Abstract
A suite of analytical techniques was used to obtain a comprehensive picture of per- and polyfluoroalkyl substances (PFAS) in selected Canadian food packaging used for fast foods (n = 42). Particle-induced gamma ray emission spectroscopy revealed that 55% of the samples contained <3580, 19% contained 3580–10 800, and 26% > 10 800 μg F/m2. The highest total F (1 010 000–1 300 000 μg F/m2) was measured in molded “compostable” bowls. Targeted analysis of 8 samples with high total F revealed 4–15 individual PFAS in each sample, with 6:2 fluorotelomer methacrylate (FTMAc) and 6:2 fluorotelomer alcohol (FTOH) typically dominating. Up to 34% of the total fluorine was released from samples after hydrolysis, indicating the presence of unknown precursors. Nontargeted analysis detected 22 PFAS from 6 different groups, including degradation products of FTOH. Results indicate the use of side-chain fluorinated polymers and suggest that these products can release short-chain compounds that ultimately can be transformed to compounds of toxicological concern. Analysis after 2 years of storage showed overall decreases in PFAS consistent with the loss of volatile compounds such as 6:2 FTMAc and FTOH. The use of PFAS in food packaging such as “compostable” bowls represents a regrettable substitution of single-use plastic food packaging.
Keywords: Fast food packaging, PFAS analysis, nontargeted PFAS analysis, regrettable substitution, plant fiber-based food packaging, PFAS precursors, hydrolysis assay, PFAS stability
Introduction
Per- and polyfluoroalkyl substances (PFAS) are used globally and comprise more than 4700 individual compounds.1,2 They have been intentionally added to food packaging for decades to confer grease and water repellency. PFAS are inherently persistent, and many are mobile, bioaccumulative, and/or toxic.3−6 As such, their use in food packaging could represent a significant issue in terms of direct human exposure and end-of-life environmental pollution.3,5−9 On December 20, 2022, the Government of Canada prohibited the manufacturing or import of single-use plastics, including “single-use plastic foodservice ware” such as polystyrene and oxo-degradable plastic clamshell containers, lidded containers, boxes, and bowls.10 This regulation, and a subsequent Canadian regulation that will restrict the use of these single-use plastics entirely by December 2023, will likely lead to greater use of plant fiber-based food packaging alternatives, to which PFAS may be added to achieve grease- and water-repellency.11 If so, this would represent a regrettable substitution of trading one harmful option for another.
Several studies have documented the widespread use of PFAS in food packaging,2,3,6,12,13 although only one was from Canada.14 The specific compounds used have changed over time, from past use of perfluorooctanesulfonic acid (PFOS) precursors such as N-ethyl perfluorooctane sulfonamido alcohol-based phosphate esters (SamPAPs) to substances based on 6:2 fluorotelomer alcohol (FTOH) and perfluoropolyethers9 (Tables S1 and S2 summarize literature data on PFAS in food packaging). PFAS can migrate from food packaging into food depending on the type of material, contact time, temperature, and PFAS chain length.3,15−18 For example, the consumption of popcorn from microwave popcorn bags containing PFAS has been related to elevated serum PFAS levels.15
An associated concern is the presence of polymeric PFAS, notably side-chain fluorinated polymers, in food packaging materials.19−21 Although these PFAS polymers are likely less mobile and bioaccessible than nonpolymeric PFAS, they contain nonpolymeric PFAS impurities and can produce lower molecular weight breakdown products such as FTOHs.12 As polymers, these substances are not subject to the same regulatory scrutiny as monomers and neither are their impurities and breakdown products.22−25
The negative health7 and environmental8 consequences of PFAS used in food packaging have led to legislative changes. In 2020, Denmark banned PFAS-containing cardboard and paper used in food packaging.26 As of 2022, 11 states in the United States have passed regulations banning the use of PFAS in food packaging.20 Industry has taken note of these actions: in 2020, three U.S. manufacturers agreed to voluntarily phase out sales of food packaging products containing 6:2 FTOH by 2023, and McDonald’s and Restaurant Brands International have committed to removing all PFAS from consumer packaging materials by 2025.27−29 Currently, PFOS, perfluorooctanoic acid (PFOA), and long-chain perfluoroalkyl carboxylic acids (PFCAs) as well as their salts and precursors are prohibited from manufacture, use, sale, or import under the Canadian Environmental Protection Act.22,23 Although the Government of Canada provides some information on the regulation of PFAS in food packaging,30 the Canadian regulatory system is far from transparent and does not include a publicly available list of PFAS that are restricted or that can be used in these materials, as is done in the U.S.
We herein used a suite of analytical techniques to obtain a comprehensive picture of PFAS in a selection of Canadian fast-food packaging, motivated by understanding the potential for exposure, Canadian regulatory requirements, and implications for the restrictions on single-use plastics for food packaging. Particle-induced gamma ray emission (PIGE) spectroscopy was used to screen samples for total fluorine (F) content followed by targeted analysis of PFAS by liquid and gas chromatography mass spectrometry (LC-MS/MS, GC-MS) for samples with high total F. We used high-resolution or HRMS-based nontarget analysis (NTA) to identify a broader set of unknown PFAS. The mass balance gap between total F and identifiable PFAS was further closed using a hydrolysis assay followed by GC-MS to detect neutral PFAS breakdown products. We chose the hydrolysis rather than the total oxidizable precursor (TOP) assay because we found that the former yielded higher concentrations of neutral PFAS when applied to textile samples.42 Finally, we shed light on changes in PFAS in fast-food packaging after storage along with associated implications.
