Highlights:
-
•
Hypoxia induced radioresistance is an under researched area in rectal cancer.
-
•
There are several preclinical trials which show promise in sensitizing hypoxic cells to radiation.
-
•
The ability to manipulate the hypoxic response of cancer cells can potentially de-escalate radiotherapy and improve outcomes.
-
•
More research is needed in hypoxia-induced radioresistance in rectal cancer.
Keywords: Rectal cancer, Tumour hypoxia, Radiotherapy, Hypoxia
Abstract
Introduction
Neoadjuvant radiotherapy is successfully used in rectal cancer to improve overall survival. However, treatment response is both unpredictable and variable. There is strong evidence to show that the phenomenon of tumour hypoxia is associated with radioresistance, however the mechanism(s) behind this are poorly understood. Consequently, there have only been a small number of studies evaluating methods targeting hypoxia-induced radioresistance. The purpose of this systematic review is to evaluate the potential effectiveness of targeting hypoxia-induced radioresistance in rectal cancer and provide recommendations for future research in this area.
Methods
A comprehensive literature search was performed following the PRISMA guidelines. This study was registered on the Prospero database (CRD42023441983).
Results
Eight articles met the inclusion criteria. All studies identified were in vitro or in vivo studies, there were no clinical trials. Of the 8 studies identified, 5 assessed the efficacy of drugs which directly or indirectly targeted hypoxia and three that identified potential targets. There was conflicting in vivo evidence for the use of metformin to overcome hypoxia induced radioresistance. Vorinostat, atovaquone, and evofosfamide showed promising preclinical evidence that they can overcome hypoxia-induced radioresistance.
Discussion
The importance of investigating hypoxia-induced radioresistance in rectal cancer is crucial. However, to date, only a small number of preclinical studies exist evaluating this phenomenon. This systematic review highlights the importance of further research to fully understand the mechanism behind this radioresistance. There are promising targets identified in this systematic review however, substantially more pre-clinical and clinical research as a priority for future research is needed.
Introduction
A diagnosis of rectal cancer is devastating, and patients are often subjected to a challenging regime of chemoradiotherapy followed by major resectional surgery. Neoadjuvant chemoradiotherapy has been successfully used to downstage the disease, reduce local recurrence rates, improve survival, and, more recently, induce a complete clinical and/or pathological response [1]. Despite significant advances in radiotherapy, the response is both unpredictable and variable, with approximately 5–30 % of patients experiencing disease progression whilst undergoing chemoradiotherapy [2], [3]. Furthermore, a significant number of patients (∼80 %) will experience adverse side effects and toxicity whilst undergoing radiotherapy, including diarrhoea, incontinence, fistulas, sexual dysfunction and pain [4]. Cumulatively, these demonstrate some of the challenges and uncertainties associated with this treatment.
There is strong evidence to show that the phenomenon of tumour hypoxia, particularly in solid tumours, is associated with radioresistance [5], [6], [7]. Tumour hypoxia is defined as a reduced oxygen availability and arises from the complex interplay of multiple factors, including inadequate blood perfusion, uncontrolled rapid tumour proliferation, and elevated metabolic needs, which collectively culminate in an unavoidable oxygen-deficient environment. It is generally accepted that the oxygen level in hypoxic rectal cancer tumour tissues averages between 1 % and 2% O2 or below [8]. Genome-wide and microRNA analyses have consistently demonstrated that colorectal cancers (CRC) exhibiting a hypoxic tumour microenvironment equate to significantly poorer clinical outcomes, particularly in terms of disease-free survival [9], [10], [11], [12]. Furthermore, there is evidence indicating that radiologically detected hypoxia employing a novel hypoxia marker 60Cu-diacetyl-bis (N4-methylthiosemicarbazone), is a predicative factor for diminished survival and reduced tumour response to neoadjuvant chemoradiotherapy in patients diagnosed with rectal cancer [13]. There are several prevailing theories as to why tumour hypoxia leads to radioresistance. The oxygen fixation hypothesis suggests that the DNA damage caused as a consequence of the free radicals generated in response to radiotherapy, is more difficult to repair when caused by the product of a radical and an oxygen molecule [14]. Conversely, the absence of oxygen means that DNA damage is not fixed and therefore can undergo repair. However, more recently research is being developed around the intrinsic cellular adaptations to hypoxia and within the tumour microenvironment. This can lead to alterations in gene expression, increased cellular signalling, and activation of survival pathways, all of which can contribute to radioresistance [15]. The hypoxia induced factors (HIFs) are a group of transcriptional factors that function as master regulators of oxygen homeostasis [16]. Activation of HIF in response to hypoxia in tumour cells has been associated with disruption in the ability of DNA repair, the inhibition of apoptosis, and alterations of the cellular metabolism [17]. However, due the complexity of the HIF signalling pathway in response to various concentrations of oxygen further research is needed to fully elucidate the mechanisms underlying the role of HIF activation in promoting radioresistance in specific tumours under hypoxic conditions. Furthermore, research into hypoxia has revealed that many HIF-independent mechanism are activated in hypoxia, including chromatin reprogramming [18], [19], inflammation [20] and control of cell cycle [21]. All of these mechanisms have the potential to contribute to radioresistance but have not been tested in vitro or in vivo.
The purpose of this systematic review is to evaluate the potential effectiveness of targeting hypoxia-induced radioresistance in rectal cancer and provide recommendations for future research in this area.
