] [Google Scholar]
- 49.McEwan P Bennett Wilton H Ong A, et al. A model to predict disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD): the ADPKD Outcomes Model. BMC Nephrol. 2018;19(1):37–12. doi: 10.1186/s12882-017-0804-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoji J, Mii A, Terasaki M, Shimizu A. Update on recurrent focal segmental glomerulosclerosis in kidney transplantation. Nephron. 2020;144(1):65–70. doi: 10.1159/000510748 [DOI] [PubMed] [Google Scholar]
- 51.US National Library of Medicine. C3 glomerulopathy. 2015. Accessed April 14, 2023. https://medlineplus.gov/genetics/condition/c3-glomerulopathy/ [Google Scholar]
- 52.Stojkovikj J Zejnel S Gerasimovska B, et al. Goodpasture syndrome diagnosed one year and a half after the appearance of the first symptoms (case report). Open Access Maced J Med Sci. 2016;4(4):683–687. doi: 10.3889/oamjms.2016.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson GJ Cho Y Teixiera-Pinto A, et al. Long-term outcomes of patients with end-stage kidney disease due to membranoproliferative glomerulonephritis: an ANZDATA registry study. BMC Nephrol. 2019;20(1):417–419. doi: 10.1186/s12882-019-1605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(6):983–997. doi: 10.2215/CJN.11761116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Tol A, Lameire N, Morton RL, Van Biesen W, Vanholder R. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol. 2019;14(1):84–93. doi: 10.2215/CJN.08150718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gandjour A, Armsen W, Wehmeyer W, Multmeier J, Tschulena U. Costs of patients with chronic kidney disease in Germany. PLoS One. 2020;15(4):e0231375. doi: 10.1371/journal.pone.0231375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cloutier M, Manceur AM, Guerin A, Aigbogun MS, Oberdhan D, Gauthier-Loiselle M. The societal economic burden of autosomal dominant polycystic kidney disease in the United States. BMC Health Serv Res. 2020;20(1):126–210. doi: 10.1186/s12913-020-4974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gagnon-Sanschagrin P, Liang Y, Sanon M, Oberdhan D, Guérin A, Cloutier M. Excess healthcare costs in patients with autosomal dominant polycystic kidney disease by renal dysfunction stage. J Med Econ. 2021;24(1):193–201. doi: 10.1080/13696998.2021.1877146 [DOI] [PubMed] [Google Scholar]
- 59.van der Tol A Stel VS Jager KJ, et al. A call for harmonization of European kidney care: dialysis reimbursement and distribution of kidney replacement therapies. Nephrol Dial Transplant. 2020;35(6):979–986. doi: 10.1093/ndt/gfaa035 [DOI] [PubMed] [Google Scholar]
- 60.Vanholder R, Conway PT, Gallego D, Scheres E, Wieringa F. The European Kidney Health Alliance (EKHA) and the decade of the kidney. Nephrol Dial Transplant. 2023;38(5):1113–1122. doi: 10.1093/ndt/gfac211 [DOI] [PubMed] [Google Scholar]
- 61.Knoers N Antignac C Bergmann C, et al. Genetic testing in the diagnosis of chronic kidney disease: recommendations for clinical practice. Nephrol Dial Transplant. 2022;37(2):239–254. doi: 10.1093/ndt/gfab218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rehm HL. Time to make rare disease diagnosis accessible to all. Nat Med. 2022;28(2):241–242. doi: 10.1038/s41591-021-01657-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tong A Chando S Crowe S, et al. Research priority setting in kidney disease: a systematic review. Am J Kidney Dis. 2015;65(5):674–683. doi: 10.1053/j.ajkd.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 64.ERKReg. The European Rare Kidney Disease Registry. 2021. Accessed April 14, 2023. https://www.erknet.org/patients-registry/registry-mission [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ERICA Coordinating Center. PROMs Repository. 2022. Accessed April 14, 2023. https://erica-rd.eu/work-packages/patient-centred-research/proms-repository/ [Google Scholar]
- 66.The European Rare Kidney Disease Reference Network (ERKNet). Overview ERKNet Guideline Projects. 2022. Accessed April 14, 2023. https://www.erknet.org/guidelines-pathways/overview-erknet-guideline-projects [Google Scholar]