Materials and Methods
Sampling
Food packaging samples were collected twice from food retailers in Toronto, Canada: February to March 2020 (42 samples) and August 2020 (resampling of 8 samples from the same retailers). Samples included molded “compostable” fiber bowls, sandwich and burger wrappers, popcorn serving bags, and dessert and bread wrappers.18,40 Detailed descriptions of the samples, sampling procedure, and all analytical methods used are in Supporting Information Sections S1–S5 and Table S3. The first round of analytical work (i.e., PIGE, targeted and nontargeted) occurred in August 2020, while the hydrolysis assay and the second round of targeted analysis occurred in August 2022 after samples were stored for about 2 years at room temperature in the dark.
PIGE Analysis
Samples (n = 50 including field and lab duplicates) were analyzed for total F using PIGE spectroscopy as described by Ritter et al.43 and Xia et al.42Section S2 and Table S9 describe study-specific quality assurance/quality control (QA/QC) analysis and sampling information.
Targeted GC-MS and LC-MS/MS
Eight products with high total F were selected for targeted analysis by LC-MS/MS and GC-MS after solvent extraction before and after 2 years of storage using analytical methods adapted from Wu et al.44 and described by Whitehead et al.45Section S3 and Tables S4–S8 summarize the 55 PFAS selected for targeted analysis, including a study-specific QA/QC analysis (including laboratory and field blanks).
Hydrolysis Assay
Subsamples from the same eight products analyzed with a targeted MS analysis were subjected to hydrolysis after 2 years of storage using the approach of Nikiforov,41 modified by Xia et al.42 Samples were mixed with a NaOH solution in methanol/water (90:10), heated at 60 °C in an oven for 16 h, and analyzed using GC/MS for a targeted analysis of FTOHs and fluorotelomer (meth)acrylates (FT(M)Acs) (Section S4).
Nontargeted LC-MS/MS
The same eight products selected for targeted analysis also underwent NTA using an extraction procedure and instrumental method adapted from Yuan et al.18 and Barrett et al.46 All samples were analyzed using a Vanquish ultrahigh-performance liquid chromatography (UHPLC) system (Thermo Fisher Scientific) with an electrospray ionization (ESI) source, coupled to a Q Exactive high-resolution mass spectrometer (Thermo Fisher Scientific). After instrumental analysis, the spectra were run through an in-house R script for peak detection and PFAS database matching.46 The identities of PFAS were then manually confirmed via MS2 analysis, and only those of PFAS supported by predicted MS2 fragments were kept. Of the tentatively identified compounds, authentic standards were commercially available for two compounds (6:2 FTUCA and 5:3 FTCA), which were used for validation. Supporting Information Section S5 and Figures S1 and S2 contain further details, including QA/QC measures (including laboratory and field blanks).
Results and Discussion
Total Fluorine Screening
55% of the samples contained no detectable F, defined here as <3580 μg F/m2. 19% of the samples contained trace levels of F ranging from 3580 to 10 800 μg F/m2, and 26% of the samples had >10 800 μg F/m2 (Figure S3, Tables S10 & S11). A similar range was reported previously using PIGE to measure total F in fast food packaging collected from the U.S. in 2014 and 2015 (Section S6 and Table S1).40,43
The PIGE analysis highlighted the relationship between material type and the amount of F. The highest concentrations of total F (1 010 000–1 300 000 μg F/m2) were detected in four samples of molded fiber bowls (also known as bagasse bowls) used for take-out food such as salads and burritos (Figure S4). These bowls are marketed as “green” alternatives to plastic bowls, since they are purportedly “compostable”. However, some molded fiber-based food packaging requires large quantities of PFAS to be mixed into the raw pulp to confer mechanical strength and prevent disintegration upon contact with liquids.13 Other samples containing quantifiable F (11 400–30 100 μg F/m2) were paper bags intended to hold oily food items such as pastries, donuts, or burgers. These bags had significantly higher total F than paper wrappers intended for use with less oily food items such as wraps (except for one paper wrapper intended for a burger, which contained no detectable F, Figure S4).
Targeted PFAS Analysis
Eight products were selected for targeted analysis (before storage) based on high F concentrations as measured by PIGE: three molded fiber bowls and five paper bags, all of which were intended to hold greasy or wet foods such as burrito bowls, salads, donuts, or popcorn. PIGE total F was 100–5000 times higher than the results from targeted total F for these samples (Table 1). ∑PFAS concentrations from targeted analysis ranged from 55 to 7180 ng/g, with the paper bags, except popcorn bag 8, containing higher ∑PFAS than the three molded fiber bowls.
Table 1. Mass Balance of Total Fluorine for Eight Products Analyzed by PIGE and Targeted Analysis of Extracts before and after Storage for 2 Years, and after Hydrolysis Assaya.
| Sample name | Burrito molded bowl 1 | Burrito molded bowl 2 | Salad molded bowl 3 | Donut paper bag 4 | Pastry paper bag 5 | Pastry paper bag 6 | Popcorn paper bag 7 | Popcorn paper bag 8 |
|---|---|---|---|---|---|---|---|---|
| PIGE | ||||||||
| Concentration | 1 300 000 | 1 010 000 | 1 180 000 | 30 100 | 23 800 | 28 000 | 11 400 | <LOQ |
| Original Targeted Analysis | ||||||||
| Concentration (original extraction) | 283 | 569 | 328 | 181 | 105 | 126 | 109 | 4.07 |
| Percent of PIGE concentration explained (%) | 0.02 | 0.06 | 0.03 | 0.60 | 0.44 | 0.45 | 0.96 | N/A |
| Concentration (re-extraction after 2 years) | 395 | 552 | 340 | 31.1 | 31.0 | 24.3 | 45.4 | 9.30 |
| Percent of PIGE concentration explained (%) | 0.03 | 0.05 | 0.03 | 0.10 | 0.13 | 0.09 | 0.40 | N/A |
| Hydrolysis | ||||||||
| Concentration | 36 100 | 24 100 | 65 700 | 3900 | 8100 | 8000 | 2900 | 7.00 |
| Difference between hydrolysis and concentration (re-extraction after 2 years) | 35 700 | 23 700 | 65 400 | 3860 | 8000 | 7960 | 2860 | |
| Percent of PIGE concentration explained (%) (F from unknown precursors released after hydrolysis) | 2.8 | 2.4 | 5.6 | 13 | 34 | 28 | 25 | N/A |
Concentrations of total F analyzed by PIGE and targeted analysis of extracts and after hydrolysis are expressed in μg F/m2. Also included are percentage contributions of the targeted analysis to total F measured by PIGE. The detailed information on conversion for concentration of total F (μg F/m2) from concentration of PFAS (ng/g) for targeted analysis of extracts and after hydrolysis was shown in Section S9. N/A indicates not applicable.
