Abstract
Osteoarthritis (OA) is a chronic joint disease that reduces quality of life for patients. Ferroptosis plays a significant role in OA. However, its underlying mechanism remains unclear. In this study, we integrated 7 OA synovial datasets from the GEO database to screen for significant ferroptosis-related genes. The top 5 ferroptosis regulators were used to construct nomogram models to predict OA prevalence. Consensus clustering was applied to classify OA patients into different ferroptosis patterns based on significant ferroptosis-related genes. Subsequently, an immune cell infiltration study was performed to investigate the relationship between the significant ferroptosis regulators and immune cells. As a result, we screened 11 ferroptosis-related genes in OA patients. Five candidate ferroptosis regulators (SLC7A11, ALOX5, SLC1A5, GOT1, and GSS) were used to predict OA risk. The nomogram model based on these 5 genes is important for assessing the occurrence of OA. Consensus clustering analysis showed that OA patients could be classified into 2 ferroptosis patterns (Clusters A and B). Immune cell infiltration levels were higher in Cluster B than in Cluster A. Two subtypes, gene Clusters A and B, were classified according to the expression of ferroptosis-related DEGs among the ferroptosis patterns. Cluster A and gene Cluster A had higher ferroptosis scores than Cluster B or gene Cluster B, whereas the expression levels of the proinflammatory cytokines interleukin (IL)-1β, tumor necrosis factor, IL-6, IL-18, and IL-10 were higher in Cluster B or gene Cluster B than those in Cluster A or gene Cluster A. Different subtypes of ferroptosis play critical roles in OA. Furthermore, immunotherapy strategies for OA treatment may be guided by our study on ferroptosis patterns.
Keywords: consensus clustering, ferroptosis, random forest, immune cell infiltration, nomogram, osteoarthritis
1. Introduction
Osteoarthritis (OA) is a widespread, disabling disease that affects people of all ages, with the majority of cases occurring in the knees.[1] According to a recent research, people over 50 years of age have a greater than 25% chance of developing knee OA.[2] Synovial inflammation, cartilage damage, bone remodeling, and bone redundancy are the pathological characteristics of OA. Pain, muscle weakness, unstable joints, morning stiffness, and functional limitations are some of the clinical signs.[3] As the disease progresses, it has a significant impact on the patients’ quality of life and places a significant burden on society. Recent evidence has gradually acknowledged the fundamental function of the synovium in OA development. In the inflamed synovium, activated synovial cells produce catabolic and proinflammatory transmitters, which cause cartilage rupture and encourage osteophyte growth.[4] Chou et al[5] determined that synovial cells (not chondrocytes) in patients with OA produce inflammatory factors, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF), which, by single-cell sequencing, were delivered into the joint space, inducing cartilage degeneration. Therefore, further understanding of the role of the synovium in the development of knee OA is important for controlling the symptoms and slowing the structural degeneration of this joint disease.
Dixon et al[6] first proposed the novel concept of ferroptosis in 2012. Unlike cell death patterns such as necrosis, apoptosis, and autophagy, ferroptosis is an iron-dependent cell death pattern.[7] Ferroptosis is caused by specific genes and is characterized by increased mitochondrial membrane density, mitochondrial shrinkage, and the accumulation of iron and lipid peroxides.[8] Recently, the relationship between ferroptosis and OA has been studied. Low expression of glutathione peroxidase 4 (GPX4), an enzyme that converts phospholipid hydroperoxides to lipid alcohols using reduced glutathione (GSH), which inhibits ferroptosis, is associated with ferroptosis in chondrocytes of patients with OA.[9] Yao et al[10] demonstrated that chondrocytes undergo ferroptosis in an inflammatory environment in the presence of excess ferrous iron, which increases MMP13 expression and inhibits collagen II expression, ultimately inducing OA. Numerous studies have also demonstrated that the inhibition of chondrocyte ferroptosis effectively alleviates OA progression.[11–13]
Recent studies have clarified the role of synovium in OA pathogenesis. Clinical imaging observations have shown that synovial inflammation exists in the early and late stages of OA and affects disease progression. In contrast, various factors (such as trauma, bioactive lipids, and cytokines) can modulate synovial cells to produce inflammatory and anti-inflammatory mediators that promote cartilage damage and inducing OA.[14] In addition, Luo et al[12] discovered that ferroptosis in synovial cells might be involved in regulating OA. However, the mechanism underlying ferroptosis regulation in OA has mainly focused on chondrocytes and has not yet been clarified in synovial cells.
