Abstract
Objective:
To quantify the association between time to colposcopy and risk of subsequent cervical cancer.
Methods:
A longitudinal analysis of patients aged 21–79 with an abnormal cervical cancer test result from healthcare systems in Dallas, Texas, Massachusetts, and Washington was performed. The outcome was a cervical cancer diagnosis ≥12 months after the abnormal result. The primary analysis compared receipt of colposcopy within 3 months (≤91 days) versus 3–12 months (92–365 days) and no colposcopy within 12 months of the abnormal test; post hoc analyses compared colposcopy within 12 months (≤365 days) to no colposcopy within 12 months. Associations were assessed using multivariable Cox proportional hazards regression controlling for age, risk status, result severity, and healthcare system.
Results:
Of 17,541 patients, 53.3% of patients received colposcopy within 3 months, 22.2% received colposcopy in 3–12 months, and 24.6% had no colposcopy within 12 months. One hundred forty-seven patients were diagnosed with cervical cancer within 12 months and removed from subsequent analyses. Sixty-five patients (0.4%) were diagnosed with cervical cancer more than one year (≥366 days) after the abnormal Pap/HPV result. The risk of cervical cancer detection more than one year after the abnormal test result was not different in patients who received colposcopy within 3–12 months (HR 1.07, 95% CI 0.54–2.12) and higher among patients with no colposcopy within 12 months (HR 2.34, 95% CI 1.33–4.14), compared to patients who had colposcopy within 3 months. Post hoc analyses showed the risk of cervical cancer diagnosis was 2.29-fold higher among those without colposcopy within 12 months compared to those who received colposcopy within 12 months (95% CI 1.37–3.83); among patients with high-grade cytology results, the risk of cervical cancer detection among those without colposcopy within 12 months was 3.12-fold higher compared to those who received colposcopy within 12 months (95% CI 1.47–6.70).
Conclusion:
There was no difference in cervical cancer risk at > 1 year between patients who received colposcopy within 3 months versus 3–12 months of an abnormal result. Patients who did not receive colposcopy within 12 months of an abnormal result had a higher risk of subsequent cervical cancer compared to those who received a colposcopy within 12 months.
Keywords: cervical cancer screening, colposcopy, timely follow-up, abnormal Pap management, guideline implementation
Précis
Time to colposcopy of ≤3 months vs 3–12 months after abnormal testing was not associated with risk of cervical cancer after 1 year.
Introduction
Cervical cancer is most often diagnosed in unscreened and under screened patients or after failure to follow-up abnormal screening results.1–3 Colposcopy should follow abnormal Pap or human papillomavirus (HPV) results to detect early incident cervical cancers and to detect and treat precancerous lesions before progression.4 However, there are limited data on how soon colposcopic follow-up should occur after an abnormal result.6,7
Despite limited evidence, several national societies suggest goals for colposcopy timing, ranging from within 2 weeks to 12 months after the abnormal result, depending on severity.8,9 The National Breast and Cervical Cancer Early Detection Program (NBCCEDP), serving low-income, under-/uninsured patients, sets two goals: 90% of abnormal Pap/HPV tests should receive colposcopic evaluation, and 75% of those colposcopies should occur within 90 days of the abnormal result.10 During the COVID-19 pandemic, the American Society for Colposcopy and Cervical Pathology (ASCCP) recommended that diagnostic evaluation occur within 3 months of a high-grade result and within 6–12 months of a low-grade screening result.11 A modeling study found that, compared to immediate follow-up, 3-month and 6-month intervals to colposcopy led to 0.8% and 1.4% reduction in life-years, respectively.12
The goal of this study was to evaluate the association between colposcopy timing and subsequent cervical cancer diagnosis, and whether this association differed by test result severity. These analyses were designed to examine the effects of recommendations for colposcopy timing after an abnormal screening result, which became particularly relevant when timely access to care was restricted during the COVID-19 pandemic.13
Methods
This was a longitudinal analysis of the MultilEvel opTimization of the ceRvIcal Cancer Screening process in diverse Settings & populations (METRICS) Research Center, part of the Population-based Research to Optimize the Screening Process (PROSPR II) consortium.14 Three healthcare systems contributed patient data: Kaiser Permanente Washington (KPWA), a mixed-model healthcare system providing care and coverage in Washington state; Parkland Health (PH), a publicly-funded, integrated safety-net healthcare system for under-/uninsured residents in Dallas County, Texas with academic oversight from the University of Texas Southwestern (UTSW); and Mass General Brigham (MGB), an integrated healthcare delivery system in the Boston area with two academic medical centers and their affiliated primary care networks. Institutional Review Boards at each site approved all study activities.
The METRICS study population was previously described.15 Electronic health record (EHR), administrative data, and central cancer registries were used to identify demographic information, cytology and HPV tests and results, procedures and results, pregnancy status, and cancer diagnoses. Data sources and collection methods at KPWA and PH have been previously described and are similar at MGB.16 For this analysis, we included METRICS cohort members who were 21–79 years old at their first qualifying abnormal Pap/HPV test from 2010–2015 (hereafter called the “index” test) and remained in the cohort without a cervical cancer diagnosis for at least one year after the abnormal result (months 0–12). Qualifying abnormal results were defined based on the 2006 and 2012 management guidelines17–20:
High-grade cytology (ASC-H, HSIL, AGC, or suspicious for cancer/adenocarcinoma in situ) or HPV 16/18+ among 21–79-year-olds;
Low-grade cytology (ASC-US / HPV-positive, ASC-US / HPV unknown, or LSIL) among 25–79-year-olds;
Persistent mild abnormality (two scenarios): a third consecutive ASC-US or LSIL cytology among 21–24-year-olds; or occurrence of a second consecutive negative cytology with positive HPV test among 25–79-year-olds.
