Abstract
Introduction
Proton pump inhibitors (PPIs) show a high level of efficacy and a high safety profile, and they have been increasingly prescribed in recent years. However, recent pharmacoepidemiological evidence has shown that PPI use has been associated with health risks and complications.
Objectives
This study aimed to assess the prescribing patterns of proton pump inhibitors and the prevalence of potential drug–drug interactions (DDIs) among patients who use PPIs.
Method
This was a retrospective analysis of electronic health records from the Ministry of National Guard Hospitals in Riyadh from January 2019 to June 2022. All adult patients who used PPIs were included to assess the prescribing patterns and drug utilization, including the number of prescriptions, duration of prescriptions, number of doses, and prescription indications. Potential DDIs were assessed based on concurrent use, which is defined as taking an interacting drug parallel to PPIs. The assessment includes complete or partial overlapping, with at least one day of overlapping.
Results
The total number of PPI prescriptions was 80,365 for a total of 9,930 patients with a mean age of 67.5. The majority of PPIs were prescribed in high doses (74%), without reporting appropriate indications (95%), and 17% were prescribed for long-term use. A total of 24,575 (33.6%) potential DDIs with PPIs were found.
Conclusion
The results showed that the majority of the PPI prescriptions were made with a high number of doses, without reporting appropriate indications, with some having potential DDIs. This might result in exposing patients to an increasing number of health risks. The findings highlight the importance of implementing a stewardship program for PPI prescription with periodic reassessments of patients’ needs for these medications.
Keywords: PPIs, Drug utilization, Patient safety
1. Introduction
Proton pump inhibitors (PPIs), including omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole, are approved medications by the US Drug and Food Administration and the Saudi Food and Drug Authority (SFDA) for treating frequent acid-related diseases in adults (Ahmed and Clarke 2022). PPIs are one of the most frequently prescribed classes of drugs (Forgacs and Loganayagam, 2008, Jarchow-Macdonald and Mangoni, 2013, Schepisi et al., 2016, Alhossan et al., 2019, Madi et al., 2019, Al-Dosari et al., 2021), and they have been increasingly prescribed in recent years because of their efficacy and safety profiles. However, they may impose health risks if they are not prescribed according to a drug use evaluation (DUE), which is a systematic approach that assesses the safety and effectiveness of a medication to improve patients’ health (Jarchow-Macdonald and Mangoni, 2013, Chen et al., 2016). The approved indications for PPI prescription include gastroesophageal reflux disease (GERD), peptic ulcer disease (PUD), erosive and ulcerative esophagitis, stress ulcer prophylaxis, and Helicobacter pylori (H. pylori) infection (Ahmed and Clarke 2022). PPIs are recommended to be prescribed for a short-term period (2–8 weeks) (Ren et al., 2019, Haastrup et al., 2021, Ahmed and Clarke, 2022).
Previous studies conducted worldwide showed that PPIs were overprescribed to polypharmacy patients, for a prolonged period in some cases, and sometimes without having an appropriate indication (Forgacs and Loganayagam, 2008, Jarchow-Macdonald and Mangoni, 2013, Schepisi et al., 2016, Alhossan et al., 2019, Madi et al., 2019, Al-Dosari et al., 2021). Despite their efficacy and clinical significance profile, long-term use of PPIs has been associated with adverse health events including hypomagnesemia, Clostridium difficile (C. difficile) infections, osteoporosis, vitamin B12 deficiency, pneumonia, and dementia (Wan et al., 2018, Ahmed and Clarke, 2022). Therefore, it is important to review the prescriptions of PPIs among patients and evaluate their use according to the recommended guidelines in terms of the indications and prescription period. Moreover, PPIs were found to have possible interactions with commonly prescribed medications including antidepressants, antiplatelets, and anticonvulsants, such as phenytoin, escitalopram, clopidogrel, warfarin, gliclazide, and atorvastatin, by increasing or decreasing the level of these medications in the blood (Jungnickel, 2000, Wedemeyer and Blume, 2014, Aljadani and Aseeri, 2018). These pharmacological interactions were found to be associated with cardiovascular and bleeding events (Tantry et al., 2011, Gjestad et al., 2015).
