Summary
Inhibitors of neprilysin improve glycemia in patients with heart failure and type 2 diabetes (T2D). The effect of weight loss by diet, surgery, or pharmacotherapy on neprilysin activity (NEPa) is unknown. We investigated circulating NEPa and neprilysin protein concentrations in obesity, T2D, metabolic dysfunction-associated steatotic liver disease (MASLD), and following bariatric surgery, or GLP-1-receptor-agonist therapy. NEPa, but not neprilysin protein, was enhanced in obesity, T2D, and MASLD. Notably, MASLD associated with NEPa independently of BMI and HbA1c. NEPa decreased after bariatric surgery with a concurrent increase in OGTT-stimulated GLP-1. Diet-induced weight loss did not affect NEPa, but individuals randomized to 52-week weight maintenance with liraglutide (1.2 mg/day) decreased NEPa, consistent with another study following 6-week liraglutide (3 mg/day). A 90-min GLP-1 infusion did not alter NEPa. Thus, MASLD may drive exaggerated NEPa, and lowered NEPa following bariatric surgery or liraglutide therapy may contribute to the reported improved cardiometabolic effects.
Subject areas: Health sciences, Obesity medicine, Diabetology
Graphical abstract

Highlights
-
•
The protease neprilysin impact the plasma levels of numerous hormones
-
•
Neprilysin activity was found to be increased by diabetes, obesity, and MASLD
-
•
Bariatric surgery and GLP-1-based therapy normalized neprilysin activity
-
•
Altered neprilysin activity may contribute to metabolic diseases
Health sciences; Obesity medicine; Diabetology
Introduction
Neprilysin or neutral endopeptidase (NEP) is a protease that exists in a soluble form (in circulation) and as an integral membrane protein in several tissues.1,2 The abundance of tissue or systemic NEP may be measured by evaluating protein concentrations or through a more functional approach by investigating NEP activity (NEPa). NEP cleaves and degrades several peptide hormones including glucagon-like peptide 1 (GLP-1), an incretin hormone important for appetite control and glucose regulation.3,4 The NEP-inhibitor sacubitril, administered in combination with an angiotensin 2 type 1 receptor inhibitor (valsartan), is approved and used for treating patients with systolic heart failure.5,6 Interestingly, patients with heart failure and the common comorbidity type 2 diabetes (T2D) significantly improved their glycemic control with sacubitril/valsartan as compared to another heart failure medication enalapril.7 Furthermore, the decrease in HbA1c was greater in individuals randomized to sacubitril/valsartan compared to valsartan alone,8 indicating that the improvements in glycemia may be driven by the inhibition of NEPa. In a single study, obesity has been found to correlate with NEPa.9 Furthermore, NEP protein levels have been reported to reduce following dietary10 or surgical (bariatric surgery)11 weight loss. However, NEPa and NEP protein levels have to our knowledge not been investigated in patients with diabetes or biopsy-verified metabolic dysfunction-associated steatotic liver disease (MASLD) before and after dietary, pharmacological or surgical weight loss interventions. Bariatric surgery is currently the most effective treatment for obesity and leads to a long-lasting, sustained weight loss12,13 and remission of MASLD and T2D.14,15 Moreover, bariatric surgery enhances the postprandial GLP-1 response with important impacts on postoperative glucose regulation.16,17,18 Acute NEP-inhibition with sacubitril/valsartan increases postprandial plasma concentrations of total GLP-1 (metabolite 9-36NH2) in individuals with4 or without diabetes,3 and the activity of NEP may therefore play an unappreciated role in metabolic disease, and following bariatric surgery. The effect of bariatric surgery on circulating NEPa remains unknown.
We hypothesized that NEPa is increased in patients with metabolic diseases (MASLD in particular), and that bariatric surgery reduces plasma NEPa. In the present study, we investigated circulating NEPa and NEP protein concentrations in separate cohorts of individuals with metabolic diseases (obesity, T2D, and/or MASLD), before and after bariatric surgery or following short- or long-term GLP-1 receptor agonism to evaluate possible predictors of NEPa and/or NEP protein levels, and how surgical, pharmacological, or dietary interventions may modulate NEPa.
Results
Study overview
Samples from nine independent studies (studies a-i summarized in Table 1) were included in the present study (baseline characteristics in Table S1). We report on baseline values from all nine studies (in a cross-sectional manner) for the purpose of evaluating circulating NEPa and NEP protein concentrations in different patient groups of metabolic disease. Furthermore, we investigate the effect of bariatric surgery, long-term liraglutide therapy, and short-term GLP-1 administration on NEPa and NEP protein concentrations. We did not perform sample size calculations prior to investigation as the present study includes post-hoc analyses on an exploratory outcome.
Table 1.
Study overview
| Identifier: clinical trials identifier (PMID) | Study design; resource; and time points | Study details |
|---|---|---|
| a) MASLD study: NCT04340817 (unpublished) | Prospective study; heparinized plasma; baseline (fasted state) | Male or female individuals with MASLD (n = 23), or MASLD and T2D (n = 13), and non-MASLD controls (n = 30) evaluated by non-invasive specialized ultrasound (FibroScan) |
| b) MASLD study: NCT04907721 (unpublished) | Cross sectional study; heparinized plasma; baseline (fasted state) | Male or female individuals with (n = 23) or without (n = 26) MASLD evaluated by magnetic resonance imaging (whole-liver quantification) |
| c) MASLD and bariatric surgery study (33163832) | Prospective study; heparinized plasma; baseline (surgery day) and 12 months post-op | Male or female individuals with or without MASLD (n = 33; of which 10 had T2D) before and after bariatric surgery (RYGB or SG). Baseline MASLD (n = 37; of which 9 have T2D), and baseline controls (n = 11 admitted for a cholecystectomy). MASLD was evaluated from liver biopsies by liver pathologists |
| d) RYGB study: NCT01945840 (32209584) | Prospective study, heparinized plasma, baseline (6–375 days pre-op), 6 months (post-op) and 12 months (post-op) | Male or female individuals with IGT (n = 4) or T2D (n = 16) before and after RYGB |
| e) RYGB study: NCT01202526 (24241533) | Prospective study; heparinized plasma; baseline (3–9 days pre-op), 1 week (post-op), 3 months (post-op) and 1 year (post-op) | Male or female individuals with NGT (n = 10), or T2D (n = 10) before and after RYGB |
| f) RYBG study: NCT01700686 (27322465) | Prospective study; serum, baseline (3–55 days pre-op), 4 weeks (post-op) and 6 months (post-op) | Male or female individuals with obesity (n = 18) before and after RYGB |
| g) Liraglutide study: NCT02094183 (33951361) | Interventional study; serum; screening (before weight loss), baseline (8 weeks after 13% diet-induced weight loss), and 52 weeks (after liraglutide- or diet-induced weight maintenance period) | Male and female individuals with overweight or obesity before and after an initial 8-week diet-induced weight loss period (800 kcal/day amounting to 13% weight loss) followed by a 52-week weight maintenance period with diet (Cambridge Weight Plan products, n = 15) or liraglutide (1.2 mg/day, supplemented with Cambridge Weight plan products, if necessary, n = 20) |
| h) Liraglutide study: NCT03520062 (32495988) | Interventional study; heparinized plasma; baseline (fasting samples), 4 weeks (after the initiation and titration of maximal liraglutide dose of 3 mg/day), 6 weeks (after steady state treatment of 3 mg liraglutide/day) and 9 weeks (follow-up 3 weeks after the discontinuation of liraglutide) | Male individuals with obesity (n = 14) treated with liraglutide (3 mg/day) for 6 weeks |
| i) GLP-1e study: NCT04186026 (unpublished) | A subgroup from a cross-over study; serum; baseline (fasting samples), 45 min (with continuous saline or GLP-1 infusion) and 90 min (with continuous saline or GLP-1 infusion) | Male individuals with overweight or obesity (n = 19) treated with GLP-1 (7–36 amide, 1 pmol/kg/min) or saline (control) for 90 min (cross-over) |
Overview of studies included in the article.
