Abstract
The ongoing evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in the emergence of new variants that are resistant to existing vaccines and therapeutic antibodies, has raised the need for novel strategies to combat the persistent global COVID-19 epidemic. In this study, a monoclonal anti-human angiotensin-converting enzyme 2 (hACE2) antibody, ch2H2, was isolated and humanized to block the viral receptor-binding domain (RBD) binding to hACE2, the major entry receptor of SARS-CoV-2. This antibody targets the RBD-binding site on the N terminus of hACE2 and has a high binding affinity to outcompete the RBD. In vitro, ch2H2 antibody showed potent inhibitory activity against multiple SARS-CoV-2 variants, including the most antigenically drifted and immune-evading variant Omicron. In vivo, adeno-associated virus (AAV)-mediated delivery enabled a sustained expression of monoclonal antibody (mAb) ch2H2, generating a high concentration of antibodies in mice. A single administration of AAV-delivered mAb ch2H2 significantly reduced viral RNA load and infectious virions and mitigated pulmonary pathological changes in mice challenged with SARS-CoV-2 Omicron BA.5 subvariant. Collectively, the results suggest that AAV-delivered hACE2-blocking antibody provides a promising approach for developing broad-spectrum antivirals against SARS-CoV-2 and potentially other hACE2-dependent pathogens that may emerge in the future.
Keywords: SARS-CoV-2, angiotensin-converting enzyme II, adeno-associated virus
Graphical abstract

Tao and colleagues developed AAV-mediated delivery of hACE2-blocking monoclonal antibody ch2H2, which enables sustained high-level antibody presence in mice. This approach offers broad-spectrum protection against SARS-CoV-2 variants infection while also ameliorating pulmonary histopathology. It holds promise as a solution to combat upcoming COVID-19 waves and emerging hACE2-dependent pathogens.
Introduction
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has ravaged the world, leading to over 760 million cases and 6.8 million deaths worldwide as of March 2023 (World Health Organization [WHO], 2023). Given the limited availability and restricted regimens of antivirals available to treat SARS-CoV-2 infection, vaccines remain the main approach to combating this disease. However, the massive propagation has allowed its rapid evolution, resulting in the constant emergence of new SARS-CoV-2 variants of concern (VOCs) with drifted antigenicity. This has largely hampered the major approach to COVID-19 vaccine development, which relies on using viral spike (S) protein as the antigen with the intent to obstruct virus-receptor interaction. A prominent example is the SARS-CoV-2 Omicron variant BA.1, which emerged in late 2021 and contained 37 mutations in the S protein, including 15 in its receptor-binding domain (S-RBD).1 Since the antigenic landscape of Omicron was reshaped, these mutations rendered BA.1 refractory to neutralizing antibodies elicited by most COVID-19 vaccines.1,2,3,4,5 The subsequent emergence of Omicron subvariants BA.4 and BA.5, which dominated the pandemic in the second half of 2022, presented further mutations and exhibited even more resistance to neutralization by triple-dosed vaccine serum and convalescent serum from BA.1 breakthrough infections.6,7,8,9,10 Moreover, the antigenically drifted Omicron variants also compromised the efficacy of therapeutic monoclonal antibodies (mAbs).2,5,6,10,11,12,13,14 Although Omicron variants appear to be less virulent,15 their higher transmission rate has inevitably led to an overwhelming number of hospitalizations and posed a serious societal threat. Therefore, there is an urgency to develop new interventions that possess broader protection to limit the transmission of continuously emerging SARS-CoV-2 variants in the future.
Using mAbs targeting viral receptors presents a practical approach to block virus-receptor interaction and thus inhibit viral entry. These mAbs can be employed for both prophylaxis and therapeutic treatment of infections. For instance, ibalizumab, an mAb that binds human CD4 to block multidrug-resistant HIV-1 infection,16 showed antiviral and immunologic responses in phase 3 clinical trial17 and was granted US Food and Drug Administration (FDA) approval in 2018. Presently, two receptors have been identified for SARS-CoV-2: human angiotensin-converting enzyme 2 (hACE2) and CD147. hACE2 acts as the major entry receptor for SARS-CoV-2 through its interaction with S-RBD. CD147, a type I transmembrane protein involved in the infection of some viruses,18 also partakes in SARS-CoV-2 entry, although the mechanism remains elusive.19 Recent findings suggest that the blockade of CD147 via anti-CD147 mAb could suppress viral replication in vitro.19,20 In phase 1 and 2 clinical trials, a humanized anti-CD147 IgG2 mAb meplazumab was associated with faster recovery from pneumonia in COVID-19 patients.21 In a similar vein, targeting the hACE2 receptor has also been considered a potential measure for COVID-19 prevention. A recent study has reported that mouse-derived polyclonal antibodies targeting hACE2 could block the entry of VSV-based pseudotyped SARS-CoV-2.22 However, this type of treatment is still in the early stages of research development and has not yet been proved effective in clinical trials.
In this study, we generated a chimerized mAb (ch2H2) that exhibited potent hACE2-blocking activity capable of inhibiting the infection of diverse SARS-CoV-2 variants, including Alpha, Beta, Gamma, Delta, and Omicron subvariants. We also demonstrated that adeno-associated virus (AAV)-mediated expression of ch2H2 in K18-hACE2 mice airways can prevent Omicron BA.5 infection and reduce pulmonary histopathology. Our study provides an alternative strategy for developing broad-spectrum antivirals against emerging SARS-CoV-2 variants and any other pathogens that use hACE2 for infection.
Results
Generation and characterization of anti-hACE2 antibody-secreting hybridoma
To develop an effective treatment against all SARS-CoV-2 variants, we decided to target the viral entry receptor hACE2 and generate blocking antibodies. Balb/c mice were first primed with recombinant AAVs encoding hACE2 (AAV6/hACE2 and AAV9/hACE2), followed by boosters with recombinant hACE2 protein (Figure 1A). This heterologous vaccination strategy combining different antigen-delivering approaches was employed intending to induce a larger anti-hACE2 antibody repertoire for subsequent screening. In mice serum, a high titer of anti-hACE2 antibody was detected after the AAV/hACE2 immunization and three boosters of recombinant hACE2 protein (Figure 1B). In vitro, 50% tissue culture infectious dose (TCID50) assay was performed using authentic SARS-CoV-2, and the mean 50% neutralizing titer (NT50) of the immune serum reached 140 at 18 weeks post primary immunization (Figure 1C). We then used the hybridoma technique to generate monoclonal anti-hACE2 antibodies. An ELISA screening of ∼900 hybridoma supernatants led to the selection of the most prominent hACE2-binding clone (2H2) and one non-binding control (2G8), which were further examined for their binding abilities. As expected, the hybridoma supernatant collected from clone 2H2, but not from clone 2G8, recognized hACE2 on hACE2-expressing 293T (293T/hACE2) cells (Figure 1D). These two mAb clones were used for subsequent experiments in this study.
Figure 1.
Generation and characterization of anti-hACE2-secreting hybridoma
(A) Schematic representation of immunization schedule. Balb/c mice were immunized with 3 × 1011 vg of AAV6/hACE2 and 1 × 1012 vg of AAV9/hACE2 via the intratracheal and intraperitoneal routes, respectively, followed by boosts of Alum-hACE2 protein (10 μg) administered via intramuscular injection at weeks 10, 12, and 14 post primary immunization. (B) The titration of anti-hACE2 antibody in the mouse sera at week 14 p.i. (mean ± SEM). (C) The neutralizing activity (NT50) of hACE2-immunized mice serum at week 18 p.i. evaluated by tissue culture infectious dose assay (mean ± SEM; n = 4 for each group). The p values were calculated by two-tailed unpaired Student’s t test. (D) The binding ability of hybridoma supernatants from clones 2H2 (middle panel, red line) and 2G8 (right panel, blue line) to hACE2 expressed on 293T/hACE2 stable cell line was assessed by flow cytometry analysis. The plain medium (left panel, black line) was included as a control. Gray-shaded histograms represent the background staining of 293T/hACE2 cells. MFI, mean fluorescence intensity.
Characterization of the hACE2-blocking ability of hybridoma clone 2H2
We first evaluated whether hybridoma clone 2H2 could block SARS-CoV-2 S-RBD binding to hACE2. To this end, 293T/hACE2 cells were pre-incubated with plain medium or supernatant collected from hybridoma clones 2H2 and 2G8. Afterward, cells were incubated with mouse Fc-conjugated S-RBD recombinant protein and subjected to fluorescence-activated cell sorting (FACS) analysis. Clone 2H2, but not 2G8, efficiently interrupted the interaction between S-RBD and hACE2, achieving nearly 100% inhibition (Figure 2A). This result indicated that clone 2H2 targets the S-RBD-binding site on hACE2. We next exploited a SARS-CoV-2 S protein-mediated cell-cell fusion assay to assess the blocking ability of clone 2H2. In this assay, the AAV8/GFP-transduced 293T cells expressing the SARS-CoV-2 S protein (293T/spike) were used as effector cells, while AAV8/mCherry-transduced 293T/hACE2 cells were used as target cells. 293T/hACE2 cells pre-incubated with irrelevant immunoglobulin (Ig) G, 2H2, or 2G8 hybridoma supernatants were co-cultured with 293T/spike cells for 72 h, followed by fluorescent microscopy examination. In the presence of isotype control and 2G8 hybridoma supernatant, the effector cells formed syncytia with the target cells (Figure 2B), a process mediated by S protein-hACE2 interaction.23 In contrast, 2H2 hybridoma supernatant significantly impaired the formation of syncytia (Figure 2B). These results demonstrated hybridoma clone 2H2’s capability of blocking S protein binding to hACE2, suggesting its potential for neutralizing SARS-CoV-2.
Figure 2.
