Abstract
This study evaluated the therapeutic effects and toxic reactions of combining transcatheter arterial chemoembolization (TACE) and intensity-modulated radiotherapy (IMRT) with sorafenib for the treatment of advanced hepatocellular carcinoma (HCC) patients with macrovascular invasion (MVI). We retrospectively analyzed the clinical data of 82 HCC patients with MVI, among whom 35 were treated with TACE plus IMRT alone, and 47 were treated with the combined therapy of TACE, IMRT, and sorafenib. The progression-free survival (PFS), overall survival (OS), and adverse events were assessed. The baseline characteristics were comparable between the 2 groups (all P > .05). In the TACE plus IMRT plus sorafenib group, the median PFS was 17.2 months (95% confidence interval, 14.1–19.9), significantly longer than the 9.4 months (95% confidence interval, 6.8–11.2) observed in the TACE plus IMRT group (P < .001). Additionally, patients treated with the TACE plus IMRT plus sorafenib showed a longer median OS than those treated with TACE plus IMRT alone (24.1 vs 17.3 months; P < .001). The occurrence rates of grade 1 to 2 hand-foot syndrome, other skin reactions, diarrhea, and hair loss were higher in the TACE plus IMRT plus sorafenib group (all P < .05). There were no grade 4 or higher adverse events in either group. The combination of TACE plus IMRT with sorafenib provided substantial clinical benefits in the treatment of HCC patients with MVI, increasing the tumor response rate and prolonging both PFS and OS. This approach demonstrated a tolerable and manageable safety profile.
Keywords: hepatocellular carcinoma (HCC), intensity-modulated radiotherapy (IMRT), macrovascular invasion (MVI), sorafenib, transcatheter arterial chemoembolization (TACE)
1. Introduction
Hepatocellular carcinoma (HCC) is a global health concern, with approximately half of all newly diagnosed cases occurring in China. Early-stage HCC can often be treated with surgical techniques such as liver transplantation, radical resection, and radiofrequency ablation.[1] Unfortunately, the majority of HCC patients are diagnosed at advanced stages, and around 20% present with macrovascular invasion (MVI) at the time of diagnosis.[2] These advanced cases, particularly those with MVI, have a poor prognosis, with median overall survival (OS) times of only 3 to 5 months when treated with best supportive care.[3,4]
Currently, sorafenib stands as the only approved first-line drug with proven efficacy for the treatment of locally advanced HCC patients with MVI. However, its effectiveness is limited, extending the median survival time by only 47 days compared to the placebo group in key phase 3 clinical trials.[5,6] The overall response rate and survival benefits of sorafenib are still far from satisfactory, and MVI is considered a poor prognostic factor for HCC patients treated with this drug.[7]
In recent years, locoregional therapies like transcatheter arterial chemoembolization (TACE) and external-beam radiotherapy (RT) have shown promising results in advanced HCC patients with MVI. This includes remarkable remission rates, durable local control, and extended survival times, whether applied alone or in combination with systemic treatments.[8–13] Specifically, unresectable HCC patients with MVI, possessing good liver function and well-developed periportal collateral circulation, may achieve substantial clinical benefits from TACE or combined TACE plus RT treatment. Moreover, the administration of sorafenib in conjunction with TACE and/or RT has demonstrated encouraging clinical outcomes for patients with locally advanced HCC.[14–16]
Despite these advancements, there remains a lack of clarity regarding the differences in clinical effects and adverse reactions between various treatment strategies. In response, our study aims to compare the therapeutic effects and toxic reactions of TACE plus intensity-modulated radiotherapy (IMRT) in combination with sorafenib versus TACE plus IMRT alone in advanced HCC patients with MVI.
2. Methods
2.1. Study design and patients
The clinical records of advanced-stage hepatocellular carcinoma (HCC) patients with macrovascular invasion (MVI), who received either TACE plus IMRT combined with sorafenib or TACE plus IMRT alone, were retrospectively collected from the Department of Gastroenterology, 3201 Hospital, Hanzhong 723000, Shaanxi, China, between July 2017 and July 2020. Inclusion criteria were: (1) age ≥ 18 years; (2) Eastern Cooperative Oncology Group (ECOG) performance score ≤ 2; (3) diagnosis of primary HCC combined with MVI through biopsy and/or imaging, and no prior receipt of anticancer treatment; (4) Child-Pugh grade A; (5) unresectable tumor status; (6) a simple nodular lesion or confluent multinodular lesions that could be considered a single lesion for the RT field; (7) leukocyte count ≥ 3000/mL; absolute neutrophil count ≥ 1500/mL; hemoglobin level ≥ 90 g/L; platelet count (PLT) ≥ 80 × 109/L; aspartate aminotransferase and alanine aminotransferase levels < 2.5 times the upper normal limit; bilirubin levels < 2 times the upper normal limit; and a prothrombin time-international normalized ratio < 1.5, except if the patients were on oral anticoagulation. Exclusion criteria were: (1) indications of extrahepatic metastasis via chest, abdomen CT scan, and/or whole-body bone scan; (2) previous history of abdominal radiotherapy. The study was approved by the Ethics Committee of 3201 Hospital, and informed consent was waived due to the retrospective nature of the design.
