Abstract
The kingdom Crenarchaeota is now known to include archaea which inhabit a wide variety of low-temperature environments. We report here lipid analyses of nonthermophilic crenarchaeotes, which revealed the presence of cyclic and acyclic dibiphytanylglycerol tetraether lipids. Nonthermophilic crenarchaeotes appear to be a major biological source of tetraether lipids in marine planktonic environments.
Archaea are a phenotypically diverse group of microorganisms whose evolutionary history distinguishes them from the two other domains of life, Eucarya and Bacteria (41–44). Although cultivated archaea once appeared to be limited to restricted “extreme” habitats (42), recent ecological surveys have suggested that novel, uncultivated archaeal groups are common inhabitants of temperate and polar seas (5, 6, 11, 21, 23, 30, 36), freshwater lake sediments (14, 22, 31), and terrestrial soils (2, 16, 26, 39). Of the newly detected but as-yet-uncultivated archaeal groups, the most frequently encountered are closely affiliated with the Crenarchaeota (37, 43, 44), a kingdom once thought to consist solely of thermophilic microorganisms.
One of the few unique, unifying phenotypic features of characterized and cultivated archaea is the presence of ether-linked isoprenoids as the dominant cell membrane lipid (9, 18, 20, 35, 38, 42). Diphytanylglycerol diether (archaeol) is the predominant membrane core lipid in most methanogens and all extreme halophiles (9, 18, 20, 35, 38). In contrast, the cell membranes of hyperthermophilic archaea and a few methanogens contain caldarchaeol, a dibiphytanyldiglycerol tetraether (20, 35, 38).
To further characterize nonthermophilic crenarchaeotes phenotypically, we collected microbial biomass from several disparate natural habitats known to harbor large numbers of them (5, 6, 11, 30). Samples were analyzed for archaeal rRNA and DNA and for lipids by gas chromatography-mass spectrometry.
Samples AM-1 and AM-7 were collected in nearshore waters of Arthur Harbor, Anvers Island, Antarctica, in early August 1996. Approximately 1,500 liters of seawater was prefiltered through a 1.6-μm-pore-size glass fiber filter, and the filtrate was concentrated by ultrafiltration through a polysulfone hollow-fiber filter with a 30,000-molecular-weight (MW) cutoff (Amicon). Concentrated cells were collected by centrifugation (4°C, 38,900 × g, 1 h) and frozen until rRNA and lipid analysis. Samples SMB3 and SMB4 were collected from a depth of 100 m in the Santa Monica Basin, on 293-mm-diameter glass fiber filters (0.7-μm nominal pore size) as previously described (40). Filters were frozen until nucleic acid extraction and lipid analysis. Enriched cell fractions of the sponge-associated archaeon Cenarchaeum symbiosum were prepared by differential and Percoll gradient centrifugation as previously described (30, 32).
Filter-collected samples were Soxhlet extracted in methylene chloride-methanol, 2:1 (vol/vol). Five percent saline was added and the sample was extracted into three aliquots of methylene chloride. The combined organic layers were dried over Na2SO4. The extracted material was saponified in 5% NaOH-methanol, 3:1, and neutral products were isolated and fractionated by column chromatography on 5% deactivated SiO2 with hexane, toluene-hexane, ethyl acetate-hexane, and methanol. Lipids which were eluted in solvent more polar than 20% ethyl acetate-hexane were acid hydrolyzed by heating for 4 h at 110°C in 57% HI (concentrate). The acid was diluted with distilled water and extracted with three aliquots of hexane. The combined organic layers were dried over NaSO4. The sample was dissolved in tetrahydrofuran under a nitrogen atmosphere, and LiAlH4 was added. The sample was then heated for 2 h at 70°C, ethyl acetate was added to quench the LiAlH4, and the solution was diluted with 5% saline. The hydrocarbons were extracted with three aliquots of ethyl acetate. The organic layers were combined and dried over NaSO4.
Antarctic picoplankton cell pellets were exhaustively extracted by sonication in five successive aliquots of methylene chloride-methanol, 2:1 (vol/vol). The cellular residue was then allowed to dry and was acid hydrolyzed with 57% HI (concentrate) by heating for 4 h at 110°C. The sample was then diluted with extracted, distilled H2O and extracted three times with hexane. The organic layers were combined and dried over Na2SO4. Reduction with LiAlH4 and subsequent analysis were performed as described above.