Characterization of hACE2-blocking ability of hybridoma clone 2H2
(A) Competitive binding of hybridoma clones 2H2 and 2G8 supernatants to 293T/hACE2 cells with mouse Fc-containing recombinant S-RBD protein, measured by FACS analysis. Plain medium (left panel, black line) was included as a control. Gray-shaded histograms represent the background staining of 293T/hACE2 cells. (B) The treatment of hybridoma supernatant from clone 2H2, but not 2G8, inhibited the fusion between 293T/spike cells (green) and 293T/hACE2 cells (red). The serum of hACE2-immunized mouse and irrelevant IgG served as positive and negative controls, respectively. Scale bar, 200 μm.
Molecular mechanism of hACE2 neutralization by clone 2H2
The epitope of clone 2H2 was probed using complementary methods. First, hydrogen-deuterium exchange mass spectrometry (HDX-MS) was used to examine the 2H2-binding residues on hACE2. For this analysis, recombinant hACE2 with an octa-His tag at the C terminus and the Fab region of 2H2 were prepared for the experiments. During the reaction, hACE2 was incubated in deuterium oxide-based buffer for different time intervals to monitor the HDX of the amide protons. Upon binding to the Fab of 2H2, the epitope would be sequestered from solvent exposure, thus reducing the corresponding HDX. By mapping the HDX difference, i.e., ΔHDX, defined by subtracting the deuterium uptake of hACE2/2H2-Fab complex from that of hACE2 alone, onto the crystal structure of hACE2, we could infer the 2H2 epitope, which corresponded to the regions that exhibited the highest ΔHDX. After 3,600 s of HDX, residues 21–29 at the N terminus of hACE2 (Figure 3A, superimposed on an S-RBD/hACE2 complex structure; (PDB: 6M0J) showed the most profound difference in ΔHDX up to 20% (blue colored in Figure 3A). These residues are implicated in direct contacts with Q24 and T27 of S-RBD (gold colored in Figure 3A).24 The other peptide downstream residues 21–29, namely residues 24–40, also exhibited significant difference in ΔHDX, although not as much as that of residues 21–29 (Figure 3B). Collectively, the HDX-MS analysis strongly indicated the localized 2H2-binding epitope within the N terminus of hACE2.
Figure 3.
2H2 epitope mapping by HDX-MS and cryo-EM analyses
(A) Structural mapping of 2H2 epitope on hACE2 by the HDX difference in percentage (%). The HDX difference (ΔHDX) is defined by subtracting the deuterium uptake of hACE2/2H2-Fab complex from that of hACE2. Color-coded ΔHDX (scale indicated by the color bar) is superimposed on a crystal structure of hACE2/S-RBD (PDB: 6M0J). The S-RBD model is shown in gold, and the N-terminal residues 21–29 of hACE2, which has the highest ΔHDX, are highlighted by the red dashed boxed. (B) Deuterium uptakes of hACE2 N-terminal peptides as a function of HDX time. Data are shown as mean ± SEM for triplicates. (C) Superimposition of cryo-EM structure of hACE2/2H2-Fab complex and crystal structure of hACE2/S-RBD complex (PDB: 6M0J). The partial cryo-EM map of hACE2/2H2-Fab is displayed in white. As for the ribbon models of proteins, the heavy chain (HC) and light chain (LC) of 2H2-Fab are shown in cyan and pink, respectively, and hACE2 is in wheat color and S-RBD in yellow. The mapped epitope of 2H2-Fab (N-terminal amino acids 21–29 of hACE2) is colored in red. (D) Enlarged panel of the competitive binding region of 2H2-Fab and S-RBD on hACE2 (highlighted by orange box in C).
We next used single-particle cryoelectron microscopy (cryo-EM) to generate an electron microscopy (EM) map of hACE2 in complex with the 2H2-Fab to a nominal resolution of 3.5 Å (Figures 3C and 3D). Due to the intrinsic dynamics of the binary complex and the small apparent size of the complex at about 100 kDa, part of the 2H2 Fab could not be resolved in the cryo-EM map. Nevertheless, the secondary structural features of hACE2 were well resolved, thus allowing rigid body docking of the crystal structure of hACE2 (PDB: 6M0J) into the EM map. Additionally, part of the 2H2 Fab could be resolved sufficiently to enable docking of a structural model into the EM map. Further manual refinement and energy minimization of the docking models removed the steric clashes while preserving the relative binding pose of hACE2 and the 2H2 Fab, as constrained by the cryo-EM map. The resulting model showed that the two loops of 2H2-Fab heavy chain (cyan colored) were bound to the N terminus of hACE2 at the region that showed the most significant changes in the HDX-MS analysis (residues 21–29, red colored in Figures 3C and 3D). The superimposition of our model on the crystal structure of S-RBD-bound hACE2 (PDB: 6M0J) illustrated how the 2H2 Fab (light chain in pink and heavy chain in cyan) sterically prevents S-RBD (yellow colored in Figures 3C and 3D) from binding to hACE2. Overall, these biophysical studies provided compelling structural evidence to explain how 2H2 targets the S-RBD-binding motif of hACE2 to neutralize SARS-CoV-2 binding to hACE2.
mAb ch2H2 broadly neutralized wild-type and the variants of SARS-CoV-2
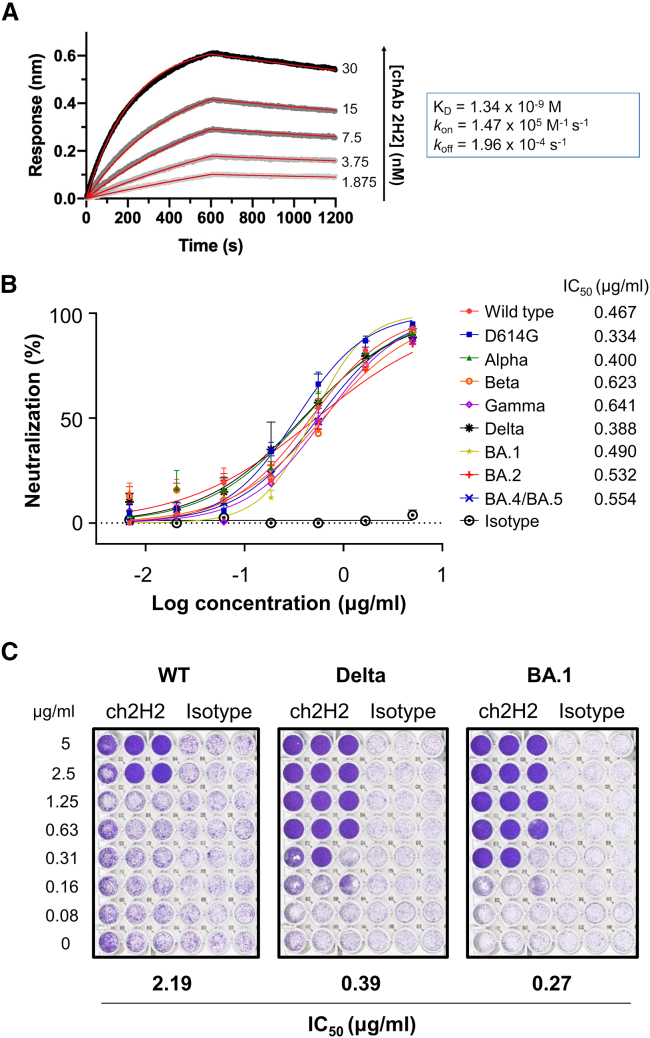
The goal of this study was to develop a broadly neutralizing antibody for COVID-19 prevention and treatment. For clinical use, the immunogenicity of the antibody should be diminished to minimize potential side effects. To this end, we decided to humanize mAb 2H2 to reduce its immunogenicity for future applications. A chimeric mAb 2H2 (ch2H2) was generated by grafting the variable regions of both heavy chain (VH) and light chain (VL) onto a human IgG1 and kappa backbone (Figure S1A). mAb ch2H2 was produced using the CHO cell expression system and then purified for further examinations and experiments. It was first confirmed that the molecular size and the hACE2-binding ability of mAb ch2H2 were comparable to the parental mouse mAb 2H2 (Figures S1B–S1E, respectively). Next, the binding affinity of mAb ch2H2 was evaluated by using biolayer interferometry (BLI). mAb ch2H2 bound to hACE2 with a dissociation constant (KD) of 1.34 nM (Figure 4A), which indicated a stronger binding affinity than the S-RBD derived from various SARS-CoV-2 variants to hACE2 (KD = 5.4–31.4 nM).25 These data suggest that mAb ch2H2 has substantial potential to outcompete SARS-CoV-2 for receptor binding.
Figure 4.
Chimeric 2H2 (ch2H2) mAb has potent neutralizing activity against diverse SARS-CoV-2 variants in vitro
(A) The kinetics of mAb ch2H2 binding to immobilized hACE2, analyzed by biolayer interferometry (BLI). Actual curves are displayed as gray lines, and the best fit of the data to a 1:1 binding model is indicated by red lines. (B) Neutralization potency of mAb ch2H2 against various pseudotyped SARS-CoV-2 variants. Data are shown as mean ± SEM for triplicates. The antibody concentrations resulting in 50% inhibition of infectivity (IC50) are also presented. (C) Neutralization of authentic SARS-CoV-2 variants by mAb ch2H2. Data are representative of two independent experiments.
