Abstract
Gangavati sona (GS) is a high-yielding, fine-grain rice variety widely grown in the Tungabhadra command area in Karnataka, India; however, it is susceptible to bacterial blight (BB). Therefore, the present study was conducted to improve the GS variety for BB resistance. Three BB-resistant genes (xa5, xa13, and Xa21) were introgressed into the genetic background of susceptible cultivar GS through marker-assisted backcrossing (MABB) by using Improved samba Mahsuri (ISM), a popular, high-yielding, bacterial blight resistant rice variety as a donor parent. Foreground selection was carried out using gene-specific markers, viz., xa5FM (xa5), xa13prom (xa13), and pTA248 (Xa21), while background selection was carried out using well-distributed 64 polymorphic microsatellite markers. The true heterozygote F1 was used as the male parent for backcrossing with GS to obtain BC1F1. The process was repeated in BC1F1 generation, and a BC2F1 plant (IGS-5-11-5) possessing all three target genes along with maximum recurrent parent genome (RPG) recovery (86.7%) was selfed to obtain BC2F2s. At BC2F2, a single triple gene homozygote plant (IGS-5-11-5-33) with 92.6% RPG recovery was identified and advanced to BC2F5 by a pedigree method. At BC2F5, the seven best entries were selected, possessing all three resistance genes with high resistance levels against bacterial blight, yield level, and grain quality features equivalent to better than GS. The improved versions of GS will immensely benefit the farmers whose fields are endemic to BB.
Keywords: Rice, Disease, Genomics, Bacterial blight, Markers, Backcrossing
Introduction
Globally, rice is one of the most important staple food crops and is a primary food source for more than half of the world’s population. It provides 21% of the energy and 15% of the protein requirements of human beings (Kennedy et al. 2002). Estimates have projected that rice production must be increased by 0.6–0.9% annually by 2050 to meet the food demand of the anticipated 9.8 billion people (Carriger and Vallee 2007). Nevertheless, rice has witnessed yield plateauing in the last two decades due to several production constraints and biotic and abiotic stress factors (Peng et al. 2004). Among the biotic stresses that affect rice crops bacterial blight (BB) disease caused by Xanthomonas oryzae pv. oryzae (Xoo) (Swings et al. 1990) is the most destructive one, which has a major impact on yield loss ranging from 50 to 90% (Ou 1985; Sere et al. 2005). The disease occurs in the host plant at the seedling, vegetative, and reproductive stages, but infection at the tillering stage causes severe blighting of leaves, resulting in yield loss (Shivalingaiah 2011). Chemical control of this disease is complicated and limited due to the concerns over health hazards (Guvvala et al. 2013). Managing the disease using host plant resistance is the most effective, environmentally safe, and economical option (Khush et al. 1989). Therefore, the preferred bacterial blight disease management strategy is developing resistant rice varieties by deploying resistance genes.
Globally, more than 45 BB resistance genes have been identified from diverse sources, which offer resistance to various strains of Xoo (Neelam et al. 2020). Many of these resistance genes have been tagged by closely linked molecular markers (Sonti 1998; Rao et al. 2002). Through conventional breeding, a few of these genes, including Xa4, have been integrated into numerous high-yielding varieties (Khush et al. 1989). However, the widespread cultivation of these varieties has led to the prevalence of isolates that overcome Xa4 (Mew et al. 1992). Therefore, deploying multiple BB resistance genes into rice cultivars results in a broad spectrum and more durable level of resistance than varieties with only a single BB resistance gene.
In the Tungabhadra command area of Karnataka state, India, rice is grown extensively in 3.5 lakhs ha. In the northern districts of Karnataka, rice is grown both in Kharif and the summer seasons by utilizing the water resources of the Tungabhadra River. Since extensive rice cultivation is carried out in these areas, this region is known as the “Rice Bowl” of Karnataka state. Gangavati sona (GS) is a major rice variety cultivated in these regions. GS is known for its high yield (Kharif: 6.5–7.0 t/ha and summer: 7.0–7.5 t/ha), excellent cooking and eating qualities, and suitability to grow in wet and dry seasons without compromising yield levels. Despite its popularity in most rice-growing regions, farmers are facing the problem of moderate to severe BB infection in GS, which leads to significant yield loss, and the Tungabhadra command area is known to be endemic for BB disease.
Previously, Joseph et al. (2004) introduced xa13 and Xa21 into the popular basmati rice variety Pusa Basmati1 and released them as Improved Pusa Basmati1 in India for commercial cultivation. Similarly, Sundaram et al. (2008) incorporated three major BB resistance genes (xa5, xa13 and Xa21) in the background of a highly popular rice variety, Samba Mahsuri. The “Improved Samba Mahsuri” (ISM) possesses high grain yield and elite grain quality traits of Samba Mahsuri with a high resistance level against BB disease. Therefore, ISM was released for commercial cultivation across India in Samba Mahsuri growing areas in 2008. With this background, in the present study, we employed a marker-assisted backcross breeding (MABB) strategy to incorporate three major BB resistance genes, viz., xa5, xa13, and Xa21, into the genetic background of Gangavati sona intending to increase the durability and spectrum of resistance of this highly preferred, premium quality rice variety.
Materials and methods
Plant materials
Gangavati sona (GS), a high-yielding, medium, slender, fine grain, BB susceptible, elite rice variety developed and released for commercial cultivation in the year 2012 by Agricultural Research Station, Gangavathi of University of Agricultural Sciences, Raichur, Karnataka, was used as a recurrent parent (RP). Improved Samba Mahsuri (ISM; also known as RPBio-226), a high yielding, medium slender fine grain, low glycemic index (50.99), BB resistant possessing three major BB resistance genes, viz., xa5, xa13 and Xa21 (Sundaram et al. 2008), developed from the cross SS1113/Samba Mahsuri through marker-assisted backcross breeding (MABB) developed by ICAR-Indian Institute of Rice Research (ICAR-IIRR) Hyderabad in association with CSIR-Centre for Cellular and Molecular Biology (CSIR-CCMB), Hyderabad, India and currently occupied more than 6,00,000 hectares was used as the donor parent (DP) for bacterial blight resistance.