Methods
Literature search strategy
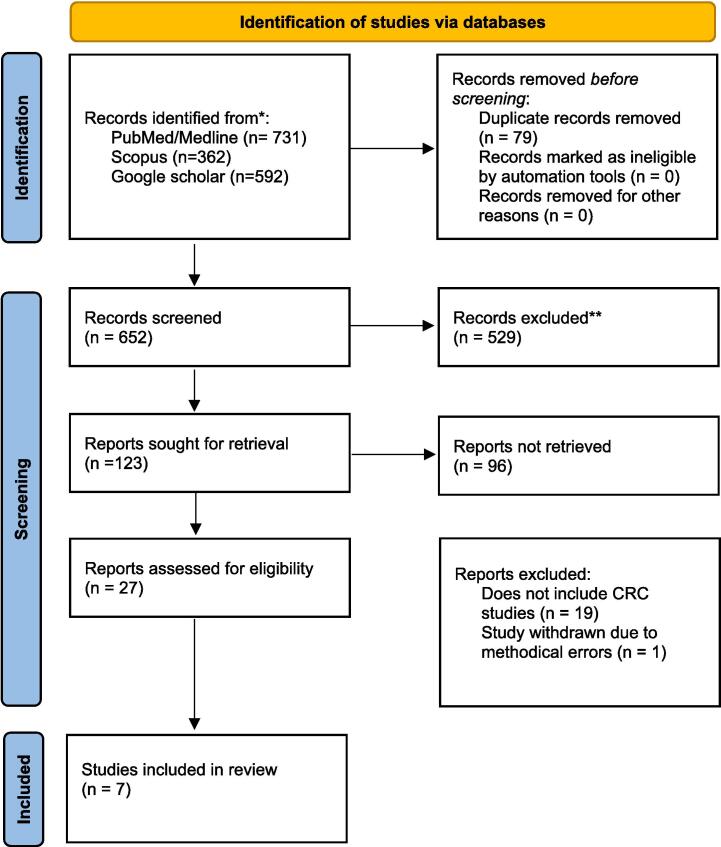
A comprehensive literature search was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A search was performed on PubMed/Medline, Scopus, and Google scholar from inception to March 2023. Search terms included; “colorectal neoplasms”, “colorectal cancer”, “rectal cancer”, “colon cancer”, “radiotherapy”, “radiation therapy”, “radiotherapeutics”, “radiooncology”, “hypoxia”, “tumour hypoxia”, “oxygen deficiency”, “low oxygen tension”, “radioresistance”, ”radiotherapy resistance“, ”radioresistant“, ”resistance to radiation“, ”hypoxia-inducible factor 1”, “HIF-1”, ”hypoxia-inducible factor-1”, “HIF-1alpha”. All search terms were combined with Boolean operators and searched with MeSH terms to ensure maximal sensitivity. After titles and abstracts were screened using the inclusion and exclusion criteria stated below, full-text article reference lists were searched for any further articles that were suitable for inclusion (Fig. 1). The full study protocol was registered on the Prospero database (CRD42023441983).
Fig. 1.
Prisma flow chart.
Inclusion and exclusion criteria
All study designs that investigate the effectiveness of targeting hypoxia-induced radioresistance in colorectal cancer radiotherapy were included to capture a broad range of evidence on the topic. Exclusion criteria included studies that were not related to the topic of interest, studies that were conducted on other types of cancer, studies that were not available in full-text format, those not available in the English language.
Quality assessment
Quality assessment was performed with a modified version of the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist was used to assess the study quality (Table 2) [22], [23].
Table 2.
Quality assessment using CAMARADES checklist.
| Num | Criteria | de Mey 2019 |
de Bruycker 2019 |
Saelen 2012 |
Haynes 2018 |
|---|---|---|---|---|---|
| 1 | Publication in peer-reviewed journal | Y | Y | Y | Y |
| 2 | Statement of control of temperature | NM | Y | NM | NM |
| 3 | Randomization of treatment or control | Y | Y | Y | Y |
| 4 | Allocation concealment | NM | NM | NM | NM |
| 5 | Blinded assessment of outcome | NM | NM | NM | NM |
| 6 | Avoidance of anesthetics with marked intrinsic properties | NM | Y | NM | Y |
| 7 | Use of animals with cancer | Y | Y | Y | Y |
| 8 | Sample size calculation | NM | NM | NM | NM |
| 9 | Statement of compliance with regulatory requirements | Y | Y | Y | Y |
| 10 | Statement regarding possible conflict of interest | Y | Y | Y | Y |
| 11 | Physiological monitoring | NM | Y | NM | Y |
| 12 | Prespecified inclusion and exclusion criteria | NM | NM | NM | NM |
| 13 | Reporting animals excluded from analysis | NM | NM | Y | NM |
| 14 | Reporting of study funding | Y | Y | NM | Y |
| Total score | 6 | 9 | 6 | 8 |
Abbreviations: Num – number in CAMARADES checklist Y = yes, NM = Not mentioned.
Results
Our literature search revealed 652 potential studies after removal of duplicates (Fig. 1). Following screening of title and abstract, 20 papers were excluded leaving 7 potential studies for which the full text was obtained and reviewed. Of these papers, the reference list was searched to look for any potential papers to include. A total of 8 papers met the inclusion criteria. All studies were in vitro or mouse in vivo studies. There were no clinical trials identified. Of the 8 studies identified, 5 assessed the efficacy of drugs which directly or indirectly targeted hypoxia and three identified potential mechanistic targets (Table 1).
Table 1.
Study characteristics.
