Abstract
Focal segmental glomerulosclerosis (FSGS) is the most common glomerular disorder causing end-stage renal diseases worldwide. Central to the pathogenesis of FSGS is podocyte dysfunction, which is induced by diverse insults. However, the mechanism governing podocyte injury and repair remains largely unexplored. Asparagine endopeptidase (AEP), a lysosomal protease, regulates substrates by residue-specific cleavage or degradation. We identified the increased AEP expression in the primary proteinuria model which was induced by adriamycin (ADR) to mimic human FSGS. In vivo, global AEP knockout mice manifested increased injury-susceptibility of podocytes in ADR-induced nephropathy (ADRN). Podocyte-specific AEP knockout mice exhibited much more severe glomerular lesions and podocyte injury after ADR injection. In contrast, podocyte-specific augmentation of AEP in mice protected against ADRN. In vitro, knockdown and overexpression of AEP in human podocytes revealed the cytoprotection of AEP as a cytoskeleton regulator. Furthermore, transgelin, an actin-binding protein regulating actin dynamics, was cleaved by AEP, and, as a result, removed its actin-binding regulatory domain. The truncated transgelin regulated podocyte actin dynamics and repressed podocyte hypermotility, compared to the native full-length transgelin. Together, our data reveal a link between lysosomal protease AEP and podocyte cytoskeletal homeostasis, which suggests a potential therapeutic role for AEP in proteinuria disease.
Keywords: focal segmental glomerulosclerosis, podocyte injury, lysosomal protease, asparagine endopeptidase, transgelin, cytoskeletal dynamics
Graphical abstract

Zhang and colleagues identify a protective role for the lysosomal protease AEP in ADRN. They report that AEP protects podocytes by cleaving actin-binding protein transgelin to attenuate actin rearrangement. This work unveils a link between lysosomal protease and cytoskeleton regulation in podocytes, suggesting a therapeutic target for proteinuria disease.
Introduction
Focal segmental glomerulosclerosis (FSGS) is the leading glomerular cause of end-stage renal diseases worldwide. FSGS refers to a pathologic symptom that is characterized by progressive glomerular scarring, which is manifested by the obliteration of capillary lumina and increase of glomerular matrix affecting at least one glomerulus and with a high risk of developing chronic kidney disease.1 Proteinuria and podocyte injuries are cardinal features of FSGS. Typically, FSGS has been recognized as the representation of podocytopathies.2 FSGS may be responsive to glucocorticoids while some cases present steroid resistance, and relapses are typical.1 Therefore, it is imperative to unveil the underlying mechanism of podocyte injuries and develop optional therapies to prevent glomerulosclerosis progression.
Generally, cytoskeletal rearrangement is a key mechanism implicated in podocyte dysfunction in nephrotic glomerular kidney diseases.3 Once the actin-based cytoskeleton of podocytes undergoes rearrangement, the sophisticated architecture and physiology that sustain the glomerular filtration barrier are predisposed to destruction. The breakdown of the actin backbone impels podocytes to abnormal hypermotility, which leads to foot processes effacement. The disturbance in actin dynamics weakens the anchoring of podocytes to the glomerular basement membrane or triggers podocyte apoptosis, which results in podocyte detachment, podocyte loss, and ultimately aggravated proteinuria. Hence, the precise regulation of the actin dynamic is essential for podocyte function, which highlights the research of actin-associated protein. Recent efforts identified mutations in gene causing FSGS have underlined a series of proteins with an important role in podocyte function, which are previously classified as actin binding protein or actin-associated protein, as they either interact directly with the cytoskeleton or are involved in regulating cytoskeletal dynamics.3
Transgelin is an actin-binding protein that is named for its potential to induce actin gelation. All transgelin isoforms compose of an N-terminal calmodulin homologous (CH) domain, actin-binding motif, and a C-terminal calmodulin-like domain, which is closely related to its actin-binding activity.4 Emerging evidence demonstrates that transgelin is associated with actin cytoskeleton remodeling and promotes the migration and invasion of cancer stem cells.5 Furthermore, transgelin regulates the Rho GTPase family,6,7 which is the master regulator of podocyte cytoskeleton.8 Although the expression of transgelin in kidney disease has been confirmed by literatures,9,10,11,12 the modification and mechanism of transgelin and its exact role in podocytes remain unclear.
Asparagine endopeptidase (AEP), also known as legumain, is a lysosomal cysteine protease with a high specificity for cleaving asparaginyl bonds.13 Similar to other cysteine proteases, pro-AEP is synthesized as an inactive form and autocatalytically activated in the acidic endolysosomal environment.14 Although AEP is mainly located in endolysosomes, it has also been demonstrated to remain active and functional in the cytoplasm, membrane, and extracellular areas.15,16 Recently, evidence of novel AEP functions and its substrates has been emerging. AEP has showed diverse functions in neurological diseases, cardiovascular diseases, cancer, and immune disorders.17,18,19,20 In kidney, AEP was abundant in proximal tubular cells and was indispensable for normal lysosomal protein degradation in proximal tubules.21 It was demonstrated to contribute to ECM remodeling through degrading matrix components such as fibronectin.22 Besides, the anti-fibrotic role of AEP was observed in unilateral ureteral obstruction model.23 However, the role of AEP in glomerular disorders, especially podocytopathies like FSGS, remains unclear. Therefore, we explored the role of AEP in FSGS to uncover the link between lysosomal proteinase and podocyte physiology.
In this study, we examined the role of AEP in podocytes on the basis of an experimental FSGS model adriamycin-induced nephropathy (ADRN). Global deletion of AEP exacerbated the ADR-induced glomerular dysfunction. More specifically, podocyte-specific AEP knockout was sufficient to worsen podocyte injury and proteinuria in mice. Furthermore, induction of AEP in podocytes rescued the podocyte injury and glomerular lesion. Similar results were also found in podocytes with AEP knockdown or overexpression in vitro. Notably, we found that AEP cleaved transgelin at N150 and removed its C terminus, which regulated the cytoskeletal dynamic in podocytes. The data provide evidence that AEP is protective for podocyte and regulates cytoskeletal dynamic in podocytes by its enzymatic characteristic. Our finding reveals that AEP could be a therapeutic target for podocyte injury.
Results
AEP was upregulated and activated in FSGS
To identify the expression profile of AEP in podocyte pathology, we induced FSGS in mice by ADR injection. By mRNA and Western blot analysis, we observed that AEP expression was increased in ADRN (Figures 1A and 1D–1F). Immunohistochemical staining revealed that AEP was both upregulated in glomeruli and tubules areas (Figures 1B and 1C). Consistently, immunofluorescence labeling with antibody against AEP colocalized with a podocyte marker synaptopodin indicated that it was highly expressed in podocytes in ADRN group compared with the control group (Figure 1G). In vitro, we treated the cultured human podocytes with ADR and puromycin aminonucleoside (PAN), both of which are known as podocyte damage agents and induce proteinuria in vivo.24 By western blot, we observed the increases of pro-AEP as well as the active-AEP after these podocyte-toxic reagents administration (Figures 1H and 1I). Specifically, the upregulation of AEP expression and activation were in a time-dependent manner. The pro-AEP and active-AEP increased synchronously in the podocytes exposed to ADR for 6 h, while in 12-h groups only active-AEP significantly upregulated (Figures 1J and 1K), which suggested the increase of AEP might be an early response to damage. In PAN-treated podocytes, the level of pro-AEP and active-AEP significantly elevated at the 12-h time point, while the active-AEP but not pro-AEP significantly increased in the 24-h groups (Figures 1L and 1M). In addition, to investigate whether AEP could respond to the prosclerotic transforming growth factor (TGF)-β1 signaling, we also exposed the podocytes to TGF-β1 for different time points and demonstrated that the endogenous signal TGF-β1 also induced the increase in AEP in podocytes (Figure S1). These results suggested that AEP was implicated in FSGS. In podocytes, AEP could be upregulated and activated in a comparatively early stage after injury.
Figure 1.