We then examined whether mAb ch2H2 could neutralize SARS-CoV-2 variants. 293T/hACE2 cells were pre-incubated with different concentrations of mAb ch2H2 or irrelevant IgG. Subsequently, cells were infected with pseudotyped wild-type SARS-CoV-2 (Wuhan strain) and other VOCs, including D614G mutant, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P1), Delta (B.1.617.2), and Omicron sublineages BA.1 (B.1.1.529), BA.2 (B.1.1.529.2), and BA.4/BA.5 (B.1.1.529.4/B.1.1.529.5). The neutralization efficacy was determined 3 days post infection (p.i.). In contrast to the isotype control, which failed to inhibit viral infection regardless of the dose, mAb ch2H2 efficiently neutralized the infections of all tested pseudotyped SARS-CoV-2, displaying similar neutralization curves of individual variants (Figure 4B). Its 50% inhibitory concentration (IC50) ranged from 0.33 to 0.64 μg/mL against respective variants without significant differences. In addition, several commercial anti-hACE2 antibodies were tested for their capability to inhibit pseudotyped wild-type SARS-CoV-2 infection, and none of them demonstrated neutralization activity comparable to that of mAb ch2H2 (Figure S2). The neutralizing activity of mAb ch2H2 was further validated using authentic SARS-CoV-2. Wild-type SARS-CoV-2 and two of the previously dominant variants, Delta and Omicron BA.1, were used in this experiment. Consistently, mAb ch2H2 neutralized all tested viruses, with notably lower IC50s against both the Delta and Omicron BA.1 variants (0.39 μg/mL and 0.27 μg/mL, respectively) (Figure 4C). Taken together, the results suggest that anti-hACE2 mAb ch2H2 is a broad-spectrum neutralizing antibody against SARS-CoV-2. This also validated the concept that broad-neutralization activity could be achieved by directly blocking the hACE2 receptor for SARS-CoV-2 entry.
Given that hACE2 plays multiple physiological roles in the signaling pathway of the renin-angiotensin system (RAS), the safety profile associated with targeting such a vital regulator of vascular function26 is of concern. Theoretically, a reagent designed to impede the S protein-hACE2 interaction should not induce cytotoxicity, as the S-RBD-binding site on hACE2 is located distally from its catalytic domain.27,28 While our structural study demonstrated that mAb ch2H2 did not target the enzymatic domain of hACE2 (Figure 3), we further examined whether mAb ch2H2 might interfere with the carboxypeptidase activity of ACE2 before proceeding to the animal experiment. A synthetic substrate was supplied to recombinant hACE2 protein incubated with mAb ch2H2 or control antibodies (purified human IgG as the negative control and the anti-hACE2 polyclonal antibody AF933, which is known to inhibit the enzymatic activity of hACE2,29 as the positive control). Even at a high concentration of 1,000 nM, mAb ch2H2 showed no allosteric inhibition of hACE2 activity, as hACE2 was still able to cleave the substrate (Figure S3). This result suggests that the treatment of mAb ch2H2 will not impede the physiological function of hACE2.
AAV-delivered mAb ch2H2 alleviated SARS-CoV-2 BA.5 sequelae in K18-hACE2 transgenic mice
Although the prophylaxis or therapeutic effect of recombinant anti-hACE2 mAb against SARS-CoV-2 infection has been previously reported,29,30,31,32,33,34 such protection was rather transitory due to the short half-life of administered antibodies (∼7 days).35 When a long-term protein expression is required, AAV vector is an advantageous transgene delivery system. Previous studies have shown that AAV vectors are capable of expressing monoclonal antibodies at constant levels for several months in vivo following a single administration.36,37,38,39,40 To further improve mAb ch2H2 for future applications, we generated an AAV6 carrying an expression construct of mAb ch2H2 (AAV6/ch2H2). Herein, mAb ch2H2 was expressed from a single open reading frame by linking the heavy and light chains with a 2A self-cleaving peptide (Figure S4A), and this construct was cloned into the AAV vector for AAV6/ch2H2 production. We then transduced 293T cells with AAV6/ch2H2 and examined the anti-hACE2 mAb generated in this manner via the cell-based hACE2-binding assay (Figure 1A). The data confirmed that the binding characteristics of mAb ch2H2 generated from this new expression cassette were similar to those from the hybridoma (Figure S4B). Next, the transduced expression of mAb ch2H2 in vivo was assessed. K18-hACE2 transgenic mice were intratracheally injected with 3 × 1011 vg of AAV6/ch2H2, and mouse serum was collected at indicated times for titration. Human IgG could be detected in the serum within 1 week p.i., reaching a concentration of 136 ng/mL (Figure 5A). The titer gradually increased, peaking at 288 ng/mL after 4 weeks, and remained at a high level for at least 5 weeks. Furthermore, the titer of mAb ch2H2 in the bronchoalveolar lavage fluid (BALF) was measured at week 2 p.i. as an indicator of antibody expression in the lungs. Remarkably, a substantial amount of mAb ch2H2 (3,042 ± 1,196 ng/mL) was found in the BALF, approximately 17 times higher than that in the serum (Figure S5A). Taken together, the results showed that we were able to induce a long-lasting expression of anti-hACE2 mAb with broad SARS-CoV-2-neutralizing capability in vivo.
Figure 5.
AAV6/ch2H2 prophylaxis protected K18-hACE2 transgenic mice from SARS-CoV-2 Omicron BA.5 challenge
(A) AAV6/ch2H2 transduction can result in long-term expression of mAb ch2H2 in mice serum (mean ± SEM). (B) Schematic overview of challenge experiment design. K18-hACE2 transgenic mice (n = 4 for each group), which received a dose of 3 × 1011 vg for both AAV6/ch2H2 and AAV6/empty, were challenged with SARS-CoV-2 Omicron BA.5 variant at a dose of 5 × 104 PFU after 1 week of AAV transduction. Mice were sacrificed on day 3 post challenge, and the lungs were collected for subsequent analyses. (C) The numbers of viral genomic RNA in the lungs of infected K18-hACE2 mice transduced with AAV6/empty (black circle) or AAV6/ch2H2 (red circle) measured by RT-QPCR. The dashed line indicates the limit of detection (LOD, 102 copies/mL of total RNA). (D) The titer of infectious virions in the lungs of infected K18-hACE2 mice transduced with AAV6/empty (black circle) or AAV6/ch2H2 (red circle), measured by TCID50 assay. The dashed line indicates the LOD (102 TCID50/mL). (E) The pulmonary pathophysiology of AAV6/empty- and AAV6/ch2H2-transduced K18-hACE2 mice shown by the H&E images of mouse lungs. Scale bar, 100 μm. (F) Histopathological scores of the lung pathology in infected AAV6/empty- and AAV6/ch2H2-transduced K18-hACE2 mice. In all analyses, p values were calculated by two-tailed unpaired Student’s t test.
K18-hACE2 mice were first transduced with AAV6/ch2H2 intratracheally for 7 days and then challenged with SARS-CoV-2 Omicron BA.5, one of the recently dominant SARS-CoV-2 variants, at an infection dose of 5 × 104 plaque-forming units (PFU). Mice that received AAV6/empty served as the control group. On day 3 p.i., the mice were sacrificed, and the copy number of viral RNA, the titer of infectious virus, and histopathology in the lungs were analyzed. The results showed a 421-fold reduction of viral RNA in the lungs of AAV6/ch2H2-transduced mice compared to the AAV6/empty mice (an average of 1.3 × 105 ± 1.8 × 105 copies/mL and 5.5 × 107 ± 3.5 × 107 copies/mL, respectively) (Figure 5C). Moreover, the expression of mAb ch2H2 led to an 84-fold reduction in the infectious virus titer in mice lungs, decreasing it from 3.2 × 105 ± 1.0 × 105 to 3.8 × 103 ± 4.9 × 103 TCID50/mL (Figure 5D). In the histopathological examination, lungs of the control group showed typical lesions caused by SARS-CoV-2 infection, including moderate peribronchial and perivascular to interstitial inflammatory infiltration with mononuclear cells, alveolar septa thickening, and fibrin and hyaline membrane formation. Additionally, multifocal to diffusive hemorrhage was observed in two cases. In contrast, the lungs of AAV6/ch2H2-transduced mice only showed mild or scattered inflammatory infiltration (Figure 5E). A histopathological scoring system was further used to quantify the pathophysiological condition of the lung. The mean score in control mice was 2.8, whereas it was lowered to 1.9 in AAV6/ch2H2 mice (Figure 5F). These data demonstrated that AAV6/ch2H2 prophylaxis could limit SARS-CoV-2 Omicron BA.5 infection and ameliorate pulmonary pathological manifestation. Altogether, the results from this study suggest that mAb ch2H2 is a prominent prophylactic agent providing broad neutralizing activity against early and current pandemic strains of SARS-CoV-2. With a proper administration strategy, such as AAV-mediated delivery, it may play a significant role in the fight against newly emerging SARS-CoV-2 variants in the future.
Discussion
SARS-CoV-2 has evolved rapidly as it spreads across global human populations, leading to the emergence of D614G substitution in the spike glycoprotein early in the pandemic and sequentially five VOCs.41 These variants are more transmissible than ancestral SARS-CoV-2 and some demonstrate high resistance to neutralization.42 For example, Omicron subvariants, owing to their heavily mutated S-RBD region, showed immune escape from vaccinated or convalescent patient serum, as well as most therapeutic mAbs.1,2,3,4,5,6,7,8,9,10,11,12,13,14,43,44,45 This prompted the development of next-generation vaccines targeting the Omicron BA.1 subvariant, which showed potent neutralizing activity at the beginning of the Omicron variant outbreak.46,47,48 However, within a few months, the pandemic was soon sequentially overtaken by Omicron BA.2, BA.4, and BA.5 subvariants. The rather low cross-neutralizing capacity of BA.1-specific mAbs raised concerns about the effectiveness of BA.1-derived vaccine boosters in achieving broad-spectrum protection.7 The recently available WT/BA.5-bivalent booster elicited high neutralizing titer against Omicron BA.4 and BA.5 subvariants but had limited neutralization ability against the newly emerged Omicron BA.2.75.2, BQ.1.1, or XBB.1 subvariants.49 Therefore, it is imperative to devise new vaccine development approaches or other prophylactic strategies that can offer cross-variant protection. Few recent approaches have focused on broadening the antigenic spectrum of S protein-derived antigens, such as the inclusion of different antigenic mutations from various SARS-CoV-2 variants to develop cross-protective vaccines or the inclusion of randomly arranged sarbecovirus spike to develop mosaic nanoparticles.46,50 In this study, we tapped into a different strategy to target the viral receptor and generated a potent chimerized hACE2-blocking mAb that can act against multiple SARS-CoV-2 VOCs, demonstrated by pseudotyped and authentic SARS-CoV-2 neutralization assays. Of note, in contrast to the results obtained from the pseudovirus neutralization assay (Figure 4B), the authentic virus neutralization assay demonstrated a rather heterogeneous outcome, with a higher IC50 of mAb ch2H2 against the wild-type SARS-CoV-2 than against other VOCs (Figure 4C). While pseudovirus-based neutralizing assays are commonly employed for their enhanced biosafety levels, it is important to acknowledge that the pseudovirus system may not fully replicate the characteristics of authentic virus infection. A pseudovirus infection system can only recapitulate the viral entry step, hence neglecting the impacts of other infection events on the evaluation of antivirals. That being said, our data still illustrated the broad inhibitory activity of mAb ch2H2.