Exclusion criteria (Appendix 1, available online at http://links.lww.com/xxx) included a cervical cancer diagnosis or hysterectomy prior to the index test, pregnancy at the time of the index test, and/or a diagnostic procedure on the same day as the index test (i.e., confirmatory Pap/HPV test concurrent with a scheduled procedure). Because we were interested in patients still “at-risk” for a cervical cancer diagnosis after receiving a colposcopy, those diagnosed with cervical cancer within 12 months (≤365 days) were enumerated and excluded from subsequent analyses. Patients’ risk status at the index test was classified based on age and prior screening and cervical procedure history using three mutually exclusive categories:
Surveillance/Alternate Risk, documentation in the EHR of abnormal Pap/HPV test results (NILM/hrHPV+ or more severe, including ASC-US/HPV-), prior cervical biopsy showing dysplasia, or prior cervical procedure (colposcopy, LEEP, cone, cryotherapy, laser, or other excisional procedure, regardless of pathology results), age 66–79 years, or immunosuppressed (specifically, patients living with HIV);
Unknown Risk, those 21–65 years old with no documented screening history in the EHR; and
Average Risk, those 21–65 years old who had a cervix, were not under alternate screening schedule, and had a documented EHR history of normal screens (all tests NILM alone or NILM/hrHPV-).
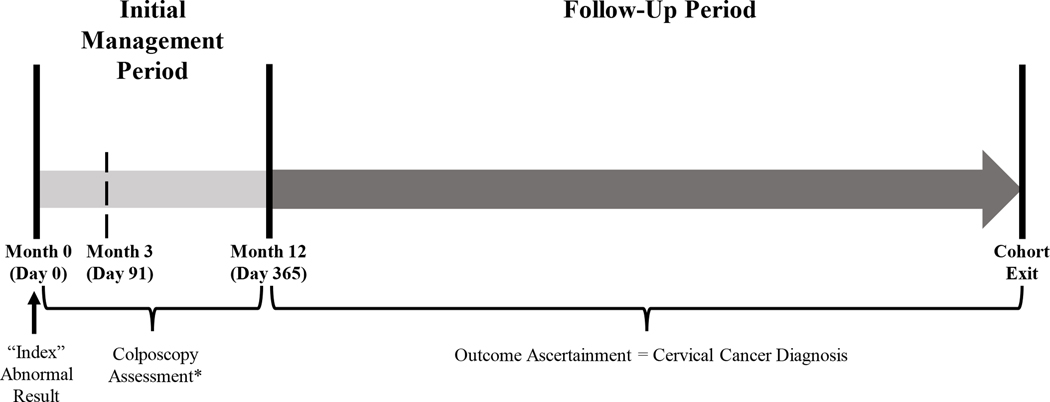
The initial 365 days following the index test was defined as the initial management period (months 0–12; Figure 1).21 Patients were classified into three categories: colposcopy performed within 3 months (≤3 months, ≤91 days) after the abnormal result; colposcopy performed 3 months and one day and up to 12 months after the abnormal result (3–12 months, 92–365 days); and no colposcopy performed within 12 months. These time frames were chosen based on the NBCCEDP recommendation.10 Primary analysis compared the latter two categories to receipt of colposcopy ≤3 months from the index test. Post-hoc analysis compared receipt of colposcopy within 12 months (≤3 months and 3–12 months) to no colposcopy within 12 months. The primary outcome was a cervical cancer diagnosis during the follow-up period, defined as from Month 13 (day 366) through cancer diagnosis or cohort exit (Figure 1), stratified by initial management period category. We distinguished patients who were evaluated (i.e., received one or more Pap/HPV test(s) or procedure(s) during the follow-up period) and were not diagnosed with cervical cancer, from patients who left the cohort prior to evaluation (i.e., no Pap/HPV test or procedure from Month 13 forward). Race/ethnicity, health insurance, comorbidity scores, BMI, and Yost quintile22 were identified as previously described.15 Statistical significance of differences in baseline characteristics by initial management period category was determined using the chi-square test.
Figure 1.
Schematic of the initial management and follow-up periods after a qualifying abnormal cervical cancer test result. *Patients with cervical cancers detected during the initial management period were not included in the analysis as they had the outcome of interest before the start of follow-up.
We used the Kaplan Meier approach to estimate the cumulative detection of cervical cancers during the follow-up period for each initial management period category, with stratification by test result severity and risk status. Patients were censored at cohort exit due to reaching the end of the study period, death, disenrollment from the healthcare system (KPWA only), moving out of the SEER catchment area (KPWA only), moving out of Dallas County, Texas (PH only), or going without a primary care or women’s health clinic visit for >37 months (MGB, PH). For those with no colposcopy within 12 months, it is possible that cancer was present but undetected; for this reason, cumulative detection includes prevalent and incident cancers. Statistical significance of differences in cumulative cervical cancer detection was determined using the log-rank test.
We used multivariable Cox proportional hazards regression to estimate hazard ratios and 95% confidence intervals for the risk of cervical cancer diagnosis more than 1 year after the index test. Patients were censored using the same criteria as for the Kaplan Meier testing above. Models were adjusted for age and risk status at index test, severity of index cytology result, and healthcare site; no patients were excluded due to missing covariate information. Because site was highly correlated with other patient characteristics including race/ethnicity, Yost quintile, BMI, and insurance status, site was the only one of these variables included in the final model. We found no violations in the proportional hazards assumption for the time-dependent covariates. In the primary analysis, patients receiving colposcopy ≤3 months served as the reference group. For post hoc analyses, the reference group were patients receiving colposcopy ≤12 months. Analyses were conducted using SAS version 9.4 and R version 4.0.3.