AEP was upregulated in injured podocytes
(A) Relative mRNA level of AEP in the kidney from control balb/c male mice and ADR-induced FSGS balb/c male mice (n = 6). (B) Representative immunohistochemistry images of AEP in the kidney from control (Ctrl) mice and ADRN mice. Scale bar, black 20 μm. (C) Quantification of AEP positive area per glomerulus (20 glomeruli per mouse were analyzed, n = 9 mice per group). (D) Representative western blot and summarized data (E, F) of AEP and nephrin expression in the kidney cortex from Ctrl and ADR mice (n = 9 blots in total). (G) Double immunofluorescence staining of AEP (green) and synaptopodin (red) in glomeruli from Ctrl mice and ADRN mice. Scale bar, 20 μm. (H and I) Representative western blotting of AEP (n = 6 blots in total) in podocytes treated with ADR (0.2 μg/mL) and puromycin (PAN, 25 μg/mL), respectively and the quantification of pro-AEP and active AEP expression (J and K for ADR-treated podocytes; L and M for PAN-treated podocytes). n = 6 per group. ∗p < 0.05, ∗∗p < 0.01. Data are mean ± SEM.
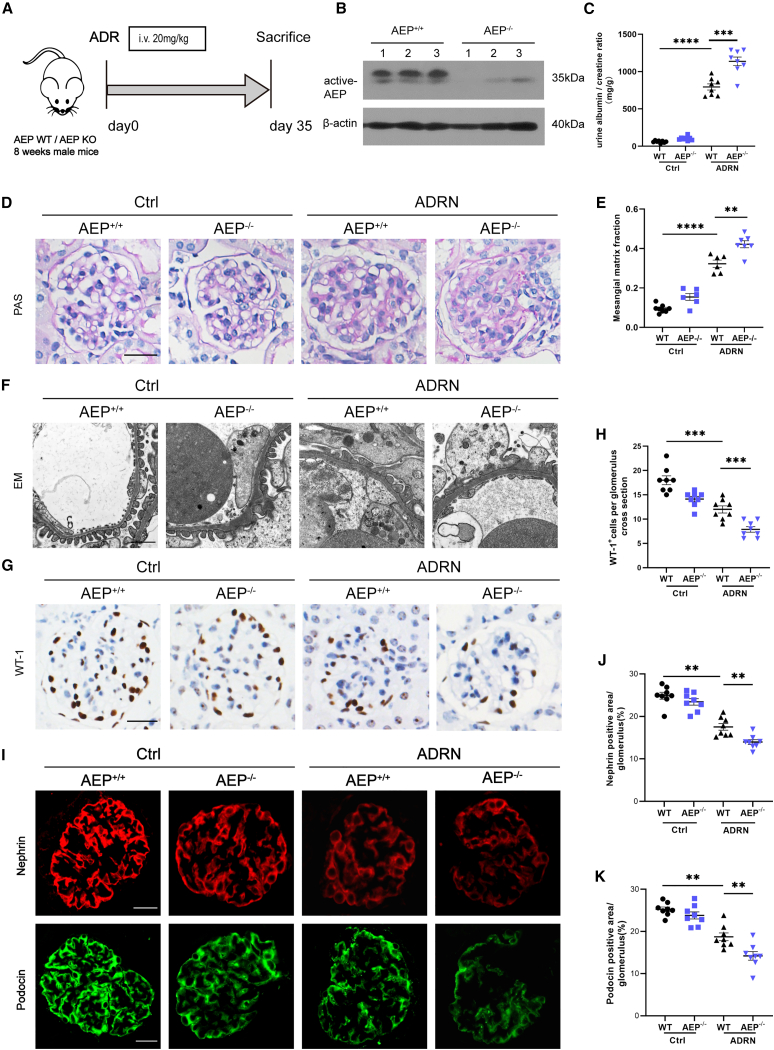
Absence of AEP increased susceptibility of glomerular disorders
We next examined the role of AEP in glomerular function with the global AEP knockout mice (AEP−/−). Groups of mice were treated by tail vein injection of ADR or saline once and were executed at day 35 (Figure 2A). The deletion of AEP in mice had been confirmed by western blot (Figure 2B). After ADR injection, the AEP−/− mice developed more severe proteinuria compared to the AEP-wild-type (WT) (AEP+/+) mice (Figure 2C). Histologically, both ADRN groups showed the development of glomerulosclerosis in comparison to control groups, while the mesangial areas of AEP knockout groups significantly increased (Figures 2D and 2E). Transmission electron microscopic analysis demonstrated that the absence of AEP resulted in aggravated foot process effacement, a characteristic that usually parallels the findings in which the glomerular filtration barrier collapse (Figure 2F). Podocyte dysfunction is a known agitator in the progression of glomerular diseases. To gain a better understanding of the consequence of these structural disruptions on podocyte, we performed WT-1 staining, which is a known podocytes marker. Quantification of the WT-1-positive cells indicated an apparent decline in podocyte number in the AEP−/− group, as compared with the AEP+/+ group (Figures 2G and 2H). Moreover, impaired podocyte function had been corroborated by the reduced nephrin and podocin expression, both of which were the key components of slit diaphragm and podocyte cytoskeleton, and increased desmin expression, which was an indicator of injured podocytes as comparing the AEP−/− with the AEP+/+mice (Figures 2I–2K and S2). Taken together, global AEP knockout mice showed the impaired glomerular function and these findings suggested a protection role of AEP for podocytes.
Figure 2.
KO of AEP exacerbated glomerular lesions in ADR-induced nephropathy
(A) The schematic diagram shows the procedure of ADR-induced FSGS of AEP WT or AEP KO mice. (B) Representative western blotting (n = 6 blots in total) of AEP expression in kidney cortex lysate from AEP+/+ and AEP−/− mice (n = 6). (C) Urine albumin-to-creatinine ratio in different groups mice (n = 8). (D) Representative images for morphological examinations of glomerular changes by Periodic acid-Schiff (PAS) staining. Scale bar, 20 μm. (E) Quantification of mesangial matrix fraction of glomeruli in PAS (20 glomeruli per mouse were analyzed; n = 8 mice per group). (F) Representative transmission electron microscopy (EM) images showing morphological changes in the podocyte foot process in different groups of mice. Scale bar, 1 μm (n = 3). (G) Representative immunohistochemistry images of WT-1 in the kidney section from different groups. Scale bar, 20 μm. (H) Quantification of WT-1 positive cells per glomerulus in the kidney (20 glomeruli per mouse were analyzed, n = 8 mice per group). (I) Representative image for nephrin and podocin staining in the kidney section from different groups, Scale bar, 20 μm. (J and K) Quantification of nephrin, podocin positive area per glomerulus in the kidney sections (20 glomeruli per mouse were analyzed; n = 8 mice per group). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Data are mean ± SEM.
AEP exhibited protection in injured podocytes
Next, we investigated the effect of AEP intervention on podocyte phenotypes and function in cultured human podocytes. Podocytes were infected with lentivirus short hairpin RNA (shRNA) (AEP shRNA) for AEP knockdown, or with adenovirus (AdAEP) for AEP overexpression. The expressions of AEP were confirmed by western blot (Figures 3A–3D). Based on the results of AEP-knockout (KO) mice, we hypothesized that AEP had an effect on podocyte actin cytoskeleton. By phalloidin staining, we observed that AEP knockdown led to F-actin disarrangement and actin filament decrease (Figures 3E and 3F). Secondary examination of the function of podocytes by western blot demonstrated that nephrin and podocin level particularly decreased in AEP shRNA podocytes, compared with the scramble podocytes. In contrast with nephrin and podocin, an elevated desmin expression was determined, suggesting a disruption of podocyte function after AEP knockdown (Figures 3G–3J). In contrast, as the AEP was overexpressed in podocytes, the actin reorganization, which was caused by ADR, was significantly restored (Figures 3K and 3L). Consistently, in the AdAEP podocytes, the nephrin and podocin level had improved partly while the desmin level was partly suppressed, as compared to the AdGFP podocytes under ADR condition (Figures 3M–3P). Together, these findings denoted that AEP was protective for podocytes, which might prevent podocyte injury through stabilizing cytoskeleton.
Figure 3.
AEP knockdown induced podocyte cytoskeleton disarrangement and cell injury and AEP overexpression reversed ADR-induced podocyte injury
(A and B) Gene silencing efficiency of AEP by Western blot in shAEP transfected podocytes (n = 6 blots in total). (C and D) Overexpression of AEP by Western blot in AdAEP transfected podocytes (n = 6 blots in total). (E and F) Representative images of F-actin by rhodamine-phalloidin staining of podocytes. Scale bar, 20 μm. Summarized data from counting the cells with distinct, longitudinal F-actin fibers was shown in (F). Scoring was determined from 100 podocytes on each slide (n = 6). (G–J) Representative western blotting (G) and summarized data (H–J) showing nephrin, podocin and desmin protein levels in podocytes transfected with scrambled shRNA or AEP shRNA (n = 6). (K and L) Representative images of F-actin by rhodamine-phalloidin staining of AdAEP podocytes. Scale bar, 20 μm. Summarized data is shown in (L) (n = 6). (M–P) Representative western blotting (M) and summarized data (N–P) showing nephrin, podocin and desmin protein levels in podocytes transfected with GFP or AEP adenovirus under ADR stimulation conditions (n = 6). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Data are mean ± SEM.