Among the 55 targeted PFAS (prestorage), 5–14 individual compounds were detected in each sample, including (FT(M)Acs), FTOHs, PFCAs, fluorotelomer phosphate monoesters and diesters (PAPs and diPAPs), and fluorotelomer sulfonic acids (FTSAs), at levels from ∼1 to 5670 ng/g for individual compounds (Figure S5, Table S12a).
Two short-chain PFAS, i.e., 6:2 FTOH and 6:2 FTMAc, were most abundant (except for one sample) from 300 to ∼5700 ng/g per individual compound. 6:2 FTMAc comprised 60–79% by weight for five of the eight samples (burrito bowl 1 and paper bags 4–7) or was second most abundant (42–46% in bowls 2 and 3). 8:2 FTMAc was not detected in any of the samples. To the authors’ knowledge, this is the first time that FTMAcs have been reported in food packaging. In two of the eight samples, FTOHs dominated at ∼55% by weight or were the second most abundant compound (five of the eight samples) with concentrations of 300–1735 ng/g. 8:2 FTOH was present (8 to ca. 100 ng/g) in 4 samples. 4:2 and 10:2 FTOH were not detected.
Yuan et al. reported finding seven individual FTOHs in 78% of food packaging from China purchased from 2013 to 2015, with the most abundant being 8:2 FTOH.18 Previous analyses of U.S. food packaging found that 6:2 FTOH was the major component in all FTOH-detectable food packaging.47Table S2 contains further comparisons with the literature.
Across the PFCAs, FTAcs, FTMAcs, and FTOHs, the six-perfluorocarbon homologues were detected with the highest abundance, aligning with the general industrial transition from longer chain to 6:2 fluorotelomer-based monomers and polymers and their abundance in numerous environmental matrices.48,49
Effect of Storage on the PFAS Profile
During storage, PFAS, especially volatile neutral ones, such as 6:2 FTOH, could be transformed and/or released from the products, changing the initial composition of the item. Before conducting the hydrolysis experiment, we reanalyzed the same fast-food packing products after they had been stored in a sealed bag in the dark for ∼2 years at room temperature. Total PFAS concentrations from the targeted analysis in re-extracted samples were lower in six of the eight samples, by up to 85%, than those in the original extracts, ranging from 130 to 2430 ng/g (Table S12b). Most pronounced were losses of 65–100% of 6:2 FTMAc and 6:2 FTOH in two samples (paper bags 4 and 6). Concentrations of 8:2 FTOH, 6:2 and 8:2 PAP, and 6:2 diPAP decreased to less than the limit of detection (<LOD) in several samples. These decreases are consistent with losses of volatile PFAS (FTOHs) observed in outdoor jacket textile samples, stored under conditions similar to those here for 3.5 years.50
The concentrations of most ionic PFAS were comparable to those from the original extractions with the exception of perfluoropropionic acid (PFPrA), perfluoropentanoic acid (PFPeA), and perfluorohexanoic acid (PFHxA), which increased by up to 8-fold in five samples (3 bowls and paper bags 7 and 8). These changes were likely due to the transformation of known (e.g., 6:2 FTOH, 6:2 FTMAc, 6:2 PAP, and 8:2 FTOH) and unknown precursors.51,52 We hypothesize that at least some mass losses of volatile FTOHs and FTMAcs were due to volatilization, whether present initially or due to transformation from precursors.
In contrast, 6:2 FTOH concentrations increased in five samples, potentially due to the transformation of the precursors. Notably, total PFAS concentrations measured by targeted analysis increased by 26 and 132% in two samples likely due to transformation of nondetected precursors to 6:2 FTOH.
Hydrolysis Assay
The hydrolysis treatment can free up chemically bound FTOHs and other volatile PFAS from the precursors, particularly from side-chain fluorinated polymers. Table 1 summarizes the data for pre- and post-hydrolysis for the eight samples with median concentrations plotted in Figure 1. After hydrolysis (focusing on neutral PFAS), 6:2 FTOH and 6:2 FTMAc had detection frequencies of 100% and 88% and median concentrations of 14 500 and 84.5 ng/g, respectively. 6:2 FTOH concentrations increased in all samples by an average of 220 times after hydrolysis, similarly to that observed in textile products.41,42 Conversely, the concentrations of 6:2 FTMAc and 6:2 FTAc decreased in all samples that contained them, likely due to their conversion to 6:2 FTOH during hydrolysis.
Figure 1.
