Abstract
Fifteen Cyanothece strains isolated from saline environments have been characterized with regard to exopolysaccharide (EPS) production. The polymers contained six to eight monosaccharides, with one or two acidic sugars. In some EPS samples, the additional presence of acetyl, pyruvyl, and/or sulfate groups was also detected.
The interest in microorganisms as producers of high-molecular-weight polysaccharides has greatly increased in recent years, since these biopolymers often show advantages over the polysaccharides currently in use, which are mostly extracted from plants or marine macroalgae (19). Cyanobacteria can also be included among the potential sources of new polymers, several species being characterized by the presence of thick capsules surrounding the cells and by the ability to release polysaccharide material into culture medium (22). Thus, research aimed at the isolation and characterization of new exopolysaccharide (EPS)-producing cyanobacterial strains has been undertaken, focusing attention on hypersaline habitats because they are generally known to harbor a large number of EPS-producing strains (3, 7). In this report, the production of exocellular polysaccharides by 15 cyanobacterial strains isolated from hypersaline and saline habitats and belonging to the Cyanothece group (14) is described, along with the chemical and rheological characterization of the polymers.
The 15 cyanobacterial strains studied (Table 1) were photoautotrophically grown in enriched seawater medium (6), except for strains ET 2 and ET 5, which were cultivated in Zarrouk medium (24); when required, the amount of NaNO3 was reduced from 1.5 to 0.35 g liter−1. The strains were axenically grown in an atmosphere of 95% air–5% CO2 for 8 days under continuous illumination (initial concentration of chlorophyll a, 1.8 mg liter−1) with mean photon fluxes of 30 μmol photon m−2 s−1 for the first 3 days of growth and 80 μmol photon m−2 s−1 for the remaining 5 days (photosynthetic active radiation). Total carbohydrates of the cultures and soluble carbohydrates in the medium were determined as previously described (21). Uronic acid and acetyl and pyruvyl contents of crude EPS samples, obtained by precipitation with 2-propanol (21), were colorimetrically estimated as described by Galambos (11), Weissman and Meyer (23), and Sloneker and Orentas (16), respectively. The presence of sulfate in EPSs was assessed by Fourier transform infrared (FT-IR) spectrometry (13). The monosaccharidic composition of hydrolyzed EPS samples (2 N trifluoroacetic acid, 120°C for 45 min) was determined by high-pressure liquid chromatography (21). The viscosity of aqueous solutions of the polymers and of commercial xanthan gum (0.1% [wt/vol]; weight determined as glucose equivalents by the method of Dubois et al. [8]) was determined with a Brookfield LVT viscosimeter.
TABLE 1.
Origin of the Cyanothece strains investigated and carbohydrate production by photoautotrophic batch cultures run for 8 days under balanced (control) or nitrogen-limited (NL) growth conditions
| Straina | Origin | Carbohydrate production (mg liter−1)b
|
|||
|---|---|---|---|---|---|
| Total
|
Soluble
|
||||
| Control | NL | Control | NL | ||
| CE 4 | Central Italyc | 800 | 1,272 | 344 | 960 |
| CE 9 | Central Italyc | 776 | 1,776 | 536 | 1,592 |
| TP 5 | Sicily, Italyc | 776 | 2,032 | 136 | 864 |
| TP 10 | Sicily, Italyc | 552 | 984 | 216 | 584 |
| CH 1 | Greecec | 1,208 | 1,736 | 480 | 1,024 |
| 16Som2 | Getzira, Republic of Somaliac | 1,056 | 2,368 | 200 | 2,184 |
| TI 4 | Sicily, Italyd | 1,376 | 1,776 | 264 | 1,048 |
| CA 3 | Sardinia, Italyd | 816 | 2,440 | 152 | 2,360 |
| VI 13 | Sardinia, Italye | 968 | 1,888 | 168 | 336 |
| VI 22 | Sardinia, Italye | 992 | 1,896 | 160 | 340 |
| PE 13 | Laconia, Greecee | 824 | 1,416 | 496 | 288 |
| PE 14 | Laconia, Greecee | 616 | 1,856 | 376 | 416 |
| IR 20 | Dead Sea, Israelf | 1,272 | 1,560 | 640 | 552 |
| ET 2 | Lake Abijata, Ethiopiag | 456 | 2,464 | 304 | 152 |
| ET 5 | Lake Abijata, Ethiopiag | 440 | 968 | 152 | 88 |
Available from the collection of the Centro di Studio dei Microrganismi Autotrofi of the Consiglio Nazionale delle Ricerche.