2.2. Treatment strategies
Before initiating TACE treatment, the systemic status of the patient was evaluated, and fasting was conducted. Sedative drugs were administered as necessary, and an iodine allergy test was performed if required. The right femoral artery was punctured using the Seldinger technique, and a 5-F RH catheter (Cook, IN) was selectively inserted into the hepatic artery. Digital subtraction angiography was used to assess tumor size, location, vascular distribution, feeding arteries, and to identify the target vessel. A microcatheter (Renegade, Boston Scientific, MA; Progreat, Terumo, Tokyo, Japan) was selectively inserted into the target vessel for superselective chemoembolization of vascular perfusion. Chemotherapy drugs included 1000 mg of 5-fluorouracil and 10 to 20 mg of mitomycin, with an embolization agent of 10 to 20 mL ultra-fluid lipiodol, and a 1 to 2 mm gelatin sponge used for hepatic artery embolization if needed. The interval between TACE treatments was 4 to 6 weeks, contingent on response.[17]
Following 4 to 6 weeks of TACE completion, patients commenced IMRT treatment.[18] The patient was fixated in the treatment position using an anthropomorphic phantom, and an enhanced CT scan was performed under calm breathing, covering the liver tissue and areas within 5 cm above and below the liver. Concurrently, an MRI scan was also executed under the same positioning conditions. The imaging results were transmitted to the MasterPlan treatment planning system, where CT and MRI images were fused to outline the gross tumor volume and the organs at risk. The gross tumor volume was expanded into the planning target volume, with expansion ranges varying based on tumor diameter. Following delineation, the prescription dose and the limited dose of organs at risk were entered. Treatment details, including divided dose, number of exposures, treatment duration, and biological effect dose, were specified, with a dose volume histogram applied for plan evaluation. During the IMRT treatment period, routine hepatic protection and supportive treatments were administered, and blood and liver function tests were conducted weekly.
For patients receiving TACE + IMRT plus sorafenib, continuous standard doses of oral sorafenib (400 mg twice daily) were administered before, during, or after IMRT treatment. If grade 3 or higher toxic reactions occurred, sorafenib was withdrawn until symptoms were reduced to grade 1 or 2, then cautiously escalated. Sorafenib treatment was maintained until clinical or radiological progression, death, or occurrences of specific toxicities or patient request, with a criterion for discontinuation being total bilirubin > 3 mg/dL 4 weeks after cessation of treatment.[19]
2.3. Therapeutic effect evaluation
The evaluation of tumor response was conducted according to the Modified Response Evaluation Criteria in Solid Tumors for HCC, and was assessed 3 months after the completion of IMRT. Responses were categorized as either complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).[20] CT or MRI scans were utilized for this therapeutic effect evaluation. Progression-free survival (PFS) was determined from the date of IMRT or sorafenib administration to the date of disease progression or the date of the last disease assessment. OS was calculated from the day of IMRT or sorafenib administration to the date of the final follow-up or death, with no patients lost to follow-up.
2.4. Follow-up and evaluation of adverse events
Follow-up was conducted every 4 weeks through regular outpatient clinic visits. These visits included a physical examination, assessment of performance status (ECOG), routine blood tests, evaluation of biochemical indicators, liver biochemistry, blood coagulation, alpha-fetoprotein level (AFP), chest radiography, and abdominal CT or MRI examinations. B-ultrasound or CT examinations were utilized to detect any occurrences of tumor progression. Adverse events (AEs) were observed and recorded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Additionally, patients were specifically evaluated for the presence of radiation-induced liver disease (RILD) 4 months after the completion of IMRT.[20,21]
2.5. Statistical analysis
The distribution of continuous data was assessed using the Kolmogorov–Smirnov test. Normally distributed data were presented as the mean ± standard deviation (SD) and analyzed using the Student t test. Non-normally distributed data are presented as the median (range) and were analyzed using the Mann–Whitney U test. Categorical variables were presented as frequencies and analyzed using Fisher exact test. Survival analysis (OS and PFS) was carried out using the Kaplan–Meier method, with the Log-rank test for comparisons. A Cox proportional hazards regression model was further conducted to analyze the association between survival and potential risk factors, including the calculation of hazard ratios (HR) and 95% confidence intervals (CIs). All analyses were performed using SPSS 17.0 (IBM, Armonk, NY). A two-sided P value of <0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics of advanced HCC patients with MVI
A total of 82 patients diagnosed with advanced HCC and MVI met the inclusion and exclusion criteria for the study. Among them, 35 patients were treated with the combination of TACE, IMRT, and sorafenib (n = 35), while the remaining 47 patients received TACE and IMRT alone (n = 47). All the included patients had liver function graded as Child-Pugh class A.
The baseline characteristics, including age, gender, ECOG score, tumor size and number, portal vein tumor thrombosis (PVTT), hepatic vein tumor thrombosis, tumor stage, AFP levels, and viral hepatitis infection, were comparable between the 2 groups (all P > .05), as shown in Table 1.
Table 1.
