Abstract
Background:
Perimenopausal insomnia (PMI) is a relatively common menopausal symptom that can cause serious problems for the women themselves and their families. Today, the world is facing the trend and challenges of an aging population. It is reported that about 1.5 million women worldwide enter menopause every year, with sleep disorder identified as a core symptom. The efficacy of acupuncture combined with traditional Chinese medicine for treating PMI has been recognized by patients and doctors.
Methods:
We searched 8 databases to identify 15 randomized controlled trials evaluating the effects of acupuncture combined with traditional Chinese medicine on sleep in patients with PMI compared with Western medicine alone. Subsequently, data extraction and analysis were performed to assess the quality and risk of bias of the study method design, and a meta-analysis of the data was performed.
Results:
This study included 15 randomized controlled trials involving 1188 patients with PMI. The results show that acupuncture combined with traditional Chinese medicine seems to be more effective than Western medicine in the treatment of PMI: efficiency (RR: 1.18; 95% CI: 1.08, 1.29; P = .001); the Pittsburgh Sleep Quality Index (PSQI) (WMD: −2.77; 95% CI: 4.15–1.39; P < .0001); follicle-stimulating hormone (FSH) (WMD: −31.45; 95% CI: 42.7–20.2; P < .001) and the Hamilton Anxiety Score (HAMA) (WMD: −2.62, 95% CI: −3.93, −1.32; P < .0001). Compared with western medicine, E2 (WMD: 5.07; 95% CI: 5.78–15.92; P = .36) and LH (WMD: −4.86; 95% CI: 11.5–1.78; P = .151) had no difference.
Conclusion:
The current analysis results show that acupuncture combined with Chinese medicine seems to have a more positive effect than western medicine alone in improving sleep and FSHF in PMI patients, but no difference has been found in improving E2 and LH. This study provides a basis for acupuncture combined with Chinese medicine to treat PMI. However, due to the higher risk of evaluation in included studies, more rigorous randomized controlled trials and higher quality studies are needed to validate included studies.
Keywords: acupuncture1, Chinese herbal medience2, hormone levels5, perimenopause insomnia3, sleep quality4
1. Introduction
Sleep disorders, such as difficulty in falling asleep, sleep deprivation or inability to fall asleep, can greatly affect the quality of life and can happen regardless of any triggers. Menopause is not only a natural process of women normal aging but also an important period in a woman life. The scope of menopause will also vary in different races, nationalities and lifestyles.[1] During menopause, many clinical symptoms that last or accompany the rest of life and heavily affect people life quality will occur, such as depression, anxiety, irritability, paranoia, insomnia, etc. Perimenopausal insomnia (PMI) is a common menopausal symptom that will bring serious problems to women and their families. PMI will also give rise to a poor quality of life, low work efficiency, anxiety and depression.[2]
Nowadays, the world is facing the challenge of population aging. It is reported that about 1.5 million women worldwide enter menopause every year,[3] and sleep disorders are identified as typical symptoms.[4] Studies have found that sleep disorders are very common in middle-aged women and the prevalence of insomnia increases significantly from premenopause to perimenopause and then to postmenopause, with the highest incidence in perimenopause.[5] Epidemiological investigation shows that the prevalence of moderate to severe insomnia among climacteric women in Japan is 50.8%, which is much higher than in Europe and North America.[6] In Korea, 14.3% of middle-aged women suffer from insomnia at least 3 times a week. This prevalence of insomnia is similar to that in Singapore and France.[7] An American sleep survey shows that 59% of perimenopausal women suffer sleep disorders at least several nights a week,[8] whereas women who have experienced other perimenopausal symptoms (hot flashes and depression) are more likely to have insomnia.[9,10] A study on insomnia in perimenopausal women from China showed that the rate of difficulty falling asleep continued to increase from the premenopausal stage (28.12%) to the postmenopausal stage (50.29%).[11] In addition, 25% of perimenopausal women and 30% of postmenopausal women said they had only a few nights or less of sleep per month.[12] It is generally believed that the occurrence or aggravation of insomnia is associated with menopause, but there is no clear or effective explanation at present. Some studies believe that hot flashes and sweating are unique vasomotor symptoms in menopause,[13] so a connection between sleep disorders and vasomotor status and mood in women during perimenopause seems to be an explanation.[14] But more importantly, the reduction of endogenous estrogen is a directly influential factor that cannot be ignored in PMI.[15] Therefore, our understanding of PMI symptoms contributes to the choice of appropriate treatment methods. Hormone replacement therapy seems to be an effective treatment and has proved helpful for reducing sleep latency and the total number of wakes at night and also for increasing the total sleep time of menopausal women.[16] However, it should be noted that hormone replacement therapy has always been controversial, with many patients stopping or refusing to use it for fear of adverse reactions. Patients are more inclined to choose natural drugs and complementary replacement therapy.[17]
Acupuncture and traditional Chinese herbs are the mainstay of traditional Chinese medicine (TCM) and also used as effective methods for treating perimenopausal symptoms.[18] Acupuncture is one of the simplest, safest and most popular alternative therapies.[19] Studies have confirmed that acupuncture is effective in the treatment of PMI.[20] A recent randomized controlled trial (RCT) claimed that acupuncture can significantly improve the clinical symptoms of PMI,[21] and can also be used as a treatment for comorbid PMI and depression.[19] In the TCM syndrome differentiation and treatment system, the treatment of PMI mainly focuses on premenopausal and postmenopausal symptoms and insomnia. TCM syndrome differentiation mostly includes: kidney deficiency and liver depression; disharmony of heart and kidney; liver depression and spleen deficiency; kidney deficiency in yin and yang; heart, gallbladder and qi deficiency; heart and spleen deficiency; and other syndromes. According to the symptoms, doctors will select the prescription and medication using the principles of syndrome differentiation and treatment, which has advantages in the clinical treatment of PMI. In order to better observe the clinical effect of acupuncture combined with TCM in the treatment of PMI compared with Western medicine, we conducted a meta-analysis of the RCT on acupuncture combined with TCM for the treatment of PMI.
2. Methods
The study was conducted in accordance with the preferred reporting item of the systematic review and meta-analysis (PRISMA)[22] and was in line with the CHARMS checklist. The study was approved by PROSPERO on May 9, 2022, with registration number of 420222323909.
2.1. Search strategy
We comprehensively searched the following 8 databases: Embase, PubMed, Web of Science, Cochrane Library, sinomed, Chinese National Knowledge Infrastructure, Wan Fang Data Knowledge Service Platform, Chinese Scientific Journal Database (VIP database) and Sinomed. During the search, we used “menopause,” “perimenopause,” “lose sleep,” “insomnia,” “acupuncture,” “randomized control,” “Random Allocation” and “clinical controlled trial” as the subject words and selected the bibliographies of qualified RCTs and controlled clinical trials published as of August 31, 2023. Finally, we adjusted the retrieval format to adapt to different databases. At the same time, the literature was supplemented by a manual search of retrospective references of the included literature and references of the same or similar topics in a systematic review report (the detailed search strategy is provided in the Supplementary Material, http://links.lww.com/MD/K617).
2.2. Inclusion and exclusion criteria
According to inclusion and exclusion criteria, eligible studies were identified, data were extracted and cross-checked and any ambiguity was resolved through discussion and consensus. The experimental groups received acupuncture (manual acupuncture, electroacupuncture, ear acupuncture, warm acupuncture) combined with Chinese herbal medicine whereas the control groups were treated with Western medicine.
The inclusion criteria were as follows: RCTs or controlled clinical trials; patients diagnosed with PMI under the internationally recognized diagnostic criteria for PMI, regardless of age, course and case origin, without other diseases; and the treatment group used acupuncture combined with Chinese herbal medicine while the control group used Western medicine.
