Abstract
Visceral artery aneurysms (VAAs) are vascular pathologies that are difficult to treat. The variable geometry of the vessels and the location of aneurysms render difficult their evaluation in radiological imaging studies. Less invasive endovascular procedures are increasingly used in common practice. Our aim was to test the feasibility of using 3D printing technology in the preparation of preoperative spatial models of visceral artery aneurysms and their impact on interventional treatment. In our observational study, we examined a group of patients with true aneurysms of the visceral arteries who were followed and who underwent endovascular procedures with the use of 3D prints for better imaging of vascular lesions. We analyzed the fused filament fabrication method of 3D printing and printable materials in the preparation of spatial vascular models. We confirmed that more accurate visualization and analysis of vascular anatomy could assist operators in attempting minimally invasive treatment with good results. Extending imaging studies using 3D printing models that allow for the assessment of the position, morphology and geometry of the aneurysm sac, particularly of vessel branches, could encourage surgeons to perform endovascular procedures.
Keywords: coil embolization, endovascular treatment, stentgrafting, stenting, 3-dimensional printing, visceral artery aneurysm
1. Introduction
Visceral artery aneurysms account for 4% of all arterial aneurysms.[1] The lack of specific symptoms renders quick detection of VAAs difficult, and the finding rate has increased due to the popularity of routinely performed diagnostic imaging examinations. The patient life is threatened by spontaneous rupture of the aneurysm sac, leading to hemorrhage, impairment, or loss of the function of the supplied organ or even death.[2,3] The most common cause of VAAs is atherosclerosis. Other etiological factors can include injuries; connective tissue diseases; pregnancy; congenital diseases; infections, including bacteria and fungi; inflammatory diseases, including those associated with cholecystitis and pancreatitis; and vasculitis.[4,5] Regarding visceral localization, the most common aneurysms are splenic artery aneurysms (SAAs), which occur in 60% to 80%, with a 2% risk of rupture and a mortality rate of 36% to 90%. Hepatic artery aneurysms (HAAs) account for 20% of cases, with a 20% risk of rupture and an estimated mortality of 21% to 35%. Coeliac trunk aneurysms account for only 4% of all visceral aneurysms, with a 20% risk of rupture and a mortality rate of 50% to 70%.[1,6] VAAs, due to the location and geometry of the vessels, make it challenging to plan the treatment and perform a repair procedure, especially endovascular procedures.[2,3] Accurate assessment of VAA morphology, location, and geometry is necessary for the appropriate selection of methods and tools, especially in planning endovascular treatment. The safety and effect of endovascular procedures depend on the surgeon experience and excellent knowledge of the anatomy of the operated artery, based solely on diagnostic imaging examinations.[4] One of the possibilities for extending diagnostics based on radiological examinations is the production of an individually prepared 3D model using 3D printing.[7–9] We aimed to test the potential of 3D printing technology in planning minimally invasive procedures and medical treatment of VAAs. We assumed the preparation of spatial 3D models based on the imaging studies of patients with VAAs, the assessment by specialists of their usefulness in further proceedings, and, in subsequent stages, the performance of repair procedures for aneurysms.
2. Materials and methods
The study was a single-center, prospective study as a proof of concept to evaluate the feasibility of 3D printing in the minimally invasive treatment of visceral artery aneurysms. Gender, age, aneurysm etiology, or comorbidities were not criteria for inclusion in the research group. All patients had previously undergone imaging examinations and consultation with a vascular surgeon and were referred for specialist consultation to qualify for invasive treatment. Further criteria for including patients in the research group were single or multiple true aneurysms of the visceral arteries visualized by CT angiography, the tortuous geometry of the visceral vessels, or aneurysms in the area of vessel divisions or sacs located in the course of angular deflections of the arteries, or the aneurysm sacs with departing arterial branches. The CT scans had to meet the minimum quality requirements, enabling the generation of a virtual 3D model, that is, without artifacts in clinically significant places, in the arterial phase, with contrast filling the blood vessels in a way that allows for their identification and differentiation from the surrounding tissues. The thickness of the layers in the computed tomography examination was assumed to be no more than 1 mm. The size of the aneurysm was close to the limit value, qualifying patients for invasive treatment or with a size of the aneurysm sac considered to require surgery. Patients requiring emergency admission and treatment of the aneurysm and whose anatomical course of the vessels and pathological changes showed uncomplicated geometry and morphology were excluded from the study group. The study covered patients observed and treated in 2019 to 2022. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethical Committee of Regional Specialist Hospital in Wroclaw, Research and Development Center, Wroclaw (protocol code: EC 8.01.2019, date of approval: 9/01/2019). The patients were divided into 2 groups: those who underwent repair procedures and those for observation. The 3D printing process is well described in the literature.