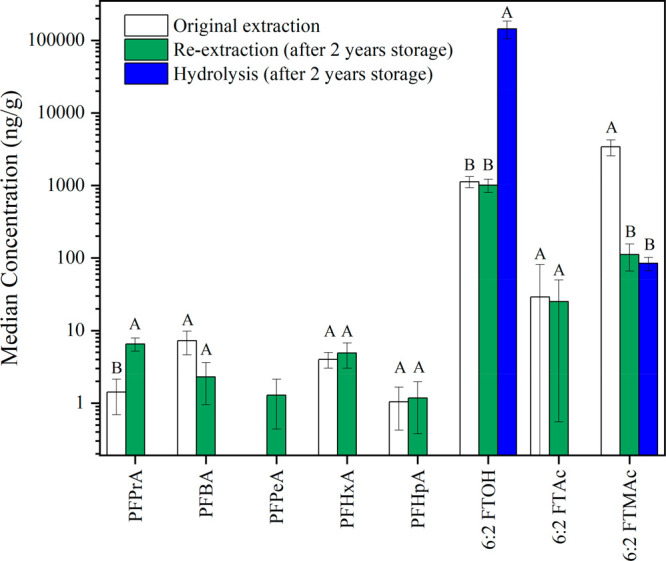
Median concentrations (ng/g) of detected PFAS in eight samples in extracts before and after 2 years of storage and after hydrolysis (which was performed on samples after 2 years of storage). For hydrolysis, only neutral PFAS were targeted in the analysis. The letters on the top of each bar indicate the results of an ANOVA test (p = 0.05) on concentrations obtained with the three sets of analyses. Different letters indicate a significant difference for a given congener.
The hydrolysis treatment provides crucial information to close the mass balance between targeted analyses and PIGE (Table 1). 6:2 FTOH, 6:2 FTMAc, and 6:2 FTAc released from hydrolysis accounted for 2.4–34% of the total F determined by PIGE for seven samples, confirming that the hydrolysis treatment is a useful tool to assess the mass of unknown precursors. However, the large gap remaining between these two techniques suggests the presence of other PFAS that have not been converted to products capable of detection by our targeted analysis.
Non-Targeted Analysis (NTA)
We also used NTA to attempt to explain the discrepancy between total F obtained by using PIGE and results from targeted analysis before and after the hydrolysis assay. Six compound groups were detected, with 22 individual compounds (Figures S6–S12, Table S13).
6:2 FTUCA was detected as the most abundant PFAS in all three bowls, with peak intensities even greater than those of the targeted PFCAs. 6:2 FTUCA has been detected previously in food packaging, including microwave popcorn bags from Europe, the U.S., and China,53,54 as well as paper and cardboard fast-food packaging from the U.S.39,40 FTUCAs can be present in materials as transformation products from the corresponding FTOHs.35,54−57
Among n:3 FTCAs, 5:3 FTCA and 6:3 FTCA were detected in two samples and with lower peak intensities than 6:2 FTUCA. As with FTUCAs, n:3 FTCAs were most likely from the transformation of FTOHs.55 6:2 FTUCA and 5:3 FTCA have been measured previously in microwave popcorn bags from around the world53,54 and North American fast-food packaging.39,40 This study is the first to detect 6:3 FTCA in food packaging, to the authors’ knowledge. 5:3 FTCA, which can be a metabolic transformation product of 6:2 FTOH and further converted into PFCAs with long in vivo half-lives, is of concern because of its biopersistence and potential toxicity.59
6:2 FTUCA and 5:3 FTCA concentrations were quantified through targeted analysis (Table S12). The concentrations of 6:2 FTUCA in the original extracts (prestorage) were comparable to those in popcorn bags previously reported.39,54 6:2 FTUCA and 5:3 FTCA concentrations increased ∼6- and ∼10-fold after storage, respectively, which suggested the presence of precursors like FTOHs.55
One multiple H-substituted-ether-substituted-perfluoroalkyl carboxylic acid (C9H5F13O3) was observed with low intensity in burrito bowl 2 (Figure S8). This compound class was first detected in influent and effluent wastewater samples from a fluorochemical manufacturing park in 201860 but has not been previously reported in food packaging.
Two (linear) PFCAs containing a C=C double bond, with the chemical formula Cn+8HF2n+13O2 where n = 3 and n = 4, were both measured only in plastic-coated popcorn bag 8 with moderate intensity (Figure S9). These compounds were reported in environmental agricultural samples and wastewater effluent samples55,60,61 but not previously in food packaging. These PFCAs could come from impurities/oligomers in the fluoropolymer used as an extrusion processing aid in the production of plastic coatings.2 Also, several single-H substituted linear PFCAs were detected in popcorn bag 8 with low to moderate intensity (Figure S10). The n = 9 compound was also detected in pastry bag 5 with low intensity. These compounds (more specifically, compounds with n = 3 to 13) have been detected preivously in wastewater samples62 and raw and treated drinking water.55,60,63,64
Seven linear perfluoroalkyl dicarboxylic acids with chain lengths from 7 to 13 were also detected in popcorn bag 8 with low to moderate intensity (Figure S11). This group of compounds was first discovered in wastewater from a fluorochemical manufacturing park.60
Implications
The suite of compounds measured here was consistent with the intentional use of side-chain fluorinated polymers, such as those with a C6 side chain, to confer grease and water repellency, with molded carboard bowls consistently having the highest concentrations of total F. Our results are also consistent with the presence (and some losses during storage) of impurities and breakdown products from these side-chain fluorinated polymers.
PFAS used in food packaging have a high potential of exposing consumers to compounds that are of toxicological concern such as 6:2 FTOH15 and some of the less known compounds identified via NTA such as FTUCAs and FTCAs.58,59 Further, our results suggest that, when stored, such food packaging could be a source of exposure to volatile PFAS such as FTOHs and FTMAcs in indoor air,49 as they appear to be released over time.
