Abstract
Phyllanthus emblica Linn, a prominent member of the euphorbiaceae family, exhibits extensive distribution across a multitude of tropical and subtropical nations. Referred to as “Balakka” in Indonesia, this plant assumes various names across regions, such as “kimalaka,” “balakka,” “metengo,” “malaka,” and “kemloko” in North Sumatra, Ternate, Sundanese, and Java respectively. Phyllanthus emblica thrives in tropical locales like Indonesia, Malaysia, and Thailand, while also making its presence felt in subtropical regions like India, China, Uzbekistan, and Sri Lanka. The fruits of Balakka are enriched with bioactive constituents recognized for their wide-ranging benefits, including antioxidant, anti-aging, anti-cholesterol, anti-diabetic, immunomodulatory, antipyretic, analgesic, anti-inflammatory, chemoprotective, hepatoprotective, cardioprotective, antimutagenic, and antimicrobial properties. Comprising a spectrum of phenolic compounds (such as tannins, phenolic acids, and flavonoids), alkaloids, phytosterols, terpenoids, organic acids, amino acids, and vitamins, the bioactive components of Malacca fruit offer a diverse array of health-promoting attributes. In light of these insights, this review aims to comprehensively examine the pharmacological activities associated with P. emblica and delve into the intricate composition of its phytochemical constituents.
Keywords: Phyllanthus emblica, phytochemical composition, pharmacological properties, natural product, bioactive substances
1 Introduction
Phyllanthus emblica Linn, a member of the euphorbiaceae family, is extensively distributed throughout the majority of tropical and subtropical nations. Phyllanthus is a very large genus containing approximately 550–750 species and 10 to 11 subgenera. It is endemic to equatorial southeast Asia and is found in the mixed forest of tropical and subtropical regions at elevations between 150 and 1,400 m. In Indonesia P. emblica is called balakka, kimalaka, kemlaka, kemloko, or malaka (Summanen, 1999; Mal and Meena, 2022). Natural products have existed since the dawn of humanity, the significance of traditional systems of medicine and particular traditional medical practices is now acknowledged worldwide. To evaluate selective pharmaceuticals of herbal origin, it is now necessary to adopt an intelligent and pragmatic approach. All parts of Phyllanthus Emblica, including its fruits, flowers, seeds, leaves, and bark, have been extensively utilized in numerous traditional remedies. Pharmacological studies reveals that P. emblica have antioxidant (Chaphalkar et al., 2017; Sheoran et al., 2019), anticancer (Ngamkitidechakul et al., 2010; Mahata et al., 2013; Gaire and Subedi, 2014; Zhao et al., 2015; Chekdaengphanao et al., 2022; Naik and David, 2023), Immunomodulatory (Jantan et al., 2019), cytoprotective (Zhang et al., 2016), anti-viral (Lv et al., 2014; Lv et al., 2015), anti-jaundice, anti-dyslipidemic (Quranayati et al., 2023), anti-aging (Wu et al., 2022), anti-apoptotic (Chekdaengphanao et al., 2022), anti-inflammatory (Wang et al., 2019), hepatoprotective (Pramyothin et al., 2006), nephroprotective (Huang et al., 2023), and anti-diabetic (Naik and David, 2023). Phyllanthus emblica has various constituents have been used in the formulation of numerous herbal and patent medicines (Dinesh et al., 2017). The majority of fixed oils, essential oils, and phosphatides are found in fruit seeds. Fruit, leaves, and bark are rich in tannins; bark also contains leucodelphinidin, while roots are abundant in lupeol and ellagic acid (Hussain et al., 2021). The yellowish-brown seeds of P. emblica contain linoleic acid, stearic acid, palmitic acid, linolenic acid, myristic acid, and oleic acid while D-myoinositol, D-fructose, and D-glucose are predominantly found in the ethanol fractions of P. emblica (Saini et al., 2022). Phyllanthus emblica contains chemopreventive compounds like lupol and glochidone, which belong to the lupine-type triterpenoids (Ramasamy et al., 2012). Additionally, it harbors antioxidant properties from compounds like mallotusinin, isomallotusinin, isostrictinin, and mallonin, along with phyllembilin, cinnamic acid, and chebulagic acid (Ahmad et al., 2021). The vitamin C content in P. emblica far surpasses that of common citrus fruits like lemons, oranges, and tangerines (Bajgai et al., 2006). Besides vitamin C, this fruit also contains other essential vitamins including carotene, niacin, riboflavin, and thiamine (Ghosal, 1996). For every 100 g of P. emblica fruit, there is an impressive vitamin C quantity ranging from 600 to 1,300 mg. Among the amino acids present, the prominent ones are glutamic acid (29.6%), proline (14.6%), aspartate (8.1%), alanine (5.4%), and lysine (5.3%) (Saini et al., 2022). Phyllanthus emblica fruit is rich in vitamin C (70%–72%) and includes a variety of components such as tannins, phembembaic acid (6.3%), gallic acid (5%), lipids (6%), emblicol, flavonoids, and mymic acid. The leaves of P. emblica contain gallic acid, chebulic acid, ellagic acid, kaempferol, kaempferol-3-o-glucoside, gallo tannin, and rutin, phosphoric acid, essential oils, linoleic acid, oleic acid, stearic acid, palmitic acid, and mystic acid. The bark of the plant contains proanthocyanidins, tannins, and leucodelphinidin. Moving on to the roots, they contain ellagic acid and lupeol (Saini et al., 2022). This thorough review focuses on the phytochemical makeup and the effects of P. emblica. Through an extensive exploration, this study aims to shed light on the numerous health advantages of P. emblica, thereby stimulating more research and progress in utilizing this herbal remedy to enhance human health.
2 Botanical description and taxonomy
Balakka is a type of plant that lives in a forest on the savanna. The forest has medium-sized trees with lots of branches, and they’re about 10–20 m tall. The fruit of the balakka plant is round and has ridges. It is divided into six parts, and each part has a stone in it. The stones are about 1.8–2.5 cm across. The fruit is small and round with a tough covering. Inside, there are six seeds. The fruit looks nice–it is round and yellow. It tastes sour and astringent, which means it makes your mouth pucker a little. The balakka plant grows slowly and can climb things. It is mostly white in color. It is found in certain parts of Sumatra, which is an island in Indonesia. It likes to grow in places that are not very wet, like yards and along roads. It can also grow in places where people plant things, like farms. The balakka plant likes specific types of soil that have a pH between 6.5 and 7. There are different kinds of soil where balakka plants grow. Some soils have a gray or yellowish top layer and a red or yellow lower layer. These soils do not have a lot of nutrients, and they’re a bit acidic. The balakka plant also grows in places where the soil has lots of clay. The land where balakka grows comes from rocks and other materials in the ground. It is usually not very high above sea level, maybe around 50–350 m. The balakka plant likes places where it rains between 2,500 and 3,500 mm every year (Gantait et al., 2021). The P. emblica Figure 1 can be seen below.
FIGURE 1.

Phyllanthus emblica.
3 Botanical description and taxonomy
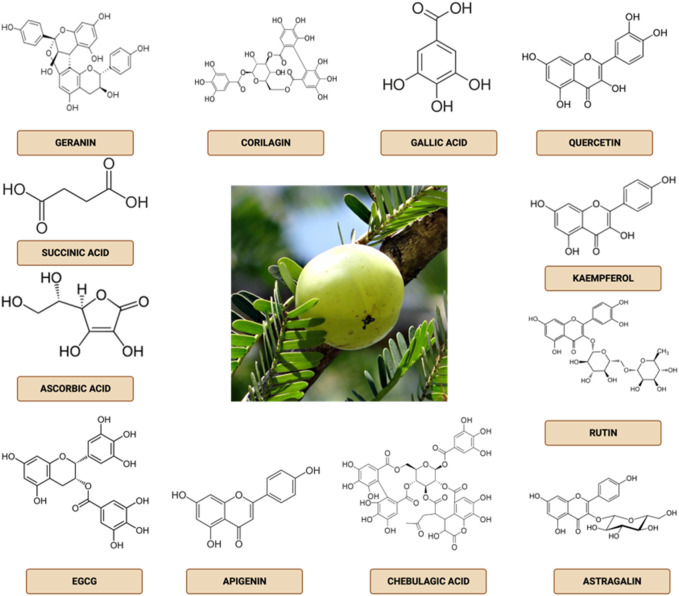
The bioactive components of natural ingredients are defined as secondary metabolites with human and animal pharmacological effects. Bioactive components of Phyllanthus emblica fruit include a group of phenolic compounds (tannins, phenolic acids, and flavonoids), alkaloids, phytosterols, terpenoids, organic acids, amino acids, and vitamins (Yang and Liu, 2014). Emblicanin A, B, punigluconin, pedunculagin, geranin, isochorylagin, corylagin, chebulanic acid, chebulagate acid, isostrictinin, gallic acid, mucous acid lactone gallate, digalloylglucose, methyl gallate, ethyl error, monogalloyl glucose, putanjivin A, galloil-HHDP-glucose, and elaeocarpusin are hydrolyzed tannins found in P. emblica fruit (Usharani et al., 2013). Other bioactive substances include gallic acid, ellagic acid, chlorogenic acid, malic acid, chebulic acid, and cinnamic acid. Additionally, P. emblica fruit contains flavonoid compounds like quercetin, kaempferol, and routine (Jagdale et al., 2021). This plant contains phytochemicals such as fixed oils, phosphatides, essential oils, tannins, minerals, vitamins, aminoacids, fatty acids, and glycosides, among others (Chanda et al., 2020). Phyllanthus emblica has been found to contain linolenic, linoleic, oleic, stearic, palmitic, and myristic acids. Sugar residues consist of D-glucose, D-fructose, D-myo-inositol, D-galacturonic acid, D-arabinosyI, D-rhamnosyl, D-xylosyI, D-glucosyI, D-mannosyl, and D-galactosyI (Ahmad et al., 2021). The results of our previous study indicate the presence of alkaloids (specifically phyllantidine and phyllantine) and tannins (including chebulagic acid, chebulinic acid, punigluconin, emblicanin A, ellagic acid, emblicanin B, 1-O-galloyl-b-D-glucose, ellagic acid, ellagotannin, 3-ethylgallic acid, corilagin, pedunculagin, trigallayl glucose, 3,6-di-O-galloyl-D-glucose, and 1,6-di-O-galloyl-b-D-glucose) as determined by chromatographic and infra-red spectral analysis. Additionally, the presence of flavonoids, including kaempferol-3-O-a-L-(600-ethyl)-rhamnopyranoside, quercetin, acylated apigenin glucoside, and kaempferol-3-O-a-L-(600-methyl)-rhamnopyranoside, was also documented (Halim et al., 2022). The chemical structure of phytochemical composition in P. emblica can be seen in Figure 2 and Table 1 below.
FIGURE 2.
Main phytochemical components in Phyllanthus emblica.
TABLE 1.