[10–12] Angio-CT data in DICOM format were processed in open-source software (3D Slicer) for segmenting and generating a virtual 3D model by automatic thresholds regarding the data resulting from Hounsfield units of the baseline examination and manual correction of the ranges covering the selected anatomical regions. Regardless of the software, manual data verification remains necessary to avoid errors that could significantly impact the geometry of the generated 3D model. Mural thrombus and calcified atherosclerotic plaques giving positive and negative remodeling of the vessel or the aneurysm sac were included in the 3D model geometry. In the next stage, the printing parameters were set in slicer software (Ultimaker Cura; Ultimaker B.V. Utrecht, Netherlands) dedicated to the fused filament fabrication (FFF) type of 3D printer (Ultimaker 2+; Ultimaker B.V. Zaltbommel, Netherlands), including the operating temperature appropriate for the material used, the layer height, parameters of the supports and the method of adhesion to the working table. The support material and any contamination were removed from the printed models. The filament used in all prints was thermoplastic polyurethane elastomer 98A. Endovascular operators analyzed the 3D printed models. Based on the comparison of angio-CT examinations and spatial 3D models, decisions were made about possible modifications of the previously agreed upon treatment plan and the intraoperative procedure. The quality of the geometric representation of anatomical details in the accurate model is crucial for the medical suitability of the prototype. The 3D printed models were subjected to digital subtraction angiography on a GE Innova 4100 angiograph to validate the process and assess its quality. Obtained images have been measured on radiological workstations with Siemens Syngo Via and Pixmeo SARL OsiriX MD software.
3. Results
The study group consisted of ten patients. Five underwent endovascular aneurysm surgery, including 3 SAAs and 2 renal artery aneurysms (RAAs). Five patients were under observation, including 1 HAA, 2 RAAs, 1 SAA, and 1 case of dolichoectasia and aneurysm in the course of the upper pancreatic-duodenal artery and the lower pancreatic-duodenal artery with an additional RAA. Due to insufficient data, 1 patient who underwent an RAA repair procedure was excluded from the research group. All 3D printed models had an empty lumen corresponding to the contrast medium flow and vessel walls on CT angiography. In 1 model, after appropriate preparation of the project, a 2-layer aortic wall was obtained [Fig. 1D]. Measurements of the 3D printed models done using an electronic digital caliper. After evaluating the metric measurements of 3D printed models and comparing the dimensions of the aneurysm sacs and other characteristic anatomical structures with the initial angio-CT imaging examinations, the differences in dimensions of aneurysm sacs were <1 mm and applied to all models and measurements. The results and discrepancies of measurements are presented in the table [Table 1]. Based on the results, the method was considered to reflect the data obtained in the angio-CT examinations accurately. In operated SAA cases, 3D models were used only to visually assess the splenic artery anatomy, aneurysm sac, and spleen supply vessels [Fig. 2A and C]. Additional model measurements were not performed to select endovascular tools, related to the insufficient flexibility and deformability of the 3D models under the rigid guidewires compared to the typical changes in the geometry of the splenic artery during procedures. That posed a risk of inappropriate length selection of stents or stentgrafts. During particular procedures, the patients were provided with: a Gore ViaBahn covered stent 6 × 50 mm in size; a Gore Viabahn 6 × 50 mm covered stent and Medtronic Concerto Helix spirals; and a Medtronic Concerto 3D detachable coil system, which after release formed a scaffold filled with additional Medtronic Concerto Helix spirals. Control arteriographies confirmed that the aneurysm sacs were supplied correctly in each case [Fig. 2B and D]. In the case of RAA, the 3D model was used to extend the imaging diagnosis to better visualize the outgoing branches of blood vessels from the aneurysm sac, their length, and further courses [Fig. 3A–C]. Venous vessels were also preserved in the model [Fig. 3A]. Based on the actual 3D model, the agreed treatment plan was changed radically. The patient was initially qualified for the classic surgical procedure involving ex vivo aneurysm sac surgery with renal autograft. After the analysis of the patient spatial 3D model, endovascular treatment was performed [Fig. 3D]. The model allowed for a better assessment of the position, length, and diameter of the segmental arteries of the kidney in relation to the aneurysm sac, showing the possibility of proper guidewire positioning and release of the vessel remodeling stent in a way that allowed for coiling of the sac by Medtronic Concerto Helix spirals without the risk of coil migration. The 3D model of the patient observed with the common hepatic artery aneurysm visualized well stenosis of the celiac trunk through the limbs of the diaphragm, its post-stenotic dilatation, and hepatic artery modeling by the aneurysm sac lying along the vessel [Fig. 1: A1–A3, B, and C]. The dolichoectasia and aneurysm model in the upper pancreatic-duodenal artery and the lower pancreatic-duodenal artery [Fig. 4A and B] helped accurately assess the dilation without other visible structures on CT angiography hindered accurate evaluation [Fig. 4C and D]. Additionally, the model preserves the coexisting right renal artery aneurysm and the stenosis of the celiac trunk in the area of its proximal part. In 2 cases of observed RAA, the aneurysm sacs were located close to the hilum on the division of the renal arteries [Fig. 5A1–A3]. Failure to inherently supply the aneurysm sacs could be associated with at least partial obstruction of the flow through the branches and renal ischemia. Due to the size of the aneurysms, the patients are under observation. The last 3D model of an SAA was made for multiple aneurysms in the distal part of the SA and the left gastro-omental artery [Fig. 5B and C].
