Abstract
Methanotrophs in enrichment cultures grew and sustained atmospheric methane oxidation when supplied with methanol. If they were not supplied with methanol or formate, their atmospheric methane oxidation came to a halt, but it was restored within hours in response to methanol or formate. Indigenous forest soil methanotrophs were also dependent on a supply of methanol upon reduced methane access but only when exposed to a methane-free atmosphere. Their immediate response to each methanol addition, however, was to shut down the oxidation of atmospheric methane and to reactivate atmospheric methane oxidation as the methanol was depleted.
Methane-oxidizing bacteria are culturable on 10 to 50% methane in air, but such cultured methanotrophs have not been found to sustain oxidation of atmospheric methane (1.8 parts per million per volume [ppmv]). Sustained oxidation of atmospheric methane in aerobic soils has been explained by the presence of uncultured methanotrophs with high affinities for methane (3, 6), cooxidation by nitrifiers (32), and mixotrophy (16, 22).
The metabolism of methane oxidation is initiated by the methane monooxygenase (MMO) (1). Methane is oxidized by the MMO to methanol with the consumption of NADH + H+ or reduced quinoles (20, 37). From methanol, a set of dehydrogenases supply reductive power by the oxidation of methanol, formaldehyde, and formate, producing carbon dioxide in a concerted pathway which offers formaldehyde to be assimilated for biosynthesis. During fortuitous oxidation this pathway is not fully operative beyond the MMO, and reductive power must be supplied endogenously from storage material as PHA (polyhydroxyalkanoates) or exogenously as methane metabolites for the sustained oxidation of various hydrocarbons such as n-alkanes, propylene, or trichloroethylene (5, 8, 10–12, 18, 21, 26, 31, 33). We hypothesized that a low availability of methane would result in a starvation effect comparable to fortuitous oxidation, with a restricted flow of reductive power back to the MMO which at a critical threshold value would bring the MMO to a halt.
Mixotrophy with parallel oxidation of methanol, formaldehyde, or formate liberated during the degradation of organic matter in soil (14, 25, 29) has the potential to feed the MMO with reductive power and sustain its methane oxidation. In this study, we tested whether addition of methanol or formate would sustain the oxidation of atmospheric methane by starved methanotrophs.
Soil collection.
The soil used in experiments with indigenous methane-oxidizing bacteria was collected at different dates in 1995 and 1996 from 5 to 10 cm below the litter layer in a stand of 33- to 34-year-old Picea abies trees 35 km north of Copenhagen, Denmark. The soil was sieved (2-mm mesh) and stored at 5°C in the dark. Enrichment cultures of methanotrophic bacteria were obtained from five different agricultural soils collected from 0- to 10-cm depths at different dates in 1995. Two soils were taken from near the Agricultural University of Norway (K2 and H1), one from about 130 km north of Oslo (Ap), and two from Stend in western Norway, near Bergen (Kr and St) (Table 1).
TABLE 1.
Characteristics of soils used as inocula for methane enrichment and indigenous (forest soil) experiments
| Code | Vegetation | Soil type | pH (in H2O) | Carbon (% of dry wt) |
|---|---|---|---|---|
| K2 | Ley (grains) | Clay loam | 6.0 | 4.77 |
| H1 | Grains | Clay loam | 6.2 | 7.41 |
| Ap | Ley (grains) | Sandy loam | 7.1 | 3.26 |
| Kr | Ley | Organic | 6.4 | 32.80 |
| St | Ley | Sandy | 5.6 | 5.38 |
| Forest | Spruce | Loamy sand | 4.2 | 13.30 |
Cultivation of methane oxidizers.