The threshold for a manufacturer to provide toxicological information to Health Canada pertaining to specific constituents and/or single additives to packaging materials for use with foods is a Probable Daily Intake of >0.025 μg kg bw–1 d–1 based on a migration test.65 Our calculations indicated that this Probable Daily Intake value could be exceeded when considering total PFAS measured here using targeted analysis (Supporting Information Section S7). This Health Canada threshold Probable Daily Intake of >0.025 μg kg bw–1 d–1 (compounds not specified) exceeds the tolerable weekly intake (TWI) of 4.4 ng kg bw–1 week–1 set by the European Food Safety Authority for the sum of PFOS, PFOA, perfluorononanoic acid (PFNA), and perfluorohexanesulfonic acid (PFHxS).66
The continued use of PFAS in food packaging should be questioned given opportunities for release and exposure and the movement by numerous government bodies and private entities to discontinue their use. In particular, the use of PFAS in plant fiber-based food packaging (e.g., molded cardboard bowls) could be seen as a regrettable substitution for single-use plastic because of the hazard posed by the use of PFAS.
Acknowledgments
Funding was provided by Environment and Climate Change Canada, Great Lakes Protection Initiative (GCXE21P039), Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2017-06654), and by charitable contributions to the Green Science Policy Institute, including from the Target Foundation. Z.W. gratefully acknowledges funding by the European Union under the Horizon 2020 Research and Innovation Programme (Project ZeroPM, Grant No. 101036756).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.2c00926.
Product sampling; PIGE analysis; sample preparation and instrumental analysis for targeted, hydrolysis and nontargeted analysis, PIGE comparison summary, discussion of nontargeted analysis; discussion of Canadian food packaging regulations and example Probably Daily Intake calculation; calculation of total F concentration (μg F/m2) from ng/g analyzed by targeted analysis; summaries of previous studies of fast food packaging using PIGE and targeted analysis; sample descriptions; lists of analytes, LODs and surrogate and internal standards for targeted analysis; comparison of duplicated PIGE and targeted analyses; summary of total F in all food packaging samples; list of samples with total F > LOQ analyzed by PIGE; full results from targeted analysis before and after storage; summary of 22 PFAS tentatively identified using nontargeted analysis; QA/QC nontargeted analysis (chromatograms and spike and recovery values); histogram of all PIGE data; box plots summarizing total F concentrations by type of food packaging; relative contributions of major PFAS categories obtained by targeted analysis; summaries of all compound groups identified using nontargeted analysis (PDF)
Summaries and data for studies on fast-food FCM, on FCM but not fast-food, and on food samples (XLSX)
Author Present Address
◇ Key Laboratory of Geographic Information Science (Ministry of Education), School of Geographic Sciences, East China Normal University, Shanghai 200241, China
Author Contributions
⌀ Heather Schwartz-Narbonne and Chunjie Xia are cofirst authors. Miriam L. Diamond and Marta Venier share equally as corresponding authors.
The authors declare no competing financial interest.
Supplementary Material
References
- OECD . Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs); Paris, 2018; Vol. 7. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en. (Accessed 2021-07-06).
- Glüge J.; Scheringer M.; Cousins I. T.; DeWitt J. C.; Goldenman G.; Herzke D.; Lohmann R.; Ng C. A.; Trier X.; Wang Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process Impacts 2020, 22 (12), 2345–2373. 10.1039/D0EM00291G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokkers B. G. H.; van de Ven B.; Janssen P.; Bil W.; van Broekhuizen F.; Zeilmaker M. J.; Oomen A. G.. Per- and polyfluoroalkyl substances (PFASs) in food contact materials; RIVM: Bilthoven, 2018; Vol. 2018. 10.21945/RIVM-2018-0181. [DOI]
- Curtzwiler G. W.; Silva P.; Hall A.; Ivey A.; Vorst K. Significance of Perfluoroalkyl Substances (PFAS) in Food Packaging. Integr Environ. Assess Manag 2021, 17 (1), 7–12. 10.1002/ieam.4346. [DOI] [PubMed] [Google Scholar]
- Lindstrom A. B.; Strynar M. J.; Libelo E. L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45 (19), 7954–7961. 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- CalEPA Department of Toxic Substances Control . Product-Chemical Profile for Food Packaging Containing Perfluoroalkyl or Polyfluoroalkyl Substances; 2020. https://dtsc.ca.gov/wp-content/uploads/sites/31/2020/07/Draft-Profile_PFASs-in-Food-Packaging_FINAL_ADA.pdf (Accessed 2021-07-07).
- Sunderland E. M.; Hu X. C.; Dassuncao C.; Tokranov A. K.; Wagner C. C.; Allen J. G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo Sci. Environ. Epidemiol 2019, 29 (2), 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C.; Kappleman W.; DiGuiseppi W. Ecological Considerations of Per- and Polyfluoroalkyl Substances (PFAS). Curr. Pollut Rep 2017, 3 (4), 289–301. 10.1007/s40726-017-0070-8. [DOI] [Google Scholar]
- Rice P. A. C6-Perfluorinated Compounds: The New Greaseproofing Agents in Food Packaging. Curr. Environ. Health Rep 2015, 2 (1), 33–40. 10.1007/s40572-014-0039-3. [DOI] [PubMed] [Google Scholar]
- Single-Use Plastics Prohibition Regulations: SOR/2022-138. Canada Gazette. June 22, 2022. https://www.gazette.gc.ca/rp-pr/p2/2022/2022-06-22/html/sor-dors138-eng.html (accessed 2022-12-06).