Gas chromatography was performed on a Carlo Erba 6180 gas chromatograph equipped with a J&W DB-5 column, on-column injection, and a flame ionization detector, by using H2 as the carrier gas. The oven was programmed with injection and a 1-min hold at 80°C, followed by a 3°C/min ramp to 320°C, and held at 320°C for 20 min. Gas chromatography-mass spectrometry analysis for all samples was performed on a Finnigan Incos 50 mass spectrometer with an HP 5890 Series II gas chromatograph by using a J&W DB-5 column and He as the carrier gas, with on-column injection. The oven was programmed from 80 to 150°C at 20°C/min and then to 320°C at 4°C/min, followed by a 20-min isothermal period at 320°C. Identifications were made by comparison of gas chromatography retention times and mass spectra with those of ether lipid-derived hydrocarbons prepared from Sulfolobus solfataricus and by comparison with previously reported data (7).
rRNA extraction, gene cloning, and hybridization experiments were performed as previously described (21), except that a different universal probe (S-*-Univ-1390-a-A-18 [45]) was used to quantify total rRNA. rRNA extracted from the symbiotic crenarchaeon C. symbiosum (30) was used as a positive control and to normalize results obtained with the marine crenarchaeotal group I probe (S-O-Cenar-0554-a-A-20) to those obtained with the domain-specific archaeal probe (21).
rRNA analyses.
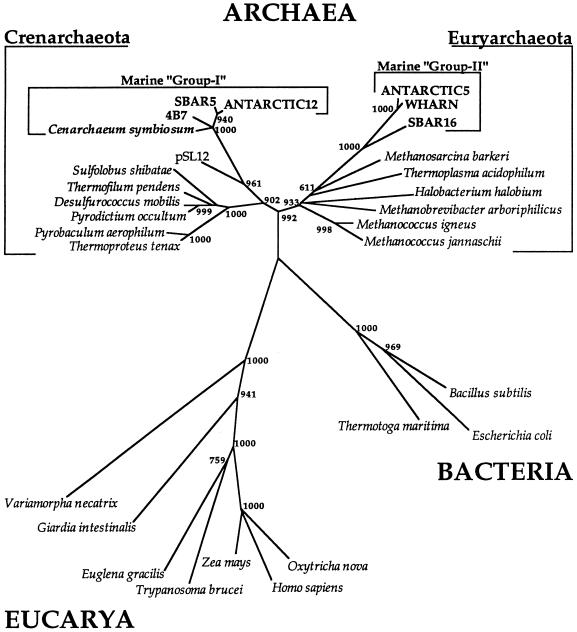
In plankton samples collected from Arthur Harbor (Anvers Island, Antarctica), approximately 11 to 18% of the total rRNA was attributable to archaea (AM-1 and AM-7 [Table 1]), consistent with previous studies (6). Cloning experiments from the same samples recovered only two major phylogenetic types of Antarctic archaea (group I crenarchaeotes and group II euryarchaeotes [Fig. 1; Table 2]). The preponderance of archaeal ribosomal DNA (rDNA) genes recovered from Antarctic samples were of the crenarchaeotal type (98% group I [Table 2]), while the remainder of the cloned rDNA genes were associated with the planktonic euryarchaeota (Table 2), as determined by group-specific rRNA probing. Quantitative rRNA hybridization experiments also indicated that the crenarchaeotal group I archaea dominated Antarctic surface water samples (Table 1). Hybridization experiments with the rRNA extracted from subsurface waters of the Santa Monica Basin (SMB3 and SMB4 [Table 1]) indicated that between 0.7 and 2.9% of the total rRNA was archaeal. There was insufficient rRNA available in the Santa Monica Basin samples to probe for archaeal subgroups. However, several previous reports (5, 6, 11, 21, 36) have indicated that nonthermophilic crenarchaeotes are the majority component of subsurface marine archaeal populations. Enriched cell preparations of the crenarchaeotal symbiont of Axinella mexicana (30, 32) yielded on average (n = 10) 22% archaeal rRNA, relative to total rRNA (data not shown).