Studies have been conducted to assess the knowledge and attitude toward the use of PPIs among healthcare providers in Saudi Arabia. These studies highlighted the need to increase the level of healthcare providers' knowledge to reduce the unnecessary prescription of PPIs (Alhossan et al., 2019, Asdaq et al., 2021). A recently published study found that PPIs were prescribed to many patients inappropriately (Al-Dosari, Binafeef and Alsolami 2021). However, there is a lack of studies assessing PPI use in terms of prescription patterns and the prevalence of potential drug–drug interactions. Therefore, this study aims to assess prescription patterns of proton pump inhibitors and the prevalence of potential drug–drug interactions (DDIs) among patients using PPIs.
2. Methods
2.1. Study design
This is a descriptive retrospective analysis of electronic health records (EHRs) of adult patients who had a prescription for PPIs from January 2019 to June 2022 to assess prescribing patterns and the prevalence of DDIs among patients on PPIs in the Ministry of National Guard Hospitals in the Riyadh region of Saudi Arabia. Ethical approval was obtained from the King Abdullah International Medical Research Center’s (KAIMRC) Institutional Review Board.
2.2. Study subjects
This study included patients aged 18 years and older among the Ministry of National Guard Hospitals’ in- and outpatients in Riyadh who had a prescription for PPIs. The PPIs included were esomeprazole and omeprazole since they were the only types used in the study setting throughout the study period. Those who had a prescription for the study drugs without a prescription date indicated in their record were excluded as this information is necessary to estimate the potential DDIs.
2.3. Measures
The patterns of PPI prescriptions were assessed using the type of PPIs prescribed, patients’ demographics, and the number of PPIs prescribed during the study period. EHRs were reviewed to determine the duration of PPI prescription, which was classified as long-term use (more than 6 weeks of PPI use) or short-term use (less than 6 weeks). Moreover, the dose level was assessed and classified as low dose (10 mg), standard dose (20 mg-30 mg), or high dose (>40 mg) (Ren et al., 2019, Haastrup et al., 2021, Ahmed and Clarke, 2022). Indications for PPI prescription include gastroesophageal reflux disease (GERD), esophagitis, peptic ulcer disease (PUD), and H. Pylori infection eradication. The prevalence of adverse health events potentially associated with PPI use including hypomagnesemia and clostridium difficile infections was also assessed.
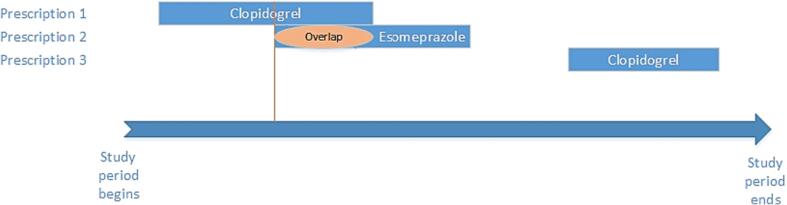
The prevalence of potential DDIs among patients using PPIs was assessed based on concurrent use, which is defined as taking one or more interacting medications in the same period as a prescription for a PPI (Fig. 1). This study included complete or partial overlapping, with at least one day of overlapping. To identify potential DDIs, we used the Summary of Product Characteristics (SPC) of the study drugs (SFDA 2020) and the online Drug Interactions Checker database (Drug Interactions Checker 2021). All DDIs included in the analysis are listed in Supplementary Table 1. These sources were also used to search for drugs that, when used with PPIs, may cause unwanted health effects (SFDA, 2020, Drug Interactions Checker, 2021). A list of potential DDIs was generated for each patient based on the provided data.
Fig. 1.