The purpose of this study was to investigate the function of ferroptosis regulators in the synovia of OA patients. To collect expression information for ferroptosis regulators, we combined 7 OA synovial datasets from the Gene Expression Omnibus (GEO) database. We performed a comprehensive evaluation of the role of ferroptosis regulators in OA diagnosis and subtype categorization. Based on the candidate ferroptosis regulators screening by random forest (RF), a nomogram model was developed and implemented using a RF screening model constructed to predict OA risk. Furthermore, we aimed to identify distinct ferroptosis patterns.
2. Materials and methods
2.1. Data of information and processing
Relevant datasets were collected in the GEO database (http://www.ncbi.nlm.nih.gov/geo/). The search details were designed as follows: (“osteoarthritis” [MeSH Terms] OR osteoarthritis [All Fields]) AND (“synovial membrane” [MeSH Terms] OR synovial membrane [All Fields]) AND “Homo sapiens” [porgn] AND (“gse” [Filter] AND “Expression profiling by array” [Filter]). Seven microarray datasets were found (GSE1919, GSE12021, GSE46750, GSE55235, GSE55457, GSE82107, and GSE89408), including 160 samples, comprising 79 OA and 81 healthy controls (HC; Table 1). To eliminate disparities among the research methods and platforms, the integrated dataset was batch-normalized using the limma package in the R software (version 4.1.3). We then extracted the expression matrix of 60 ferroptosis regulators[15] and examined their differential expression in OA and HC groups.
Table 1.
Datasets of OA microarray analysis included in this study.
| Number | Series | Platforms | OA | HC |
|---|---|---|---|---|
| 1 | GSE1919 | GPL91 | 5 | 5 |
| 2 | GSE12021 | GPL96 | 10 | 9 |
| 3 | GSE46750 | GPL10558 | 12 | 12 |
| 4 | GSE55235 | GPL96 | 10 | 10 |
| 5 | GSE55457 | GPL96 | 10 | 10 |
| 6 | GSE82107 | GPL570 | 10 | 7 |
| 7 | GSE89408 | GPL11154 | 22 | 28 |
OA = osteoarthritis.
2.2. Model building with support vector machines and random forests
The RF model combines the concepts of the bagging algorithm and randomized feature algorithm, and is an effective supervised learning method.[16] Support-vector machines (SVM) are supervised learning models that use associated learning algorithms to analyze data for classification and regression.[17] In this study, RF and SVM models were created using “RandomForest” and “Kernlab” packages respectively, and the prevalence of OA was predicted using candidate ferroptosis regulators among differentially expressed ferroptosis regulators. Ferroptosis regulators with top 5 mean decrease Gini index were used as candidate genes for follow-up analysis. The model was comprehensively evaluated by plotting “Reverse cumulative distribution of residuals,” “Boxplots of residuals” and a “receiver operating characteristic (ROC) curve.”
2.3. Establishing the nomogram model
To accurately predict the probability of each outcome, an integrated set of predictions based on unique patient features was combined into a nomogram model based on multivariate analysis.[18] To predict OA, we used the “regression modeling techniques” to build a nomogram model based on candidate ferroptosis regulators. We also developed a “calibration curve” to verify the correctness of the model, as well as a “decision analysis curve (DCA)” and “clinical impact curve” to determine whether decisions made using the model were beneficial for patients.
2.4. Consensus clustering analysis and principal component analysis
Consensus clustering is a method that provides quantitative analysis results for identifying possible subclasses in a dataset.[19] The “ConsensusClusterPlus” package provides the number of clusters and their respective stability.[20] Considering the considerable differences in the expression of ferroptosis regulators, consensus clustering analysis was used to distinguish between the various OA ferroptosis patterns by the “ConsensusClusterPlus” package in R. Dimensionality reduction was accomplished using principal component analysis (PCA) to check the reliability of clustering to quantify the ferroptosis pattern and further determine whether the clustering typology was correct.
2.5. Gene ontology functional enrichment analysis of differentially expressed genes between different ferroptosis pattern subtypes
Using the adjusted P < .05, logFC ≥ 1 as the threshold, the “limma” package in R (version 4.1.3) was used to identify DEGs between the distinct ferroptosis patterns. Gene ontology (GO) enrichment analysis of ferroptosis-related DEGs was performed using the online database DAVID. Enrichment results were visualized using a free online platform (https://www.bioinformatics.com.cn) for data analysis.
2.6. Immune cell infiltration analysis
To determine the ferroptosis score of immune cells in each sample and gauge their abundance in OA samples, single sample gene set enrichment analysis (ssGSEA) was performed using the “GSEABase” and “GSVA” programs. Furthermore, correlation tests were conducted to determine whether the expression of ferroptosis regulators correlated with immune cell infiltration.