Enrichment cultures of methane oxidizers were made by adding 5 g of soil to 10 ml of NMS (a modified version of the mineral medium used by Whittenbury et al. [34]: MgSO4 · 7H2O [1.0 g liter−1], CaCl2 · 2H2O [134.0 mg liter−1], EDTA ferric monosodium salt [4.0 mg liter−1], KNO3 [1.0 g liter−1] [stock solution adjusted to pH 6.8], trace element solution [0.5 ml], KH2PO4 [272.0 mg liter−1], and Na2HPO4 · 2H2O [356.0 mg liter−1] [this P buffer was adjusted separately to pH 6.8 before addition] [trace element solution contained EDTA disodium salt {500.0 mg liter−1}, FeSO4 · 7H2O {200.0 mg liter−1}, ZnSO4 · 7H2O {10.0 mg liter−1}, MnCl2 · 4H2O {3.0 mg liter−1}, H3BO3 {30.0 mg liter−1}, CoCl2 · 6H2O {20.0 mg liter−1}, CaCl2 · 2H2O {1.0 mg liter−1}, NiCl2 · 6H2O {2.0 mg liter−1}, and Na2MoO4 · 2H2O {3.0 mg liter−1}]) in 120-ml serum flasks, which were capped with butyl rubber stoppers and supplied with a mixture of 17% CH4 and 1.7% CO2. The flasks were incubated at 30°C with shaking at 170 rpm in a Maxi-Shake (Heto Holten AS, Allerød, Denmark). Subculturing was done regularly as the cultures became visibly turbid. These cultures were, early in their turbidity, used for methane oxidation experiments.
Extraction of indigenous soil bacteria.
Indigenous methane oxidizers were extracted and separated from the soil particles by the density gradient centrifugation method described by Priemé et al. (24). To prevent growth of protozoa, we added cycloheximide to a final concentration of 100 mg liter−1. The effect of cycloheximide on methane oxidation was found to be tolerable (up to 13,000 mg of cycloheximide liter−1 added to soil slurries produced <15% inhibition of methane oxidation activity [data not shown]).
Gas chromatography.
Methane oxidation rates were determined by flame ionization gas chromatography of headspace samples collected from serum flasks closed with butyl rubber septa. Methanotrophic enrichment cultures in flasks were sampled for analysis of 1 ml of gas (13). Slurries or extracts of indigenous bacteria in 120-ml flasks were sampled likewise for analysis of 3 ml of gas (23).
Atmospheric methane oxidation in the presence of methanol or formate.
In experiments with diluted methane enrichments, 10-ml cultures were incubated with laboratory air in 120-ml capped serum flasks incubated in the Maxi-Shake at 170 rpm and 30°C. Pulses (0.1 ml) of methanol (high-performance liquid chromatography grade; Rathburn, Walkerburn, United Kingdom) were added from a 1-ml syringe fixed by the flask septum. In a prolonged starvation experiment in which 60 ml of St culture (1.2 × 108 cells ml−1) was incubated at room temperature with magnetic stirring (Variomag, Munich, Germany), intermittent flask closures for periods of 10 to 20 h allowed monitoring of the effect of methanol on atmospheric methane oxidation. In a follow-up experiment with 30 ml of St culture (6.0 × 107 cells ml−1) in 60-ml serum flasks, methanol or formate was added after the atmospheric methane oxidation had declined to very low levels.
In experiments involving soil slurries (10 g of soil and 10 ml of water) or suspensions of extracted bacteria (20 ml), 0.2 ml of methanol (99.8%) or water was added with a syringe through the septum. In some experiments, with cultures or suspensions exposed to atmospheric methane for extended periods, the flasks were kept uncapped (loosely covered with aluminum foil or parafilm) and incubated at 22°C in a vertical position on the shaker (100 cycles min−1). At intervals, the flasks were capped and placed horizontally and the shaker was adjusted to 125 cycles min−1 to allow aerobic measurements of methane oxidation. Where the organisms were to be deprived of methane, the septa were not removed and the flasks were flushed daily with a methane-free mixture of 20% O2 and 80% N2. To measure the methane oxidation in these treatments, the methane-free atmosphere was replaced with normal air for a brief period (5 h) and then restored as soon as the measurements were completed.
Cell counts.
Total cell counts were determined in a Helber counting chamber (depth, 0.02 mm; 1/400 mm3 [Mellige]). Viable methanotrophs were estimated as most probable numbers on microtiter plates (35). Serial dilutions in NMS (1:10 per step) were incubated at 30°C in 2% CO2- and 20% CH4-enriched air. A well with visible turbidity not seen in the control plate incubated without added CH4 after 3 weeks was considered a positive well.
Data analysis.