Phytochemical components of Phyllanthus emblica.
| No. | Extraction method | Analytic | Bioactiv compund identified | Part of the plant | References |
|---|---|---|---|---|---|
| Technique | |||||
| 1 | etylacetate | GC-MS | - Citronellyl Propionate | fruit | Acharya (2016) |
| - 1-Methyl-4 Isopropyl-Cyclohexyl 2-Hydroperfluorobutanoate | |||||
| - Citronellyl Acetate | |||||
| −3,7,11,15-Tetra Methyl-2 Hexadecen-1-Ol | |||||
| - Bicyclo (2.2.1) - Heptane,1,3,3-Trimethyl- | |||||
| - 7-Octadecyne,2-Methyl | |||||
| - Bicyclo(2.2.1) Heptane, 1,7,7-Trimethyl- | |||||
| - Bicyclo(3.1.1) Heptane,2,6,6-Trimethyl- | |||||
| - Bicyclo(2.2.1) Heptane,2,2,3-Trimethyl-Endo- | |||||
| - Cyclohexane, 1-Methyl-4-(1-Methylethenyl)- Cis- | |||||
| 2 | petroleum ether | GC-MS | - Hentriacontane | Leaf | Elangovan and Irulappan (2015) |
| - Dotriacontane | |||||
| - Tetracontane | |||||
| - Tritetracontane | |||||
| - Pentacosane | |||||
| - Octadecanoic Acid | |||||
| - Methyl Ester | |||||
| - Vitamin E | |||||
| 3 | n-hexane, ethyl acetate, and methanol solvents | GC-MS | - 9-Octadecene | Stem bark | Quranayati et al. (2023) |
| - Methyl Palmitate | |||||
| - 1-Octadecene | |||||
| - 1-Tetracosanol | |||||
| - Docosanoic Acid | |||||
| - Cyclopropane, 1-(1-Hydroxy-1-Heptyl)-2-Methylene-3-Pentyl | |||||
| - 1-Pentadecene | |||||
| - 1-Hexadecene | |||||
| - 9-Eicosene, (E)- | |||||
| - Neophytadiene | |||||
| 4 | methyl salicylate | GC-MS | - 2-Methyl Butyl Acetate | fruit | Amir, et al. (2014) |
| - Isopropyl,2-Methyl Butyrate | |||||
| −2,4-Hexadienol | |||||
| - Benzaldehyde | |||||
| - Menthane | |||||
| - Decane | |||||
| - Butyl Cyclohexane | |||||
| - Butyl Cyclohexene | |||||
| - Acetophenone | |||||
| - Undecane | |||||
| 5 | N-Hexane, Ethyl Acetate, and Ethanol | GC-MS | - 2-Furanmethanol | Leaf | Asmilia et al. (2020) |
| - 1h-Cyclopropaanaphthalene | |||||
| - Trans-Caryophyllene | |||||
| - Cyclohexane | |||||
| - Caryophyllene | |||||
| - Sativen | |||||
| - Delta-Guaiene | |||||
| - Tetradecanoic Acid | |||||
| - Octadecanal | |||||
| - 3-Eicosyne | |||||
| 6 | Methanol | GC-MS | - Octyl-Β-D-Glucopyranoside | fruit | Dinesh et al. (2016) |
| 7 | Ethanol | GC-MS | - Galacturonic Acid | fruit | Li et al. (2019) |
| - Glucuronic Acid | |||||
| - The Monosaccharide Standard | |||||
| - Hydroxylamine Hydrochloride N-Propylamine | |||||
| - 3-Phenylphenol | |||||
| - Trifluoroacetic Acid | |||||
| 8 | Ethanol and methanol | GC-MS | −1,2,3-Benzenetriol | fruit | Nair et al. (2020) |
| - Hexadecanoic Acid | |||||
| - 2-Tert-Butyl-4-Iso Propyl-5 Methyl Phenol | |||||
| - 9-Octadecenoic Acid | |||||
| - Octadecanoic Acid | |||||
| 9 | Methanol | GC-MS | −1,5-Hexanediol | leaf | Abdel-Hady et al. (2022) |
| - D-Mannose | |||||
| −1,3-Dimethylindole | |||||
| −24,25-Dihydroxy Vitamin D | |||||
| - Pyrrolizin-1,7-Dione-6- Carboxylic Acid, Methyl (Ester) | |||||
| - Phenol,2,4-Di-Tert-Butyl- | |||||
| - Menthol,1'-[Butyln-3-One-1- Yl)-, (1r, 2s, 5r) | |||||
| −10-Heptadecen-8-Ynoic Acid, Methyl Ester. (E) | |||||
| - Vitamin A Palmitate | |||||
| - Cis-11-Eicosenoic Acid | |||||
| 10 | Methanol | GC-MS | −2,4-Dimethylfuran | fruit | Akter et al. (2022) |
| - 4-(2-Hydroxyethyl)-3-Methyl-2-Pyrazolin-5-One | |||||
| - Trans-2,3-Epoxyoctane | |||||
| - 2-Furancarboxylic Acid, 2-Ethylhexyl Ester | |||||
| - Heptanoic Acid, 3-Hydroxy-, Methyl Ester | |||||
| - 2-(3-Methylguanidino)Ethanol D-Glycero-D-Ido-Heptose | |||||
| - Paromomycin | |||||
| - Octadecanoic Acid | |||||
| −9,9-Dimethoxybicyclo[3.3.1 ]Nona-2,4-Dione | |||||
| - N-Propyl Nonyl Ether | |||||
| 11 | Ethanol, acetone, n-hexane | GC-MS | - Succinic Acid Dimethyl Ester | fruit | Harahap et al. (2019) |
| - Dimethyl Pentanedioate | |||||
| - Dimethyl Adipate | |||||
| - Methyl Adipate | |||||
| −1,3-Dioxolane-4-Methanol | |||||
| 12 | Ethanol | HPLC | - Quinic Acid | fruit, bark, leaf | Kumar et al. (2017a) |
| - Caffeic Acid | |||||
| - Gallic Acid | |||||
| - Vanillic Acid | |||||
| - Gentisic Acid-O-Hexoside - | |||||
| - (+)-Catechin | |||||
| - Brevifolincarboxylic Acid | |||||
| - Epicatechin | |||||
| - Ellagic Acid-O-Dihexoside | |||||
| - Ellagic Acid-O-Hexoside | |||||
| 13 | Ethanol | HPLC | - Mucic Acid- 1,4-Lactone- 3-0-Gallate | fruit | Li et al. (2019) |
| - Hamamelitannin | |||||
| - Isocorilagin | |||||
| - Ethyl Gallate | |||||
| - Methyl Gallate | |||||
| - Ellagic Acid | |||||
| - Quercetin-3-O-Rhamnoside | |||||
| - Undefined | |||||
| 14 | methanol | HPLC | - B-Glucogallin | fruit | Kumar et al. (2015) |
| - Trigalloylglucose | |||||
| - Geraniin | |||||
| - Gallic Acida | |||||
| - Castalin | |||||
| - Trigalloylglucose (Isomer) | |||||
| - Corilagin | |||||
| - Protocatechuic Acida | |||||
| - Methylgallate | |||||
| - P-Coumaric Acid | |||||
| 15 | methanol | HPLC | - Digallic Acid | fruit | Balusamy et al. (2019) |
| 16 | Ethanol | HPLC | - Gallic Acid | fruit | Li et al. (2020) |
| - Fisetin | |||||
| 17 | Ethanol | LC/MS | - 5-Hydroxyisophthalic Acid | fruit | Wu et al. (2022a) |
| - Amlaic Acid | |||||
| −3,5-Dihydroxybenzoic Acid | |||||
| −10-Gingerdione | |||||
| - 6-Methylgingediacetate | |||||
| - Ethyl Gallate | |||||
| - (-)-1,10-Epoxy-Guaia-11-Ene | |||||
| - 7alpha-Hydroxycholesterol | |||||
| - Irisoquin F | |||||
| −2′-Hydroxycinnamaldehyde | |||||
| 18 | Ethanol | HPLC | - Gallic | fruit | Kumnerdkhonkaen et al. (2018) |
| - Phydroxybenzoic | |||||
| - Vanillic | |||||
| - Syringic | |||||
| - P-Coumaric | |||||
| - Ferulic | |||||
| - Sinapinic Acids | |||||
| 19 | Methanol | HPLC | - L-Ornithine | fruit | Luo et al. (2022) |
| - Taurine | |||||
| - Glucose 1-Phosphate | |||||
| - Deoxycytidine | |||||
| - Methylmalonic Acid | |||||
| - 3-Hydroxy-3- Methylbutanoic Acid | |||||
| - 5-Hydroxytryptamine | |||||
| - N-Lactoyl-Phenylalanine | |||||
| - 2-(3,4-Dimethoxyphenyl) Ethanamine | |||||
| - Indoleacrylic Acid | |||||
| 20 | Methanol | LC/MS | - L-Ornithine | leaf | Kiran et al. (2021) |
| - Taurine | |||||
| - Glucose 1-Phosphate | |||||
| - Deoxycytidine | |||||
| - Methylmalonic Acid | |||||
| - 3-Hydroxy-3- Methylbutanoic Acid | |||||
| - 5-Hydroxytryptamine | |||||
| - N-Lactoyl-Phenylalanine | |||||
| - 2-(3,4-Dimethoxyphenyl) Ethanamine | |||||
| - Indoleacrylic Acid | |||||
| 21 | Ethanol | HPLC | - Gallic Acid | fruit | Gong et al. (2020) |
| - Methyl Gallate | |||||
| 22 | Ethanol and Methanol | LC/MS | - Arbamoyl Phosphate | fruit | Kumar et al. (2017b) |
| - L-Methionine; Methionine; L-2-Amino-4methylthiobutyric Acid | |||||
| - Pyridoxamine Phosphate | |||||
| - Sulfate Derivative Of Norepinephrine | |||||
| - Nicotinate D-Ribonucleoside | |||||
| - 4-Hydroxy-All-Trans-Retinyl Acetate | |||||
| −5(S),6(S)-Epoxy-15(S)-Hydroxy-7e,9e,112,13e- Eicosatetraenoic Acid | |||||
| - Prostaglandin B1 | |||||
| - N6-D-Biotinyl-L-Lysine; Biocytin; Epsilon-N-Biotinyl- L-Lysine | |||||
| −12-Oxo-20-Dihydroxy-Leukotriene B4 | |||||
| 23 | Ethanol | GC-MS | - Fatty Acids And Fatty Acyl Esters | fruit | Khaled et al. (2018) |
| - Fatty Acyl Esters | |||||
| - Phthalides | |||||
| - Terpenes | |||||
| - Sterol | |||||
| - Palmitic Acid And Palmitic Acid Methyl Ester | |||||
| - Linoleic Acid | |||||
| - Stearic Acid | |||||
| - Elaidic Acid | |||||
| 24 | Ethanol | HPLC | - Hypophyllanthin | fruit, leaf | Kumar et al. (2017c) |
| - Phyllanthin | |||||
| - Gallic Acid | |||||
| - Ellagic Acid | |||||
| - Quinic Acid | |||||
| - Chebulinic Acid | |||||
| 25 | Methanol | HPLC | - Mucic Acid | fruit | Yang et al. (2012) |
| - Mucic Acid Lactone | |||||
| - Malic Acid | |||||
| - Mucic Acid Gallate | |||||
| - Chebulic Acid | |||||
| - Mucic Acid Digallate | |||||
| - Mucic Acid Lactone Gallate | |||||
| - Galloylglucose | |||||
| - Mucic Acid Methyl Ester Gallate | |||||
| - Gallic Acid | |||||
| 26 | Ethanol | HPLC | - Quercetine | fruit | Halim et al. (2022) |
| - Betaine | |||||
| - Trigonelline | |||||
| - Stearamide | |||||
| - Ellagic Acid | |||||
| - Myricitrin | |||||
| - Myricetin | |||||
| - Leucine | |||||
| - Kaempferol | |||||
| - Α-Linoleic Acid | |||||
| 27 | Methanol | HPLC, LC/MS | - Ellagic Acid | fruit | Muthusamy et al. (2017) |
| - Phyllanthin | |||||
| - Hypophyllanthin | |||||
| - Niranthin |
4 Traditional medicine use
Balakka is a component that is frequently used in traditional medicine. This plant has been used in India to treat cancer, diabetes, liver, cardiac problems, and anemia. The fruit of the Balakka tree contains chromium, zinc, and copper. Chromium exhibits substantial antidiabetic activity in a variety of experimental diabetes models. Additionally, chromium compounds can enhance the fat metabolism of diabetic rats. The fruit of balakka is used as a treatment for tuberculosis and as an anti-aging agent. The fruit of balakka contains tannin, which has antibacterial properties, and vitamin C, which has antioxidant properties. In addition, it has been demonstrated and investigated that balakka is one of the anti-cancer plants. Balakka plant flavonoids and phenols have antioxidant properties because they can capture free radicals (Vauzour et al., 2008). One of the benefits of antioxidants is their ability to prevent degenerative diseases such as diabetes mellitus caused by oxidative stress caused by the deterioration of organ cells or body systems. Besides having medicinal properties, the natural benefits of balakka include its fruit for cars, candy, jelly jam, leather contains colorants that can run out as a blue dye in various fabrics only, tanneries, furniture and agricultural implements, and firewood. So balakka can be used for a wide variety of applications healthcare or herbal medicine, food and beverage, cosmetic, industry, dyeing, tanning, etc. According to the Wealth of India, Phyllanthus emblica seeds have been documented as a potential remedy for asthma, bronchitis. The juice that is released during the harvesting of fruit is also utilized as an ocular rinse and for the management of ocular inflammation. According to Chopra P. emblica has been recognized for its notable wound healing capabilities, making it a potential treatment option for snakebites and scorpion stings. The fixed oil included in FPE has been utilized in traditional formulations as a hair tonic to enhance hair growth and pigmentation (Chopra, 1958; Bruno, 1984).