- Ackerman J.; McRobert D.; Sears M.. PFAS on food contact materials: Consequences for human health, compost, and the food chain and prospects for regulatory action in Canada and beyond. McGill Journal of Sustainable Development Law. https://www.mcgill.ca/mjsdl/article/pfas-food-contact-materials-consequences-human-health-compost-and-food-chain-and-prospects (accessed 2021-07-06).
- OECD . PFASs and Alternatives in Food Packaging (Paper and Paperboard) Report on the Commercial Availability and Current Uses; 2020. https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/PFASs-and-alternatives-in-food-packaging-paper-and-paperboard.pdf (Accessed 2021-07-06).
- Glenn G.; Shogren R.; Jin X.; Orts W.; Hart-Cooper W.; Olson L. Per- and Polyfluoroalkyl Substances and Their Alternatives in Paper Food Packaging. Compr Rev. Food Sci. Food Saf 2021, 20 (3), 2596–2625. 10.1111/1541-4337.12726. [DOI] [PubMed] [Google Scholar]
- D’eon J. C.; Crozier P. W.; Furdui V. I.; Reiner E. J.; Libelo E. L.; Mabury S. A. Observation of a Commercial Fluorinated Material, the Polyfluoroalkyl Phosphoric Acid Diesters, in Human Sera, Wastewater Treatment Plant Sludge, and Paper Fibers. Environ. Sci. Technol. 2009, 43 (12), 4589–4594. 10.1021/es900100d. [DOI] [PubMed] [Google Scholar]
- Susmann H. P.; Schaider L. A.; Rodgers K. M.; Rudel R. A. Dietary Habits Related to Food Packaging and Population Exposure to PFASs. Environ. Health Perspect 2019, 127 (10), 107003. 10.1289/EHP4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreta C.; Tena M. T. Determination of Perfluorinated Alkyl Acids in Corn, Popcorn and Popcorn Bags before and after Cooking by Focused Ultrasound Solid-Liquid Extraction, Liquid Chromatography and Quadrupole-Time of Flight Mass Spectrometry. J. Chromatogr A 2014, 1355, 211–218. 10.1016/j.chroma.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Begley T. H.; Hsu W.; Noonan G.; Diachenko G. Migration of Fluorochemical Paper Additives from Food-Contact Paper into Foods and Food Simulants. Food Addit Contam Part A Chem. Anal Control Expo Risk Assess 2008, 25 (3), 384–390. 10.1080/02652030701513784. [DOI] [PubMed] [Google Scholar]
- Yuan G.; Peng H.; Huang C.; Hu J. Ubiquitous Occurrence of Fluorotelomer Alcohols in Eco-Friendly Paper-Made Food-Contact Materials and Their Implication for Human Exposure. Environ. Sci. Technol. 2016, 50 (2), 942–950. 10.1021/acs.est.5b03806. [DOI] [PubMed] [Google Scholar]
- Schultes L.; Peaslee G. F.; Brockman J. D.; Majumdar A.; McGuinness S. R.; Wilkinson J. T.; Sandblom O.; Ngwenyama R. A.; Benskin J. P. Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform?. Environ. Sci. Technol. Lett. 2019, 6 (2), 73–78. 10.1021/acs.estlett.8b00700. [DOI] [Google Scholar]
- Schellenberger S.; Jönsson C.; Mellin P.; Levenstam O. A.; Liagkouridis I.; Ribbenstedt A.; Hanning A. C.; Schultes L.; Plassmann M. M.; Persson C.; Cousins I. T.; Benskin J. P. Release of Side-Chain Fluorinated Polymer-Containing Microplastic Fibers from Functional Textiles during Washing and First Estimates of Perfluoroalkyl Acid Emissions. Environ. Sci. Technol. 2019, 53 (24), 14329–14338. 10.1021/acs.est.9b04165. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Miller G. Z.; Gearhart J.; Peaslee G.; Venier M. Side-Chain Fluorotelomer Based Polymers in Children Car Seats. Environ. Pollut. 2021, 268, 115477. 10.1016/j.envpol.2020.115477. [DOI] [PubMed] [Google Scholar]
- Fall 2016 amendments to the prohibition of certain toxic substances regulations. Government of Canada. https://www.canada.ca/en/environment-climate-change/services/canadian-environmental-protection-act-registry/fall-2016-amendments-prohibition-toxic-substances.html (accessed 2021-08-30).
- Portal on per and poly fluorinated chemicals: Canada. Organisation for Economic Co-operation and Development. https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/countryinformation/canada.htm (accessed 2021-06-09).
- Packaging materials. https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/packaging-materials.html (accessed 2021-08-30).
- ECHA . Guidance for Monomers and Polymers - Guidance for the Implementation of REACH; Helsinki, 2012. https://echa.europa.eu/guidance-documents/guidance-on-reach?panel=registration#registration%3EGuidance-on-Registration%3C/a%3E%3C/li%3E%3Cli%3E%3Ca-data-cke-saved. (accessed 2021-06-09).
- Boucher J.Denmark moves ahead with PFAS ban in FCMs. Food Packaging Forum. https://www.foodpackagingforum.org/news/denmark-moves-ahead-with-pfas-ban-in-fcms (accessed 2021-06-09).
- FDA announces the voluntary phase-out by industry of certain PFAS used in food packaging. CFSAN Constituent Updates. https://www.fda.gov/food/cfsan-constituent-updates/fda-announces-voluntary-phase-out-industry-certain-pfas-used-food-packaging (accessed 2021-08-15).
- Packaging, toys & waste. https://corporate.mcdonalds.com/corpmcd/our-purpose-and-impact/our-planet/packaging-toys-and-waste.html (accessed 2022-08-30).
- Packaging & recycling. https://www.rbi.com/English/sustainability/packaging-and-recycling/default.aspx (accessed 2022-08-30).