| Author | Year | Type of study | Cell lines used | Patients samples | Drug tested/Target identified | Major Findings |
|---|---|---|---|---|---|---|
| de Mey | 2019 | in vitro/in vivo | HCT116, DLD-1, HT29, SW480, and CT26 | no | Metformin and phenformin | Metformin improved the hypoxic radiosensitivity of CRC cells with enhancement ratios of 1.72- and 2.86-fold. CT26 tumour-bearing mice treated with metformin or phenformin with radiation substantially delayed tumour growth with a 1.3 and 1.5 fold enhancement |
| De Bruycker | 2019 | in vivo | colo205-tumours | no | Metformin | No difference in tumour doubling time between colo205 tumour bearing mice that received radiation with control or metformin |
| Saelen | 2012 | in vitro/in vivo | HCT116, HT29, and SW620, and KM20L2 | no | Vorinostat | Tumour growth delay in mice treated with a combination of ionising radiation and Vorinostat was significantly higher compared to control |
| Ashton | 2016 | in vitro / spheroids | HCT116 | no | Atovaquone, metformin and phenformin | Spheroids treated with metformin and phenformin showed reduction of hypoxia with EF5 immunostaining but not to the extent of atovaquone. Xenografts showed that treatment with atovaquone was able to abolish hypoxia. |
| Haynes | 2018 | in vivo | Patient-derived colorectal cancer xenografts | no | Evofosfamide | Evofosfamide increased the number of γH2AX foci in xenografts treated with either, 5-FU, radiation or a combination of both |
| Classen | 2019 | in vitro | HCT116, HEK293T | no | Autophagy | Inhibition of autophagy does not generally increase radiotherapy success and may also lead to an unfavourable outcomex especially under amino acid and oxygen restriction. |
| Sun | 2014 | in vitro | SW480, SW620 | no | Autophagy | Under hypoxia, HIF-1α induces miRNA-210 which in turn enhances autophagy and reduces radiosensitivity by downregulating Bcl-2 expression |
| Kawai | 2016 | in vitro 2d and clinical tissue samples | HCT116 LoVo HT29 SW480 DLD-1 KM12SM | 222 samples | ALODA | In vitro, ALDOA expression was negatively associated with chemo- and radiosensitivity and positively associated with proliferation, sphere formation and invasion in both normoxia and hypoxia. |
Drugs
Metformin
Three studies assessed the effect of the anti-diabetic biguanide drugs metformin or phenformin to try and overcome hypoxic-induced radioresistance in vitro and mouse in vivo experiments (Table 1) [24], [25]. De Mey et al [26] utilised one murine and four human cell lines (CT26, HCT116, DLD-1, HT29, SW480 respectively) and cultured them in aerobic conditions or subjected them to metabolic hypoxia in a micropellet model (this model cannot give a percentage of oxygen the cells were exposed to). Cells were then dosed with metformin (1–9 µM) or phenformin (10–100 µM) before exposed to radiation (0–9 Gy). The use of both metformin and phenformin significantly improved the radiosensitivity of hypoxic colorectal cancer cells. In this study, phenformin overcame hypoxic radioresistance with enhancement ratios of 1.75 and 2.87 for CT26 and HCT116 tumour cells. Metformin improved the hypoxic radiosensitivity of CT26 and HCT116 with enhancement ratios of 1.72- and 2.86-fold. This was potentially explained through the inhibition of complex 1 activity and impaired oxygen consumption. They further validated their experiments with CT26 tumour-bearing mice. The addition of metformin (300 mg/kg) or phenformin (200 mg/kg) with radiation (9 Gy) substantially delayed tumour growth with a 1.3 and 1.5 fold enhancement, as well as significantly increasing the survival rate.
De Bruycker et al [25] studied colo205-bearing mice who received intravenous metformin before radiation. This study exhibited no difference in tumour doubling time between mice that received radiation (15 Gy) with control or metformin (100 mg/kg). The authors scanned the mice with [18F]HX4 hypoxia μPET/computed tomography (CT) scan at baseline and post-metformin dosing. In mice treated with metformin, there was a significant mean intratumoral reduction in [18F]HX4 tumour-to-background ratio compared to saline treated mice. The [18F]HX4 hypoxia μPET technique holds promise for accurately measuring tumour hypoxia. This is because [18F]HX4, when exposed to low oxygen levels, undergoes reduction, and produces reactive intermediates that are subsequently accumulated by viable hypoxic cells [27].
These two studies have shown conflicting evidence with regards to metformin as a radiosensitiser, De Mey et al showed that in several CRC cell lines they were able to achieve significant radiosensitisation with metformin [24]. This was further confirmed in vivo with CT26 tumour-bearing mice. Conversely, De Bruycker et al used Colo205-bearing mice but found metformin made no difference in the radiosensitivity of the tumours [25]. Ashton et al also evaluated metformin and radiotherapy in 3D HCT-116 spheroids, however, except for showing that hypoxia was relieved on admission of metformin they did not report how it effects spheroid growth.
Histone deacetylase inhibition
There is increasing evidence that histone deacetylase inhibition (HDACi) lower the cell's capacity to repair IR-induced DNA damage, both at the level of damage signalling and by affecting the major DNA repair pathways (NHEJ and HR), in many different cell types in vitro [28]. Saelen et al [29] investigated reversing the radioresistant hypoxic phenotype in vitro and in vivo with the pan-HDACi vorinostat. The authors used four different colorectal cancer cell lines (HCT116, HT29, and SW620 and KM20L2) and athymic Balb/c mice with colorectal cancer xenografts. Vorinostat (1 or 2 μM) was able to reverse the hypoxia-induced radioresistance (0 – 5 Gy) in hypoxia-cultured cells (1 % O2). The authors report the results in surviving fractioning (SF) of cells following clonogenic assay (HT29, SF 0.24 ± 0.02 versus 0.51 ± 0.05; p = 0.005; SW620, SF 0.052 ± 0.03 versus 0.36 ± 0.10; p = 0.04; KM20L2, SF 0.040 ± 0.006 versus 0.20 ± 0.02; p = 0.002). In vivo studies compared tumour growth delay at 2-fold increase of relative tumour volume (TGD2x) in mice treated with a combination of ionising radiation and vorinostat. TGD2x was significantly higher in mice with hypoxic tumours treated with ionising radiation 6.07 ± 2.5 versus hypoxic tumours treated with ionising radiation and vorinostat − 1.43 ± 3.6; p = 0.015. In this study hypoxia in xenografts was achieved by clamping the tumour during and before irradiation for a total of five minutes. Radiation exposure inhibited the growth of normoxic SW620 xenografts but not of hypoxic tumours. Vorinostat was able to significantly enhance the radiosensitivity of hypoxic xenografts.