2.7. Expression of the characteristic proinflammatory cytokines of OA
Inflammatory mediator secretion is crucial for dysfunctional processes involved in OA. IL-1β, TNF, IL-6, IL-15, IL-13, IL-18, IL-10, leukemia inhibitory factor, and chemokines (e.g., CCL5) are associated with OA and are potential therapeutic targets.[21] We further explored the expression of proinflammatory cytokines among the different subtypes of ferroptosis to clarify the role of ferroptosis patterns in OA.
2.8. Statistical analysis
R (version 4.1.3) was used for all statistical analysis. The Wilcoxon test was used to compare the differences between the groups. The area under the ROC curve (AUC) was calculated and visualized using the “pROC” package in R. When a two-tailed test was used to assess all parameters, the results were considered statistically significant at P < .05.
3. Results
3.1. Expression of 60 ferroptosis regulators in osteoarthritis
Wilcoxon test was used to analyze the expression of 60 ferroptosis regulators in the synovial tissues of OA and HC. Eleven ferroptosis regulators (ALOX5, CS, GLS2, GSS, CRYAB, SLC7A11, PHKG2, FADS2, SLC1A5, GOT1, and IREB2) were identified. Compared to HC, ALOX5, GLS2, GSS, SLC1A5, and IREB2 were upregulated in OA, whereas CS, CRYAB, SLC7A11, PHKG2, FADS2, and GOT1 were downregulated (Fig. 1A and B). The positions of the significant ferroptosis regulators on the chromosomes were shown in Figure 1C.
Figure 1.
Differential expression of ferroptosis regulators in synovial tissue of OA vs HC. (A) Box plot of differential expression of ferroptosis regulators. (B) Heatmap showed differential expression of ferroptosis regulators. (C) Location of significant ferroptosis regulators in chromosomes. *P < .05, **P < .01, ***P < .001. OA = osteoarthritis.
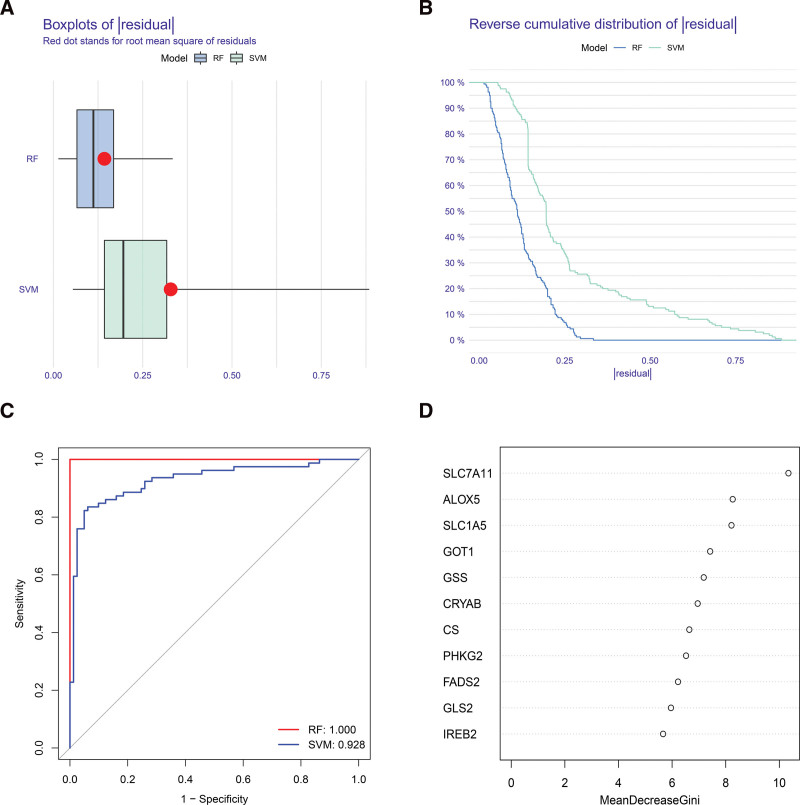
3.2. Model building with support-vector machines and random forests
The occurrence of OA was predicted by RF and SVM models using differentially expressed ferroptosis regulators. The residuals of the RF model are smaller than those of the SVM model, according to both the “Boxplots of residual” (Fig. 2A) and the “Reverse cumulative distribution of residual” (Fig. 2B), suggesting that the prediction of OA occurrence using the RF model is of greater significance. Therefore, in this study, the RF model was identified as the most effective for predicting OA occurrence. Similarly, the ROC curve (Fig. 2C) showed that it was more accurate than the SVM model. Subsequently, we ranked differentially expressed ferroptosis regulators based on their importance (Fig. 2D). In our research, the top 5 genes by mean reduction in Gini index were SLC7A11, ALOX5, SLC1A5, GOT1, and GSS which were used as candidate genes for subsequent analysis.