The use of hACE2-blocking mAb carries several advantages over other analogous strategies, such as hACE2-Fc fusion proteins that act as a “decoy” to trap SARS-CoV-2.23,51,52,53 One advantage is that ACE2-blocking mAb has a higher binding affinity (KD = 1.34 nM for mAb ch2H2). Another is that it can circumvent the potential induction of immune responses by the engineered decoy transgene, which may affect the safety and efficacy in clinical use. Anti-hACE2 antibodies have been previously tested as a strategy to block SARS-CoV-2 infection.30,31,32,33,34 In this study, however, AAV was used to administer anti-hACE2 mAb, which distinguishes our approach from previous efforts. One of the benefits of AAV-expressed mAbs is that they can be maintained at constant levels in vivo following a single vector administration.36,37,40,54 This feature represents a significant advantage over the traditional protein-based therapies, which have a short half-life and require frequent and repeated administration to maintain the proteinaceous reagents at therapeutic levels. Our findings clearly indicated that a single intratracheal administration of AAV6/ch2H2 could effectively establish sustained transgene expression in mice for at least 7 weeks, resulting in elevated titers of AAV-expressed mAb ch2H2 in the circulation (Figure 5A). Moreover, we observed even higher levels of mAb ch2H2 in mouse lungs (Figure S5A). These mAb expression levels may persist for more than 1 year, potentially even being lifelong, as reported by some studies on the AAV delivery system.37,38,55 We further demonstrated that this enduring expression of mAb ch2H2 did possess prophylactic potential in vivo against Omicron BA.5, the most antigenically drifted SARS-CoV-2 variant and prone to cause breakthrough infections.6,7,8,9,10 Nonetheless, it could be argued that our challenge experiment did not demonstrate a complete blocking of SARS-CoV-2 infection. There are two plausible explanations for this outcome. First, the SARS-CoV-2 challenge took place rather early after the AAV6/ch2H2 transduction (day 7), which could have led to relatively low mAb ch2H2 titers in the lungs at the time of the challenge. It is plausible that an enhanced protective effect of mAb ch2H2 might have been observed if the SARS-CoV-2 challenge occurred after the antibody titers in the lungs reached a plateau, typically after day 28 post transduction. Second, inadequate antibody levels in the nasal cavity, a major site of SARS-CoV-2 infection,56 might have allowed some cells to become infected, preventing the achievement of sterilizing immunity. To explore this possibility, we conducted additional experiments to examine the nasal lavage fluid (NALF) and found a discrepancy in antibody titers between the upper and lower respiratory airways. The mAb ch2H2 concentration in the nasal cavity was approximately 200 ng/mL, which was 14-fold lower than that in the lung, with significant variation among individuals (Figure S5B). Therefore, improving the antibody expression in the nasal cavity may be approached to escalate the protective effect of mAb ch2H2. Potential strategies for this enhancement could include intranasal delivery of AAV or the use of other AAV serotypes with enhanced transduction efficiency in the respiratory tract. AAV9, AAV6.2, AAV6.2FF, and AAVhu68, which have been reported to transduce airways,57,58,59 could be useful alternatives in this context. For example, a recent study on vectored immunoprophylaxis utilizing AAV6.2FF to deliver humanized anti-respiratory syncytial virus antibodies demonstrated high antibody titers in the serum and mucosal surfaces, leading to potential sterilizing immunity.60
While we have demonstrated the prophylactic function of mAb ch2H2 and its potential for preventing SARS-CoV-2 infection, there remain challenges to be addressed and areas for improvement before it can be transitioned into clinical trials. For instance, the AAV6/ch2H2 dose used to transduce mice (3 × 1011 vg) may be considered high in this scenario. In an additional test using a reduced AAV6/ch2H2 dose of 1 × 1011 vg/mouse to evaluate the circulating antibody titer level, we found that the mAb titer in mouse serum was actually comparable to that achieved with the higher dose of 3 × 1011 vg/mouse (Figure S6). Although further animal experiments could not be performed to verify the protective efficacy of a low-AAV6/ch2H2-dose regimen due to limited access to the animal biological safety level 3 (ABSL-3) facility, this could be a valuable direction for future research. Moreover, the untapped therapeutic potential of mAb ch2H2 is worthy of further investigation. Last but not least, the types of the chimeric mAb 2H2 for clinical applications also deserve further exploration. In this study, mAb ch2H2 was built on the base of IgG. However, recent studies have proved the importance of the two mucosal antibodies, IgM and IgA, which constitute the first line of defense against mucosal pathogens, in anti-SARS-CoV-2 immune response.61,62 Typically, IgM assembles into pentamers and IgA assembles into dimers in the presence of the joining chain (J chain), facilitating the effective mucosal transcytosis of these antibodies. Owing to their avidity effects, these multivalent antibodies can exhibit enhanced neutralization of SARS-CoV-263,64 and offer broader protection against SARS-CoV-2 variants.65,66 Therefore, the generation of ch2H2 IgM and IgA mAb presents a promising avenue to explore to increase the efficacy of this anti-hACE2 antibody.
In conclusion, this study proposed a gene-therapy-like strategy to achieve long-term immunoprophylaxis against the infection of SARS-CoV-2 variants. The continuous emergence of SARS-CoV-2 variants outpaces the speed of vaccine development. While booster doses are intended to maintain the immunity, people’s willingness to receive them may decrease due to the concerns about the side effects, accessibility, or the perceived necessity of boosters.67,68 As an alternative approach to preventing or treating COVID-19, this promising preclinical study on AAV-delivered anti-hACE2 mAb ch2H2 provides a potentially more effective intervention. Furthermore, we anticipate that such anti-hACE2 mAb would be particularly beneficial for immunocompromised or immunosuppressed populations, who are especially vulnerable to the severe consequences of SARS-CoV-2 infection, and for those who do not generate vaccine- or infection-elicited protective immunity. It may very well serve as a more universal solution in combating against hACE2-dependent pathogens.
Materials and methods
Ethics statement
All mouse works were conducted according to the Guideline for the Care and Use of Laboratory Animals as defined by the Council of Agriculture, Taiwan. Mouse work was approved by the Institutional Animal Care and Use Committee (IACUC) of Academia Sinica (protocol ID: 20-05-1471 and 22-01-1798). The Institutional Biosafety Committee of Academia Sinica approved experiments with infectious SARS-CoV-2 virus under BSL3 conditions. All sample processes were conducted in accordance with Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19) recommended by the Taiwan Centers for Disease Control (CDC).
Animals
Balb/c mice were purchased from National Laboratory Animal Center (Taipei, Taiwan). B6.Cg-Tg(K18-hACE2) 2Prlmn/J transgenic mice (stock number: 034860) were purchased from Jackson Laboratory (ME, USA). All mice were maintained as small breeding colonies in a specific pathogen-free environment in the animal facilities of the Institute of Biomedical Sciences, Academia Sinica. All experimental procedures were reviewed and approved by the Animal Care and Use Committee of Academia Sinica.
Cells and viruses
The monkey kidney epithelial Vero-E6 cells, parental human embryonic kidney 293T cells, and 293T cells stably expressing hACE2 (293T/hACE2) or S protein (293T/spike) (provided by National RNAi Core Facility, Academia Sinica, Taiwan) were grown at 37°C under 5% CO2 in the growth medium (DMEM, Gibco, Thermo Fisher Scientific, MA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S, Gibco, Thermo Fisher Scientific, MA, USA)].
AAV6/hACE2, AAV9/hACE2, AAV8/GFP, AAV8/mCherry, and AAV6/ch2H2 were produced by AAV core facility (Academia Sinica, Taipei, Taiwan). Lentiviral-based pseudotyped SARS-CoV-2 variants expressing luciferase were provided by National RNAi Core Facility (Academia Sinica, Taipei, Taiwan). Wild-type (hCoV-19/Taiwan/4/2020), Delta variant (hCoV-19/Taiwan/1144/2021), and Omicron BA.1 variant (hCoV-19/Taiwan/16804/2021) of SARS-CoV-2 virus were obtained from the Taiwan CDC. Omicron BA.5 (hCoV-19/Taiwan/TSGH-8189/2022) was obtained from Tri-Service General Hospital, Taipei, Taiwan. Vero-E6 cells were used for the propagation of SARS-CoV-2 stocks. The preparation of wild-type, Delta variant, and Omicron BA.1 variant, as well as the cell-based experiments involving these viruses, was conducted in the biosafety level 3 (BSL-3) facility in the Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan. Omicron BA.5 variant was propagated in the BSL-3 facility in the Institute of Preventive Medicine, National Defense Medical College, Taipei, Taiwan.
Plasmid construction
Engineering of chimeric anti-hACE2 (ch2H2) mAb was conducted by Human Therapeutic Antibody Development Platform (BioTReC, Academia Sinica). The signal peptides and variable regions of mouse mAb 2H2 were synthesized and fused with the coding sequence of human kappa light chain constant region, and human IgG1 heavy chain constant region into pcDNA5/FRT, named pcDNA/ch2H2-LC and pcDNA/ch2H2-HC, respectively. For AAV vector construction, the coding sequence of ch2H2 light chain (forward primer, 5′-GGAATTGTACCCGCGGCCGCCATGGAGACAGACACACTCC-3′; reverse primer, 5′-CTCCGGATCCACACTCTCCCCTGTTGAAGCTCTTTAC-3′), heavy chain (forward primer, 5′-CTGGACCTATGGAGGTGAAGCTGGTG-3′; reverse primer, 5′-GATAAGCTTGATGCGGCCGCTCATTTACCCGGAGACAGG-3′), and P2A (forward primer, 5′-GGGAGAGTGTGGATCCGGAGCTACTAACTT-3′; reverse primer, 5′- GAGGAGAACCCTGGACCTATGGAGGTGAAG-3′) were amplified by PCR and cloned into a NotI-linearized ssAAV vector, downstream of a global cytomegalovirus (CMV) enhancer/beta-actin (CB) promoter In-Fusion HD Cloning Plus kit (Takara Bio, Shiga, Japan). The recombinant AAV vectors were produced by a triple transfection method as previously described69 and purified by cesium chloride sedimentation. The physical vector titers were assessed by real-time PCR using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, MA, USA).