Results
A total of 17,541 patients with a qualifying abnormal result (index test) between 2010 and 2015 were evaluated after applying exclusion criteria (2.3% of total METRICS cohort; Appendix 1 [http://links.lww.com/xxx]). Overall, 53.3% (n = 9,353) of eligible patients received colposcopy within 3 months of their index test, while 22.2% (n = 3,901) received colposcopy within 3–12 months and 24.6% (n = 4,287) did not undergo colposcopy within 12 months (Table 1). During the initial management period, 0.8% (n = 147) were diagnosed with cervical cancer within 12 months of the index test; most (81.6%) were diagnosed within 3 months of the index test.
Table 1.
Demographic and Test Characteristics of PROSPR METRICS Cohort Members at Index Abnormal Cervical Cancer Test, by Initial Management Period Category
| Total | Initial Management Period Category1 |
|||
|---|---|---|---|---|
| Colposcopy ≤3 mos after abnormal result | Colposcopy 3–12 mos after abnormal result | No Colposcopy within 12 mos of abnormal result | ||
|
| ||||
| Total Patients with Qualifying Abnormal Test | 17,541 | 9,353 | 3,901 | 4,287 |
| Total Cancers Diagnosed in Months 0–12 2 | 147 | 120 | 22 | 5 |
|
| ||||
| Total Eligible Patients for Analysis 3 | 17,394 | 9,233 | 3,879 | 4,282 |
|
| ||||
| Patient Characteristics at Abnormal Test | n (Col %) | n (Row %) | n (Row %) | n (Row %) |
|
| ||||
| Site | ||||
| KPWA | 3,840 (22.1) | 2,926 (76.2) | 375 (9.8) | 539 (14.0) |
| PH | 7,045 (40.5) | 2,670 (37.9) | 2,550 (36.2) | 1,825 (25.9) |
| MGB | 6,509 (37.4) | 3,637 (55.9) | 954 (14.7) | 1,918 (29.5) |
| Age (years) | ||||
| 21–29 | 5,027 (28.9) | 2,498 (49.7) | 1,240 (24.7) | 1,289 (25.6) |
| 30–39 | 5,795 (33.3) | 3,245 (56.0) | 1,313 (22.7) | 1,237 (21.4) |
| 40–49 | 3,466 (19.9) | 1,928 (55.6) | 765 (22.1) | 773 (22.3) |
| 50–59 | 2,087 (12) | 1,072 (51.4) | 389 (18.6) | 626 (30.0) |
| 60–69 | 879 (5.1) | 421 (47.9) | 143 (16.3) | 315 (35.8) |
| 70–79 | 140 (0.8) | 69 (49.3) | 29 (20.7) | 42 (30.0) |
| Race/Ethnicity | ||||
| Asian / Pacific Islander, Non-Hispanic | 897 (5.2) | 596 (66.4) | 117 (13.0) | 184 (20.5) |
| Black, Non-Hispanic | 2,887 (16.7) | 1,095 (37.9) | 755 (26.2) | 1,037 (35.9) |
| Hispanic | 6,114 (35.4) | 2,867 (46.9) | 2,051 (33.6) | 1,196 (19.6) |
| White, Non-Hispanic | 6,944 (40.3) | 4,334 (62.4) | 879 (12.7) | 1,731 (24.9) |
| None of the above / multiple races | 407 (2.4) | 262 (64.4) | 61 (15.0) | 84 (20.6) |
| Unknown | 145 | 79 | 16 | 50 |
| Health Insurance | ||||
| Commercial | 8,051 (46.4) | 5,212 (64.7) | 959 (11.9) | 1,880 (23.4) |
| Medicare | 860 (5.0) | 385 (44.8) | 169 (19.7) | 306 (35.6) |
| Medicaid/Other/Uninsured | 8,423 (48.6) | 3,611 (42.9) | 2,734 (32.5) | 2,078 (24.7) |
| Unknown | 60 | 25 | 17 | 18 |
| Comorbidity Score | ||||
| 0–1 | 14,048 (84.1) | 7,496 (53.4) | 3,244 (23.1) | 3,308 (23.6) |
| 2+ | 2,655 (15.9) | 1,254 (47.2) | 5,73 (21.6) | 828 (31.2) |
| Unknown | 691 | 484 | 61 | 146 |
| BMI (kg/m 2 ) | ||||
| <18.5 | 340 (2.0) | 178 (52.4) | 51 (15.0) | 111 (32.7) |
| 18.5–24.9 | 6,532 (37.8) | 3,731 (57.1) | 1,220 (18.7) | 1,581 (24.2) |
| 25.0–29.9 | 4,936 (28.6) | 2,550 (51.7) | 1,181 (23.9) | 1,205 (24.4) |
| ≥30.0 | 5,455 (31.6) | 2,722 (49.9) | 1,410 (25.9) | 1,323 (24.3) |
| Unknown | 131 | 52 | 17 | 62 |
| Yost Quintile (State) | ||||
| 1 | 4,804 (30.1) | 2,266 (47.2) | 1,379 (28.7) | 1,159 (24.1) |
| 2 | 3,405 (21.3) | 1,665 (48.9) | 943 (27.7) | 797 (23.4) |
| 3 | 2,451 (15.3) | 1,386 (56.6) | 486 (19.8) | 579 (23.6) |
| 4 | 2,765 (17.3) | 1,612 (58.3) | 464 (16.8) | 689 (24.9) |
| 5 | 3,145 (19.7) | 1,905 (60.6) | 404 (12.9) | 836 (26.6) |
| Unknown | 824 | 399 | 203 | 222 |
|
| ||||
| Abnormal Test Characteristics | ||||
|
| ||||
| Risk Status at Abnormal Test | ||||
| Surveillance/Alternate Risk | 6,367 (36.6) | 2,667 (41.9) | 1,772 (27.8) | 1,928 (30.3) |