Figure 2.
Construction of the RF model. (A) Boxplot exhibited the residuals of the RF and the SVM models. (B) Reverse cumulative distribution of residuals displayed the distribution of residuals between the RF model and the SVM models. (C) ROC curves of the RF and SVM models. (D) Ranking of the 11 significant ferroptosis factors according to importance. RF = random forest, ROC = receiver operating characteristic, SVM = support vector machine.
3.3. Establishment of the nomogram model
Scoring of 5 candidate ferroptosis regulators using a nomogram model to determine the OA scores (Fig. 3A). The prevalence of OA was predicted on the basis of these scores. Calibration curves showed the high accuracy of this nomogram model for diagnosing OA (Fig. 3B). As shown in the DCA plot, the gray and black lines are consistently below the red line in the range 0 to 1. These results suggested that the nomogram model appeared to be a useful decision-making tool for assessing OA (Fig. 3C). The clinical effect curve (Fig. 3D) also demonstrated the predictive power of the nomogram model.
Figure 3.
Construction of the nomogram model. (A) Nomogram model created using candidate genes. (B) The calibration curve of the nomogram model, which indicated the high accuracy of the model. (C) DCA curves, which indicated that the nomogram model had a decision-making role in the predictive assessment of OA. (D) Clinical impact curves were employed to evaluate the nomogram model’s clinical impact. DCA = decision analysis curve.
3.4. Identification of distinct ferroptosis patterns
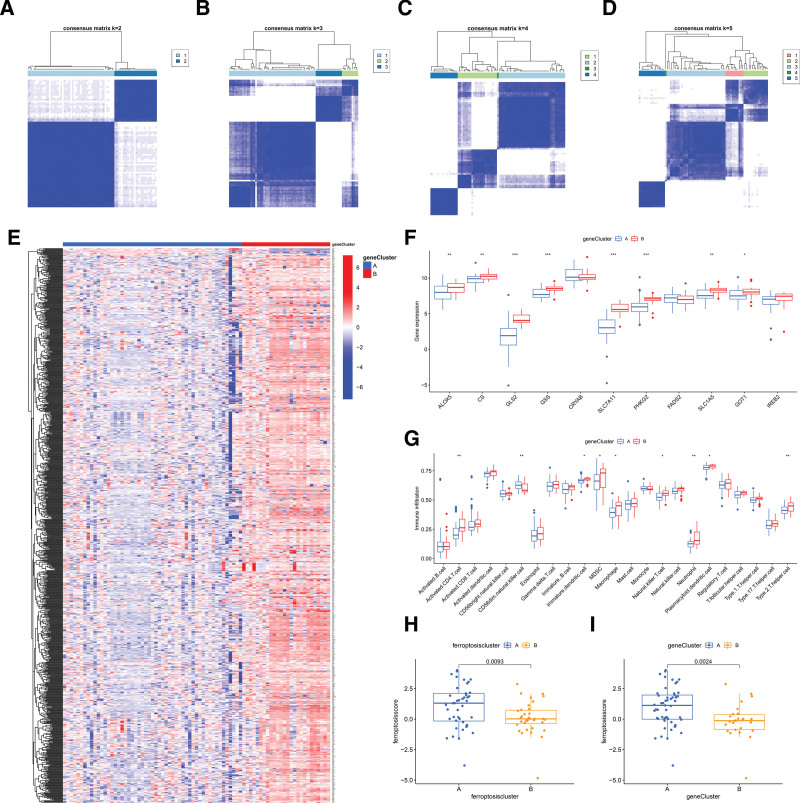
To investigate the patterns classification of ferroptosis patterns, 11 significant ferroptosis regulators (Fig. 4A–D) were used to separated OA into 2 subtypes (Clusters A and B) using the consensus clustering method. There were 44 and 35 samples in Clusters A and B, respectively. In these 2 completely different ferroptosis patterns, the expression of ferroptosis regulators (ALOX5, CS, GLS2, GSS, SLC7A11, PHKG2, SLC1A5, and GOT1) was higher in Cluster B than in Cluster A. There were no differences in CRYAB, FADS2, or IREB2 between the 2 patterns. (Fig. 4E and F). PCA of these OA ferroptosis patterns showed significant differences between the 2 patterns distinguished by these significant ferroptosis regulators (Fig. 4G). We subsequently identified 901 DEGs between the 2 ferroptosis patterns. According to GO function enrichment analyses, these DEGs were primarily associated with immunity (Fig. 4H); for instance, GO:0006954 (inflammatory response), GO:0070098 (chemokine-mediated signaling pathway), and GO:0030593 (neutrophil chemotaxis).