Baseline characteristics of patients in TACE plus IMRT plus sorafenib group versus TACE plus IMRT group.
| Characteristics | TACE plus IMRT plus sorafenib (n = 35) | TACE plus IMRT (n = 47) | P |
|---|---|---|---|
| Age (year) | 56.2 (36–72) | 55.5 (35–80) | .624 |
| Male | 29 (82.9%) | 41 (87.2%) | .579 |
| Tumor size (cm) | 7.3 (2.0–15.3) | 7.5 (1.6–17.0) | .921 |
| Tumor number | .926 | ||
| Single | 19 (54.3%) | 26 (55.3%) | |
| Multiple | 16 (45.7%) | 21 (44.7%) | |
| PVTT | 31 | 39 | .372 |
| Vp2 | 3 (9.7%) | 2 (5.1%) | |
| Vp3 | 14 (45.2%) | 24 (61.5%) | |
| Vp4 | 14 (45.2%) | 13 (33.3%) | |
| HVTT | |||
| Yes | 7 (20%) | 9 (19.1%) | .923 |
| No | 28 (80%) | 38 (80.9%) | |
| T stage (T3b/T4) | .573 | ||
| T3b | 33 (94.3%) | 46 (97.9%) | |
| T4 | 2 (5.7%) | 1 (2.1%) | |
| N stage | .221 | ||
| N0 | 27 (77.1%) | 42 (89.4%) | |
| N1 | 8 (22.9%) | 5 (10.6%) | |
| Underlying disease | .471 | ||
| HBV | 31 (88.6%) | 39 (83.0%) | |
| HCV | 1 (2.9%) | 4 (8.5%) | |
| No | 3 (8.6%) | 4 (8.5%) | |
| AFP (ng/L) | .978 | ||
| ≤400 | 20 (57.1%) | 27 (57.4%) | |
| >400 | 15 (42.9%) | 20 (42.6%) | |
| ECOG | .999 | ||
| ≤1 | 33 (94.3%) | 45 (95.7%) | |
| >1 | 2 (5.7%) | 2 (4.3%) |
Data are presented as median (range) or n (%) unless otherwise indicated.
AFP = alpha-fetoprotein, ECOG = Eastern Cooperative Oncology Group, HBV = hepatitis B virus, HCV = hepatitis C virus, HVTT = hepatic vein tumor thrombosis, IMRT = intensity-modulated radiotherapy, PVTT = portal vein tumor thrombosis, TACE = transcatheter arterial chemoembolization.
3.2. Tumor response and failure pattern
As outlined in Table 2, in the group of 35 patients treated with TACE plus IMRT in combination with sorafenib, 3 patients (8.6%) achieved a CR, 13 patients (37.1%) achieved a PR, 11 patients (31.4%) exhibited SD, and the remaining 8 patients (22.9%) had PD. In contrast, among the 47 patients treated with TACE plus IMRT alone, 21 patients (44.7%) achieved PR, 15 patients (31.9%) showed SD, and the other 11 patients (23.4%) had PD.
Table 2.
Comparisons of tumor responses between the treatment groups.
| Tumor response | TACE plus IMRT plus sorafenib (n = 35) | TACE plus IMRT (n = 47) | P |
|---|---|---|---|
| CR | 3 (8.6%) | 0 | .074 |
| PR | 13 (37.1%) | 21 (44.7%) | .493 |
| SD | 11 (31.4%) | 15 (31.9%) | .963 |
| PD | 8 (22.9%) | 11 (23.4%) | .954 |
| ORR (CR + PR) | 16 (45.7%) | 21 (44.7%) | .926 |
| DCR (CR + PR + SD) | 27 (77.1%) | 36 (76.6%) | .954 |
CR = complete response, DCR = disease control rate, IMRT = intensity-modulated radiotherapy, ORR = overall response rate, PD = progressive disease, PR = partial response, SD = stable disease, TACE = transcatheter arterial chemoembolization.
Twenty-two failures (62.9%) were observed in the group treated with TACE plus IMRT in combination with sorafenib, compared to 34 failures (72.3%) in the TACE plus IMRT group. Specifically, in the TACE plus IMRT in combination with sorafenib group, local progression within the RT field occurred in 4 patients (11.4%), intrahepatic metastases or new lesions outside the RT field were detected in 10 patients (28.6%), and extrahepatic failures (including distant metastases) were observed in 10 patients (28.6%). In the TACE plus IMRT group, local progression within the RT field occurred in 7 patients (14.9%), intrahepatic metastases or new lesions outside the RT field were detected in 27 patients (57.4%), and extrahepatic failures (including distant metastases) were observed in 12 patients (25.5%). Notably, patients receiving TACE plus IMRT in combination with sorafenib experienced fewer intrahepatic metastases outside the RT field compared to those receiving TACE plus IMRT alone (P = .018).