MASLD, Non-alcoholic fatty liver disease; T2D, type 2 diabetes; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; op, operation; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; CAP, controlled attenuation parameter.
Neprilysin activity is increased in patients with overweight, obesity, and/or type 2 diabetes, and hepatic steatosis may partially drive the increase in neprilysin activity
Circulating NEPa was significantly increased in overweight and obesity compared to lean individuals (Figure 1A). In individuals with type 2 diabetes, NEPa was increased by 42% compared to controls with overweight or obesity with normal glucose tolerance (1621 [753:2975] vs. 1168 [663:1981] pmol/mL/min, p = 0.04, Figure 1B). We investigated NEPa and NEP protein concentrations in patients with MASLD, as obesity constitutes the main risk factor for the progression of MASLD and found NEPa to be increased by 213% in individuals with overweight or obesity with MASLD compared to controls with or without obesity without MASLD (1582 [984:3689] vs. 677 [376:1162], p < 0.0001, Figure 1C). When only evaluating individuals with overweight or obesity with (n = 98) compared to without (n = 48) MASLD, NEPa remained significantly increased in individuals with MASLD (p = 0.0007). NEPa and hepatic steatosis (assessed by FibroScan using the controlled attenuated parameter (CAP) value) were positively associated in study a (p < 0.0001, R2 = 0.24) (Figure S1A). Similarly, in study b, a positive correlation (p = 0.02, R2 = 0.11) was found when evaluating NEPa and hepatic steatosis (whole liver quantification) evaluated by MRI (Figure S1B). In study c, patients reduced their plasma levels of triglycerides (mean ± SD; 1.4 ± 0.7 vs. 1 ± 0.6, p = 0.002) in addition to a reduction in NEPa following bariatric surgery. Overweight, obesity, type 2 diabetes, and MASLD did not affect plasma or serum concentrations of NEP protein. MASLD and age were significant predictors of NEPa outcome (p < 0.01) independently of BMI, HbA1c and sex, and explained 15% of the variation in NEPa (model 1 in Table 2; added variable plots in Figure S1C).
Figure 1.
Circulating NEPa, but not NEP protein, are increased in overweight, obesity, type 2 diabetes and MASLD
(A–F) NEPa in (A) lean (n = 48), overweight (n = 41) or obesity (n = 232), in (B) individuals with normal glucose tolerance (NGT, n = 226) or type 2 diabetes (T2D, n = 58), and in (C) individuals with (n = 98) or without (n = 89) MASLD. NEP protein concentrations in (D) lean (n = 43), overweight (n = 31), and obesity (n = 158), in (E) individuals with NGT (n = 200) or T2D (n = 41), and in (F) individuals with (n = 63) or without (n = 56) MASLD. Data are presented as mean ± SEM as Tukey boxplots and compared using (A and D) one-way ANOVA or (B, C, E, and F) unpaired t-tests in GraphPad Prism 9.4.1. ns means non-significant, ∗ indicates p ≤ 0.05, ∗∗ indicates p < 0.01 and ∗∗∗∗ indicates p < 0.0001.
Table 2.
Multiple linear regression analysis
| Coefficient (beta) | Standard Error | T value | Significance | [95% Conf. Interval, 2.5%] | [95% Conf. Interval, 97.5%] | |
|---|---|---|---|---|---|---|
| Constant | ||||||
| NEPa | −411.82 | 1290.63 | −0.319 | 0.75 | −2961.85 | 2138.21 |
| Independent variables | ||||||
| Sex | −214.16 | 395.84 | −0.541 | 0.59 | −996.26 | 567.94 |
| Age | 42.44 | 16.35 | 2.595 | 0.01 | 10.13 | 74.75 |
| BMI | 21.55 | 24.66 | 0.874 | 0.38 | −27.16 | 0.27 |
| HbA1c | −24.77 | 32.45 | −0.763 | 0.45 | −88.88 | 39.34 |
| MASLD | 1325.16 | 477.72 | 2.774 | 0.006 | 381.28 | 2269.04 |
| R2 | 0.18 | |||||
| Adjusted R2 | 0.15 | |||||
| N | 157 | |||||
Statistics performed in R (lm function in base package) with age, BMI, HbA1c as continuous data, and MASLD and sex as categorical data (model 1). This analysis included measurements from studies a-c (Table 1).