5 Pharmacological activity of balakka (Phyllanthus emblica)
5.1 Antioxidant activity
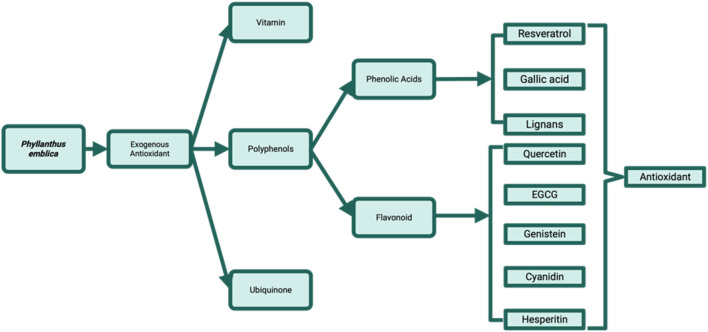
Oxidative stress is associated with an increased generation of free radicals or a reduction in antioxidant content. The observed phenomenon indicates a disturbance in the equilibrium between pro-oxidant and antioxidant molecules. Pro-oxidants and free radicals are characterized by the presence of several unpaired electrons, rendering them unstable and highly reactive towards other substances. Phyllanthus emblica has antixoidant capacity, according to previosuly study showed that methanol extract of P. emblica exhibited appreciable in vitro antioxidant activity for scavenging the DPPH radical (IC50 = 39.73 ± 2.12 μg/mL), nitric dioxide (IC50 = 39.14 ± 2.31 μg/mL) and moderate antioxidant activity for lipid peroxidation (IC50 = 84,10 ± 3.04 μg/mL). Phyllanthus emblica also demonstrated recovery ability in CCl4 treated rats by elevating the level of catalase, superoxide dismutase, glutathione peroxidase and glutathione in the rats’ pulmonary. The pharmaceutical activities of P. emblica might be attributed to by active phyto-constituents such as gallic acid, caffeic acid, kaempferol and rutin (Tahir et al., 2016). Another study also revelaed that the IC50 of the P. emblica branches, leaves and barks aqueous extract were respectively (6.92 ± 0.22) μg/mL), (7.72 ± 0.25) μg/mL), and (6.54 ± 0.27) μg/mL). These results showed slightly lower than the IC50 of the ascorbic acid (8.06 ± 0.01) μg/mL) (Iamsaard et al., 2014). The antioxidant capacity of P. emblica were addressed by phytochemical components such as reseveratrol, gallic acid, lignans, quercetin, EGCG, genistein, cyanidin, and hesperitin (Figure 3).
FIGURE 3.
Antioxidant of Phyllanthus emblica.
5.2 Antiinflammatory activity
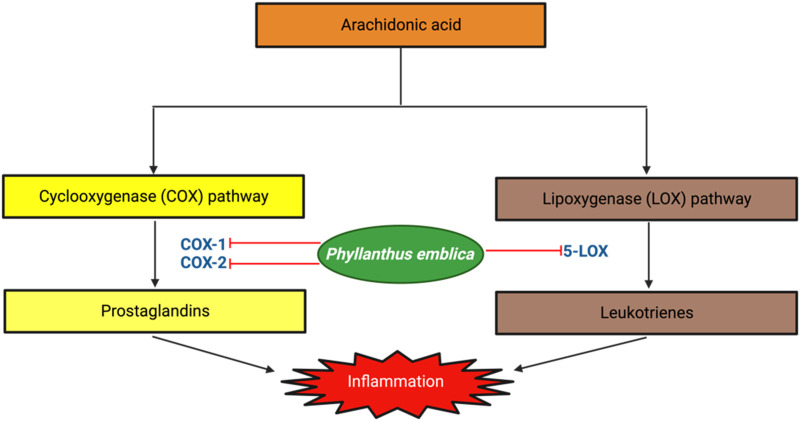
Inflammation serves as a crucial innate immune response of the organism to external stimuli, such as injury or infection caused by pathogens (Jubaidi et al., 2021). Inflammation is an essential immunological response that enables the body to endure and recover from injury. Inflammation is recognized as a beneficial pathological process due to its significant involvement in restorative, healing, and aggressive mechanisms, particularly in resisting stress generated by pathogens and harmful situations (Rakha et al., 2022). The phenomenon of inflammation is intricate and encompasses a multitude of cellular responses, mostly categorized as acute and chronic inflammation. Acute inflammation serves as a protective mechanism for the body, facilitating the healing of injuries and defending against microbial invasion. In contrast, chronic inflammation specifically targets essential cells, molecules, and organs, thereby contributing to the onset and progression of diverse chronic pathologies such as cardiovascular disease, skeletal muscle disorders, inflammatory bowel disease, diabetes, cancer, and neurological diseases (Ferraz et al., 2020). Consequently, chronic inflammation also exacerbates the aging process The fruit extracts of Phyllanthus emblica showed a significantly hight dose-dependent inhibition of Nitric Oxide (NO) and COX-2. NO is a vital immune-signaling pathways molecule. NO overproduction could cause numerous pathological disorders and abnormalities, such as serious inflammation, cardiovascular related injury, and oxidative stress. Phyllanthus emblica extracts exhibited dose-dependent NO inhibition in LPS-stimulated RAW264.7. At 50 and 100 μg/mL concentration, the 95% ethanolic extract exhibited significantly higher NO inhibition (49.1%) compared to hot water and commercial extract. The extracts of P. emblica exhibited significantly higher COX-2 inhibition compared to hot water and commercial extract. The highest COX-2 inhibition was shown at 10 μg/mL concentration (46.4%). COX-2 inhibition may control inflammation in inflammatory diseases and abnormalities. Phyllanthus emblica exhibited its anti-inflammatory activities by inhibiting NO production to avoid excess NO production in macrophage cells and COX-2 enzyme (Li et al., 2022). The mechanism by which Phyllanthus emblica exerts its anti-inflammatory effects involves the inhibition of key enzymes involved in the inflammatory process, namely, COX-1, COX-2, and 5-LOX. These enzymes play pivotal roles in the synthesis of pro-inflammatory mediators, and their suppression by P. emblica contributes to the reduction of inflammation. This multifaceted mechanism underscores the potential therapeutic value of Phyllanthus emblica in alleviating inflammatory conditions (Figure 4).
FIGURE 4.
Antiinflammatory of Phyllanthus emblica.
5.3 Immunomodulatory activity
An immunomodulator is a constituent that possesses the ability to modulate the immune system, encompassing both innate and adaptive immunological responses (Oo et al., 2021). At now, there is a growing interest in investigating the potential of bioactive chemicals obtained from medicinal plants as agents for modulating the immune system in scientific research. Inflammation can be described as a physiological process that occurs within the human body as a result of immunological responses to combat pathogens (Zaragozá et al., 2020). The human body consists of mechanisms that serve to protect against external pathogens, such as bacteria and viruses, as well as to facilitate tissue repair and initiate the healing process following an injury. Nevertheless, an overabundance of inflammation exacerbates functional impairment, causes tissue damage, and leads to pain and discomfort (Bayat et al., 2021; Masad et al., 2021; Han et al., 2022). Phyllanthus emblica enhanced the effectiveness of the immunomodulatory system by raising blood levels of CD4, CD8, CD16, CD19, IgM, and IgG as well as albumin and globulin levels in the serum. In comparison to all experimental groups, the P. emblica group at a dose of 250 mg/kg b.wt. exhibited the most appreciable outcomes to increase immunity (Bakr and Naga, 2020). The aqueous extract of P. emblica fruit possessed a dose-dependent immunomodulatory activity to albino rats with a dose of 100 and 200 mg/kg for 19 days. The fruit extracts significantly increased the hemagglutination antibody titer, leukocytes count, the percentage of lymphocytes distribution, and delayed hypersensitivity in mice (Nirala et al., 2020).
5.4 Antidiabetic activity
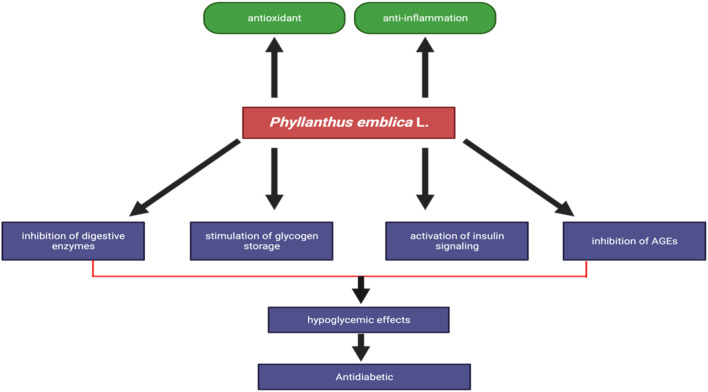
Diabetes mellitus, a prevalent endocrine metabolic illness, has resulted in substantial morbidity and death as a consequence of both microvascular consequences (retinopathy, neuropathy, and nephropathy) and macrovascular complications (heart attack, stroke, and peripheral vascular disease) (Hussain et al., 2021). The human body is equipped with both enzymatic and non-enzymatic antioxidative systems that serve to mitigate the production of reactive oxygen species, which have been implicated in the development of several degenerative disorders, such as diabetes. The prevalence of the disease is experiencing a rapid escalation on a global scale, impacting various regions across the globe (Shamsudi et al., 2022). Individuals with diabetes experience elevated levels of blood glucose due to a lack of insulin. Type 2 diabetes, also known as non-insulin-dependent diabetes mellitus, is the predominant manifestation of the condition, including approximately 90%–95% of cases characterized by insufficient insulin production or utilization (Bitew et al., 2021). According to the World Health Organization, it is projected that the diabetic population would experience a substantial growth, potentially reaching 300 million or more individuals by the year 2025 (Ansari et al., 2022). Presently, the therapeutic options for diabetes encompass insulin as well as a range of oral antidiabetic medications, including sulfonylureas, biguanides, and glinides (Kalaitzoglou et al., 2019). The presence of numerous significant unfavorable effects necessitates the exploration of more efficacious and less hazardous hypoglycemic drugs, rendering it a crucial field of inquiry (Islam et al., 2023). According to Bashir et al. (2018), the ethanolic extract of P. emblica is a potent remedy to reduce glucose level. In diabetic rats, the glucose level significantly decreased (166 ± 0.7 mg/dL) after being treated with 80 mg/kg P. emblica compared to untreated diabetic rats (380 ± 0.7 mg/dL). The ethanolic extract of P. emblica contains tannin, an effective agent to prevent adipogenesis and increase glucose uptake by increasing insulin sensitivity towards peripheral tissues (Gul et al., 2022). Phyllanthus emblica has several mechanism causes hypoglycemia which are inhibition of digestive enzymes, stimulation of glycogen storage, acativation of insulin signaling, and inhibition of AGEs (Figure 5.)