| Average Risk | 6,103 (35.1) | 3,698 (60.6) | 1,198 (19.6) | 1,207 (19.8) |
| Unknown Risk | 4,924 (28.3) | 2,868 (58.3) | 909 (18.5) | 1,147 (23.3) |
| Abnormal Test Result | ||||
| High-Grade (≥HSIL) | 3,662 (21.1) | 2,560 (69.9) | 505 (13.8) | 597 (16.3) |
| Low-Grade (≤LSIL) | 12,525 (72.0) | 6,320 (50.5) | 3,115 (24.9) | 3,090 (24.7) |
| Persistent Mild Abnormality | 1,207 (6.9) | 353 (29.3) | 259 (21.5) | 595 (49.3) |
|
| ||||
| Initial Management Period Characteristics | ||||
|
| ||||
| Most Severe Pathology in Initial Management Period 4 | ||||
| AIS / CIN III / CIN II / HSIL | 2,197 (12.6) | 1,634 (74.4) | 563 (25.6) | 0 |
| LSIL / CIN I | 4,082 (23.5) | 2,519 (61.7) | 1,563 (38.3) | 0 |
| HPV / Condylomata / Atypia | 1,414 (8.1) | 1,069 (75.6) | 345 (24.4) | 0 |
| Normal | 4,532 (26.1) | 3,382 (74.6) | 1,150 (25.4) | 0 |
| Insufficient / Unknown / No Biopsy | 887 (5.1) | 629 (70.9) | 258 (29.1) | 0 |
| No Procedure | 4,282 (24.6) | 0 | 0 | 4,282 (100.0) |
| Treatment Completed in Initial Management Period 5 | ||||
| No | 14,925 (85.8) | 7,301 (48.9) | 3,342 (22.4) | 4,282 (28.7) |
| Yes | 2,469 (14.2) | 1,932 (78.2) | 537 (21.8) | 0 |
All patient characteristics, test characteristics, and initial management characteristics (including total cancers diagnosed months 0–12) were significantly different (p<0.001) by initial management.
Cancer diagnoses were identified through pathology reports and central cancer registries. Cancer diagnoses made among patients for whom a procedure was not documented in during the Initial Management Period (Months 0–12) were identified exclusively from central cancer registries.
Patients diagnosed with cancer in Months 0–12 were excluded from Total Eligible Patients for Analysis.
Most severe pathology result recorded for all procedures that occurred in Initial Management Period (Months 0–12).
Treatment included LEEP, cone, or unspecified excisional procedure as well as cryotherapy or laser ablation.
Table 1 shows differences in patient demographic characteristics and index test characteristics by colposcopy receipt and timing among the 17,394 eligible patients not diagnosed with cervical cancer during the initial management period. Colposcopy timing differed by site: while most PH patients completed a colposcopy within 12 months of an abnormal test (74.1%), only half were completed within 3 months. By contrast, most KPWA and MGB patients (76.2% and 55.9%, respectively) had a coloscopy within 3 months. Across sites, most patients with high-grade or low-grade results received colposcopy within 3 months (69.9% and 50.5%, respectively), compared to fewer than a third (29.3%) of patients with persistent mild abnormalities. Appendix 2, available online at http://links.lww.com/xxx, shows demographic characteristics by site. Most demographic characteristics that were associated with initial management were no longer associated when stratified by site.
During the follow-up period, most patients (80.6%) had at least one negative evaluation for cancer and were not diagnosed with cancer, while 19.0% left the cohort prior to evaluation (Table 2). Sixty-five patients (0.4%) were diagnosed with cervical cancer during the follow-up period, approximately half of whom (n = 32) had high-grade index test results. Patients who did not receive a colposcopy within 12 months (0.6%) had proportionately twice as many cervical cancers diagnosed after Month 12 compared to those receiving colposcopy within 12 months. Stage of cervical cancer diagnosed during the follow-up period was worse for those who did not receive a colposcopy within 12 months of the index test compared to those either within 3 months or 3–12 months (regional and distant stages, 43.8% vs. 6.3% and 14.3%, respectively; Appendix 3, available online at http://links.lww.com/xxx).
Table 2.