TABLE 1.
Percentage of rRNA hybridization signal from Eucarya, Bacteria, and Archaea in filtered picoplankton samples
| Sample | % of group-specific
rRNA:
|
|||
|---|---|---|---|---|
| Normalized to total rRNA
|
Normalized to archaeal rRNA (group I) | |||
| Eucarya | Bacteria | Archaea | ||
| AM-1 | 10.9 | 93.5 | 11.3 | 74.5 |
| AM-7 | 2.4 | 84.4 | 18.2 | 80.1 |
| SMB3 | 32.4 | 80.4 | 2.9 | NDa |
| SMB4 | 54.5 | 23.1 | 0.7 | ND |
ND, not determined.
FIG. 1.
Evolutionary relationships of uncultivated marine planktonic Archaea (groups I and II) relative to known and cultivated Crenarchaeota and Euryarchaeota. Evolutionary relationships were inferred from 687 unambiguous aligned residues by transversion distance analysis as previously described (5). Numbers appearing at each bifurcation indicate the number of bootstrapped replicates which supported that bifurcation, out of 1,000 bootstrap replicates. pSL12 represents a cloned rRNA fragment recovered from a hot spring microbial community, which appears to be specifically related to the rRNA sequences of marine nonthermophilic crenarchaeotes (1).
TABLE 2.
Phylogenetic identification of archaeal rDNA clones in libraries derived from Antarctic plankton samples
| Sample | No. of clones in:
|
% of
clones in:
|
|||
|---|---|---|---|---|---|
| Archaea | Group I | Group II | Group I | Group II | |
| AM-1 | 276 | 273 | 3 | 98.9 | 1.1 |
| AM-7 | 283 | 277 | 6 | 97.9 | 2.1 |
Lipid analyses.
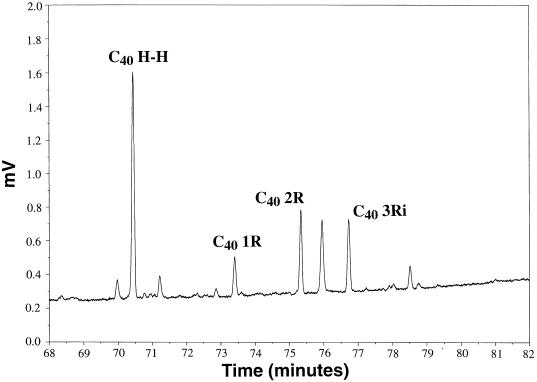
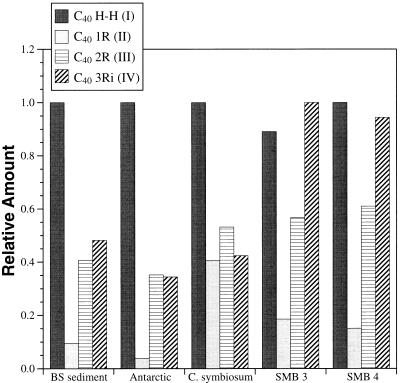
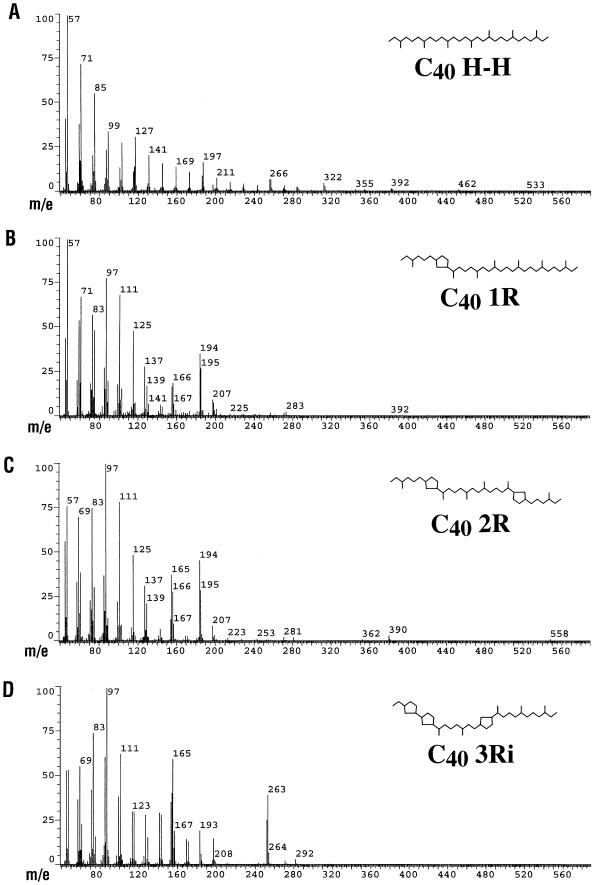
Marine picoplankton samples from Antarctica and the Santa Monica Basin, as well as the crenarchaeal sponge symbiont C. symbiosum, all contained caldarchaeol-derived acyclic and cyclic dibiphytanes (Fig. 2 to 4). Several isoprenoids were common to Antarctic plankton, Santa Monica Basin plankton, and the symbiotic crenarchaeon C. symbiosum. These ether-derived lipids also corresponded to those recently found in Black Sea sediments and suspended particulate material (15, 19); data are shown for comparison in Fig. 4 (e.g., BS sediments). The acyclic head-to-head-linked biphytane was the most abundant isoprenoid identified in most samples (C40 H H [Fig. 2 and 3]). In all samples, significant amounts of cyclic biphytanes containing one, two, or three cyclopentane rings (Fig. 2 and 3) were evident (Fig. 4). Isoprenoids containing one and two rings were identical to those found in S. solfataricus (7), while the three-ring biphytane is a structural isomer of the three-ring isoprenoid found in S. solfataricus. Other possible isomers of cyclic biphytanes, identified based on their retention times, were also observed in most samples, but their structures have not yet been confirmed. In one Santa Monica Basin plankton sample (SMB3), the most abundant caldarchaeol-derived isoprenoid was a three-ring cyclic biphytane (Fig. 4).