3.3. Survival outcomes
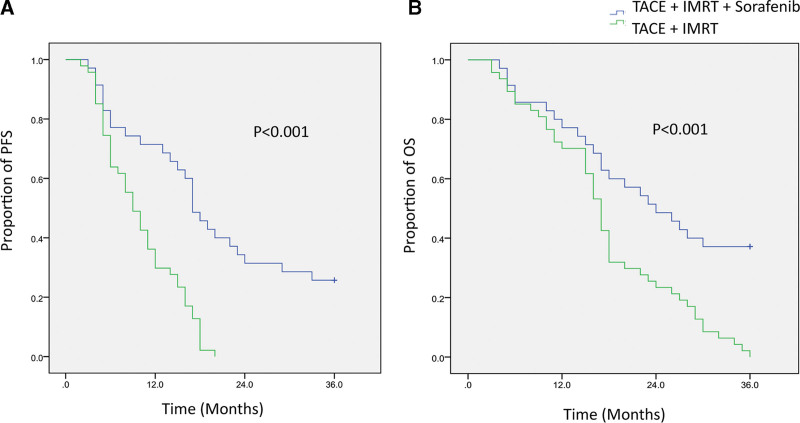
The median follow-up time was 13.2 months (range, 3.2–36.0) for the TACE plus IMRT in combination with sorafenib group, and 14.4 months (range, 3.4–36.0) for the TACE plus IMRT group. During the follow-up period, there were no treatment-related deaths in either group. The Kaplan–Meier curves for PFS and OS for both groups are illustrated in Figure 1. The median PFS was 17.2 months (95% confidence interval, 14.1–19.9) for the TACE plus IMRT in combination with sorafenib group, significantly longer than the 9.4 months (95% CI, 6.8–11.2) observed in patients treated with TACE plus IMRT alone (P < .001; Fig. 1A). Additionally, the combination therapy group achieved a significant OS benefit, with a median OS of 24.1 months (95% CI, 15.9–32.1), compared to 17.3 months (95% CI, 15.5–18.5) for the TACE plus IMRT alone group (P < .001; Fig. 1B).
Figure 1.
Kaplan–Meier survival curves comparing treatment outcomes between 2 groups. (A) Kaplan–Meier curves depicting the PFS of patients treated with TACE plus IMRT in combination with sorafenib (n = 35) versus those treated with TACE plus IMRT alone (n = 47). (B) Kaplan–Meier curves illustrating the OS of patients in the TACE plus IMRT in combination with sorafenib group compared to the TACE plus IMRT group. IMRT = intensity-modulated radiotherapy, OS = overall survival, PFS = progression-free survival, TACE = transcatheter arterial chemoembolization.
3.4. Multivariate analyses
In Table 3, Cox multivariate regression analyses demonstrated that several factors were independently associated with OS in patients with advanced HCC combined with MVI. These factors include tumor size >10 cm (HR = 1.394, 95% CI: 1.100–1.767, P = .006), the presence of PVTT at levels Vp3 (HR = 1.032, 95% CI: 1.007–1.058, P = .013) and Vp4 (HR = 2.015, 95% CI: 1.191–3.409, P = .009) compared to Vp2, and hepatic vein tumor thrombosis (HR = 1.472, 95% CI: 1.045–2.074, P = .027). Other significant associations include AFP levels above 400 ng/L (HR = 1.513, 95% CI: 1.114–2.055, P = .008), and the treatment strategy of TACE plus IMRT in combination with sorafenib (HR = 0.488, 95% CI: 0.277–0.860, P = .013).
Table 3.
Multivariate analysis of prognostic factors for OS in HCC patients with MVI.
| Variables | Multivariate analyses | |||
|---|---|---|---|---|
| HR | 95%CI | P | ||
| Age (year) | <65 | 1 | Reference | – |
| ≥65 | 1.216 | 0.784–1.885 | .382 | |
| Gender | Male | 1 | Reference | – |
| Female | 0.892 | 0.777–1.024 | .105 | |
| Tumor size | ≤10 cm | 1 | Reference | – |
| >10 cm | 1.394 | 1.100–1.767 | .006 | |
| Tumor number | Single | 1 | Reference | – |
| Multiple | 1.307 | 0.894–1.911 | .167 | |
| PVTT | Vp2 | 1 | Reference | – |
| Vp3 | 1.032 | 1.007–1.058 | .013 | |
| Vp4 | 2.015 | 1.191–3.409 | .009 | |
| HVTT | No | 1 | Reference | – |
| Yes | 1.472 | 1.045–2.074 | .027 | |
| T stage | T3b | 1 | Reference | – |
| T4 | 1.028 | 0.987–1.071 | .186 | |
| N stage | N0 | 1 | Reference | – |
| N1 | 1.013 | 0.990–1.037 | .275 | |
| Underlying disease | No | 1 | Reference | – |
| HBV | 1.005 | 0.997–1.013 | .218 | |
| HCV | 1.228 | 0.797–1.893 | .352 | |
| AFP | ≤400 ng/L | 1 | Reference | – |
| >400 ng/L | 1.513 | 1.114–2.055 | .008 | |
| ECOG | 0–1 | 1 | Reference | – |
| >1 | 1.029 | 0.993–1.066 | .117 | |
| Treatment | TACE plus IMRT | 1 | Reference | – |
| TACE plus IMRT plus sorafenib | 0.488 | 0.277–0.860 | .013 | |
AFP = alpha-fetoprotein, ECOG = Eastern Cooperative Oncology Group, HBV = hepatitis B virus, HCV = hepatitis C virus, HVTT = hepatic vein tumor thrombosis, IMRT = intensity-modulated radiotherapy, PVTT = portal vein tumor thrombosis, TACE = transcatheter arterial chemoembolization.