FIGURE 5.
Antiinflammatory of Phyllanthus emblica.
5.5 Hepatoprotective activity
One of the most prevalent chronic liver diseases, NAFLD (Non-alcoholic fatty liver disease) is closely associated with metabolic syndrome and refers to the accumulation of hepatic steatosis that is not brought on by excessive alcohol consumption. Phyllanthus emblica, a rich source of gallic acid and many known medically phytochemicals and acids. Phyllanthus emblica fruit exhibits in vitro inhibitory activity on hepatic steatosis and liver fibrosis (Paik et al., 2020). The gallic acid content is also in vivo proven to improve high fat diet (HFD)-induced dyslipidemia, hepatosteatosis, and oxidative stress. Huang et al. initiated a research project aiming to evaluate the hepatoprotective effect of the aqueous extract of P. emblica L. fruit (WEPE) on NAFLD in an animal model. The findings revealed that WEPE significantly reduce body weight, peritoneal fat and epididymal fat in rats treated with HFD, as well as increase antioxidant enzyme activities and improve steatosis by increasing adiponectin in adipocytes and PPAR-α in the liver and decreasing SREBP-1c in the liver. This could be the reason for WEPE’s ability to reduce hepatic fat deposition. These findings exhibited that WEPE could be beneficial for treating HFD-induced steatosis (Tung et al., 2018).
5.6 Neuroprotective activity
Flavonoids have garnered attention for their ability to alter neuronal activity and prevent age-related neurodegeneration (Dajas et al., 2003). Flavonoid-rich plant or food extracts may preserve fragile neurons, enhance neuronal function, or stimulate neuronal regeneration to improve cognition in people and animals (Hwang et al., 2012). Their neuroprotective properties have been demonstrated in oxidative stress and Aβ-induced neuronal death models. Ginkgo biloba extracts high in flavonoids have been shown to benefit and modulate the brain, especially in Alzheimer’s disease and age-related dementia (Calderaro et al., 2022). Individual flavonoids, such as the citrus flavanone tangeretin, have been shown to maintain nigro-striatal integrity and functionality after 6-hydroxydopamine lesioning, suggesting that it may protect against Parkinson’s disease pathology (Vauzour et al., 2008). In a recent study conducted by Rajalakshmi et al. (2019), The results of this study on Phyllanthus emblica (Indian gooseberry) offer valuable insights into its potential neuroprotective and antioxidant properties, shedding light on its traditional medicinal uses. This research focused on assessing the radical scavenging capabilities of P. emblica through various assays and its impact on neuroprotection using human neural cell lines (PC12) subjected to glutamate-induced cellular inhibition.
One of the key findings of this investigation was the remarkable antioxidant activity exhibited by P. emblica extract. The DPPH and hydroxyl radical scavenging assays revealed IC50 values of 73.21 μg/mL and 0.426 mg/mL, respectively. This indicates its capacity to effectively neutralize free radicals, which are known to contribute to oxidative stress and various health issues. Moreover, the study observed significant lipid peroxidation activity (IC50: 73.21 μg/mL), further highlighting the potential of P. emblica in preventing oxidative damage to cellular membranes, a crucial factor in maintaining cellular integrity. The neuroprotective effects of P. emblica were also explored, and the results demonstrated its ability to safeguard PC12 cells against glutamate-induced cytotoxicity. This was confirmed through cell viability assays, which showed that P. emblica extract had a protective effect on neural cells. Additionally, monitoring LDH activity, GSH levels, and ROS levels provided further evidence of its neuroprotective properties. According to Li et al. (2011), the extracts of P. emblica were found to provide protection to PC12 cells against cell death triggered by H2O2. All samples derived from P. emblica exhibited a protective effect on H2O2-induced PC12 cell death, which was both dose-dependent and consistent across all extracts. The results indicate that the hot water and ethanol extracts exhibited superior PC12 cell protection percentages compared to the commercial extracts. According to Li et al. (2022), the neuroprotective benefits of hydroalcoholic extracts derived from P. emblica were shown in rat models with kainic acid-induced seizures. These effects may be attributed to the antioxidant and anti-inflammatory properties of the extracts.
5.7 Cardioprotective potential
Globally, there is a significant escalation in the prevalence of chronic diseases, including cardiovascular diseases, cancer, diabetes, and obesity. In the year 2001, chronic diseases accounted for nearly 59% of the total recorded deaths worldwide, amounting to 56.5 million fatalities. Furthermore, these disorders were responsible for 46% of the overall burden of disease on a global scale. Cardiovascular diseases (CVD) encompass a range of conditions that affect the heart and blood arteries, such as hypertension (elevated blood pressure) and coronary heart disease (myocardial infarction) (Mayakrishnan et al., 2013). According to Ekta in Pria et al, oral administration of P. emblica fruit extract at a dose of 50 and 100 mg/kg BW twice a day for 2 weeks significantly reversed the effects of IRI (ischaemia reperfusion injury), a disease occurs due to oxidative stress. This cardioprotective effect occurs due to the emblicanin A and B contents of P. emblica fruit extract (Rajak et al., 2004).
Globally, there has been a concerning surge in the prevalence of chronic diseases, encompassing conditions such as cardiovascular diseases, cancer, diabetes, and obesity. In 2001, chronic diseases accounted for a staggering 59% of total recorded deaths worldwide, resulting in 56.5 million fatalities. Additionally, these ailments imposed a substantial burden, constituting 46% of the overall global disease burden. Among chronic diseases, cardiovascular diseases (CVD) comprise a spectrum of conditions affecting the heart and blood vessels, including hypertension (elevated blood pressure) and coronary heart disease (myocardial infarction) (Mayakrishnan et al., 2013). In a noteworthy study by Ekta in Pria et al., it was demonstrated that the oral administration of P. emblica fruit extract at doses of 50 and 100 mg/kg body weight, twice a day for 2 weeks, led to a significant reversal of the effects of ischemia-reperfusion injury (IRI), a condition arising from oxidative stress. This observed cardioprotective effect is attributed to the presence of emblicanin A and B within P. emblica fruit extract (Rajak et al., 2004).
5.8 Anticancer activity
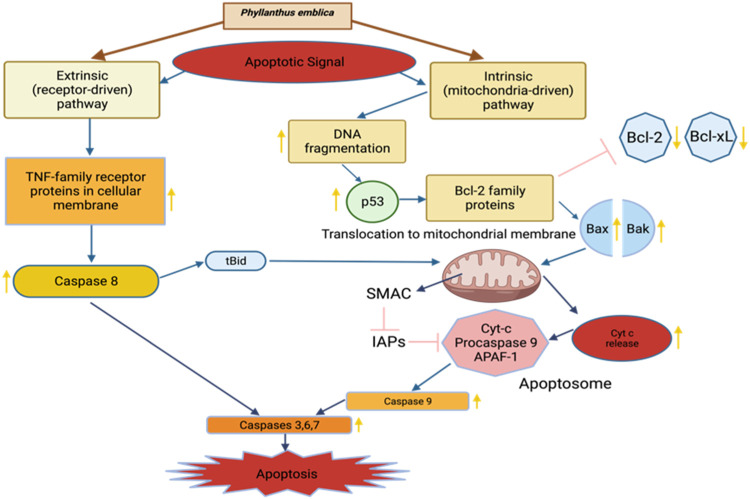
Cancer remains a prominent global health challenge, compelling researchers to explore novel substances and treatments aimed at diminishing cancer cell survival, impeding angiogenesis, thwarting proliferation, and restraining metastasis. In the past decade, specific phytochemical compounds and flavonoids have emerged as promising candidates for cancer therapy. In a study conducted by Mahata et al. (2013), the mechanism of action of Phyllanthus emblica fruit extract was meticulously examined. The investigation focused on its impact on activator protein-1 (AP-1) activity and its relevance to cervical cancer cells driven by human papillomavirus (HPV). The findings unveiled a compelling pattern: the Phyllanthus emblica fruit extract exhibited dose- and time-dependent inhibition of DNA binding in both constitutively active AP-1 HPV16-positive (SiHa) and HPV18-positive (HeLa) cervical cancer cells. This AP-1 inhibition, accompanied by the suppression of viral transcription, resulted in the inhibition of cervical cancer cell growth. Moreover, the growth-inhibitory effect of Phyllanthus emblica was primarily attributed to the induction of apoptotic cell death. These findings collectively suggest that Phyllanthus emblica demonstrates its anticancer efficacy by concurrently inhibiting AP-1 and targeting the transcription of viral oncogenes responsible for the development of cervical cancer, thereby indicating its potential utility in the treatment of HPV-induced cervical cancer cells (Mahata et al., 2013; Quranayati et al., 2022). In a separate investigation by Samatiwat et al. (2021), the anti-proliferative activity of the ethanolic extract of Phyllanthus emblica bark was assessed in the context of cholangiocarcinoma. The results highlighted the extract’s cytotoxic potential on the KKU-452 CCA cell line, with an IC50 value of 52.2 μg/mL, accompanied by a significant induction of apoptosis. Furthermore, the ethanolic extract of Phyllanthus emblica bark substantially inhibited cell migration at concentrations of 25 and 50 μg/mL, with reductions of 425% and 32.9%, respectively, compared to untreated cells. These anticancer effects were attributed to the phenolic acid and flavonoid content present in the bark extract of Phyllanthus emblica (Samatiwat et al., 2021). The anticancer potential of Phyllanthus emblica is graphically illustrated in Figure 6.
FIGURE 6.
Anticancer of Phyllanthus emblica.
5.9 Antihyperlipidemic and atherogenic activity
In recent years, hyperlipidemia and oxidative stress have emerged as significant health concerns, acknowledged as primary risk factors in the development and progression of atherosclerosis, as well as cardiovascular and cerebrovascular diseases (Li et al., 2011). Consequently, there has been a growing interest in exploring the potential of new natural antioxidants, predominantly of plant origin (Sun et al., 2021). Flavonoids and phenolic compounds derived from plants have gained recognition for their multifaceted pharmacological properties, including antioxidant and anti-hyperlipidemic effects (Gong et al., 2020). The powdered extract of Phyllanthus emblica has demonstrated remarkable anti-hyperlipidemic, hypolipidemic, and anti-atherogenic effects, substantiated by statistically significant differences when compared to control groups (Santoshkumar et al., 2013). These effects can be conceivably attributed to the flavonoid content found in Phyllanthus emblica, which exhibits a hypolipidemic effect in atherogenic albino rats. The flavonoids in Phyllanthus emblica function as hypolipidemic agents by inhibiting the activity of HMG-CoA reductase while concurrently increasing the activity of plasma lecithin cholesterol acyl transferase (LCAT) (Sobhani et al., 2017).