Development and selection of improved BB-resistant lines through the MABB strategy
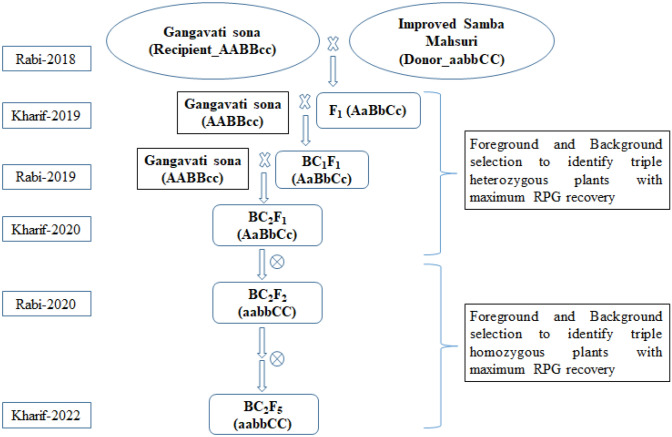
The recurrent parent GS was crossed with Improved Samba Mahsuri, and the F1s were analyzed for their heterozygosity (i.e. true F1s) using the target resistance gene-specific markers, namely, xa5FM specific for xa5, xa13prom specific for xa13 (Hajira et al. 2016) and pTA248 specific for Xa21 (Ronald et al. 1992) (Fig. 1). True F1s thus identified were then backcrossed to the recurrent parent, GS, to generate BC1F1s. Then, they were subjected to foreground selection using gene-specific markers, and plants that are triple gene heterozygous were subjected to background selection to identify a single plant with maximum recurrent parent genome (RPG) recovery using a set of 64 SSR parental polymorphic markers, which are evenly distributed across the 12 rice chromosomes (Table 1). The extent of recurrent parent genome (RPG) recovery among the selected backcross-derived plants was assessed utilizing the software tool Graphical Genotype V 2.0 (Van Berloo 1999).
Fig. 1.
Outline of the marker-assisted backcross breeding scheme to develop BB resistance in Gangavati sona
Table 1.
Microsatellite markers polymorphic between Gangavati sona (recurrent parent) and Improved Samba Mahsuri (donor parent)
| S.N. | Chromosome no. | Total no. of markers analysed | No. of polymorphic markers | Polymorphic markers |
|---|---|---|---|---|
| 1 | 1 | 65 | 8 | RM 10009, 4554, 10167, 10078, 6324, 4959, 10115, 10695 |
| 2 | 2 | 39 | 5 | RM 13155, 14140, 13131, 13045, RMES2-1 |
| 3 | 3 | 38 | 5 | RM 14735, 15630, 15004, 15404, HRM15679 |
| 4 | 4 | 94 | 8 | RM 16606, 17345, 17377, 16868, 3524, 16373, 16855, 6997 |
| 5 | 5 | 61 | 5 | RM 169, 18516, 592, 17920, 19147 |
| 6 | 6 | 75 | 5 | RM 19367, 19410, 1369, 589, 20583 |
| 7 | 7 | 106 | 4 | RM 6697, 3583, 20923), chr7-1.2 |
| 8 | 8 | 88 | 3 | RM 6925, 5933, 22565 |
| 9 | 9 | 50 | 3 | 3RM 3769, 3808), RMES9-2 |
| 10 | 10 | 45 | 3 | RM 5271, chr10-22.1, ESSR 10–22.7 |
| 11 | 11 | 78 | 8 | RM 21, 26352, 27369, 26558, 26868), chr11-8.9, chr11-25.5, chr11-28.1 |
| 12 | 12 | 50 | 7 | RM 2877, 28481, 28441, RMS 10060, SSR12-7.4, JGT12-20.24, ESSR12-20.2 |
| Total no. of markers | 789 | 64 | ||
A single positive BC1F1 plant with maximum RPG recovery was selected and backcrossed with GS to generate BC2F1s, and the process of marker-assisted backcrossing was repeated as mentioned above, and a single BC2F1 plant with all the three target resistance genes with maximum RPG recovery was selfed to develop BC2F2s. Finally, a BC2F2 plant possessing xa5, xa13, and Xa21 genes in homozygous condition along with maximum RPG and closely resembling GS based on morphological features along with a high level of resistance against BB was identified and advanced through the pedigree method of breeding to BC2F5. The BC2F5 lines were also further evaluated for BB resistance under controlled conditions and for grain and cooking quality, key agro morphological traits, including yield parameters under field conditions.
The background genome recovery ‘G’ was calculated using the below formula (Sundaram et al. 2008).
where N = total number of parental polymorphic markers screened, X = number of markers showing homozygosity for recurrent parent allele, Y = number of markers showing heterozygosity for parental alleles.
Phenotypic screening for bacterial blight
The seven pyramided breeding lines of GS at BC2F5 generation and parents were challenged with BB pathogen. IXo-20, a virulent isolate of Xanthomonas oryzae pv. oryzae (Xoo) (collected from Telangana) during Kharif 2022 in the experimental field of ICAR-IIRR farm located at Rajendranagar, Hyderabad, India, to assess their resistance against BB. The pathogen was multiplied on modified Wakimoto’s agar media at 280C for 72–96 h, harvested after incubation, and diluted with sterile distilled water to get a final concentration of 108 cfu/ml. Inoculation was done at the maximum tillering stage following the leaf clip method of Kauffman et al. (1973) by clipping the leaf tip (about 1 to 2 cm) of the uppermost leaves with a sterilized scissor dipped in the bacterial suspension. Disease reactions were recorded 15 days post-inoculation by measuring the lesion length and following the SES scale (standard evaluation system for rice) (IRRI 2013) for resistance/susceptibility.