Podocyte-specific AEP KO exacerbated podocyte injury in ADRN
To determine if AEP is specifically involved in ADR-induced podocyte injury in vivo, we generated podocyte-specific AEP KO mice (Cre+/AEP-flox) with the Cre-Loxp recombination system (Figure 4A) and confirmed this by genotyping (Figure S3A). Successful deletion of AEP in Cre+/AEP-flox mice was confirmed by western blotting using isolated glomerulus (Figures 4B and 4C). Meanwhile, immunofluorescence staining showed a significantly decreased colocalization of AEP with the podocyte marker synaptopodin (Figure S3B). There was no significant difference in body weight and kidney weight between Cre+/AEP-flox mice and Cre−/AEP-flox mice (Figure S3C).
Figure 4.
Podocyte-specific AEP deletion aggravated renal injury in ADR-induced nephropathy
(A) Generation of conditional KO mice in which AEP was specifically ablated in podocytes by using Cre-LoxP recombination system. Exons 2 and 3 were deleted upon NPHS2-Cre-mediated recombination. Genotyping was confirmed by tail preparation and PCR at 3 weeks of age. (B and C) Representative Western blot (n = 6 blots in total) and quantification of AEP expression in isolated glomeruli lysate from AEP-flox/Cre- and AEP-flox/Cre+ mice. (D) Urine albumin-to-creatinine ratio in mice (n = 8). (E) Representative images of Periodic acid-Schiff (PAS) staining and electron microscopy (EM) showing glomerular morphological changes in different groups of mice. Scale bar, 20 μm in PAS, 1 μm in EM. (F) Quantification of mesangial matrix fraction of glomeruli in PAS (n = 8). (G) Representative images of nephrin, podocin, desmin, and WT-1 staining in different groups. Scale bar, 20 μm. (H) Quantification of WT1 positive cells per glomerulus in the kidney (20 glomeruli per mouse were analyzed; n = 8 mice per group). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are mean ± SEM.
We next investigated whether reduced AEP expression alone in podocytes imparts proteinuria directly. We found that the ratio of urine albumin to creatine of the Cre+/AEP-flox with ADR mice was significantly higher than that of Cre−/AEP-flox with ADR mice (Figure 4D). In addition, exacerbation of mesangial proliferation and glomerulosclerosis was observed in Cre+/AEP-flox with ADR mice when compared to the Cre−/AEP-flox with ADR mice (Figures 4E and 4F). To investigate whether the apparent albuminuria was caused by damage of the glomerular filtration barrier, we performed electron microcopy analysis and not surprisingly observed the aggravated foot processes effacement in Cre+/AEP-flox with ADR mice (Figure 4E).
Podocytes provide structural support to build the filtration barrier with the slit diaphragm proteins, such as nephrin and podocin.3 As shown in Figure 4G, compared to the Cre−/AEP-flox with ADR mice, the level of nephrin and podocin were suppressed while desmin expression increased in Cre+/AEP-flox with ADR mice, which indicated a phenotypic alteration of podocytes. Consistently, the podocytes number remarkably decreased in Cre+/AEP-flox with ADR mice, which was determined by quantification of WT-1-positive cells (Figures 4G and 4H). These results revealed that AEP KO in podocytes exacerbated podocytes injury and affected the filtration barrier function in the FSGS model.
Augmentation of AEP expression in podocytes in mice alleviated podocyte cytoskeletal disorder
To further explore whether the induction of AEP influences podocyte or not in vivo, we generated the podocyte-specific AEP overexpression mice (cre+/AEP-rosa-flox mice), which were confirmed by tail genotyping (Figures 5A and S4A). Immunofluorescence staining and western blot confirmed the markedly increased expression of AEP in the podocytes and glomeruli (Figures 5B and S4B). Podocyte injury was induced with ADR injection. We observed the augmentation of AEP in glomeruli with immunofluorescence staining (Figure S4B). As compared with the cre-/AEP-rosa-flox group, the cre+/AEP-rosa-flox mice showed lower urinary albumin excretion after ADR injection (Figure 5C). By morphological examination, we confirmed the less glomerular matrix deposition and alleviated ultrastructure changes, compared to their controls (Figures 5D and 5E). Most important, the cytoskeletal markers of podocytes, nephrin, and podocin were strikingly rescued in AEP overexpression group, while the expression of desmin was partly downregulated compared to the control group with ADR injection (Figure 5F). In addition, podocyte-specific AEP overexpression in mice reduced ADR-induced podocyte loss (Figures 5F and 5G). These findings showed a relief of podocyte injury in AEP overexpression mice after ADR injection and indicated that AEP was beneficial for podocyte survival with a causative influence in cytoskeleton balance.
Figure 5.
Podocyte-specific AEP overexpression ameliorated podocyte injury in ADR-induced nephropathy
(A) Generation of conditional gene-edit mice in which AEP was specifically overexpressed in podocytes by using Cre–LoxP recombination system. AEP gene CDS was inserted after Rosa26 exon 1 which was in company with the stop element. The stop element was deleted upon NPHS2-Cre-mediated recombination. Genotyping was confirmed by tail preparation and PCR at 3 weeks of age. (B) Western blot and summarized data of AEP expression in glomeruli from Cre-/AEP-rosa-flox and Cre+/AEP-rosa-flox mice (n = 6). (C) Urine albumin-to-creatinine ratio in mice (n = 8). (D) Representative images of Periodic acid-Schiff (PAS) staining and electron microscopy analysis showing glomerular morphological changes in different groups of mice. Scale bar, 20 μm in the PAS, 1 μm in the EM. (E) Quantification of mesangial matrix fraction of glomeruli in PAS (n = 8). (F) Representative images of nephrin, podocin, desmin, and WT-1 staining in different groups. Scale bar, 20 μm. (G) Quantifications of WT1 per glomerulus in the kidney (20 glomeruli per mouse were analyzed; n = 8 mice per group). Scale bar, 20 μm ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are mean ± SEM.
AEP degraded transgelin
Transgelin is an actin-binding protein that regulates the podocyte cytoskeleton in response to podocyte injury12 and contributes to the development of proteinuria.25 The endogenous interaction of AEP and transgelin was confirmed by co-immunoprecipitation assay, as shown in Figures 6A and 6B. Consistently, we confirmed the interaction of AEP with transgelin in kidney lysate which was disrupted by AEP KO (Figure 6C). To explore the potential role for transgelin of the interaction with a lysosomal protease, we assessed the protein level of full-length transgelin in podocytes with either AEP knockdown or AEP overexpression (Figures 6D–6G). Strikingly, AEP knockdown in podocytes elevated transgelin protein level, while AEP overexpression suppressed transgelin level, suggesting a direct regulatory of AEP in transgelin protein. Accordingly, we hypothesized that the alteration of full-length transgelin level might be caused by proteolysis by the protease AEP. In vivo, compared with AEP+/+ mice, AEP KO mice displayed enhanced transgelin staining, which colocalized with podocyte marker synaptopodin. Similarly, transgelin expression increased in cre+/AEP-flox mice, while the transgelin level was downregulated in cre+/AEP-rosa-flox mice glomeruli (Figures 6H–6K). Together, these data suggest that AEP targets transgelin in podocytes and exerts a proteolytic role on transgelin protein.
Figure 6.
AEP degraded transgelin
(A and B) An endogenous interaction of AEP and transgelin in podocytes was confirmed by co-immunoprecipitation (co-IP), using IgG group as a negative control. (C) An endogenous interaction of AEP and transgelin in kidney lysates from AEP KO and AEP WT mice was confirmed by co-IP. (D and E) Representative western blot and quantification of transgelin in podocytes transfected with scrambled shRNA or AEP shRNA (n = 6). (F and G) Representative western blot and quantification of transgelin in podocytes transfected with AdGFP or AdAEP (n = 6). (H–K) Representative immunofluorescence images of transgelin (green) and synaptopodin (red) in glomeruli from AEP+/+ and AEP−/− mice (the first and second row), AEP-flox/cre- and AEP-flox/cre+ mice (the third and fourth row), cre-/AEP-rosa-flox and cre+/AEP-rosa-flox mice (the fifth and sixth row). Scale bar, 20 μm. The quantification data were shown in (I, J, and K) ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are mean ± SEM.