Immunization
For the generation of anti-hACE2 hybridoma, 6- to 8-week-old female Balb/c mice were immunized with 3 × 1011 vg of AAV6/hACE2 and 1 × 1012 vg of AAV9/hACE2 via the intratracheal and intraperitoneal (i.p.) routes, respectively, as previously described.70 This was followed by boosts of Alum-hACE2 protein (10 μg) administered via intramuscular injection at weeks 10, 12, and 14 post primary immunization.
ELISA analysis of antibody binding to hACE2
For the detection of mouse anti-hACE2 antibodies, 96-well NUNC Maxisorp plates (Thermo Fisher Scientific, MA, USA) were coated overnight with 0.5 μg/well of recombinant his-tagged hACE2 (homemade, 5 μg/mL, 50 μL). Plates were blocked overnight with 3% skim milk in PBS. Hybridoma supernatant or serum samples collected from hACE2-immunized Balb/c mice were serially diluted in 3% skim milk in PBS and incubated in coated wells for 1 h at room temperature. After incubation, bound antibodies were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, A, and M antibody (Cappel, NC, USA).
For the detection of chimerized anti-hACE2 (ch2H2) antibodies, the plates were coated with goat anti-human IgG, A, and M (Jackson Immuno Research, PA, USA). Serum collected from AAV6/ch2H2-transduced K18-hACE2 mice were added to the blocked plates and HRP-conjugated goat anti-human IgG (Cappel, NC, USA) was used as detection antibody. Serial diluted Chrompure human IgG (Jackson Immuno Research, PA, USA) was included in each plate as the standard to ensure the accurate quantification of mAb ch2H2 titers. Plates were developed by TMB substrate (BD Biosciences, NJ, USA) and the reactions were stopped by adding 2 N H2SO4. The OD450 absorbance was detected by EMax Microplate reader (Molecular Devices, CA, USA).
Flow cytometry analysis
To test the binding ability of anti-hACE2 antibodies, 4 × 105 293T/hACE2 cells were resuspended in 100 μL of Dulbecco’s phosphate buffered saline (DPBS) containing 1% FBS and then incubated with hybridoma supernatant, purified mAb 2H2, mAb ch2H2, or isotype IgG for 1 h. Next, the cells were incubated with 100 μL of PE-labeled goat anti-mouse IgG-Fc antibody (Jackson Immuno Research, PA, USA) or PE-labeled goat anti-human IgG Fc (Jackson Immuno Research, PA, USA) for 30 min. To test the ability of antibodies to block the binding between hACE2 and S-RBD, 4 × 105 293T/hACE2 cells were first incubated with hybridoma supernatant for 1 h, followed by a subsequent incubation with 0.2 μg of recombinant S-RBD containing a human Fc region at the C terminus. Next, the cells were stained with PE-conjugated goat anti-human IgG Fc (Jackson Immuno Research, PA, USA) for 30 min. After the staining procedure, cells were resuspended in 300 μL of 1% FBS-DPBS containing 0.25 μg of 7-amino-actinomycin D (Thermo Fisher Scientific, CA, USA) for the exclusion of non-viable cells. The cells were analyzed by using Attune NxT-14 color analyzer (Thermo Fisher Scientific, MA, USA) and FlowJo software (Tree Star, OR, USA).
For testing the transduced expression of mAb, 1 × 106 293T cells were transduced with recombinant AAV6/ch2H2 at the MOI of 105. At day 2 post transduction, the culture supernatant was collected and serially diluted for treating 4 × 105 293T/hACE2 cells. The incubation and staining procedures were identical to those described above.
In vitro cell-cell fusion assay
293T/hACE2 cells transduced by AAV8/mCherry were used as the target cells, and 293T/spike cells transduced by AAV8/EGFP were used as the effector cells. At 24 h post AAV transduction, target 293T/hACE2 cells and effector 293T/spike cells were detached with Versene solution (Gibco, Thermo Fisher Scientific, MA, USA) and co-cultured in a ratio of 1:1 in 24-well plates. Hybridoma supernatant was added at the time of co-culture, and, 24 h later, the syncytia images were acquired by inverted fluorescence microscope (ImageXpress Micro XLS, Molecular Device, USA).
Production and purification of mouse 2H2 and chimeric 2H2 antibodies
The mouse 2H2 antibody was produced by hybridoma cells. To produce the chimeric 2H2 (ch2H2) antibody, ExpiCHO cells were transfected with plasmids pcDNA/ch2H2-HC and pcDNA/ch2H2-LC according to the Max Titer protocol on ExpiCHOTM Expression System user guide (Gibco, MA, USA). The hybridoma or transfected ExpiCHO cells were centrifuged at 1,500 rpm for 10 min at 4°C for the collection of the supernatant after 3 or 12 days of transfection, respectively. The supernatant was centrifuged again at 2,000 rpm for 60 min at 10°C and then filtered through a 0.45-μm filter. The filtrate was then purified on Protein A Sepharose 4 Fast Flow (GE healthcare, IL, USA) and Econo column (Bio-Rad, CA, USA). The purity and degree of assembly were analyzed by SDS-PAGE and western blot analysis.
SDS-PAGE and western blot
The 100-ng purified mAb 2H2 and mAb ch2H2 was loaded on to 4%–12% Bis-Tris gradient gel (NuPAGE, Thermo Fisher Scientific, MA, USA) and transferred to the polyvinylidene fluoride (PVDF) membranes (Bio-Rad, CA, USA). The transferred membranes were blocked with 5% skim milk in TBS containing 0.05% Tween 20 (TBST) for 2 h at room temperature. For mAb 2H2, the light chain was recognized by rabbit anti-mouse IgG k light chain antibody (Pharmingen, BD Biosciences, NJ, USA) and the heavy chain was recognized by goat anti-mouse IgG (Cappel, MP Biomedicals, OH, USA). For ch2H2, the light chain was recognized by goat anti-human kappa chain (Cappel, MP Biomedicals, OH, USA) and the heavy chain was recognized by goat anti-human IgG Fc (Cappel, MP Biomedicals, OH, USA). The detection antibodies were diluted in 5% skim milk in TBST, and the transferred PVDF membranes were incubated in diluted antibodies overnight at 4°C. HRP-conjugated donkey-anti-goat IgG (Santa Cruz, TX, USA) and HRP-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, PA, USA) served as the secondary antibody. The blots were visualized with ECL western blot substrate (Millipore, MA, USA) and analyzed by LAS 3000 (Fujifilm, Tokyo, Japan).
Preparation of hACE2-8H and mAb 2H2 for structure analyses
The hACE2 tagged with 8 his (hACE2-8H) construct was constructed in pcDNA3.1 followed by transiently expressed in Expi293 cell. hACE2-8H protein was purified by a HiTrap 5-mL QFF column (GE healthcare, IL, USA) followed by Superdex 200 16/600 GL column (GE healthcare, IL, USA) in PBS buffer. Fab fragments of 2H2 were digested from IgG using papain (Thermo Fisher Scientific, MA, USA) in digestion buffer (PBS, 20 mM EDTA, 20 mM cysteine) for 6 h at 37°C. Stop reaction buffer (PBS with 0.3 M iodoacetamide) was used to stop papain digestion. Purified hACE2-8H and digested 2H2-Fab were mixed at 1:1.1 molar ratio for 30-min incubation on ice before further purification by Superdex 200 Increase 10/300 GL (GE healthcare, IL, USA) in 20 mM Tris/HCl, pH 7.4, 150 mM NaCl. The complex peak was pooled and concentrated to 3 mg/mL.
HDX-MS
HDX-MS measurements were carried out using an SYNAPT G2-HDMS system equipped with a LEAP robotic liquid handler (Waters Corporation, MA, USA). The stock solutions of hACE2-8H, 2H2-Fab or hACE2-8H/2H2-Fab complex (100 μM) were diluted 20-fold by the labeling buffer (50 mM Tris, 150 mM NaCl [(pD7.4]) to trigger HDX for 0.5, 10, 30, 60, 120, and 240 min at 25°C (in triplicates), followed by a 1:1 mixing with the quenching buffer (50 mM Tris, 200 mM TCEP, 4 M guanidine hydrochloride [pH 2.0]). Online digestion using an immobilized pepsin digestion column (Waters Enzymate BEH Pepsin, 2.1 × 30 mm) followed by trapping using a C18 trapping column (Acquity BEH VanGuard 1.7 μm, 2.1 × 5.0 mm) was performed. The pepsin digests were separated by a linear acetonitrile gradient of 5%–40%. Protein Lynx Global Server (PLGS) and DynamX (Waters Corporation, MA, USA) were used to identify the individual peptides and subsequently data processing using the following parameters: minimum intensity of 1,000, minimum product ions per amino acid of 0.1, maximum MH + error of 5 ppm, and file threshold of three. A reference molecule ((Glu1)-fibrinopeptide B human [Merck Millipore, MA, USA]) was used to lock mass with an expected molecular weight of 785.8426 Da. The fractional deuterium uptakes of individual peptides and common peptides were extracted by DynamX to generate heatmaps as a function of residue number and to facilitate structural mapping onto the structure of ACE2-8H/2H2-Fab complex using PyMOL (Schrödinger, NY, USA).