3.5. Adverse events
The most common grade 1 to 2 AE was skin reactions, which occurred in 33 (94.3%) patients treated with TACE plus IMRT in combination with sorafenib, and 32 (68.1%) patients treated with TACE plus IMRT alone. The difference was statistically significant (P = .004). However, no grade 3 or higher skin reactions occurred in patients of either group. The occurrence rates of grade 1 to 2 hand-foot syndrome, other skin reactions, diarrhea, and hair loss in patients treated with TACE plus IMRT in combination with sorafenib were higher than those observed in patients treated with TACE plus IMRT alone, and the differences were statistically significant (all P < .05). However, the treatments were not terminated for patients due to all manageable grade 1 to 2 toxic reactions. Among 82 HCC patients with MVI, 13 had grade 3 hematologic toxic reactions, including 5 (14.3%) in the TACE plus IMRT in combination with sorafenib group and 8 (17.0%) in the TACE plus IMRT group; no significant difference was observed between the 2 groups (P = .737). The most common hematological toxic reactions in both groups were leukopenia and thrombocytopenia. Additionally, a total of 9 patients had grade 3 hepatotoxic reactions, including 4 (11.4%) in the TACE plus IMRT in combination with sorafenib group and 5 (10.6%) in the TACE plus IMRT group, with increased γ-glutamyl transferase levels being the most common hepatotoxicity. There were no grade 4 or 5 AEs, and no patients experienced RILD. All toxic reactions were within the manageable range, and the results are shown in Table 4.
Table 4.
Comparison of adverse events between treatment groups.
| AEs | TACE plus IMRT plus sorafenib (n = 35) | TACE plus IMRT (n = 47) | P * | P † | ||
|---|---|---|---|---|---|---|
| Grade 1 to 2 | Grade 3 | Grade 1 to 2 | Grade 3 | |||
| Hematological | 26 (74.3%) | 5 (14.3%) | 34 (72.3%) | 8 (17.0%) | .844 | .737 |
| Leukopenia | 25 (71.4%) | 5 (14.3%) | 28 (59.6%) | 8 (17.0%) | .267 | .976 |
| Anemia | 6 (17.1%) | 0 (0%) | 9 (19.1%) | 1 (2.1%) | .816 | 1.0 |
| Thrombocytopenia | 22 (62.9%) | 5 (14.3%) | 25 (53.2%) | 7 (14.9%) | .381 | .939 |
| Hepatotoxicity | 28 (80.0%) | 4 (11.4%) | 31 (66.0%) | 5 (10.6%) | .161 | 1.0 |
| Increased ALT level | 6 (17.1%) | 0 | 7 (14.9%) | 0 | .783 | 1.0 |
| Increased AST level | 24 (68.6%) | 0 | 24 (51.1%) | 1 (2.1%) | .111 | 1.0 |
| Increased ALP level | 8 (22.9%) | 0 | 12 (25.5%) | 0 | .780 | 1.0 |
| Increased bilirubin level | 23 (65.7%) | 0 | 30 (63.8%) | 0 | .860 | 1.0 |
| Hypoproteinemia | 9 (25.7%) | 0 | 7 (14.9%) | 0 | .221 | 1.0 |
| Increased GGT level | 27 (77.1%) | 4 (11.4%) | 29 (61.7%) | 4 (8.5%) | .137 | .718 |
| Dermatological | ||||||
| Hand-foot syndrome | 6 (17.1%) | 0 | 0 | 0 | .005 | 1.0 |
| Other skin reactions | 33 (94.3%) | 0 | 32 (68.1%) | 0 | .004 | 1.0 |
| Gastrointestinal | ||||||
| Nausea | 5 (14.3%) | 0 | 4 (8.5%) | 0 | .408 | 1.0 |
| Vomit | 1 (2.9%) | 0 | 0 (0%) | 0 | .244 | 1.0 |
| Anorexia | 7 (20.0%) | 0 | 7 (14.9%) | 0 | .543 | 1.0 |
| Diarrhea | 10 (28.6%) | 0 | 0 (0%) | 0 | <.001 | 1.0 |
| Other | ||||||
| Fatigue | 9 (28.6%) | 0 | 11 (23.4%) | 0 | .810 | 1.0 |
| Hair loss | 5 (14.3%) | 0 | 0 (0%) | 0 | .012 | 1.0 |
Data are median (range) or n (%)
AEs = adverse events; ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; GGT = γ-glutamyl transferase; IMRT = intensity-modulated radiotherapy; TACE = transcatheter arterial chemoembolization.
Comparison of the occurrence rate of grade 1 to 2 AEs between the 2 groups.
Comparison of the occurrence rate of grade 3 AEs between the 2 groups.
4. Discussion
Our results demonstrated that, compared with TACE plus IMRT alone, the combination of TACE, IMRT, and sorafenib significantly improved the median PFS (17.2 vs 9.4 months; P < .001) and the median OS (24.1 vs 17.3 months; P < .001). These findings suggest that a comprehensive, multidisciplinary collaboration may represent a novel approach for treating advanced HCC patients with MVI.
Sorafenib efficacy and safety in advanced HCC patients have been established through international multicenter, randomized controlled clinical trials.[1,22] These trials showed that sorafenib could enhance disease control rate, prolong patients’ median time to radiological progression, time to disease progression, and OS in both Euro-American and Asia-Pacific populations.[23] However, the response rate of sorafenib alone in advanced HCC patients with PVTT was only 2% to 3.3%, and the extension of median survival was about 3 months compared to the placebo group.[24]
Additionally, previous studies had demonstrated that the combination of sorafenib with local tumor treatments, such as TACE or EBRT, could achieve superior therapeutic effects in HCC patients with PVTT, compared to sorafenib alone.[25–27] A pivotal phase III clinical trial contrasted the efficacy of sorafenib monotherapy (s-m group) with sorafenib plus local treatment (including intra-arterial chemotherapy and radiation therapy, referred to as the s-lrts group) in advanced HCC patients.[28] Among the 290 patients in this trial, those in the s-lrts group experienced both a median PFS of 5.3 months and a median OS of 8.5 months, exceeding the outcomes in the s-m group. These improvements were observed regardless of the presence of extrahepatic metastases or regional lymph node metastases. Specifically, the median OS in the s-lrts group was longer than that in the s-m group, both without metastases (18.0 months versus 7.8 months) and with extrahepatic or regional lymph node metastases (8.3 months versus 4.8 months). Therefore, the combination of TACE and IMRT with sorafenib could have provided relatively long-term local regional control for advanced HCC patients with MVI.