Prescriptions with overlapping days. An example illustrating three prescriptions for the same patient during the study period. Prescription 2 (Esomeprazole) has overlapping days with prescription 1 (Clopidogrel). While prescription 3 (Clopidogrel) has no interaction with prescription 2 (esomeprazole).
Eligible patients’ records were reviewed, and data on the following were collected: 1) patient demographics, including age and gender; 2) active drug prescriptions during the study period and dates of prescription, duration of the prescription, and prescription dose and unit; 3) prescriptions of the study drugs (esomeprazole and omeprazole) during the study period and dates of prescription, duration of the prescription, prescription dose and unit, and route of administration; and 4) diagnosis according to the International Classification of Diseases 10th Revision (ICD-10) codes, approved indications for PPI prescription including GERD (K21. 9), H. Pylori infection (B96. 81), esophagitis (K20. 9), and PUD (K27. 9), dates of diagnosis, and first diagnosis date.
2.4. Statistical analysis
Prescription patterns of PPIs were assessed based on patients' age and gender, types of PPIs prescribed, duration, dose, PPI prescription indications, and potential DDIs. Categorical data were described using descriptive analysis. Continuous data were expressed as the mean and standard deviation (±SD). A 95 % CI was used to report the precision of the results. The prevalence of potential DDIs among PPI users was expressed in percentage with a 95 % confidence interval. Statistical analysis was performed using version 16 of the STATA statistical software program (StataCorp, College Station, TX, USA).
3. Results
Over the study period (January 2019–June 2022), the total number of PPI prescriptions was 80,365 for a total of 9,930 patients with a mean age of 67.5 (±SD 15.2). The majority of the sample were males (64.2 %), and the majority were outpatients (67 %), followed by inpatients (31 %) and emergency patients (2.1 %) (Table 1). Among the patients on PPIs (n = 9,930), hypomagnesemia (n = 9, 0.1 %) and C. difficile infections (n = 43, 0.4 %) were the most frequently observed long-term adverse events potentially associated with PPI use. Almost 1 % (n = 84) of the patients on PPIs had at least one GI bleeding event (Table 1).
Table 1.
Demographic and clinical characteristics of patients using PPIs (n = 9,930).
| Variable | Mean | ±SD | |
|---|---|---|---|
| Age (years) | 67.5 | 15.2 | |
| Variable | n | % | 95 % CI |
| Age groups | |||
| 18–29 | 232 | 2.3 | 2.05–2.65 |
| 30–44 | 796 | 8 | 7.49–8.57 |
| 45–59 | 2251 | 22.7 | 21.85–23.50 |
| 60–74 | 3615 | 36.4 | 35.46–37.36 |
| >74 | 3036 | 30.6 | 29.67–31.49 |
| Gender | |||
| Female | 3554 | 35.8 | 29.67–31.49 |
| Male | 6376 | 64.2 | 63.26–65.15 |
| Encounter type | |||
| Emergency | 210 | 2.1 | 1.84–2.42 |
| Inpatient | 3100 | 31.2 | 30.31–32.14 |
| Outpatient | 6620 | 66.7 | 65.73–67.59 |
| GI bleeding | |||
| At least one event | 84 | 0.8 | 0.68–1.05 |
| None | 9846 | 99.2 | 98.95–99.32 |
| Clostridium difficile | |||
| At least one event | 43 | 0.4 | 0.31–0.58 |
| None | 9887 | 99.6 | 99.42–99.69 |
| Hypomagnesemia | |||
| At least one event | 9 | 0.1 | 0.04–0.17 |
| None | 9921 | 99.9 | 99.83–99.96 |
*n: total number of observations; CI: confidence interval; SD: standard deviation; GI: gastrointestinal.