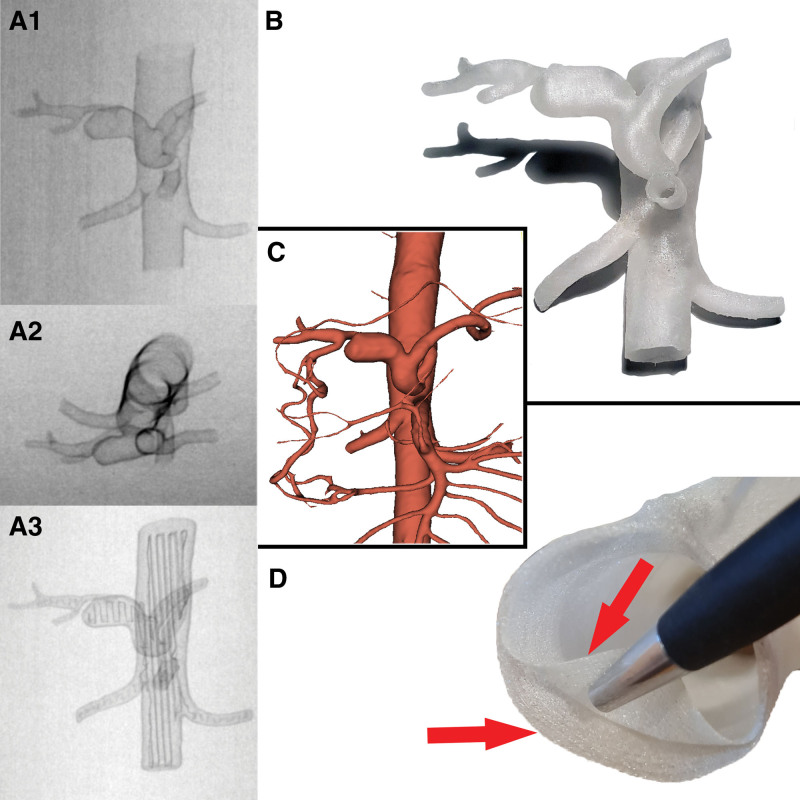
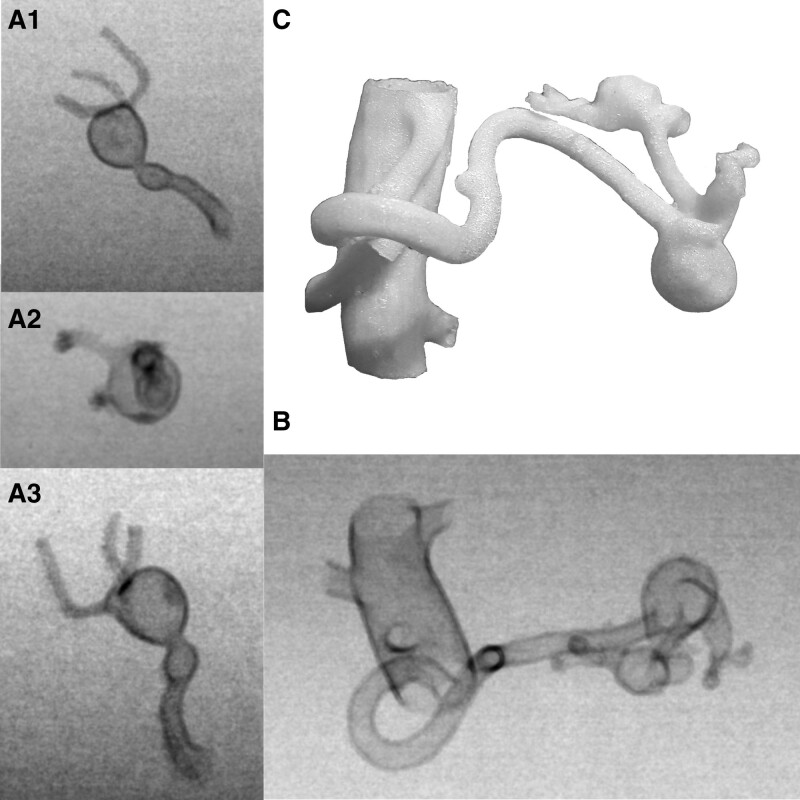
Figure 1.