Atovaquone
Atovaquone, a naphthoquione with broad spectrum antiprotozoal activity, was investigated by Ashton et al [30] in HCT116 colorectal cancer spheroids. In an avascular 3D tumour environment, atovaquone was able to alleviate spheroid hypoxia. Immunostaining of athymic BALB/c nude female mice HCT116 xenografts showed that treatment with atovaquone was able to abolish hypoxia. The authors also assessed the effects of metformin (2 mM) and phenformin (10 μM) on spheroid hypoxia with EF5 immunostaining and found that they were able to significantly reduce hypoxia but not to the extent of atovaquone. Spheroid growth was not assessed post-irradiation in the HCT116 model.
Evofosfamide
Haynes et al [31] investigated the hypoxia-activated prodrug evofosfamide with patient-derived colorectal cancer xenografts and spheroids. Cells were cultured in 2 % O2 before being treated with a combination of 5-fluorouracil (5-FU), evofosfamide and radiation. Under hypoxic conditions, the number of colorectal cancer-initiating cells increased, exhibiting a canonical trait of heightened capacity for self-renewal. The addition of evofosfamide was able to significantly increase the radiosensitivity of spheroids and patient-derived colorectal cancer xenografts as well as decreasing the fraction of colorectal cancer-initiating cells. This was demonstrated with immunofluorescent staining to γH2AX of spheroids. Treatment with 5-FU or radiation in combination with evofosfamide resulted in a further increase in the proportion of cells with γH2AX foci (97 % for 5-FU + evofosfamide or 91 % for radiation + evofosfamide). This included a 2.0- to 5.2-fold increase in the proportion of cells with > 50 foci compared with either agent given alone. Finally, the authors tested [18F]-FAZA-PET/CT imaging to assess for tumour hypoxia in mice with implanted patient-derived colorectal cancer xenografts. In those with high uptake of [18F]-FAZA (increased intratumoral hypoxia), evofosfamide had the greatest effect on tumour growth rate.
Targets
Autophagy
Autophagy is a highly conserved cellular process that involves the degradation and recycling of intracellular components through the formation of double-membrane vesicles, known as autophagosomes [32]. Two papers have assessed how manipulating autophagy affects radiosensitivity in colorectal cancer (Table 1). Classen et al [33] described culturing HCT116 colorectal cancer cells in vitro under 1 % O2 hypoxic conditions. The authors impaired autophagic flux by and siRNA knock down of two key proteins, ATG7 and Beclin 1, and autophagic flux was further impaired with glutamine starvation. However, under hypoxic conditions radiosensitivity was unchanged with Beclin 1 gene knockdown, and furthermore, survival increased with an ATG7 targeted knockdown. The authors concluded that these results highlight that inhibition of autophagy does not generally increase radiotherapy success and may also lead to an unfavourable outcome especially under amino acid and oxygen restriction.
Sun et al [34] experimented with colon cancer cell lines SW480 and SW620 under 2 % O2 hypoxic conditions. The authors silenced HIF-1α to manipulate miR-210, which promotes migration and invasion, and highly expressed under hypoxic conditions. The inhibition of HIF-1α decreased miR-210 expression and autophagy, furthermore siRNA-mediated silencing of miR-210 upregulated Bcl-2 expression. Autophagy-related proteins including Beclin-1, ATG12 and LC3II as well as the ratio of LC3II/LC3I were decreased after miRNA-210 siRNA treatment under hypoxic treatment compared with hypoxic treatment only group. The authors conclude that upregulation of Bcl-2 reduced the survival fraction of colon cancer cells after radiation treatment (4 Gy) which was not related to apoptosis. However, the results underpinning this conclusion are difficult to interpret. They report that under hypoxic conditions, apoptosis induced by X-ray irradiation (RT) increased after miR-210 siRNA transfections, compared with RT alone. The rate of apoptosis did not differ when cells were co-transfected with miRNA-210 and Bcl-2 siRNA. The authors suggest that the inhibition of miR-210 leads to increased radiosensitivity by upregulating Bcl-2 expression under hypoxic conditions (although not back to control, no values are provided). They further conclude that under hypoxia, HIF-1α induces miRNA-210 which in turn enhances autophagy and reduces radiosensitivity by downregulating Bcl-2 expression in colon cancer cells. It should be noted that the relationship between upregulation of Bcl-2 expression and autophagy is complex and can vary depending on cellular context [35], [36].
These two studies assessing how autophagy relates to radiosensitivity in CRC depicts its complexity and heterogeneity [37]. Classen et al used HCT-116 CRC cell line in 1 % O2 which is different to Sun et al who use the SW480 and SW620 cell lines in 2 % O2. This makes comparisons very difficult to make, however there is good evidence that hypoxia drives the promotion of autophagy. The radiobiological response to autophagy activation in hypoxia however is likely to be cell line and tumour dependant and there is still a lot of work required to evaluate how this can translate clinically.
Fructose-bisphosphate aldolase A
Other than autophagy, an association between glycolysis targets and hypoxia-induced radioresistance in rectal cancer has been investigated. Here, Kawai et al [38] used hypoxic tumour cells of metastatic liver tissue from patients with colorectal cancer (CRC) as an ‘in vivo’ hypoxia culture model. The authors investigated the expression of fructose-bisphosphate aldolase A (ALDOA), one of the glycolytic enzymes that catalyzes the reversible conversion of fructose-1,6-bisphosphate to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate, which is a downstream target of HIF-1α. They used six colorectal cancer cell lines, HCT116, LoVo, HT29, SW480, DLD-1 and KM12SM and utilised 1 % O2 hypoxic conditions. In vitro, ALDOA expression was negatively associated with radiosensitivity and positively associated with proliferation, sphere formation and invasion in both normoxia and hypoxia. They validated their findings through univariate and multivariate analyses of microarray data from 222 resected colorectal cancer samples and revealed that ALDOA was an independent prognostic factor.
Discussion
This systematic review has highlighted the importance of further investigating tumour hypoxia in colorectal cancer, as it remains an unexplored area with significant potential as a target for improving treatment efficacy. The review has identified that targeting hypoxia-induced radioresistance could be a promising approach to enhance radiotherapy outcomes in patients with rectal cancer. Only pre-clinical experimentation currently exists investigating this important area. One possible explanation for this is given by Fletcher et al who described that the majority of studies assessing hypoxia and radiotherapy were performed at the end of the twentieth century when radiotherapy for rectal cancer was not routinely employed [39]. To bridge the gap between pre-clinical experimentation and clinical translation, further understanding of the key molecular mechanisms underlying hypoxia-induced radioresistance is needed.