FIG. 2.
Gas chromatogram of an Antarctic sample which corresponds to the mass spectral analysis shown in Fig. 3, in the region of biphytane elution. Subscripts denote the number of carbon atoms in the biphytane. R, pentacyclic ring; i, isomer.
FIG. 4.
Caldarchaeol-derived biphytanes from diverse marine samples. Compounds correspond to the structures shown in Fig. 3, as identified by comparison to known standards. Relative abundances of cyclic and acyclic biphytanes in diverse marine samples are shown. The value for most abundant of the four components was arbitrarily set at a relative value of 1. BS, Black Sea sediment; Antarctic, Antarctic picoplankton lipids; C. symbiosum, C. symbiosum lipids; SMB3 and SMB4, Santa Monica Basin suspended particulate lipid samples.
FIG. 3.
Mass spectra of the four identified caldarchaeol derivatives that were common to all samples analyzed. Lipids were extracted, derivatized, and analyzed as described in the text. The mass spectra are derived from the Antarctic picoplankton lipid sample used for the GC trace in Fig. 2. R, pentacyclic ring; i, isomer.
Origins of dibiphytanyl ether lipids in marine plankton.
Although diverse in origin, the archaeal samples from Antarctica, the Santa Monica Basin, and the sponge A. mexicana had at least two properties in common. They all appear to be dominated by nonthermophilic crenarchaeotes (e.g., group I [Fig. 1]), and they all contained caldarchaeol-derived acyclic and cyclic dibiphytanes. Cyclopentane ring-containing phytanyl ether lipids have been previously identified only in thermophilic archaea (18, 25, 33). Additionally, some thermophilic archaea increase the proportion of cyclic and acyclic tetraethers in respose to increasing growth temperatures and pressure (8, 17, 34). It is therefore somewhat surprising that identical lipids are present in nonthermophilic crenarchaeotes, some of which thrive at temperatures as low as −1.5°C (6). However, recent phylogenetic studies (1) suggest that nonthermophilic crenarchaeotes share a most recent common ancestor with contemporary thermophiles (1) (pSL12 in Fig. 1), so the presence of these lipids could indeed represent the remnants of a thermophilic ancestry. However, crenarchaeotal psychrophiles and mesophiles likely contain other membrane lipids not detected in our analyses, which may differentiate them from their hyperthermophilic close relatives and which might be adaptive for lower temperature growth.