The exclusion criteria were as follows: articles with repeated experiments; articles with no clear diagnostic criteria for PMI or irregular patient evaluation methods; articles with treatment other than acupuncture combined with traditional Chinese herbal medicine (such as moxibustion and acupoint injection) in the treatment group; and the control group used treatment other than Western medicine. The automated tool used to exclude literature was Endnote-X9.
2.3. Outcomes
The main results of the study included the Pittsburgh Sleep Quality Index (PSQI) and the total effective rate of treatment (the total effective rate is (the number of people with marked effect plus the number of cured people)/the total number of people). The secondary outcomes of the study included estradiol (E2), luteinizing hormone (LH), follicle-stimulating hormone (FSH) and the Hamilton Anxiety Rating Scale (HAMA) score.
2.4. Data extraction
Two reviewers extracted the data respectively, including study design, diagnostic criteria, PMI duration, sample size, age, intervention strategy, control method, treatment duration, follow-up duration and adverse events. Incomplete data were queried or followed-up with the original author by telephone and email.
2.5. Risks of bias
Two researchers used the Cochran Risk of Bias Tool 2.0 and the Newcastle-Ottawa Scale to evaluate the quality of the included studies and made judgments for each item, such as “high risk,” “low risk” and “ambiguous.” Each randomized controlled trial was evaluated according to the following 6 items: the randomization process; interventions that deviate from expectations; lack of result data; measurement results; select report results; and overall. If the methodology used is appropriate and properly and clearly described, the study is considered low risk; Otherwise, if the method cannot be accurately judged, it is rated as high risk, or there are some problems. Two investigators independently assessed these factors and, if necessary, consulted a third investigator (JF) to resolve differences.
2.6. Data analysis
The 2 researchers used stata16.0 for statistical analysis of the data. Secondary categorical variables are expressed as the risk ratio (RR) and the corresponding 95% CI. Continuous data are expressed as the weighted mean difference (WMD) or standardized mean difference and the corresponding 95% CI. To determine whether there is heterogeneity in the evaluation statistics, I2 and P are used: I2 > 50% or P < .01 indicates significant heterogeneity and that the random effect model is required; otherwise, the fixed effect model is used. If significant heterogeneity exists, subgroup analysis or meta-regression is performed to find the source of heterogeneity. For the statistical analysis, P < .05 is considered statistically significant. In addition, a sensitivity analysis was conducted to test the reliability of the results by excluding low-quality tests. A funnel plot and Egger diagram were used to detect publication bias. If significant publication bias is found, the stability of the results is tested by the trim-and-fill method.
3. Results
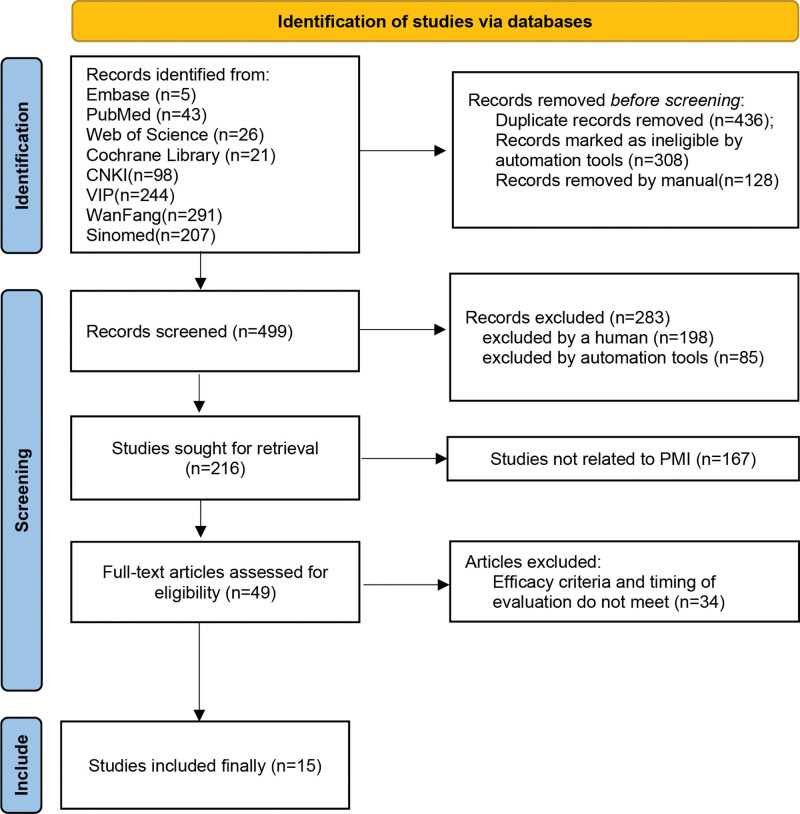
A total of 935 articles were retrieved, all of which were published in journals; 436 duplicates were excluded; 85 articles were excluded by automation tool for ineligible;198 articles are not RCT; 167 articles are not related for PMI; after careful reading, 34 articles were further excluded because the treated patients did not meet the inclusion requirements. The subsequent analysis included 15 articles (Fig. 1).
Figure 1.
Flow diagram of the included and excluded studies in the systematic review.
3.1. Basic characteristics of eligible studies
The main characteristics of the included study are shown in Table 1 (see end of article): study location, sample size of the treatment group and the control group, treatment method selected by the treatment group, treatment method used by the control group, time process of efficacy evaluation, efficacy evaluation indicators and adverse events.
Table 1.
characteristics of included studies.