Atmospheric methane oxidation rates were calculated according to first-order kinetics (dC/dt = Ck, where C is the methane concentration and k is the rate constant) by estimating k through linear regression of ln (C) against time (t). The regression coefficient (k) estimates the methane oxidation in parts per million per volume per hour at 1 ppmv in the gas phase. This was further transformed to nanograms of CH4 per milliliter or per gram (dry weight) of soil, taking into account the molecular weight of methane, the headspace volume, the liquid volume, and the solubility of methane in water (36). The reported rates are thus estimates of the methane oxidation at 1 ppmv in the gas phase; comparison with ambient in situ flux measurements implies multiplication by 1.8.
Atmospheric methane oxidation by cultured methanotrophs.
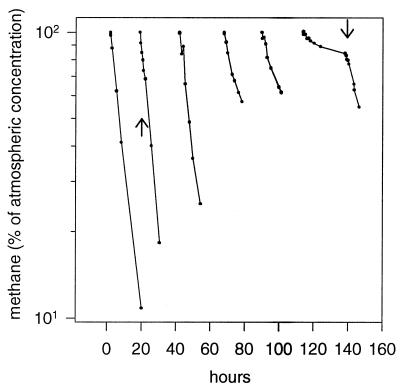
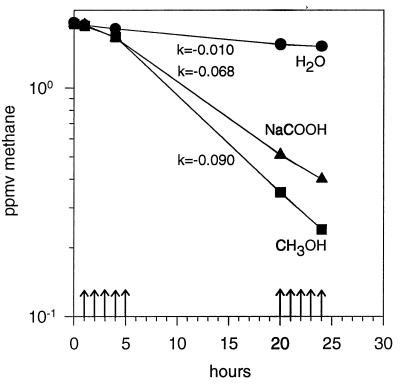
The mixotrophic oxidation of atmospheric methane was investigated in methanotrophic enrichment cultures from five agricultural soils. Diluted cultures exposed to air and supplemented with methanol grew and initiated oxidation of atmospheric methane after 40 to 70 h; maximum oxidation occurred in the presence of 0.5 to 5 mM methanol (data not shown). Undiluted cultures exposed to air maintained high levels of atmospheric methane oxidation during an initial starvation period of about 0 to 90 h, at which point PHA or a similar reserve material was visible by phase contrast microscopy as large refractive bodies in the cells. A decline in oxidation was quickly restored by addition of methanol, as seen for the St culture containing a minimum of 2.0 × 106 methanotrophs ml−1 (Fig. 1). Prolonged exposure to air did not hinder the ability of methanol or formate to restore the oxidation of atmospheric methane. As shown in Fig. 2, a threshold value below which methane oxidation was halted was not observed when the starved methanotrophs were supplemented with methanol or formate.
FIG. 1.
Oxidation of atmospheric methane by the St culture. At 0 h, laboratory air replaced the 17% CH4 and 1.7% CO2 growth condition. Single pulses of methanol were added after 20.1 h (833 μM final concentration) and after 139.3 h (83.3 μM final concentration), as indicated by the arrows. Note the logarithmic vertical axis.
FIG. 2.
Oxidation of atmospheric methane in the St culture after 10 days of exposure to laboratory air. Equal volumes of CH3OH, NaCOOH (166.7 μM pulses), or water were added as indicated by the arrows. Uptake rates are denoted by k. Note the logarithmic vertical axis (k of 1 equals 30 nl of CH4 oxidized h−1 at 1 ppmv of CH4).
Atmospheric methane oxidation by indigenous methane oxidizers.