Neprilysin activity reduces following bariatric surgery, but not after diet-induced body weight loss
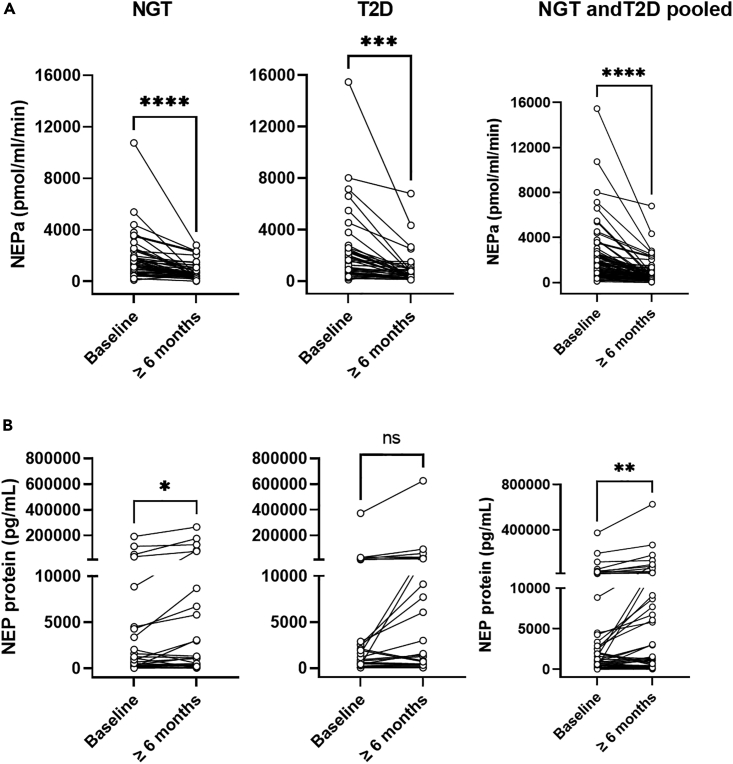
To investigate the effect of bariatric surgery on plasma or serum NEPa and concentrations of NEP protein, we analyzed samples obtained from individuals before (mean ± SD; age: 43 ± 11 years, BMI: 42 ± 6 kg/m2, HbA1c: 45 ± 12 mmol/mol, type 2 diabetes yes/no: 39/46) and ≥6 months after bariatric surgery from studies c-f (Table 1). NEPa declined by 57% (1385 [727:2068] vs. 523 [348:1008] pmol/mL/min, p < 0.0001) in individuals with NGT (n = 46) and by 63% (1504 [604:2512] vs. 387 [239:882] pmol/mL/min, p = 0.0001) in individuals with T2D (n = 39) ≥ 6 months after bariatric surgery, while plasma or serum concentrations of NEP protein increased or were unchanged in patients with NGT (p = 0.04) and T2D (Figures 2A and 2B). As expected, body weight (Figure S2A) and plasma concentrations of glucose (Figure S2B) reduced following bariatric surgery (≥6 months). Sex, age, delta body weight, and delta HbA1c levels did not associate with delta NEPa following bariatric surgery (model 2, F statistic: p = 0.35, adjusted R2 = 0.008) (added variable plots in Figure S3).
Figure 2.
Circulating NEPa decreases following bariatric surgery in individuals with normal glucose tolerance (NGT) or type 2 diabetes (T2D), whereas NEP protein concentrations are unaltered (NGT) or even increased (T2D) following bariatric surgery
(A and B) (A) NEPa (n = 46 NGT, n = 39 T2D) and (B) NEP protein (n = 26 NGT, n = 27 T2D) before (baseline) and ≥6 months after bariatric surgery. Studies c-f are used (Table 1). Data are presented as mean ± SEM as spaghetti plots and compared in GraphPad Prism 9.4.1 using paired t-tests. ∗ indicates p ≤ 0.05, ∗∗ indicates p ≤ 0.01, and ∗∗∗∗ indicates p ≤ 0.0001.
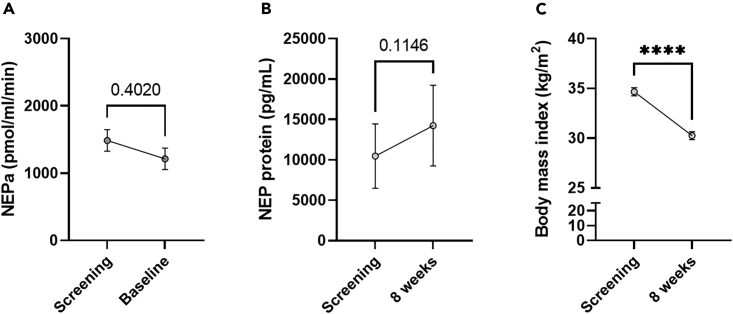
To investigate the effect of SG vs. RYGB on NEPa, we compared NEPa before and after SG in study c (n = 18) with NEPa before and after RYGB in studies c-f (n = 65). Both SG and RYGB decreased NEPa, and the effect on NEPa did not depend on surgery type (evaluated by two-way ANOVA, Figure S4A). Baseline NEPa values were numerically higher in the RYGB group (albeit not significantly, p = 0.2). In study e, plasma samples were collected before (mean ± SD, 6 ± 5 days before) RYGB, and 1 week, 3 months and 1 year after RYGB from ten individuals with NGT and ten individuals with T2D. NEPa was unaltered 1 week after RYGB, but decreased by 49% 3 months after RYGB (with a mean weight loss of 16%) and by 73% 1 year after RYGB (with a mean weight loss of 25%) (Figure S4B, p < 0.05 by two-way ANOVA). Furthermore, NEPa decreased by 55% 4 weeks after RYGB (study f in Table 1, 788 ± 508 vs. 1739 ± 1227 pmol/mL/min). As this may suggest that the weight loss component is important for reducing NEPa with bariatric surgery, we evaluated the effect of a diet-induced weight loss on NEPa (study g in Table 1). NEPa and plasma concentrations of NEP protein were unaltered (Figures 3A and 3B) in individuals subjected to a low-calorie diet for 8 weeks amounting to a considerable weight loss of 13% (mean ± SD; n = 50, BMI: 34.7 ± 2.9 vs. 30.3 ± 2.8 kg/m2, p < 0.0001, Figure 3C).
Figure 3.
Circulating NEPa does not reduce following a diet-induced reduction in body weight
(A–C) (A) NEPa (n = 50), (B) NEP protein (n = 45) and (C) body mass index from study g (Table 1). Data are presented as mean ± SEM and compared in GraphPad Prism 9.4.1 using paired t-tests. ∗∗∗∗ indicates p ≤ 0.0001.
We found increases in postprandial peak levels of total GLP-1 (mean ± SD for baseline, 3 months and 1 year; 15 ± 4 vs. 105 ± 38 vs. 75 ± 27) with concurrent reductions in NEPa in patients following bariatric surgery in study e.
