Thirst is such a powerful physiologic driver of fluid intake. A driver so powerful, it has caused men at sea to go literally mad, caused a lost hiker to beg his best friend to kill him because of his thirst-induced hysteria (which he did),1 and even caused Queen Cersei to suck up water off a filthy prison floor with her mouth on her hands and knees in season 5, episode 8, of the HBO series Game of Thrones.
Thirst is such a ubiquitous irritant and nuisance to comfort in humans that individuals who do not have neurologic impairments and have access to water or fluids will drink fluids in response to the smallest elevations in serum osmolality.2 Serum sodium is the largest contributor to serum osmolality, and thus, it is rare for anyone who has mental capacity and access to fluids to become hypernatremic because of the overwhelming innate impulse of thirst, regulated by neurons in the lamina terminalis circumventricular organs. Plasma sodium concentrations are tightly controlled by thirst and arginine vasopressin.2 Thus, in health, serum osmolality and serum sodium are easily kept in the normal range below concentrations that would qualify as hypernatremia. Indeed, individuals with central diabetes insipidus who miss doses of desmopressin, or even individuals with the much harder to manage nephrogenic diabetes insipidus, do not become hypernatremic because they can generally keep up consuming large volumes of water to prevent marked increases in serum sodium and tonicity despite the inability to concentrate their urine.
The etiology of hospital-acquired hypernatremia is generally multifactorial and related to the net balance of insufficient water intake with increased loss of free water. Insufficient water intake is often due to intubation (decreased access to free water), whereas most patients who develop hypernatremia without intubation have impaired mental status thus interfering with normal thirst perception.3 In addition, the vast majority of patients with hospital-acquired hypernatremia have impaired kidney concentrating capacity (primarily due to diuretics or osmotic diuresis) or increased extrarenal fluid losses (enteral and insensible).3
In the study by Arzhan et al.,4 published in this issue of CJASN, the authors studied more than 1.7 million patients using the Cerner Health Facts national clinical database and examined hypernatremia severity as a graded exposure and conducted thorough analyses considering categories of acute and chronic kidney dysfunction. They examined these exposures and assessed the odds of in-hospital mortality, discharge to hospice, and discharge to a nursing facility compared with patients who remained normonatremic throughout hospitalization. They reported some very large adjusted odds ratios (ORs). In some strata (severity of hypernatremia, baseline level of eGFR, AKI versus CKD, AKI on CKD), the ORs for death were massive (e.g., OR of 179.49 for severe hypernatremia overall versus normonatremia, OR of 108.5 for moderate hypernatremia with eGFR ≥90 ml/min per 1.73 m2, or OR of 718.23 for severe hypernatremia and eGFR ≥90 ml/min per 1.73 m2).
The authors admit some limitations, importantly on the ability to adjust for severity of illness. Indeed, the investigators only adjusted for age, sex, race, and the modified Quan–Charlson Comorbidity Score. This score is derived from chronic comorbidities that are present before admission. I would posit that there is still significant residual confounding that is present in these analyses. Indeed, the authors mention they were unable to adjust for acute disease severity scores (including Acute Physiology, Age, Chronic Health Evaluation, or Sequential Organ Failure Assessment) and admit potential for residual confounding. However, they suggest that the strength of associations observed suggests that hypernatremia should be viewed as an important risk factor for poor outcomes in hospitalized patients and that efforts should be employed to minimize the adverse outcomes from hypernatremia.
However, the potential that interventions for hypernatremia will result in less mortality or discharge to nursing or hospice facilities is close to nil for the following reasons.
The ORs observed for the primary and secondary analyses by various subgroups exceed any reasonable effect size that would be attributable to an electrolyte-based risk factor.
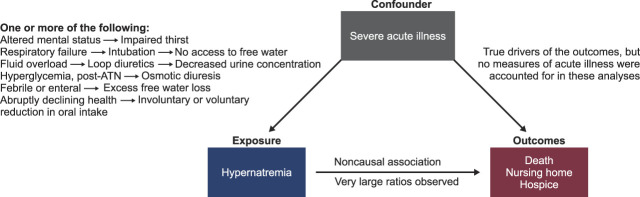
From a Bayesian point of view, most clinicians and scientists would find it improbable that hypernatremia would be the driver of poor outcomes, rather than a confounder. Hypernatremia is in most cases not the primary cause of morbidity. It is exceedingly more common to be the consequence of many processes discussed above (intubation, altered mental status, pharmacologic or osmotic diuresis, insensible and enteral losses) and outlined in the left causal pathway of Figure 1 that all coalesce to impair free water intake and increase free water losses. All those processes leading to either decreased free water intake or increased free water losses are representative features of the most severely ill of the hospitalized patients. Furthermore, patients who transition from hospitalization to hospice/comfort care likely have diminished intake of food and water. The large administrative database used for these analyses was inadequate in granularity to allow for appropriate control for the severity of illness not only on admission but throughout the hospital stay (pathway on right in Figure 1).