The most frequently prescribed PPI was esomeprazole (88 %), followed by omeprazole (12 %). Around 70 % of the prescribed PPIs were orally administered, 63.5 % were administered as tablets, 3.8 % were administered as suspensions, 2.3 % were administered as capsules, and 0.6 % were administered as sachets. About 30 % of the prescribed PPIs were non-orally administered through injections. The mean duration of PPI use was 28 (±SD 61.7) days. The duration of PPI prescription was classified as long-term in 16.97 % of the cases (more than 6 weeks of PPI use), while it was classified as short-term in 83.03 % (less than 6 weeks) (Table 2).
Table 2.
PPIs utilization prescriptions patterns per prescription (n = 80,365).
| Variable | n | % | 95 % CI |
|---|---|---|---|
| Types of prescribed PPIs | |||
| Omeprazole | 9681 | 12.0 | 11.82–12.27 |
| Esomeprazole | 70,684 | 88.0 | 87.73–88.18 |
| Duration of PPIs use | |||
| 7 days or less | 27,265 | 33.9 | 33.60–34.25 |
| >7–21 days | 6495 | 8.1 | 7.89–8.27 |
| >21–42 | 5680 | 7.1 | 6.89–7.24 |
| More than 42 days | 8061 | 10.0 | 9.82–10.24 |
| Not reported | 32,864 | 40.9 | 40.55–41.23 |
| Administration routes | |||
| Oral | 56,446 | 70.2 | 69.92–70.55 |
| Sachet | 515 | 0.6 | 00.59–00.70 |
| Tablet | 51,069 | 63.5 | 63.21–63.88 |
| Suspension | 3016 | 3.8 | 3.62–3.89 |
| Capsule | 1846 | 2.3 | 2.19–2.40 |
| Non-oral (Injection) | 23,919 | 29.8 | 29.45–30.08 |
| PPIs combination use (per prescription time) | |||
| One type only | 80,227 | 99.8 | 99.80–99.86 |
| Combination of PPIs | 138 | 0.17 | 00.14–00.20 |
| Strength of dose | |||
| Low dose | 51 | 0.1 | 00.05–00.08 |
| Standard dose | 20,865 | 26.0 | 25.66–26.27 |
| High dose | 59,449 | 73.9 | 73.67–74.28 |
| PPIs indications | |||
| Peptic ulcer disease | 909 | 1.1 | 1.06–1.21 |
| Gastroesophageal reflux disease | 2217 | 2.8 | 2.65–2.87 |
| H. pylori | 78 | 0.1 | 00.08–00.12 |
| Esophagitis | 370 | 0.5 | 00.41–00.51 |
| Not reported | 76,791 | 95.5 | 95.41–95.69 |
| Drug-drug interactions | |||
| Potential DDIs with PPIs | 24,575 | 30.6 | 30.26–30.90 |
| PPIs with Atorvastatin | 12,006 | 48.9 | 48.23–49.48 |
| PPIs with Clopidogrel | 8387 | 34.1 | 33.54–34.72 |
| Other drugs | 4182 | 17.0 | 16.55–17.49 |
| No potential DDIs with PPIs | 55,790 | 69.4 | 69.10–69.74 |
*n: total number of observations; CI: confidence interval; SD: standard deviation; PPIs: proton pump inhibitors; H. pylori: Helicobacter pylori; DDIs: drug-drug interactions.
Out of the total 80,365 prescriptions, only 3,574 (4.45 %) PPIs were prescribed with an appropriate indication reported. Among these appropriate indications, reflux disease was the most frequently reported indication (n = 2217; 2.8 %), followed by peptic ulcer (n = 909; 1.1 %), esophagitis (n = 370; 0.5 %), and H. pylori infection (n = 78; 0.1 %). PPIs were most often prescribed in a high dose (73.9 %), followed by a standard dose (26 %) (Table 2).
During the study period, a total of 24,575 (33.58 %; 95 % Cl 30.26 – 30.90) potential drug–drug interactions with PPIs were found. The most frequently observed potential drug–drug interaction was between PPIs and atorvastatin (n = 12,006; 48.85 %), followed by clopidogrel (n = 8,387; 34.13 %) (Table 2).