Neprilysin activity reduces after long-term liraglutide therapy, but not after a short-term glucagon-like peptide-1 infusion
To investigate the effect of GLP-1 on NEPa and concentrations of NEP protein, we included individuals with overweight or obesity subjected to 6 or 52 week of liraglutide therapy (studies g and h in Table 1) or acute administration of native GLP-1 (7–36 amide; study i in Table 1). In study g, a 52-week weight maintenance period with liraglutide therapy of 1.2 mg/day (after an initial 8-week calorie restriction period amounting to a 13% weight loss) reduced NEPa by 44% (p = 0.04, Figure 4A) without altering plasma concentrations of NEP protein (Figure 4B). NEPa (p = 0.4) and NEP protein were unchanged during the 52-week weight maintenance with diet only (Figures 4C and 4D). Both groups equally maintained their weight loss. Next, we evaluated the effect of liraglutide (at a higher dose of 3 mg/day) in individuals with overweight or obesity (mean ± SD; age 38 ± 11 years, body mass index 32 ± 4 kg/m2) for 6 weeks (study h in Table 1). Liraglutide (3 mg/day) significantly reduced NEPa by 33% from baseline to 4 weeks (p = 0.03) and continued to be 28% reduced at 6 weeks (p = 0.04). At follow-up (3 weeks after liraglutide therapy was discontinued), NEPa had returned to baseline (Figure 4A). Body weight was reduced by 3% after 4- and 6-week liraglutide therapy (p < 0.0001 and p = 0.002) and returned to baseline at follow-up (p = 0.4, one-way ANOVA) (mean ± SD for baseline vs. follow-up; 103 ± 13 vs. 101 ± 16). NEP protein levels were unaltered with the high dose liraglutide therapy of 3 mg/day (Figure 4B). Finally, to investigate the acute effects of GLP-1 on NEPa, we included samples from a subgroup of participants from a currently unpublished study (study i in Table 1). Male individuals with overweight or obesity (n = 19) completed two experimental days consisting of a 90-min infusion of saline or GLP-1 (7–36 amide, 1 pmol/kg/min) completed in randomized order. NEPa did not change following the infusion of saline or GLP-1 (Figures 4G and 4H).
Figure 4.
Therapy with the GLP-1 analogue, liraglutide, reduces NEPa in obesity
(A and B) (A) NEPa and (B) NEP protein concentrations in individuals with obesity (n = 20) following a 52-week liraglutide-induced (1.2 mg/day) weight maintenance period.
(C and D) (C) NEPa and (D) NEP protein concentrations in individuals with obesity (n = 15) following a 52-week diet-induced weight maintenance period.
(E and F) (E) NEPa and (F) NEP protein in male individuals with overweight or obesity (n = 14) treated with liraglutide (3 mg/day) for 6 weeks.
(G) NEPa and delta NEPa (baseline subtracted) in male individuals with overweight or obesity (n = 19) after a 90-min saline or GLP-1 (7–36 amide, 1 pmol/kg/min) infusion completed in randomized order. Data are presented as mean ± SEM and compared in GraphPad Prism 9.4.1 using paired t-test (A–D) and one-way repeated measures ANOVA (E and F). ∗ indicates p < 0.05.
Discussion
Here, we show that circulating NEPa, but not NEP protein levels, are increased in patients with overweight, obesity, diabetes and MASLD, and provide evidence that hepatic steatosis may drive the augmented NEPa in obesity and type 2 diabetes. Unaltered plasma or serum concentrations of NEP protein suggests that the alterations in NEPa are qualitative rather than quantitative in terms of NEP protein and may be due to altered post-translational modifications. Fat accumulation in the liver appeared to be a driver of increased circulating NEPa, as shown here, and since NEP is highly expressed in liver tissue, improvements in liver health may reduce NEPa in the circulation. However, our linear regression model only explained 15% of the variance in NEPa, whereas other factors such as dyslipidemia,19 estrogen,20,21 substrate inhibition (B-type natriuretic peptide22), whole-body insulin resistance, heart failure or chronic kidney disease may influence soluble NEPa as shown by others.9,23,24,25,26,27,28
The effect of bariatric surgery on systemic neprilysin
We included four independent cohorts of participants who underwent bariatric surgery. Importantly, samples for most baseline measurements in these studies were collected on or very close to the day of surgery, yet 15 (of the 87) patients had a baseline value taken >21 days prior to surgery. A ∼60% reduction in NEPa with bariatric surgery is substantial and may support a surgery-related effect rather than an effect of the initial weight loss. The reduction in NEPa with bariatric surgery is comparable to that obtained by the pharmacological inhibition of NEPa by sacubitril/valsartan, which induces a 92% reduction in plasma NEPa in individuals with type 2 diabetes.29 Bariatric surgery also induced a 25% weight loss in patients examined ≥6 months post-surgery, but the reduction in NEPa with bariatric surgery appeared to be independent of changes in body weight and HbA1c levels. Thus, factors other than changes in body weight and HbA1c modulate NEPa. To further investigate the effect of weight loss on NEPa, we examined individuals subjected to an 8-week very-low-calorie diet (amounting to a weight loss of 13%), which did not reduce (or normalize) NEPa underlining that the attenuating effects of bariatric surgery on NEPa is not a weight loss phenomenon per se. However, we cannot exclude that the 13% weight loss induced by diet may be insufficient to alter NEPa compared with the 25% weight loss induced by bariatric surgery. To further support that obesity per se is not the main driver of increased NEPa we show that MASLD, but not BMI or HbA1c, was significantly associated with NEPa. Obesity is the main risk factor for fatty liver disease and increased NEPa in obesity as shown here and by others9 is therefore not surprising.
Sleeve gastrectomy and Roux-en-Y gastric bypass are considered metabolic surgeries as they initially affect metabolism independently of weight loss. Glucose homeostasis is significantly improved after surgery, and medication(s) for treating T2D can be discontinued in most patients. The degree to which GLP-1 is responsible for the metabolic success following bariatric surgery is continually debated.30 However, as evidenced in the literature, metabolic improvements are at least initially (especially after RYGB) largely induced by GLP-1.18,31 We hypothesized that the reduction in NEPa contributes to augmented postprandial GLP-1 concentrations following bariatric surgery. Indeed, NEPa was reduced following bariatric surgery together with a concomitant increase in postprandial GLP-1 concentrations. These data suggest that NEPa, in addition to body weight loss, contribute to the exaggerated postprandial GLP-1 response following bariatric surgery,30 which also aligns with postprandial increases of GLP-1 in individuals with4 or without3 type 2 diabetes following the pharmacological inhibition of NEP. However, other mechanisms than altered NEPa, such as changes in gastrointestinal tract anatomy causing rapid nutrient delivery, must be of greater importance for the exaggerated GLP-1 secretion following bariatric surgery. Dipeptidyl peptidase-4 (DPP-4), another important regulator of GLP-1, also reduces following bariatric surgery,32 and co-inhibition of DPP-4 and NEP have synergistic effects on augmenting GLP-1 levels, which may suggest that both proteases impact on GLP-1 levels after bariatric surgery.
Neprilysin in metabolic disease
As shown here, and by others, NEPa is increased in obesity and type 2 diabetes. Altered levels of glucagon-like-peptides are evident in individuals with obesity and type 2 diabetes,33,34 and, considering NEP’s peptide-degrading properties,35,36,37 NEPa may therefore contribute to the altered plasma concentrations of GLP-1 in individuals with obesity and type 2 diabetes. Indeed, long-term sacubitril/valsartan therapy augments GLP-1 in individuals with chronic heart failure.38 The clinical employment of NEP-inhibitors (sacubitril) may therefore add additional pathophysiological relevance regarding glucose control. Future studies evaluating the importance of NEP on the impaired GLP-1 response observed in individuals with obesity and/or type 2 diabetes, but also the long-term effects of NEP inhibition on postprandial GLP-1 levels, are warranted, and we propose that augmented NEPa in metabolic disease may partially explain the observed changes in plasma concentrations of GLP-1.