| Study | Country | Outcome measure | Experimental treatment | Control treatment | Sample size (I/C) | Age(y) [mean (SD)] (I/C) | Acupuncture points | Duration | Adverse events (I/C) |
|---|---|---|---|---|---|---|---|---|---|
| Chen2019 | China | Efficiency | ① and Chai Hu Jia Long Gu Mu Li Tang |
Diazepam | 60/60 | 46.6 (3.2)/46.2 (2.8) | GV20, GV23, LI4, EX-HN1, HT7, SP6 | ||
| Yang2018 | China | Efficiency, PSQI | ② and Shu Gan Bu Shen Tang |
Diazepam | 80/82 | 51.2 (10.25)/50.7 (9.76) | CO18, TF4, CO12, CO10 | 4w | |
| Chen2018 | China | Efficiency | ③ And Huang Lian E Jiao Tang |
Estazolam | 40/40 | - | BL15, BL23, KI7, HT7, PC7, LR3 | ||
| Kang2021 | China | HAMA, PSQI, Efficiency, FSH, LH, E2 | ① and Xiang Fu Tang |
Estazolam | 43/43 | 50.45 (3.92)/49.3 (3.15) | BL62, KI6, SP6, BL20, ST36 | 4w | |
| Lai2015 | China | Efficiency | ② and Chai Shao Long Mu Zao Ren Tang |
Eszopiclone | 35/32 | - | EX-HN1, HT7, SP6, LR3 | ||
| Lu2022 | China | PSQI, FSH, E2 | ① and Bai Zi Yang Xin Tang |
Estazolam | 46/46 | 46.13/46.13 | GV20, EX-HN1, BL15, BL23, ST25, CV4, EX-CA1 | 4w | 0/10 (lethargy, fatigue) |
| Qiao2022 | China | Efficiency, PSQI | ① and Ning Shen Tang |
Eszopiclone | 35/35 | 48.6 (1.5)/48.2 (1.0) | GV20, GB20, GV29, SP6, HT7, PC7, ST36, KI3 | 30d | |
| Chen2015 | China | FSH, LH, E2 | ① and Jia Wei Huang Lian Ejiao Tang |
Alprazolam | 30/30 | 47.87 (4.12)/47.03 (3.84) | EX-HN1, PC7, HT7, SP6, KI3, BL15, BL23 | 30d | |
| Du2017 | China | PSQI, FSH, E2 | ① and Wu Mei Wan |
Estazolam | 42/41 | 50.61 (2.62)/50.45 (3.19) | EX-HN1, PC6, SP6 | 4w | 8 Mild nervousness, gastrointestinal discomfort/26 drowsiness, dizziness |
| Fan2020 | China | Efficiency, FSH, LH, E2 | ① and Bu Shen Shu Gan Ning Xin Tang |
Nilestriol Tablets | 40/40 | 50.16 (4.27)/49.88 (4.15) | GV20, EX-HN1, HT7, PC6, BL23, BL17, LR3, BL15, ST36, SP6, CV4 | 3m | 2/6 |
| Lai2018 | China | Efficiency, PSQI | ② and Geng Nian Ning Shen Tang |
Eszopiclone | 40/40 | 52.35 (5.39)/51.13 (5.58) | EX-HN1, HT7, SP6, LR3, GB20 | 28d | |
| Qiao2021 | China | Efficiency | ① and Chai Hu Jia Long Gu Mu li Tang |
Diazepam, oryzanol | 19/19 | - | GV20, EX-HN1, HT7, PC7, SP6, BL62, KI6 | 28d | |
| Sun2013 | China | Efficiency | ① and Ning Shen Tang |
Diazepam, oryzanol | 28/28 | 48.32 (1.33)/48.97 (1.42) | GV20, SP6, ST36, HT7 | 3w | |
| Yan2020 | China | PSQI, HAMA, FSH, LH, E2 | ① and Xiang Fu Tang |
Estazolam | 59/57 | 50.8 (7.6)/49.6 (7.2) | EX-HN1, HT7, SP6, BL18, BL13, GB20, ST36 | 16w | 1 Dizziness/dizzy 3, dry mouth 2, exhausted 1, Multilingualism 1, rash 1 |
| Zhang2021 | China | HAMA, FSH, LH, E2, PSQI | ① and Bai He Di Huang Tang |
Estazolam | 39/39 | 52.76 (2.81)/52.14 (2.63) | GV20, HT7, GV24, EX-HN1, GB13, PC6, SP6 | 4w | 3/10 |
Experimental treatment: ①:acupuncture; ②: ear acupuncture;③: Electroacupuncture;④: lift needle.
E2 = estradiol, FSH = follicle stimulating hormone, HAMA = Hamilton Anxiety Scale, LH = luteinizing hormone, PSQI = Pittsburgh Sleep Quality Index.
1188 patients were involved in the 15 studies: 666 received acupuncture combined with traditional Chinese medicine and 582 received Western medicine. All were single center control experiments.
For the diagnosis of PMI, clear diagnostic criteria are found in 16 articles. For the efficacy criteria of PMI, PSQI was evaluated in 7 studies.[23–29] The effective rate was used to evaluate the treatment effect[24,25,28,30–36]in 10 studies. The extent of E2 and FSH changes was recorded in 7 studies.[23,24,26,27,29,32,37] Five studies recorded the changes of LH,[24,27,29,32,37] and 3 studies used HAMA[24,27,29]to identify the anxiety state of patients. Five reported adverse events[23,26,27,29,32]
3.2. Risks of bias
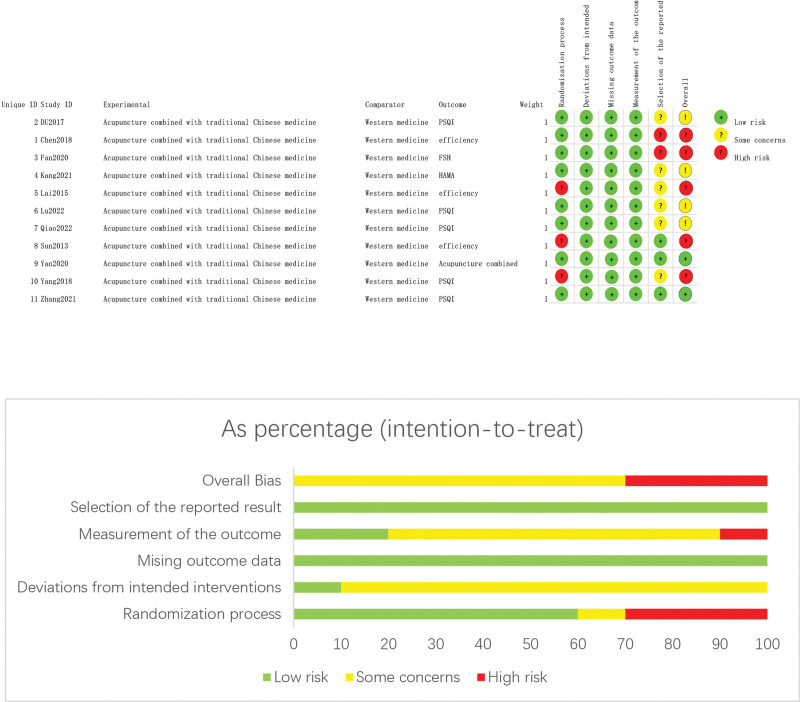
The results of methodological evaluation are shown in Figure 2 and Table 2. Of the 15 studies, random grouping was mentioned in all 15, of which 8[23,24,27–29,31,32,34] used the random number table method but the other 7[25,26,30,33,35–37] did not mention the specific randomization method. None of the articles described the blind method or allocation concealment method.
Figure 2.
Risk of bias graph.
Table 2.
Results of quality assessment using the Newcastle-Ottawa Scale for case-control studies.
| Study | Selection | Comparability | Exposure | Scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate definition of cases | Representativeness of the cases | Selection of controls | Definition of controls | Control for important factors | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | ||
| Chen, 2015 | ※ | ※ | - | ※ | ※※ | - | ※ | - | 6 |
| Chen, 2019 | ※ | ※ | - | ※ | ※※ | -- | ※ | - | 6 |
| Lai, 2018 | ※ | ※ | - | ※ | ※※ | - | ※ | - | 6 |
| Qiao, 2021 | ※ | ※ | - | ※ | ※ | ※ | - | 5 | |
※Scoring point.
3.3. Results of individual studies
3.3.1. Efficiency.
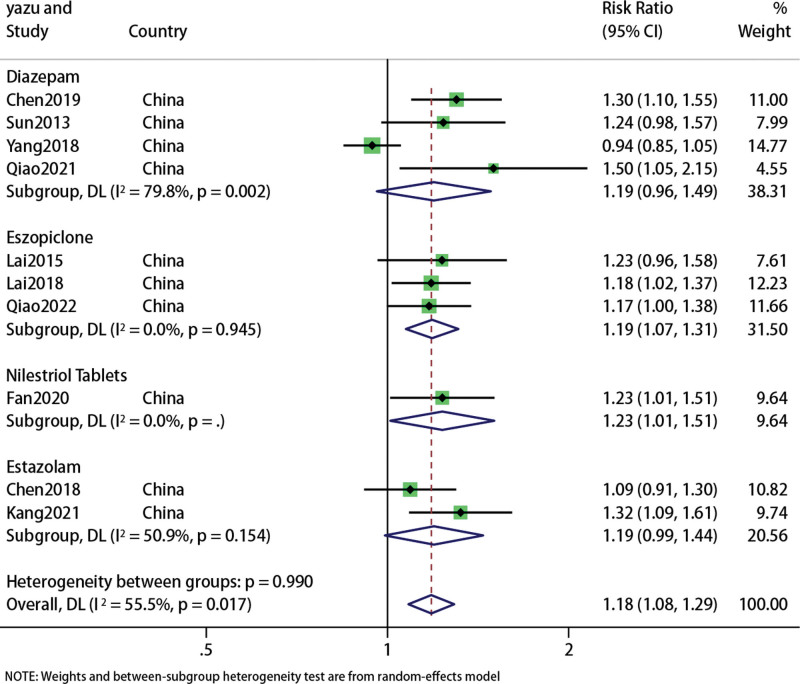
A total of 839 patients in 10 studies[24,25,28,30–36] reported the effective rate of acupuncture combined with traditional Chinese medicine in the treatment of PMI. The results of meta-analysis were shown in the forest figure (Fig. 3). The overall results showed that acupuncture combined with traditional Chinese medicine was superior to Western medicine alone in the effective treatment of PMI (RR: 1.18; 95% CI: 1.08, 1.29; P = .001), but the heterogeneity was significant (I2 = 55.5%, P = .017), so we used a random effects model.