Evaluation of improved GS lines for agro-morphological traits
Seven improved backcross derived lines of BC2F5 generation, possessing BB-resistant genes in the background of GS along with recurrent parent and donor parent, were space planted in the main fields during the wet season of 2021–22 of ICAR-IIRR farm, Hyderabad, India, at a spacing of 15 × 20 cm in four replicates. The field was maintained with the recommended dose of fertilizers (120:60:60 kg NPK per hectare). Phenotypic data of the selected plants for key agro-morphological traits were collected, including days to 50% flowering (DFF), plant height (cm), number of productive tillers per plant, panicle length (cm), number of grains per panicle, grain yield per plant (g), 1000-grain weight (g), L/B ratio, grain type and type of panicle exertion among ten plants for each replication (n = 4) as explained in Abhilash et al. (2016). The data were statistically analyzed for genetic parameters, and the Coefficient of variation (CV) was calculated using standard errors of the mean (S.Em. ±) as per the procedure described by Freeman (1973). An analysis of variance (ANOVA) was performed using the R Studio version 4.3.1 to determine the variation among the improved breeding lines of GS.
Results
Introduction of xa5, xa13 and Xa21 genes into Gangavati sona background
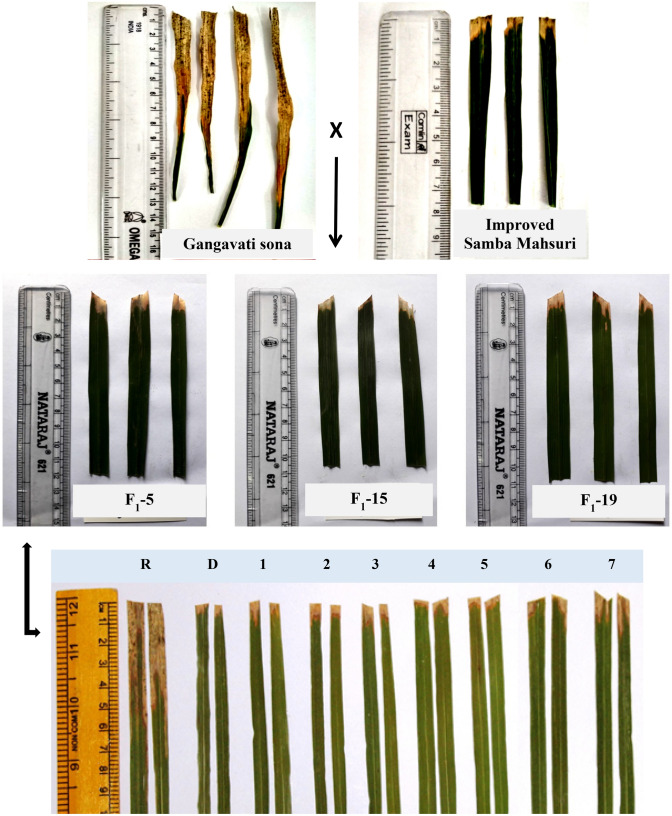
The male parent (ISM) and the female parent (Gangavati sona) were sown staggered to match the flowering period for crossing to obtain F1 seeds. The 46 F1 plants were inoculated with bacterial culture and scored for their disease reactions. The F1 plants had disease lesion lengths ranging from 0.8 to 1.2 cm, indicating a resistant nature (Fig. 2). A total of 36 F1 plants were found to be triple heterozygous (i.e. true F1s) with respect to the three targets BB-resistant genes (Table 2). Among 142 BC1F1s screened, 13 plants were triple heterozygous for target-resistant genes (i.e. xa5, xa13, and Xa21). These plants were analyzed through background selection to assess the RPG recovery using a set of 64 parental polymorphic markers (Table 1). The plant (IGS-5-11) with the highest RPG of 74.2% was identified and used as a pollen donor to produce BC2F1s. A total of 137 BC2F1s were raised and subjected to foreground selection employing gene-specific markers to identify true heterozygous plants. Eight out of 137 plants were confirmed to possess all three target genes, and they were then subjected to background selection using parental polymorphic markers. A single plant (IGS-5-11-5) with maximum RPG recovery (86.7%) was identified (Fig. 3). This plant was selfed to produce BC2F2 plants. A total of 286 BC2F2 plants were raised and inoculated with bacterial culture in the field for phenotypic screening with a local isolate of Xoo, IX0-20 at the maximum tillering stage. The disease lesion lengths varied from 0.9 to 2.2 cm. The phenotypically resistant plants were subjected to marker analysis with the help of gene-specific markers. Of the 286 plants, a total of 4 plants (1 out of 71) were identified as triple homozygous for all three target genes.
Fig. 2.
Disease reaction of parents, F1’s and derived BC2F5 for Xoo strain. R: Recurrent Parent; D: Donor Parent; 1–7: Derived BC2F5 plants (1: IGS-5-11-5-33-24-06-06, 2: IGS-5-11-5-33-24-06-24, 3: IGS-5-11-5-33-24-06-30, 4: IGS-5-11-5-33-24-06-30, 5: IGS-5-11-5-33-24-06-93, 6: IGS-5-11-5-33-24-06-184, 7-IGS-5-11-5-33-24-06-214)
Table 2.