5.10 Antihypertensive activity
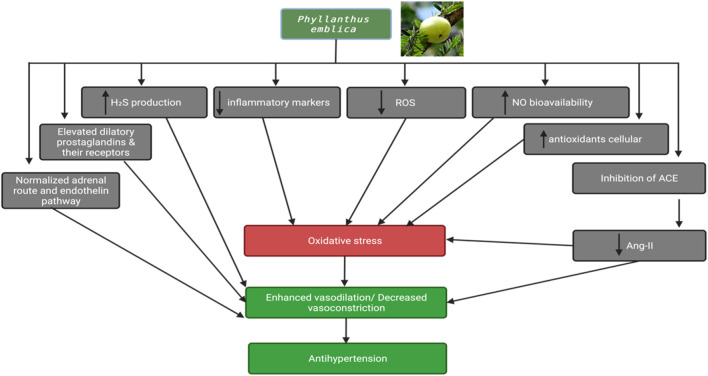
In a rigorously conducted study by Ghaffari et al. (2020), a randomized, triple-blind, and placebo-controlled research approach was employed to investigate the antihypertensive effects of P. emblica. The study included 92 patients who were randomly assigned to two groups. Each group received either P. emblica at a dosage of 500 mg/three times daily after meals or a placebo, in addition to their standard antihypertensive treatments. The findings of this study were quite compelling. After 8 weeks of treatment, the systolic blood pressure exhibited a significant reduction of 15.6% ± 8.23% in the P. emblica group, as compared to a 6.3% ± 7.49% decrease in the placebo group. Likewise, diastolic blood pressure showed a notable decrease of 12.3% ± 7.87% in the P. emblica group, in contrast to a 3.88% ± 7.98% decrease in the placebo group. Importantly, throughout the study duration, no toxic side effects were observed in any of the patients, underscoring the safety profile of P. emblica in this context (Ghaffari et al., 2020). For a visual representation of the mechanism, please refer to Figure 7.
FIGURE 7.
Antihypertension activity of Phyllanthus emblica.
5.11 Antimicrobial activity
Jahan and Akter (2015) conducted research to evaluate the antimicrobial activity of Phyllanthus emblica. This research was conducted utilizing disc diffusion method. The methanolic extract of P. emblica was assayed at 500 µg/disc concentration with standard kanamycin disc against gram positive, gram negative and multi drug resistant strains. The results demonstrated that the ethanolic extract of PE exhibited significant antimicrobial effect againts some microorganisms especially, Bacillus subtilis (25 mm), Staphylococcus aureus (20 mm) and Shigella dysenteriae (17 mm). Both aqueous and ethanolic extract of P. emblica was proven effective against Pseudomonas aeruginosa in disc diffusion method (Farhana et al., 2022). The methanolic extract of P. emblica at a dose rate of 50 mg/mL and 25 mg/mL, respectively, had a complete bactericidal effect on AMR (antimicrobial-resistant) S. Typhi and S. Enteritidis (Nair et al., 2020).
5.12 Anti-diarrheal activity
According to Afrin et al. (2016), P. emblica extract exhibited a significant anti-diarrheal effect. These authors conducted research to evaluate the anti-diarrheal activity towards diarrheal (castor oil induced) mice at a dose of 2 mL/mouse. The extracts of P. emblica at a dose of 500 mg/kg BW were given 1 h before the mice were induced with castor oil orally. The results showed that P. emblica significantly inhibited the defecation mean number compared to control group administrated with standard anti-diarrheal drug. The methanolic extract of P. emblica at a dose of 25% exhibited 42.86% inhibition while at a dose of 500 mg/kg BW, it showed 64.29% inhibition. The anti-diarrheal standard drug (loperamide) at a dose of 5 mg/kg exhibited 71.43% inhibition. As P. emblica succesfully inhibited the castor oil induced diarrhea, it might have exhibited its antidiarrheal effect by inhibiting the biosynthesis of prostaglandin through antisecretory mechanism (Afrin et al., 2016).
5.13 Anti-aging activity
In a study conducted by Wu et al. (2022), it was observed that the polyphenols found in the fruit of P. emblica demonstrated a notable protective effect against the aging process in the Caenorhabditis elegans model. The anti-aging properties were evidenced through the augmentation of thermal resistance, as well as the significant reduction in the activity levels of acetylcholinesterase (AChE) by 34.71% and butyrylcholinesterase (BuChE) by 45.38%. These findings were accompanied by a significant increase in the activity of antioxidant enzymes, specifically superoxide dismutase (SOD) by 30.74% and catalase (CAT) by 8.42%. Additionally, there was a notable decrease in the amount of malondialdehyde (MDA) by 36.25%. The presence of rich flavonols and phenolic acids, including quercetin, myricetin, ellagic, gallic, and chlorogenic acids, together with their glycosides, may potentially be associated with the interrelation of these qualities (Wu et al., 2022).
5.14 Laxative
Mehmood et al. (2013) reported that the crude extract derived from P. emblica exhibited prokinetic and laxative properties in mice. The extract facilitated the movement of charcoal meal through the small intestine and resulted in an increase in the production of wet feces. These effects were comparable to the effects observed with carbachol, a commonly used cholinergic agonist known for its ability to accelerate intestinal contents. The extract’s gut stimulatory activities were observed to be slightly influenced by atropine, a blocker of muscarinic receptors. The mice exhibited wet feces at a rate of 35.7% and 44.1% when administered with a crude extract of P. emblica at doses of 100 and 300 mg/kg, respectively. The experimental group receiving the positive control, CCh (1 mg/kg), exhibited a moist feces percentage of 48.2%, whereas the group treated with saline solution displayed a wet feces percentage of just 10.5%. The laxative effect was determined by calculating the percentage increase in moist feces compared to the total fecal production, as described by Mehmood et al. (2013). The pharmacological activity of P. emblica can be seen in Table 2.
TABLE 2.
Pharmacological effects and mechanisms of action of extract from Phyllanthus emblica.
| No. | Dose | Pharmacology activity | Mechanism | References |
|---|---|---|---|---|
| 1 | 50 and 100 μg/mL | Antioxidant | - enhancing reducing power and total antioxidant capacity, scavenging hydroxil radical and superoxide anion | Uddin et al. (2016); Li et al. (2020) |
| - Reduced peroxidation level | ||||
| - Induced defense system (GSH, SOD, CAT, GPx, GSH reductase, and GSH S-transferase) | ||||
| 2 | 50 and 100 μg/mL | Antiinflammatory | - Inhibited Nitric Oxide (NO) and COX-2 (dose-dependent) | Li et al. (2022) |
| 3 | 100; 200; 500 mg/kg BW | Immunomodulatory | - Induced the activity of GSH, CAT, and SOD in the thymus of mice | Ghosh et al. (2013); Saini et al. (2022) |
| - increased the hemagglutination antibody titer, leukocytes count, the percentage of lymphocytes distribution, and delayed hypersensitivity in mice | ||||
| 4 | 500 mg/kg BW | Hepatoprotective | - Gallic acid content improves high fat diet induced dyslipidemia, hepatosteatosis, and oxidative stress | Huang et al. (2017) |
| - increasing adiponectin in adipocytes and PPAR-α in the liver and decreasing SREBP-1c in the liver | ||||
| 5 | Dose dependent | Neuroprotective | - Conferred PC12 cells protection against H2O2-induced cell death | Li et al. (2022) |
| 6 | 50 and 100 mg/kg BW | Cardioprotective | - The emblicanin A and B contents reversed the effects of IRI (ischaemia reperfusion injury) | Priya and Islam (2019) |
| 7 | 25 and 50 μg/mL | Anticancer | - Phenolic acid and flavonoid content inhibited cell migration | Samatiwat et al. (2021) |
| 8 | 2 mL/kg/day | Antihyperlipidemic | - Activated PPARα and carnitine palmitoyl transferase (involved in lipid oxidation) | Variya et al. (2016) |
| - Reduced the serum triglycerides | ||||
| 9 | 500 mg/TDS | Antihypertensive | - Reduced systolic and diastolic blood pressure | Ghaffari et al. (2020) |
| 10 | 50 and 25 mg/mL | Antimicrobial | - Methanolic extract showed potent antimicrobial activity to antimicrobial-resistant (AMR) Salmonella Typhi and Salmonella Enteritidis; Bacillus subtilis, Staphylococcus aureus and Shigella dysenteriae; Pseudomonas aeruginosa | Nair et al. (2020); Jahan and Akter (2015); Farhana et al. (2022) |
| 11 | 250 and 500 mg/kg BW | Anti-diarrheal | - Inhibited the biosynthesis of prostaglandin through antisecretory mechanism | Afrin et al. (2016) |
| 12 | 1.2 mg/mL | Anti-aging | - Reduced the activity of AchE, BuChE; enhanced antioxidant enzymes activity of SOD and CAT; decrease MDA level | Wu et al. (2022b) |
| 13 | 100 and 300 mg/kg BW | Laxative | - The crude extract increased the production of wet feces at a mechanism similar to the effect of standard drug carbachol | Mehmood et al. (2013) |
6 Safety and toxicity
Plants especially medicinal plants have been used for treatments in humans. Since the prehistoric era, raw parts or extract of Phyllanthus emblica have been used to treat various diseases and in vitro and in vivo studies have proven its effectiveness to inhibit various pathogenesis. Chronic toxicity studies by inducting P. emblica oral doses of 300, 600 and 1,200 mg/kg for 270 days resulted in no evident changes in treated animals pathologically. Previous studies reported the absence of toxicity of P. emblica fruit extract at doses of 200, 400, 300, and 500 mg/kg (Golechha et al., 2014; Middha et al., 2015; Anto et al., 2022). Furthermore, Uddin et al. (2016) reported that the ethanolic extract of Phyllanthus emblica is safe with a dose up to 2000 mg/kg b.w. in rats. The hematological examination, behavioral observation, and biochemical test of P. emblica demonstrated the absence of toxic effect. Thiennimitr et al. (2018) reported that consuming Lactobacillus sp. mediated fermented P. emblica fruit juice up to 9 mL/kg/day for 60 days to both male and female rats is safe and no rat exhibited any remarkable changes in the body weights, internal organs, hematology, and biochemical parameters.
7 Future perspective and challaenges
Plants have been used as remedial agents throughout history. In the last two centuries, scientists have found a way to utilize secondary metabolites contents in medicinal plants for drug manufacturing. Phyllanthus emblica, is a widely used herb in the Indian medicinal system. Numerous researches have proven therapeutic properties using different extracts and herbal preparations of P. emblica. A variety of well-established beneficial health effects and pharmacological activities was ascribed to P. emblica, namely, antioxidant, anti-cancer, hepatoprotective, neuroprotective, immunomodulatory, anti-inflammatory, anti-diabetic, and anti-hyperlipidemic effects. Phyllanthus emblica is traditionally used to address numerous disorders along with food ingredients. In spite of the fact that various modern research techniques have been established to validate the medicinal uses of P. emblica traditionally, some aspects including its contents and its applications need to be further investigated scientifically. For example, only a few studies reported P. emblica antimalarial, antiviral, anti-venom, and insecticidal properties. Some of its properties were also reported with other parts of P. emblica. Accordingly, it is imperative that the agents, molecules or parts mediating its therapeutic activities be identified. Additionally, more extensive research, such large-scale evidence-based trials, must be done to examine the medical benefits of P. emblica.