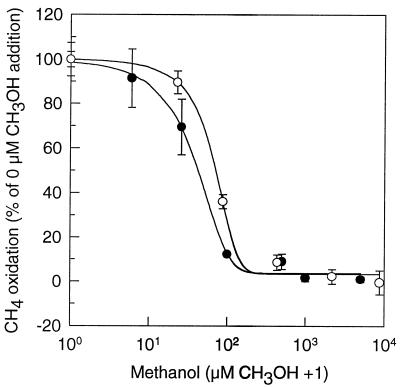
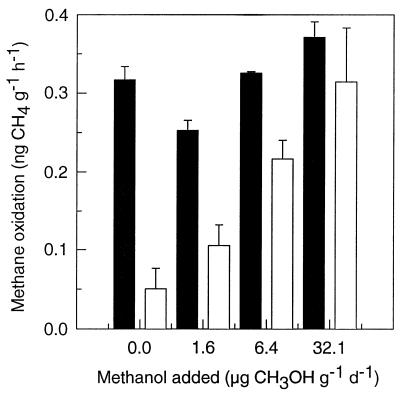
Methanol was also explored as a cosubstrate for the oxidation of atmospheric methane by indigenous forest soil bacteria. Addition of 0 to 5 mM methanol in daily to weekly doses for up to 7 months did not enhance the atmospheric methane oxidation by indigenous soil bacteria, either in soil slurries or in suspensions of extracted bacteria (data not shown). Also, we did not observe any immediate stimulation of oxidation following the addition of methanol to methane-starved slurries or suspensions (data not shown). On the contrary, the oxidation of atmospheric methane in slurries or suspensions was inhibited by the addition of methanol (Fig. 3). However, the inhibitory effect of methanol was transient. The recovery period depended on the initial concentration of added methanol; for example, at 8.8 mM CH3OH, methane oxidation rates recovered completely within 8 days (data not shown). The CO2 evolution was monitored during a similar experiment by which methanol depletion could be quantified as the difference in accumulated CO2 between amended and unamended slurries. We found that the recovery of methane oxidation coincided with the exhaustion of the methanol. In soil slurries with no methanol addition, methane starvation caused a decrease in methane oxidation compared to slurries kept at 1.8 ppmv of methane (Fig. 4).
FIG. 3.
Oxidation of atmospheric methane in soil slurries (○) and suspensions (•) of extracted soil bacteria. The curves represent fits to sigmoid functions. Error bars correspond to ±1 standard error (n = 3). Note the logarithmic horizontal axis.
FIG. 4.
Oxidation of atmospheric methane by soil slurries supplemented with daily doses of methanol for 17 days and supplied with atmospheric methane (solid bars) or deprived of methane (open bars). A recovery period of 4 days followed the last methanol dose before methane oxidation rates were measured. The addition of 32.1 mg of CH3OH g−1 day−1 corresponds to an initial CH3OH concentration of 0.44 mM. Error bars correspond to 1 standard error (n = 3).
Conclusions.
Many characteristics of in situ oxidation of methane by soils have been reproduced by cultured methanotrophs (2, 9, 17). However, cultured methanotrophs do not sustain methane oxidation during prolonged exposure to atmospheric methane concentrations (28, 30). This has been an important contribution to the assumption that as-yet-uncultured methanotrophs with superior affinities for methane are the dominant oxidizers of atmospheric methane in aerobic soils (3, 6, 7, 9, 15, 17, 19, 27). However, the present investigation demonstrates that culturable methanotrophs sustain high rates of atmospheric methane oxidation if they are supplied with methanol or formate.
Bender and Conrad (3, 4) have stated that cultured methanotrophs are characterized by a high threshold value for methane as a consequence of their low methane affinity. We propose that this minimum value, beyond which no methane is oxidized during prolonged incubation for 3 to 7 days, is caused by a restricted electron flow to the MMO. A threshold value regulated by energy metabolism would be dependent on production and consumption of reductive equivalents. Hence, during prolonged oxidation to methane concentrations too low to support a balanced supply of reductive power, the MMO will halt as a result of starvation and depleted energy reserves (and thus be comparable to fortuitous oxidation).
The indigenous methane oxidizers in soil slurries or suspensions of extracted bacteria contrasted with the cultured methane oxidizers in their response to an external supply of methanol. The indigenous bacteria reduced their atmospheric methane oxidation to a minimum in response to moderate (10 to 100 μM) concentrations of methanol. Inhibition was reversible, however, and restoration of the original activity appeared to coincide with the depletion of methanol. This effect is likely to have been exerted through (i) product inhibition of the MMO, (ii) competitive inhibition of the MMO by methanol (5), or (iii) varying starvation statuses among the individual soil bacteria. Further, we were unable to detect any methane threshold for these bacteria, as the methane concentration of the slurries declined at a log linear scale to below the detection limit of the gas chromatograph (0.1 ppmv). The absence of a threshold does not mean that the indigenous methane oxidizers were independent of a minimum substrate supply, as was demonstrated by the significant decline in the atmospheric methane oxidation after exposure to a methane-free atmosphere. Such loss of methane-oxidizing capacity during methane starvation is comparable to the loss observed by Schnell and King (30) in a mixed hardwood-conifer soil. In contrast, soil from a coniferous forest showed unaltered methane oxidation activity upon methane starvation (27). This contrast can be explained by differing availabilities of soil methanol, hence emphasizing the regulative importance of alternative C1 compounds on the oxidation of atmospheric methane in soils.