Liraglutide as a regulator of neprilysin activity
We show that long-term (≥6 weeks) liraglutide may regulate systemic NEPa. Liraglutide is similar (98% homologues in molecular structure) to native GLP-1 (amide 7–36), and both peptides are substrates for NEP cleavage.39 However, liraglutide is cleaved at a slower rate by NEP39 (and DPP-4) thereby extending its half-life. Substrate inhibition by liraglutide may modulate NEPa as shown with other NEP substrates,22 but studies investigating the regulation of NEPa are warranted. Interestingly, acute administration of GLP-1 did not significantly alter NEPa, which may suggest the following: Firstly, the inhibiting effect of GLP-1 on NEPa may not happen acutely. Secondly, GLP-1 (7–36 amide) was infused at 1 pmol/kg/min (resulting in the plasma concentration of GLP-1 close to the physiological range of endogenous GLP-1), while liraglutide was administered in doses of 1.2–3 mg/day, resulting in supraphysiological plasma concentrations of 20–30 nmol/L and the attenuating effect of liraglutide/GLP-1 on NEPa may be dose-dependent. Thirdly, liraglutide has other properties (e.g., albumin-binding/or substrate inhibition) compared to native GLP-1 that may affect NEPa by for example augmented substrate-induced inhibition of NEPa.
To conclude, we demonstrate that NEPa is increased in metabolic disease and that MASLD partially drives the increase in circulating NEPa in obesity and type 2 diabetes. However, whether altered NEPa contributes to the cardiometabolic profile in patients with MASLD and type 2 diabetes may not be concluded from the presented data. Given that reduced NEPa by NEP inhibition associates with improvements in cardiometabolic control it may be speculated that a reduction in NEPa with gastric bypass or sleeve gastrectomy may add to the metabolic improvements following bariatric surgery, possibly through a reduction in NEP-induced cleavage of proglucagon-derived hormones such as GLP-1. Conversely, increased NEPa in obesity and type 2 diabetes may partially explain the impaired GLP-1 response observed in these conditions. Chronic augmentation of GLP-1 in plasma may attenuate NEPa (perhaps through substrate inhibition) as shown in our studies of liraglutide therapy. The reduction in NEPa (with bariatric surgery or liraglutide) does not appear to be a weight loss phenomenon. Finally, systemic NEP protein concentrations do not dictate NEPa, and modulation of NEPa may therefore happen post-translationally. Therefore, NEPa may play a role for the cardiometabolic risk in patients with metabolic diseases and potentially contribute to the improved cardiovascular profile in patients following bariatric surgery or GLP-1 based therapies.
Limitations of the study
A caveat apparent with studies of NEP is large biological variation. We show that especially plasma or serum concentrations of NEP protein vary greatly, consistent with findings from a previous study.19 In fact, following bariatric surgery, NEPa reduced while NEP protein levels increased in individuals with NGT indicating a compensatory increase in NEP protein. Other studies find that NEP protein levels reduce following bariatric surgery11,40 at least in patients with remission of hypertension 15 months post-surgery.40 Therefore, caution should be advised regarding NEP protein as a surrogate marker for NEPa. However, differences in specificity between ELISAs may also play a role. Large biological variation was also apparent for our measurements of NEPa, exceeding the analytical variation of NEPa which was 8% for intra-assay variation and 13% for inter-assay variation. Importantly, (and similarly to DPP-441), NEP is a transmembrane protein, and systemic NEPa may not correlate with tissue levels. We do not know the degree to which NEPa is affected in tissues with high expression levels of NEP (liver, kidney, or gastrointestinal tract) in individuals with metabolic disease, and the reported NEPa and NEP protein concentrations in serum or plasma may therefore not depict the entire story. However, concurrent with the literature,9 we find NEPa (but not NEP protein) to be a biomarker of metabolic disease. Many factors have previously been associated with NEP including dyslipidemia, estrogen, B-type natriuretic peptide, insulin resistance, heart failure and chronic kidney disease, which have not been investigated in the present study and may therefore confound results. We aimed at evaluating the connection between NEPa and postprandial GLP-1 levels in the context of bariatric surgery, but we only had OGTT-stimulated plasma levels of GLP-1 from study e (n = 20). Finally, we cannot exclude that the reduction in NEPa following liraglutide therapy is not due to a competitive inhibition of NEPa by liraglutide for substrate cleavage in the activity assay. However, liraglutide-induced inhibition of NEPa in the assay must then mimic liraglutide’s substrate-inhibiting properties of NEP in vivo.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Human plasma or serum was collected from 9 studies (8 of which have a clinical trials registration numbers) |
NCT04340817 NCT04907721 NCT01945840 NCT01202526 NCT01700686 NCT02094183 NCT03520062 NCT04186026 |
N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Suc-Ala-Ala-Phe-AMC (NEPa assay) | Bachem | Cat # 71973-79-0 |
| Aminopeptidase M (NEPa assay) | Merck | Cat # 9054-63-1 |
| Phe-AMC (NEPa assay) | Bachem | Cat # 98516-72-4 |
| Phosphoramidon (NEPa assay) | Merck | Cat #R7385 |
| Critical commercial assays | ||
| Human Neprilysin DuoSet ELISA (NEP protein assay) | R&D Systems | Cat # DY1182 |
| Software and algorithms | ||
| R version 4.2.2 | R | N/A |
| GraphPad Prism version 9.4.1 | GraphPad software | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nicolai J Wewer Albrechtsen (nicolai.albrechtsen@regionh.dk).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Study approvals
The studies conducted in Denmark (8 out of 9; study overview in Table 1) were approved by the regional scientific ethical committee (approval numbers: H-17029039 (study a), H-20023717 (study b), H-16030784 and H-16030782 (study c), H-3-2010-041 (study e), H-2-2010-064 (study f), H-4-2010-134 (study g), H-16050115 (study h), H-19013060 (study i)) and by the Danish data protection board. Ethical approval for study d was obtained from the West London National Research Ethics Committee (13/L0/1510). All studies were conducted in accordance with the Declaration of Helsinki.
Data processing
This paper reports on nine independent studies, some of which have previously been published,42,43,44,45,46,47 outlined in Table 1. We report on studies a and b in a cross-sectional manner, studies c-f are prospective studies with bariatric surgery as an intervention, and studies g-i are have GLP-1 receptor agonist therapy or native GLP-1 as an intervention. MASLD status was evaluated in studies a, b, and c. In study a, MASLD was evaluated by a specialized ultrasound (FibroScan), while whole liver quantification with magnetic resonance imaging (MRI) technology was used in study b. In study c, MASLD was assessed by experienced liver pathologists from liver biopsy samples taken at baseline after which 33 of 70 individuals underwent either sleeve gastrectomy (SG, n = 18) or Roux-en-Y gastric bypass (RYGB, n = 13).