Because there is no other time-updated variable, the primary exposure of hypernatremia in these analyses is biased and thus has an inflated probability to demonstrate an association with any untoward outcome. Anyone whose clinical course was complicated or worsened during the hospitalization has the chance to become hypernatremic over time (ascertainment bias) and thus has the potential to be captured as part of the 6% of the primary exposure group that was at such high risk for the adverse outcomes.
Figure 1.
Major confounding by severity of acute illness on associations observed between hospital-acquired hypernatremia and the three outcomes in the study by Arzhan et al.4 ATN, acute tubular necrosis.
The findings reported by Arzhan et al. are reminiscent of the lessons learned from Plato's Allegory of the Cave, in his legendary work Republic. Plato describes a dialogue between Socrates and Plato's brother Glaucon in which he imagines a group of people chained together inside an underground cave as prisoners. Behind the prisoners, there is a fire, and between the prisoners and the fire are moving puppets and real objects on a raised walkway with a low wall. However, the prisoners are unable to see anything behind them because they have been chained and stuck looking in one direction—at the cave wall—their whole lives. As they look at the wall before them, they believe the shadows of objects cast by the moving figures are real things—and the only things that exist. They are unable to see the objects or the flames that are casting the shadows. They devote their entire lives attempting to make sense of the shadows because they believe that is all there is to reality. One of the inmates is eventually released from his chains and makes his way outside the cave. He cannot see much at first because of the high sunshine blinding him. Nevertheless, when his eyes adjust, he begins to recognize the actual items that were responsible for the cave's shadows. For the first time, he is able to view the fire, the items, and the outside world. The newly released prisoner understands that the cave's shadows were simply a weak representation of the outside world.
All the analyses that were conducted in this article for the outcomes associated with the severity of hypernatremia and various strata of kidney function are just mere shadows on the wall, reflecting the underlying downward health spiral of the patient. Although the adjusted ORs between hypernatremia with the three outcomes of interest are numerically large, it is clear that the hypernatremia is not the causal mediator of the demise of these hospitalized patients. Because the hypernatremia is not the causal mediator, it is unlikely that efforts to prevent and minimize subsequent adverse outcomes of hypernatremia would succeed. It should be clear, however, that in hospitalized patients who have intact thirst perception and are hypernatremic, there may be some value of efforts to correct hydration status as symptom relief or palliation. Otherwise, we should not waste time and efforts chasing the shadows of severe illness and end-of-life trajectories.
Acknowledgments
The content of this article reflects the personal experience and views of the author and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author.
Footnotes
See related article, “Outcomes of Hospital-Acquired Hypernatremia,” on pages 1396–1407.
Disclosures
S.G. Coca reports employment with Icahn School of Medicine at Mount Sinai. Mount Sinai owns part of Renalytix. S.G. Coca reports consultancy for 3ive, Axon, Bayer, Boehringer-Ingelheim, Nuwellis, Renalytix, Reprieve Cardiovascular, Takeda, and Vifor; ownership interest in pulseData and Renalytix; research funding from ProKidney, Renal Research Institute, Renalytix, and XORTX; patents or royalties from Renalytix; role as an Associate Editor for Kidney360; and role on the Editorial Boards of CJASN, JASN, and Kidney International.
Funding
None.
Author Contributions
Conceptualization: Steven G. Coca.
Writing – original draft: Steven G. Coca.
Writing – review & editing: Steven G. Coca.
References
- 1.Death of David Coughlin. Wikipedia. Accessed September 4, 2023. https://en.wikipedia.org/wiki/Death_of_David_Coughlin
- 2.Seay NW, Lehrich RW, Greenberg A. Diagnosis and management of disorders of body tonicity-hyponatremia and hypernatremia: core curriculum 2020. Am J Kidney Dis. 2020;75(2):272–286. doi: 10.1053/j.ajkd.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 3.Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med. 1996;124(2):197–203. doi: 10.7326/0003-4819-124-2-199601150-00002 [DOI] [PubMed] [Google Scholar]
- 4.Arzhan S Roumelioti M-E Litvinovich I, et al. Outcomes of hospital-acquired hypernatremia. Clin J Am Soc Nephrol. 2023;18(11):1396–1407. doi: 10.2215/CJN.0000000000000250 [DOI] [PMC free article] [PubMed] [Google Scholar]