- Minet L.; Wang Z.; Bruton T. A.; Blum A.; Peaslee G.; Shalin A.; Schwartz-Narbonne H.; Venier M.; Whitehead H.; Wu Y.; Diamond M. L. Use and Release of Per- and Polyfluoroalkyl Substances (PFAS) in Consumer Food Packaging in U.S. and Canada. Environ. Sci. Proc. Impacts 2022, 24, 2032–2042. 10.1039/D2EM00166G. [DOI] [PubMed] [Google Scholar]
- Xia C.; Diamond M. L.; Peaslee G. F.; Peng H.; Blum A.; Wang Z.; Shalin A.; Whitehead H. D.; Green M.; Schwartz-Narbonne H.; Yang D.; Venier M. Per- and Polyfluoroalkyl Substances in North American School Uniforms. Environ. Sci. Technol. 2022, 56 (19), 13845–13857. 10.1021/acs.est.1c07876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaider L. A.; Balan S. A.; Blum A.; Andrews D. Q.; Strynar M. J.; Dickinson M. E.; Lunderberg D. M.; Lang J. R.; Peaslee G. F. Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett. 2017, 4, 105–111. 10.1021/acs.estlett.6b00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter E. E.; Dickinson M. E.; Harron J. P.; Lunderberg D. M.; DeYoung P. A.; Robel A. E.; Field J. A.; Peaslee G. F. PIGE as a Screening Tool for Per- and Polyfluorinated Substances in Papers and Textiles. Nuclear Instr Methods Physics Res. B 2017, 407, 47–54. 10.1016/j.nimb.2017.05.052. [DOI] [Google Scholar]
- Wu Y.; Romanak K.; Bruton T.; Blum A.; Venier M. Per- and Polyfluoroalkyl Substances in Paired Dust and Carpets from Childcare Centers. Chemosphere 2020, 251, 126771. 10.1016/j.chemosphere.2020.126771. [DOI] [PubMed] [Google Scholar]
- Whitehead H. D.; Venier M.; Wu Y.; Eastman E.; Urbanik S.; Diamond M. L.; Shalin A.; Schwartz-Narbonne H.; Bruton T. A.; Blum A.; Wang Z.; Green M.; Tighe M.; Wilkinson J. T.; Mcguinness S.; Peaslee G. F. Fluorinated Compounds in North American Cosmetics. Environ. Sci. Technol. Lett. 2021, 8 (7), 538–544. 10.1021/acs.estlett.1c00240. [DOI] [Google Scholar]
- Nikiforov V. A. Hydrolysis of FTOH Precursors, a Simple Method to Account for Some of the Unknown PFAS. Chemosphere 2021, 276, 130044. 10.1016/j.chemosphere.2021.130044. [DOI] [PubMed] [Google Scholar]
- Barrett H.; Du X.; Houde M.; Lair S.; Verreault J.; Peng H. Suspect and Nontarget Screening Revealed Class-Specific Temporal Trends (2000–2017) of Poly- and Perfluoroalkyl Substances in St. Lawrence Beluga Whales. Environ. Sci. Technol. 2021, 55 (3), 1659–1671. 10.1021/acs.est.0c05957. [DOI] [PubMed] [Google Scholar]
- Rewerts J. N.; Morré J. T.; Massey Simonich S. L.; Field J. A. In-Vial Extraction Large Volume Gas Chromatography Mass Spectrometry for Analysis of Volatile PFASs on Papers and Textiles. Environ. Sci. Technol. 2018, 52 (18), 10609–10616. 10.1021/acs.est.8b04304. [DOI] [PubMed] [Google Scholar]
- Buck R. C; Franklin J.; Berger U.; Conder J. M; Cousins I. T; de Voogt P.; Jensen A. A.; Kannan K.; Mabury S. A; van Leeuwen S. P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr Environ. Assess Manag 2011, 7 (4), 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Mcdevitt M. E.; Becanova J.; Blum A.; Bruton T. A.; Vojta S.; Woodward M.; Lohmann R. The Air That We Breathe: Neutral and Volatile PFAS in Indoor Air. Environ. Sci. Technol. Lett. 2021, 8 (10), 897–902. 10.1021/acs.estlett.1c00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremmel C.; Frömel T.; Knepper T. P. Systematic Determination of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Outdoor Jackets. Chemosphere 2016, 160, 173–180. 10.1016/j.chemosphere.2016.06.043. [DOI] [PubMed] [Google Scholar]
- van der Veen I.; Schellenberger S.; Hanning A.-C.; Stare A.; de Boer J.; Weiss J. M.; Leonards P. E. G. Fate of Per- and Polyfluoroalkyl Substances from Durable Water-Repellent Clothing during Use. Environ. Sci. Technol. 2022, 56 (9), 5886–5897. 10.1021/acs.est.1c07876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen I.; Hanning A. C.; Stare A.; Leonards P. E. G.; de Boer J.; Weiss J. M. The Effect of Weathering on Per- and Polyfluoroalkyl Substances (PFASs) from Durable Water Repellent (DWR) Clothing. Chemosphere 2020, 249, 126100. 10.1016/j.chemosphere.2020.126100. [DOI] [PubMed] [Google Scholar]