(A) 3D models in the DSA study, with an empty light (A1, A2) and, for comparison, a 3D model filled with support material (A3). (B) 3D model of the printed HAA. (C) The collateral vascular circulation from the gastroduodenal artery was well visualized in the prepared virtual model of the HAA aneurysm. It is possible to select individual arteries for printing depending on the needs. (D) RAA model with a double-layered aortic wall (each layer 0.28mm thick) and empty vessel lumen. HAA = hepatic artery aneurysm, RAA = renal artery aneurysm.
Table 1.
Characteristics of the research group.
| Sex | Age | Aneurysm location | Dimensions of the aneurysm sac (angio-CT) | The longest measurement of the aneurysm sac (3D printed model) | Dimensional deviation | Qualification for the research group | Impact of 3D model analysis | Treatment applied | Additional aneurysms |
|---|---|---|---|---|---|---|---|---|---|
| M | 40 | RAA | 27 × 26 × 25 mm | 27.29 mm | 0.29 mm | Aneurysm located in the renal hilum, complicated morphology of the aneurysm, initial qualification for aneurysm repair with renal autograft | Improvement of the aneurysm morphology analysis, change of treatment method | Endovascular treatment - protection of the renal artery with a 5 × 30 mm stent and aneurysm sac coiling | N/A |
| F | 58 | SAA | 20 × 12 × 11 mm | 20.41 mm | 0.41 mm | Aneurysm in the splenic hilum with 2 branches, complicated morphology of the aneurysm | Improvement of the aneurysm morphology analysis, selection of treatment method | Endovascular treatment - coiling of the aneurysm sac with the additional use of a 3D coil | N/A |
| F | 62 | SAA | 17 × 12 × 14 mm | 17.67 mm | 0.67 mm | The tortuous course of the SA, complicated morphology of the aneurysm | Improvement of the aneurysm morphology analysis, selection of treatment method | Endovascular treatment - aneurysm supply with a 6 × 50 mm stent graft | In the splenic hilum, branch of SA, Ø 3 mm |
| F | 42 | SAA | 25 × 22 × 17 mm | 24.92 mm | 0.08 mm | The tortuous course of the SA, complicated morphology of the aneurysm | Improvement of the aneurysm morphology analysis, selection of treatment method | Endovascular treatment - aneurysm supply with a 6 × 50 mm stent graft and coiling of the aneurysm sac. | In the splenic hilum, SA, Ø 4 mm. |
| M | 44 | HAA | 22 × 11 × 10 mm | 22.57 mm | 0.57 mm | Aneurysm in the proximal section of the HA constricts the HA; MALS | Improvement of the aneurysm morphology analysis and communication with the patient | Observation | Poststenotic dilatation of the celiac trunk, up to 10 mm |
| F | 74 | SPDA, IPDA | 18 × 13 × 12 mm | 18.43 mm | 0.43 mm | Multiple dilatations of SPDA and IPDA arteries | Improvement of the aneurysm morphology analysis | Observation | RRAA, Ø 5 mm |
| M | 68 | RAA | 17 × 13 × 11 mm | 17.28 mm | 0.28 mm | Aneurysm on the RA division with 2 branches | Improvement of the aneurysm morphology analysis | Observation | N/A |
| F | 46 | RAA | 19 × 16 × 15 mm, 9 × 9 × 8 mm | 18.24 mm, 9.36 mm | 0.76 mm, 0.36 mm | Two RAAs, one of them on a RA division with 3 branches | Improvement of the aneurysms morphology analysis | Observation | Celiac trunk, up to 16 mm |
| F | 55 | SAA | 18 × 15 × 19 mm | 19.60 mm | 0.60 mm | Aneurysm on the SA division with branches | Improvement of the aneurysm morphology analysis | Observation | Left gastroomental artery, up to 13 mm |
F = female, M = male, HA = hepatic artery, HAA = hepatic artery aneurysm, IPDA = inferior pancreatic-duodenal artery, MALS = median arcuate ligament syndrome, N/A = not applicable, RAA = renal artery aneurysm, RRAA = right renal artery aneurysm, SA = splenic artery, SAA = splenic artery aneurysm, SPDA = superior pancreatic-duodenal artery.