In studies d-f, patients underwent RYGB, and in study g, participants underwent a diet-induced weight loss intervention for 8 weeks (800 kcal per day, Cambridge Weight Plan, Corby, UK) resulting in a weight loss of 13% (n = 50) followed by either a 52-week diet (n = 15) or liraglutide (n = 20, 1.2 mg/day)-weight maintenance period. In study h, participants were subjected to liraglutide therapy for 6 weeks (3 mg/day) with additional blood samples obtained at week 4 (during liraglutide therapy) and 9 (3 weeks after liraglutide discontinuation), and finally, in study i, saline or GLP-1 (7–36 amide, 1 pmol/kg/min) was infused for 90 min on two separate days in randomized order. Many of the patients in studies c-e with type 2 diabetes were medicated with glucose-lowering drugs such as metformin, which lowered overall glucose levels in these patients.
Method details
Biochemical analysis
Plasma samples were collected in heparinized tubes (addition of an anti-coagulant), or as serum (following blood clotting) and used for the biochemical analyses.
NEP protein concentrations were measured using the Human Neprilysin DuoSet ELISA (R&D Systems Denmark) following the manufacturer’s instructions. NEP activity was measured using a newly in-house-developed two-step enzymatic assay modified from a previous report.48 Here, plasma (decanted from heparinized blood collection tubes after centrifugation), or serum was incubated with the substrate Suc-Ala-Ala-Phe-AMC (5 mM, Bachem cat. no. 4006306). The assay was designed as a two-stage enzymatic assay: in the first reaction (60 min incubation at 37°C), the NEP available in heparinized plasma or serum cleaves the substrate (Suc-Ala-Ala-Phe-AMC) between Ala and Phe, thereby generating Phe-AMC. Aminopeptidase M (Merck cat. no L5006) is added to initiate the second reaction (30 min incubation at 37°C) a cleavage between Phe and AMC. The fluorophore AMC is measured with emission at 440 nm and excitation at 360 nm. Known substrate amounts of Phe-AMC (Bachem cat. no. 4001559) were used to generate the calibrator curve. Intra- and inter-assay variations were 8 and 13%, respectively. Sample availability differed from cohort to cohort, and it was therefore not possible to investigate exclusively plasma or serum. In plasma samples blood clotting is prevented with the addition of anti-coagulants, whereas in serum samples blood is allowed to coagulate prior to centrifugation. In the literature, reports on NEPa and NEP protein concentrations have been evaluated in serum or plasma samples.22,26,27,38To test whether NEPa can be measured using serum and heparin plasma we obtained serum and heparinized blood from five healthy individuals using four technical replicates and by linear regression found R2 value of 0.88 supporting that NEPa using our in-house assay can be obtained independent if serum or heparin plasma is used.
Quantification and statistical analysis
Significance was assessed by unpaired t-tests or one-way analysis of variance (ANOVA) for between-group comparisons, and paired t-tests or repeated one-way ANOVA for the interventional studies. Tukey post-hoc analysis was employed to correct for multiple comparisons. A p value below or equal to 0.05 was considered significant. Linear regression analyses were performed to identify determinants of NEPa. To evaluate the primary driver of NEPa in metabolic disease, we performed multiple linear regression on a subset of individuals (n = 157, from studies a-c in Table 1) with no missing values for NEPa, MASLD (categorical data, yes/no), HbA1c (continuous data), BMI (continuous data), age (continuous data), and sex (categorical data, male/female) (model 1 in Table 2). Categorical variables were recoded as binary variables. To investigate possible associations with the bariatric surgery-induced NEPa reduction, we performed multiple linear regression using sex, age, delta (post-pre surgery) body weight and delta HbA1c as independent variables (n = 61 from studies c-f) (model 2). Statistical calculations (t-tests, one-way ANOVA, and two-way ANOVA) and graphs were created using GraphPad Prism (version 9.4.1 for Windows; GraphPad Software). Simple and multiple linear regressions were performed using the built-in lm function (base package) in R (version R 4.2.2). Data are reported as median and interquartile range [IQR] if not otherwise stated.
Additional resources
We include biological material from 9 separate studies with the clinicaltrials.gov registration numbers (or PMID id): NCT04340817, NCT04907721, PMID: 33163832, NCT01945840, NCT01202526, NCT01700686, NCT02094183, NCT03520062, NCT04186026. These studies are highlighted in Table 1.
Acknowledgments
This study was supported by Novo Nordisk Foundation, Excellence Emerging Investigator Grant – Endocrinology and Metabolism (Application No. NNF19OC0055001), Future Leader Award European Foundation for the Study of Diabetes (NNF21SA0072746) and Sapere Aude Independent Research Fund Denmark (1052-00003B). Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation (Grant agreement NNF14CC0001). We thank Christine Rasmussen, Department of Clinical Biochemistry, Bispebjerg University Hospital, Denmark for excellent technical assistance in the biochemical analyses.
Author contributions
SASK and NJWA conceived the idea of investigating systemic NEPa and NEP protein levels in metabolic disease and following the surgical and pharmaceutical modulation of metabolism. SASK wrote the first draft of the article. LLG, MW, JSP, FB, KA, TT, SST, EWI, NJJ, MMR, JPG, JR, BH, JJH, BH, JH, FG, SM, MSS, and KNB-M contributed with samples, and all authors revised and approved the final version of the article.
Declaration of interests
The authors declare no competing interests.
Published: October 12, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108190.