AEP directly cleaved transgelin at N150 and removed the C-terminal domain of transgelin
According to AEP function as an AEP in diabetic nephropathy,26 we hypothesized that transgelin was the substrate of AEP. To further explore whether AEP proteolytically processes transgelin, we conducted an in vitro cleavage assay that transgelin was co-incubated with kidney lysates prepared from AEP+/+ and AEP−/− mice under pH 7.4 or 6.0. At pH 6.0, AEP displayed high activity in cleaving transgelin, whereas at pH 7.4 AEP was inactive (Figures 7A and 7B). With GST-transgelin plasmids and recombined AEP protein (rAEP), we determined the cleavage of transgelin by active AEP in 293T cells (Figures 7C and 7D). AEP proteins with mutations that abolished the cysteine protease activity of AEP (C189S) were unable to cleave transgelin, indicating AEP is responsible for the transgelin proteolytic cleavage (Figures 7E and 7F). Moreover, a biochemical assay with purified GST-transgelin and rAEP revealed that AEP directly cleaved transgelin independently (Figure 7G). We validated the fragmental transgelin by transgelin N antibody, but not transgelin C antibody, suggesting that AEP had removed transgelin C terminus (Figure 7G). AEP is known to be strictly selective in cleaving asparagine.13 To investigate the cleavage site in transgelin, we compared the amino acid sequences of different species and speculated the 141 and 150 asparagine to be the cleavage site by AEP (Figure 7H). By generating transgelin N141 or N150 point mutant plasmids, we observed that N150A not N141A abolished the cleavage of transgelin by AEP (Figure 7I). Notably, cleavage by AEP removed transgelin C terminus and cut off the actin binding motif, while the transgelin N terminus CH domain remained unaffected (Figure 7H). Hence, we demonstrated that transgelin was a substrate of AEP, which cleaved transgelin at N150 site thereafter removed the C terminus of transgelin.
Figure 7.
AEP cleaved transgelin at N150 site in C terminus
(A) Western blot analysis of the cell lysates of 293T cells overexpressing GST-transgelin, which were incubated with kidney lysate from AEP+/+ or AEP−/− mice at pH 6.0 or pH 7.4 AEP buffer for 30 min, respectively. (B) The enzymatic activities of AEP in different groups in (A) were determined by AEP activity assay. (C) Western blot showing the cleavage of transgelin by recombined AEP at pH 6.0 and pH 7.4. The cleavage fragments were recognized by transgelin antibody and GST-tag antibody. (D) The enzymatic activity of AEP in different groups in (C) were determined using AEP activity assay. (E) Western blot showing the cleavage of transgelin by AEP WT that was abolished by mutant AEP C189S. (F) The enzymatic activity of AEP in different groups in (E) were determined using AEP activity assay. (G) Western blot showing the processing of purified GST-transgelin by recombinant AEP. The AEP-derived transgelin fragments were detected using anti-transgelin N-terminal antibody and anti-transgelin C-terminal antibody. (H) Transgelin amino acid sequence alignment among different species. ABM, actin-binding motif; CLR: C-terminal calponin-like repeat. (I) Cleavage of point mutant transgelin by AEP. The cleavage was analyzed by western blot after GST-transgelin wide-type, N150A and N141A were incubated with active recombined AEP.
Cleavage of transgelin regulated podocyte actin cytoskeleton
To investigate the role of truncated transgelin in cytoskeleton, we generated plasmids expressing different fragmental transgelin (Figure 8A) and transfected them into podocytes in vitro. As AEP removed transgelin C terminus, which functioned as regulator of actin binding for transgelin,4 we hypothesized that the transgelin fragment generated by AEP attenuated podocyte cytoskeleton rearrangement. In support of our understanding of the role of transgelin in podocytes,25 podocytes with full-length transgelin overexpression displayed aggravated injury after ADR administration as the expression of desmin and cleaved caspase 3 (a marker of podocyte apoptosis) were upregulated significantly, and the podocyte marker podocin decreased. However, the injury in podocytes with overexpressed fragment transgelin 1–137 or transgelin 138–175 were restored while transgelin C terminus 176–201 overexpressing had no significant effect (Figure 8B). RhoA belongs to the small GTPase family, whose abnormal upregulation in proteinuria disease was demonstrated to be detrimental to podocyte8 and transgelin was reported to regulate actin dynamic through the RhoA/ROCK pathway in ovarian cancer progression.7 Of interest, the transgelin 1–137 fragment attenuated abnormal RhoA accumulation, while the 138–175 fragment and 176–201 fragment had no significant effect on RhoA expression. These finding suggested that fragmental transgelin had the mitigated podocyto-toxicity compared to its full-length and C terminus-harboring forms. In addition, we observed podocytes F-actin and focal adhesion numbers with immunofluorescence assay. Paxillin is a known marker of focal adhesions in podocytes, which is tightly associated with podocytes actin cytoskeleton and reflects the actin cytoskeleton dynamic. The F-actin within podocytes and the focal adhesions number count by paxillin staining remarkably decreased after ADR administration, indicating a hypermotility status of podocyte, which contributes to actin rearrangement. Surprisingly, podocyte expressing fragmental transgelin showed diverse change in the actin organization. The transgelin full-length groups displayed comparable loss of F-actin in contrast with ADR-vector group. However, after removing the C terminus of transgelin, in the podocytes expressing transgelin 1–137 and 138–175, the restoration of F-actin and paxillin were observed, comparing to the ADR-vector group and full-length group, while isolated C terminus unit displayed no significant amelioration in cytoskeleton rearrangement. Overall, the cleavage of transgelin by AEP, which directly cut off its C-terminal regulatory domain, attenuated cytoskeleton rearrangement.
Figure 8.
Cleavage of transgelin C terminus affected podocytes cytoskeleton
(A) Schematic diagram of transgelin domains and its fragments with different length. (B–F) Western blot showing the expression of desmin, podocin, cleaved caspase 3 and RhoA in podocytes with different fragments overexpression. Summarized data were shown in (C–F) (n = 3). (G–I) Representative immunofluorescence images of F-actin staining and paxillin staining and quantification of F-actin and paxillin (H and I) in podocytes transfected with different plasmids. Scare bar, 20 μm ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Data are mean ± SEM.
Discussion
Over the past two decades, despite research has revealed the molecular basis of podocytopathies (mostly represented by FSGS), the mechanism of effective therapy remains obscure. In the present study, we identify the role of a lysosomal protease AEP in podocytes on the background of a primary podocyte injury model ADRN. The current work provides the first evidence that the deletion of AEP in podocytes in vivo increases the susceptibility of podocytes to damage, leading to more severe glomerular injury. In contrast, as AEP had been induced in podocytes, the glomerular lesion and podocyte damage were attenuated. By in vitro intervention, we confirmed that AEP was reno-protective that specifically improved the viability of podocytes. Furthermore, AEP, as a protease, regulated the actin-binding protein transgelin through cleavage, and thereby affected the podocyte actin dynamic.
One of our findings is that the expression of AEP increased after podocyte injury, which could alleviate podocyte injury. In our study, the systemic AEP KO, conditional podocyte KO, and overexpression mice had almost normal phenotypes at birth, suggesting that AEP does not affect the renal filtration function under physiological conditions, which could be explained by the low expression of AEP in glomeruli under normal conditions. However, after the onset of podocyte injury, induced by exogenous ADR, PAN, or endogenous TGF-β, respectively, the expression of AEP was significantly increased and activated, which indicated that AEP may be a relatively responder and participates in the process of disease. In view of existing theories, the intricated podocyte protease network is an integrative part of the podocyte’s damage response.27 Proteases in podocytes display diverse functions. Lysosomal protease cathepsin L has been delineated to contribute to the development of podocyte injury,28 while cathepsin D seems to have a protective effect.29,30 The current knowledge of AEP has described extensively that it is implicated in neurodegenerative diseases, cardiovascular diseases, aging, senescence, and, of our interest, in kidney homeostasis.16 In kidney, Tan's team reported that AEP could degrade extracellular matrix and reduced renal tubular injury in model like unilateral ureteral obstruction model.23 Considering the main process that lysosome mediated in podocyte pathology is to promote cytoprotection through autophagy,31 the AEP, from the perspective of lysosomal enzyme, is likely to be beneficial. Therefore, we hypothesized that AEP was a protective enzyme that was responsible for maintaining homeostasis and promoted repair after injury. As expected, in our ADRN experimental model, AEP KO aggravated glomerular injury, while the overexpression of AEP alleviated the damage, consistently in vivo and vitro. These results suggest that AEP functions as a disease defender, which is similar to cathepsin D. Podocytes apoptosis, anoikis, or detachment may lead to decreased podocyte number, failing to maintain the integrity of glomerular basement membrane and this could result in massive protein leakage into primary filtrate, developing pathological conditions such as FSGS.3 Based on morphological results, AEP could reduce the loss of podocytes caused by ADR, further indicating that AEP can improve the resistance of podocytes to injury, maintain the function and structure of podocytes. Once we confirmed AEP was a protective part of cellular response to stimuli, its increased expression after the onset of disease seems to be contradictory. In the process of the disease, the simultaneous action of multiple enzymes and molecules makes the balance of the cell tilt to the injury state. The role of AEP shows relatively insufficient, which also explains why overexpression of AEP can only partially improve the damage, not completely reversed it, which shows the complexity of the disease.