EBRT enhanced the therapeutic effects in advanced HCC patients with PVTT. Three-dimensional conformal radiotherapy was one method within EBRT, aimed at achieving portal vein recanalization, and preventing tumor growth or intrahepatic spreading in advanced HCC patients with PVTT. IMRT technology represented a further development of three-dimensional conformal radiotherapy, refining the irradiation field for precision and improving the accuracy of the irradiation dose delivery.[12] Despite the administration of higher irradiation doses to tumors, IMRT technology helped reduce the occurrence rates of AEs during radiation therapy.[29,30] In our study, neither RILD nor notable radiotherapy-related complications were observed. The radiation was directed mainly at the tumor thrombi rather than the primary HCC, capitalizing on the high radiation tolerances of large blood vessels. Consequently, systemic damage caused by radiation was limited and minimized.[31]
Advanced HCC patients with MVI exhibited poor tumor biological behavior, a high risk of postoperative recurrence, and a low long-term survival rate.[32] Several studies have explored different treatment strategies for this patient group. Yoon et al conducted an open-label randomized clinical trial involving 90 advanced HCC patients with MVI, all of whom had portal vein invasion and liver function of Child-Pugh grade A. Patients were randomly assigned to receive either sorafenib alone or TACE plus radiotherapy. By the 12th week, the PFS rate in the TACE-RT group was significantly higher than that in the sorafenib group (86.7% versus 34.3%; P < .001), and the median OS was also significantly prolonged in patients undergoing combination treatment (55.0 vs 43.0 weeks; P = .04).[33] Similarly, Kim et al demonstrated that patients treated with TACE in combination with radiotherapy achieved longer median time-to-progression (TTP) (5.1 vs 1.6 months, P < .001) and median OS (8.2 vs 3.2 months; P < .001) compared to those treated with sorafenib alone.[34]
For advanced HCC patients with MVI, TACE was found to be a safe and acceptable treatment when the patient’s hepatic function was tolerable, and collateral circulation of the portal vein had formed in the hepatic hilar region.[27,35] However, considering factors such as the blood supply of the tumor and the formation of collateral circulation, the complete necrosis rate of TACE alone was relatively low in these patients. Moreover, ischemia and hypoxia induced by TACE embolization might elevate the expression of HIF-1α, leading to increased VEGF expression and subsequent tumor neovascularization. As a result, the long-term clinical efficacy of TACE alone was often suboptimal, and a clinical cure could remain elusive.[36,37] Combining radiotherapy with TACE could enhance outcomes. A meta-analysis conducted by Huo et al, which included 25 clinical studies and analyzed 2577 advanced HCC patients, demonstrated that the radiotherapy plus TACE group exhibited a significantly higher probability of achieving CR or PR than the TACE alone group (P < .001).[38] Additionally, the median OS was 22.7 versus 13.5 months in the combined treatment group and the TACE alone group, respectively. These findings suggested that radiotherapy plus TACE might be suitable for advanced HCC patients without surgical options.[38] In recent years, the combination of TACE, IMRT, and sorafenib emerged as a promising treatment strategy for HCC patients with PVT. The theoretical foundation of this combined approach lay in the stimulation of VEGF-2 up-regulation through necrotic tissue after TACE, fostering tumor neovascularization. Sorafenib, with its multi-target properties, could inhibit this neovascularization and block tumor growth, while precision radiotherapy targeted the PVTT. Together, these therapies worked in concert to achieve a complementary effect[10].
Previous studies had established that the tumor response was a critical predictive factor for survival in HCC patients with MVI.[39,40] This underscores the importance of early response to treatment, as it might be indicative of a favorable survival outcome. A meta-analysis that compared various radiation therapy modalities, including 3DCRT, SIRT, and SBRT, specifically for HCC patients with PVT, provided further insight. The results revealed that among these methods, SBRT achieved the highest pooled response rates (51.3%; 95% CI: 45.7–57.0).[41] Interestingly, the 1-year OS rates were similar across all 3 modalities, suggesting that HCC patients with tumor thrombus could derive benefits from comprehensive treatment approaches.[41,42] The findings of our study corroborated these previous insights, reinforcing the value of selecting the appropriate treatment strategy based on individual patient characteristics.