Figure 4.
Consensus clustering analysis of significant ferroptosis regulators. (A–D) Consensus matrices of the 11 significant ferroptosis regulators for k = 2–5. (E) Heatmap of the expression of ferroptosis regulators in different ferroptosis patterns. (F) Boxplot of ferroptosis regulators in different ferroptosis subtypes. (G) Principal component analysis revealed significant differences in the transcriptome between the 2 ferroptosis patterns. (H) Enrichment circle diagram displayed the functional enrichment of ferroptosis-related DEGs between Cluster A and Cluster B. *P < .05, **P < .01, ***P < .001.
3.5. Analysis of immune cell infiltration
To estimate the immune cells in OA tissues and assess the relationship between 22 immune cells and 11 significant ferroptosis regulators, we applied ssGSEA for further validation. The results demonstrated that the expression of ALOX5, which encodes a key enzyme that mediates lipid peroxidation reactions,[22] was positively correlated with the levels in many immune cells (Fig. 5A). We compared immune cell infiltration in OA with high and low expression of ALOX5. The results showed that immune cell types were upregulated in patients with high ALOX5 expression than in patients with low ALOX5 expression (Fig. 5B). Finally, immune cell infiltration by various ferroptosis patterns was analysed. Compared with Cluster A, immune cells (activated CD4 + T cell, activated dendritic cell, etc.) were increased in Cluster B (Fig. 5C).
Figure 5.
ssGSEA enrichment analysis. (A) Heat map of expression of 11 significant ferroptosis regulators correlated with 22 immune cells. (B) Boxplot of the difference in immune cells abundance between high and low ALOX5 expression groups. (C) Boxplot of the difference in immune cells abundance between Cluster A and Cluster B. *P < .05, **P < .01, ***P < .001. ssGSEA = single sample gene set enrichment analysis.
3.6. Identification of distinct ferroptosis gene patterns and generation of the ferroptosis gene signature
To further verify the pattern of ferroptosis patterns, 901 ferroptosis-related DEGs were used to classify patients with OA into several genetic subtypes. Consistent with the typing of ferroptosis patterns, there were 2 completely different ferroptosis gene patterns, gene Cluster A and gene Cluster B (Fig. 6A–D). A heatmap of the expression levels of 901 ferroptosis-related DEGs in gene Clusters A and B was shown in Figure 6E. Figure 6F and G revealed that the immune cell infiltration level and the expression of 11 significant ferroptosis regulators between gene Cluster A and gene Cluster B were similar to ferroptosis patterns, which indicated the correctness of the grouping. To quantify ferroptosis patterns, we calculated the corresponding ferroptosis scores for each sample between the different ferroptosis patterns and ferroptosis gene patterns using the PCA algorithm. The results demonstrated that both Cluster A and gene Cluster A had higher ferroptosis scores than Cluster B and gene Cluster B (Fig. 6H and I), indicating that Cluster A was more closely related to ferroptosis than Cluster B. We used a Sankey diagram to show relationships between ferroptosis scores, ferroptosis gene patterns, and ferroptosis patterns. (Fig. 7A).
Figure 6.
Consensus cluster analysis of 901 ferroptosis-related DEGs in OA. (A–D) Consensus matrices for the 11 significant ferroptosis-related genes for k = 2–5. (E) Heatmap showed 901 ferroptosis-related DEGs involving gene Clusters A and B. (F) Boxplot displayed the expression of 11 significant ferroptosis regulators in gene Clusters A and B. (G) Immune cell infiltration between gene Clusters A and B. (H) Differences in ferroptosis scores between Clusters A and B. (I) Differences in ferroptosis score between gene Clusters A and B. *P < .05, **P < .01, ***P < .001.
Figure 7.
The role of ferroptosis patterns in distinguishing OA. (A) Sankey diagram illustrated the connection between the ferroptosis score, ferroptosis gene patterns, and ferroptosis patterns. (B) Differential expression of proinflammatory cytokines between Clusters A and B. (C) Differential expression of proinflammatory cytokines between gene Clusters A and B. *P < .05, **P < .01, ***P < .001. OA = osteoarthritis.
3.7. The role of ferroptosis patterns in distinguishing OA
We investigated the correlation between ferroptosis patterns and the proinflammatory cytokines associated with OA (IL-1β, TNF, IL-6, IL-15, IL-18, LIF, CCL5, IL-10, and IL13) in order to further reveal the relationship between these 2 conditions. Figure 7B and C showed that the expression levels of proinflammatory cytokines were higher in cluster B or gene cluster B than those in cluster A or gene cluster A, which indicated that cluster B or gene cluster B is linked to OA characterized by immune response.