Several studies have recently reported the occurrence of tetraether lipids in suspended particulates and sediments from the Indian Ocean, the Cariaco Trench, and the Black Sea (15, 19). However, the majority of the samples from these prior studies was derived from anaerobic sediments, and the types of archaea present in these samples were not determined. The possibility that methanogens are responsible for the ether lipids observed in these predominantly anaerobic sediment samples cannot be ruled out (15). In contrast, archaea in the marine samples which we examined consisted predominantly of nonthermophilic crenarchaeotes, and they also contained acyclic and cyclic dibiphytanes. No previously cultivated types of archaea, including methanogens, were detected in any of our samples. The combined rRNA and lipid analyses reported here strongly suggest that nonthermophilic crenarchaeotes (group I [Fig. 1]) are the predominant biological source of biphytanyl tetraether lipids in temperate and polar marine plankton.
Archaebacterial lipids are potentially useful biogeochemical tracers. Acyclic and cyclic ether lipids have been detected in a wide variety of contemporary (3, 4, 15, 19, 27, 28) and ancient (12, 24) marine sediment samples. Archaebacterial lipids have also been reported to be more resistant to degradation and turnover than are the ester-linked lipids of bacteria and eucarya (10, 13, 29). In coastal marine sediments, archaeal diphytanyl glycerol diether degraded significantly more slowly than did bacterial ester-linked phospholipids (13). The presence of archaeal tetraethers as “molecular fossils” in ancient sediments and petroleum deposits also attests to the inherent resistance of these lipids to decomposition (3, 12, 24). Considering the relative stability of such ether lipids, and the ubiquity and abundance of nonthermophilic crenarchaeotes in marine habitats (5, 6, 11, 21, 23, 30, 36), planktonic archaea may well contribute to carbon deposition in the sea in part through production, sedimentation, and accumulation of their biphytanyl tetrather lipids in marine sediments.
Acknowledgments
We thank Antarctic Support Associates staff for their helpful assistance at Palmer Station and Shane Anderson and Chris Gottschalk for collection of A. mexicana. We thank Tom Langworthy for helpful advice and discussion.
This work was supported by NSF grants OCE95-29804 and OPP94-18442 to E.F.D. and OPP-95310364 and OCE-9310364 to S.G.W. The Santa Monica Basin samples were collected during research funded by the U.S. Department of Energy’s (DOE) National Institute for Global Environmental Change (NIGEC) through the NIGEC Southeast Regional Office at the University of Alabama, Tuscaloosa (DOE Cooperative Agreement No. DE-FC03-90ER61010).
REFERENCES
- 1.Barns S M, Delwiche C F, Palmer J D, Pace N R. Perspectives on archaeal diversity, thermophily, and monophyly from environmental rRNA sequences. Proc Natl Acad Sci USA. 1996;93:9188–9193. doi: 10.1073/pnas.93.17.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bintrim S B, Donohue T J, Handelsman J, Roberts G P, Goodman R M. Molecular phylogeny of Archaea from soil. Proc Natl Acad Sci USA. 1997;94:277–282. doi: 10.1073/pnas.94.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chappe B, Albrecht P, Michaelis W. Polar lipids of archaebacteria in sediments and petroleums. Science. 1982;217:65–66. doi: 10.1126/science.217.4554.65. [DOI] [PubMed] [Google Scholar]
- 4.Chappe B, Michaelis W, Albrecht B, Ourisson G. Fossil evidence for a novel series of archaebacterial lipids. Naturwissenschaften. 1979;66:522–523. [Google Scholar]
- 5.DeLong E F. Archaea in coastal marine environments. Proc Natl Acad Sci USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLong E F, Wu K Y, Prezelin B B, Jovine R V M. High abundance of Archaea in Antarctic marine picoplankton. Nature. 1994;371:695–697. doi: 10.1038/371695a0. [DOI] [PubMed] [Google Scholar]