8 Conclusion
In conclusion, our comprehensive review of P. emblica, commonly known as Indian gooseberry or Amla, has shed light on its remarkable phytochemical composition and extensive pharmacological properties. This indigenous fruit, deeply rooted in traditional medicine, has proven to be a valuable source of bioactive compounds that hold immense potential for various therapeutic applications. The phytochemical analysis of Phyllanthus emblica revealed a rich array of secondary metabolites, including flavonoids, tannins, polyphenols, and ascorbic acid. These compounds collectively contribute to the antioxidant, anti-inflammatory, and antimicrobial activities of the plant, making it a promising candidate for the development of natural remedies and pharmaceutical agents. Regarding its pharmacological properties, Phyllanthus emblica has exhibited a wide spectrum of effects, ranging from its hepatoprotective and immunomodulatory actions to its antidiabetic, anticancer, and cardioprotective potentials. The extensive research conducted in this area has highlighted the versatility of this botanical treasure, providing avenues for future investigations and applications in the realm of medicine and healthcare. However, it is essential to acknowledge the limitations of our review. Firstly, while we have compiled a comprehensive overview of the available literature, the field of P. emblica research is continuously evolving, and new discoveries may have emerged since the completion of this review. Additionally, the majority of studies have been conducted in vitro or in animal models, necessitating further clinical trials to establish the efficacy and safety of Phyllanthus emblica-based interventions in humans. Moreover, variations in the phytochemical composition of P. emblica due to geographical and environmental factors pose a challenge in standardizing its therapeutic applications. Phyllanthus emblica holds great promise as a source of bioactive compounds with diverse pharmacological properties. While this review serves as a valuable resource for understanding its potential benefits, further research and clinical investigations are warranted to fully harness the therapeutic potential of this remarkable botanical species. Despite the limitations, the compelling evidence presented in this review underscores the significance of Phyllanthus emblica in the realm of natural medicine and inspires continued exploration in the pursuit of improved healthcare solutions.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Author contributions
AP: Funding acquisition, Methodology, Writing–original draft. AD: Software, Supervision, Writing–original draft. UH: Conceptualization, Visualization, Writing–original draft. YS: Formal analysis, Writing–review and editing. EP: Validation, Writing–review and editing. NK: Investigation, Writing–original draft. PH: Formal analysis, Writing–review and editing. RS: Conceptualization, Data-curation, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. PS: Formal analysis, Writing–original draft. FN: Data-curation, Formal analysis, Supervision, Visualization, Writing–review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Hady H., Abdallah morsi E., Ahmed El-wakil E. (2022). In-vitro antimicrobial potentialities of phylunthus emblica leaf extract against some human pathogens. Egypt. J. Chem. 65 (7), 705. 10.21608/EJCHEM.2021.109577.4998 [DOI] [Google Scholar]
- Acharya C. D. (2016). Ethnicity and Scientific validation of West Bengal Amla (Phyllanthus emblica L.) with special reference to GC-MS screening. Int. J. Exp. Res. Rev. 3 (1), 54. 10.52756/ijerr.2016.v03.006 [DOI] [Google Scholar]
- Afrin F., Banik S., Hossain M. S. (2016). Pharmacological activities of methanol extract of Phyllanthus acidus pulp. J. Med. Plants Res. 10 (43), 790–795. 10.5897/JMPR2015.5806 [DOI] [Google Scholar]
- Ahmad B., Hafeez N., Rauf A., Bashir S., Linfang H., Rehman M. U., et al. (2021). Phyllanthus emblica: a comprehensive review of its therapeutic benefits. South Afr. J. Bot. 138, 278–310. 10.1016/j.sajb.2020.12.028 [DOI] [Google Scholar]
- Akter R., Khan S. S., Kabir M. T., Halder S. (2022). GC-MS-employed phytochemical characterization, synergistic antioxidant, and cytotoxic potential of Triphala methanol extract at non-equivalent ratios of its constituents. Saudi J. Biol. Sci. 29 (1), 103287. 10.1016/j.sjbs.2022.103287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir D. (2014). Composition of the essential oil of the fruits of Phyllanthus emblica cultivated in Egypt. J. Pharm. Chem. Biol. Sci. 2 (3), 205. [Google Scholar]
- Ansari P., Akther S., Hannan J. M. A., Seidel V., Nujat N. J., Abdel-Wahab Y. H. (2022). Pharmacologically active phytomolecules isolated from traditional antidiabetic plants and their therapeutic role for the management of diabetes mellitus. Molecules 27 (13), 4278. 10.3390/molecules27134278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anto E. J., Syahputra R. A., Silitonga H. A., Situmorang P. C., Nugaraha S. E. (2022). Oral acute toxicity study extract ethanol of balakka fruit (Phyllanthus emblica). Pharmacia 69 (1), 187–194. 10.3897/pharmacia.69.e81280 [DOI] [Google Scholar]
- Asmilia N., Fahrimal Y., Abrar M., Rinidar R. (2020). Chemical compounds of Malacca leaf(Phyllanthus emblica) after triple extraction with N-hexane, ethyl acetate, and ethanol. e Sci. World J. 1 (1), 2739056. 10.1155/2020/2739056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgai T. R., Hashinaga F., Isobe S., Raghavan G. V., Ngadi M. O. (2006). Application of high electric field (HEF) on the shelf-life extension of emblic fruit (Phyllanthus emblica L.). J. Food Eng. 74 (3), 308–313. 10.1016/j.jfoodeng.2005.03.023 [DOI] [Google Scholar]
- Bakr E. H., Naga M. (2020). Immunomodulatory efficacy of Phyllanthus emblica and Costus speciosus aqueous extracts for immunosuppressive rats. Egypt. J. Nutr. 35 (2), 101–123. 10.21608/ENJ.2020.144766 [DOI] [Google Scholar]
- Balusamy S. R., Veerappan K., Ranjanm A. (2019). Phyllanthus emblica fruit extract attenuates lipid metabolism in 3T3-L1 adipocytes via activating apoptosis mediated cell death. J. Pre-proof 1 (1), 6. 10.1016/j.phymed.2019.153129 [DOI] [PubMed] [Google Scholar]
- Bashir A. S. I. F. A., Mushtaq A. A. M. I. R., Mehboob T. O. O. B. A. (2018). Evaluation of antioxidant and antidiabetic activity of Phyllanthus emblica (fruit). Biol. Pak. 64 (1), 85–91. [Google Scholar]
- Bayat P., Farshchi M., Yousefian M., Mahmoudi M., Yazdian-Robati R. (2021). Flavonoids, the compounds with anti-inflammatory and immunomodulatory properties, as promising tools in multiple sclerosis (MS) therapy: a systematic review of preclinical evidence. Int. Immunopharmacol. 95, 107562. 10.1016/j.intimp.2021.107562 [DOI] [PubMed] [Google Scholar]
- Bitew M., Desalegn T., Demissie T. B., Belayneh A., Endale M., Eswaramoorthy R. (2021). Pharmacokinetics and drug-likeness of antidiabetic flavonoids: molecular docking and DFT study. Plos one 16 (12), e0260853. 10.1371/journal.pone.0260853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M. (1984). Raw materials, profits, and the productivity slowdown. Q. J. Econ. 99 (1), 1–29. 10.2307/1885718 [DOI] [Google Scholar]
- Calderaro A., Patanè G. T., Tellone E., Barreca D., Ficarra S., Misiti F., et al. (2022). The neuroprotective potentiality of flavonoids on alzheimer’s disease. Int. J. Mol. Sci. 23 (23), 14835. 10.3390/ijms232314835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda S., Biswas S. M., Sarkar P. K. (2020). Phytochemicals and antiviral properties of five dominant medicinal plant species in Bankura district, West Bengal: an overview. J. Pharmacogn. Phytochemistry 9 (6), 1420–1427. 10.22271/phyto.2020.v9.i6u.13146 [DOI] [Google Scholar]
- Chaphalkar R., Apte K. G., Talekar Y., Ojha S. K., Nandave M. (2017). Antioxidants of Phyllanthus emblica L. bark extract provide hepatoprotection against ethanol-induced hepatic damage: a comparison with silymarin. Oxidative Med. Cell. Longev. 2017, 3876040. 10.1155/2017/3876040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekdaengphanao P., Jaiseri D., Sriraj P., Aukkanimart R., Prathumtet J., Udonsan P., et al. (2022). Anticancer activity of Terminalia chebula, Terminalia bellirica, and Phyllanthus emblica extracts on cholangiocarcinoma cell proliferation and induction of apoptosis. J. Herb. Med. 35, 100582. 10.1016/j.hermed.2022.100582 [DOI] [Google Scholar]
- Chopra R. N. (1958). Chopra's indigenous drugs of India, 284. Academic Publishers, 1994, 503–505. [Google Scholar]
- Dajas F., Rivera F., Blasina F., Arredondo F., Echeverry C., Lafon L., et al. (2003). Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox. Res. 5, 425–432. 10.1007/BF03033172 [DOI] [PubMed] [Google Scholar]
- Dinesh M., Roopan S. M., Selvaraj C. I. (2016). Photocatalytic degradation of nitrophenol using biologically active Phyllanthus emblica seed extract. J. Photochem. Photobiol. 1 (1), 273–278. 10.1016/j.jphotobiol.2016.05.033 [DOI] [PubMed] [Google Scholar]
- Dinesh M., Roopan S. M., Selvaraj C. I., Arunachalam P. (2017). Phyllanthus emblica seed extract mediated synthesis of PdNPs against antibacterial, heamolytic and cytotoxic studies. J. Photochem. Photobiol. B Biol. 167, 64–71. 10.1016/j.jphotobiol.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Elangovan M., Irulappan E. (2015). Determination of bioactive compounds from the petroleum ether leaf extract of moringa oleifera and Phyllnthus emblica using GC-MS analysis. World J. Pharm. Res. 4 (3), 1290. [Google Scholar]
- Farhana F., Mosaddek A. S. M., Joynal B. J., Sharmin H., Mosaddek N. (2022). Antibacterial effect of Amlaki (Phyllanthus emblica) extract against Pseudomonas aeruginosa . J. Clin. images Med. case Rep. 3, 6. 10.52768/2766-7820/1886 [DOI] [Google Scholar]
- Ferraz C. R., Carvalho T. T., Manchope M. F., Artero N. A., Rasquel-Oliveira F. S., Fattori V., et al. (2020). Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 25 (3), 762. 10.3390/molecules25030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaire B. P., Subedi L. (2014). Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin. J. Integr. Med. 2, 014. 10.1007/s11655-014-1984-2 [DOI] [PubMed] [Google Scholar]
- Gantait S., Mahanta M., Bera S., Verma S. K. (2021). Advances in biotechnology of Emblica officinalis Gaertn. syn. Phyllanthus emblica L.: a nutraceuticals-rich fruit tree with multifaceted ethnomedicinal uses. 3 Biotech. 11, 62–25. 10.1007/s13205-020-02615-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari S., Navabzadeh M., Ziaee M., Ghobadi A., Ghods R., Hashem-Dabaghian F. (2020). A randomized, triple-blind, placebo-controlled, add-on clinical trial to evaluate the efficacy of Emblica officinalis in uncontrolled hypertension. Evidence-Based Complementary Altern. Med. 2020, 8592869. 10.1155/2020/8592869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S. (1996). Active constituents of Emblica officinalis: Part I. The chemistry and antioxidative effects of two new hydrolysable tannins. Emblicanin A B. Indian J. Chem. 35, 941–948. 10.1002/chin.199647279 [DOI] [Google Scholar]
