Abstract
Ventriculoperitoneal (VP) shunt infections are associated with increased risk of morbidity and mortality from complications such as meningitis, ventriculitis, shunt malfunction and in some cases, recurrence of infection. Brevibacterium and Corynebacterium are gram positive organisms that are rarely implicated in VP shunt infections but are more commonly associated with colonization of dialysis and central venous catheters. Typical microbiological isolates in VP shunt infections include Staphylococcus aureus, Staphylococcus epidermidis and gram-negative rods. Here, we describe the case of a young woman who had VP shunt placement for over a decade without any history of infection, and now presented with new-onset VP shunt co-infection with Brevibacterium and Corynebacterium organisms.
Keywords: Cerebrospinal fluid, Brevibacterium, Ventriculoperitoneal shunt, Corynebacterium, Lumboperitoneal shunt
Introduction
Ventriculoperitoneal (VP) shunt placement is a very common neurosurgical procedure in the United States with over 30,000 procedures performed yearly [1], [2]. Annual incidence of VP shunt infections has been estimated to range from 5%− 31% with over 60% associated mortality rate [1], [2], [3]. Current medical literature suggests that most VP shunt infections occur within the first 2–6 months of placement, and typical colonizing organisms are commonly those with low virulence such as Staphylococcus aureus, Staphylococcus epidermidis and gram-negative rods [1], [2], [3], [4], [5], [6], [7], [8] Clinical implications of shunt infections include shunt malfunction, meningitis, ventriculitis, increased shunt revision rates and high infection recurrence rates [2], [4], [8].
Corynebacterium species, a group of gram-positive bacilli have been implicated in very small cases of VP shunt infections [9]. Majority of Corynebacterium infections occur with peritoneal dialysis and central venous catheters [2], [3], [4], [7]. These group of organisms are notably resistant to various antibiotics, and, in most cases, definitive treatment will require shunt removal [2], [3], [4], [7]. On the other hand, Brevibacterium species, which are nonmotile, catalase positive, gram-positive bacilli, previously thought to be nonpathogenic, are now emerging as recognizable causes of blood stream infections in patients with central venous catheters and in immunocompromised patients [7], [8]. These organisms are rarely associated with VP shunt infections [7], [8], [10].
Here, we describe the case of a young woman who had VP shunt placement for over a decade without any history of infection, and now presented with new-onset VP shunt co-infection with Brevibacterium and Corynebacterium minutissimum organisms. To the best of our knowledge, this is the first case report in the literature that highlights co-infection of VP shunt with Brevibacterium and Corynebacterium minutissimum organisms in an adult patient.
Case presentation
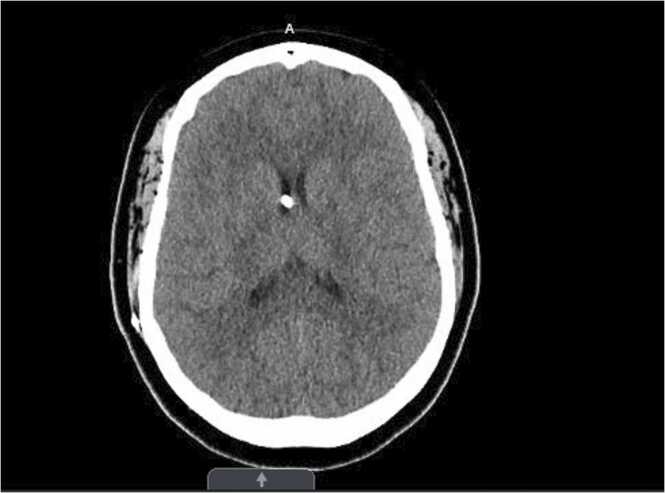
A 43-year-old African American female with a past medical history of seizures, morbid obesity, hypertension, and obstructive hydrocephalus status post ventriculoperitoneal shunt (VP) placement over 10 years ago, presented to the neurosurgery clinic in May 2022 due to a non-healing painful pimple-like lesion at the site of VP shunt insertion (right frontal area), with unknown duration of onset. This was treated with levofloxacin at the time. On follow-up visit three months on, patient had complaints of headache in addition to the persistent pimple-like lesion. She had no fever, neck stiffness or blurry vision. Shunt series done showed no abnormality. CT-scan of the head without contrast done, revealed well decompressed ventricles (Fig. 1). However, given patient’s complaint of a non-healing persistent pimple like lesion, now with new-onset drainage, there was a high index of suspicion of shunt infection. Patient was admitted for an elective removal of the VP shunt.