Detection of Cervical Cancer Beginning 12 months After Index Abnormal Cervical Cancer Test by Initial Management Period Category, Stratified by Result Severity
| Index Test Result | Initial Management Period Category | |||
|---|---|---|---|---|
|
|
|
|||
| Total | Colposcopy ≤3 mos after abnormal result | Colposcopy 3–12 mos after abnormal result | No Colposcopy within 12 mos of abnormal result | |
|
| ||||
| All Results 1 | n (Col %) | n (Col %) | n (Col %) | n (Col %) |
|
| ||||
| Outcome by End of Follow-Up2 | 17,394 | 9,233 | 3,879 | 4,282 |
| Patients diagnosed with cancer | 65 (0.4) | 27 (0.3) | 13 (0.3) | 25 (0.6) |
| Patients whose evaluation did not show cancer | 14,019 (80.6) | 7,747 (83.9) | 3,290 (84.8) | 2,982 (69.6) |
| Patients who left the cohort prior to evaluation | 3,310 (19.0) | 1,459 (15.8) | 576 (14.9) | 1,275 (29.8) |
|
| ||||
| High-Grade Results 3 | ||||
|
| ||||
| Outcome by End of Follow-Up2 | 3,662 | 2,560 | 505 | 597 |
| Patients diagnosed with cancer | 32 (0.9) | 17 (0.7) | <5 (0.8) | 11 (1.8) |
| Patients whose evaluation did not show cancer | 2,805 (76.6) | 2,019 (78.9) | 406 (80.4) | 380 (63.7) |
| Patients who left the cohort prior to evaluation | 825 (22.5) | 524 (20.5) | 95 (18.8) | 206 (34.5) |
|
| ||||
| Low-Grade Results 4 | ||||
|
| ||||
| Outcome by End of Follow-Up2 | 12,525 | 6,320 | 3,115 | 3,090 |
| Patients diagnosed with cancer | 29 (0.2) | 10 (0.2) | 8 (0.3) | 11 (0.4) |
| Patients whose evaluation did not show cancer | 10,172 (81.2) | 5,405 (85.5) | 2,652 (85.1) | 2,115 (68.5) |
| Patients who left the cohort prior to evaluation | 2,324 (18.6) | 905 (14.3) | 455 (14.6) | 964 (31.2) |
|
| ||||
| Persistent Mild Abnormality Results 5 | ||||
|
| ||||
| Outcome by End of Follow-Up2 | 1,207 | 353 | 259 | 595 |
| Patients diagnosed with cancer | <5 (0.3) | 0 | <5 (0.4) | <5 (0.5) |
| Patients whose evaluation did not show cancer | 1,042 (86.3) | 323 (91.5) | 232 (89.6) | 487 (81.9) |
| Patients who left the cohort prior to evaluation | 161 (13.3) | 30 (8.5) | 26 (10.0) | 105 (17.7) |
Among patients who received a colposcopy within 3 months, the median time to cohort exit from the abnormal test result was 76.7 months (IQR 57.6–100.8 months). Among patients who received a colposcopy within 3–12 months, the median time to cohort exit from the abnormal test result was 82.4 months (IQR 62.9–104.8 months). Among patients who did not receive a colposcopy within 12 months, the median time to cohort exit from the abnormal test result was 76.7 months (IQR 49.2–101.7 months).
Indicates the number of patients who were diagnosed with cancer after Month 12, the number of patients who were not diagnosed with cancer and received at least one Pap/HPV test or procedure after Month 12 prior to cohort exit, and the number of patients who were not diagnosed with cancer after Month 12 and did not receive a Pap/HPV test or procedure prior to cohort exit. Patients whose evaluation did not show cancer may include people who completed a Pap/HPV test and/or a procedure but not the entire evaluation (i.e., had an abnormal Pap test result and did not go on to complete a colposcopy or other diagnostic evaluation). Among patients diagnosed with cancer, the median time to diagnosis from the abnormal test result was 40.0 months (IQR 25.2–70.8 months). Among patients not diagnosed with cancer and evaluated at least once prior to cohort exit, the median time to cohort exit from the abnormal test result was 84.9 months (IQR 66.9–106.3 months). Among patients not diagnosed with cancer and not evaluated prior to cohort exit, the median time to cohort exit from the abnormal test result was 40.9 months (IQR 37.0–54.9 months).
Among patients diagnosed with cancer who had high-grade cytology results, the median time to diagnosis from the abnormal test result was 32.8 months (IQR 21.0–61.0 months). Among patients not diagnosed with cancer and evaluated at least once prior to cohort exit, the median time to cohort exit from the abnormal test result was 79.6 months (IQR 63.7–102.5 months). Among patients not diagnosed with cancer and not evaluated prior to cohort exit, the median time to cohort exit from the abnormal test result was 42.6 months (IQR 33.1–62.3 months). Among patients who received a colposcopy within 3 months, the median time to cohort exit from the abnormal test result was 73.4 months (IQR 53.6–97.8 months). Among patients who received a colposcopy from 3–12 months, the median time to cohort exit from the abnormal test result was 75.4 months (IQR 56.7–99.8 months). Among patients who did not receive a colposcopy within 12 months, the median time to cohort exit from the abnormal test result was 70.8 months (IQR 40.3–91.3 months).
Among patients diagnosed with cancer who had low-grade cytology results, the median time to diagnosis from the abnormal test result was 54.6 months (IQR 30.8–79.2 months). Among patients not diagnosed with cancer and evaluated at least once prior to cohort exit, the median time to cohort exit from the abnormal test result was 87.4 months (IQR 67.9–108.1 months). Among patients not diagnosed with cancer and not evaluated prior to cohort exit, the median time to cohort exit from the abnormal test result was 40.5 months (IQR 37.0–53.2 months). Among patients who received a colposcopy within 3 months, the median time to cohort exit from the abnormal test result was 78.2 months (IQR 58.5–102.6 months). Among patients who received a colposcopy from 3–12 months, the median time to cohort exit from the abnormal test result was 84.6 months (IQR 63.5–105.4 months). Among patients who did not receive a colposcopy within 12 months, the median time to cohort exit from the abnormal test result was 78.5 months (IQR 48.2–104.1 months).
Among patients diagnosed with cancer who had a persistent mild abnormality result, the median time to diagnosis from the abnormal test result was 44.3 months (IQR 25.4–67.0 months). Among patients not diagnosed with cancer and evaluated at least once prior to cohort exit, the median time to cohort exit from the abnormal test result was 79.8 months (IQR 65.6–97.1 months). Among patients not diagnosed with cancer and not evaluated prior to cohort exit, the median time to cohort exit from the abnormal test result was 43.7 months (IQR 37.0–60.5 months). Among patients who received a colposcopy within 3 months, the median time to cohort exit from the abnormal test result was 73.9 months (IQR 62.8–90.3 months). Among patients who received a colposcopy from 3–12 months, the median time to cohort exit from the abnormal test result was 79.8 months (IQR 62.8–99.7 months). Among patients who did not receive a colposcopy within 12 months, the median time to cohort exit from the abnormal test result was 76.7 months (IQR 61.6–95.9 months).