In contrast, we identified that the protective effect of AEP was due to maintaining the cytoskeleton homeostasis of podocytes. This is concluded by observing the important actin-related proteins of podocytes, which include nephrin, podocin, and desmin, as well as the phalloidin staining of podocytes. Mounting evidence demonstrated a strong correlation between AEP and cytoskeleton regulation. For example, AEP cleaves tau, which is physically regulating microtubules polymerization, thereby changes the tau character, and results in neuron pathology.20 Remarkably, actin dynamic is also regulated by AEP through gamma adducin and tropomodulin-3, both of which are actin-binding proteins and change their characters after AEP processing.17,32 In this study, we found that when AEP was interfered in podocytes in vivo and in vitro, the expression of its essential structural molecules nephrin and podocin were positively correlated with AEP, and F-actin also visually changed, compared to the control groups. Podocytes cytoskeleton reflects their unique function and morphology, and is commonly the final target of multiple hits.3 Moreover, the defect of lysosomal protease cathepsin D has also been confirmed to affect the cytoskeleton of neurons,29 suggesting our hypothesis that AEP regulates the podocyte cytoskeleton is reasonable. To date, there is little evidence regarding how lysosomal enzymes participate in the regulation of podocyte cytoskeleton. Our research has enriched the knowledge of this field.
Specifically, how does AEP regulate the podocyte cytoskeleton? We identified that AEP could cleave transgelin, which is an actin-binding protein. By performing the proteomic analyses of AEP-KO and AEP-WT mice tissues, we focused on transgelin (data not shown). First of all, through co-immunoprecipitation and immunofluorescence experiments, we determined a correlation between AEP and full-length transgelin in podocytes and confirmed AEP as an upstream regulator within the regulatory unit. In view of the enzymatic characteristics of AEP, AEP may directly degrade the full length transgelin. Next, in vitro cleavage assay confirmed our hypothesis, and we identified the cleave site of AEP within transgelin through site mutation experiments. In fact, TGF-β may trigger podocyte injury by transgelin expression during the development of diseases. The transcription of transgelin has been demonstrated to be induced by the TGF-β/Smad3 pathway.33 And TGF-β signaling is the most important participant that mediates the progression of glomerular diseases.2 Namely, AEP might block this pathway by cleaving transgelin protein. Our results showed that AEP protectively degraded transgelin and alleviated cytoskeleton rearrangement to maintain the homeostasis within podocytes. Overall, these findings provide evidence for the direct relationship between the lysosomal protease AEP and podocyte cytoskeletal regulatory protein, and indicate that podocyte has mobilized functional enzyme through its self-repair mechanism probably to adjust the actin cytoskeleton through cleaving actin-binding proteins, such as transgelin, to resist the attack, which is reminiscent of the effect of cathepsin D in previous studies.30 In contrast with our results, it has been reported that lysosomal protease cathepsin L can process GTPase dynamin and podocyte actin-associated protein synaptopodin, threatening the actin cytoskeleton and aggravating cell damage.28 This reflects the diversity of enzymes in podocytes and is also suggestive of a complicated regulatory network of cytoskeleton.3,27,34
Furthermore, after cleavage by AEP, the C-terminal domain of transgelin was removed, probably changing the binding affinity of transgelin to actin. Accordingly, the C-terminal domain of transgelin is the regulatory unit for its binding ability with actin. After removing the C-terminal domain, the affinity of transgelin with actin decreases.4,6,35 Podocytes were transfected with transgelin or its fragments and exposed to ADR to induce damage. Phalloidin and paxillin staining showed that the number of actin filaments and focal adhesions in podocytes with fragmental transgelin expression were restored, which indicated that the architecture of podocytes had partly recovered and showed the performance of reducing cell migration and deformation.36,37,38,39 After injury caused by diverse cues, the cytoskeleton undergoes rearrangement, perhaps underlining podocytes motility change, leading to protein leakage and glomerular disease. The current consensus points out that any factor that causes the cytoskeleton to be either too tight or too loose might pose a threat to the survival of podocytes.40 For example, mutation of ACTN4 exposes a hidden actin binding site, which makes it more tightly bound to the actin filaments, resulting in the actin cytoskeleton becoming too tight and rigid, eventually causing dysfunction.41 According to the results, we proposed that the binding ability of transgelin that lost the C-terminal domain to actin was weakened. This blocked the cytoskeleton rearrangement, thereby the anchoring of foot process was enhanced, which prevented podocyte from migrating, presenting podocytes a more stable state. In addition, transgelins has been determined to be involved in the regulation of RhoA/ROCK,5,6,7,35,42 and the role of Rho family in the regulation of podocyte cytoskeleton has been illustrated by a large number of literatures.43 These findings support our results that the fragmentation of transgelin may attenuate RhoA accumulation in podocytes. The detail mechanism underlying the effect of truncated transgelin on Rho family needs to be further studied.
The experiment on mechanism has not been further explored in animals because of the limitation of conditions, which is a limitation of this study. We will improve this factor in future research. In addition, AEP enzyme not only plays a role in lysosome, but also plays a role in nucleus, cytoplasm and even extracellular, the next research should also focus on the role of AEP in different parts.
Collectively, our research proposes a lysosomal protease AEP, which plays a protective role in glomerular podocytes and can delay the process of primary FSGS to a certain extent. These data reveal that AEP participates in the regulation of podocyte cytoskeleton rearrangement by cleaving the actin-binding protein transgelin. Our findings enrich the knowledge of AEP substrate library in kidney. Of note, podocyte proteases regulation is highly delicate and this will complement ongoing efforts to explore podocyte repair function and provide a new direction for seeking effective treatment for kidney disease.
Materials and methods
Animals
C57BL/6 J male mice that were 7 weeks old were obtained from Charles River Laboratories (Beijing, China). AEP global KO mice were provided by Dr. Keqiang Ye (Emory University School of Medicine, Atlanta, GA, USA). All animals were raised in a pathogen-free environment with a 12-h light and dark cycle and allowed free access to water and standard chow. The mice numbers of animal experiments were according to our previous study. Mice with poor physical condition before grouping and mice that died during the experiment were excluded. We used a random number method for random allocation and were blinded to allocate animals and assess the results during the experiments. All animal studies were reviewed and approved by the Ethics Committee of Huazhong University of Science and Technology.
Generation of podocyte-specific AEP KO mice
AEPfl/fl mice (C57BL/6J) were generated by Beijing Viewsolid Biotechnology Company, Ltd. (Beijing, China). In these mice, AEP exons 2 and 3 were flanked by loxP sequences. AEPfl/fl mice (C57BL/6J) were hybridized with Podocin-Cre mice (Jackson Laboratories) to generate podocyte-specific AEP-KO mice (Nphs2-Cre+/AEPfl/fl mice, simplifed as cre+/AEP-flox). Genotyping was confirmed by tail DNA and PCR at 4 weeks of age with primers: flox genotyping primers (forward5′-CAATTCTCAGCAACCACATG-3′; reverse 5′-GATCAGAACAATCTCCTAAACTG-3′) and Cre genotyping primers (forward 5′-GCGCTGCTGCTCCAG-3′; reverse 5′- CGGTTAT TCAACTTGCACCA-3′). WT presented only a 201-bp band; homozygous (AEPfl/fl) presented only a 235-bp band; heterozygous (AEPfl/+) presented both bands. Cre positive (Cre+) presented at 100 bp, but Cre negative (Cre-) had no band. The Cre negative mice (homozygous for the floxed AEP allele without Podocin-Cre) were used as control group.