This review identified four drugs which have been used to overcome radioresistance caused by hypoxia. Metformin and phenformin, two widely used anti-diabetic biguanide drugs, have been recently explored for their potential anti-cancer effects [40]. However, there is ongoing debate regarding their mechanisms of action and efficacy as cancer therapies. Within the context of this review, it was found that metformin can modulate intra-tumoural hypoxia, a key driver of tumour progression and treatment resistance. However, the studies that assessed the impact of metformin on cell survival in cancer cells produced conflicting results indicating the need for further research to better understand its potential anti-cancer effects. Whilst the modulation of hypoxia by metformin is an interesting finding, more studies are needed to elucidate the specific mechanisms of action and the potential clinical utility of these drugs as cancer therapies.
There is accumulating preclinical evidence that HDACi can radiosensitise cancer cells both in vitro and in vivo [28], [41]. Changes on chromatin structure are expected to affect radiosensitivity. HDACi facilitates chromatin relaxation by increasing the levels of histone acetylation, nevertheless this relaxed structure is not more susceptible to DNA Double Strand Breaks (DSBs) [42]. However, HDACi effectively maintains histones in a hyperacetylated state and hinders the natural process of chromatin refolding into a more condensed structure during repair [43]. There is evidence that elevated histone acetylation levels in the vicinity of DSBs are subsequently replaced by histone post-translational modifications (PTMs) associated with a more condensed chromatin structure. Consequently, HDACi impede these rapid epigenetic transitions, resulting in an altered chromatin state and heightened sensitivity to radiation. This is further compounded by a relationship of HDACis with DNA repair efficacy through the downregulation of key DNA repair proteins such as Ku70, Ku86, Rad51 and DNA-PKc [44]. Finally, a complex relationship exists between, chromatin and its structure in hypoxia, and how this is affected with HDACi, DNA repair and radiotherapy [45], [46]. For example, Vorinostat, a pan-HDACi, commonly used in resistant or recurrent cutaneous T cell lymphoma has showed promise in overcoming the hypoxic-induced radioresistance in vitro and in vivo experiments [29], [47]. There is only one study assessing the impact of Vorinostat, on hypoxia induced radiosensitivity in colorectal cancer and showed its ability to significantly enhance the radiosensitivity of hypoxic xenografts [29]. More recent literature has shown its potential in altering intratumoural hypoxia signalling not solely attributed to changes in chromatin structure [47].
Evofosfamide is a hypoxia activated prodrug, that in the presence of hypoxia, is reduced to release a cytotoxic bromo-isophosphoramide moiety. It has been tested and shown promise in a variety of different cancers including sarcoma, advanced solids tumours, leukaemia and multiple myeloma as a single agent and not with radiotherapy [48]. Atovaquone is a naphthoquione with broad spectrum antiprotozoal activity [49]. It is used currently as an antimicrobial medication for the prevention and treatment of Pneumocystis jirovecii pneumonia, malaria and babesiosis. Its mechanism of action for the treatment of infectious diseases is largely unknown, but is thought to be related to the inhibition of the mitochondrial electron transport chain leading to cell death [49]. However, emerging evidence suggests that it has many other targeted effects which may make it an attractive candidate for cancer treatment [50].
Hypoxia-inducible factor 1 (HIF-1) is a heterodimeric transcription factor composed of an oxygen-sensitive alpha subunit (HIF-1α) and a constitutively expressed beta subunit (HIF-1β). In response to low oxygen levels (hypoxia), HIF-1α protein levels increase and the HIF-1α/HIF-1β complex binds to hypoxia response elements (HREs) in the promoter regions of multiple target genes to activate their transcription [51]. Despite extensive research, a comprehensive understanding of the interplay between hypoxia-inducible factor (HIF) signalling and radiotherapy in the tumour microenvironment is still elusive [16]. This is due in part to the intricate complexity of both the upstream and downstream mechanisms involved in HIF signalling within the tumour microenvironment. This is reflected in a study assessing 86 biopsies of patients with rectal cancer before they underwent long course chemoradiotherapy [52]. Immunohistological staining for HIF-1α and GLUT-1 expression showed no predictive impact regarding response to chemoradiotherapy measured by tumour regression grade (TRG) and was not associated with overall survival. However, despite Kawai et al identifying ALODA (a downstream target of HIF-1α) as an independent prognostic factor for colorectal cancer, it is unlikely that a single target would improve hypoxia-induced radiosensitivity in patients with rectal cancer [38]. This is because the molecular mechanisms that contribute to hypoxia-induced radioresistance are complex and multifactorial, involving multiple signalling pathways and cellular processes.
There is an interest in autophagy and its interplay with HIF, which has been shown to play a critical role in cancer cell survival and adaptation to hypoxic conditions [53]. There is debate as to its role in response to radiotherapy as autophagy can promote cell survival and proliferation by recycling nutrients and eliminating damaged organelles or conversely lead to the destruction of essential cellular components and the initiation of cell death pathways [54]. Despite the papers reviewed in this study, it is unclear whether targeting autophagy combined with radiotherapy promotes cell survival or leads to cell death. Further research is needed to fully understand the mechanisms involved in the interplay between autophagy and radiotherapy and to determine the optimal approach to targeting autophagy for therapeutic benefit.