Few studies have focused on the combination of TACE plus IMRT with sorafenib for treating HCC patients with MVI, and no standardized treatment strategy has been established. The safety profile of this treatment strategy requires further investigation. In our study, the occurrence rates of diarrhea, hand-foot syndrome, hair loss, and other skin reactions were increased due to the combination of sorafenib and locoregional treatments, but all these adverse events were controllable. Patients in both treatment groups exhibited comparable levels of hepatotoxicity and hematological toxicity. The inclusion of sorafenib in the treatment regimen did not significantly increase either the incidence or severity of these toxicities. No toxic reactions of grade 4 or higher were observed in either group, making TACE plus IMRT in combination with sorafenib a feasible and tolerable treatment option.[43]
However, this study has limitations. First, all enrolled patients were treated with IMRT after TACE, and the reasonable dose of sorafenib and the optimal treatment schedule remain uncertain. Second, the study design was retrospective, possibly introducing selection bias. Third, the sample size was relatively small, and multicenter randomized controlled clinical trials with a larger sample size and long-term follow-up are still required to verify these results.
5. Conclusion
The combination of TACE plus IMRT with sorafenib has demonstrated significant clinical benefits for the treatment of HCC patients with MVI. This therapeutic approach not only enhances the tumor response rate but also extends both PFS and OS, all within a safety profile that is both tolerable and manageable.
Author contributions
Conceptualization: Dan Yang, Zhufang Ma.
Data curation: Dan Yang, Jiaojiao Du.
Formal analysis: Jiaojiao Du, Weijie Nie, Chaozhi Wang.
Funding acquisition: Chaozhi Wang.
Methodology: Weijie Nie.
Writing – original draft: Dan Yang.
Writing – review & editing: Dan Yang, Zhufang Ma.
Abbreviations:
- AEs
- adverse events
- AFP
- alpha-fetoprotein
- ALT
- alanine aminotransferase
- CI
- confidence interval
- CR
- complete response
- ECOG
- Eastern Cooperative Oncology Group
- HCC
- hepatocellular carcinoma
- IMRT
- intensity-modulated radiotherapy
- MVI
- macrovascular invasion
- OS
- overall survival
- PD
- progressive disease
- PFS
- progression free survival
- PLT
- platelet count
- PR
- partial response
- PVTT
- portal vein tumor thrombosis
- RILD
- radiation-induced liver disease
- RT
- radiotherapy
- SD
- stable disease; standard deviation
- TACE
- transcatheter arterial chemoembolization
The study was approved by the Ethics Committee of 3201 Hospital, Hanzhong 723000, Shaanxi, China.
The informed consent of patient was waived due to the nature of retrospective design.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Yang D, Du J, Nie W, Wang C, Ma Z. Combination treatment of transcatheter arterial chemoembolization, intensity-modulated radiotherapy, and sorafenib for hepatocellular carcinoma with macrovascular invasion. Medicine 2023;102:45(e35713).
Contributor Information
Dan Yang, Email: yangdan17@yandex.com.
Jiaojiao Du, Email: 17839224058@163.com.
Weijie Nie, Email: 1946838046@qq.com.
Chaozhi Wang, Email: 15724773@qq.com.
References
- [1].Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 22021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:541–65. [DOI] [PubMed] [Google Scholar]
- [2].Yang X, Kang Y, Qiao Y, et al. Magnetic resonance imaging evaluation of characteristics of vascular invasion in intermediate and advanced hepatic alveolar echinococcosis. Exp Ther Med. 2019;17:4197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ma L, Deng K, Zhang C, et al. Nomograms for predicting hepatocellular carcinoma recurrence and overall postoperative patient survival. Front Oncol. 2022;12:843589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hu Y, You S, Yang Z, et al. Nomogram predicting survival of hepatocellular carcinoma with portal vein tumour thrombus after curative resection. ANZ J Surg. 2019;89:E20–5. [DOI] [PubMed] [Google Scholar]
- [5].Yang J, Liang H, Hu K, et al. The effects of several postoperative adjuvant therapies for hepatocellular carcinoma patients with microvascular invasion after curative resection: a systematic review and meta-analysis. Cancer Cell Int. 2021;21:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang Y, Zhang Z, Zhou Y, et al. Should we apply sorafenib in hepatocellular carcinoma patients with microvascular invasion after curative hepatectomy? Onco Targets Ther. 2019;12:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ma D, Liu M, Zhai X, et al. Development and validation of prognostic risk prediction models for hepatocellular carcinoma patients treated with immune checkpoint inhibitors based on a systematic review and meta-analysis of 47 cohorts. Front Immunol. 2023;14:1215745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu J, Cheng D, Liao Y, et al. Development of a magnetic resonance imaging-derived radiomics model to predict microvascular invasion in patients with hepatocellular carcinoma. Quant Imaging Med Surg. 2023;13:3948–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim JW, Kim DY, Han KH, et al. Phase I/II trial of helical IMRT-based stereotactic body radiotherapy for hepatocellular carcinoma. Dig Liver Dis. 2019;51:445–51. [DOI] [PubMed] [Google Scholar]
- [10].Zhao Y, Zhu X, Wang H, et al. Safety and efficacy of transcatheter arterial chemoembolization plus radiotherapy combined with sorafenib in hepatocellular carcinoma showing macrovascular invasion. Front Oncol. 2019;9:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun J, Yang L, Shi J, et al. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: an open-label randomized controlled trial. Radiother Oncol. 2019;140:20–5. [DOI] [PubMed] [Google Scholar]