Generation of podocyte-specific AEP overexpression mice
AEP (Rosa26)fl/fl mice (C57BL/6J) were generated by Beijing Viewsolid Biotechnology Company, Ltd. (Beijing, China). In these mice, conditional regulatory read framework Promoter-Floxp-Stop-Floxp-AEP gene were knocked in between Rosa26 exon1 and exon2. AEP (Rosa26)fl/fl mice (C57BL/6J) were hybridized with Podocin-Cre mice (Jackson Laboratories) to generate podocyte-specific AEP overexpression mice (Nphs2-Cre+/AEP (Rosa26)fl/fl mice, simplified as cre+/AEP-rosa-flox). Genotyping was confirmed by tail DNA and PCR at 4 weeks of age with primers: flox genotyping primers (forward 5′-CCTCGTCGTCTGATTGGCTCTC-3′; reverse 5′-GGACAGGATAAGTATGACATCATCA-3′) and Cre genotyping primers (forward 5′-GCGCTGCTGCTCCAG-3′; reverse 5′- CGGTTAT TCAACTTGCACCA-3′). WT presented only a 300-bp band, homozygous (AEPfl/fl) presented only a 635-bp band, heterozygous (AEPfl/+) presented both bands. Cre positive (Cre+) presented at 100 bp, but Cre negative (Cre-) had no band. The Cre negative mice (homozygous for the floxed AEP allele without Podocin-Cre) were used as a control group.
ADR nephropathy
In the ADR model, which is analogous to human FSGS, ADR was purchased from Aladdin (Shanghai, China). Adult male mice (8 weeks of age with Balb/C background) weighing 21–24 g were obtained from Charles River Laboratories. Mice were administered with ADR (15 mg/kg) intravenously by tail vein injection. For C57BL/6J background mice, high dose of ADR for 20 mg/kg were applied. At the end of the study, blood and urine samples were collected for biochemical testing. Simultaneously, the mice were euthanized and the kidney tissue samples were harvested for histopathological analysis.
Glomeruli isolation
To identify the target protein expression profile, mouse glomeruli were isolated as described previously.44 Mice were anesthetized and perfused slowly with inactivated Dynabeads M-450 (2 × 107 beads/mouse, M-450; Invitrogen, Carlsbad, CA, USA) diluted in PBS through renal intravenous injection. The kidneys were removed and the cortex, medulla, and papilla were separated by dissection. The cortex was minced into 1-mm3 pieces and digested in collagenase (Sigma-Aldrich, Cat No. C6885) at 37°C for 15 min. Then, the tissue was pressed gently through a 100-μm cell strainer and suspended by ice-cold PBS, and the cell suspension was centrifuged at 1,500 rpm at 4°C for 5 min for collecting the pellet. The pellet was resuspended in PBS, and the glomeruli containing Dynabeads were gathered using a magnetic particle concentrator and washed at least three times with PBS. The purity of glomeruli was estimated by using a light microscope to inspect glomeruli suspensions on glass slides.
Urine albumin and creatinine measurements
Urine creatinine levels were measured using an auto-chemistry analyzer according to the creatinine assay kit. Urine albumin was detected using an ELISA kit (Abcam, Cambridge, UK; Cat No. ab108792) according to the manufacturer’s instructions. The urine albumin excretion rate was expressed as the ratio of albumin to creatinine.
RNA isolation and quantitative RT-PCR
Total RNAs were isolated from cultured cells and mouse glomeruli with FastPure Cell/Tissue Total RNA Isolation Kit (RC112-01, Vazyme, Nanjing) according to directions of the manufacturer. Quantitative RT-PCR (qRT-PCR) (Q311-02, Vazyme, Nanjing, China) was conducted with the StepOne Plus qRT-PCR System (Thermo Fischer Scientific, Waltham, MA, USA). The specific primers sequences used in this study are listed: Lgmn F: GCTGATGGTCCCAGTGAATA, R: CGTTACAGTGGAGGGATAGTTAG; synpo: F: ATGGAGGGGTACTCAGAGGAG, R: CTCTCGGTTTTGGGACAGGTG; desmin: F: GACGTGGATGCAGCTACTCTA; R: GGAACGCGATCTCCTCGTTG.
Cell culture and treatments
A conditionally immortalized human podocyte cell line was cultured and maintained as described previously.45 In brief, podocytes were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin at 33°C for proliferation. Upon reaching appropriate confluence, the cells were maintained at 37°C for 10–14 days to induce differentiation. Differentiated podocytes were exposed to different stimuli as follows: ADR (0.2 μg/mL) and PAN (25 μg/mL; Abcam). HEK293T cells were cultured for plasmid transfection experiments in DMEM containing 10% fetal bovine serum. The cultured cells were regularly screened for mycoplasma contamination by our research group.
RNA interference and gene overexpression of podocyte
A lentivirus vector harboring a shRNA sequence targeting AEP was synthesized by Jikai Gene (Shanghai, China), and scrambled shRNA was used as a control. Adenovirus vectors containing the DNA target sequence for AEP were obtained from Vigene Biosciences (Shandong, China). Podocytes were transiently transfected with lentivirus or adenovirus according to the manufacturer’s instructions, to interfere with AEP expression or to induce AEP. The overexpression of transgelin full length and transgelin 1–137, 138–175, and 176–201 fragment were synthesized by HanBio (Shanghai, China).
In vitro transgelin cleavage assay and AEP activity assay
To assess the cleavage of transgelin by AEP in vitro, HEK293T cells were transfected with GST-tagged transgelin plasmids using Lipofectamine 2000 (Invitrogen; Cat No. 11668019). Forty-eight hours after transfection, the cells were collected, washed with PBS, lysed in lysis buffer (Beyotime Biotechnology, Shanghai, China; Cat No. P0013), and centrifuged for 15 min at 12,000 rpm at 4°C to obtain the supernatant. Then, the supernatant was incubated with recombinant AEP in the indicated pH buffer at 37°C for 30 min. In addition, cells were co-transfected GST-transgelin with WT AEP or mutant AEP (C189S) with abolished cysteine protease activity, and the supernatant was incubated at pH 6.0. To measure the cleavage of purified transgelin by AEP, GST-tagged protein was purified with glutathione beads (Invitrogen; Cat No. G2879) according to the manufacturer’s instructions. The purified transgelin was incubated with recombinant AEP. To test the effect of endogenous AEP on the cleavage of transgelin, the supernatant was incubated with mouse kidney lysates at pH 7.4 or 6.0 at 37°C for 30 min. The samples were then boiled in 1× SDS loading buffer and analyzed using immunoblotting. To identify the transgelin cleavage site by AEP, the recombinant AEP incubated purified transgelin point mutant fragment, the indicated asparagine site of the transgelin plasmid was mutated to alanine to validate the cleavage.
Western blot
The total protein of cultured cells and isolated renal glomeruli were extracted with RIPA lysis buffer (Beyotime). The total protein lysates were measured by a BCA protein assay kit (Beyotime). After being boiled for 5 min at 95°C in SDS protein-loading buffer, proteins were separated by SDS-PAGE and transferred onto polinylvidene fluoride membranes (Millipore Corp., Bedford, MA, USA). The membranes were blocked in 5% non-fat milk for 1 h at room temperature and then incubated with primary antibodies overnight at 4°C. The following primary antibodies were used in this study: AEP (R&D; Cat No. AF2199, 1:1,000); Nephrin (Abcam; 1:1,000); Podocin (Sigma-Aldrich; P0372, 1:1,000); Desmin (Bioworld Technology; Cat No. BS1712, 1:1,000); cleaved Caspase 3 (Cell Signaling; 1:1,000); GST (Protein Tech Group; Cat No. 10000-0-AP, 1:3,000); full-length transgelin (Protein Tech Group;10493-1-AP); transgelin N (Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-271719,1:1000); transgelin C (Sigma; ZEB1211,1:1000); RhoA (Protein Tech Group; 10749-1-AP); and β-actin (Protein Tech Group; Cat No. 66031-1-Ig, 1:3000).
Co-immunoprecipitation
The whole-cell lysates were prepared just as described above. Then equal amounts (1 mg) of total protein samples from three groups (control, HG, and IgG) were incubated with 1 μg primary antibody overnight at 4°C on a rotating device. The next day, 30 μL Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) that had been washed with ice-cold PBS and centrifuged at 2,500 rpm for 5 min at 4°C to remove supernatant, was then added to the mixture and rotated for 2 h at 4°C. The pellets were collected by centrifugation at 2,500 rpm for 5 min at 4°C and washed three times with 1.0 mL PBS for 5 min each time on a rotating device. Then the pellets were resuspended in 2× SDS sample buffer and boiled in 98°C for precipitated proteins were 5 min. The immunodetected by western blotting analysis.