Expressed as final minus initial carbohydrate concentration.
Saltworks.
Hypersaline ponds.
Tidal pools.
Hypersaline lake.
Alkaline lake.
The release of soluble carbohydrates into the culture medium during an 8-day incubation by the 15 Cyanothece strains was tested first under balanced growth conditions (Table 1). Four strains (IR 20, CH 1, PE 13, and CE 9) showed sustained production of soluble carbohydrates that, depending on the strain, accounted for 40 to 70% of the total carbohydrates produced. For the other strains, the soluble fraction represented 17 to 70% of the total carbohydrates but never exceeded 400 mg liter−1. When the cultures were carried out under conditions of nitrogen limitation, the carbohydrate production pattern that resulted was significantly modified (Table 1). Eight strains reacted by increasing the amount of soluble carbohydrates released into the culture medium, as already observed in many other EPS-producing microalgae and cyanobacteria (1, 6, 10, 20). Two strains (VI 13 and VI 22) produced higher amounts of both total and soluble carbohydrates, but in such a way that their ratio did not change. The other five strains showed a dramatic decrease in the soluble-to-total-carbohydrate ratio. Since microscopic observations of these strains showed that the thickness of the capsules did not increase, this behavior has to be linked to the accumulation of intracellular carbohydrate reserves, as was previously observed in the EPS-producing cyanobacterium Cyanospira capsulata under conditions of nitrogen limitation (5).
All of the EPSs produced by the Cyanothece strains showed the presence of uronic acids at concentrations ranging from 10 to 80% of the carbohydrate content of crude polymers (Table 2). Nine polymers contained O-acetyl groups, but only two of them, produced by strains ET 2 and ET 5, contained these groups at concentrations higher than 2% of the total carbohydrates, an amount comparable to the degree of acetylation reported for xanthan (18). Ketal-linked pyruvyl groups were found in 14 of the 15 polysaccharides, the highest contents amounting to more than 2% of the total carbohydrates. All of the polymers, except the EPSs obtained from strains ET 2 and ET 5, also contained sulfate groups, but they were abundantly present in only six EPS samples (Table 2). The number of constitutive monosaccharides of the EPS samples ranged, in various combinations, from six to eight (Table 3). Rhamnose and fucose were found in all of the EPS samples, whereas glucose and mannose were present in 14 and 13 of the EPSs, respectively; ribose was found only in the EPSs produced by strains IR 20 and PE 13. Quantitative sugar composition analysis of the polysaccharides showed that glucose was the most abundant monosaccharide in about half of the cases. The anionic nature of the polymers was ensured by the presence of one (in eight EPSs) or two acidic sugars, namely, glucuronic and galacturonic acids. However, it has to be stressed that it is quite difficult to obtain reliable quantitative data for uronic acids from hydrolyzed polymers, owing to their easy degradation (4). The presence of uronic acids in cyanobacterial EPSs may be considered quite usual (22), whereas there are too few data concerning the presence of sulfate and pyruvate groups to draw a general picture. Indeed, for many years, it was believed that sulfated EPSs could be produced only by eukaryotes, but now this opinion has been ruled out and sulfate groups have also been recognized in cyanobacterial EPSs, most, but not all (2, 12, 15), of them produced by strains isolated from saline or hypersaline environments (9, 12, 17). From the results obtained in this study, it seems that the presence of sulfate groups is a distinctive feature of the EPSs produced by the Cyanothece strains isolated from marine or hypersaline environments, sulfate groups being absent in the polymers produced by the two strains isolated from alkaline Lake Abijata. In this connection, it is worth mentioning that the EPS produced by C. capsulata, the only other alkaliphilic EPS-producing cyanobacterium so far studied, is also devoid of sulfate groups in the macromolecule (21).
TABLE 2.