Figure 3.
Forest plot of Efficiency.
Compared with Diazepam, acupuncture and moxibustion combined with traditional Chinese medicine had no advantages (RR: 1.19; 95% CI: 0.96, 1.49; P = .112), (I2 = 79.8%, P = .002), the difference was not statistically significant.
Compared with Eszopiclone, acupuncture combined with Chinese medicine appeared to be more effective (RR: 1.19; 95% CI: 1.07, 1.31; P = .001), (I2 = 0, P = .945);
Compared with Nilestriol Tablets, acupuncture combined with Chinese medicines is more effective (RR: 1.23; 95% CI: 1.01, 1.51; P = .039), (I2 = 0, P = 0), the difference was statistically significant.
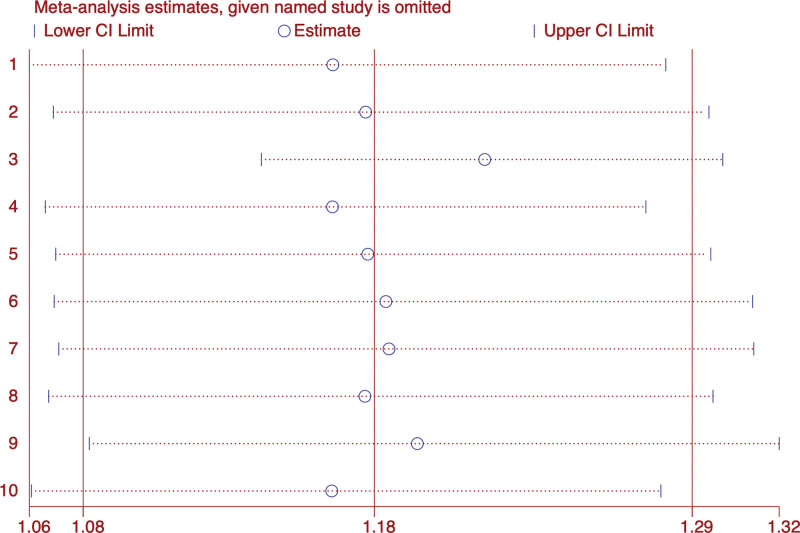
Compared with Estazolam, acupuncture combined with Chinese medicine showed no difference (RR: 1.19; 95% CI: 0.99, 1.44; P = .064), (I2 = 50.9%, P = .154).Sensitivity analysis showed that the results were stable, but egger diagram showed that there might be publication bias (Fig. 4).
Figure 4.
Efficiency sensitivity analysis.
3.4. PSQI
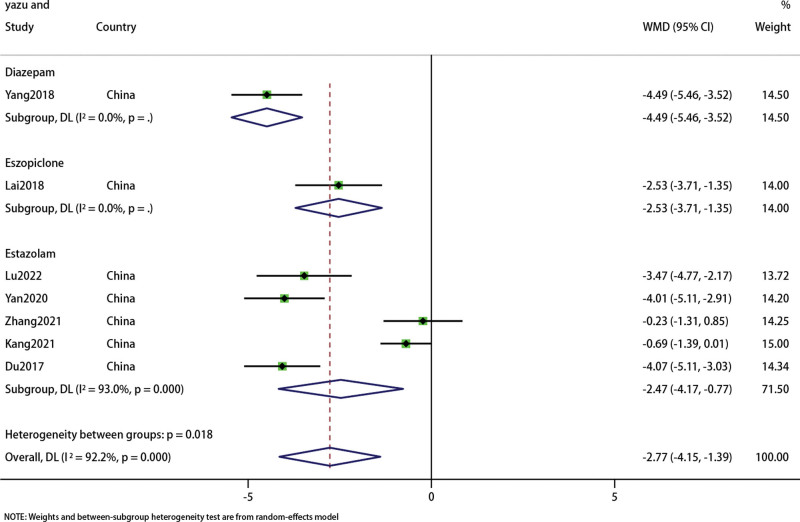
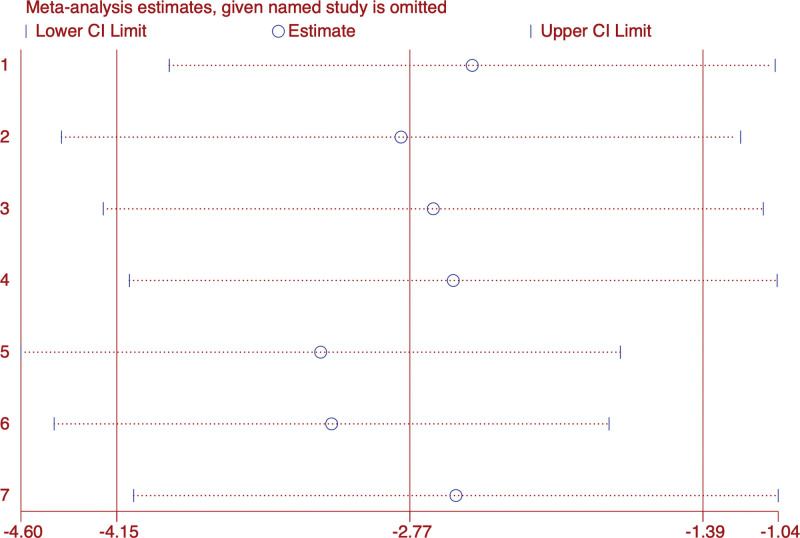
We conducted a meta-analysis on the PSQI results of 697 patients in 7 studies.[23–29] Subgroup analysis showed (Fig. 5) that acupuncture combined with Chinese medicine was superior to Diazepam in improving PSQI (WMD: −4.49; 95% CI: 5.46–3.52; P < .0001) (I2 = 0%, P < .00001), Eszopiclone(WMD: −2.53; 95% CI: 3.71–1.35; P < .0001) (I2 = 0%, P < .00001), Estazolambut(WMD: −2.47; 95% CI: 4.17–0.77; P = .004) (I2 = 93%, P = 0); The overall results were (WMD: −2.77; 95% CI: 4.15–1.39; P < .0001),the heterogeneity was high (I2 = 92.2%, P = 0). Therefore, we adopted the random response model. Sensitivity analysis shows that the structure is stable (Fig. 6).
Figure 5.
Forest plot of PSQI. PSQI = Pittsburgh Sleep Quality Index.
Figure 6.
Sensitivity analysis of PSQI. PSQI = Pittsburgh Sleep Quality Index.