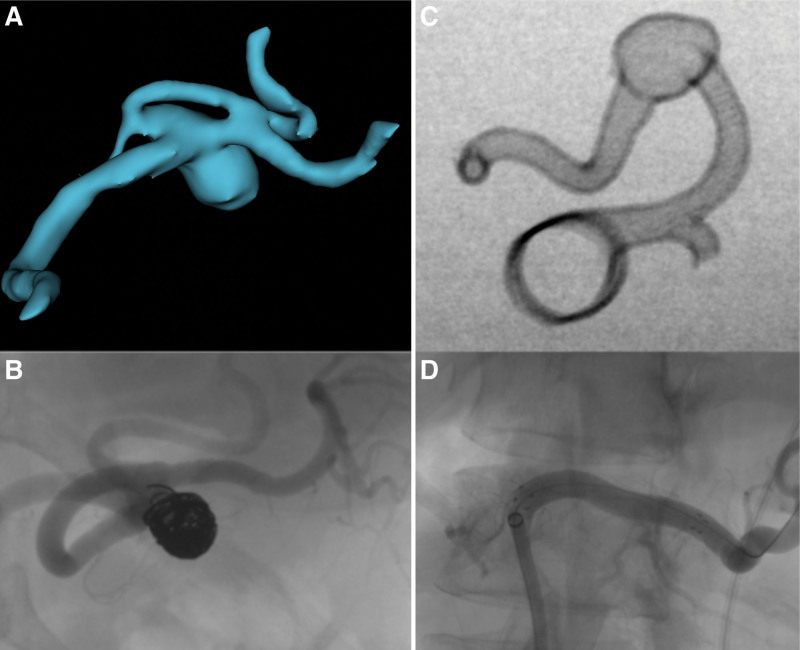
Figure 2.
(A) Virtual 3D model of the SAA sac. (B) Embolized SAA sac. (C) DSA study of the SAA 3D model. (D) Stentgraft in place of SA aneurysm sac. SAA = splenic artery aneurysm.
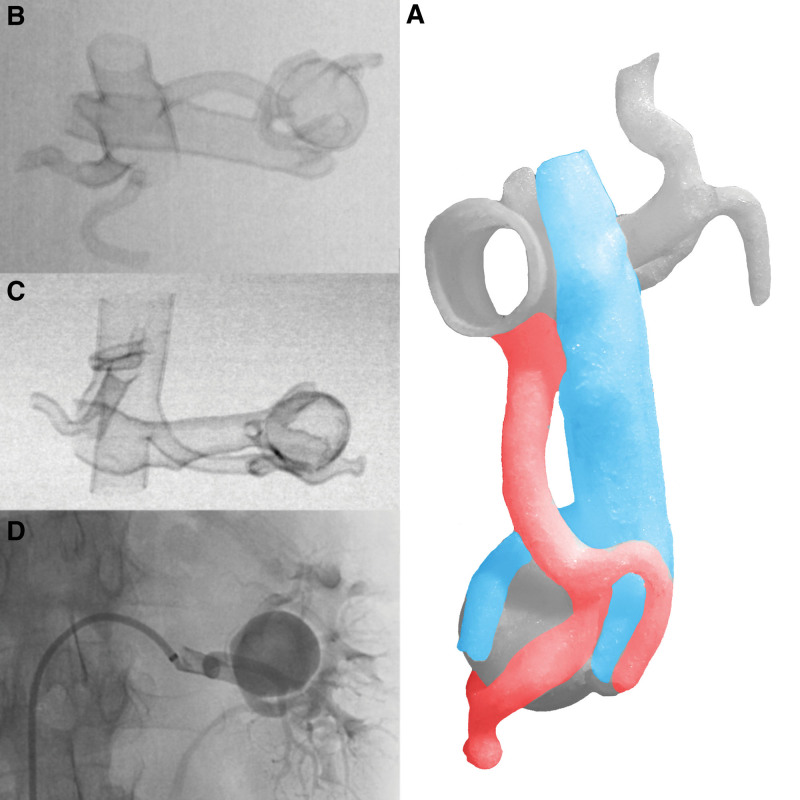
Figure 3.
(A) Photo of the RAA 3D model with the preserved system of arteries and veins prepared regardless of the final treatment method. Printed 3D models can also help in visualizations for classic treatments. (B) DSA of the RAA 3D model with a clearly visible separation of the segmental artery from the aneurysm sac. (C) DSA of the RAA 3D model. (D) DSA of the RAA during the procedure. RAA = renal artery aneurysm.