Fig. 1.
Initial CT head/Brain without contrast showing ventriculoperitoneal shunt in place, with well decompressed ventricles.
Initial vital signs on admission, showed a blood pressure of 118/61, heart rate of 66 bpm, respiratory rate of 16 bpm, temperature of 36.7 ℃ (98.1 ℉), body mass index of 69.1 kg/m2 and an oxygen saturation of 95% on room air. Physical examination of the head showed presence of a pustular lesion in the right frontal area of the VP shunt placement, there was no associated swelling, erythema, or drainage. Physical examination of the remainder of systems were unremarkable. Complete blood count, urinalysis, comprehensive metabolic panel, and coagulation panel were normal. Blood culture showed no growth of organisms. Patient had elevated erythrocyte sedimentation rate of 83 mm/hr, and C-reactive protein of 1.91 mg/dl. Blood glucose was mildly elevated at 129. The presence of a significant amount of pus upon removal of the scab lesion during surgery further increased the suspicion of infection. VP shunt was removed with multiple samples sent for microbiological cultures including the ventricular catheter, shunt valve and cerebrospinal fluid (CSF). Fluoroscopic diagnostic lumbar puncture done status post shunt removal revealed normal opening pressure and negative CSF findings.
Gram stain of the shunt valve revealed gram-positive cocci in pairs and clusters and few gram-positive rods. Catheter tip culture revealed growth of gram-positive rods. Gram stain of the incision site swab showed a few gram-positive cocci in pairs and clusters. CSF culture revealed no growth of organisms. Patient was started on intravenous aztreonam, metronidazole, and vancomycin for empiric antibiotic coverage pending final culture results. Final catheter tip culture showed isolates of Corynebacterium minutissimum. Two sets of blood culture were obtained which showed no growth of organisms. Final culture results of the VP shunt valve showed isolates of Brevibacterium and Corynebacterium minutissimum organisms.
Antibiotic susceptibility report showed Corynebacterium to be susceptible to vancomycin, while Brevibacterium was sensitive to ceftriaxone and cefotaxime only. Given patient’s penicillin allergy with unknown reaction, a penicillin desensitization was planned, and patient was upgraded to the intensive care unit for close monitoring for signs of hypersensitivity reaction and anaphylaxis. Patient had a successful penicillin desensitization and thereafter was started on long term antibiotic regimen of IV vancomycin and ceftriaxone for 6-weeks duration.
While on the medical floors, patient complained of new onset rashes on the medial surface of both thighs with itching sensation, associated with intravenous vancomycin infusion. Physical examination showed several maculopapular rashes. Patient had no fever at this time. Due to the possibility of “Red Man” syndrome, the infusion time of vancomycin was reduced, with considerations for diphenhydramine and possible antibiotic change. The next day, patient endorses resolution of rashes on the medial thighs with infusion halving time, but stated she developed new rashes on the left cubital fossa, for which diphenhydramine was given. Vancomycin was continued at this time, with plans to discontinue if new rash develops afterwards. The next day, all rashes were resolved. Upon further reassessment, patient was determined to be clinically stable for discharge and arrangement for home health services and outpatient antibiotic infusion was made. Patient was discharged on the 15th day of hospital stay.
Upon discharge, patient was scheduled to follow-up outpatient with her primary care physician, infectious disease, and neurosurgery for VP shunt replacement, pending resolution of infection with completion of antibiotic therapy. During follow-up neurosurgery clinic visits, patient had complaints of headache and vision issues. Therapeutic lumbar puncture was done for patient which revealed findings of elevated opening pressure of 21 cm H2O with otherwise clear CSF fluid. Given patient’s morbid obesity, a new diagnosis of idiopathic intracranial hypertension was made.
Following completion of long-term antibiotic treatment with unremarkable follow-up baseline laboratory results, patient underwent another lumbar puncture with indication to check clearance of infection. Patient was then scheduled for elective re-insertion of the VP shunt, however CT scan with BrainLAB protocol done intraoperatively, showed severely collapsed ventricles. Associated risks of VP shunt placement with this finding includes shunt malfunction and collapse of ventricles around the VP catheter. At this point, lumboperitoneal (LP) shunt placement was a more clinically appropriate alternative. This option was proposed to patient along with a discussion on the risks including LP shunt infection, CSF leak, and post op hematoma. Patient had a successful LP shunt placement with laparoscopic abdominal access for distal shunt placement in December 2022. Patient was discharged from the hospital on post-op day 1 with instructions to follow up outpatient with neurosurgery.