- [12].Yang Y, Xiong L, Li M, et al. Advances in radiotherapy and immunity in hepatocellular carcinoma. J Transl Med. 2023;21:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hu L, Lin J, Shi X, et al. Efficacy of transarterial therapy combined with first-line tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: a network meta-analysis. World J Surg Oncol. 2023;21:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127:3782–93. [DOI] [PubMed] [Google Scholar]
- [15].Zhang YL, Cui XJ, Xing H, et al. Molecular targeted therapy and immunotherapy in advanced hepatocellular carcinoma: a systematic review and Bayesian network meta-analysis based on randomized controlled trials. Ann Med. 2023;55:2242384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:1164–74. [DOI] [PubMed] [Google Scholar]
- [17].Zhang T, Zhao YT, Wang Z, et al. Efficacy and safety of intensity-modulated radiotherapy following transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Medicine (Baltim). 2016;95:e3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang WH, Wang Z, Wu JX, et al. Survival benefit with IMRT following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels. Liver Int. 2015;35:2603–10. [DOI] [PubMed] [Google Scholar]
- [19].Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol. 2016;65:1140–7. [DOI] [PubMed] [Google Scholar]
- [20].Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. [DOI] [PubMed] [Google Scholar]
- [21].Koay EJ, Owen D, Das P. Radiation-induced liver disease and modern radiotherapy. Semin Radiat Oncol. 2018;28:321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II–III trial. J Clin Oncol. 2021;39:3002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheng AL, Guan Z, Chen Z, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer. 2012;48:1452–65. [DOI] [PubMed] [Google Scholar]
- [24].Gu W, Tong Z. Sorafenib in the treatment of patients with hepatocellular carcinoma (HCC) and microvascular infiltration: a systematic review and meta-analysis. J Int Med Res. 2020;48:300060520946872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kawamura Y, Akuta N, Shindoh J, et al. Efficacy of the combination of systemic sequential therapy and locoregional therapy in the long-term survival of patients with BCLC stage C hepatocellular carcinoma. Cancers (Basel). 2023;15:3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cho Y, Choi JW, Kwon H, et al. Transarterial chemoembolization for hepatocellular carcinoma: 2023 expert consensus-based practical recommendations of the Korean Liver Cancer Association. Clin Mol Hepatol. 2023;29:521–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Duan R, Gong F, Wang Y, et al. Transarterial chemoembolization (TACE) plus tyrosine kinase inhibitors versus TACE in patients with hepatocellular carcinoma: a systematic review and meta-analysis. World J Surg Oncol. 2023;21:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee S, Kim BK, Kim SU, et al. Efficacy of sorafenib monotherapy versus sorafenib-based loco-regional treatments in advanced hepatocellular carcinoma. PLoS One. 2013;8:e77240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fan Z, Zhou P, Jin B, et al. Recent therapeutics in hepatocellular carcinoma. Am J Cancer Res. 2023;13:261–75. [PMC free article] [PubMed] [Google Scholar]
- [30].Lee SJ, Kim M, Kwak YK, et al. MRI-guided radiotherapy for PVTT in HCC patients: evaluation of the efficacy and safety. J Cancer Res Clin Oncol. 2022;148:2405–14. [DOI] [PubMed] [Google Scholar]
- [31].Li W, Pei Y, Wang Z, et al. Efficacy of transarterial chemoembolization monotherapy or combination conversion therapy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2022;12:930868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim N, You M-W. Hepatocellular carcinoma and macrovascular tumor thrombosis: treatment outcomes and prognostic factors for survival. Jpn J Radiol. 2019;37:781–92. [DOI] [PubMed] [Google Scholar]
- [33].Li L, Zhao W, Wang M, et al. Transarterial chemoembolization plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2018;18:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim GA, Shim JH, Yoon SM, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320–9.e6. [DOI] [PubMed] [Google Scholar]
- [35].Liu JN, Li JJ, Yan S, et al. Transarterial chemoembolization combined with lenvatinib versus transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2023;13:1074793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Qiu G, Xie K, Jin Z, et al. The multidisciplinary management of hepatocellular carcinoma with portal vein tumor thrombus. Biosci Trends. 2021;15:148–54. [DOI] [PubMed] [Google Scholar]
- [37].Cerrito L, Annicchiarico BE, Iezzi R, et al. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: beyond the known frontiers. World J Gastroenterol. 2019;25:4360–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015;1:756–65. [DOI] [PubMed] [Google Scholar]
- [39].Cannella R, Taibbi A, Porrello G, et al. Hepatocellular carcinoma with macrovascular invasion: multimodality imaging features for the diagnosis. Diagn Interv Radiol. 2020;26:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang JC, Xia AL, Xu Y, et al. Comprehensive treatments for hepatocellular carcinoma with portal vein tumor thrombosis. J Cell Physiol. 2019;234:1062–70. [DOI] [PubMed] [Google Scholar]
- [41].Rim CH, Kim CY, Yang DS, et al. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis and systematic review. Radiother Oncol. 2018;129:112–22. [DOI] [PubMed] [Google Scholar]
- [42].Lee J, Shin IS, Yoon WS, et al. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: Meta-analyses and a systematic review. Radiother Oncol. 2020;145:63–70. [DOI] [PubMed] [Google Scholar]
- [43].Ai L, Xu Z, Yang B, et al. Sorafenib-associated hand-foot skin reaction: practical advice on diagnosis, mechanism, prevention, and management. Expert Rev Clin Pharmacol. 2019;12:1121–7. [DOI] [PubMed] [Google Scholar]