4. Discussion
The use of PPIs has grown in recent decades, which increases concerns about the overuse or inappropriate use of these medications. This study found that, over the last three and a half years, more than 80,000 PPIs were prescribed to 9,930 patients. Studies have found that the PPI prescription rate has increased over the last few years due to the effectiveness of PPIs in multiple treatment areas (Heidelbaugh et al., 2012, Madi et al., 2019). There were more than 35 million prescriptions for omeprazole in the United Kingdom and more than 52 million prescriptions in the US (Shanika et al., 2023).
This study found that the majority of PPI users (67 %) were above 60 years old, which is consistent with other studies' findings as PPI indications are commonly diagnosed among this particular population (Heidelbaugh et al., 2012, Madi et al., 2019). PPIs are common in older populations, and this is because they are at higher risk of gastrointestinal diseases, esophagitis, and other diseases than younger populations (AlMutairi et al., 2018). The use of PPIs may expose patients to an increasing number of risks, especially among the elderly and inpatients who are more vulnerable to multiple comorbidities and polypharmacy (Heidelbaugh et al., 2012, Madi et al., 2019). A meta-analysis of 18 studies showed an increased risk of hip, spine, and any-site fracture among PPI users (Zhou et al., 2016). As for gender, this study found that the prevalence of PPI utilization was higher among males than females (64.2 % vs. 35.8). Other studies found different gender results (Hálfdánarson et al., 2018, Torres-Bondia et al., 2022). These discrepancies in results might be due to the varying prevalence of GI diseases in different communities. Studies found that the prevalence of reflux esophagitis in men increased over a 10-year period, while female rates remained constant (Adachi et al., 2015). Moreover, a population-based study found that being a male was a risk factor for erosive esophagitis and non-erosive reflux disease (NERD) (Kim et al., 2008). Also, this study found that two thirds (66.7 %) of the PPIs were prescribed during outpatient encounters. This might be explained by the fact that PPIs can be initiated by general practitioners (GPs), who can prescribe PPIs as gastroenterologists (Matuz et al., 2020). Prescriptions for diagnoses that are undocumented or unsubstantiated cause overuse in the outpatient context (Heidelbaugh et al., 2012).
Over eighty-thousand prescriptions of PPIs were observed during the study period. Esomeprazole was more prevalent in this study than omeprazole. Although these medications have the same effects, studies found that esomeprazole provides more effective gastric acid control than omeprazole (Lind et al., 2000, Richter et al., 2001, Röhss et al., 2004).
Even though it is required to report the indications of prescriptions according to drug prescription guidelines and the healthcare system, this study highlights that the majority of PPI prescriptions lack indications (95.5 %). Therefore, it is important to assess the reasons behind this issue and improve the practice of recording the prescription information in the health system. Prescriptions without reported indications can impose harm on patients, including a lack of mentoring of patients’ history and mal-use of these medications. Some studies highlighted the need for training health providers on the prescribing guidelines (Ross and Loke 2009). Moreover, it is important to give healthcare providers ample time to deliver the best healthcare as well as the use of technologies and friendly-use health system interfaces to ease and shorten this process as time pressure can influence their adherence to these guidelines (Tsiga et al., 2013).
Almost three quarters of the patients (73.9 %) were prescribed a high dose. High-dose PPI therapy is commonly used to treat patients with ulcers to prevent bleeding. However, it is recommended not to prescribe high doses without regularly monitoring the need for PPI therapy, especially for long-term use (Jarchow-Macdonald and Mangoni, 2013, Chen et al., 2016). Among the reported durations of use, nearly one sixth of the patients (16.97 %) were prescribed PPIs for long-term use. In similar studies, it was found that prolonged and high-dose use of PPIs was associated with adverse risks including C. difficile infection and hypomagnesemia (Heidelbaugh et al., 2009, Wan et al., 2018, Jaynes and Kumar, 2019). However, this study found a small number of patients on PPIs diagnosed with these conditions, including 43 patients with C. difficile infection and 9 patients with hypomagnesemia. Therefore, there is a need to educate healthcare providers in continually assessing patients' need for PPI prescriptions with appropriate indications and using the lowest possible dose for the shortest possible duration to meet patients' therapeutic needs (Jarchow-Macdonald and Mangoni, 2013, Chen et al., 2016).