Uronic acid, acetate, pyruvate, and sulfate contents of EPSs released by Cyanothece strains investigateda
| Strain | Uronic acids | Acetate | Pyruvate | Sulfate |
|---|---|---|---|---|
| CE 4 | 80.1 | 0.66 | 0.36 | Tr |
| CE 9 | 35.7 | 0.0 | 1.17 | Tr |
| TP 5 | 40.4 | 0.0 | 1.10 | ++ |
| TP 10 | 31.3 | ND | 3.86 | + |
| CH 1 | 27.4 | 0.52 | 1.04 | Tr |
| 16Som2 | 20.6 | 0.0 | 0.0 | ++ |
| TI 4 | 58.2 | 0.98 | 1.37 | Tr |
| CA 3 | 66.8 | 0.62 | 2.72 | Tr |
| VI 13 | 32.1 | 0.0 | 0.34 | ++ |
| VI 22 | 40.8 | 0.55 | 0.23 | ++ |
| PE 13 | 20.9 | 0.0 | 2.08 | Tr |
| PE 14 | 21.7 | 0.32 | 0.17 | Tr |
| IR 20 | 9.8 | 0.75 | 2.11 | ++ |
| ET 2 | 63.1 | 4.2 | 2.28 | − |
| ET 5 | 29.4 | 2.5 | 0.39 | − |
Expressed as a percentage of the total carbohydrates. ND, not determined; ++, abundant; +, present; −, absent; Tr, trace.
TABLE 3.
Monosaccharidic composition of EPSs produced by Cyanothece strains grown under balanced growth conditionsa
| Strain | GlcAc | GalAc | Gal | Glc | Man | Ara | Xyl | Rib | Fuc | Rha |
|---|---|---|---|---|---|---|---|---|---|---|
| CE 4 | − | + | 0.02 | 1.26 | 0.0 | 0.35 | 0.46 | 0.0 | 0.30 | 1.0 |
| CE 9 | − | + | 0.31 | 2.79 | 0.68 | 0.0 | 0.0 | 0.0 | 1.12 | 1.0 |
| TP 5 | − | + | 0.0 | 0.93 | 0.0 | 0.44 | 0.28 | 0.0 | 1.16 | 1.0 |
| TP 10 | + | + | 0.0 | 0.64 | 0.10 | 1.75 | 0.0 | 0.0 | 0.77 | 1.0 |
| CH 1 | − | + | 1.42 | 0.0 | 0.63 | 0.0 | 0.87 | 0.0 | 3.06 | 1.0 |
| 16Som2 | + | + | 0.13 | 1.75 | 0.38 | 0.0 | 1.22 | 0.0 | 0.96 | 1.0 |
| TI 4 | + | − | 1.17 | 2.87 | 0.40 | 0.0 | 0.70 | 0.0 | 2.70 | 1.0 |
| CA 3 | + | + | 0.0 | 1.40 | 0.01 | 9.15 | 0.0 | 0.0 | 1.96 | 1.0 |
| VI 13 | + | + | 0.07 | 2.01 | 0.46 | 0.0 | 1.51 | 0.0 | 1.48 | 1.0 |
| VI 22 | + | − | 0.25 | 2.75 | 1.00 | 0.0 | 1.79 | 0.0 | 1.75 | 1.0 |
| PE 13 | + | − | 11.59 | 22.0 | 5.88 | 0.0 | 5.88 | 0.29 | 3.76 | 1.0 |
| PE 14 | + | + | 0.0 | 4.94 | 2.94 | 61.3 | 0.0 | 0.0 | 1.88 | 1.0 |
| IR 20 | + | − | 0.01 | 0.01 | 0.23 | 0.0 | 0.0 | 0.01 | 0.15 | 1.0 |
| ET 2 | + | + | 1.44 | 1.34 | 0.75 | 5.76 | 0.0 | 0.0 | 1.51 | 1.0 |
| ET 5 | + | + | 2.18 | 3.08 | 1.77 | 0.0 | 3.30 | 0.0 | 2.10 | 1.0 |
Expressed as a molar ratio with respect to rhamnose content.
GlcA, glucuronic acid; GalA, galacturonic acid; Glc, glucose; Gal, galactose; Man, mannose; Ara, arabinose; Xyl, xylose; Rib, ribose; Fuc, fucose; Rha, rhamnose; +, present; −, absent.