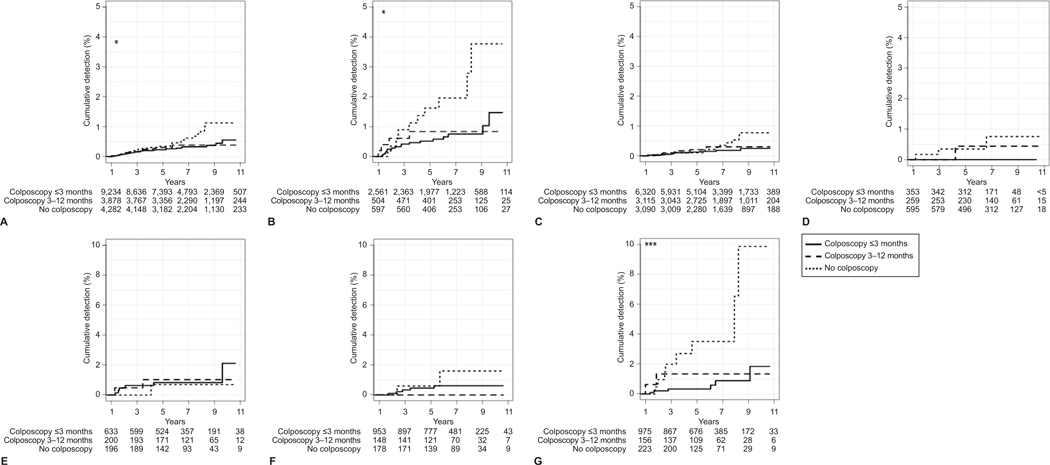
The cumulative detection of cervical cancer during the follow-up period differed based on the timing of initial management among those with high-grade index results (Figure 2A). The highest detection of cervical cancer was observed among those with high-grade cytology results and unknown risk status (Figure 2B), with 10% of these patients diagnosed with cervical cancer by the end of follow-up (up to 11 years). There were no significant differences in cervical cancer detection based on colposcopy timing for low-grade results (overall [Figure 2A], when stratified by risk status [Appendix 4, available online at http://links.lww.com/xxx]), or persistent mild abnormalities (Figure 2A).
Figure 2.
Time to cervical cancer diagnosis after index abnormal cervical cancer test and initial management period, stratified by cytology result severity and risk status. Kaplan-Meier estimates for cumulative detection of cervical cancer after an abnormal cervical cancer test and the initial management period, stratified by result severity (n=17,394) (A–D). Cumulative detection estimates stratified by risk status are shown separately for high-grade abnormalities (n=3,662) (E–G). All results (A), high-grade (B), low-grade (C), persistent mild (D), high-grade results for patients under surveillance (E), high-grade results for patients at average risk (F), and high-grade results for patients of unknown risk (G). *P<.05; ***P<.001 based on the log-rank test.
Primary analyses showed that, after adjusting for age, risk status, result severity, and site, the risk of cervical cancer diagnosis during follow-up was similar in patients who had colposcopy 3–12 months after the index test compared to patients who had colposcopy in ≤3 months, regardless of cytology result severity (Table 3). However, there was a 2.34-fold higher risk of a cervical cancer diagnosis during follow-up (95% CI 1.33–4.14) among patients who did not receive colposcopy within 12 months compared to those who underwent colposcopy in ≤3 months.
Table 3.
Association between Initial Management Period Category and Cancer Diagnosis Beginning 12 months after Index Abnormal Cervical Cancer Test
| Initial Management Period | n | Primary Analysis1 Hazard Ratio (95% CI) |
Post Hoc Analysis2 Hazard Ratio (95% CI) |
| All Abnormal Tests 3 | 17,394 | ||
| Colposcopy ≤3 mos | 9,233 | Reference | Reference |
| Colposcopy 3–12 mos | 3,879 | 1.07 (0.54–2.12) | |
| No Colposcopy within 12 mos | 4,282 | 2.34 (1.33–4.14) | 2.29 (1.37, 3.83) |
| High-Grade Abnormalities 4 | 3,662 | ||
| Colposcopy ≤3 mos | 2,560 | Reference | Reference |
| Colposcopy 3–12 mos | 505 | 1.02 (0.34–3.09) | |
| No Colposcopy within 12 mos | 597 | 3.14 (1.47–6.70) | 3.12 (1.50, 6.49) |
| Low-Grade and Persistent Mild Abnormalities 5 | 13,732 | ||
| Colposcopy ≤3 mos | 6,673 | Reference | Reference |
| Colposcopy 3–12 mos | 3,374 | 1.02 (0.40–2.57) | |
| No Colposcopy within 12 mos | 3,685 | 1.78 (0.79–4.06) | 1.78 (0.88, 3.58) |
Primary analysis examined whether receipt of colposcopy within 3 months (≤91 days) vs. 3–12 months (92–365 days) of an abnormal result was associated with cervical cancer diagnosis >12 months (>365 days) after an abnormal cervical cancer test. Models were adjusted for patient age, risk status, and site.
Post hoc analysis examined whether receipt of colposcopy within 12 months (≤365 days) vs. not within 12 months of an abnormal result was associated with cervical cancer diagnosis >12 months (>365 days) after an abnormal cervical cancer test. Models were adjusted for patient age, risk status, and site.
Post hoc analyses showed that the risk of cervical cancer diagnosis was 2.29-fold higher among those without colposcopy within 12 months compared to those who received colposcopy within 12 months of the index test (95% CI 1.37–3.83). Among the subset of patients with high-grade cytology results, the risk of cervical cancer detection was 3.12-fold higher (95% CI 1.47–6.70). There was no association between the initial management timing and cervical cancer detection among patients with low-grade results, which included persistent mild abnormalities.