- Ghosh A. R. K. A., Laloo D. A. M. I. K. I., Singh N. K. (2013). Comparative estimation and chemical standardization of new and old sample of Chyawanprash. Int. J. Pharm. Pharm. Sci. 5, 801–804. [Google Scholar]
- Golechha M., Sarangal V., Ojha S., Bhatia J., Arya D. S. (2014). Anti-inflammatory effect of Emblica officinalis in rodent models of acute and chronic inflammation: involvement of possible mechanisms. Int. J. Inflamm. 2014, 178408. 10.1155/2014/178408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Li X., Xia Y., Xu J., Li Q., Zhang C., et al. (2020). Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends food Sci. Technol. 103, 304–320. 10.1016/j.tifs.2020.07.026 [DOI] [Google Scholar]
- Gul M., Liu Z. W., Rabail R., Faheem F., Walayat N., Nawaz A., et al. (2022). Functional and nutraceutical significance of Amla (Phyllanthus emblica L.): a review. Antioxidants 11 (5), 816. 10.3390/antiox11050816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim B., Syahputra R. A., Adenin I., Lubis H., Mendrofa F., Lie S., et al. (2022). Determination of phytochemical constituent, antioxidant activity, total phenol and total flavonoid of extract ethanol Phyllanthus emblica fruit. Pharmacogn. J. 14 (1), 63–67. 10.5530/pj.2022.14.9 [DOI] [Google Scholar]
- Han L., Fu Q., Deng C., Luo L., Xiang T., Zhao H. (2022). Immunomodulatory potential of flavonoids for the treatment of autoimmune diseases and tumour. Scand. J. Immunol. 95 (1), e13106. 10.1111/sji.13106 [DOI] [Google Scholar]
- Harahap F. S., Atifah Y., Ginting N. (2019). Comparison of the chemical compounds of Malacca bark and Malacca fruit (Phyllanthus emblica) with gas chromatography-mass spectrometer (GC-MS). J. Phys. Conf. Ser. 1 (1), 4. 10.1088/1742-6596/1477/7/072011 [DOI] [Google Scholar]
- Huang C. Z., Tung Y. T., Hsia S. M., Wu C. H., Yen G. C. (2017). The hepatoprotective effect of Phyllanthus emblica L. fruit on high fat diet-induced non-alcoholic fatty liver disease (NAFLD) in SD rats. Food & Funct. 8 (2), 842–850. 10.1039/C6FO01585A [DOI] [PubMed] [Google Scholar]
- Huang Y. N., Chen S. Y., Lin J. A., Chiang I. C., Yen G. C. (2023). Phyllanthus emblica L. extract alleviates leptin resistance and lipid accumulation by inhibiting methylglyoxal production. Food Biosci. 53, 102619. 10.1016/j.fbio.2023.102619 [DOI] [Google Scholar]
- Hussain N., Kakoti B. B., Rudrapal M., Sarwa K. K., Celik I., Attah E. I., et al. (2021a). Bioactive antidiabetic flavonoids from the stem bark of Cordia dichotoma Forst.: identification, docking and ADMET studies. Molbank 2021 (2), M1234. 10.3390/m1234 [DOI] [Google Scholar]
- Hussain S. Z., Naseer B., Qadri T., Fatima T., Bhat T. A. (2021b). “Anola (emblica officinalis): morphology, taxonomy, composition and health benefits,” in Fruits grown in highland regions of the himalayas: nutritional and health benefits (Cham: Springer International Publishing; ), 193–206. [Google Scholar]
- Hwang S. L., Shih P. H., Yen G. C. (2012). Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 60 (4), 877–885. 10.1021/jf204452y [DOI] [PubMed] [Google Scholar]
- Iamsaard S., Arun S., Burawat J., Sukhorum W., Wattanathorn J., Nualkaew S., et al. (2014). Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllanthus emblica L.) aqueous extracts. J. Zhejiang Univ. Sci. B 15 (4), 405–408. 10.1631/jzus.B1300284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. R., Akash S., Jony M. H., Alam M. N., Nowrin F. T., Rahman M. M., et al. (2023). Identifying novel therapeutic inhibitors to target FMS-like tyrosine kinase-3 (FLT3) against acute myeloid leukemia: a molecular docking, molecular dynamics, and DFT study. Mol. Cell. Biochem. 7, 12–16. 10.1080/07391102.2023.2192798 [DOI] [PubMed] [Google Scholar]
- Jagdale Y. D., Mahale S. V., Zohra B., Nayik G. A., Dar A. H., Khan K. A., et al. (2021). Nutritional profile and potential health benefits of super foods: a review. Sustainability 13 (16), 9240. 10.3390/su13169240 [DOI] [Google Scholar]
- Jahan N., Akter S. (2015). Assessment of the antimicrobial activity of the ethanolic extract of Phyllanthus emblica in combination with different classes of antibiotics against single and multi-drug resistant strains. J. Pharmacogn. Phytochemistry 4 (4), 142–155. [Google Scholar]
- Jantan I., Haque M. A., Ilangkovan M., Arshad L. (2019). An insight into the modulatory effects and mechanisms of action of phyllanthus species and their bioactive metabolites on the immune system. Front. Pharmacol. 10, 878. 10.3389/fphar.2019.00878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubaidi F. F., Zainalabidin S., Taib I. S., Hamid Z. A., Budin S. B. (2021). The potential role of flavonoids in ameliorating diabetic cardiomyopathy via alleviation of cardiac oxidative stress, inflammation and apoptosis. Int. J. Mol. Sci. 22 (10), 5094. 10.3390/ijms22105094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzoglou E., Fowlkes J. L., Popescu I., Thrailkill K. M. (2019). Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes/metabolism Res. Rev. 35 (2), e3100. 10.1002/dmrr.3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled S. E., Hashem F. A., Shabana M. H. (2018). A biochemometric approach for the assessment of Phyllanthus emblica female fertility effects as determined via UPLC-ESI-qTOFMS and GC-MS. food Funct. 1 (1), 9. 10.1039/c9fo00767a [DOI] [PubMed] [Google Scholar]
- Kiran K. R., Swathy P. S., Paul B., Shama Prasada K., Radhakrishna Rao M., Joshi M. B., et al. (2021). Untargeted metabolomics and DNA barcoding for discrimination of Phyllanthus species. J. Ethnopharmacol. 1 (1), 113928. 10.1016/j.jep.2021.113928 [DOI] [PubMed] [Google Scholar]
- Kumar S., Chandra P., Bajpai V., Singh A., Srivastava M., Mishra D., et al. (2015). Rapid qualitative and quantitative analysis of bioactive compounds from Phyllanthus amarus using LC/MS/MS technique, s. Industrial Crops Prod. 69 (1), 143–152. 10.1016/j.indcrop.2015.02.012 [DOI] [Google Scholar]
- Kumar S., Singh A., Bajpai V. (2017c). Development of a UHPLC–MS/McS method for the quantitation of bioactive compounds in Phyllanthus species and its herbal formulations. J. Sep. Sci. 1 (1), 5. 10.1002/jssc.201601361 [DOI] [PubMed] [Google Scholar]
- Kumar S., Singh A., Kumar C. (2017b). Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Analysis 7 (1), 214–222. 10.1016/j.jpha.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Aneesh K. A., Kshemada K., Ajith K. G. S., Binil R. S. S., Deora N., et al. (2017a). Amalaki rasayana, a traditional Indian drug enhances cardiac mitochondrial and contractile functions and improves cardiac function in rats with hypertrophy. Scientific 1 (1), 8588. 10.1038/s41598-017-09225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumnerdkhonkaen P., Saenglee S., Asgar M. A., Senawong G., Khongsukwiwat K., Senawong T. (2018). Antiproliferative activities and phenolic acid content of water and ethanolic extracts of the powdered formula of Houttuynia cordata Thunb. fermented broth and Phyllanthus emblica Linn. fruit. BMC Complementary Altern. Med. 18 (130), 130. 10.1186/s12906-018-2185-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H. E. N., Xin-Bo M. A., Liang Y. H., Shi-Cheng P. E. I., Yi-Ping F. E. N. G., Min W. E. I. (2011). Effects of persimmon leaf total flavonoid on enzyme of lipoprotein metabolism and antioxidation in hyperlipidemia rats. Chin. J. Nat. Med. 9 (1), 74–77. 10.1016/s1875-5364(11)60024-1 [DOI] [Google Scholar]
- Li P. H., Wang C. W., Lu W. C., Song T. Y., Wang C. C. (2022). Antioxidant, anti-inflammatory activities, and neuroprotective behaviors of Phyllanthus emblica L. Fruit extracts. Agriculture 12 (5), 588. 10.3390/agriculture12050588 [DOI] [Google Scholar]
- Li W., Zhu H. W., Chen Y. J., Xiao H., Ge Y. Z., Hu H. E., et al. (2020). Bioactivity‐guided isolation of anti‐inflammatory components from Phyllanthus emblica . Food Sci. Nutr. 8 (6), 2670–2679. 10.1002/fsn3.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Guo B., Wang W., Li L., Cao L., Yang C., et al. (2019). Characterization of phenolic compounds from Phyllanthus emblica fruits using HPLC-ESI-TOF-MS as affected by an optimized microwave-assisted extraction. Int. J. Food Prop. 22 (1), 330–342. 10.1080/10942912.2019.1583249 [DOI] [Google Scholar]
- Luo X., Zhang B., Pan Y., Gu J., Tan R., Gong P. (2022). Phyllanthus emblica aqueous extract retards hepatic steatosis and fibrosis in NAFLD mice in association with the reshaping of intestinal microecology. Front. Pharmacol. 1 (1), 893561. 10.3389/fphar.2022.893561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J. J., Wang Y. F., Zhang J. M., Yu S., Wang D., Zhu H. T., et al. (2014). Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica . Org. Biomol. Chem. 12 (43), 8764–8774. 10.1039/c4ob01196a [DOI] [PubMed] [Google Scholar]
- Lv J. J., Yu S., Xin Y., Cheng R. R., Zhu H. T., Wang D., et al. (2015). Anti-viral and cytotoxic norbisabolane sesquiterpenoid glycosides from Phyllanthus emblica and their absolute configurations. Phytochemistry 117, 123–134. 10.1016/j.phytochem.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Mahata S., Pandey A., Shukla S., Tyagi A., Husain S. A., Das B. C., et al. (2013). Anticancer activity of Phyllanthus emblica Linn (Indian gooseberry): inhibition of transcription factor AP-1 and HPV gene expression in cervical cancer cells. Nutr. cancer 65 (1), 88–97. 10.1080/01635581.2013.785008 [DOI] [PubMed] [Google Scholar]
- Mal A., Meena D. S. (2022). Phyllanthus emblica: a herbal remedy for healthy life. ECS Trans. 107 (1), 3199–3206. 10.1149/10701.3199ecst [DOI] [Google Scholar]
- Masad R. J., Haneefa S. M., Mohamed Y. A., Al-Sbiei A., Bashir G., Fernandez-Cabezudo M. J., et al. (2021). The immunomodulatory effects of honey and associated flavonoids in cancer. Nutrients 13 (4), 1269. 10.3390/nu13041269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayakrishnan V., Kannappan P., Abdullah N., Ahmed A. B. A. (2013). Cardioprotective activity of polysaccharides derived from marine algae: an overview. Trends Food Sci. Technol. 30 (2), 98–104. 10.1016/j.tifs.2013.01.007 [DOI] [Google Scholar]
- Mehmood M. H., Rehman A., Rehman N. U., Gilani A. H. (2013). Studies on prokinetic, laxative and spasmodic activities of Phyllanthus emblica in experimental animals. Phytotherapy Res. 27 (7), 1054–1060. 10.1002/ptr.4821 [DOI] [PubMed] [Google Scholar]