4. Discussion
OA is one of the most prevalent causes of joint pain and disability in older patients. Ferroptosis is associated with OA. However, the mechanism by which ferroptosis in the synovium affects OA remains unknown.[14] We identified 11 significant ferroptosis regulators in the synovial membranes of OA patients. Five ferroptosis regulators (SLC7A11, ALOX5, SLC1A5, GOT1, and GSS) were screened using RF models to predict OA occurrence. A nomogram model was constructed using these 5 significant ferroptosis regulators, and both DCA and clinical impact curves indicated that the model has unique clinical diagnostic advantages.
SLC7A11, a member of solvate carrier family 7, is believed to be the primary cause of ferroptosis. SLC7A11 alleviates lipid reactive oxygen species (ROS) stress by mediating cysteine synthesis by GSH and activating the essential enzyme GPX4, which reduces lipid hydroperoxides.[23] Luo et al[12] found low expression of SLC7A11 in lipopolysaccharide-induced synovial cells compared with untreated synovial cells, suggesting that SLC7A11-mediated ferroptosis in synovial cells was involved in OA. Glutamic oxaloacetic transaminase 1 (GOT1) knockdown results in impaired mitochondrial oxidative phosphorylation and promotes a catabolic state. GOT1 cooperates with inhibitors of lipid antioxidant mechanisms and cysteine import to promote ferroptosis. Thus, the destabilized iron pool and cell susceptibility to ferroptosis are increased by suppression of GOT1.[24] Solute carrier family 1 member 5 (SLC1A5) is a cell surface transporter that mediates glutamine uptake. The uptake and metabolism of glutamine results in the formation of lipid ROS and promotes cellular ferroptosis.[25] Cystine (Cys2) is oxidized to cysteine (Cys), which is utilized by glutathione synthetase (GSS) to produce glutathione.[26] If the GSH-dependent ability of GPX4 to convert lipid peroxides to lipid alcohols is lower than the iron-dependent accumulation of lipid ROS, cellular ferroptosis occurs.[27] Thus, GSS may be involved in ferroptosis by affecting GSH production, thereby regulating lipid ROS accumulation. The key enzyme ALOX5 mediates lipid peroxidation by generating lipid peroxides.[22] Lipid peroxidation is an important feature of ferroptosis.[28] According to prior research, ALOX5 is not only a ferroptosis target, but inhibition of ALOX5 can also prevent ferroptosis.[29] Another important aspect of the involvement of ALOX5 in ferroptosis is that ALOX5 is an iron-containing enzyme and iron accumulation often occurs during ferroptosis.[30] We first identified 3 up-regulated candidate ferroptosis regulators (ALOX5, GOT1, and GSS) and 2 down-reguated candidate ferroptosis regulators (SLC7A11 and SLC1A5), which collaborated with each other was crucial for predicting the occurrence of OA. However, recent studies have not clarified the relationship between these 5 ferroptosis regulators and OA. In this study, we aimed to provide new insights on how these ferroptosis regulators function in the synovial membranes of OA patients.
Based on the 11 significant ferroptosis regulators, we distinguished 2 functionally distinct subtypes of OA in ferroptosis: Clusters A and B. The ferroptosis score of Cluster A was higher than that of Cluster B, suggesting that Cluster A was more closely related to the ferroptosis. The levels of immune infiltration and proinflammatory cytokines (IL-1β, TNF, IL-6, IL-15, IL-18, and IL-10) were higher in Cluster B than in Cluster A suggesting that Cluster B may be involved in immunity.
Iron levels in the synovium have been linked to OA. He et al[31] found that synovial tissues from OA contained iron deposits, whereas normal synovial tissues from healthy controls did not. In addition, hemosiderin and iron levels were higher in the synovium and synovial fluid, respectively, in patients.[32] However, it is uncertain whether ferroptosis occurs in the synovium of OA patients, causing inflammation, cartilage degeneration, and other pathological changes. The role of the synovium in OA should be studied further in the future.