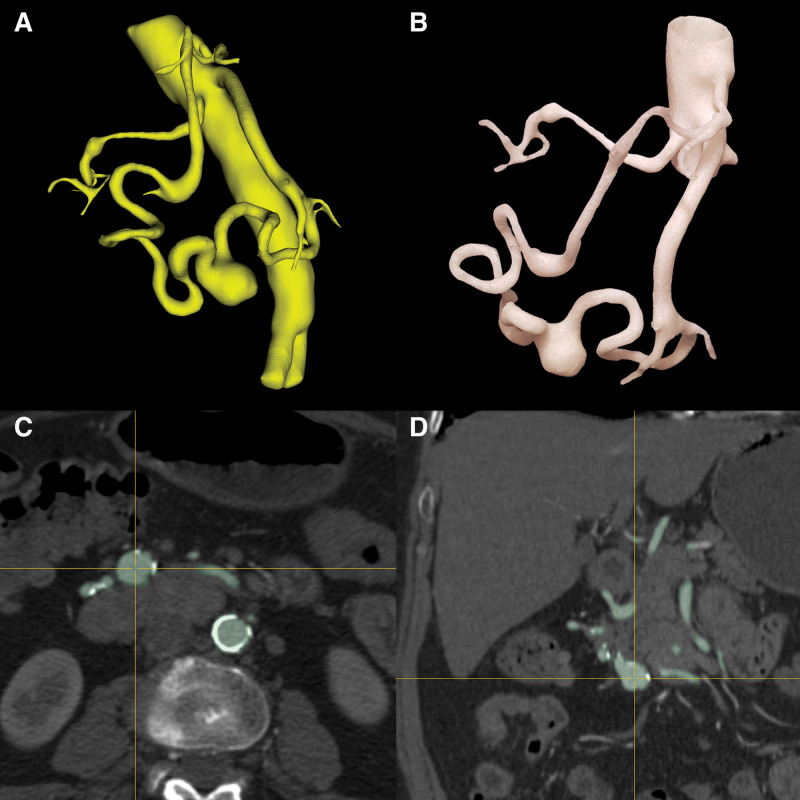
Figure 4.
The printed 3D model on the 1:1 scale (B) enables the accurate assessment of many changes without other anatomical structures visible in the angio-CT examination (C, D). In the figures, dolichoectasia and an aneurysm in the course of the upper pancreatic-duodenal artery and the lower pancreatic-duodenal artery with additional RRAA are shown. Virtual model (A). RRAA = right renal artery aneurysm.
Figure 5.
DSA examination of RAA (A1–A3) and SAA (B) 3D models and spatial 3D model of SAA (C) with different complexity of departures and vessel curvatures. RAA = renal artery aneurysm, SAA = splenic artery aneurysm.
4. Discussion
3D reconstructions depend on the quality of diagnostic imaging examinations, the absence of artifacts, good blood vessel contrast, the examination protocol, and obtaining data at the low heights of the examination layers. A radiologist with appropriate clinical knowledge should prepare the virtual model, preferably cooperating with a surgeon performing endovascular procedures. Small anatomical details, including vessels with fine diameters, might be poorly visible in imaging examinations and impossible to accurately 3D print. The precision of the obtained 3D models requires a repeatable, uniform procedure and periodic validation. The use of individualized preoperative diagnostics based on 3D printing could affect the planning of the treatment process, including reducing the invasiveness of the selected surgical method. The spatial models produced in 3D printing technology are instrumental in making decisions for a treatment plan in anatomically complex vascular pathologies. 3D printing can be useful in high-risk operations, in patients with comorbidities, and in cases in which the information obtained from imaging examinations leaves doubts as to the determination of an unequivocal treatment plan and the acceptable margin of error during the procedure is slight. 3D models can also help in the development of novel treatment procedures or the implementation of new treatment techniques.[13,14] The usefulness of 3D printing in educating and training surgeons with little practical experience has been demonstrated.[8,15] FFF was the chosen method of 3D printing due to the availability of the method, the possible simplicity of its implementation in everyday practice, and the relatively simple and clean technological process for the user. An important aspect of the selected method is its wide range of available filaments and 3D printers and low cost. Due to angular departures, diameters, lengths, and often tortuous courses, visceral arteries deform differently under the pressure of guidewires. For this reason, we attempted to select a material that was flexible, easily accessible, and partially translucent, potentially enabling benchmark tests of the printed materials. Printed 3D models made of materials with higher stiffness and hardness are suitable for precisely marking the exit sites of visceral arteries in individually fenestrated stent grafts of abdominal aortic aneurysms.[16] However, the vascular models made of hard materials on the Shore scale described in the literature cannot be used as VAA models for accurate testing of endovascular instruments. The bovine aorta is graded with a hardness of approximately 41 Shore OO, corresponding to less than 5 Shore A.[17] The thermoplastic polyurethane elastomer 98A used is too hard to perform highly accurate benchmark tests of visceral lesions; therefore, we did not use the models as simulators. Currently, there is no commercially available material for printing in FFF or fused deposition modeling (FDM) technologies with the desired low hardness corresponding to the vessels’ mechanical properties. The technology has significant limitations in extruding highly flexible filaments less than 60 Shore A.