3.5. E2 and FSH
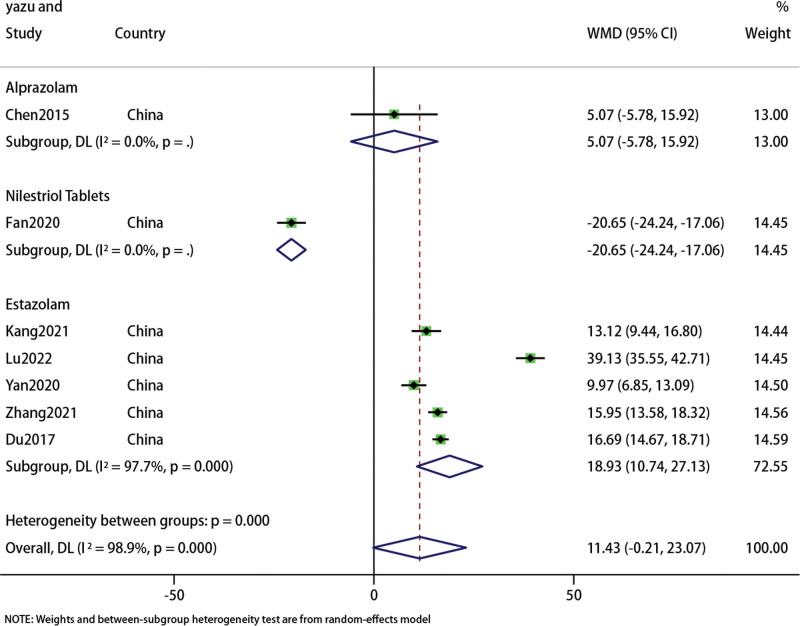
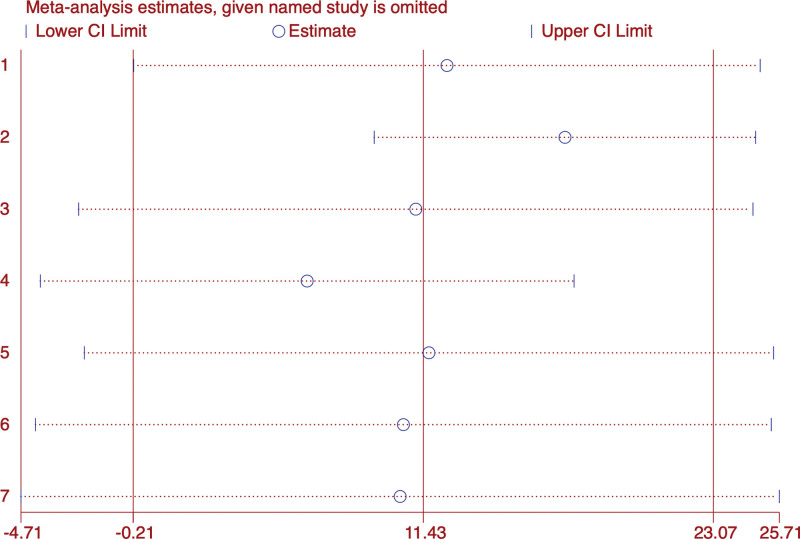
A total of 7 studies[23,24,26,27,29,32,37] included E2 and FSH data of 595 patients. We conducted a meta-analysis of E2 and FSH respectively. The forest map results of E2 subgroup analysis showed that there was no difference in the results of acupuncture combined with TCM compared with Alprazolam (WMD: 5.07; 95% CI: 5.78–15.92; P = .36) (I2 = 0, P = 0), but better than Nilestriol Tablets(WMD: −20.56; 95% CI: 24.24–17.06; P < .001) (I2 = 0, P = 0) and Estazolam(WMD: 18.93; 95% CI: 10.74–27.13; P < .001) (I2 = 97.7%, P = 0). Overall, acupuncture combined with traditional Chinese medicine showed no difference with western medicine in improving E2 levels in PMI patients (WMD: 11.43; 95% CI: 0.21–23.07; P = .054).However, the heterogeneity of the results was significant (I2 = 98.9%, P = 0), so we used the random effects model (Fig. 7). Sensitivity analysis showed that the results were stable (Fig. 8).
Figure 7.
Forest plot of E2.
Figure 8.
Sensitivity analysis of E2.
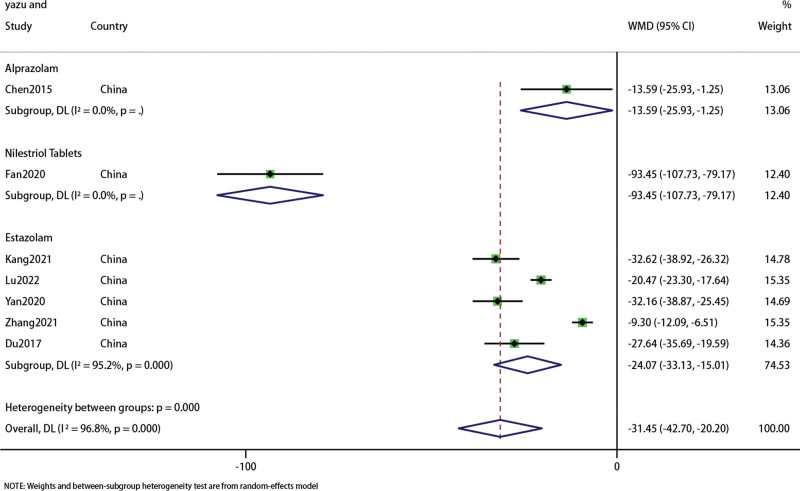
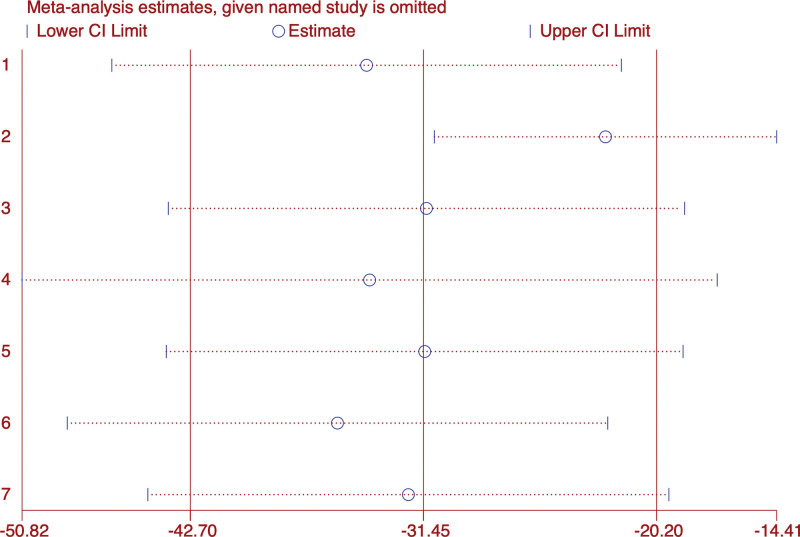
Subgroup analysis was performed on FSH data, and the results were shown in the forest diagram (Fig. 9). The overall results indicated that acupuncture combined with Chinese medicine was superior to western medicine in improving FSH level in PMI patients (WMD: −31.45; 95% CI: 42.7–20.2; P < .001), the heterogeneity was significant (I2 = 96.8%, P = 0). Sensitivity analysis showed that the results were stable (Fig. 10).
Figure 9.
Forest plot of FSH. FSH = follicle-stimulating hormone.
Figure 10.
Sensitivity analysis of FSH. FSH = follicle-stimulating hormone.