- Middha S. K., Goyal A. K., Lokesh P., Yardi V., Mojamdar L., Keni D. S., et al. (2015). Toxicological evaluation of Emblica officinalis fruit extract and its anti-inflammatory and free radical scavenging properties. Pharmacogn. Mag. 11 (Suppl. 3), S427. 10.4103/0973-1296.168982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy A., Sanjay E. R., Prasad N. (2017). Quantitative analysis of Phyllanthus species for bioactive molecules using high-pressure liquid chromatography and liquid chromatography–mass spectrometry. Quantitative Analysis Phyllanthus 1 (1), 2. 10.1007/s40011-017-0839-y [DOI] [Google Scholar]
- Naik J., David M. (2023). Phytofabrication of silver and zinc oxide nanoparticles using the fruit extract of Phyllanthus emblica and its potential anti-diabetic and anti-cancer activity. Part. Sci. Technol. 41 (6), 761–773. 10.1080/02726351.2022.2141668 [DOI] [Google Scholar]
- Nair A., Balasaravanan T., Jadhav S., Mohan V., Kumar C. (2020). Harnessing the antibacterial activity of Quercus infectoria and Phyllanthus emblica against antibiotic-resistant Salmonella Typhi and Salmonella Enteritidis of poultry origin. Veterinary World 13 (1), 1388–1396. 10.14202/vetworld.2020.1388-1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamkitidechakul C., Jaijoy K., Hansakul P., Soonthornchareonnon N., Sireeratawong S. (2010). Antitumour effects of Phyllanthus emblica L.: induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytotherapy Res. 24 (9), 1405–1413. 10.1002/ptr.3127 [DOI] [PubMed] [Google Scholar]
- Nirala R. K., Raj P., Anjana K., Mandal K. G. (2020). A review on immunomodulatory activity of amla and Aloe vera. J. Pharmacogn. Phytochemistry 9 (5), 2014–2016. [Google Scholar]
- Oo A. M., Mohd Adnan L. H., Nor N. M., Simbak N., Ahmad N. Z., Lwin O. M. (2021). Immunomodulatory effects of flavonoids: an experimental study on natural-killer-cell-mediated cytotoxicity against lung cancer and cytotoxic granule secretion profile. Proc. Singap. Healthc. 30 (4), 279–285. 10.1177/2010105820979006 [DOI] [Google Scholar]
- Paik J. M., Golabi P., Younossi Y., Mishra A., Younossi Z. M. (2020). Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 72 (5), 1605–1616. 10.1002/hep.31173 [DOI] [PubMed] [Google Scholar]
- Pramyothin P., Samosorn P., Poungshompoo S., Chaichantipyuth C. (2006). The protective effects of Phyllanthus emblica Linn. extract on ethanol induced rat hepatic injury. J. Ethnopharmacol. 107 (3), 361–364. 10.1016/j.jep.2006.03.035 [DOI] [PubMed] [Google Scholar]
- Priya F. F., Islam M. S. (2019). Phyllanthus emblica Linn.(Amla)—a natural gift to humans: an overview. J. Dis. Med. Plants 5, 1–9. 10.11648/j.jdmp.20190501.11 [DOI] [Google Scholar]
- Quranayati Q., Iqhrammullah M., Saidi N., Nurliana N., Idroes R., Nasution R. (2022). Cytotoxicity and phytochemical profiles of Phyllanthus emblica stem barks with in silico drug-likeliness: focusing on antidiabetic potentials. J. Adv. Pharm. Technol. Res. 13 (4), 281–285. 10.4103/japtr.japtr_319_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quranayati Q., Saidi N., Nurliana N., Idroes R., Gusti N., Nasution R. (2023). Effect of Phyllanthus emblica L. stem bark extract on diabetic nephropathy and hyperlipidemia in rats. J. Pharm. Pharmacogn. Res. 11 (2), 308–314. 10.56499/jppres23.1595_11.2.308 [DOI] [Google Scholar]
- Rajak S., Banerjee S. K., Sood S., Dinda A. K., Gupta Y. K., Gupta S. K., et al. (2004). Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in ratsficinalis causes myocardial adaptation and protects against oxidative stress in ischemic‐reperfusion injury in rats. Phytotherapy Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 18 (1), 54–60. 10.1002/ptr.1367 [DOI] [PubMed] [Google Scholar]
- Rajalakshmi S., Vijayakumar S., Praseetha P. K. (2019). Neuroprotective behaviour of Phyllanthus emblica (L) on human neural cell lineage (PC12) against glutamate-induced cytotoxicity. Gene Rep. 17, 100545. 10.1016/j.genrep.2019.100545 [DOI] [Google Scholar]
- Rakha A., Umar N., Rabail R., Butt M. S., Kieliszek M., Hassoun A., et al. (2022). Anti-inflammatory and anti-allergic potential of dietary flavonoids: a review. Biomed. Pharmacother. 156, 113945. 10.1016/j.biopha.2022.113945 [DOI] [PubMed] [Google Scholar]
- Ramasamy S., Abdul Wahab N., Zainal Abidin N., Manickam S., Zakaria Z. (2012). Growth inhibition of human gynecologic and colon cancer cells by Phyllanthus watsonii through apoptosis induction. PLoS One 7 (4), e34793. 10.1371/journal.pone.0034793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R., Sharma N., Oladeji O. S., Sourirajan A., Dev K., Zengin G., et al. (2022). Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: a comprehensive review. J. Ethnopharmacol. 282, 114570. 10.1016/j.jep.2021.114570 [DOI] [PubMed] [Google Scholar]
- Samatiwat P., Tabtimmai L., Suphakun P., Jiwacharoenchai N., Toviwek B., Kukongviriyapan V., et al. (2021). The effect of the EGFR-targeting compound 3-[(4-Phenylpyrimidin-2-yl) amino] benzene-1-sulfonamide (13f) against cholangiocarcinoma cell lines. Asian Pac. J. Cancer Prev. APJCP 22 (2), 381. 10.31557/APJCP.2021.22.2.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoshkumar J., Devarmani M. S., Sajjanar M., Pranavakumar M. S., Dass P. J. M. I. (2013). A study of anti-inflammatory activity of fruit of Emblica officinalis (Amla) in Albino rats. Medica Innov. 2 (1), 17–26. [Google Scholar]
- Shamsudin N. F., Ahmed Q. U., Mahmood S., Shah S. A. A., Sarian M. N., Khattak M. M. A. K., et al. (2022). Flavonoids as antidiabetic and anti-inflammatory agents: a review on structural activity relationship-based studies and meta-analysis. Int. J. Mol. Sci. 23 (20), 12605. 10.3390/ijms232012605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheoran S., Nidhi P., Kumar V., Singh G., Lal U. R., Sourirajan A., et al. (2019). Altitudinal variation in gallic acid content in fruits of Phyllanthus emblica L. and its correlation with antioxidant and antimicrobial activity. Vegetos 32, 387–396. 10.1007/s42535-019-00048-x [DOI] [Google Scholar]
- Sobhani Z., Reza Nami S., Ahmad Emami S., Sahebkar A., Javadi B. (2017). Medicinal plants targeting cardiovascular diseases in view of Avicenna. Curr. Pharm. Des. 23 (17), 2428–2443. 10.2174/1381612823666170215104101 [DOI] [PubMed] [Google Scholar]
- Summanen J. O. (1999). A chemical and ethnopharmacological study on Phyllanthus emblica L.(Euphorbiaceae). Zhongguo Zhong Yao Za Zhi 22, 515–8. [Google Scholar]
- Sun J., Wang Z., Chen L., Sun G. (2021). Hypolipidemic effects and preliminary mechanism of chrysanthemum flavonoids, its main components luteolin and luteoloside in hyperlipidemia rats. Antioxidants 10 (8), 13CC09. 10.3390/antiox10081309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir I., Khan M. R., Shah N. A., Aftab M. (2016). Evaluation of phytochemicals, antioxidant activity and amelioration of pulmonary fibrosis with Phyllanthus emblica leaves. BMC complementary Altern. Med. 16, 406–41C2. 10.1186/s12906-016-1387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiennimitr P., Yasom S., Tunapong W., Chunchai T., Wanchai K., Pongchaidecha A., et al. (2018). Lactobacillus paracasei HII01, xylooligosaccharides, and synbiotics reduce gut disturbance in obese rats. Nutrition 54, 40–47. 10.1016/j.nut.2018.03.005 [DOI] [PubMed] [Google Scholar]
- Tung Y. T., Huang C. Z., Lin J. H., Yen G. C. (2018). Effect of Phyllanthus emblica L. fruit on methionine and choline-deficiency diet-induced nonalcoholic steatohepatitis. J. food drug analysis 26 (4), 1245–1252. 10.1016/j.jfda.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M. S., Mamun A. A., Hossain M. S., Akter F., Iqbal M. A., Asaduzzaman M. (2016). Exploring the effect of Phyllanthus emblica L. on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: promising natural gift for the mitigation of Alzheimer's disease. Ann. Neurosci. 23 (4), 218–229. 10.1159/000449482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usharani P., Fatima N., Muralidhar N. (2013). Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: a randomized, double-blind, controlled study. Diabetes, Metabolic Syndrome Obes. Targets Ther. 6, 275–284. 10.2147/DMSO.S46341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Variya B. C., Bakrania A. K., Patel S. S. (2016). Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 111, 180–200. 10.1016/j.phrs.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J. P. (2008). The neuroprotective potential of flavonoids: a multiplicity of effects. Genes. & Nutr. 3 (3), 115–126. 10.1007/s12263-008-0091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M. D., Fu L., Cheng C. C., Gao R., Lin M. Y., Su H. L., et al. (2019). Inhibition of LPS-induced oxidative damages and potential anti-inflammatory effects of Phyllanthus emblica extract via down-regulating NF-κB, COX-2, and iNOS in RAW 264.7 cells. Antioxidants 8 (8), 270. 10.3390/antiox8080270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Cai J., Fang Z., Li S., Huang Z., Tang Z., et al. (2022b). The composition and anti-aging activities of polyphenol extract from Phyllanthus emblica L. fruit. Nutrients 14 (4), 857. 10.3390/nu14040857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Liu X., Sun Z. (2022a). Fruit of Phyllanthus emblica L. suppresses macrophage foam-cell genesis and vascular lipid deposition using in vivo and in vitro models of early atherosclerosis development. Food Sci. Technol. Res. 28 (4), 325. 10.3136/fstr.FSTR-D-22-00002 [DOI] [Google Scholar]
- Yang B., Kortesniemi M., Liu P., Karonen M., Salminen J. P. (2012). Analysis of hydrolyzable tannins and other phenolic compounds in emblic leafflower (Phyllanthus emblica L.) fruits by high performance liquid Chromatography−Electrospray ionization mass spectrometry. J. Agric. Food Chem. 1 (1), 8672–8683. 10.1021/jf302925v [DOI] [PubMed] [Google Scholar]
- Yang B., Liu P. (2014). Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica . J. Agric. food Chem. 62 (3), 529–541. 10.1021/jf404703k [DOI] [PubMed] [Google Scholar]
- Zaragozá C., Villaescusa L., Monserrat J., Zaragozá F., Álvarez-Mon M. (2020). Potential therapeutic anti-inflammatory and immunomodulatory effects of dihydroflavones, flavones, and flavonols. Molecules 25 (4), 1017. 10.3390/molecules25041017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhao L., Guo X., Li C., Li H., Lou H., et al. (2016). Chemical constituents from Phyllanthus emblica and the cytoprotective effects on H 2 O 2-induced PC12 cell injuries. Archives Pharmacal Res. 39, 1202–1211. 10.1007/s12272-014-0433-2 [DOI] [PubMed] [Google Scholar]
- Zhao T., Sun Q., Marques M., Witcher M. (2015). Anticancer properties of Phyllanthus emblica (Indian gooseberry). Oxid. Med. Cell. Longev. 2015, 950890. 10.1155/2015/950890 [DOI] [PMC free article] [PubMed] [Google Scholar]