Number of plants confirmed for foreground and background selection in each backcross population
| S.N. | Generation | Total no. of plants analysed | Total No. of plants with all three genes | % Recurrent parent genome (RPG) recovery | Plant with maximum RPG% |
|---|---|---|---|---|---|
| 1 | F1 | 46 | 36 | – | |
| 2 | BC1F1 | 142 | 13 | 74.2% | IGS-5-11 |
| 3 | BC2F1 | 137 | 8 | 86.7% | IGS-5-11-5 |
| 4 | BC2F2 | 286 | 4 | 92.6% | IGS-5-11-5-33 |
| 5 | BC2F5 | 98.3% | IGS-5-11-5-33-24-06-06 | ||
| IGS-5-11-5-33-24-06-24 | |||||
| IGS-5-11-5-33-24-06-30 | |||||
| IGS-5-11-5-33-24-06-91 | |||||
| IGS-5-11-5-33-24-06-93 | |||||
| IGS-5-11-5-33-24-06-184 | |||||
| IGS-5-11-5-33-24-06-214 |
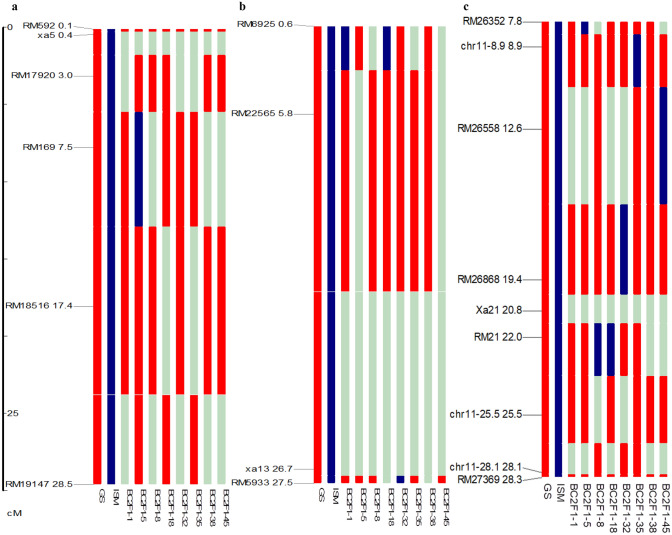
Fig. 3.
Graphical representation of genome recovery in selected BC2F1 population through graphical genotype analysis. a Extent of background genome recovery in the genomic regions in the vicinity of xa5 on chromosome 5. b Extent of background genome recovery in the genomic regions in the vicinity of xa13 on chromosome 8. c Extent of background genome recovery in the genomic region in the vicinity ofXa21 on Chromosome 11
Further, background selection was performed using the parental polymorphic SSR markers to identify a single plant with maximum RPG. The Plant, IGS-5-11-5-33 (Fig. 4), had the highest RPG recovery (92.6%). It was then advanced through the pedigree method till the BC2F5 generation. Seven promising BC2F5 lines similar to GS, i.e. IGS-5-11-5-33-24-06-06, IGS-5-11-5-33-24-06-24, IGS-5-11-5-33-24-06-30, IGS-5-11-5-33-24-06-91, IGS-5-11-5-33-24-06-93, IGS-5-11-5-33-24-06-184, and IGS-5-11-5-33-24-06-214 were selected and evaluated for BB resistance, agro morphological traits, and yield analysis.
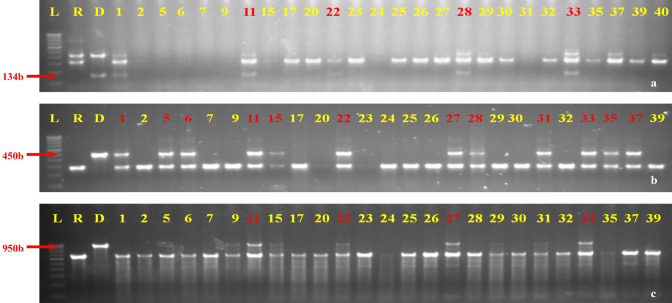
Fig. 4.
Identification of homozygous BC2F2 (IGS-5-11-5-33) plants possessing xa5 + xa13 + Xa21. a Analysis of xa5 gene using xa5FM marker b Analysis of xa13 gene using xa13prom marker. c Analysis of Xa21 gene using pTA248 marker. L Ladder, R recurrent parent GS; D, donor parent ISM; 1-39, 40 test samples
Evaluation of pyramided lines for BB resistance
The pyramided lines were evaluated for BB reaction under field conditions using IXo-20 strain (Table 3). The donor parent ISM was highly resistant to BB with a disease lesion length of 0.5 ± 0.13 cm, and the recurrent parent, GS, was highly susceptible, with a disease lesion length of 8.0 ± 0.39 cm. All seven pyramided BC2F5 lines were witnessed to be resistant to BB, with average lesion lengths ranging from 1.1 to 1.7 cm.
Table 3.
Disease reaction of improved pyramided lines of GS with bacterial blight pathogen
| S.N. | Entry | Reactions against BB (IX0-20) | |
|---|---|---|---|
| Score (cm) | Reaction | ||
| 1 | GS | 8.0 ± 0.39 | S |
| 2 | ISM | 0.5 ± 0.13 | R |
| 3 | IGS-5-11-5-33-24-06-06 | 1.1 ± 0.21 | R |
| 4 | IGS-5-11-5-33-24-06-24 | 1.7 ± 0.79 | R |
| 5 | IGS-5-11-5-33-24-06-30 | 1.2 ± 0.08 | R |
| 6 | IGS-5-11-5-33-24-06-91 | 1.4 ± 0.19 | R |
| 7 | IGS-5-11-5-33-24-06-93 | 1.2 ± 0.11 | R |
| 8 | IGS-5-11-5-33-24-06-184 | 1.3 ± 0.18 | R |
| 9 | IGS-5-11-5-33-24-06-214 | 1.3 ± 0.22 | R |
S susceptible, R resistant
Assessment of pyramid lines for yield and agro-morphological characters
Among the seven BC2F5 pyramided lines, the IGS-5-11-5-33-24-06-30 exhibited significantly better performance over the RP, and GS concerning yield and other agro-morphological traits (Table 4). IGS-5-11-5-33-24-06-06 was early (93.4 days to 50% flowering) compared to others, and GS and also exhibited the highest value for plant height (101.2 cm). IGS-5-11-5-33-24-06-214 also produced more productive tillers per plant (12.1). All the pyramided lines were found on par or better than both parents in terms of panicle length, number of grains per panicle, grain yield per plant, and 1000-grain weight. Further, all the improved lines had an LB ratio equivalent to GS (~ 3.8), and all the lines had full exerted panicles similar to the recurrent parent.