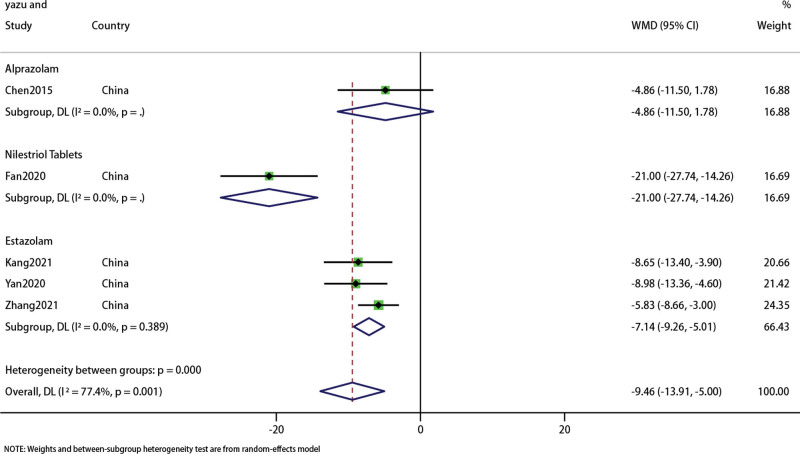
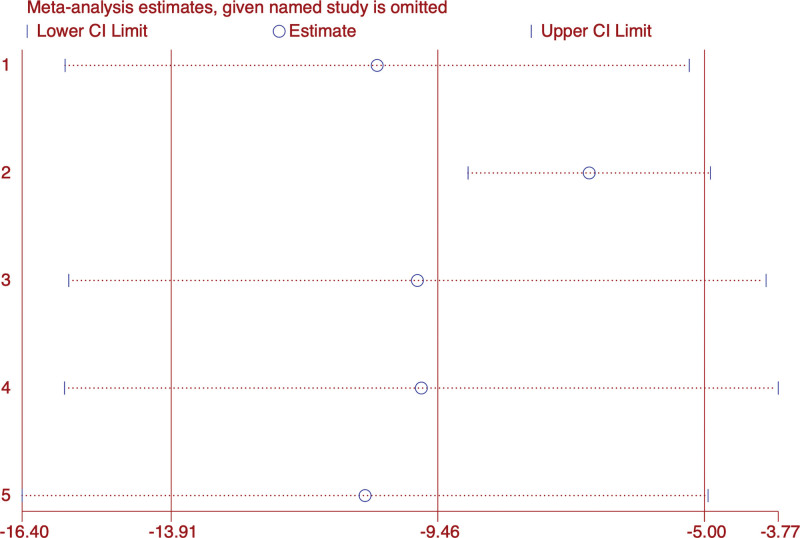
3.6. LH
In the 5 studies recording LH changes, there were 420 patients.[24,27,29,32,37] The forest map results of data analysis showed that the treatment effect of acupuncture combined with traditional Chinese medicine group was better than Western medicine group (WMD: −9.46; 95% CI: 13.91 to 5; P < .001), but heterogeneity was high (I2 = 77.4%, P = 0). Subgroup analysis did not indicate that acupuncture combined with traditional Chinese medicine was different from Alprazolam (WMD: −4.86; 95% CI: 11.5–1.78; P = .151) (Fig. 11). Sensitivity analysis showed that the results were stable (Fig. 12).
Figure 11.
Forest plot of LH. LH = luteinizing hormone.
Figure 12.
Sensitivity analysis of LH. LH = luteinizing hormone.
3.7. HAMA
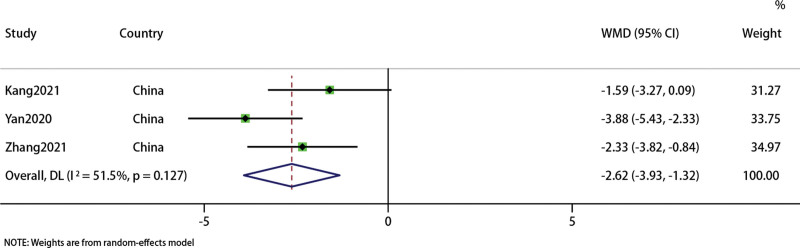
Three studies involving 280 patients recorded HAMA[23,26,28] to evaluate patients’ anxiety. Sensitivity analysis indicated that the results were stable. Forest map results showed that acupuncture combined with traditional Chinese medicine group had advantages than Western medicine group (WMD: −2.62, 95% CI: −3.93, −1.32; P < .0001). The heterogeneity was high (I2 = 51.5%, P = .127) (Fig. 13).
Figure 13.
Forest plot of HAMA. HAMA = Hamilton Anxiety Score.
3.8. AE
Adverse reactions were reported in 5 studies of 74 patients.[23,26,27,29,32] Among them, 11 patients in acupuncture combined with traditional Chinese medicine group (1.49%) and 63 patients in Western medicine group (9.66%). The incidence of adverse events in acupuncture combined with traditional Chinese medicine group was clearly lower than that in Western medicine group.
4. Discussion
PMI is common in climacteric women and their quality of life and working status will be heavily affected.[38]PMI is mostly considered to be caused by the decline in estrogen but hormone replacement therapy as a first-line treatment cannot be accepted or relied on by most patients due to risks, side effects and other problems.[39] Acupuncture combined with TCM is effective in the treatment of PMI. At present, there is no systematic evaluation or meta-analysis of acupuncture combined with TCM in the treatment of PMI. The meta-analysis in this paper included 15 studies, involving 1188 patients, to summarize and analyze the clinical efficacy of acupuncture combined with TCM in the treatment of PMI.
It is worth noting that, compared with insomnia in the general population, PMI exerts a more negative influence on mental health than on the body, which can be a very dangerous factor characterized by a sluggish female heart response.[40] There may be different triggers for PMI (hot flashes, hormonal changes). Studies have shown that hot flashes can predict the awakening times on a polysonograph of sleep per hour. The frequency of hot flashes may also influence the levels of E2 and FSH.[41] The number of hot flashes at night is inversely proportional to the level of E2 and is directly proportional to the level of FSH.[42] Other studies have shown that hot flashes during menopause are a symptom caused by vasomotor contraction. The vasomotor mechanism is believed to be as follows: the decrease of estrogen concentration leads to a decrease of endorphin concentration in the hypothalamus, which results in an increase of norepinephrine and serotonin release, thus causing hot flashes,[43,44] and affecting sleep quality. There is evidence that the reproductive hormone levels are associated with the subjective and objective sleep quality of perimenopausal women.[45] Significant changes in estrogen levels in menopausal women will cause decreased sensitivity of urethral smooth muscle and atrophy of urethral epithelium, increasing nocturia and producing other urinary tract symptoms that induce early awakening and affect sleep quality.[46,47] In addition, climacteric women often suffer from depression, which aggravates their mental and psychological symptoms and takes its toll on their sleep quality.[48]
In TCM, PMI belongs to the category of premenopausal and postmenopausal symptoms and insomnia, which is mainly caused by a deficiency of kidney essence. As mentioned in the Yellow Emperor Canon of Internal Medicine: “when a woman is 7 years old, her kidney Qi is full. When a woman is about 49 years old, because her kidney essence has been exhausted and her menstruation has stopped, she loses her fertility and her physical function begins to decline.” It is generally believed that the kidney-Tiangui-Chongren (Chong and Ren meridians in TCM 8 extraordinary meridians)-uterus system of TCM is similar to the hypothalamic-pituitary-ovarian axis system of Western medicine. Based on TCM theory and treatment principles, TCM has clear advantages in the treatment of PMI. However, acupuncture and TCM are more commonly used in the treatment of PMI. Studies have shown that acupuncture can improve the sleep quality of perimenopausal women, as measured by the PSQI.[49] The night sleep time of postmenopausal women after acupuncture treatment was notably prolonged.[50] Recent RCTs on acupuncture and moxibustion in the treatment of PMI show that acupuncture and moxibustion are safe and effective for the treatment of PMI.[21,51] Some try to explain the mechanism of acupuncture and moxibustion in the treatment of PMI: acupuncture and moxibustion can reduce oxidative stress in the rostral ventrolateral medulla and regulate the excitability of the sympathetic nerve, where the sympathetic nerve control center is located.[52] Electroacupuncture can regulate the hyperactivity of the hypothalamic-pituitary-adrenal axis by improving the expression of RNA.[53] In addition, acupuncture can also increase the E2, FSH and LH levels to stabilize the hormones.[19,54] TCM is a kind of comprehensive treatment that follows the principle of syndrome differentiation and treatment and integrity, and its treatment effect is often for the whole body. Studies have shown that Chaihu Guizhi Ganjiang decoction can improve depression and reduce the concentrations of plasma IL-6 and sIL-6R, thus it can alleviate stress in perimenopausal and postmenopausal women and reduce the incidence rate of PMI.[55] Pharmacological research shows that in a rat model with a low serum E2 level, Erxian decoction can increase the production of ovarian E2 by regulating ovarian aromatase and activating the detoxification pathway of liver catalase.[56] Early published studies also showed that the use of Chinese herbs is a well-tolerated and valuable short-term alternative treatment for climacteric women with hot flashes, especially for perimenopausal women with palpitations, emotional disorders, insomnia and other symptoms.