Anti-angiogenic therapies such as bevacizumab, an anti-VEGF monoclonal antibody have been prescribed second line in metastatic colorectal cancer. These drugs have the ability to modify tumour hypoxia. Despite early initial success in clinical trials, prolonged treatment led to resistance to anti-VEGF therapy and cessation of therapy not only led to tumour regrowth but with a more aggressive phenotype [55]. One possible explanation for this is that prolonged anti-VEGF therapy induces intra-tumoural hypoxia and in turn the surviving cells are extremely resistant to therapy. This has been seen in breast cancer xenografts and pancreatic islet tumours [56]. Despite a growing interest in investigating the potential synergistic effects of combining anti-VEGF therapy with radiotherapy there have not been any studies assessing anti-VEGF and radiotherapy [39], [56]. The only clinical trials assessing hypoxic modification in radiotherapy are on-going in head and neck cancer utilising nimorazole as a hypoxic radiosensitiser [57]. The Danish Head and Neck Cancer Study Group (DAHANCA) have been performing several phase I and II trials and have conducted a randomised controlled trial specifically for nimorazole. The results of these trials have shown that nimorazole can significantly enhance the effectiveness of radiotherapeutic management for supraglottic and pharynx tumours, whilst also being well-tolerated by patients. There are also multiple novel hypoxic radiosensitisers in development [58]. There are many significant challenges that need to be addressed, including the considerable heterogeneity of intra-tumoral hypoxia, which makes it difficult to determine which patients will benefit the most from a hypoxic radiosensitizer. Nonetheless, these ongoing studies offer hope for improving the treatment outcomes for head and neck cancer patients, and in the near future, targeting hypoxia in radiotherapy will begin to be trialled in other cancers [59].
Future perspectives and conclusions
There is an obvious scarcity of knowledge with regards to hypoxia-induced radioresistance in rectal cancer where radiotherapy is commonly employed. However, there are some compelling potential drugs and specific pathways which deserve attention and further research. The are several avenues in which this can be explored further.
-
o
Development of novel hypoxia-activated prodrugs (HAPs) that can specifically target hypoxic tumours and either increase tumour reoxygenation or target intrinsic cellular mechanisms making tumours more sensitive to radiotherapy. An example of a novel HAPs in development includes CP-506 [60].
-
o
Repurposing of existing drugs that show potential in targeting hypoxia-induced radioresistance which have been identified above.
-
o
Understanding the response of the tumour and its microenvironment to hypoxia and radiation to target specific pathways to increase its radiosensitivity.
-
o
The type of radiation delivered to a tumour may potentially alter a hypoxic tumours response. Proton beam therapy has a better relative biological effectiveness (RBE) than photon radiotherapy in both normoxia and hypoxia [61]. Furthermore, it can specifically target hypoxic areas with minimal damage to normal surrounding tissues [62], [63]. Other particle ions (such as carbon ion therapy) may also hold promise considering their higher RBE and lower oxygen enhancement ratio compared to both photons and protons[64].
Overall, investigating the role of hypoxia in colorectal cancer has the potential to significantly improve treatment outcomes for patients and should be a priority area for future research.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Matthew Fok: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Rhianna Hill: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft. Hayley Fowler: Formal analysis, Investigation, Writing – original draft. Jayanma Uzzi-Daniel: Investigation, Data curation, Writing – original draft. Sonia Rocha: Writing – review & editing, Supervision. Gabrielle Grundy: Writing – review & editing, Supervision. Jason Parsons: Writing – review & editing, Supervision. Dale Vimalachandran: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Feeney G., Sehgal R., Sheehan M., Hogan A., Regan M., Joyce M., et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25(33):4850–4869. doi: 10.3748/wjg.v25.i33.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuremsky J.G., Tepper J.E., McLeod H.L. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74(3):673–688. doi: 10.1016/j.ijrobp.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Xiao Q, Venkatachalam N, Hofheinz RD, Veldwijk MR, Herskind C, et al. Predicting response to neoadjuvant chemoradiotherapy in rectal cancer: from biomarkers to tumor models. Ther Adv Med Oncol. 2022;14:17588359221077972. [DOI] [PMC free article] [PubMed]
- 4.Joye I., Haustermans K. Early and late toxicity of radiotherapy for rectal cancer. Recent Results Cancer Res. 2014;203:189–201. doi: 10.1007/978-3-319-08060-4_13. [DOI] [PubMed] [Google Scholar]
- 5.Evans S.M., Koch C.J. Prognostic significance of tumor oxygenation in humans. Cancer Lett. 2003;195(1):1–16. doi: 10.1016/s0304-3835(03)00012-0. [DOI] [PubMed] [Google Scholar]
- 6.Vaupel P., Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 7.Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 8.Muz B., de la Puente P., Azab F., Azab A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (auckl) 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y.-f., Yu Z.-l., Lv M.-y., Zheng B., Tan Y.-x., Ke J., et al. Genome-Wide analysis reveals hypoxic microenvironment is associated with immunosuppression in poor survival of stage II/III colorectal cancer patients. Front Med. 2021;8 doi: 10.3389/fmed.2021.686885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y., Qu A., Wu Q., Zhang X., Wang L., Li C., et al. Prognostic value of a hypoxia-related microRNA signature in patients with colorectal cancer. Aging (Albany NY) 2020;12(1):35. doi: 10.18632/aging.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao Y., Jiang X., Li Y., Wang K., Chen R., Liu J., et al. Identification of a hypoxia-related gene prognostic signature in colorectal cancer based on bulk and single-cell RNA-seq. Sci Rep. 2023;13(1):2503. doi: 10.1038/s41598-023-29718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Y.