Table 4.
Performance of parents and improved pyramided lines for agro-morphological traits
| S.N. | Plant identity | Days to 50% flowering (DFF) | Plant height (cm) | No. of productive tillers/plant | Panicle length (cm) | Number of grains per panicle | Grain yield per plant (g) | 1000 seed weight (g) | L/B ratio | Grain type | Panicle exertion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GS | 95.3 ± 0.50 | 98.4 ± 0.62 | 8.2 ± 0.11 | 22.6 ± 0.13 | 254.8 ± 6.65 | 22.0 ± 0.39 | 14.2 ± 0.15 | 3.8 ± 0.05 | MS | FE |
| 2 | ISM | 104.8 ± 1.26 | 92.9 ± 0.50 | 8.2 ± 0.11 | 21.7 ± 0.34 | 224.5 ± 5.32 | 18.0 ± 0.29 | 12.4 ± 0.30 | 3.7 ± 0.07 | MS | PE |
| 3 | IGS-5-11-5-33-24-06-06 | 93.9 ± 0.75 | 98.1 ± 0.88 | 8.2 ± 0.28 | 22.5 ± 0.08 | 260.9 ± 2.81 | 22.3 ± 0.55 | 14.0 ± 0.27 | 3.7 ± 0.01 | MS | FE |
| 4 | IGS-5-11-5-33-24-06-24 | 94.6 ± 0.25 | 97.0 ± 2.37 | 8.5 ± 0.59 | 22.8 ± 0.16 | 264.8 ± 3.52 | 22.7 ± 0.61 | 14.1 ± 0.18 | 3.7 ± 0.02 | MS | FE |
| 5 | IGS-5-11-5-33-24-06-30 | 97.4 ± 0.25 | 97.8 ± 1.87 | 7.8 ± 0.41 | 22.7 ± 0.14 | 253.5 ± 0.82 | 23.3 ± 0.78 | 14.0 ± 0.04 | 3.8 ± 0.01 | MS | FE |
| 6 | IGS-5-11-5-33-24-06-91 | 95.3 ± 0.65 | 93.1 ± 0.80 | 11.6 ± 0.99 | 22.6 ± 0.21 | 266.8 ± 2.18 | 23.1 ± 1.30 | 13.9 ± 0.21 | 3.7 ± 0.04 | MS | FE |
| 7 | IGS-5-11-5-33-24-06-93 | 95.5 ± 1.22 | 95.4 ± 0.87 | 11.6 ± 1.71 | 22.6 ± 0.16 | 252.9 ± 1.60 | 22.5 ± 0.34 | 14.0 ± 0.05 | 3.7 ± 0.01 | MS | FE |
| 8 | IGS-5-11-5-33-24-06-184 | 99.8 ± 1.04 | 101.2 ± 1.28 | 11.0 ± 1.51 | 22.8 ± 0.14 | 264.9 ± 3.68 | 23.1 ± 0.57 | 14.1 ± 0.10 | 3.7 ± 0.01 | MS | FE |
| 9 | IGS-5-11-5-33-24-06-214 | 102.0 ± 0.71 | 98.9 ± 1.27 | 12.1 ± 0.52 | 22.7 ± 0.00 | 260.0 ± 5.60 | 23.8 ± 0.33 | 14.0 ± 0.06 | 3.7 ± 0.02 | MS | FE |
| Mean | 98 | 96.97 | 9.69 | 22.57 | 256 | 22.31 | 13.86 | 3.71 | |||
| CV (%) | 3.76 | 2.93 | 19.75 | 1.60 | 5.00 | 7.73 | 3.96 | 1.11 | |||
| CD (5%) | 1.28 | 1.87 | 1.35 | 0.26 | 5.94 | 0.90 | 0.27 | 0.05 | |||
| Mean SS | 70.94** | 18.04** | 15.59** | 15.53** | 40.36** | 30.62** | 33.83** | 6.81** | |||
| GCV | 3.75 | 2.73 | 18.27 | 1.48 | 4.98 | 7.53 | 3.88 | 1.04 | |||
| PCV | 3.86 | 3.03 | 20.63 | 1.68 | 5.23 | 8.02 | 4.11 | 1.35 | |||
| h2b (%) | 94.59 | 80.99 | 78.48 | 78.41 | 90.78 | 88.11 | 89.14 | 60.00 | |||
| GAM@5% | 7.52 | 5.06 | 33.35 | 2.71 | 9.78 | 14.55 | 7.54 | 1.66 |
CV coefficient of variation, CD critical difference, Mean SS Mean sum of squares, GCV genotypic coefficient of variance, PCV phenotypic coefficient of variance, h2b broad sense of heritability, GAM genetic advance in % of mean, MS medium slender, FE full exerted, PE partially exerted
Estimation of genetic variability parameters
Genotypic coefficient of variation (GCV) and phenotypic coefficient of variation (PCV) estimates (Table 4) were found to be lower (1.04–8.02%) for traits days to 50% flowering, plant height, panicle length, number of grains per panicle, grain yield per plant, 1000 seed weight, L/B ratio and were moderate (18.27–20.63%) for number of productive tillers per plant revealed the presence of variability for the traits under study. Similarly, estimates for heritability in a broad sense were high (78.41–94.59%) for all the traits except for the trait L/B ratio (moderate-60%). The genetic advance as a per cent of mean (GAM) was recorded to be lower (1.66–9.78%) for days to 50% flowering, plant height, panicle length, number of grains per panicle, 1000 seed weight, L/B ratio; moderate (14.55%) for grain yield per plant and was high (33.35%) for number of productive tillers per plant.