- 7.De Rosa M, de Rosa S, Gambacorta A. Lipid structures in the Caldariella group of extreme thermoacidophile bacteria J. Chem Soc Chem Commun. 1977;1977:514–515. [Google Scholar]
- 8.De Rosa M, Esposito E, Gambacorta A, Nicolaus B, Bu’Lock J D. Effects of temperature on ether lipid composition of Caldariella acidophila. Phytochemistry. 1980;19:827–831. [Google Scholar]
- 9.De Rosa M, Gambacorta A, Gliozzi A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol Rev. 1986;50:70–80. doi: 10.1128/mr.50.1.70-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickins H D, Van Vleet E S. Archaebacterial activity in the Orca Basin determined by the isolation of characteristic isopranyl ether-linked lipids. Deep-Sea Res. 1992;39:521–536. [Google Scholar]
- 11.Fuhrman J A, McCallum K, Davis A A. Novel major archaebacterial group from marine plankton. Nature. 1992;356:148–149. doi: 10.1038/356148a0. [DOI] [PubMed] [Google Scholar]
- 12.Hahn J, Haug P. Traces of archaebacteria in ancient sediments. Syst Appl Microbiol. 1986;7:178–183. [Google Scholar]
- 13.Harvey H R, Fallon R D, Patton J S. The effect of organic matter and oxygen on the degradation of bacterial membrane lipids in marine sediments. Geochim Cosmochim Acta. 1986;50:795–804. [Google Scholar]
- 14.Hershberger K L, Barns S M, Reysenbach A L, Dawson S C, Pace N R. Crenarchaeota in low-temperature terrestrial environments. Nature. 1996;384:420. doi: 10.1038/384420a0. [DOI] [PubMed] [Google Scholar]
- 15.Hoefs M J L, Schouten S, de Leeuw J W, King L L, Wakeham S G, Damsté J S S. Ether lipids of planktonic archaea in the marine water column. Appl Environ Microbiol. 1997;63:3090–3095. doi: 10.1128/aem.63.8.3090-3095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurgens G, Lindström K, Saano A. Novel group within the kingdom Crenarchaeotafrom boreal forest soil. Appl Environ Microbiol. 1997;63:803–805. doi: 10.1128/aem.63.2.803-805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneshiro S M, Clark D S. Pressure effects on the composition and thermal behavior of lipids from the deep-sea thermophile Methanococcus jannaschii. J Bacteriol. 1995;177:3668–3672. doi: 10.1128/jb.177.13.3668-3672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kates M. Membrane lipids of archaea. In: Kates M, Kushner D J, Matheson A T, editors. The biochemistry of archaea (Archaebacteria). New York, N.Y: Elsevier Science Publishing; 1993. pp. 261–296. [Google Scholar]
- 19.King, L. L., and S. G. Wakeham. Submitted for publication.
- 20.Koga Y, Nishihara M, Morii H, Akagawa-Matsushita M. Ether polar lipids of methanogenic bacteria: structures, comparative aspects, and biosyntheses. Microbiol Rev. 1993;57:164–182. doi: 10.1128/mr.57.1.164-182.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massana R, Murray A E, Preston C M, DeLong E F. Vertical distribution and phylogenetic characterization of marine planktonic Archaeain the Santa Barbara Channel. Appl Environ Microbiol. 1997;63:50–56. doi: 10.1128/aem.63.1.50-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGregor B J, Moser D P, Alm E W, Nealson K H, Stahl D A. Crenarchaeota in Lake Michigan sediment. Appl Environ Microbiol. 1997;63:1178–1181. doi: 10.1128/aem.63.3.1178-1181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McInerney J O, Wilkinson M, Patching J W, Embley T M, Powell R. Recovery and phylogenetic analysis of novel archaeal rRNA sequences from a deep-sea deposit feeder. Appl Environ Microbiol. 1995;61:1646–1648. doi: 10.1128/aem.61.4.1646-1648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaelis W, Albrecht P. Molecular fossils of archaebacteria in kerogen. Naturwissenschaften. 1979;66:420–422. [Google Scholar]