Cryo-EM sample preparation, data collection, processing, and model building
Three microliters of the purified hACE2-8H/2H2-Fab complex protein was applied onto 300-mesh Quantifoil R1.2/1.3 holy carbon grid. The grid was glow discharged at 20 mA for 30 s and was blotted for 2.5 s under 4°C with 100% humidity and vitrified using a Vitrobot Mark IV (Thermo Fisher Scientific, MA, USA). Data acquisition was performed on a Titan Krios G3 at 300 keV equipped with a Gatan K3 detector. Videos were acquired in the super-resolution mode using EPU v2.10 (Thermo Fisher Scientific, MA, USA). Data were processed with C1 symmetry in CryoSPARC 3.0. An initial model of Fab was generated based on PDB 6EV2 and 7CZR, and the hACE2 structure template was generated from PDB 7W6R by using Swiss-Model. Model fitting to the map was done by Chimera X 1.5.
BLI
For BLI kinetics assay, hACE2-sfGFP was cloned, expressed, and purified as hACE2-8H. A stock solution of biotinylated hACE2-sfGFP was diluted to 10 μg/mL for immobilization onto high-precision streptavidin (SAX) biosensors (Sartorius, Gottingen, Germany) in assay buffer (PBS, 0.1% BSA, 0.02% Tween 20). Stock solutions of mAb 2H2 were serially diluted to 30, 15, 7.5, 3.75, and 1.875 nM in assay buffer for independent binding using an OctetRED 96 biolayer interferometer (Pall ForteBio, CA, USA). The association and dissociation were monitored over 600 and 600 s, respectively. The six independent BLI sensorgrams with different mAb 2H2 protein concentrations were baseline corrected using double reference. The data were globally fit to a 1:1 binding model using Data Analysis v.10.0 software (ForteBio). The results were exported and replotted by Prism v.9 (GraphPad Software, CA, USA).
Pseudotyped SARS-CoV-2 neutralization assay
One day before neutralization assay, 293T/hACE2 at a density of 1 × 104 cells/well were seeded into 96-well black plate (PerkinElmer, MA, USA). Purified mAb ch2H2 and commercial anti-hACE2 antibodies were serially diluted 3-fold in 50 μL DMEM supplemented with 2% FBS (ranging from 5 to 0.006 μg/mL) and used to treat 293T/hACE2 cells for 1 h at 37°C. Then, the pseudotyped SARS-CoV-2 carrying the luciferase reporter were added to each well. After 6 h of incubation, antibody-treated 293T/hACE2 cells were supplemented with 150 μL DMEM/2% FBS and incubated for another 3 days. Luciferase Assay kit (Promega, WI, USA) was used to detect luciferase activity in infected cells and the IC50 was calculated by using the nonlinear regression module of Prism software version v.9 (GraphPad Software, CA, USA).
Authentic SARS-CoV-2 variants micro-neutralization assay
Wild-type (hCoV-19/Taiwan/4/2020), Delta variant (hCoV-19/Taiwan/1144/2021), and Omicron BA.1 variant (hCoV-19/Taiwan/16804/2021) of SARS-CoV-2 were used for authentic virus micro-neutralization assay. Purified mAb ch2H2 or isotype control was serially diluted 2-fold (ranging from 5 to 0.08 μg/mL) in DMEM supplemented with 2% FBS and then added to pre-seeded Vero-E6 cells in triplicate. After 1 h of incubation, cells were infected with 100 TCID50 of wild-type SARS-CoV-2 or variants for 4 days. Then, cells were fixed with 10% formaldehyde and stained with 0.5% crystal violet for 20 min. The plates were washed with distilled water and scored for the cytopathic effects. The 50% neutralizing titer was calculated by the Reed and Muench method.
ACE2 enzymatic activity assay
The enzymatic activities of ACE2 were assessed following the user’s guide of ACE2 inhibitor screening kit (BioVision, MA, USA). Three-fold serially diluted mAb ch2H2, Chrompure human IgG, (Jackson Immuno Research, PA, USA), or goat anti-hACE2 antibody AF933 (R&D system, MN, USA) were incubated with recombinant hACE2 at room temperature for 15 min. After the incubation, the ACE2 substrate was added to the cells and the relative fluorescence units (RFU) were measured on a SpectraMax Gemini EM Microplate Reader (Molecular Devices, CA, USA) with an excitation wavelength set at 320 nm and an emission wavelength set at 420 nm in kinetic mode for 1 h. The relative activity (%) was calculated as (ΔRFU of sample/ΔRFU of enzyme control) × 100.
AAV infection in mice
Eight- to 10-week-old Balb/c or K18-hACE2 mice were anesthetized by i.p. injection of a mixture of atropine (0.4 mg/mL, Astar Pharmaceutical, Hsinchu, Taiwan)/ketamine (20 mg/mL, Ketalar, Pfizer, PA, USA)/xylazine (0.4%, Rompun, Bayer, PA, USA). AAV immunization was described in section “immunization.” For in vivo prophylaxis study of SARS-CoV-2 infection, K18-hACE2 mice were intratracheally administrated with 3 × 1011 vg AAV6/ch2H2.
Collection of BALF
After sacrifice, blood was collected from the mice’s hearts using a 27G needle with a 1-mL syringe. The neck area was then disinfected with alcohol and a longitudinal incision was made along the neck to expose the underlying tissues. By carefully cutting through the muscles and connective tissues in the neck, the trachea was revealed. Another incision was made approximately halfway up the trachea, and then 1 mL of PBS was injected through the incision into the lung using a 24G indwelling needle attached to a 1-mL syringe. Care was taken during this process to slowly extract the maximal volume of BALF. The collected fluid was then centrifuged at 8,000 × g for 10 min, and the resulted supernatant was stored at −80°C for further analysis.
Collection of NALF
After the collection of BALF, a 29G insulin needle with a PE20 tubing (∼1 cm in length, BD Intramedic, NJ, USA) was inserted into the same tracheal incision but in the opposite direction to the BALF collection. Then, 300 μL of PBS was flushed through the nasal passages. The fluid obtained from the nasal cavity was subjected to centrifugation at 8,000 × g for 10 min. The resulting supernatant was carefully collected and stored at −80°C for further analysis.
SARS-CoV-2 challenge experiment
Six- to eight-week-old K18-hACE2 mice were anesthetized and intranasally (i.n.) challenged with 5 × 104 PFU of SARS-CoV-2 Omicron BA.5 variant in a volume of 50 μL. Mice were sacrificed for the sample collection on day 3 p.i. Mouse lungs were harvested for the quantification of viral genomic RNA, titration of infectious virions, and the histopathological analysis. All mouse challenge experiments were evaluated and approved by the IACUC of Academia Sinica, Taiwan, and conducted in the animal biological safety level 4 (ABSL-4) facility at Institute of Preventive Medicine, National Defense Medical College, Taipei, Taiwan.
RNA extraction and RT-QPCR for SARS-CoV-2 genomic RNA quantification
To measure the copy number of SARS-CoV-2 genomic RNA, one-half of the right mouse lung was homogenized. To ensure the nucleic acid stability during sample transportation, DNA/RNA shield (Zymo research, CA, USA) was added into tissue homogenates. Homogenates were centrifuged at 3,000 rpm for 5 min at 4°C. The supernatant was collected, and total RNA was then extracted by using LabTurbo AIO Viral DNA/RNA Extraction Kit (Taigen Bioscience, TPE, TW). RT-qPCR was performed using the TaqMan Fast Virus 1-Step Master Mix (Applied Biosystems, CA, USA) with primer-probe sets targeting the SARS-CoV-2 N gene. Forward primer N_Sarbeco_F1 (5′-CACATTGGCACCCGCAATC-3′) and the reverse primer N_Sarbeco_R1 (5′-GAGGAACGAGAAGAGGCTTG-3′), in addition to the probe N-Sarbeco-P1 (5′- FAM-ACTTCCTCAAGGAACAACATTGCCA-BBQ-3′), were used in the assay. Thermal cycles of RT-QPCR were set as the following conditions: 50°C for 5 min, 95°C for 30 s, followed by 45 cycles of 95°C for 5 s and 58°C for 30 s. Both RNA extraction and RT-qPCR were performed using LabTurbo AIO 48 SP-QPCR System (Taigen Bioscience, TPE, TW), an all-in-one automated device, according to the manufacturer’s instructions. To estimate the copy numbers of viral genome, in vitro transcribed RNA standards containing SARS-CoV-2 N gene sequences were synthesized by using HiScribe T7 Quick High Yield RNA Synthesis Kit (New England Biolabs, MA, USA). RNA standards were reverse transcribed by QIAcuity One-Step Viral RT-PCR Kit (Qiagen, NRW, GER) and quantified by QIAcuity Digital PCR system (Qiagen, NRW, GER).
Quantification of viral titer by the tissue cell culture infectious assay
To quantify infectious SARS-CoV-2 virions, one-half of the right mouse lung was homogenized in 1 mL of MEM supplemented with 1% FBS and 1% P/S. After centrifugation at 13,000 rpm for 10 min, the supernatant was collected for virus titration. Vero-E6 cell monolayers in quadruplicate were incubated with 100 μL of serial 10-fold dilutions of samples in MEM containing 1% FBS for 1 h at 37°C. Cells were then incubated for 4 days for the observation of cytopathic effects via crystal violet staining. The 50% tissue culture infectious dose (TCID50) per milliliter was calculated by the Reed and Muench method.
Histopathological examination of the tissue
Mice lung were collected and fixed in 4% paraformaldehyde. After 1 week of fixation, the tissues were processes for paraffin embedding, sectioning, and hematoxylin and eosin (H&E) staining followed by microscopy examination. The histopathology of infected mouse lungs was evaluated according to a lung histopathological scoring system as previously reported.71,72
Graphical illustrations
Graphical illustrations were created by using Biorender.com.
Statistical analysis
Results are presented as the mean ± standard error of the mean (SEM). Differences between experimental groups of animals were analyzed by Student’s t test; p < 0.05 was considered as statistically significant.