It has been shown that the presence of proinflammatory cytokines in the joint tissues of patients with OA can lead to pathological inflammation. These cytokines promote the development of metabolic disorders.[21] The 2 main cytokines that promote inflammation in OA are IL-1β and TNF, which are mainly produced by the synovial tissue. The expression levels of IL-1β and TNF are increased in synovial tissue and synovial fluid during OA, thereby inducing increased production of proinflammatory mediators and catabolic substances.[21] When IL-1β and TNF are triggered by chondrocytes, proteolytic enzymes are generated, which can damage the cartilage and exacerbate the inflammatory response. Additionally, IL-1β and TNF cause ROS synthesis and exacerbates cartilage deterioration.[33]
IL-6 is a crucial proinflammatory cytokine associated with OA and is involved in cartilage degradation and inflammation.[34] In the synovial fluid of patients with OA, IL-6 is elevated and plays a vital role in reducing expression of type II collagen.[21] IL-18, a member of the IL-1 family, is highly expressed in the synovial fluid. IL-18 induces a synovial inflammatory response and promotes the production of MMP1, MMP-3, MMP-13, and proinflammatory cytokines, thereby inducing chondrocyte apoptosis and autophagy.[35] Unlike the proinflammatory cytokines described above, IL-10 has a chondroprotective role. IL-10 can protect the cartilage by downregulating the expression of matrix metalloproteinases and inhibiting chondrocyte apoptosis.[36] However, another study found that cytokines and MMPs in synovial fibroblasts, which are associated with OA, were not affected by IL-10 induction, but the combined induction of IL-10 and TNF-α increased cytokine and MMPs expression in synovial fibroblasts.[37] Therefore, the role of IL-10 in the synovium of patients requires further exploration. In addition, our study found that 901 ferroptosis-related DEGs in gene Clusters A and B were mainly enriched in immune-related biological processes, which verifies the reliability of the above results.
5. Conclusions
In this study, 5 candidate regulators of ferroptosis were identified. A nomogram model for predicting OA occurrence was established, which has been beneficial for patients with OA. Consensus cluster analysis divided OA patients into 2 ferroptosis patterns, Cluster A and Cluster B, where Cluster A associated with ferroptosis, and Cluster B was associated with immunity. These findings may help to develop a potential treatment strategy for OA.
Author contributions
Conceptualization: JinFu Liu.
Data curation: Lihua Chen, Bo Xiong, GuanYu Lu, Cai Chen.
Formal analysis: Bo Xiong, Cai Chen.
Writing – original draft: Yue Huang, JinFu Liu.
Writing – review & editing: JinFu Liu.
Abbreviations:
- DAVID
- Database for Annotation, Visualization and Integrated Discovery
- GEO
- Gene Expression Omnibus
- GO
- gene ontology
- GOT1
- glutamic oxaloacetic transaminase 1
- GPX4
- glutathione peroxidase 4
- GSH
- glutathione
- GSS
- glutathione synthetase
- IL-1β
- interleukin-1β
- OA
- osteoarthritis
- PCA
- principal component analysis
- RF
- random forest
- ROC
- receiver operating characteristic
- ROS
- reactive oxygen species
- SLC1A5
- solute carrier family 1 member 5
- ssGSEA
- single sample gene set enrichment analysis
- SVM
- support vector machine
- TNF
- tumor necrosis factor
This work was supported by the Middle-aged and Young Teachers’ Basic Ability Promotion Project of Guangxi (2022KY0282) and Project of Guangxi University of Traditional Chinese Medicine (XYJ21127, XYJ21128).
The authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors and participants of this article were informed of the content of the article, signed a written informed consent form, and agreed to its publication.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Huang Y, Chen L, Xiong B, Lu G, Chen C, Liu J. Integrating multiple microarray datasets to explore the significance of ferroptosis regulators in the diagnosis and subtype classification of osteoarthritis. Medicine 2023;102:45(e35917).
Contributor Information
Yue Huang, Email: huangyue037@163.com.
Lihua Chen, Email: chenc19941016@163.com.
Bo Xiong, Email: xionby@163.com.
GuanYu Lu, Email: 18107794322@163.com.
Cai Chen, Email: chenc19941016@163.com.