Acknowledgments
We thank the Commission of the European Union for financial support of A. Priemé through Environmental Program contract ENV4-CT95-0035 and the Norwegian Research Council for financial support of S. Jensen through “Soil Biology Program” contract 103130/120.
REFERENCES
- 1.Anthony C. The biochemistry of methylotrophs. London, England: Academic Press Ltd.; 1982. [Google Scholar]
- 2.Bédard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender M, Conrad R. Kinetics of CH4 oxidation in oxic soils exposed to ambient air or high CH4 mixing ratios. FEMS Microbiol Ecol. 1992;101:261–270. [Google Scholar]
- 4.Bender M, Conrad R. Kinetics of methane oxidation in oxic soils. Chemosphere. 1993;26:687–696. [Google Scholar]
- 5.Colby J, Stirling D I, Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath) Biochem J. 1977;165:395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad R. Capacity of aerobic microorganisms to utilize and grow on atmospheric trace gases (H2, CO, CH4) In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C: American Society for Microbiology; 1984. pp. 461–467. [Google Scholar]
- 7.Conrad R. Soil microbial processes involved in production and consumption of atmospheric trace gases. Adv Microb Ecol. 1995;14:207–250. [Google Scholar]
- 8.Green J, Dalton H. Substrate specificity of soluble methane monooxygenase. J Biol Chem. 1989;264:17698–17703. [PubMed] [Google Scholar]
- 9.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henrysson T, McCarty P L. Influence of the endogenous storage lipid poly-β-hydroxybutyrate on the reducing power availability during cometabolism of trichloroethylene and naphthalene by resting methanotrophic mixed cultures. Appl Environ Microbiol. 1993;59:1602–1606. doi: 10.1128/aem.59.5.1602-1606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins I J, Best D J, Scott D. Generation of products by methanotrophs. Basic Life Sci. 1982;19:383–402. doi: 10.1007/978-1-4684-4142-0_29. [DOI] [PubMed] [Google Scholar]
- 12.Hou C T, Patel R N, Laskin A I, Barnabe N. Microbial oxidation of gaseous hydrocarbons: oxidation of lower N-alkenes and N-alkanes by resting cell suspensions of various methylotrophic bacteria, and the effect of methane metabolites. FEMS Microbiol Lett. 1980;9:267–270. [Google Scholar]
- 13.Jensen, S., and R. A. Olsen. Atmospheric methane consumption in adjacent arable and forest soil systems. Soil Biol. Biochem., in press.