Supplemental information
References
- 1.Kerr M.A., Kenny A.J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem. J. 1974;137:477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalivaeva N.N., Zhuravin I.A., Turner A.J. Neprilysin expression and functions in development, ageing and disease. Mech. Ageing Dev. 2020;192:111363. doi: 10.1016/j.mad.2020.111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wewer Albrechtsen N.J., Mark P.D., Terzic D., Hansen L.H., Andersen U.Ø., Hartmann B., Carr R.D., Gustafsson F., Deacon C.F., Holst J.J., et al. Sacubitril/valsartan augments postprandial plasma concentrations of active GLP-1 when combined with sitagliptin in men. J. Clin. Endocrinol. Metab. 2019;104:3868–3876. doi: 10.1210/jc.2019-00515. [DOI] [PubMed] [Google Scholar]
- 4.Wewer Albrechtsen N.J., Møller A., Martinussen C., Gluud L.L., Rashu E.B., Richter M.M., Plomgaard P., Goetze J.P., Kjeldsen S., Hansen L.H., et al. Acute effects on glucose tolerance by neprilysin inhibition in patients with type 2 diabetes. Diabetes Obes. Metab. 2022;24:2017–2026. doi: 10.1111/dom.14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray J.J.V., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J., Shi V.C., Solomon S.D., Swedberg K., et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur. J. Heart Fail. 2013;15:1062–1073. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurray J.J.V., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Solomon S.D., Swedberg K., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 7.Seferovic J.P., Claggett B., Seidelmann S.B., Seely E.W., Packer M., Zile M.R., Rouleau J.L., Swedberg K., Lefkowitz M., Shi V.C., et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5:333–340. doi: 10.1016/s2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijkman M.O., Claggett B., Vaduganathan M., Cunningham J.W., Rørth R., Jackson A., Packer M., Zile M., Rouleau J., Swedberg K., et al. Effects of sacubitril/valsartan on glycemia in patients with diabetes and heart failure: the PARAGON-HF and PARADIGM-HF trials. Cardiovasc. Diabetol. 2022;21:110. doi: 10.1186/s12933-022-01545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Standeven K.F., Hess K., Carter A.M., Rice G.I., Cordell P.A., Balmforth A.J., Lu B., Scott D.J., Turner A.J., Hooper N.M., Grant P.J. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes. 2011;35:1031–1040. doi: 10.1038/ijo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henke C., Haufe S., Ziehl D., Bornstein S.R., Schulz-Menger J., Heni M., Engeli S., Jordan J., Birkenfeld A.L. Low-fat hypocaloric diet reduces neprilysin in overweight and obese human subjects. ESC Heart Fail. 2021;8:938–942. doi: 10.1002/ehf2.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghanim H., Monte S., Caruana J., Green K., Abuaysheh S., Dandona P. Decreases in neprilysin and vasoconstrictors and increases in vasodilators following bariatric surgery. Diabetes Obes. Metab. 2018;20:2029–2033. doi: 10.1111/dom.13320. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe B.M., Kvach E., Eckel R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016;118:1844–1855. doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 14.Sheng B., Truong K., Spitler H., Zhang L., Tong X., Chen L. The Long-Term Effects of Bariatric Surgery on Type 2 Diabetes Remission, Microvascular and Macrovascular Complications, and Mortality: a Systematic Review and Meta-Analysis. Obes. Surg. 2017;27:2724–2732. doi: 10.1007/s11695-017-2866-4. [DOI] [PubMed] [Google Scholar]
- 15.Hafeez S., Ahmed M.H. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J. Obes. 2013;2013 doi: 10.1155/2013/839275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nannipieri M., Baldi S., Mari A., Colligiani D., Guarino D., Camastra S., Barsotti E., Berta R., Moriconi D., Bellini R., et al. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J. Clin. Endocrinol. Metab. 2013;98:4391–4399. doi: 10.1210/jc.2013-2538. [DOI] [PubMed] [Google Scholar]
- 17.Hindsø M., Svane M.S., Hedbäck N., Holst J.J., Madsbad S., Bojsen-Møller K.N. The role of GLP-1 in postprandial glucose metabolism after bariatric surgery: a narrative review of human GLP-1 receptor antagonist studies. Surg. Obes. Relat. Dis. 2021;17:1383–1391. doi: 10.1016/j.soard.2021.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Hindsø M., Hedbäck N., Svane M.S., Møller A., Martinussen C., Jørgensen N.B., Dirksen C., Gasbjerg L.S., Kristiansen V.B., Hartmann B., et al. The Importance of Endogenously Secreted GLP-1 and GIP for Postprandial Glucose Tolerance and β-Cell Function After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy Surgery. Diabetes. 2023;72:336–347. doi: 10.2337/db22-0568. [DOI] [PubMed] [Google Scholar]
- 19.Reddy Y.N.V., Iyer S.R., Scott C.G., Rodeheffer R.J., Bailey K., Jenkins G., Batzler A., Redfield M.M., Burnett J.C., Jr., Pereira N.L. Soluble Neprilysin in the General Population: Clinical Determinants and Its Relationship to Cardiovascular Disease. J. Am. Heart Assoc. 2019;8 doi: 10.1161/jaha.119.012943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J., Guan H., Booze R.M., Eckman C.B., Hersh L.B. Estrogen regulates neprilysin activity in rat brain. Neurosci. Lett. 2004;367:85–87. doi: 10.1016/j.neulet.2004.05.085. [DOI] [PubMed] [Google Scholar]
- 21.O'Hagan T.S., Wharton W., Kehoe P.G. Interactions between oestrogen and the renin angiotensin system - potential mechanisms for gender differences in Alzheimer's disease. Am. J. Neurodegener. Dis. 2012;1:266–279. [PMC free article] [PubMed] [Google Scholar]
- 22.Vodovar N., Séronde M.F., Laribi S., Gayat E., Lassus J., Januzzi J.L., Jr., Boukef R., Nouira S., Manivet P., Samuel J.L., et al. Elevated Plasma B-Type Natriuretic Peptide Concentrations Directly Inhibit Circulating Neprilysin Activity in Heart Failure. JACC. Heart Fail. 2015;3:629–636. doi: 10.1016/j.jchf.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Esser N., Barrow B.M., Choung E., Shen N.J., Zraika S. Neprilysin inhibition in mouse islets enhances insulin secretion in a GLP-1 receptor dependent manner. Islets. 2018;10:175–180. doi: 10.1080/19382014.2018.1502521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esser N., Zraika S. Neprilysin inhibition: a new therapeutic option for type 2 diabetes? Diabetologia. 2019;62:1113–1122. doi: 10.1007/s00125-019-4889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutta S., Grobe N., Kumbaji M., Osman H., Saklayen M., Li G., Elased K.M. Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes. Am. J. Physiol. Renal Physiol. 2018;315 doi: 10.1152/ajprenal.00565.2017. F263-f274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayés-Genís A., Barallat J., Galán A., de Antonio M., Domingo M., Zamora E., Urrutia A., Lupón J. Soluble neprilysin is predictive of cardiovascular death and heart failure hospitalization in heart failure patients. J. Am. Coll. Cardiol. 2015;65:657–665. doi: 10.1016/j.jacc.2014.11.048. [DOI] [PubMed] [Google Scholar]