Quantitative data are not reported because hydrolysis is known to degrade uronic acids to various extents, depending on the polymer structure (4).
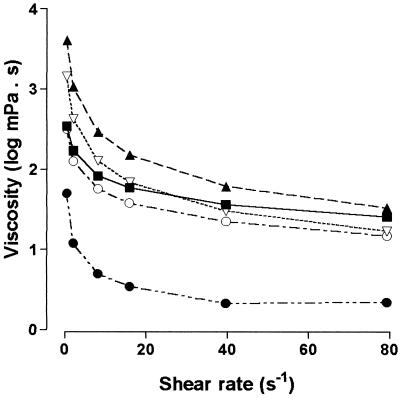
The viscosity dependence on shear rate of 0.1% aqueous solutions of the EPSs produced by the Cyanothece strains, compared with that of xanthan gum, showed that the EPSs can be divided into four different groups (in Fig. 1 are reported the curves of one representative polymer from each group). The first group, including the EPSs produced by strains CA 3, VI 22, and CE 4, showed a viscosity dependence on shear rate similar to that of xanthan gum, but with viscosity values constantly higher than that of the reference polymer. A second group, composed of the polymers produced by strains IR 20 and VI 13, showed the same behavior, but with viscosity values lower than that of xanthan gum. The polymers produced by strains PE 14, PE 13, TP 5, TP 10, TI 4, CH 1, and 16Som2 showed a shear thinning behavior more accentuated than that of xanthan gum, with higher viscosities at low shear rates and lower viscosities at high shear rates, a property that could be of particular interest for some applications, e.g., for the formulation of oil drilling muds (19). Finally, three EPSs, produced by strains ET 2, ET 5, and CE 9, showed very low viscosity values at all of the shear rates tested. In this connection, it has to be stressed that these three EPSs are produced by strains unable to form a true capsule (14). A possible explanation for this behavior could be the absence of a component that is essential for EPS binding to the cell surface, as well as for establishing interactions among the macromolecules in solution.
FIG. 1.
Viscosity dependence on shear rate of 0.1% (wt/vol) aqueous solutions of xanthan gum (▪) and the EPSs produced by Cyanothece strains CA 3 (▴), PE 14 (▿), IR 20 (○), and ET 2 (•).
In conclusion, examination of all of the data obtained shows that at least three Cyanothece strains (CA 3, CE 4, and 16Som2) can be considered quite promising for industrial exploitation and are worthy of further investigations to determine the most suitable fields of application.
Acknowledgments
This work was partially supported by the Consiglio Nazionale delle Ricerche (CNR) in the framework of the Progetto Coordinato POLISA (Nuovi polisaccaridi microbici di potenziale interesse per l’industria alimentare) and by the Istituto Nazionale di Coordinamento Agroindustria of the CNR.
We are indebted to M. Roussomoustakaki, University of Athens, who kindly supplied strain CH 1; to Luca Calamai, University of Florence, who determined FT-IR spectra and shared his experience in the field; to Nino Feminò, who isolated strain TI 4; and to Veronica Delfino for technical assistance.