Other 3D printing technologies than FFF/FDM allow printing with materials with higher flexibility and high translucency. Before starting our work in the FFF technology, publications on VAAs 3D printing mainly described using rigid and inflexible polymers. Due to the possible usefulness in planning precise minimally invasive procedures, in subsequent works, materials and 3D printing technologies that enable fabrication with high flexibility of vascular model walls should be used, which will enable benchmark tests, selection of endovascular tools, and pretreatment training.
The virtual model preparation time and the time of 3D printing limit cases that must be operating from the emergency department. Nevertheless, models of visceral aneurysms produced by 3D printing technology can significantly facilitate the assessment of aneurysm sacs and vessel branches, especially in cases of VA division aneurysms and aneurysms of the renal arteries in the hilum area. Changing the treatment plan into an endovascular, as well as the surgeon preparation for the procedure based on an actual 3D model, can significantly reduce the time of the procedure and its invasiveness.[9,18,19] This advantage is important in procedures in which patients with comorbidities are at risk for ex vivo supply of distal renal artery or hilum aneurysms, which are considered the gold standard.[20,21] The use of 3D printing for assessing renal artery aneurysms previously described in the literature resulted in the qualification of patients for surgical procedures.[22,23] In the case of several branches spreading from the sac of splenic artery aneurysms, using 3D coils in combination with the earlier good visualization of the sac in the 3D printing model might also contribute to attempts at the endovascular method. In our cases, we confirmed that more accurate visualization and analysis of vascular anatomy could assist operators in attempting minimally invasive treatment with good results.[24,25]
5. Conclusions
3D printing is an accessible and easy to implement method that could be widely used in treating VAAs. The extension of radiological diagnostics with spatial models produced by 3D printing could be helpful for operators and affect the treatment choice. The FFF 3D printing technology has limitations in the direct production of highly flexible models, especially useful as preoperative stimulators. Further research is necessary on 3D printing in treating aneurysms of the renal arteries, especially in the distal segment of the renal arteries and those lying in the kidney hilum.
Author contributions
Conceptualization: Daniel Grzegorz Soliński.
Data curation: Daniel Grzegorz Soliński, Marcin Celer.
Formal analysis: Daniel Grzegorz Soliński, Marcin Celer, Krzysztof Dyś.
Investigation: Daniel Grzegorz Soliński, Krzysztof Dyś, Maciej Wiewióra.
Methodology: Daniel Grzegorz Soliński, Krzysztof Dyś, Maciej Wiewióra.
Project administration: Daniel Grzegorz Soliński, Krzysztof Dyś, Wojciech Witkiewicz, Maciej Wiewióra.
Resources: Daniel Grzegorz Soliński, Marcin Celer.
Software: Daniel Grzegorz Soliński.
Supervision: Krzysztof Dyś, Wojciech Witkiewicz, Maciej Wiewióra.
Validation: Daniel Grzegorz Soliński, Marcin Celer, Krzysztof Dyś.
Visualization: Daniel Grzegorz Soliński, Krzysztof Dyś.
Writing – original draft: Daniel Grzegorz Soliński, Marcin Celer.
Writing – review & editing: Daniel Grzegorz Soliński, Marcin Celer, Maciej Wiewióra.
Abbreviations:
- CT
- computed tomography
- FDM
- fused deposition modeling
- FFF
- fused filament fabrication
- HAA
- hepatic artery aneurysm
- RAA
- renal artery aneurysm
- SAA
- splenic artery aneurysm
- VAA
- visceral artery aneurysm
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Soliński DG, Celer M, Dyś K, Witkiewicz W, Wiewióra M. 3D printing in the endovascular treatment of visceral artery aneurysms. Medicine 2023;102:45(e35844).
Contributor Information
Marcin Celer, Email: celermarcin@gmail.com.
Krzysztof Dyś, Email: kdys@vp.pl.
Wojciech Witkiewicz, Email: witkiewicz@wssk.wroc.pl.
Maciej Wiewióra, Email: mwiewiora@sum.edu.pl.