5. Limitation
This study was evaluated in strict accordance with the PRISMA report list, but there were also some deficiencies. First of all, the editorial board of international medical journals requires that all clinical trials must be registered before they can be published. However, all articles included in this paper have not been registered. Secondly, only 8 studies mentioned the generation of random sequences in detail, and no study specifically described allocation concealment, which may lead to selection bias. Secondly, none of the articles point out that the blind method is included in the result evaluation, which may lead to detection bias. Furthermore, we tried to ensure that all relevant studies (including those in the West and the East) were similar, but all the included studies and RCTs were conducted in China, which may limit the universality of the survey. More studies are needed to make the conclusion more applicable to other fields, which may contribute to publication bias, and also the corresponding conclusions may not be applicable to other fields as the lack of high-quality RCTs inevitably hindered a sound evaluation of the efficacy of TCM. Finally, TCM usually produces curative effects through the comprehensive combination of a variety of herbs. Because of its complex composition and mechanism, TCM has not been widely recognized abroad. Although acupuncture and moxibustion have been popularized in many countries, the relevant data on acupuncture and moxibustion combined with TCM in the treatment of PMI cannot be widely accessed abroad at present. In addition, the heterogeneity of several results is high, which may be caused by the characteristics of the TCM. Under the guiding principles of syndrome differentiation and treatment and a holistic view, acupoint selection and medication adjustment should be carried out according to the symptoms of the different patients in the treatment process, which may be one of the reasons for the high heterogeneity. Furthermore, if the patient feels pain during acupuncture or the taste of the prescription is unacceptable this can change the treatment effect and evaluation of the patient, which will also result in high heterogeneity.
Therefore, it is necessary to conduct more rigorous RCTs on acupuncture combined with TCM in the treatment of PMI. There are several suggestions: clinical trials should be registered on the international platform; the quality of study design should be improved, including randomization, allocation concealment and blinding; and international cooperation should be carried out to obtain more research and ensure the universality of the research results.
6. Conclusion
The current analysis results show that acupuncture combined with Chinese medicine seems to have a more positive effect than western medicine alone in improving sleep and FSHF in PMI patients, but no difference has been found in improving E2 and LH. This study provides a basis for acupuncture combined with Chinese medicine to treat PMI. However, due to the higher risk of evaluation in included studies, more rigorous randomized controlled trials and higher quality studies are needed to validate included studies.
Author contributions
Methodology: Jie Feng.
Project administration: Xu Gao.
Software: Shao Yin.
Visualization: Qicheng Yang.
Writing – original draft: Zhao Li.
Writing – review & editing: Fengya Zhu.
Supplementary Material
Abbreviations:
- E2 =
- estradiol
- FSH
- follicle-stimulating hormone
- HAMA
- Hamilton Anxiety Score
- LH
- luteinizing hormone
- PMI
- perimenopausal insomnia
- PSQI
- Pittsburgh Sleep Quality Index
- RCT
- randomized controlled trial
- RR
- risk ratio
- VIP
- Chinese Scientific Journal Database
- WMD
- weighted mean difference
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Li Z, Yin S, Feng J, Gao X, Yang Q, Zhu F. Acupuncture combined with Chinese herbal medicine in the treatment of perimenopausal insomnia: A systematic review and meta-analysis. Medicine 2023;102:45(e35942).
Contributor Information
Zhao Li, Email: lizhao@stu.cdutcm.edu.cn.
Shao Yin, Email: yinshao@stu.cdutcm.edu.cn.
Jie Feng, Email: fengjie@stu.cdutcm.edu.cn.
Xu Gao, Email: 1019031459@qq.com.
Qicheng Yang, Email: 1201838@163.com.
References
- [1].Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yazdi Z, Sadeghniiat-Haghighi K, Ziaee A, et al. Influence of sleep disturbances on quality of life of Iranian menopausal women. Psychiatry J. 2013;2013:907068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–50. [DOI] [PubMed] [Google Scholar]
- [4].National Institutes of Health. National Institutes of Health State-of-the-Science Conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142(12 Pt 1):1003–13. [PubMed] [Google Scholar]
- [5].Punyahotra S, Dennerstein L, Lehert P. Menopausal experiences of Thai women Part 1: symptoms and their correlates. Maturitas. 1997;26:1–7. [DOI] [PubMed] [Google Scholar]
- [6].Terauchi M, Obayashi S, Akiyoshi M, et al. Insomnia in Japanese peri- and postmenopausal women. Climacteric. 2010;13:479–86. [DOI] [PubMed] [Google Scholar]
- [7].Shin C, Lee S, Lee T, et al. Prevalence of insomnia and its relationship to menopausal status in middle-aged Korean women. Psychiatry Clin Neurosci. 2005;59:395–402. [DOI] [PubMed] [Google Scholar]
- [8].Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110(2 Pt 1):230–40. [DOI] [PubMed] [Google Scholar]
- [9].Arakane M, Castillo C, Rosero MF, et al. Factors relating to insomnia during the menopausal transition as evaluated by the insomnia severity index. Maturitas. 2011;69:157–61. [DOI] [PubMed] [Google Scholar]
- [10].Blümel JE, Cano A, Mezones-Holguín E, et al. A multinational study of sleep disorders during female mid-life. Maturitas. 2012;72:359–66. [DOI] [PubMed] [Google Scholar]
- [11].Luo M, Li J, Tang R, et al. Insomnia symptoms in relation to menopause among middle-aged Chinese women: findings from a longitudinal cohort study. Maturitas. 2020;141:1–8. [DOI] [PubMed] [Google Scholar]
- [12].Baker FC, Wolfson AR, Lee KA. Association of sociodemographic, lifestyle, and health factors with sleep quality and daytime sleepiness in women: findings from the 2007 National Sleep Foundation “Sleep in America Poll”. J Womens Health (Larchmt). 2009;18:841–9. [DOI] [PubMed] [Google Scholar]
- [13].Rebar RW, Spitzer IB. The physiology and measurement of hot flushes. Am J Obstet Gynecol. 1987;156:1284–8. [DOI] [PubMed] [Google Scholar]
- [14].Joffe H, Soares CN, Thurston RC, et al. Depression is associated with worse objectively and subjectively measured sleep, but not more frequent awakenings, in women with vasomotor symptoms. Menopause. 2009;16:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pengo MF, Won CH, Bourjeily G. Sleep in women across the life span. Chest. 2018;154:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–55. [PubMed] [Google Scholar]
- [17].Wang Y, Lou XT, Shi YH, et al. Erxian decoction, a Chinese herbal formula, for menopausal syndrome: an updated systematic review. J Ethnopharmacol. 2019;234:8–20. [DOI] [PubMed] [Google Scholar]
- [18].Peng W, Sibbritt DW, Hickman L, et al. A critical review of traditional Chinese medicine use amongst women with menopausal symptoms. Climacteric. 2014;17:635–44. [DOI] [PubMed] [Google Scholar]