-f., Rong Y.-m., Tan Y.-x., Xiao J., Yu Z.-l., Chen Y.-f., et al. A signature of hypoxia-related factors reveals functional dysregulation and robustly predicts clinical outcomes in stage I/II colorectal cancer patients. Cancer Cell Int. 2019;19(1):243 doi: 10.1186/s12935-019-0964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietz D.W., Dehdashti F., Grigsby P.W., Malyapa R.S., Myerson R.J., Picus J., et al. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing Neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum. 2008;51(11):1641–1648. doi: 10.1007/s10350-008-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimes D.R., Partridge M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed Phys Eng Express. 2015;1(4) doi: 10.1088/2057-1976/1/4/045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouleftour W., Rowinski E., Louati S., Sotton S., Wozny A.S., Moreno-Acosta P., et al. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med Sci Monit. 2021;27:e934116. doi: 10.12659/MSM.934116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziello J.E., Jovin I.S., Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80(2):51–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Xia Y., Jiang L., Zhong T. The role of HIF-1α in chemo-/radioresistant tumors. Onco Targets Ther. 2018;11:3003–3011. doi: 10.2147/OTT.S158206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batie M., Frost J., Frost M., Wilson J.W., Schofield P., Rocha S. Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science. 2019;363(6432):1222–1226. doi: 10.1126/science.aau5870. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty A.A., Laukka T., Myllykoski M., Ringel A.E., Booker M.A., Tolstorukov M.Y., et al. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science. 2019;363(6432):1217–1222. doi: 10.1126/science.aaw1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Ignazio L, Batie M, Rocha S. Hypoxia and Inflammation in Cancer, Focus on HIF and NF-κB. Biomedicines. 2017;5(2). [DOI] [PMC free article] [PubMed]
- 21.Druker J., Wilson J.W., Child F., Shakir D., Fasanya T., Rocha S. Role of Hypoxia in the Control of the Cell Cycle. Int J Mol Sci. 2021;22(9) doi: 10.3390/ijms22094874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
- 23.Macleod M.R., O'Collins T., Howells D.W., Donnan G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 24.de Mey S., Jiang H., Corbet C., Wang H., Dufait I., Law K., et al. Antidiabetic Biguanides Radiosensitize Hypoxic Colorectal Cancer Cells Through a Decrease in Oxygen Consumption. Front Pharmacol. 2018;9:1073. doi: 10.3389/fphar.2018.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Bruycker S., Vangestel C., Staelens S., Wyffels L., Detrez J., Verschuuren M., et al. Effects of metformin on tumor hypoxia and radiotherapy efficacy: a [18F]HX4 PET imaging study in colorectal cancer xenografts. EJNMMI Research. 2019;9(1):74. doi: 10.1186/s13550-019-0543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssens M.Y., Verovski V.N., Van den Berge D.L., Monsaert C., Storme G.A. Radiosensitization of hypoxic tumour cells by S-nitroso-N-acetylpenicillamine implicates a bioreductive mechanism of nitric oxide generation. Br J Cancer. 1999;79(7–8):1085–1089. doi: 10.1038/sj.bjc.6690173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanduleanu S., Wiel A., Lieverse R.I.Y., Marcus D., Ibrahim A., Primakov S., et al. Hypoxia PET Imaging with [18F]-HX4-A Promising Next-Generation Tracer. Cancers (basel) 2020;12(5) doi: 10.3390/cancers12051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groselj B., Sharma N.L., Hamdy F.C., Kerr M., Kiltie A.E. Histone deacetylase inhibitors as radiosensitisers: effects on DNA damage signalling and repair. Br J Cancer. 2013;108(4):748–754. doi: 10.1038/bjc.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saelen M.G., Ree A.H., Kristian A., Fleten K.G., Furre T., Hektoen H.H., et al. Radiosensitization by the histone deacetylase inhibitor vorinostat under hypoxia and with capecitabine in experimental colorectal carcinoma. Radiat Oncol. 2012;7:165. doi: 10.1186/1748-717X-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashton T.M., Fokas E., Kunz-Schughart L.A., Folkes L.K., Anbalagan S., Huether M., et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat Commun. 2016;7(1):12308. doi: 10.1038/ncomms12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haynes J., McKee T.D., Haller A., Wang Y., Leung C., Gendoo D.M.A., et al. Administration of Hypoxia-Activated Prodrug Evofosfamide after Conventional Adjuvant Therapy Enhances Therapeutic Outcome and Targets Cancer-Initiating Cells in Preclinical Models of Colorectal Cancer. Clin Cancer Res. 2018;24(9):2116–2127. doi: 10.1158/1078-0432.CCR-17-1715. [DOI] [PubMed] [Google Scholar]
- 32.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Classen F., Kranz P., Riffkin H., Pompsch M., Wolf A., Göpelt K., et al. Autophagy induced by ionizing radiation promotes cell death over survival in human colorectal cancer cells. Exp Cell Res. 2019;374(1):29–37. doi: 10.1016/j.yexcr.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y., Xing X., Liu Q., Wang Z., Xin Y., Zhang P., et al. Hypoxia-induced autophagy reduces radiosensitivity by the HIF-1α/miR-210/Bcl-2 pathway in colon cancer cells. Int J Oncol. 2015;46(2):750–756. doi: 10.3892/ijo.2014.2745. [DOI] [PubMed] [Google Scholar]