- 27.Núñez J., Núñez E., Barallat J., Bodí V., Miñana G., Pastor M.C., Sanchis J., Lupón J., Bayes-Genis A. Serum Neprilysin and Recurrent Admissions in Patients With Heart Failure. J. Am. Heart Assoc. 2017;6 doi: 10.1161/jaha.117.005712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parilla J.H., Hull R.L., Zraika S. Neprilysin Deficiency Is Associated With Expansion of Islet beta-Cell Mass in High Fat-Fed Mice. J. Histochem. Cytochem. 2018;66:523–530. doi: 10.1369/0022155418765164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wewer Albrechtsen N., Martinussen C., Plomgaard P., Gøtze J., Kjeldsen S., Deacon C.F., Holst J.J., Madsbad S., Bojsen-Møller K. Program of the 56th Annual Meeting of the European Association for the Study of Diabetes, Online 63 (September 2020Abstract 625), Abstract 625. 2020. Acute effects on glucose tolerance by neprilysin inhibition in patients with type 2 diabetes; p. 621. [DOI] [Google Scholar]
- 30.Hutch C.R., Sandoval D. The Role of GLP-1 in the Metabolic Success of Bariatric Surgery. Endocrinology. 2017;158:4139–4151. doi: 10.1210/en.2017-00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirksen C., Hansen D.L., Madsbad S., Hvolris L.E., Naver L.S., Holst J.J., Worm D. Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care. 2010;33:375–377. doi: 10.2337/dc09-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herz C.T., Brix J.M., Ludvik B., Schernthaner G., Schernthaner G.H. Decrease of dipeptidyl peptidase 4 activity is associated with weight loss after bariatric surgery. Obes. Surg. 2021;31:2545–2550. doi: 10.1007/s11695-020-05200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madsbad S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 2014;16:9–21. doi: 10.1111/dom.12119. [DOI] [PubMed] [Google Scholar]
- 34.Stern J.H., Smith G.I., Chen S., Unger R.H., Klein S., Scherer P.E. Obesity dysregulates fasting-induced changes in glucagon secretion. J. Endocrinol. 2019;243:149–160. doi: 10.1530/joe-19-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjeldsen S.A.S., Hansen L.H., Esser N., Mongovin S., Winther-Sørensen M., Galsgaard K.D., Hunt J.E., Kissow H., Ceutz F.R., Terzic D., et al. Neprilysin Inhibition Increases Glucagon Levels in Humans and Mice with Potential Effects on Amino Acid Metabolism. J. Endocr. Soc. 2021;5:bvab084. doi: 10.1210/jendso/bvab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hupe-Sodmann K., McGregor G.P., Bridenbaugh R., Göke R., Göke B., Thole H., Zimmermann B., Voigt K. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7-36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul. Pept. 1995;58:149–156. doi: 10.1016/0167-0115(95)00063-h. [DOI] [PubMed] [Google Scholar]
- 37.Trebbien R., Klarskov L., Olesen M., Holst J.J., Carr R.D., Deacon C.F. Neutral endopeptidase 24.11 is important for the degradation of both endogenous and exogenous glucagon in anesthetized pigs. Am. J. Physiol. Endocrinol. Metab. 2004;287:E431–E438. doi: 10.1152/ajpendo.00353.2003. [DOI] [PubMed] [Google Scholar]
- 38.Nougué H., Pezel T., Picard F., Sadoune M., Arrigo M., Beauvais F., Launay J.M., Cohen-Solal A., Vodovar N., Logeart D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur. J. Heart Fail. 2019;21:598–605. doi: 10.1002/ejhf.1342. [DOI] [PubMed] [Google Scholar]
- 39.Malm-Erjefält M., Bjørnsdottir I., Vanggaard J., Helleberg H., Larsen U., Oosterhuis B., van Lier J.J., Zdravkovic M., Olsen A.K. Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab. Dispos. 2010;38:1944–1953. doi: 10.1124/dmd.110.034066. [DOI] [PubMed] [Google Scholar]
- 40.Salman A.A., Salman M.A., Shawkat M., Hassan S.A., Saad E.H., Hussein A.M., Refaie O.R.M., Tourky M.S., Shaaban H.E.D., Abd Allah N., et al. Effect of laparoscopic sleeve gastrectomy on vasoactive mediators in obese hypertensive patients: A prospective study. Clin. Endocrinol. 2021;94:193–203. doi: 10.1111/cen.14352. [DOI] [PubMed] [Google Scholar]
- 41.Mulvihill E.E., Drucker D.J. Pharmacology, Physiology, and Mechanisms of Action of Dipeptidyl Peptidase-4 Inhibitors. Endocr. Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen J.S., Rygg M.O., Kristiansen V.B., Olsen B.H., Serizawa R.R., Holst J.J., Madsbad S., Gluud L.L., Bendtsen F., Wewer Albrechtsen N.J. Nonalcoholic Fatty Liver Disease Impairs the Liver-Alpha Cell Axis Independent of Hepatic Inflammation and Fibrosis. Hepatol. Commun. 2020;4:1610–1623. doi: 10.1002/hep4.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bojsen-Møller K.N., Dirksen C., Jørgensen N.B., Jacobsen S.H., Serup A.K., Albers P.H., Hansen D.L., Worm D., Naver L., Kristiansen V.B., et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014;63:1725–1737. doi: 10.2337/db13-1307. [DOI] [PubMed] [Google Scholar]
- 44.Alexiadou K., Cuenco J., Howard J., Wewer Albrechtsen N.J., Ilesanmi I., Kamocka A., Tharakan G., Behary P., Bech P.R., Ahmed A.R., et al. Proglucagon peptide secretion profiles in type 2 diabetes before and after bariatric surgery: 1-year prospective study. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2019-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wewer Albrechtsen N.J., Hornburg D., Albrechtsen R., Svendsen B., Toräng S., Jepsen S.L., Kuhre R.E., Hansen M., Janus C., Floyd A., et al. Oxyntomodulin Identified as a Marker of Type 2 Diabetes and Gastric Bypass Surgery by Mass-spectrometry Based Profiling of Human Plasma. EBioMedicine. 2016;7:112–120. doi: 10.1016/j.ebiom.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svane M.S., Johannesen H.H., Martinussen C., Bojsen-Møller K.N., Hansen M.L., Hansen A.E., Deacon C.F., Hartmann B., Keller S.H., Klausen T.L., et al. No effects of a 6-week intervention with a glucagon-like peptide-1 receptor agonist on pancreatic volume and oedema in obese men without diabetes. Diabetes Obes. Metab. 2020;22:1837–1846. doi: 10.1111/dom.14106. [DOI] [PubMed] [Google Scholar]
- 47.Iepsen E.W., Lundgren J., Dirksen C., Jensen J.E., Pedersen O., Hansen T., Madsbad S., Holst J.J., Torekov S.S. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int. J. Obes. 2015;39:834–841. doi: 10.1038/ijo.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yandle T., Richards M., Smith M., Charles C., Livesey J., Espiner E. Assay of endopeptidase-24.11 activity in plasma applied to in vivo studies of endopeptidase inhibitors. Clin. Chem. 1992;38:1785–1791. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.