- [19].Zhao FY, Fu QQ, Spencer SJ, et al. A promising approach for comorbid depression and insomnia in perimenopause. Nat Sci Sleep. 2021;13:1823–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193–200. [DOI] [PubMed] [Google Scholar]
- [21].Fu C, Zhao N, Liu Z, et al. Acupuncture improves peri-menopausal insomnia: a randomized controlled trial. Sleep. 2017;40. [DOI] [PubMed] [Google Scholar]
- [22].Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Du J, Fan W, Du H. Clinical observation of Jin’s three-needle combined with Jiawei Wumei Pill in the treatment of perimenopausal insomnia. Chin Pharm. 2017;28:1104–7. [Google Scholar]
- [24].Zhenxia K. Clinical study of acupuncture combined with modified Xiangfu decoction in the treatment of perimenopausal insomnia with liver depression and qi stagnation syndrome. Pract Chin West Med Clin. 2021;21:35–6. [Google Scholar]
- [25].Lai M, Hu J, Chen X, et al. Curative effect observation of Gengnian Ningshen Decoction combined with electroacupuncture in the treatment of menopausal insomnia. J Pract Chin Med. 2018;34:905. [Google Scholar]
- [26].Lu Y. Clinical observation of Baizi Yangxin Decoction combined with acupuncture in the treatment of perimenopausal insomnia. J Pract Chin Med. 2022;36:8–10. [Google Scholar]
- [27].Xueli Y, Yuandong Y, Dandan Y, et al. Clinical study on the treatment of perimenopausal insomnia with liver depression and qi stagnation syndrome by acupuncture combined with Xiangfu decoction. China J Tradit Chin Med. 2020;45:1460–4. [DOI] [PubMed] [Google Scholar]
- [28].Yang G. Clinical observation of self-made Shugan Bushen Decoction combined with ear acupuncture in the treatment of insomnia in perimenopausal women. Chin Foreign Med Res. 2018;16:130–1. [Google Scholar]
- [29].Zhang H, Zhou Q. Therapeutic effect of Baihe Dihuang decoction combined with sedative and calming acupuncture in the treatment of female perimenopausal insomnia and its effect on anxiety and depression. World J Integr Med. 2021;16:405–9. [Google Scholar]
- [30].Chen L. Analysis of the therapeutic effect of acupuncture combined with Chaihu Jia Longgu Muli Decoction in the treatment of menopausal insomnia. Clin Res Tradit Chin Med. 2019;11:99–100. [Google Scholar]
- [31]., Xi C. Clinical observation on the treatment of perimenopausal insomnia with kidney deficiency and liver stagnation by pressing acupuncture combined with Huanglian Ejiao Decoction. New Chin Med. 2018;50:115–8. [Google Scholar]
- [32].Fan H, Wu Q, Zhu L. Observation on the effect of Bushen Shugan Ningxin Decoction combined with acupuncture in the treatment of menopausal syndrome. China J Pract Med. 2020;47:115–8. [Google Scholar]
- [33].Ming L. Chaishaolongmuzaoren decoction combined with electroacupuncture in the treatment of 35 cases of menopausal insomnia. J Pract Chin Med. 2015;31:278–9. [Google Scholar]
- [34].Qiao H. Clinical effect of Ningshen decoction combined with acupuncture therapy on menopausal insomnia. Da Doctor. 2022;7:25–7. [Google Scholar]
- [35].Qiao S. Analysis of the effect of Chaihu Jia Longgu Muli Decoction on Shaoyang Zhishu combined with acupuncture in the treatment of insomnia in perimenopausal patients. World Latest Med Inf Dig. 2021;21:277–8. [Google Scholar]
- [36].Sun P, Xiao B, He X. Clinical observation of 28 cases of climacteric insomnia treated with Ningshen decoction combined with acupuncture. World Latest Med Inf Dig (Electron Ed). 2013;11:83–4. [Google Scholar]
- [37].Chen Y, Liu H, Yang B, et al. Analysis of the therapeutic effect of acupuncture combined with modified Huanglian Ejiao decoction in the treatment of perimenopausal insomnia with heart-kidney incompatibility. Mod Integr Chin West Med. 2015;25:2902–4. [Google Scholar]
- [38].Tal JZ, Suh SA, Dowdle CL, et al. Treatment of insomnia, insomnia symptoms, and obstructive sleep apnea during and after menopause: therapeutic approaches. Curr Psychiatry Rev. 2015;11:63–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dobkin RD, Menza M, Bienfait KL, et al. Ramelteon for the treatment of insomnia in menopausal women. Menopause Int. 2009;15:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Terauchi M, Hiramitsu S, Akiyoshi M, et al. Associations between anxiety, depression and insomnia in peri- and post-menopausal women. Maturitas. 2012;72:61–5. [DOI] [PubMed] [Google Scholar]
- [41].Baker FC, Willoughby AR, Sassoon SA, et al. Insomnia in women approaching menopause: beyond perception. Psychoneuroendocrinology. 2015;60:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Randolph JF, Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–12. [DOI] [PubMed] [Google Scholar]
- [43].Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. [DOI] [PubMed] [Google Scholar]
- [44].Freedman RR, Norton D, Woodward S, et al. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80:2354–8. [DOI] [PubMed] [Google Scholar]
- [45].Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- [46].Jones HJ, Zak R, Lee KA. Sleep disturbances in midlife women at the cusp of the menopausal transition. J Clin Sleep Med. 2018;14:1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Russell JK, Jones CK, Newhouse PA. The role of estrogen in brain and cognitive aging. Neurotherapeutics. 2019;16:649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kravitz HM, Kazlauskaite R, Joffe H. Sleep, health, and metabolism in midlife women and menopause: food for thought. Obstet Gynecol Clin North Am. 2018;45:679–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Avis NE, Coeytaux RR, Isom S, et al. Acupuncture in Menopause (AIM) study: a pragmatic, randomized controlled trial. Menopause. 2016;23:626–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Borud EK, Alraek T, White A, et al. The Acupuncture on Hot Flushes Among Menopausal Women (ACUFLASH) study, a randomized controlled trial. Menopause. 2009;16:484–93. [DOI] [PubMed] [Google Scholar]
- [51].Li S, Wang Z, Wu H, et al. Electroacupuncture versus sham acupuncture for perimenopausal insomnia: a randomized controlled clinical trial. Nat Sci Sleep. 2020;12:1201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang XR, Yang JW, Ji CS, et al. Inhibition of NADPH oxidase-dependent oxidative stress in the rostral ventrolateral medulla mediates the antihypertensive effects of acupuncture in spontaneously hypertensive rats. Hypertension. 2018;71:356–65. [DOI] [PubMed] [Google Scholar]
- [53].Zhu J, Chen Z, Meng Z, et al. Electroacupuncture alleviates surgical trauma-induced hypothalamus pituitary adrenal axis hyperactivity Via microRNA-142. Front Mol Neurosci. 2017;10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhu H, Nan S, Suo C, et al. Electro-acupuncture affects the activity of the hypothalamic-pituitary-ovary axis in female rats. Front Physiol. 2019;10:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ushiroyama T, Ikeda A, Sakuma K, et al. Chai-hu-gui-zhi-gan-jiang-tang regulates plasma interleukin-6 and soluble interleukin-6 receptor concentrations and improves depressed mood in climacteric women with insomnia. Am J Chin Med. 2005;33:703–11. [DOI] [PubMed] [Google Scholar]
- [56].Sze SC, Tong Y, Zhang YB, et al. A novel mechanism: Erxian Decoction, a Chinese medicine formula, for relieving menopausal syndrome. J Ethnopharmacol. 2009;123:27–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.