Among the total PPI prescriptions, a total of 24,575 potential drug–drug interactions with PPIs were found, mostly observed between PPIs and atorvastatin (48.85 %), followed by clopidogrel (34 %). PPIs are commonly prescribed with statins and antiplatelet medications to cardiovascular disease patients for gastroprotection (Tantry et al., 2011, Barkas et al., 2015). However, recent pharmacoepidemiologic studies found that PPIs intervene with the metabolism of these medications by affecting the metabolism of the hepatic enzyme CYP2C19. This can expose patients to health risks that are similarly associated with the overdosing or underdosing of these medications (Supplementary Table 1) (Tantry et al., 2011, Barkas et al., 2015, Cardoso et al., 2015).
This study is subject to retrospective observational study design limitations. These include relying on pre-existing data (secondary data from electronic health records) that are prone to missing, unconnected, and incomplete data. An important piece of missing information for assessing the use of PPIs was whether they were used for stress ulcer prophylaxis prevention. This study excluded any records with the main measurements missing, such as the date of PPI prescriptions; there were very few of these records (n < 20). The temporal relationship is more difficult to determine due to the nature of the study design. Thus, conducting a future prospective cohort study might help in reducing bias and obtaining more precise results.
5. Conclusions
The results of this study were used to explore PPI use, showing that the majority of PPIs were prescribed in high doses without reporting appropriate indications, with some having potential DDIs, which may expose patients to an increasing number of health risks. Therefore, this study highlights the need to implement a stewardship program for PPI prescription and educate healthcare providers on the appropriate utilization of PPIs with periodic reassessment of patients’ needs for these medications to encourage more appropriate PPI prescribing patterns. As awareness of the potential harms increases, it is now more important than ever for healthcare professionals to be vigilant when prescribing PPIs and to ensure that they are only prescribed when there is an appropriate indication for them. Moreover, the study’s findings will help policymakers in assessing the need to improve the current prescribing guidelines and drug safety communication of PPIs with the involvement of healthcare providers.
Ethics approval
Ethical approval was obtained from the King Abdullah International Medical Research Center’s (KAIMRC) Institutional Review Board (Ref. No SP22R/036/04). Very minimal risk as no intervention is involved in the study design. Subjects’ privacy and confidentiality were assured, and no identifiers were collected.
Disclaimer
Conclusions reached in this study are based on the scientific interpretations of the authors and do not necessarily represent the opinion of their institutes.
Funding
No fund was received to conduct this study. Publication fees will be covered by KAIMRC research funding program.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Aljoharah M. Algabbani: Conceptualization, Methodology, Data curation, Writing – original draft, Writing – review & editing. Abdulaziz S. Alangari: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Abeer A. Alqahtani for her role in data cleaning and management and Muhammed Alamri for his help in extracting the requested data.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2023.101841.
Contributor Information
Aljoharah M. Algabbani, Email: aljoharahalgab@gmail.com.
Abdulaziz S. Alangari, Email: angaria@ksau-hs.edu.sa.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Adachi K., Mishiro T., Tanaka S., et al. Gender differences in the time-course changes of reflux esophagitis in Japanese patients. Intern. Med. 2015;54:869–873. doi: 10.2169/internalmedicine.54.4083. [DOI] [PubMed] [Google Scholar]
- Ahmed, Clarke, 2022. Proton Pump Inhibitors (PPI). Retrieved 9.15.22, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK557385/.