Immunohistochemistry
Kidney tissues were fixed using 4% paraformaldehyde and embedded in paraffin. Four-micrometer sections were deparaffinized and rehydrated for immunohistochemical staining. Briefly, endogenous peroxidase was blocked with 3% H2O2 for 30 min at room temperature, sections were then blocked in 5% BSA in PBS for 1 h at room temperature and then incubated with the primary antibody for AEP (Santa Cruz Biotechnology; Cat No. sc-133234, 1:50), WT1 (Abcam; Cat No. ab89901, 1:100) overnight at 4°C. The next day, sections were washed three times with PBS and then incubated with secondary antibody for 60 min, sections were washed in PBS. Finally, sections were counterstained with hematoxylin before the inspection. For quantification of WT1 positive cells, at least 20 glomeruli sections per slide were counted.
Immunofluorescence staining
The fixed kidneys were frozen at −80°C. Frozen sections (3 μm) were blocked with 5% donkey serum and incubated with primary antibodies and developed with secondary antibodies. The following primary antibodies were used: AEP (Santa Cruz; Cat No. sc-133234, 1:50); Synaptopodin (Synaptic Systems; Cat No. 163004, 1:200); Nephrin (R&D; Cat No. AF3159, 1:100); Podocin (Sigma-Aldrich; P0372, 1:100); Desmin (Bioworld Technology; Cat No. BS1712, 1:100); transgelin (Santa Crus Biotechnology; 1:100). The secondary antibodies were Alexa Fluor 488 IgG (Invitrogen; Cat No. S11223, 1:200) and Alexa Fluor 594 IgG (Invitrogen; Cat No. A10438, 1:200). Nuclear was counterstained with Hoechst (Beyotime Biotechnology; Cat No. C1011).
F-Actin staining
To assess podocyte cytoskeleton arrangement, podocytes were cultured on glass coverslips and subjected to indicated treatments. Then, the cells were fixed using 4% paraformaldehyde solution for 30 min and incubated with rhodamine-phalloidin (Sigma-Aldrich; Cat No. P1951) in PBS containing 5% donkey serum and 0.1% Triton X-100 for 60 min at room temperature before observation. Fifty cells were counted to calculate the ratio of cells retaining distinct F-actin fibers in different groups.
Transmission electron microscopy
Tissues were collected and fixed with 2.5% glutaraldehyde at 4°C. Electron microscopic sample handling and detection were performed using an electron microscopic core as described in our previous studies.26
Morphological examination
PAS staining of 4-μm paraffin sections was used to examine the mesangial composition in the glomeruli. Mesangial and glomerular cross-sectional areas were quantified using image-pro plus 6.0 software (Media Cybernetics, Rockville, MD, USA) and a minimum of 20 glomeruli per section were measured.
Statistics
All experiments in this study were repeated at least three times and representative experiments are shown. The results are reported as the mean ± SEM. Normally distributed data were analyzed and graphed by GraphPad Prism software (GraphPad, San Diego, CA, USA). The Student t-test was used to compare two experimental groups, and all tests were two tailed, and a p value of <0.05 was considered statistically significant.
Data and code availability
The authors declare that all relevant data of this study are available within the article or from the corresponding author upon reasonable request.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China grants 81974096, 81961138007, 81770711.
Author contributions
C.Z. and H.S. conceived and supervised the study. Y.Q., J.-Y.Z., Y.-R.X, Y.-L.C., L.-L.G., and X.-Y.C performed the experiments. C.-T.L. analyzed the data. Q.Y. revised a draft of manuscript. Y.Q designed the study and wrote the paper. Z.-T.Z. provided expertise.
Declaration of interests
All authors declare no conflict of interest.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.09.003.
Supplemental information
References
- 1.Rosenberg A.Z., Kopp J.B. Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2017;12:502–517. doi: 10.2215/CJN.05960616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fogo A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol. 2015;11:76–87. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perico L., Conti S., Benigni A., Remuzzi G. Podocyte-actin dynamics in health and disease. Nat. Rev. Nephrol. 2016;12:692–710. doi: 10.1038/nrneph.2016.127. [DOI] [PubMed] [Google Scholar]
- 4.Fu Y., Liu H.W., Forsythe S.M., Kogut P., McConville J.F., Halayko A.J., Camoretti-Mercado B., Solway J. Mutagenesis analysis of human SM22: characterization of actin binding. J. Appl. Physiol. 2000;89:1985–1990. doi: 10.1152/jappl.2000.89.5.1985. [DOI] [PubMed] [Google Scholar]
- 5.Liu J., Zhang Y., Li Q., Wang Y. Transgelins: Cytoskeletal Associated Proteins Implicated in the Metastasis of Colorectal Cancer. Front. Cell Dev. Biol. 2020;8:573859. doi: 10.3389/fcell.2020.573859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H.R., Kwon M.S., Lee S., Mun Y., Lee K.S., Kim C.H., Na B.R., Kim B.N.R., Piragyte I., Lee H.S., et al. TAGLN2 polymerizes G-actin in a low ionic state but blocks Arp2/3-nucleated actin branching in physiological conditions. Sci. Rep. 2018;8:5503. doi: 10.1038/s41598-018-23816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei X., Lou H., Zhou D., Jia Y., Li H., Huang Q., Ma J., Yang Z., Sun C., Meng Y., et al. TAGLN mediated stiffness-regulated ovarian cancer progression via RhoA/ROCK pathway. J. Exp. Clin. Cancer Res. 2021;40:292. doi: 10.1186/s13046-021-02091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda J., Asano-Matsuda K., Kitzler T.M., Takano T. Rho GTPase regulatory proteins in podocytes. Kidney Int. 2021;99:336–345. doi: 10.1016/j.kint.2020.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Daniel C., Lüdke A., Wagner A., Todorov V.T., Hohenstein B., Hugo C. Transgelin is a marker of repopulating mesangial cells after injury and promotes their proliferation and migration. Lab. Invest. 2012;92:812–826. doi: 10.1038/labinvest.2012.63. [DOI] [PubMed] [Google Scholar]
- 10.Marshall C.B., Krofft R.D., Blonski M.J., Kowalewska J., Logar C.M., Pippin J.W., Kim F., Feil R., Alpers C.E., Shankland S.J. Role of smooth muscle protein SM22alpha in glomerular epithelial cell injury. Am. J. Physiol. Ren. Physiol. 2011;300:F1026–F1042. doi: 10.1152/ajprenal.00187.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito S., Pippin J.W., Shankland S.J. The glomerular parietal epithelial cell's responses are influenced by SM22 alpha levels. BMC Nephrol. 2014;15:174. doi: 10.1186/1471-2369-15-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamaki Y., Sakatsume M., Wang X., Inomata S., Yamamoto T., Gejyo F., Narita I. Injured kidney cells express SM22alpha (transgelin): Unique features distinct from alpha-smooth muscle actin (alphaSMA) Nephrology (Carlton) 2011;16:211–218. doi: 10.1111/j.1440-1797.2010.01322.x. [DOI] [PubMed] [Google Scholar]
- 13.Dall E., Brandstetter H. Mechanistic and structural studies on legumain explain its zymogenicity, distinct activation pathways, and regulation. Proc. Natl. Acad. Sci. USA. 2013;110:10940–10945. doi: 10.1073/pnas.1300686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D.N., Matthews S.P., Antoniou A.N., Mazzeo D., Watts C. Multistep autoactivation of asparaginyl endopeptidase in vitro and in vivo. J. Biol. Chem. 2003;278:38980–38990. doi: 10.1074/jbc.M305930200. [DOI] [PubMed] [Google Scholar]