- 14.Killham K. Soil ecology. Cambridge, England: Cambridge University Press; 1994. [Google Scholar]
- 15.King G M. Ecological aspects of methane oxidation, a key determinant of global methane dynamics. Adv Microb Ecol. 1992;12:431–468. [Google Scholar]
- 16.King G M. Ecophysiological characteristics of obligate methanotrophic bacteria and methane oxidation in situ. In: Murrell J C, Kelly D P, editors. Microbial growth on C1 compounds. Adover, United Kingdom: Intercept Ltd.; 1993. pp. 303–313. [Google Scholar]
- 17.King G M. Physiological limitations of methanotrophic activity in situ. In: Murrell J C, Kelly D P, editors. Microbiology of atmospheric trace gases. NATO ASI series. I 39. Berlin, Germany: Springer-Verlag; 1996. pp. 17–32. [Google Scholar]
- 18.Leak D J, Dalton H. In vivo studies of primary alcohols, aldehydes and carboxylic acids as electron donors for the methane mono-oxygenase in a variety of methanotrophs. J Gen Microbiol. 1983;129:3487–3497. [Google Scholar]
- 19.Lidstrom M E. Environmental molecular biology approaches: promises and pitfalls. In: Murrell J C, Kelly D P, editors. Microbiology of atmospheric trace gases. NATO ASI series. I 39. Berlin, Germany: Springer-Verlag; 1996. pp. 121–134. [Google Scholar]
- 20.Lipscomb J D. Biochemistry of the soluble methane monooxygenase. Annu Rev Microbiol. 1994;48:371–399. doi: 10.1146/annurev.mi.48.100194.002103. [DOI] [PubMed] [Google Scholar]
- 21.McFarland M J, Vogel C M, Spain J C. Methanotrophic cometabolism of trichloroethylene (TCE) in a two stage bioreactor system. Water Res. 1992;26:259–265. [Google Scholar]
- 22.Nesbit S P, Breitenbeck G A. A laboratory study of factors influencing methane uptake by soils. Agric Ecosyst Environ. 1992;41:39–54. [Google Scholar]
- 23.Priemé A, Christensen S. Seasonal and spatial variation of methane oxidation in a Danish spruce forest. Soil Biol Biochem. 1997;29:1165–1172. [Google Scholar]
- 24.Priemé A, Sitaula I B, Klemedtsson Å K, Bakken L R. Extraction of methane oxidizing bacteria from soil particles. FEMS Microbiol Ecol. 1996;21:59–68. [Google Scholar]
- 25.Reddy C A. Physiology and biochemistry of lignin degradation. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C: American Society for Microbiology; 1984. pp. 558–571. [Google Scholar]
- 26.Romanovskaya V A, Solokov I G, Malashenko Y R. Coupling of organotroph and lithotroph metabolism in methane-utilizing bacteria. Mikrobiologiya. 1985;54:11–16. [Google Scholar]
- 27.Roslev P, Iversen N, Henriksen K. Oxidation and assimilation of atmospheric methane by soil methane oxidizers. Appl Environ Microbiol. 1997;63:874–880. doi: 10.1128/aem.63.3.874-880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roslev P, King G M. Aerobic and anaerobic starvation metabolism in methanotrophic bacteria. Appl Environ Microbiol. 1995;61:1563–1570. doi: 10.1128/aem.61.4.1563-1570.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schink B. Microbial degradation of pectin in plants and aquatic environments. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Washington, D.C: American Society for Microbiology; 1984. pp. 580–587. [Google Scholar]
- 30.Schnell S, King G M. Stability of methane oxidation capacity to variations in methane and nutrient concentrations. FEMS Microbiol Ecol. 1995;17:285–294. [Google Scholar]
- 31.Stanley S H, Dalton H. The biotransformation of propylene to propylene oxide by Methylococcus capsulatus (Bath). 1. Optimization of rates. Biocatalysis. 1992;6:163–175. [Google Scholar]
- 32.Steudler P A, Jones R D, Castro M S, Melillo J M, Lewis D L. Microbial controls of methane oxidation in temperate forest and agricultural soils. In: Murrell J C, Kelly D P, editors. Microbiology of atmospheric trace gases. NATO ASI series. I 39. Berlin, Germany: Springer-Verlag; 1996. pp. 69–84. [Google Scholar]
- 33.Stirling D I, Dalton H. The fortuitous oxidation and cometabolism of various carbon compounds by whole-cell suspensions of Methylococcus capsulatus (Bath) FEMS Microbiol Lett. 1979;5:315–318. [Google Scholar]
- 34.Whittenbury R, Phillips K C, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 35.Woomer P L. Most probable number counts. In: Mickelson S H, Weaver R W, Angle S, Bottomley P, Bezdicek D, Smith S, Tabatabai A, Wollum A, editors. Methods of soil analysis, p. 2: microbiological and biochemical properties. SSSA book series no. 5. Madison, Wis: Soil Science Society of America, Inc.; 1994. pp. 59–80. [Google Scholar]
- 36.Yamamoto S, Alcauskas J B, Crozier T E. Solubility of methane in distilled water and seawater. J Chem Eng Data. 1976;21:78–80. [Google Scholar]
- 37.Zahn J A, DiSpirito A A. Membrane-associated methane monooxygenase from Methylococcus capsulatus (Bath) J Bacteriol. 1996;178:1018–1029. doi: 10.1128/jb.178.4.1018-1029.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]