Discussion
Colposcopic evaluation after an abnormal Pap/HPV test can identify prevalent cervical cancer as well as prevent cervical cancer through the detection and removal of precancerous lesions. In this study, colposcopy within one year detected 147 prevalent cervical cancers (0.8% of our population). Among women diagnosed with cervical cancer within one year of the index test, most were diagnosed within 3 months of the index test. Quicker evaluation may stem from clinicians communicating the importance of colposcopy to these patients because clinicians were more concerned about cancer due to more severe cytology/HPV results, a worrisome clinical exam, or symptoms. However, limited data exist to guide clinicians on a safe timeframe for colposcopy after abnormal Pap/HPV testing in order to prevent subsequent invasive cervical cancer. Our primary objective was therefore to assess the association between timing of colposcopy within an initial management period (0–12 months) and subsequent cancer diagnoses.
We found that patients who did not have colposcopy performed within 12 months were more than twice as likely to be diagnosed with cervical cancer during the follow-up period compared to those who underwent colposcopy earlier (during the initial 12-month management period). Risk of cervical cancer was three times greater for patients with a high-grade index result among those who did not have colposcopy within 12 months compared to those receiving colposcopy, while cumulative cervical cancer detection was highest among those with high-grade index results and unknown prior screening history. It is unclear if this higher risk of diagnosing cervical cancer is due to timely colposcopy facilitating earlier diagnosis of a prevalent cervical cancer or due to patients undergoing excisional procedures preventing cancer progression. However, most cancers were detected at earlier stages (Appendix 3, http://links.lww.com/xxx), supporting the idea that the screening process works both to prevent cancer and detect early, more curable cancers.
Current metrics for colposcopy timing are based largely on expert opinion. Our findings suggest that the recommendation for colposcopy within ≤3 months may be somewhat arbitrary.10,11 We did not observe differences in the risk of subsequent cervical cancer detection among patients who received colposcopy ≤3 months vs. 3–12 months after the abnormal test, indicating that both timeframes are likely safe windows. While clinicians may prefer short interval follow-up after all abnormal screening results, our results suggest that prioritizing high-grade cytology and those with unknown risk status may be prudent. Receiving colposcopy 3–12 months after the abnormal result is likely safe and appropriate for those with low-grade or persistent mild abnormalities, as per ASCCP guidance published during the COVID-19 pandemic.11 Our findings should be further investigated with data collected during the COVID-19 pandemic.23 Future research should also study if colposcopy in pregnant individuals can be deferred to postpartum unless high-grade cytology results are noted and/or there are concerning clinical findings. Prior studies support that most cancers are found among patients with high-grade cytology results, and as precancers are not generally treated during pregnancy, this might be an acceptable group to delay evaluation of low-grade abnormalities, after more research on the subject.
Our results should be interpreted with two factors in mind. First, by classifying patients based on initial management in months 0–12 and follow-up in months 13 onward, our results pertain only to patients without a cancer diagnosis in months 0–12. Any effects of early follow-up on cancer prevention within months 0–12 are not captured, nor are the potential effects of detection within 0–12 months on subsequent mortality. Second, our study outcome – detection of cervical cancer more than 12 months after an abnormal result – is not a pure measure of incidence. It includes cancers prevalent at the index test, particularly for those not receiving a timely colposcopy (i.e., in months 0–12). Therefore, we cannot exclude the possibility that some of the cancers diagnosed after the initial management period – particularly in the no colposcopy within 12 months group – were delays in detection and not failures of prevention.
One limitation to this study is that residual confounding may remain, although we controlled for several covariates. It is also possible that we missed some cervical cancer diagnoses among people who did not undergo diagnostic evaluation or have symptoms evaluated; however, this limitation is mitigated by obtaining cancer diagnostic information from central cancer registries and censoring patients upon cohort exit from analyses. Additionally, we lacked power to adequately assess the effects of colposcopy timing after persistent mild abnormalities, given relatively few patients in our cohort had these index results. Finally, we were unable to examine why patients received colposcopy at different times (or not at all), though risk factors for delayed colposcopy were evaluated in prior work.15 Future studies should explore if failure to receive colposcopy is attributable to system versus patient factors. Despite these limitations, our study is valuable because it used data from three healthcare systems with diverse patient populations from different U.S. regions, used central cancer registry data, and thoughtfully explored the association between timing of colposcopy and cervical cancer detection.
In summary, we found that patients who did not receive colposcopy within 12 months of an abnormal Pap/HPV test had a higher risk of subsequent cervical cancer detected during the follow-up period compared to patients receiving colposcopy within 12 months. Patients with high-grade results were at the highest risk for subsequent cervical cancer detection when colposcopy did not occur within 12 months. We found no evidence that the risk of cervical cancer detection differed among patients who received colposcopy within 3–12 months vs. ≤3 months of an abnormal result. Further, colposcopy timing was not associated with subsequent cervical cancer detection among patients with low-grade cytology. These data suggest clinicians should prioritize patients with high-grade cytology and unknown risk status for colposcopy within 12 months to optimize cervical cancer prevention and control.
Supplementary Material
Acknowledgements:
This work was supported by National Cancer Institute grant UM1CA221940 awarded to Jennifer S. Haas, Jasmin A. Tiro, and Aruna Kamineni. Jennifer S. Haas received funding from the American Cancer Society grant CRP-22-080-01-CTPS. The views expressed here are those of the authors only and do not necessarily represent the views of the National Cancer Institute or National Institutes of Health. The authors wish to thank the participating METRICS sites for the data provided for this study. A comprehensive list of METRICS investigators and research staff is available at the PROSPR METRICS research site (https://utsouthwestern.edu/labs/prospr-metrics/about/team.html).
Financial Disclosure
Stephanie Alimena reports receiving payment from Roche Diagnostics. Sarah Feldman reports money was paid to her institution from the Society for Improving Diagnosis in Medicine. She received payment from UptoDate.