References
- [1].Hosn MA, Xu J, Sharafuddin M, et al. Visceral artery aneurysms: decision making and treatment options in the new era of minimally invasive and endovascular surgery. Int J Angiol. 2019;28:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].González J, Esteban M, Andrés G, et al. Renal artery aneurysms. Curr Urol Rep. 2014;15:376. [DOI] [PubMed] [Google Scholar]
- [3].Hans SS, Shepard A, Weaver M, Bove P, Long G. (Eds.). Endovascular and Open Vascular Reconstruction: A Practical Approach. 1st ed. CRC Press. 2017. [Google Scholar]
- [4].Venturini M, Piacentino F, Coppola A, et al. Visceral artery aneurysms embolization and other interventional options: state of the art and new perspectives. J Clin Med. 2021;10:2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Corey MR, Ergul EA, Cambria RP, et al. The natural history of splanchnic artery aneurysms and outcomes after operative intervention. J Vasc Surg. 2016;63:949–57. [DOI] [PubMed] [Google Scholar]
- [6].Takeuchi N, Soneda J, Naito H, et al. Success-fully-treated asymptomatic celiac artery aneurysm: a case report. Int J Surg Case Rep. 2017;33:115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ballard DH, Trace AP, Ali S, et al. Clinical applications of 3D printing: primer for radiologists. Acad Radiol. 2018;25:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tam CHA, Chan YC, Law Y, et al. The role of three-dimensional printing in contemporary vascular and endovascular surgery: a systematic review. Ann Vasc Surg. 2018;53:243–54. [DOI] [PubMed] [Google Scholar]
- [9].Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic re-view. Surgery. 2016;159:1485–500. [DOI] [PubMed] [Google Scholar]
- [10].Chang D, Tummala S, Sotero D, et al. Three-dimensional printing for procedure rehearsal/simulation/planning in inter-ventional radiology. Tech Vasc Interv Radiol. 2019;22:14–20. [DOI] [PubMed] [Google Scholar]
- [11].Ganguli A, Pagan-Diaz GJ, Grant L, et al. 3D printing for preoperative planning and surgical training: a review. Biomed Microdevices. 2018;20:65. [DOI] [PubMed] [Google Scholar]
- [12].Takao H, Amemiya S, Shibata E, et al. 3D printing of preoperative simulation models of a splenic artery aneurysm: precision and accuracy. Acad Radiol. 2017;24:650–3. [DOI] [PubMed] [Google Scholar]
- [13].Jędrzejczak T, Rynio P, Lewandowski M, et al. Externalized transapical guidewire technique after artificial aortic valve replacement during complete endovas-cular aortic arch repair. Wideochir Inne Tech Maloinwazyjne. 2021;16:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rynio P, Kazimierczak A, Jedrzejczak T, et al. A 3D printed aortic arch template to facilitate decision-making regarding the use of an externalized transapical wire during thoracic endovascular aneurysm repair. Ann Vasc Surg. 2019;54:336.e5–8. [DOI] [PubMed] [Google Scholar]
- [15].Mafeld S, Nesbitt C, McCaslin J, et al. Three-dimensional (3D) printed endovascular simulation models: a feasibility study. Ann Transl Med. 2017;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rynio P, Kazimierczak A, Jedrzejczak T, et al. A 3-dimensional printed aortic arch template to facilitate the creation of physician-modified stent-grafts. J Endovasc Ther. 2018;25:554–8. [DOI] [PubMed] [Google Scholar]
- [17].Maier J, Weiherer M, Huber M, et al. Optically tracked and 3D printed haptic phantom hand for surgical training system. Quant Imaging Med Surg. 2020;10:340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Torres IO, De Luccia N. A simulator for training in endovascular aneurysm repair: the use of three dimensional printers. Eur J Vasc Endovasc Surg. 2017;54:247–53. [DOI] [PubMed] [Google Scholar]
- [19].Torres I, De Luccia N. Artificial vascular models for endovascular training (3D printing). Innov Surg Sci. 2020;3:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marone EM, Mascia D, Kahlberg A, et al. Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg. 2011;25:936–46. [DOI] [PubMed] [Google Scholar]
- [21].Orion KC, Abularrage CJ. Renal artery aneurysms: movement toward endovascular repair. Semin Vasc Surg. 2013;26:226–32. [DOI] [PubMed] [Google Scholar]
- [22].Lin JC, Myers E. Three-dimensional printing for preoperative planning of renal artery aneurysm surgery. J Vasc Surg. 2016;64:810. [DOI] [PubMed] [Google Scholar]
- [23].Holzem KM, Jayarajan S, Zayed MA. Surgical planning with three-dimensional printing of a complex renal artery aneurysm. J Vasc Surg Cases Innov Tech. 2018;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Soliński DG, Celer M, Dyś K, et al. 3D printing in the preoperative planning and endovascular treatment of splenic artery aneurysm Own clinical experience and literature review. Wideochir Inne Tech Maloinwazyjne. 2022;17:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soliński D, Celer M, Dyś K, et al. The use of 3D printing in the treatment of renal artery aneurysms. ECR. 2020. [Google Scholar]