Data and code availability
The datasets produced or analyzed during the present study are accessible upon reasonable request.
Acknowledgments
We thank the following facilities for their support on the experiments: Biosafety Level 3 Facility in Institute of Biomedical Sciences (grant AS-CFII-108-102), DNA Sequencing Core Facility (grant AS-CFII-108-115), AAV Core Facility (grant AS-CFII109-103), Flow Cytometry Core Facility (grant AS-CFII108-111), Biophysics Core Facility (grant AS-CFII-108-111), Protein Clinic (grant AS-CFII-111-206), Common Mass Spectrometry Facilities for Proteomics and Protein Modification Analysis (grant AS-CFII-111-209), and Cryo-EM Center (grant AS-CFII-108-111), all of which are funded by the Academia Sinica Core Facility and Innovative Instrument Project. We thank the Human Therapeutic Antibody R&D Platform of the Biomedical Translation Research Center, Academia Sinica, and Light Microscopy Core Facility and Pathology Core Facility of the Institute of Biomedical Sciences, Academia Sinica. We also thank the Core Facility Platform for Emerging Infectious Diseases (National Core Facility for Biopharmaceuticals, National Science and Technology Council, Taiwan) for the help with BSL-3 and ABSL-3 experiments. This research was funded by Ministry of Science and Technology, Taiwan (MOST-111-2320-B-001-024-MY3), Academia Sinica Biomedical Translation Research Center (AS-KPQ-110-EIMD and AS-KPQ-111-KNT), Infectious Disease Research Supporting Grant (AS-IDR-112-04 to M.H.T. and AS-IDR-112-08 to S.T.D.H), and Career Development Award (AS-CDA-109-L08 to S.T.D.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
M.H.T. conceived and designed the project. M.H.T. provided overall instruction for the study, and he and I.H.W. supervised the project. P.Y.W. coordinated the experiments. P.Y.W., T.Z.K, Y.H.L., L.Y.C., H.Y.N., H.N.L., and I.J.L. generated and characterized anti-hACE2 antibody-secreting hybridoma clones. M.F.H., Y.C.C., S.P., P.D., J.S.H., and S.T.D.H. analyzed the molecular mechanism for neutralization of hACE2 by mAbs. M.J.C. and H.C.W. cloned and generated chimerized mAb. C.W.C. and S.Y.T. characterized recombinant mAbs and conducted pseudovirus neutralization assays. C.W.C. and S.C.T. constructed AAV vectors and characterized mAb expression. C.C.L., C.S.C., and J.J.L. performed authentic virus neutralization assays. C.W.H., C.C.C., and J.H.K. designed and performed animal experiments in ABSL-3 facilities. W.C.L. and S.C.T. performed the analysis of mAb titers in the respiratory tracts of mice. Y.H.C. conducted histopathological examination. M.H.T., C.P.S., C.W.C., and S.I.T. analyzed the data. C.P.S., I.J.L., S.I.T., C.H.L., and Y.C.C. wrote the first draft. M.H.T., I.H.W., S.T.D.H., and H.C.W. reviewed and edited the manuscript. All authors reviewed and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.09.002.
Contributor Information
I-Hsuan Wang, Email: ihwang@ibms.sinica.edu.tw.
Mi-Hua Tao, Email: bmtao@ibms.sinica.edu.tw.
Supplemental information
References
- 1.Cameroni E., Bowen J.E., Rosen L.E., Saliba C., Zepeda S.K., Culap K., Pinto D., VanBlargan L.A., De Marco A., di Iulio J., et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. doi: 10.1038/s41586-021-04386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejnirattisai W., Huo J., Zhou D., Zahradník J., Supasa P., Liu C., Duyvesteyn H.M.E., Ginn H.M., Mentzer A.J., Tuekprakhon A., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caniels T.G., Bontjer I., van der Straten K., Poniman M., Burger J.A., Appelman B., Lavell A.H.A., Oomen M., Godeke G.J., Valle C., et al. Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccination. Sci. Adv. 2021;7:eabj5365. doi: 10.1126/sciadv.abj5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., Bolland W.H., Porrot F., Staropoli I., Lemoine F., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 6.Tuekprakhon A., Nutalai R., Dijokaite-Guraliuc A., Zhou D., Ginn H.M., Selvaraj M., Liu C., Mentzer A.J., Supasa P., Duyvesteyn H.M.E., et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422–2433.e13. doi: 10.1016/j.cell.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan K., Karim F., Ganga Y., Bernstein M., Jule Z., Reedoy K., Cele S., Lustig G., Amoako D., Wolter N., et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat. Commun. 2022;13:4686. doi: 10.1038/s41467-022-32396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora P., Kempf A., Nehlmeier I., Schulz S.R., Cossmann A., Stankov M.V., Jäck H.M., Behrens G.M.N., Pöhlmann S., Hoffmann M. Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5. The Lancet. Lancet Infect. Dis. 2022;22:1117–1118. doi: 10.1016/S1473-3099(22)00422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., Guo Y., Iketani S., Nair M.S., Li Z., Mohri H., Wang M., Yu J., Bowen A.D., Chang J.Y., et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608:603–608. doi: 10.1038/s41586-022-05053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanBlargan L.A., Errico J.M., Halfmann P.J., Zost S.J., Crowe J.E., Jr., Purcell L.A., Kawaoka Y., Corti D., Fremont D.H., Diamond M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022;28:490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann M., Krüger N., Schulz S., Cossmann A., Rocha C., Kempf A., Nehlmeier I., Graichen L., Moldenhauer A.S., Winkler M.S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456.e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L., Iketani S., Guo Y., Chan J.F.W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 15.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair H.A. Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection. Drugs. 2020;80:189–196. doi: 10.1007/s40265-020-01258-3. [DOI] [PubMed] [Google Scholar]
- 17.Emu B., Fessel J., Schrader S., Kumar P., Richmond G., Win S., Weinheimer S., Marsolais C., Lewis S. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N. Engl. J. Med. 2018;379:645–654. doi: 10.1056/NEJMoa1711460. [DOI] [PubMed] [Google Scholar]
- 18.Xiong L., Edwards C.K., 3rd, Zhou L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int. J. Mol. Sci. 2014;15:17411–17441. doi: 10.3390/ijms151017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng J., Chen L., Yuan Y., Wang K., Wang Y., Qin C., Wu G., Chen R., Zhang Z., Wei D., et al. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants delta, alpha, beta, and gamma. Signal Transduct. Target. Ther. 2021;6:347. doi: 10.1038/s41392-021-00760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bian H., Zheng Z.H., Wei D., Wen A., Zhang Z., Lian J.Q., Kang W.Z., Hao C.Q., Wang J., Xie R.H., et al. Safety and efficacy of meplazumab in healthy volunteers and COVID-19 patients: a randomized phase 1 and an exploratory phase 2 trial. Signal Transduct. Target. Ther. 2021;6:194. doi: 10.1038/s41392-021-00603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang K.Y., Lin M.S., Kuo T.C., Chen C.L., Lin C.C., Chou Y.C., Chao T.L., Pang Y.H., Kao H.C., Huang R.S., et al. Humanized COVID-19 decoy antibody effectively blocks viral entry and prevents SARS-CoV-2 infection. EMBO Mol. Med. 2021;13:e12828. doi: 10.15252/emmm.202012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang T.J., Yu P.Y., Chang Y.C., Liang K.H., Tso H.C., Ho M.R., Chen W.Y., Lin H.T., Wu H.C., Hsu S.T.D. Effect of SARS-CoV-2 B.1.1.7 mutations on spike protein structure and function. Nat. Struct. Mol. Biol. 2021;28:731–739. doi: 10.1038/s41594-021-00652-z. [DOI] [PubMed] [Google Scholar]
- 25.Han P., Li L., Liu S., Wang Q., Zhang D., Xu Z., Han P., Li X., Peng Q., Su C., et al. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185:630–640.e10. doi: 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 28.Towler P., Staker B., Prasad S.G., Menon S., Tang J., Parsons T., Ryan D., Fisher M., Williams D., Dales N.A., et al. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y., Zhang Y.N., Yan R., Wang G., Zhang Y., Zhang Z.R., Li Y., Ou J., Chu W., Liang Z., et al. ACE2-targeting monoclonal antibody as potent and broad-spectrum coronavirus blocker. Signal Transduct. Target. Ther. 2021;6:315. doi: 10.1038/s41392-021-00740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaouat A.E., Ilija B., Paola Kucan B., Nofar A., Kliker L., Alfi O., Mandelboim M., Wolf D., Tafish L., Kol I., et al. Anti-human ACE2 antibody neutralizes and inhibits virus production of SARS-CoV-2 variants of concern. iScience. 2022 doi: 10.1016/j.isci.2022.104935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du W., Hurdiss D.L., Drabek D., Mykytyn A.Z., Kaiser F.K., González-Hernández M., Muñoz-Santos D., Lamers M.M., van Haperen R., Li W., et al. An ACE2-blocking antibody confers broad neutralization and protection against Omicron and other SARS-CoV-2 variants of concern. Sci. Immunol. 2022;7:eabp9312. doi: 10.1126/sciimmunol.abp9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Y., Shi R., Zhang Y., Duan X., Li L., Zhang J., Wang F., Zhang R., Shen H., Wang Y., et al. A broadly neutralizing humanized ACE2-targeting antibody against SARS-CoV-2 variants. Nat. Commun. 2021;12:5000. doi: 10.1038/s41467-021-25331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou J., Zhang Y., Wang Y., Zhang Z., Wei H., Yu J., Wang Q., Wang G., Zhang B., Wang C. ACE2-Targeting antibody suppresses SARS-CoV-2 Omicron and Delta variants. Signal Transduct. Target. Ther. 2022;7:43. doi: 10.1038/s41392-022-00913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F., Jenkins J., de Carvalho R.V.H., Nakandakari-Higa S., Chen T., Abernathy M.E., Baharani V.A., Nyakatura E.K., Andrew D., Lebedeva I.V., et al. Pan-sarbecovirus prophylaxis with human anti-ACE2 monoclonal antibodies. Nat. Microbiol. 2023;8:1051–1063. doi: 10.1038/s41564-023-01389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieira P., Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 36.Fang J., Qian J.J., Yi S., Harding T.C., Tu G.H., VanRoey M., Jooss K. Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 37.Balazs A.B., Bloom J.D., Hong C.M., Rao D.S., Baltimore D. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat. Biotechnol. 2013;31:647–652. doi: 10.1038/nbt.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balazs A.B., Chen J., Hong C.M., Rao D.S., Yang L., Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2011;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balazs A.B., Ouyang Y., Hong C.M., Chen J., Nguyen S.M., Rao D.S., An D.S., Baltimore D. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat. Med. 2014;20:296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Navio J.M., Fuchs S.P., Pantry S.N., Lauer W.A., Duggan N.N., Keele B.F., Rakasz E.G., Gao G., Lifson J.D., Desrosiers R.C. Adeno-Associated Virus Delivery of Anti-HIV Monoclonal Antibodies Can Drive Long-Term Virologic Suppression. Immunity. 2019;50:567–575.e5. doi: 10.1016/j.immuni.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs J.L., Haidar G., Mellors J.W. COVID-19: Challenges of Viral Variants. Annu. Rev. Med. 2023;74:31–53. doi: 10.1146/annurev-med-042921-020956. [DOI] [PubMed] [Google Scholar]
