Recent breakthroughs in embryo modeling have paved the way for the creation of so-called stem cell-based embryonic models (SEMs), mirroring key stages in early human embryonic development. This groundbreaking innovation offers scientists an unprecedented platform to delve into the hallmark processes that regulate human embryonic development. Previously, SEMs resembling the post-implantation human embryo have been established using various protocols, such as human embryoids,1 iDiscoids,2 gastruloids,3,4,5 E-assembloids,6 and human extra-embryoids.7 However, the attainment of human post-implantation-like SEMs exhibiting complex embryo-like and extra-embryonic morphological features (including a yolk sac, chorionic sac, and the bilaminar embryonic disk) has remained elusive.8
Recently, Oldak and colleagues successfully generated a self-organizing SEM that closely replicates the 3D structure and essential developmental milestones observed in in utero-developed human embryos between 7 and 8 days post-fertilization (dpf) and 13 and 14 dpf.8 This includes the formation of the bilaminar embryonic disk, as well as the development of the yolk and chorionic sacs.8 In contrast to previous protocols, this novel protocol utilized genetically unmodified naive human embryonic stem cells (hESCs) to develop SEMs, and all extra-embryonic lineages were present in the resulting structures, which has not been previously accomplished.
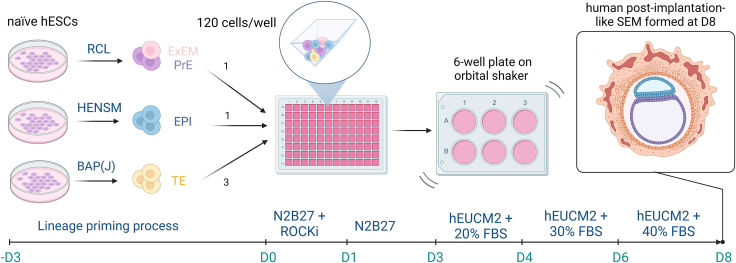
Starting with naive hESCs (WIBR1 or WIBR3) cultured under FGF/TGF/ACTIVIN/MEF-independent and serum-free human enhanced naive stem cell medium (HENSM) conditions,9 extra-embryonic-like cells, trophoblast-like cells (TEs), and epiblast-like cells (EPIs) were differentiated through culture under specific conditions for 3 days, constituting a process of lineage priming.8 To induce primitive endoderm (PrE) and extra-embryonic mesoderm (ExEM), naive hESCs were exposed to RCL conditions, a modification of the RPMI 640 glutamax/activin A/CHIR/LIF (RACL) medium conditions,10 omitting activin A, since this compound inhibits differentiation of naive hESCs toward ExEM.8 Conversely, the TE lineage was generated using BMP4/A8301/PD173074/JAKi (BAP(J)) conditions,11 allowing regulation of BMP4, ALK4/5/7, FGF, and JAK signaling to direct differentiation toward the TE lineage.8 The EPI-like lineage was formed through an extended culture of the hESCs in HENSM conditions.8 Subsequently, 120 cells were seeded in a 1:1:3 ratio (hESC:PrE/ExEM:TE) into a pyramidal-shaped microwell and cultured in differential culture conditions for 8 days (Figure 1).8 On the 3rd day of aggregation, the SEMs were transferred to a non-adherent 6-well plate and placed on an orbital shaker to prevent attachment, thereby preserving the proper morphology of the SEMs.8
Figure 1.
Protocol used by Oldak and colleagues8 to create SEMs exhibiting complex embryo-like morphological features (yolk/chorionic sacs, embryonic/bilaminar disks) that resemble the human post-implantation embryo, starting from naive human embryonic stem cells (hESCs) (WIBR1 or WIBR3)
ExEM, extra-embryonic mesoderm; EPI, epiblast; TE, trophoblast; HENSM, human enhanced naive stem cell medium; RCL, RACL medium conditions omitted of activin A; BAP(J), BMP4-A83-01-PD173074 (JAK inhibitor) culture conditions; N2B27, basal medium conditions; hEUCM2, human ex-utero culture media 2; FBS, fetal bovine serum; ROCKi, ROCK inhibitor; D, day. RCL media conditions are a modification of RACL media conditions.10 BAP(J) media conditions were composed by Io and colleagues,11 whereas HENSM media conditions originate from Bayerl et al.9 Image created with www.biorender.com.
While this approach is groundbreaking, it is important to consider that self-organization showed low efficiency, as only 1.63% of the structures formed from WIBR1 naive hESCs, and 1.09% of structures formed from WIBR3 naive hESCs exhibited the correct post-implantation organization at day 6.8 Additionally, it is worth noting that across various experimental batches and cell lines, there was a noticeable degree of asynchrony in the formed SEMs.8 This resulted in a 2-day difference in developmental stages for SEMs on days 6–8.8 Consequently, when assessing SEMs at a specific time point, more and less advanced structures were observed,8 posing a challenge for standardized evaluation. Addressing these issues is essential to use SEM models in a consistent and reliable manner. However, further development of SEMs will accelerate research and will reduce the number of human embryos used for these purposes.