- 15.Lunde N.N., Bosnjak T., Solberg R., Johansen H.T. Mammalian legumain - A lysosomal cysteine protease with extracellular functions? Biochimie. 2019;166:77–83. doi: 10.1016/j.biochi.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Solberg R., Lunde N.N., Forbord K.M., Okla M., Kassem M., Jafari A. The Mammalian Cysteine Protease Legumain in Health and Disease. Int. J. Mol. Sci. 2022;23:15983. doi: 10.3390/ijms232415983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B., Wang M., Qiu J., Liao K., Zhang W., Lv Q., Ma C., Qian Z., Shi Z., Liang R., et al. Cleavage of tropomodulin-3 by asparagine endopeptidase promotes cancer malignancy by actin remodeling and SND1/RhoA signaling. J. Exp. Clin. Cancer Res. 2022;41:209. doi: 10.1186/s13046-022-02411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia D., Chen S., Bai P., Luo C., Liu J., Sun A., Ge J. Cardiac Resident Macrophage-Derived Legumain Improves Cardiac Repair by Promoting Clearance and Degradation of Apoptotic Cardiomyocytes After Myocardial Infarction. Circulation. 2022;145:1542–1556. doi: 10.1161/CIRCULATIONAHA.121.057549. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X., Zou L., Meng L., Xiong M., Pan L., Chen G., Zheng Y., Xiong J., Wang Z., Duong D.M., et al. Amphiphysin I cleavage by asparagine endopeptidase leads to tau hyperphosphorylation and synaptic dysfunction. Elife. 2021;10:e65301. doi: 10.7554/eLife.65301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z., Song M., Liu X., Kang S.S., Kwon I.S., Duong D.M., Seyfried N.T., Hu W.T., Liu Z., Wang J.Z., et al. Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer's disease. Nat. Med. 2014;20:1254–1262. doi: 10.1038/nm.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller G., Matthews S.P., Reinheckel T., Fleming S., Watts C. Asparagine endopeptidase is required for normal kidney physiology and homeostasis. FASEB J. 2011;25:1606–1617. doi: 10.1096/fj.10-172312. [DOI] [PubMed] [Google Scholar]
- 22.Morita Y., Araki H., Sugimoto T., Takeuchi K., Yamane T., Maeda T., Yamamoto Y., Nishi K., Asano M., Shirahama-Noda K., et al. Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett. 2007;581:1417–1424. doi: 10.1016/j.febslet.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 23.Wang D., Xiong M., Chen C., Du L., Liu Z., Shi Y., Zhang M., Gong J., Song X., Xiang R., et al. Legumain, an asparaginyl endopeptidase, mediates the effect of M2 macrophages on attenuating renal interstitial fibrosis in obstructive nephropathy. Kidney Int. 2018;94:91–101. doi: 10.1016/j.kint.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 24.D'Agati V.D. Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts) Kidney Int. 2008;73:399–406. doi: 10.1038/sj.ki.5002655. [DOI] [PubMed] [Google Scholar]
- 25.Ding Y., Diao Z., Cui H., Yang A., Liu W., Jiang L. Bioinformatics analysis reveals the roles of cytoskeleton protein transgelin in occurrence and development of proteinuria. Transl. Pediatr. 2021;10:2250–2268. doi: 10.21037/tp-21-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei C., Li M., Qiu Y., Xie Y., Hao Z., Yin X., Zhang Z., Su H., Yang L., Lin J., et al. Asparaginyl endopeptidase protects against podocyte injury in diabetic nephropathy through cleaving cofilin-1. Cell Death Dis. 2022;13:184. doi: 10.1038/s41419-022-04621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinschen M.M., Huesgen P.F., Koch R.E. The podocyte protease web: uncovering the gatekeepers of glomerular disease. Am. J. Physiol. Ren. Physiol. 2018;315:F1812–F1816. doi: 10.1152/ajprenal.00380.2018. [DOI] [PubMed] [Google Scholar]
- 28.Garsen M., Rops A.L.W.M.M., Dijkman H., Willemsen B., van Kuppevelt T.H., Russel F.G., Rabelink T.J., Berden J.H.M., Reinheckel T., van der Vlag J. Cathepsin L is crucial for the development of early experimental diabetic nephropathy. Kidney Int. 2016;90:1012–1022. doi: 10.1016/j.kint.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Koch S., Scifo E., Rokka A., Trippner P., Lindfors M., Korhonen R., Corthals G.L., Virtanen I., Lalowski M., Tyynelä J. Cathepsin D deficiency induces cytoskeletal changes and affects cell migration pathways in the brain. Neurobiol. Dis. 2013;50:107–119. doi: 10.1016/j.nbd.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto-Nonaka K., Koike M., Asanuma K., Takagi M., Oliva Trejo J.A., Seki T., Hidaka T., Ichimura K., Sakai T., Tada N., et al. Cathepsin D in Podocytes Is Important in the Pathogenesis of Proteinuria and CKD. J. Am. Soc. Nephrol. 2016;27:2685–2700. doi: 10.1681/ASN.2015040366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fougeray S., Pallet N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat. Rev. Nephrol. 2015;11:34–45. doi: 10.1038/nrneph.2014.201. [DOI] [PubMed] [Google Scholar]
- 32.Xiong M., Zou L., Meng L., Zhang X., Tian Y., Zhang G., Yang J., Chen G., Xiong J., Ye K., Zhang Z. A gamma-adducin cleavage fragment induces neurite deficits and synaptic dysfunction in Alzheimer's disease. Prog. Neurobiol. 2021;203:102074. doi: 10.1016/j.pneurobio.2021.102074. [DOI] [PubMed] [Google Scholar]
- 33.Chen S., Kulik M., Lechleider R.J. Smad proteins regulate transcriptional induction of the SM22alpha gene by TGF-beta. Nucleic Acids Res. 2003;31:1302–1310. doi: 10.1093/nar/gkg224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kistler A.D., Peev V., Forst A.L., El Hindi S., Altintas M.M., Reiser J. Enzymatic disease of the podocyte. Pediatr. Nephrol. 2010;25:1017–1023. doi: 10.1007/s00467-009-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Na B.R., Kim H.R., Piragyte I., Oh H.M., Kwon M.S., Akber U., Lee H.S., Park D.S., Song W.K., Park Z.Y., et al. TAGLN2 regulates T cell activation by stabilizing the actin cytoskeleton at the immunological synapse. J. Cell Biol. 2015;209:143–162. doi: 10.1083/jcb.201407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koehler S., Odenthal J., Ludwig V., Unnersjö Jess D., Höhne M., Jüngst C., Grawe F., Helmstädter M., Janku J.L., Bergmann C., et al. Scaffold polarity proteins Par3A and Par3B share redundant functions while Par3B acts independent of atypical protein kinase C/Par6 in podocytes to maintain the kidney filtration barrier. Kidney Int. 2022;101:733–751. doi: 10.1016/j.kint.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Majumder S., Thieme K., Batchu S.N., Alghamdi T.A., Bowskill B.B., Kabir M.G., Liu Y., Advani S.L., White K.E., Geldenhuys L., et al. Shifts in podocyte histone H3K27me3 regulate mouse and human glomerular disease. J. Clin. Invest. 2018;128:483–499. doi: 10.1172/JCI95946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie K., Xu C., Zhang M., Wang M., Min L., Qian C., Wang Q., Ni Z., Mou S., Dai H., et al. Yes-associated protein regulates podocyte cell cycle re-entry and dedifferentiation in adriamycin-induced nephropathy. Cell Death Dis. 2019;10:915. doi: 10.1038/s41419-019-2139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou L., Liu Y. Wnt/beta-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat. Rev. Nephrol. 2015;11:535–545. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Focal segmental glomerlosclerosis. New Engl. J. Med. 2011 [Google Scholar]
- 41.Weins A., Schlondorff J.S., Nakamura F., Denker B.M., Hartwig J.H., Stossel T.P., Pollak M.R. Disease-associated mutant alpha-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity. Proc. Natl. Acad. Sci. USA. 2007;104:16080–16085. doi: 10.1073/pnas.0702451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin L.M., Xu Y.D., Peng L.L., Duan T.T., Liu J.Y., Xu Z., Wang W.Q., Guan N., Han X.J., Li H.Y., et al. Transgelin-2 as a therapeutic target for asthmatic pulmonary resistance. Sci. Transl. Med. 2018;10:eaam8604. doi: 10.1126/scitranslmed.aam8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W., Wang Y., Long J., Wang J., Haudek S.B., Overbeek P., Chang B.H.J., Schumacker P.T., Danesh F.R. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocca C.J., Ur S.N., Harrison F., Cherqui S. rAAV9 combined with renal vein injection is optimal for kidney-targeted gene delivery: conclusion of a comparative study. Gene Ther. 2014;21:618–628. doi: 10.1038/gt.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei C.T., Su H., Ye C., Tang H., Gao P., Wan C., He F.F., Wang Y.M., Zhang C. The classic signalling and trans-signalling of interleukin-6 are both injurious in podocyte under high glucose exposure. J. Cell. Mol. Med. 2018;22:251–260. doi: 10.1111/jcmm.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all relevant data of this study are available within the article or from the corresponding author upon reasonable request.