- Al-Dosari B.S., Binafeef B.M., Alsolami S.A. Prescribing pattern of proton pump inhibitors among patients admitted to medical ward at King Abdulaziz University Hospital, Jeddah, Saudi Arabia: a retrospective study. Saudi Med. J. 2021;42:1313–1319. doi: 10.15537/smj.2021.42.12.20210488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhossan A., Alrabiah Z., Alghadeer S., et al. Attitude and knowledge of Saudi community pharmacists towards use of proton pump inhibitors. Saudi Pharm J. 2019;27:225–228. doi: 10.1016/j.jsps.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljadani R., Aseeri M. Prevalence of drug-drug interactions in geriatric patients at an ambulatory care pharmacy in a tertiary care teaching hospital. BMC. Res. Notes. 2018;11:234. doi: 10.1186/s13104-018-3342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlMutairi H., O'Dwyer M., McCarron M., et al. The use of proton pump inhibitors among older adults with intellectual disability: a cross sectional observational study. Saudi Pharm. J. 2018;26:1012–1021. doi: 10.1016/j.jsps.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asdaq S.M.B., ALbasha M., Almutairi A., et al. Use of proton pump inhibitors: an exploration of awareness, attitude and behavior of health care professionals of Riyadh, Saudi Arabia. Saudi Pharm. J. 2021;29:713–718. doi: 10.1016/j.jsps.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkas F., Elisaf M., Rizos C.V., et al. Proton pump inhibitors and statins: a possible interaction that favors low-density lipoprotein cholesterol reduction? Hippokratia. 2015;19:332–337. [PMC free article] [PubMed] [Google Scholar]
- Cardoso R.N., Benjo A.M., DiNicolantonio J.J., et al. Incidence of cardiovascular events and gastrointestinal bleeding in patients receiving clopidogrel with and without proton pump inhibitors: an updated meta-analysis. Open Heart. 2015;2:e000248. doi: 10.1136/openhrt-2015-000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Chen Y., Li B. The efficacy and safety of proton-pump inhibitors in treating patients with non-erosive reflux disease: a network meta-analysis. Sci. Rep. 2016;6:32126. doi: 10.1038/srep32126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Interactions Checker. 2021. Drug Interactions Checker. Retrieved 9.15.21, 2021, from https://www.drugs.com/drug_interactions.html.
- Forgacs I., Loganayagam A. Overprescribing proton pump inhibitors. BMJ. 2008;336:2–3. doi: 10.1136/bmj.39406.449456.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjestad C., Westin A.A., Skogvoll E., et al. Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline. Ther. Drug Monit. 2015;37:90–97. doi: 10.1097/ftd.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haastrup P.F., Jarbøl D.E., Thompson W., et al. When does proton pump inhibitor treatment become long term? A Scoping Review. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2020-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hálfdánarson Ó., Pottegård A., Björnsson E.S., et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap. Adv. Gastroenterol. 2018;11 doi: 10.1177/1756284818777943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelbaugh J.J., Goldberg K.L., Inadomi J.M. Adverse risks associated with proton pump inhibitors: a systematic review. Gastroenterol. Hepatol. (n y). 2009;5:725–734. [PMC free article] [PubMed] [Google Scholar]
- Heidelbaugh J.J., Kim A.H., Chang R., et al. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap. Adv. Gastroenterol. 2012;5:219–232. doi: 10.1177/1756283x12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarchow-Macdonald A.A., Mangoni A.A. Prescribing patterns of proton pump inhibitors in older hospitalized patients in a Scottish health board. Geriatr. Gerontol. Int. 2013;13:1002–1009. doi: 10.1111/ggi.12047. [DOI] [PubMed] [Google Scholar]
- Jaynes M., Kumar A.B. The risks of long-term use of proton pump inhibitors: a critical review. Ther. Adv. Drug Saf. 2019;10 doi: 10.1177/2042098618809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel P.W. Pantoprazole: a new proton pump inhibitor. Clin. Ther. 2000;22:1268–1293. doi: 10.1016/s0149-2918(00)83025-8. [DOI] [PubMed] [Google Scholar]