Each author has confirmed compliance with the journal’s requirements for authorship.
Footnotes
Data Statement:
PROSPR II data are available for collaboration and sharing after appropriate approvals and agreements are completed. Additional details are provided at: https://healthcaredelivery.cancer.gov/prospr/datashare.html
The other authors did not report any potential conflicts of interest.
References
- 1.Janerich DT, Hadjimichael O, Schwartz PE, et al. The screening histories of women with invasive cervical cancer, Connecticut. Am J Public Health. 1995;85(6):791–794. doi: 10.2105/ajph.85.6.791 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao CR, Chubak J, Beaber EF, et al. Gaps in the screening process for women diagnosed with cervical cancer in four diverse US health care settings. Cancer Med. 2022. doi: 10.1002/cam4.5226 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramaniam A, Fauci JM, Schneider KE, et al. Invasive cervical cancer and screening: what are the rates of unscreened and underscreened women in the modern era? J Low Genit Tract Dis. 2011;15(2):110–113. doi: 10.1097/LGT.0b013e3181f515a2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan MJ, Werner CL, Darragh TM, et al. ASCCP Colposcopy Standards: Role of Colposcopy, Benefits, Potential Harms, and Terminology for Colposcopic Practice. J Low Genit Tract Dis. 2017;21(4):223–229. doi: 10.1097/LGT.0000000000000338 [doi]. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 6.Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: A systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68(3):199–216. doi: 10.3322/caac.21452 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan MJ, Werner CL, Darragh TM, et al. ASCCP Colposcopy Standards: Role of Colposcopy, Benefits, Potential Harms, and Terminology for Colposcopic Practice. J Low Genit Tract Dis. 2017;21(4):223–229. doi: 10.1097/LGT.0000000000000338 [doi]. [DOI] [PubMed] [Google Scholar]
- 8.Ciavattini A, Delli Carpini G, Giannella L, et al. European Federation for Colposcopy (EFC) and European Society of Gynaecological Oncology (ESGO) joint considerations about human papillomavirus (HPV) vaccination, screening programs, colposcopy, and surgery during and after the COVID-19 pandemic. Int J Gynecol Cancer. 2020;30(8):1097–1100. doi: 10.1136/ijgc-2020-001617 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail M, Hsu S, Alkhalifa M, et al. Evaluation of Different Guidelines for Cervical Cancer Screening and Management of Abnormal Cervical Cytology. Ann Cytol Pathol. 2020;5(1):001–012. [Google Scholar]
- 10.Yancy B, Royalty JE, Marroulis S, Mattingly C, Benard VB, DeGroff A. Using data to effectively manage a national screening program. Cancer. 2014;120 Suppl 16(0 16):2575–2583. doi: 10.1002/cncr.28821 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawaya GF, Holt HK, Lamar R, Perron-Burdick M, Smith-McCune K. Prioritizing cervical cancer screening services during the COVID-19 pandemic: Response of an academic medical center and a public safety net hospital in California. Prev Med. 2021. Oct;151:106569. doi: 10.1016/j.ypmed.2021.106569 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutter CM, Kim JJ, Meester RGS, et al. Effect of Time to Diagnostic Testing for Breast, Cervical, and Colorectal Cancer Screening Abnormalities on Screening Efficacy: A Modeling Study. Cancer Epidemiol Biomarkers Prev. 2018;27(2):158–164. doi: 10.1158/1055-9965.EPI-17-0378 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MA, Powell AM, Coleman JS, Keller JM, Livingston A, Anderson JR. Special ambulatory gynecologic considerations in the era of coronavirus disease 2019 (COVID-19) and implications for future practice. Am J Obstet Gynecol. 2020;223(3):372–378. doi: S0002-9378(20)30621-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaber EF, Kamineni A, Burnett-Hartman AN, et al. Evaluating and Improving Cancer Screening Process Quality in a Multilevel Context: The PROSPR II Consortium Design and Research Agenda. Cancer Epidemiol Biomarkers Prev. 2022;31(8):1521–1531. doi: 10.1158/1055-9965.EPI-22-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman S, Lykken JM, Haas JS, et al. Factors associated with timely colposcopy following an abnormal cervical cancer test result. Prev Med. 2022;164:107307. doi: S0091-7435(22)00356-5 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamineni A, Tiro JA, Beaber EF, et al. Cervical cancer screening research in the PROSPR I consortium: Rationale, methods and baseline findings from a US cohort. Int J Cancer. 2019;144(6):1460–1473. doi: 10.1002/ijc.31940 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright TC, Massad LS, Dunton CJ, et al. 2006 Consensus Guidelines for the Management of Women with Abnormal Cervical Cancer Screening Tests. Am J Obstet Gynecol. 2007;197(4):346–355. doi: S0002-9378(07)00930-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 18.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyer VA U S Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–91, W312. doi: 10.7326/0003-4819-156-12-201206190-00424 [doi]. [DOI] [PubMed] [Google Scholar]
- 20.Massad LS, Einstein MH, Huh WK, et al. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27. doi: 10.1097/LGT.0b013e318287d329 [doi]. [DOI] [PubMed] [Google Scholar]
- 21.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. doi: 10.1161/CIRCOUTCOMES.110.957951 [doi]. [DOI] [PubMed] [Google Scholar]
- 22.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–711. doi: 10.1023/a:1011240019516 [doi]. [DOI] [PubMed] [Google Scholar]
- 23.Marcondes FO, Cheng D, Warner ET, Kamran SC, Haas JS. The trajectory of racial/ethnic disparities in the use of cancer screening before and during the COVID-19 pandemic: A large U.S. academic center analysis. Prev Med. 2021;151:106640. doi: 10.1016/j.ypmed.2021.106640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.