Discussion
Recently, there has been a dramatic shift in rice production due to several biotic and abiotic stress factors, which not only reduces yield and quality but also potentially negatively impact the livelihood of millions of rice farmers. Many biotic and abiotic stress factors constantly challenge Rice cultivation. Among the biotic stresses, bacterial blight (BB) is caused by the Gram-negative bacteria Xanthomonas oryzae pv. oryzae (Xoo) causes significant yield loss in irrigated and rainfed lowland areas, accounting for 60% of rice-growing areas in India (Ismail et al. 2013). BB disease caused by the Xoo pathogen is highly dynamic, leading to effective resistance overcoming after a few years of cultivation. This has necessitated breeders to search for novel resistance (R) genes. Extensive cultivation of single R gene-containing cultivars leads to increased selection pressure on the pathogen, which results in the breakdown of resistance (Chukwu et al. 2019). Thus, the pyramiding of multiple R genes over time and space is an effective strategy to enhance the durability and spectrum of resistance. Pyramiding multiple R genes with conventional breeding is challenging, owing to potential linkage drag with other undesirable traits that are arduous to break even after several generations of backcrossing (Tanksley et al. 1989). In addition, when more than two genes are incorporated, it is difficult to differentiate the effect of each gene precisely through phenotype-based selection, which is adopted in conventional breeding.
Further, in the presence of a dominant and a recessive allele, the effect of the recessive gene is masked. The rapid advances and low cost of molecular markers, which are tightly linked to gene(s) of interest, have precisely made it possible to identify plants with multiple R genes. The past efforts to incorporate multiple genes with the help of molecular markers in the elite but susceptible rice cultivars PR106 (Singh et al. 2001), Pusa Basmati (Joseph et al. 2004), and Samba Mahsuri (Sundaram et al. 2008) have been very successful.
Resistance breeding for developing improved lines by pyramiding multiple resistance genes with the help of molecular markers is effective for BB disease (Joseph et al. 2004; Sundaram et al. 2008, 2009). At least 47 genes (Sundaram et al. 2014; Neelam et al. 2020) conferring resistance to BB have been identified in rice germplasm. Among them, a combination of three major genes was found to be more effective against the disease, viz., major recessive genes xa5 and xa13 located on chromosome 5 and 8, respectively, and a major dominant, broad-spectrum resistant gene Xa21 located on Chr. 11 derived from O. longistaminata are known to confer durable resistance against BB disease (Sundaram et al. 2008; Lalitha et al. 2013; Pradhan et al. 2015; Ramalingam et al. 2017; Rekha et al. 2018).
Gangavati sona is a premium fine grain medium slender rice variety known for its high yield and year-round cultivation in high-productivity regions of the Northern Karnataka region of India. It is highly preferred by farmers of the Gangavati area, popularly known as the “rice bowl of Karnataka” due to its medium slender (MS) grain type, where MS grain type is the dominant market segment in south India. However, the premium quality rice variety is highly susceptible to BB disease. In this context, we have introgressed three major BB resistance genes i.e., xa5, xa13, and Xa21, into GS to develop durable BB-resistant lines using marker-assisted backcross breeding (MABB) strategy without comprising GS yield and quality characteristics.
In modern breeding programs, molecular markers linked tightly to target genes are utilized to improve the selection efficiency of target traits or genes in a backcross breeding program (Jena and Mackill 2008). This involves foreground selection, where gene-specific markers are used to track target trait introgression (Hospital and Charcosset 1997), and background selection to identify the backcross-derived plant possessing maximum recovery of the recurrent parent genome (RPG) using co-dominant markers, which are polymorphic among the parents. This study used three co-dominant markers: xa5FM for the gene xa5, xa13prom for xa13 (Hajira et al. 2016), and pTA248 for Xa21 (Ronald et al. 1992) for foreground selection. These markers were also used successfully by Ramalingam et al. (2017); Rekha et al. (2018) and Dasari et al. (2022). Further, 64 parental polymorphic SSR markers were used for background selection.
The improved lines containing a combination of three resistant genes revealed a high level of resistance nature upon inoculation with the Xoo strain. The lines with only the xa5 gene introgressed exhibited a little to partial resistance due to the presence of only one resistant gene, whereas lines with more resistant gene combinations (xa13 or Xa21) exhibited a good resistance level. Even though the lines with single xa13 or Xa21 and/or other lines with two gene combinations are resistant, we have characterized only the three gene pyramided lines, considering the resistance they provided would be more durable and long-lasting. The data on disease lesion lengths of improved lines (Table 3) were recorded post-15 days after inoculation (DAI). The inoculated leaves were also examined at 21 DAI, wherein the lesion lengths had expanded in single- and two-gene introgressed lines but not in three-gene pyramided lines, indicating that there is a kind of quantitative complementation and additive nature of the resistance genes deployed. A higher level of resistance was also observed in improved introgression lines with multiple BB resistance genes, compared to those with fewer resistance genes in studies by Sundaram et al. (2008) and Dasari et al. (2022).
The genes xa5, xa13, and Xa21 introduced into the GS background are well characterized. xa5, a recessive resistance gene, encodes a variant form of transcription factor γIIa (Iyer and McCouch 2004) and the xa13 gene is also recessive which has shown a mutation in the promoter region of a gene that is a homolog of the nodulin MtN3 (Chu et al. 2006), while Xa21, a dominant gene that encodes NBS-LRR domains of a receptor kinase (Song et al. 1995).