- 42.Carabelli A.M., Peacock T.P., Thorne L.G., Harvey W.T., Hughes J., COVID-19 Genomics UK Consortium. Peacock S.J., Barclay W.S., de Silva T.I., Towers G.J., Robertson D.L. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023;21:162–177. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu L., Mok B.W.Y., Chen L.L., Chan J.M.C., Tsang O.T.Y., Lam B.H.S., Chuang V.W.M., Chu A.W.H., Chan W.M., Ip J.D., et al. Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Variant by Sera From BNT162b2 or CoronaVac Vaccine Recipients. Clin. Infect. Dis. 2022;75:e822–e826. doi: 10.1093/cid/ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rössler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheward D.J., Kim C., Ehling R.A., Pankow A., Castro Dopico X., Dyrdak R., Martin D.P., Reddy S.T., Dillner J., Karlsson Hedestam G.B., et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: a cross-sectional study. Lancet Infect. Dis. 2022;22:813–820. doi: 10.1016/S1473-3099(22)00129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee I.J., Sun C.P., Wu P.Y., Lan Y.H., Wang I.H., Liu W.C., Yuan J.P.Y., Chang Y.W., Tseng S.C., Tsung S.I., et al. A booster dose of Delta x Omicron hybrid mRNA vaccine produced broadly neutralizing antibody against Omicron and other SARS-CoV-2 variants. J. Biomed. Sci. 2022;29:49. doi: 10.1186/s12929-022-00830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gagne M., Moliva J.I., Foulds K.E., Andrew S.F., Flynn B.J., Werner A.P., Wagner D.A., Teng I.T., Lin B.C., Moore C., et al. mRNA-1273 or mRNA-Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell. 2022;185:1556–1571.e18. doi: 10.1016/j.cell.2022.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ying B., Scheaffer S.M., Whitener B., Liang C.Y., Dmytrenko O., Mackin S., Wu K., Lee D., Avena L.E., Chong Z., et al. Boosting with variant-matched or historical mRNA vaccines protects against Omicron infection in mice. Cell. 2022;185:1572–1587.e11. doi: 10.1016/j.cell.2022.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurhade C., Zou J., Xia H., Liu M., Chang H.C., Ren P., Xie X., Shi P.Y. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023;29:344–347. doi: 10.1038/s41591-022-02162-x. [DOI] [PubMed] [Google Scholar]
- 50.Cohen A.A., van Doremalen N., Greaney A.J., Andersen H., Sharma A., Starr T.N., Keeffe J.R., Fan C., Schulz J.E., Gnanapragasam P.N.P., et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science. 2022;377:eabq0839. doi: 10.1126/science.abq0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higuchi Y., Suzuki T., Arimori T., Ikemura N., Mihara E., Kirita Y., Ohgitani E., Mazda O., Motooka D., Nakamura S., et al. Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nat. Commun. 2021;12:3802. doi: 10.1038/s41467-021-24013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L., Dutta S., Xiong S., Chan M., Chan K.K., Fan T.M., Bailey K.L., Lindeblad M., Cooper L.M., Rong L., et al. Engineered ACE2 decoy mitigates lung injury and death induced by SARS-CoV-2 variants. Nat. Chem. Biol. 2022;18:342–351. doi: 10.1038/s41589-021-00965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L., Narayanan K.K., Cooper L., Chan K.K., Skeeters S.S., Devlin C.A., Aguhob A., Shirley K., Rong L., Rehman J., et al. An ACE2 decoy can be administered by inhalation and potently targets omicron variants of SARS-CoV-2. EMBO Mol. Med. 2022;14:e16109. doi: 10.15252/emmm.202216109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Limberis M.P., Adam V.S., Wong G., Gren J., Kobasa D., Ross T.M., Kobinger G.P., Tretiakova A., Wilson J.M. Intranasal antibody gene transfer in mice and ferrets elicits broad protection against pandemic influenza. Sci. Transl. Med. 2013;5:187ra72. doi: 10.1126/scitranslmed.3006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Lieshout L.P., Rghei A.D., Cao W., He S., Soule G., Zhu W., Thomas S.P., Sorensen D., Frost K., Tierney K., et al. AAV-monoclonal antibody expression protects mice from Ebola virus without impeding the endogenous antibody response to heterologous challenge. Mol. Ther. Methods Clin. Dev. 2022;26:505–518. doi: 10.1016/j.omtm.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cevik M., Kuppalli K., Kindrachuk J., Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 57.van Lieshout L.P., Domm J.M., Rindler T.N., Frost K.L., Sorensen D.L., Medina S.J., Booth S.A., Bridges J.P., Wootton S.K. A Novel Triple-Mutant AAV6 Capsid Induces Rapid and Potent Transgene Expression in the Muscle and Respiratory Tract of Mice. Mol. Ther. Methods Clin. Dev. 2018;9:323–329. doi: 10.1016/j.omtm.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Limberis M.P., Wilson J.M. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sims J.J., Greig J.A., Michalson K.T., Lian S., Martino R.A., Meggersee R., Turner K.B., Nambiar K., Dyer C., Hinderer C., et al. Intranasal gene therapy to prevent infection by SARS-CoV-2 variants. Plos Pathog. 2021;17:e1009544. doi: 10.1371/journal.ppat.1009544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rghei A.D., Yates J.G.E., Lopes J.A., Zhan X., Guilleman M.M., Pei Y., van Lieshout L.P., Santry L.A., Bridle B.W., Karimi K., et al. Antibody-based protection against respiratory syncytial virus in mice and their offspring through vectored immunoprophylaxis. Gene Ther. 2023 doi: 10.1038/s41434-023-00385-2. [DOI] [PubMed] [Google Scholar]
- 61.Gasser R., Cloutier M., Prévost J., Fink C., Ducas É., Ding S., Dussault N., Landry P., Tremblay T., Laforce-Lavoie A., et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS-CoV-2. Cell Rep. 2021;34:108790. doi: 10.1016/j.celrep.2021.108790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., Quentric P., Fadlallah J., Devilliers H., Ghillani P., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar N., Arthur C.P., Ciferri C., Matsumoto M.L. Structure of the secretory immunoglobulin A core. Science. 2020;367:1008–1014. doi: 10.1126/science.aaz5807. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Wang G., Li N., Wang Y., Zhu Q., Chu H., Wu W., Tan Y., Yu F., Su X.D., et al. Structural insights into immunoglobulin M. Science. 2020;367:1014–1017. doi: 10.1126/science.aaz5425. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Viant C., Gaebler C., Cipolla M., Hoffmann H.H., Oliveira T.Y., Oren D.A., et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 2021;13:eabf1555. doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ejemel M., Li Q., Hou S., Schiller Z.A., Tree J.A., Wallace A., Amcheslavsky A., Kurt Yilmaz N., Buttigieg K.R., Elmore M.J., et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat. Commun. 2020;11:4198. doi: 10.1038/s41467-020-18058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su Z., Cheshmehzangi A., McDonnell D., da Veiga C.P., Xiang Y.T. Mind the "Vaccine Fatigue". Front. Immunol. 2022;13:839433. doi: 10.3389/fimmu.2022.839433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attwell K., Hannah A., Leask J. COVID-19: talk of 'vaccine hesitancy' lets governments off the hook. Nature. 2022;602:574–577. doi: 10.1038/d41586-022-00495-8. [DOI] [PubMed] [Google Scholar]
- 69.Chen C.C., Sun C.P., Ma H.I., Fang C.C., Wu P.Y., Xiao X., Tao M.H. Comparative study of anti-hepatitis B virus RNA interference by double-stranded adeno-associated virus serotypes 7, 8, and 9. Mol. Ther. 2009;17:352–359. doi: 10.1038/mt.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun C.P., Jan J.T., Wang I.H., Ma H.H., Ko H.Y., Wu P.Y., Kuo T.J., Liao H.N., Lan Y.H., Sie Z.L., et al. Rapid generation of mouse model for emerging infectious disease with the case of severe COVID-19. PLoS Pathog. 2021;17:e1009758. doi: 10.1371/journal.ppat.1009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., Tang H., Nishiura K., Peng J., Tan Z., et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI insight. 2019;4:e123158. doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang R.D., Liu M.Q., Chen Y., Shan C., Zhou Y.W., Shen X.R., Li Q., Zhang L., Zhu Y., Si H.R., et al. Pathogenesis of SARS-CoV-2 in Transgenic Mice Expressing Human Angiotensin-Converting Enzyme 2. Cell. 2020;182:50–58.e8. doi: 10.1016/j.cell.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets produced or analyzed during the present study are accessible upon reasonable request.