Discussion
Evidence from the literature suggests that ventriculoperitoneal (VP) shunt infection with Brevibacterium is uncommon, more so alongside co-infection with Corynebacterium [2], [3], [4], [5], [6], [7], [8], [9], [11]. The case presentation above, describes a patient with co-infection of her VP shunt with these microorganisms. This is a patient who has had a VP shunt for over a decade with no events or complications, and now presents with subtle clinical symptoms of headache and pustule on the forehead. These presenting symptoms could easily be misdiagnosed as a skin condition or stress induced headache, as patient did not exhibit typical signs of inflammation such as fever, swelling, erythema or drainage. Possible explanation for the atypical presentation could be due to the mild activation of the immune host response by Brevibacterium species [12], making presentation and diagnosis quite challenging. This emphasizes the need for physicians to develop a high index of suspicion in the management of clinical conditions, especially those with subtle signs and symptoms.
Similar with other studies, our patient had a VP shunt removal done as part of definitive therapeutic management [12], [13]. Studies have shown that the combination of findings of fever and increased levels of white cell count, particularly neutrophil count of > 10–12% from CSF culture, has > 97% specificity, hence an important predictor for VP shunt infections [3], [10]. In contrast, other studies have shown that CSF culture only or a negative CSF culture, does not rule out VP shunt infections as CSF cultures are likely to be negative in cases of infected VP shunts and as such, culture of VP shunt valve and catheter tip are recommended [5], [6]. Consistent with latter studies, Brevibacterium and Corynebacterium minutissimum organisms were isolated from the microbiological culture of our patient’s VP shunt valve [3], [6], while CSF culture showed no growth of microorganisms. Given that both organisms are part of the normal skin flora [3], [6], it is possible that infection in this case, could be due to bacterial colonization of the VP shunt valve.
Species determination for Corynebacterium showed the subtype to be C. minutissimum, and while numerous subtypes of species exist for Brevibacterium, the culture results for this patient did not yield any species subtype, a common finding with a similar case in the literature [12]. E-test antimicrobial susceptibility testing was used to determine the minimum inhibitory concentration (MIC) for both organisms. Brevibacterium were interpreted in accordance with the Clinical Laboratory Standards Institute, as susceptible to only Ceftriaxone (MIC ≤ 0.02 µg/mL), and Cefotaxime (MIC = 0.01 µg/mL), but resistant to Vancomycin (MIC > 256 µg/mL). Corynebacterium minutissimum was susceptible to Vancomycin (MIC = 0.25 µg/mL) and resistant to Ciprofloxacin (MIC >32 µg/mL) and erythromycin (MIC =24 µg/mL).
Since Brevibacterium was highly susceptible to ceftriaxone, and patient had penicillin allergy, desensitization had to be done given the limited choice of antibiotic therapy. Afterwards, our patient was treated with long term intravenous antibiotic therapy, vancomycin, and ceftriaxone, for 6 weeks duration. Patient had biweekly follow-up visits with outpatient infectious disease clinic to monitor response to therapy. At the end of antibiotic treatment, baseline labs obtained including complete blood count, comprehensive metabolic panel, inflammatory biomarkers, and CSF culture were unremarkable. Given the limited sensitivities of these microorganisms to antibiotic as evidenced by the minimum inhibitory concentration, it calls into perspective the impact of antibiotic stewardship and its role in preventing antibiotic resistance in the clinical management of patients. Therefore, adhering to the antibiotic stewardship program as clinicians and health professionals, is necessary to prevent antibiotic resistance, reduce mortality and foster high value patient care.
Conclusion
Brevibacterium is a rare but possible cause of ventriculoperitoneal shunt infections, as shown in this case report, and coinfection with Corynebacterium can occur. Here, we highlighted a case of VP shunt coinfection with above mentioned organisms in a patient who had her VP shunt removed, treated with long term antibiotic regimen, and successfully underwent placement of an alternative shunt, without any relapse or reinfection after nine months of follow-up. In addition, given the subtle signs associated with certain microorganisms particularly Brevibacterium, the need for comprehensive clinical assessment of patients and early intervention becomes paramount to achieve proper and effective management of patients.