- 35.Decuypere J.P., Parys J.B., Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells. 2012;1(3):284–312. doi: 10.3390/cells1030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H.D., Wu D., Gu J.H., Ge J.B., Wu J.C., Han R., et al. The pro-survival role of autophagy depends on Bcl-2 under nutrition stress conditions. PLoS One. 2013;8(5):e63232. doi: 10.1371/journal.pone.0063232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill R.M., Fok M., Grundy G., Parsons J.L., Rocha S. The Role of Autophagy in Hypoxia-Induced Radioresistance. Radiother Oncol. 2023;109951 doi: 10.1016/j.radonc.2023.109951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawai K., Uemura M., Munakata K., Takahashi H., Haraguchi N., Nishimura J., et al. Fructose-bisphosphate aldolase A is a key regulator of hypoxic adaptation in colorectal cancer cells and involved in treatment resistance and poor prognosis. Int J Oncol. 2017;50(2):525–534. doi: 10.3892/ijo.2016.3814. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher T., Thompson A.J., Ashrafian H., Darzi A. The measurement and modification of hypoxia in colorectal cancer: overlooked but not forgotten. Gastroenterol Rep (oxf) 2022;10:goac042. doi: 10.1093/gastro/goac042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clifford R.E., Gerrard A.D., Fok M., Vimalachandran D. Metformin as a radiosensitiser for pelvic malignancy: A systematic review of the literature. Eur J Surg Oncol. 2021;47(6):1252–1257. doi: 10.1016/j.ejso.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Wong P., Radany E., Wong J.Y. HDAC inhibitor, valproic acid, induces p53-dependent radiosensitization of colon cancer cells. Cancer Biother Radiopharm. 2009;24(6):689–699. doi: 10.1089/cbr.2009.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrier F. Chromatin Modulation by Histone Deacetylase Inhibitors: Impact on Cellular Sensitivity to Ionizing Radiation. Mol Cell Pharm. 2013;5(1):51–59. [PMC free article] [PubMed] [Google Scholar]
- 43.Falk M., Lukasova E., Gabrielova B., Ondrej V., Kozubek S. Chromatin dynamics during DSB repair. Biochim Biophys Acta. 2007;1773(10):1534–1545. doi: 10.1016/j.bbamcr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Munshi A., Kurland J.F., Nishikawa T., Tanaka T., Hobbs M.L., Tucker S.L., et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11(13):4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- 45.Collier H., Albanese A., Kwok C.-S., Kou J., Rocha S. Functional crosstalk between chromatin and hypoxia signalling. Cell Signal. 2023;106 doi: 10.1016/j.cellsig.2023.110660. [DOI] [PubMed] [Google Scholar]
- 46.Melvin A., Rocha S. Chromatin as an oxygen sensor and active player in the hypoxia response. Cell Signal. 2012;24(1):35–43. doi: 10.1016/j.cellsig.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C., Yang C., Feldman M.J., Wang H., Pang Y., Maggio D.M., et al. Vorinostat suppresses hypoxia signaling by modulating nuclear translocation of hypoxia inducible factor 1 alpha. Oncotarget. 2017;8(34):56110–56125. doi: 10.18632/oncotarget.18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Zhao L., Li X.F. The Hypoxia-Activated Prodrug TH-302: Exploiting Hypoxia in Cancer Therapy. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.636892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baggish A.L., Hill D.R. Antiparasitic agent atovaquone. Antimicrob Agents Chemother. 2002;46(5):1163–1173. doi: 10.1128/AAC.46.5.1163-1173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta N., Srivastava S.K. Atovaquone: An Antiprotozoal Drug Suppresses Primary and Resistant Breast Tumor Growth by Inhibiting HER2/β-Catenin Signaling. Mol Cancer Ther. 2019;18(10):1708–1720. doi: 10.1158/1535-7163.MCT-18-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 52.Havelund B.M., Sørensen F.B., Lindebjerg J., Spindler K.L., Jakobsen A. Pretreatment HIF-1α and GLUT-1 expressions do not correlate with outcome after preoperative chemoradiotherapy in rectal cancer. Anticancer Res. 2011;31(5):1559–1565. [PubMed] [Google Scholar]
- 53.Tan Q., Wang M., Yu M., Zhang J., Bristow R.G., Hill R.P., et al. Role of Autophagy as a Survival Mechanism for Hypoxic Cells in Tumors. Neoplasia. 2016;18(6):347–355. doi: 10.1016/j.neo.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Jing X., Yang F., Shao C., Wei K., Xie M., Shen H., et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18(1):157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen T.F., Qvortrup C., Pfeiffer P. Angiogenesis Inhibitors for Colorectal Cancer. A Review of the Clinical Data. Cancers (basel) 2021;13(5) doi: 10.3390/cancers13051031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyazaki S., Kikuchi H., Iino I., Uehara T., Setoguchi T., Fujita T., et al. Anti-VEGF antibody therapy induces tumor hypoxia and stanniocalcin 2 expression and potentiates growth of human colon cancer xenografts. Int J Cancer. 2014;135(2):295–307. doi: 10.1002/ijc.28686. [DOI] [PubMed] [Google Scholar]
- 57.Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, et al. A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5-85. Radiother Oncol. 1998;46(2):135-46. [DOI] [PubMed]
- 58.Bonnet M., Hong C.R., Wong W.W., Liew L.P., Shome A., Wang J., et al. Next-Generation Hypoxic Cell Radiosensitizers: Nitroimidazole Alkylsulfonamides. J Med Chem. 2018;61(3):1241–1254. doi: 10.1021/acs.jmedchem.7b01678. [DOI] [PubMed] [Google Scholar]
- 59.Hill R.M., Rocha S., Parsons J.L. Overcoming the Impact of Hypoxia in Driving Radiotherapy Resistance in Head and Neck Squamous Cell Carcinoma. Cancers (basel) 2022;14(17) doi: 10.3390/cancers14174130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Wiel A.M.A., Jackson-Patel V., Niemans R., Yaromina A., Liu E., Marcus D., et al. Selectively Targeting Tumor Hypoxia With the Hypoxia-Activated Prodrug CP-506. Mol Cancer Ther. 2021;20(12):2372–2383. doi: 10.1158/1535-7163.MCT-21-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong J., Deasy J.O. Proton Tumor Control Relative Biological Effectiveness (RBE) Estimated for Various Fractionation Schedules and Hypoxia Levels. Int J Radiat Oncol*Biol*Phys. 2022;114(3, Supplement):e507. [Google Scholar]
- 62.Fok M., Toh S., Easow J., Fowler H., Clifford R., Parsons J., et al. Proton beam therapy in rectal cancer: A systematic review and meta-analysis. Surg Oncol. 2021;38 doi: 10.1016/j.suronc.2021.101638. [DOI] [PubMed] [Google Scholar]
- 63.Köthe A., Bizzocchi N., Safai S., Lomax A.J., Weber D.C., Fattori G. Investigating the potential of proton therapy for hypoxia-targeted dose escalation in non-small cell lung cancer. Radiat Oncol. 2021;16(1):199. doi: 10.1186/s13014-021-01914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melia E., Parsons J.L. DNA damage and repair dependencies of ionising radiation modalities. Biosci Rep. 2023;43(10) doi: 10.1042/BSR20222586. [DOI] [PMC free article] [PubMed] [Google Scholar]