Microsatellite markers (SSR) that are found to be polymorphic among the parents, ISM, and GS were utilized for background selection in our study. Marker-assisted background selection is beneficial in recovering RPG by reducing the number of backcrosses (Hospital and Charcosset 1997), and background selection in early backcross generations was found to help recover RPG quickly (Chen et al. 2001; Joseph et al. 2004). Many studies (Bai et al. 2006; Hasan et al. 2015; Sundaram et al. 2008; 2009) suggest that a minimum of 3–4 backcrosses are required to recover RPG completely, while recent studies indicate that with stringent MABB, it is possible to get maximum RPG recovery even with two rounds of backcrossing (Singh et al. 2001; Basavaraj et al. 2010; Miah et al. 2015; Abhilash Kumar et al. 2017; Rekha et al. 2018; Swathi et al. 2019).
In the present study, the backcrossing was restricted to two generations since both parents used to share the same Samba Mahsuri lineage. The plant with the highest RPG recovery in BC1F1 was 74.2% in IGS-5-11 and 86.7% in BC2F1 (IGS-5-11-5). In BC2F2 generation, the line IGS-5-11-5-33 exhibited 92.6% RPG recovery and 98.3% in the selected best improved BC2F5 (IGS-5-11-5-33-24-06-30) line. The high RPG recovery obtained in this study can be attributed to the fact that the stringent phenotypic selection was carried out at every backcross generation and selfing. This helps in the reduction of time and resources used as well. Earlier, Singh et al. (2012) followed a similar strategy and reported increased RPG recovery. The results obtained in the present study are in accordance with the earlier reports (Sundaram et al. 2008; Pradhan et al. 2015; Dasari et al. 2022).
When challenged with the Xoo pathogen under field conditions, GS's improved, bacterial blight-resistant lines revealed that all the pyramided lines were resistant to bacterial strain IXo-20. Similar results were also observed in earlier studies (Yoshimura et al. 1995; Huang et al. 1997; Sanchez et al. 2000; Singh et al. 2001; Sundaram et al. 2008; Dokku et al. 2013; Gitishree and Rao 2015; Dasari et al. 2022) wherein xa5, xa13, and Xa21 were introgressed in combinations or singly.
The main objective of this study was to develop improved lines of GS with durable resistance to BB. Many studies (Sanchez et al. 2000; Sundaram et al. 2008) have reported the quantitative complementation of resistance genes when they are introgressed in different combinations. Deploying three resistant genes into the GS background may help the farmers to reap the yield advantages in disease-affected areas by providing durable resistance. Parallel results were reported by Sundaram et al. 2009 and Dasari et al. 2022 in other genetic backgrounds of Indica rice cultivars. The improved lines using GS as a recurrent parent are expected to show high resistance against BB. This disease is widely prevalent, limiting rice production in Karnataka (Raichur, Koppal, and Ballari) and other Indian states (Aruna Kumari et al. 2016).
From the agro morphological data, the improved lines of GS possessing resistance against BB disease performed better when compared to GS without yield penalty. We observed that some lines were superior to Recurrent parent GS. Further, the selected lines possessing BB resistance were found to retain medium slender grain types and other grain quality traits as of GS. All the improved lines exhibited full panicle exertion and plant height similar to GS. It can be anticipated that the improved GS lines for BB resistance developed through marker-assisted backcrossing will benefit the farmers who regularly grow GS as they are similar in quality and yield, with an added advantage of three resistance genes against BB disease.
Conclusions
Through this MABB strategy, we have successfully introgressed three major BB resistance genes, i.e. xa5, xa13, and Xa21, into the background of the susceptible variety Gangavati sona (GS), without yield penalty and retaining better grain quality and other related traits of the elite variety. The identified lines possessing resistance genes have very good phenotypes and high yield levels. Hence, a few of these lines have been nominated for All India Co-ordinated Crop Improvement (AICRIP) trails for varietal release to farmers. These introgressed lines may help the farmers of GS, whose fields are affected by BB disease. In addition, these improved lines can be used in future disease-resistance breeding programs as donors. Our work demonstrated the importance of marker-assisted backcrossing for improving the popular varieties to BB resistance while retaining all the other desirable attributes for which farmers have been growing for many years with enhanced durable broad-spectrum resistance to BB.
Acknowledgements
The authors would like to thank Indian Council of Agricultural Research (ICAR)-Indian Institute of Rice Research, Hyderabad, India and University of Agricultural Sciences, Raichur, Karnataka for providing all the necessary facilities to carry out the research work.
Author contributions
Experimental idea and design: MSA, CAM, CG, PMS, RMS; execution and performing experiment: MSA, CAM, CG, BM, PSB, RMS; infrastructural facilities: LVSR, KB, GSL, RMS; data analysis: SR, CAM; manuscript preparation: CAM, MSA; editing manuscript: PS, KBK, RMK, RL, JD, JMN, MG; field selections, data collection and genotyping: CAM, MH, VA, DA, VGI, GU.
Funding
No funding was received from any institute for this study.
Data availability
All the data has been provided in the manuscript.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors have approved the manuscript for publication.