- Zabaleta I.; Bizkarguenaga E.; Bilbao D.; Etxebarria N.; Prieto A.; Zuloaga O. Fast and Simple Determination of Perfluorinated Compounds and Their Potential Precursors in Different Packaging Materials. Talanta 2016, 152, 353–363. 10.1016/j.talanta.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Zabaleta I.; Negreira N.; Bizkarguenaga E.; Prieto A.; Covaci A.; Zuloaga O. Screening and Identification of Per- and Polyfluoroalkyl Substances in Microwave Popcorn Bags. Food Chem. 2017, 230, 497–506. 10.1016/j.foodchem.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Robel A. E.; Marshall K.; Dickinson M.; Lunderberg D.; Butt C.; Peaslee G.; Stapleton H. M.; Field J. A. Closing the Mass Balance on Fluorine on Papers and Textiles. Environ. Sci. Technol. 2017, 51 (16), 9022–9032. 10.1021/acs.est.7b02080. [DOI] [PubMed] [Google Scholar]
- Jacob P.; Barzen-Hanson K. A.; Helbling D. E. Target and Nontarget Analysis of Per- and Polyfluoralkyl Substances in Wastewater from Electronics Fabrication Facilities. Environ. Sci. Technol. 2021, 55 (4), 2346–2356. 10.1021/acs.est.0c06690. [DOI] [PubMed] [Google Scholar]
- Heydebreck F.; Tang J.; Xie Z.; Ebinghaus R. Emissions of Per- and Polyfluoroalkyl Substances in a Textile Manufacturing Plant in China and Their Relevance for Workers’ Exposure. Environ. Sci. Technol. 2016, 50 (19), 10386–10396. 10.1021/acs.est.6b03213. [DOI] [PubMed] [Google Scholar]
- Martin J. W.; Mabury S. A.; O’Brien P. J. Metabolic Products and Pathways of Fluorotelomer Alcohols in Isolated Rat Hepatocytes. Chem. Biol. Interact 2005, 155 (3), 165–180. 10.1016/j.cbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Rice P. A.; Aungst J.; Cooper J.; Bandele O.; Kabadi S. v. Comparative Analysis of the Toxicological Databases for 6:2 Fluorotelomer Alcohol (6:2 FTOH) and Perfluorohexanoic Acid (PFHxA). Food Chem. Toxicol. 2020, 138, 111210. 10.1016/j.fct.2020.111210. [DOI] [PubMed] [Google Scholar]
- Kabadi S.; Fisher J. W.; Doerge D. R.; Mehta D.; Aungst J.; Rice P. Characterizing Biopersistence Potential of the Metabolite 5:3 Fluorotelomer Carboxylic Acid after Repeated Oral Exposure to the 6:2 Fluorotelomer Alcohol. Toxicol. Appl. Pharmacol. 2020, 388, 114878. 10.1016/j.taap.2020.114878. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yu N.; Zhu X.; Guo H.; Jiang J.; Wang X.; Shi W.; Wu J.; Yu H.; Wei S. Suspect and Nontarget Screening of Per- and Polyfluoroalkyl Substances in Wastewater from a Fluorochemical Manufacturing Park. Environ. Sci. Technol. 2018, 52 (19), 11007–11016. 10.1021/acs.est.8b03030. [DOI] [PubMed] [Google Scholar]
- Washington J. W.; Jenkins T. M.; Weber E. J. Identification of Unsaturated and 2H Polyfluorocarboxylate Homologous Series and Their Detection in Environmental Samples and as Polymer Degradation Products. Environ. Sci. Technol. 2015, 49 (22), 13256–13263. 10.1021/acs.est.5b03379. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Pereira A. D. S.; Martin J. W. Discovery of C5-C17 Poly- and Perfluoroalkyl Substances in Water by in-Line Spe-HPLC-Orbitrap with in-Source Fragmentation Flagging. Anal. Chem. 2015, 87 (8), 4260–4268. 10.1021/acs.analchem.5b00039. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yu N.; Qian Y.; Shi W.; Zhang X.; Geng J.; Yu H.; Wei S. Non-Target and Suspect Screening of Per- and Polyfluoroalkyl Substances in Chinese Municipal Wastewater Treatment Plants. Water Res. 2020, 183, 115989. 10.1016/j.watres.2020.115989. [DOI] [PubMed] [Google Scholar]
- Tröger R.; Ren H.; Yin D.; Postigo C.; Nguyen P. D.; Baduel C.; Golovko O.; Been F.; Joerss H.; Boleda M. R.; Polesello S.; Roncoroni M.; Taniyasu S.; Menger F.; Ahrens L.; Yin Lai F.; Wiberg K. What’s in the Water? - Target and Suspect Screening of Contaminants of Emerging Concern in Raw Water and Drinking Water from Europe and Asia. Water Res. 2021, 198, 117099. 10.1016/j.watres.2021.117099. [DOI] [PubMed] [Google Scholar]
- Rand A. A.; Rooney J. P.; Butt C. M.; Meyer J. N.; Mabury S. A. Cellular Toxicity Associated with Exposure to Perfluorinated Carboxylates (PFCAs) and Their Metabolic Precursors. Chem. Res. Toxicol. 2014, 27 (1), 42–50. 10.1021/tx400317p. [DOI] [PubMed] [Google Scholar]
- Information requirements for food packaging submissions. December 30, 2020. https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/information-requirements-food-packaging-submissions.html (accessed 2021-08-28).
- Schrenk D.; Bignami M.; Bodin L.; Chipman J. K.; del Mazo J.; Grasl-Kraupp B.; Hogstrand C.; Hoogenboom L.; Leblanc J. C.; Nebbia C. S.; Nielsen E.; Ntzani E.; Petersen A.; Sand S.; Vleminckx C.; Wallace H.; Barregård L.; Ceccatelli S.; Cravedi J. P.; Halldorsson T. I.; Haug L. S.; Johansson N.; Knutsen H. K.; Rose M.; Roudot A. C.; van Loveren H.; Vollmer G.; Mackay K.; Riolo F.; Schwerdtle T.. Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA Journal 2020, 18 ( (9), ). 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