- 25.Nishihara M, Koga Y. Hydroxyarchaetidylserine and hydroxyarchaetidyl-myo-inositol in Methanosarcina barkeri: polar lipids with a new ether core portion. Biochim Biophys Acta. 1991;1082:211–217. doi: 10.1016/0005-2760(91)90196-o. [DOI] [PubMed] [Google Scholar]
- 26.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 27.Pauly G G, Van Vleet E S. Archaebacterial ether lipids: natural tracers of biogeochemical processes. Org Geochem. 1986;10:859–867. [Google Scholar]
- 28.Pauly G G, Van Vleet E S. Acyclic archaebacterial lipids in swamp sediments. Geochim Cosmochim Acta. 1986;50:1117–1125. [Google Scholar]
- 29.Pease T K, Van Vleet E S, Barre J S. Diphytanyl glycerol ether distributions in sediments of the Orca Basin. Geochim Cosmochim Acta. 1992;56:3469–3479. doi: 10.1016/0016-7037(92)90391-u. [DOI] [PubMed] [Google Scholar]
- 30.Preston C M, Wu K Y, Molinski T F, DeLong E F. A psychrophilic crenarchaeon inhabits a marine sponge: Cenarchaeum symbiosumgen. nov., sp. nov. Proc Natl Acad Sci USA. 1996;93:6241–6246. doi: 10.1073/pnas.93.13.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleper C, Holben W, Klenk H-P. Recovery of crenarchaeotal ribosomal DNA sequences from freshwater-lake sediments. Appl Environ Microbiol. 1997;63:321–323. doi: 10.1128/aem.63.1.321-323.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schleper C, Swanson R V, Mathur E J, DeLong E F. Characterization of a DNA polymerase from the uncultivated psychrophilic archaeon Cenarchaeum symbiosum. J Bacteriol. 1997;179:7803–7811. doi: 10.1128/jb.179.24.7803-7811.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprott G D, Eikel I, Dicaire C. Novel acid-labile hydroxydiether lipid cores in methanogenic bacteria. J Biol Chem. 1990;265:13735–13740. [PubMed] [Google Scholar]
- 34.Sprott G D, Meloche M, Richards J C. Proportions of diether, macrocyclic diether, and tetraether lipids in Methanococcus jannaschiigrown at different temperatures. J Bacteriol. 1991;173:3907–3910. doi: 10.1128/jb.173.12.3907-3910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprott G D. Structures of archaebacterial membrane lipids. J Bioenerg Biomembr. 1992;24:555–566. doi: 10.1007/BF00762348. [DOI] [PubMed] [Google Scholar]
- 36.Stein J L, Marsh T L, Wu K Y, Shizuya H, DeLong E F. Characterization of uncultivated prokaryotes: isolation and analysis of a 40-kilobase-pair genome fragment from a planktonic marine archaeon. J Bacteriol. 1996;178:591–599. doi: 10.1128/jb.178.3.591-599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stetter K O. Hyperthermophilic prokaryotes. FEMS Microbiol Rev. 1996;18:149–158. [Google Scholar]
- 38.Tornabene T G, Langworthy T A. Diphytanyl and dibiphytanyl glycerol ether lipids of methanogenic archaea. Science. 1978;203:51–53. doi: 10.1126/science.758677. [DOI] [PubMed] [Google Scholar]
- 39.Ueda T, Suga Y, Matsuguchi T. Molecular phylogenetic analysis of a soil microbial community in a soybean field. Eur J Soil Sci. 1995;46:415–421. [Google Scholar]
- 40.Wakeham, S. G., and J. A. Beier. 1991. Fatty acid and sterol biomarkers as indicators of particulate organic matter and alteration processes in the water column of the Black Sea. Deep-Sea Res. 38(Suppl. 2):S943–S968.
- 41.Woese C R, Fox G E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA. 1977;74:5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woese C R, Magrum L J, Fox G E. Archaebacteria. J Mol Evol. 1978;11:245–252. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- 43.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woese C R, Kandler O, Wheelis M L. Towards a system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng D, Alm E W, Stahl D A, Raskin L. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl Environ Microbiol. 1996;62:4504–4513. doi: 10.1128/aem.62.12.4504-4513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]