Furthermore, the emergence of SEMs raises ethical questions and concerns. On one hand, using SEMs to study human post-implantation stages may appear less ethically sensitive than using human embryos for this purpose. Yet, it is crucial to note that although SEMs bear a striking similarity to human embryos at the post-implantation stage, they are not originating from blastocyst-like structures and did not undergo the typical embryonic developmental process observed in human embryos or any of the epigenetic processes that occur during the pre-implantation period (such as zygote genome activation or genome-wide DNA methylation). This prompts us to consider whether these SEMs should be viewed as models of human embryonic development, as they miss parts of the developmental trajectory. Additionally, SEMs are genetic duplicates of the donor stem cells, in contrast to human embryos that have a unique genome. Finally, legal concerns are also a significant aspect, as it is not clear whether existing regulations governing the use of human embryos in scientific research (e.g., the 14-day rule of human embryo culture) apply to SEMs. Nonetheless, it’s important to mention that the International Society of Stem Cell Research has prohibited the transfer of human-derived SEMs in both animal and human uteri and stated that the culture of SEMs should be constrained to the shortest necessary duration. In our opinion, it becomes imperative to establish clear boundaries for the use of SEMs to prevent their misuse as a means to circumvent existing regulations related to human embryo research.
Despite ethical and legal considerations, in addition to the low efficiency and inherent variability observed in the SEM model developed by Oldak and colleagues, this SEM model offers valuable potential for investigating the post-implantation stage of human embryonic development. Given that the post-implantation stage is associated with a significant rate of in utero embryo loss during pregnancy, there is a compelling need to enhance our understanding of hallmark processes occurring during the critical phase of human embryogenesis. Therefore, this work is of great relevance in this regard.
References
- 1.Weatherbee B.A., Carlos W.G., Lisa K.I., Riza M.D., Nobuhiko H., Jay S., Zernicka-Goetz M. A model of the post-implantation human embryo derived from pluripotent stem cells. Nature. 2023;1–3 [Google Scholar]
- 2.Hislop J., Amir A., Qi S., Rayna Leann S., Kamyar K.F., Ryan L., Jeremy J.V., Tahere M., Mohammad N.T., Matthew R., et al. Modelling human post-implantation development via extra-embryonic niche engineering. bioRxiv. 2023 doi: 10.1101/2023.06.15.545118. Preprint at. [DOI] [Google Scholar]
- 3.Liu L., Oura S., Markham Z., Hamilton J.N., Skory R.M., Li L., Sakurai M., Wang L., Pinzon-Arteaga C.A., Plachta N., et al. Modeling post-implantation stages of human development into early organogenesis with stem-cell-derived peri-gastruloids. Cell. 2023;186:3776–3792.e16. doi: 10.1016/j.cell.2023.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Mantziou V., Baillie-Benson P., Jaklin M., Kustermann S., Arias A.M., Moris N. In vitro teratogenicity testing using a 3D, embryo-like gastruloid system. Reprod. Toxicol. 2021;105:72–90. doi: 10.1016/j.reprotox.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan G., Jiachen W., Zhaode L., Mengqi C., Pinmou Z., Hao Z., Zhibin H., Yiqiang C., Yan Y., Jiahao S. Establishment of a novel non-integrated human pluripotent stem cell-based gastruloid model. bioRxiv. 2023 doi: 10.1101/2023.06.28.546720. Preprint at. [DOI] [Google Scholar]
- 6.Ai Z., Niu B., Yin Y., Xiang L., Shi G., Duan K., Wang S., Hu Y., Zhang C., Zhang C., Rong L., Kong R., Chen T., Guo Y., Liu W., Li N., Zhao S., Zhu X., Mai X., Li Y., Wu Z., Zheng Y., Fu J., Ji W., Li T. Dissecting peri-implantation development using cultured human embryos and embryo-like assembloids. Preprint at bioRxiv. 2023 doi: 10.1101/2023.06.15.545180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedroza M., Gassaloglu S.I., Dias N., Zhong L., Hou T.C.J., Kretzmer H., Smith Z.D., Sozen B. Self-patterning of human stem cells into post-implantation lineages. Nature. 2023;1–3 doi: 10.1038/s41586-023-06354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldak B., Emilie W., Vladyslav B., Mehmet-Yunus C., Cheng Z., Aguilera-Castrejon A., Shadi T., Sergey V., Thi Xuan Ai P., Shahd A., Dmitry L., et al. Complete human day 14 post-implantation embryo models from naïve ES cells. Nature. 2023;1–3 doi: 10.1038/s41586-023-06604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayerl J., Ayyash M., Shani T., Manor Y.S., Gafni O., Massarwa R., Kalma Y., Aguilera-Castrejon A., Zerbib M., Amir H., et al. Principles of signaling pathway modulation for enhancing human naive pluripotency induction. Cell stem cell. 2021;28:1549–1565.e12. doi: 10.1016/j.stem.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linneberg-Agerholm M., Wong Y.F., Romero Herrera J.A., Monteiro R.S., Anderson K.G.V., Brickman J.M. Naïve human pluripotent stem cells respond to Wnt, Nodal and LIF signalling to produce expandable naïve extra-embryonic endoderm. Development. 2019;146:dev180620. doi: 10.1242/dev.180620. [DOI] [PubMed] [Google Scholar]
- 11.Io S., Kabata M., Iemura Y., Semi K., Morone N., Minagawa A., Wang B., Okamoto I., Nakamura T., Kojima Y., et al. Capturing human trophoblast development with naive pluripotent stem cells in vitro. Cell stem cell. 2021;28:1023–1039.e13. doi: 10.1016/j.stem.2021.03.013. [DOI] [PubMed] [Google Scholar]