Ethical approval
Written Consent was obtained from patient prior to development of manuscript.
Consent
Written Consent was obtained from patient prior to development of manuscript.
Funding
All authors declared that no financial support was received from any organization for the submitted manuscript.
CRediT authorship contribution statement
MLO was involved in all aspects of the case report preparation including case identification, literature review, preparation and editing of case report. CI was involved in case identification and editing. CM and MA performed critical review of case report.
Declaration of Competing Interest
All authors declared that no financial support was received from any organization for the submitted manuscript.
References
- 1.Ferras M., McCauley N., Stead T., Ganti L., Desai B. Ventriculoperitoneal shunts in the emergency department: a review. Cureus. 2020;12(2) doi: 10.7759/cureus.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutierrez-Murgas Y., Snowden J.N. Ventricular shunt infections: immunopathogenesis and clinical management. J Neuroimmunol. 2014;276(1–2):1–8. doi: 10.1016/j.jneuroim.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paff M., Alexandru-Abrams D., Muhonen M., Loudon W. Ventriculoperitoneal shunt complications: a review. Interdiscip Neurosurg. 2018;13:66–70. doi: 10.1016/j.inat.2018.04.004. [DOI] [Google Scholar]
- 4.Sarguna P., Lakshmi V. Ventriculoperitoneal shunt infections. Indian J Med Microbiol. 2006;24(1):52–54. doi: 10.4103/0255-0857.19896. [DOI] [PubMed] [Google Scholar]
- 5.Vanaclocha V., Sáiz-Sapena N., Leiva J. Shunt malfunction in relation to shunt infection. Acta Neurochir. 1996;138(7):829–834. doi: 10.1007/BF01411261. PMID: 8869711. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shudifat A., Alsabbagh Q., Al-Matour B., Alkhlaifat A., Suleiman D., Jumaah M. Analysis of the rate and pattern of ventriculoperitoneal shunt infection and ventricular catheter culture yield: a 10-year single-institute experience. Pedia Neurosurg. 2020;55(2):81–85. doi: 10.1159/000508331. Epub 2020 Jul 1. PMID: 32610322. [DOI] [PubMed] [Google Scholar]
- 7.Asai N., Suematsu H., Yamada A., Watanabe H., Nishiyama N., Sakanashi D., et al. Brevibacterium paucivorans bacteremia: case report and review of the literature. BMC Infect Dis. 2019;19(1) doi: 10.1186/s12879-019-3962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reece R.M., Cunha C.B., Rich J.D. Corynebacterium minutissimum vascular graft infection: case report and review of 281 cases of prosthetic device-related Corynebacterium infection. Scand J Infect Dis. 2014;46(9):609–616. doi: 10.3109/00365548.2014.918650. [DOI] [PubMed] [Google Scholar]
- 9.Miura F.K., Andrade A.F., Randi B.A., Amato V.S., Nicodemo A.C. Cerebrospinal fluid shunt infection caused by Corynebacterium sp: case report and review. Brain Inj. 2014;28(9) doi: 10.3109/02699052.2014.919535. 1223- [DOI] [PubMed] [Google Scholar]
- 10.Benson C.E., Jr., Tatem L. Successful treatment of brevibacterium bacteremia solely with antimicrobial therapy. Cureus. 2021;13(6) doi: 10.7759/cureus.16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shweta F.N.U., Gurram P.R., O’Horo J.C., Khalil S. <em>Brevibacterium</em> Species: an emerging opportunistic cause of bloodstream infections. Mayo Clin Proc. 2021;96(4):1093–1094. doi: 10.1016/j.mayocp.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Øvsthus K.K., Sjåvik K., Lier T., Klingenberg C. Antibiotic therapy of an infant with a brevibacterium casei ventriculoperitoneal shunt infection. Pediatr Infect Dis J. 2021;40(12):e519–e520. doi: 10.1097/INF.0000000000003267. [DOI] [PubMed] [Google Scholar]
- 13.Pelegrín I., Lora-Tamayo J., Gómez-Junyent J., Sabé N., García-Somoza D., Gabarrós A., et al. Management of ventriculoperitoneal shunt infections in adults: analysis of risk factors associated with treatment failure. Clin Infect Dis: Publ Infect Dis Soc Am. 2017;64(8):989–997. doi: 10.1093/cid/cix005. [DOI] [PubMed] [Google Scholar]