References
- [1].Lv Z, Yang YX, Li J, et al. Molecular classification of knee osteoarthritis. Front Cell Dev Biol. 2021;9:725568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goff AJ, Elkins MR. Knee osteoarthritis. J Physiother. 2021;67:240–1. [DOI] [PubMed] [Google Scholar]
- [3].Dantas LO, Salvini TF, McAlindon TE. Knee osteoarthritis: key treatments and implications for physical therapy. Brazilian J Phys Ther. 2021;25:135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthr Res Ther. 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chou CH, Jain V, Gibson J, et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci Rep. 2020;10:10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li Y, Feng D, Wang Z, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miao Y, Chen Y, Xue F, et al. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine. 2022;76:103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yao X, Sun K, Yu S, et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J Orthop Transl. 2021;27:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo Z, Lin J, Sun K, et al. Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway. Front Pharmacol. 2022;13:791376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Luo H, Zhang R. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis. Exp Ther Med. 2021;21:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan J, Feng G, Ma L, et al. Metformin alleviates osteoarthritis in mice by inhibiting chondrocyte ferroptosis and improving subchondral osteosclerosis and angiogenesis. J Orthop Surg Res. 2022;17:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sanchez-Lopez E, Coras R, Torres A, et al. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liang JY, Wang DS, Lin HC, et al. A novel ferroptosis-related gene signature for overall survival prediction in patients with hepatocellular carcinoma. Int J Biol Sci. 2020;16:2430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kamińska JA. A random forest partition model for predicting NO(2) concentrations from traffic flow and meteorological conditions. Sci Total Environ. 2019;651(Pt 1):475–83. [DOI] [PubMed] [Google Scholar]
- [17].Huang S, Cai N, Pacheco PP, et al. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Gen Proteomics. 2018;15:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang L, Wei S, Zhou B, et al. A nomogram model to predict the venous thromboembolism risk after surgery in patients with gynecological tumors. Thromb Res. 2021;202:52–8. [DOI] [PubMed] [Google Scholar]
- [19].Brière G, Darbo E, Thébault P, et al. Consensus clustering applied to multi-omics disease subtyping. BMC Bioinf. 2021;22:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang Y, Liu N, Wang S. A differential privacy protecting K-means clustering algorithm based on contour coefficients. PLoS One. 2018;13:e0206832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- [22].Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin W, Wang C, Liu G, et al. SLC7A11/xCT in cancer: biological functions and therapeutic implications. Am J Cancer Res. 2020;10:3106–26. [PMC free article] [PubMed] [Google Scholar]
- [24].Kremer DM, Nelson BS, Lin L, et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun. 2021;12:4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yao X, Li W, Fang D, et al. Emerging roles of energy metabolism in ferroptosis regulation of tumor cells. Adv Sci (Weinh). 2021;8:e2100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen X, Kang R, Kroemer G, et al. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96. [DOI] [PubMed] [Google Scholar]
- [27].Jin M, Shi C, Li T, et al. Solasonine promotes ferroptosis of hepatoma carcinoma cells via glutathione peroxidase 4-induced destruction of the glutathione redox system. Biomed Pharmacother. 2020;129:110282. [DOI] [PubMed] [Google Scholar]
- [28].Zhang HL, Hu BX, Li ZL, et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24:88–98. [DOI] [PubMed] [Google Scholar]
- [29].Shan K, Feng N, Zhu D, et al. Free docosahexaenoic acid promotes ferroptotic cell death via lipoxygenase dependent and independent pathways in cancer cells. Eur J Nutr. 2022;61:4059–75. [DOI] [PubMed] [Google Scholar]
- [30].Sun QY, Zhou HH, Mao XY. Emerging roles of 5-Lipoxygenase phosphorylation in inflammation and cell death. Oxid Med Cell Longevity. 2019;2019:2749173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen H, Wang B, Chen X, et al. [Effect of body mass index on short-term effectiveness of high tibial osteotomy in treatment of varus knee arthritis]. Zhongguo Xiufu Chongjian Waike Zazhi. 2023;37:670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cai C, Hu W, Chu T. Interplay between iron overload and osteoarthritis: clinical significance and cellular mechanisms. Front Cell Dev Biol. 2021;9:817104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liao CR, Wang SN, Zhu SY, et al. Advanced oxidation protein products increase TNF-α and IL-1β expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2020;28:101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu JF, Chi MC, Lin CY, et al. PM25 facilitates IL-6 production in human osteoarthritis synovial fibroblasts via ASK1 activation. J Cell Physiol. 2021;236:2205–13. [DOI] [PubMed] [Google Scholar]
- [35].Bao J, Chen Z, Xu L, et al. Rapamycin protects chondrocytes against IL-18-induced apoptosis and ameliorates rat osteoarthritis. Aging (Milano). 2020;12:5152–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Watkins LR, Chavez RA, Landry R, et al. Targeted interleukin-10 plasmid DNA therapy in the treatment of osteoarthritis: toxicology and pain efficacy assessments. Brain Behav Immun. 2020;90:155–66. [DOI] [PubMed] [Google Scholar]
- [37].Mrosewski I, Jork N, Gorte K, et al. Regulation of osteoarthritis-associated key mediators by TNFα and IL-10: effects of IL-10 overexpression in human synovial fibroblasts and a synovial cell line. Cell Tissue Res. 2014;357:207–23. [DOI] [PubMed] [Google Scholar]