- Kim N., Lee S.W., Cho S.I., et al. The prevalence of and risk factors for erosive oesophagitis and non-erosive reflux disease: a nationwide multicentre prospective study in Korea. Aliment. Pharmacol. Ther. 2008;27:173–185. doi: 10.1111/j.1365-2036.2007.03561.x. [DOI] [PubMed] [Google Scholar]
- Lind T., Rydberg L., Kylebäck A., et al. Esomeprazole provides improved acid control vs. omeprazole In patients with symptoms of gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 2000;14:861–867. doi: 10.1046/j.1365-2036.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- Madi L., Ahmed Elhada A.H., Alrawashdeh H., et al. Prescribing pattern of proton pump inhibitors in Qatar rehabilitation institute: a retrospective study. J Res Pharm Pract. 2019;8:101–104. doi: 10.4103/jrpp.JRPP_18_79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuz M., Benkő R., Engi Z., et al. Use of proton pump inhibitors in Hungary: mixed-method study to reveal scale and characteristics. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, D., Gurney, E., Hornecker, J., 2019. Appropriate Use and Stewardship of Proton-Pump Inhibitors. Retrieved 9.18.22, 2022, from https://www.uspharmacist.com/article/appropriate-use-and-stewardship-of-protonpump-inhibitors.
- Richter J.E., Kahrilas P.J., Johanson J., et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am. J. Gastroenterol. 2001;96:656–665. doi: 10.1016/S0002-9270(00)02393-5. [DOI] [PubMed] [Google Scholar]
- Röhss K., Lind T., Wilder-Smith C. Esomeprazole 40 mg provides more effective intragastric acid control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux symptoms. Eur. J. Clin. Pharmacol. 2004;60:531–539. doi: 10.1007/s00228-004-0804-6. [DOI] [PubMed] [Google Scholar]
- Ross S., Loke Y.K. Training good prescribers: what are the best methods? Clin. Med. (Lond.) 2009;9:478–480. doi: 10.7861/clinmedicine.9-5-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepisi R., Fusco S., Sganga F., et al. Inappropriate use of proton pump inhibitors in elderly patients discharged from acute care hospitals. J. Nutr. Health Aging. 2016;20:665–670. doi: 10.1007/s12603-015-0642-5. [DOI] [PubMed] [Google Scholar]
- SFDA, 2020. Templates for Labelling Information, SPC and PIL. from https://www.sfda.gov.sa/sites/default/files/2020-11/TemplateLabelingSPCandPILv1.pdf.
- Shanika L.G.T., Reynolds A., Pattison S., et al. Proton pump inhibitor use: systematic review of global trends and practices. Eur. J. Clin. Pharmacol. 2023;79:1159–1172. doi: 10.1007/s00228-023-03534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantry U.S., Kereiakes D.J., Gurbel P.A. Clopidogrel and proton pump inhibitors: influence of pharmacological interactions on clinical outcomes and mechanistic explanations. J. Am. Coll. Cardiol. Intv. 2011;4:365–380. doi: 10.1016/j.jcin.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Torres-Bondia F., de Batlle J., Galván L., et al. Evolution of the consumption trend of proton pump inhibitors in the Lleida Health Region between 2002 and 2015. BMC Public Health. 2022;22:818. doi: 10.1186/s12889-022-13217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiga E., Panagopoulou E., Sevdalis N., et al. The influence of time pressure on adherence to guidelines in primary care: an experimental study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan A., Halpape K., Talkhi S.C., et al. Evaluation of prescribing appropriateness and initiatives to improve prescribing of proton pump inhibitors at Vancouver General Hospital. Can. J. Hosp. Pharm. 2018;71:308–315. [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer R.-S., Blume H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf. 2014;37:201–211. doi: 10.1007/s40264-014-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Huang Y., Li H., et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos. Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.