REFERENCES
- 1.Arad S M, Friedman O D, Rotem A. Effect of nitrogen on polysaccharide production in a Porphyridium sp. Appl Environ Microbiol. 1988;54:2411–2414. doi: 10.1128/aem.54.10.2411-2414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar-Or Y, Shilo M. Characterization of macromolecular flocculants produced by Phormidium sp. strain J-1 and by Anabaenopsis circularis PCC 6720. Appl Environ Microbiol. 1987;53:2226–2230. doi: 10.1128/aem.53.9.2226-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell S, Golubic S T. Benthic cyanophytes (cyanobacteria) of Solar Lake (Sinai) Algol Stud. 1985;38/39:311–329. [Google Scholar]
- 4.Cesaro A, Liut G, Bertocchi C, Navarini L, Urbani R. Physicochemical properties of the exocellular polysaccharide from Cyanospira capsulata. Int J Biol Macromol. 1990;12:79–84. doi: 10.1016/0141-8130(90)90057-h. [DOI] [PubMed] [Google Scholar]
- 5.De Philippis R, Sili C, Vincenzini M. Response of an exopolysaccharide-producing heterocystous cyanobacterium to changes in metabolic carbon flux. J Appl Phycol. 1996;8:275–281. [Google Scholar]
- 6.De Philippis R, Margheri M C, Pelosi E, Ventura S. Exopolysaccharide production by a unicellular cyanobacterium isolated from a hypersaline habitat. J Appl Phycol. 1993;5:387–394. [Google Scholar]
- 7.Dor I, Ehrlich A. The effects of salinity and temperature gradients on the distribution of littoral microalgae in experimental solar ponds, Dead Sea area, Israel. Mar Ecol. 1987;8:193–205. [Google Scholar]
- 8.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 9.Filali Mouhim R, Cornet J F, Fontane T, Fournet B, Dubertret G. Production, isolation and preliminary characterization of the exopolysaccharide of the cyanobacterium Spirulina platensis. Biotechnol Lett. 1993;15:567–572. [Google Scholar]
- 10.Fresnedo O, Serra J L. Effect of nitrogen starvation on the biochemistry of Phormidium laminosum (Cyanophyceae) J Phycol. 1992;20:253–257. [Google Scholar]
- 11.Galambos J T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967;28:350–356. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- 12.Gloaguen V, Morvan H, Hoffmann L. Released and capsular polysaccharides of Oscillatoriaceae (Cyanophyceae, Cyanobacteria) Algol Stud. 1995;78:53–69. [Google Scholar]
- 13.Lloyd A G, Dodgson K S. Infrared spectra of carbohydrate sulphate esters. Nature. 1959;184:548–549. doi: 10.1038/184548a0. [DOI] [PubMed] [Google Scholar]
- 14.Materassi, R., R. De Philippis, M. C. Margheri, V. Delfino, and M. Vincenzini. Characterization of exopolysaccharide-producing “Cyanothece” strains from hypersaline environments. In Proceedings of 3rd Asia Pacific Conference on Algal Biotechnology, in press.
- 15.Panoff J M, Priem B, Morvan H, Joset F. Sulphated exopolysaccharides produced by two unicellular strains of cyanobacteria, Synechocystis PCC 6803 and 6714. Arch Microbiol. 1988;150:558–563. [Google Scholar]
- 16.Sloneker J H, Orentas D G. Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature. 1962;194:478–479. doi: 10.1038/194478a0. [DOI] [PubMed] [Google Scholar]
- 17.Sudo H, Burgess J G, Takemasa H, Nakamura N, Matsunaga T. Sulfated exopolysaccharide production by the halophilic cyanobacterium Aphanocapsa halophytica. Curr Microbiol. 1995;30:219–222. [Google Scholar]
- 18.Sutherland I W. Structure-function relationships in microbial exopolysaccharides. Biotech Adv. 1994;12:393–448. doi: 10.1016/0734-9750(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland I W. Extracellular polysaccharides. In: Rehm H J, Reed G, Pühler A, Stadler P, editors. Biotechnology–VI. Weinheim, Germany: VCH; 1996. pp. 615–657. [Google Scholar]
- 20.Van Rijn J, Shilo M. Nitrogen limitation in natural populations of cyanobacteria (Spirulina and Oscillatoria spp.) and its effect on macromolecular synthesis. Appl Environ Microbiol. 1986;52:340–344. doi: 10.1128/aem.52.2.340-344.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincenzini M, De Philippis R, Sili C, Materassi R. Studies on exopolysaccharide release by diazotrophic batch cultures of Cyanospira capsulata. Appl Microbiol Biotechnol. 1990;34:392–396. [Google Scholar]
- 22.Vincenzini M, De Philippis R, Sili C, Materassi R. A novel exopolysaccharide from a filamentous cyanobacterium: production, chemical characterization and rheological properties. In: Dawes E A, editor. Novel biodegradable microbial polymers. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 295–310. [Google Scholar]
- 23.Weissman B, Meyer K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J Am Chem Soc. 1954;76:1753–1757. [Google Scholar]
- 24.Zarrouk C. Ph.D. thesis. Paris, France: University of Paris; 1966. [Google Scholar]