Footnotes
C. A. Manoj and M. S. Anantha have contributed equally to this work.
References
- Abhilash Kumar V, Balachiranjeevi CH, Bhaskar Naik S, Rambabu R, Rekha G, Harika G, Sundaram RM. Development of gene-pyramid lines of the elite restorer line, RPHR-1005 possessing durable bacterial blight and blast resistance. Front Plant Sci. 2016;7:1–15. doi: 10.3389/fpls.2016.01195. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Abhilash Kumar V, Balachiranjeevi CH, Bhaskar Naik S, Rekha G, Rambabu R, Harika G, Sundaram RM. Marker-assisted pyramiding of bacterial blight and gall midge resistance genes into RPHR-1005, the restorer line of the popular rice hybrid DRRH-3. Mol Breed. 2017;37(7):1–14. doi: 10.1007/s11032-017-0687-8. [DOI] [Google Scholar]
- Aruna Kumari K, Durgarani CV, Satturu V, Sarikonda KR, Chittoor PDR, Vutukuri B, Sundaram RM. Marker-assisted pyramiding of genes conferring resistance against bacterial blight and blast diseases into indian rice variety MTU1010. Rice Sci. 2016;23(6):306–316. doi: 10.1016/j.rsci.2016.04.005. [DOI] [Google Scholar]
- Bai JY, Zhang Q, Jia XP, Chuan Y, Bao X. Comparison of different foreground and background selection methods in marker assisted introgression. Acta Genet Sin. 2006;33:1073–1080. doi: 10.1016/S0379-4172(06)60144-3. [DOI] [PubMed] [Google Scholar]
- Basavaraj SH, Singh VK, Singh A, Singh A, Singh A, Yadav S, Ellur RK, Singh D, Gopala Krishnan S, Nagarajan M, Mohapatra T, Prabhu KV, Singh AK. Marker-assisted improvement of bacterial blight resistance in parental lines of PusaRH10, a superfine grain aromatic rice hybrid. Mol Breed. 2010;2:293–305. doi: 10.1007/s11032-010-9407-3. [DOI] [Google Scholar]
- Carriger S, Vallee D. More crop per drop: rice today. Int Rice Res Inst. 2007;6:10–13. [Google Scholar]
- Chen SC, Xu G, Lin XH, Zhang Q. Improving bacterial blight resistance of 6078, an elite restorer line of hybrid rice, by molecular marker-aided selection. Plant Breed. 2001;120:133–137. doi: 10.1046/j.1439-0523.2001.00559.x. [DOI] [Google Scholar]
- Chu Z, Fu B, Yang H, Xu C, Li Z, Sanchez A, Park YJ, Bennetzen JL, Zhang Q, Wang S. Targeting xa13, a recessive gene for bacterial blight resistance in rice. Theor Appl Genet. 2006;112:455–461. doi: 10.1007/s00122-005-0145-6. [DOI] [PubMed] [Google Scholar]
- Chukwu SC, Rafii MY, Ramlee SI, Ismail SI, Hasan MM, Oladosu YA, et al. Bacterial leaf blight resistance in rice: a review of conventional breeding to molecular approach. Mol Biol Rep. 2019;46:1519–1532. doi: 10.1007/s11033-019-04584-2. [DOI] [PubMed] [Google Scholar]
- Dasari A, Vemulapalli P, Gonuguntla R, Thota DK, Elumalai P, Muppavarapu K, Butam LP, Kulkarni SR, Sinha P, Gunukula H, et al. Improvement of bacterial blight resistance of the popular variety, Nellore Mahsuri (NLR34449) through marker-assisted breeding. J Genet. 2022;101:7. doi: 10.1007/s12041-021-01340-z. [DOI] [PubMed] [Google Scholar]
- Dokku P, Das KM, Rao GJN. Genetic enhancement of host plant-resistance of the Lalat cultivar of rice against bacterial blight employing marker-assisted selection. Biotechnol Lett. 2013;35:1339–1348. doi: 10.1007/s10529-013-1212-8. [DOI] [PubMed] [Google Scholar]
- Freeman GH. Statistical methods for the analysis of genotype-environment interactions. Heredity. 1973;31:339–354. doi: 10.1038/hdy.1973.90. [DOI] [PubMed] [Google Scholar]
- Gitishree D, Rao GJN. Molecular marker assisted gene stacking for biotic and abiotic stress resistance genes in an elite rice cultivar. Front Plant Sci. 2015;698(6):1–18. doi: 10.3389/fpls.2015.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guvvala D, Lalitha PK, Vinay S, Lalitha SM. Improvement of resistance to bacterial blight through marker assisted backcross breeding and field validation in rice (Oryza sativa) Res J Biol. 2013;1:82–83. [Google Scholar]
- Hajira SK, Sundaram RM, Laha GS, Yugander A, Balachandran SM, Viraktamath BC, et al. A single-tube, functional marker-based multiplex PCR assay for simultaneous detection of major bacterial blight resistance genes in rice, Xa21, xa13 and xa5. Rice Sci. 2016;23(3):144–151. doi: 10.1016/j.rsci.2015.11.004. [DOI] [Google Scholar]
- Hasan MM, Rafii MY, Ismail MR, Mahmood M, Rahim HA, Alam MA, et al. Marker assisted backcrossing: a useful method for rice improvement. Biotechnol Equip. 2015;29:237–254. doi: 10.1080/13102818.2014.995920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hospital F, Charcosset M. Marker-assisted introgression of quantitative trait loci. Genetics. 1997;147:1469–1485. doi: 10.1093/genetics/147.3.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS. Pyramiding of bacterial blight resistance genes in rice: marker-aided selection using RFLP and PCR. Theor Appl Genet. 1997;95:313–320. doi: 10.1007/s001220050565. [DOI] [Google Scholar]
- IRRI (2013) Standardization evaluation system for rice. International Rice Research Institute, P.O. Box 933, 1099 Manila, Philippines 5–18
- Ismail AM, Singh US, Singh S, Dar MH, Mackill DJ. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rain-fed lowland areas in Asia. Field Crops Res. 2013;152:83–93. doi: 10.1016/j.fcr.2013.01.007. [DOI] [Google Scholar]
- Iyer AS, McCouch SR. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol Plant Microb Interac. 2004;17:1348–1354. doi: 10.1094/MPMI.2004.17.12.1348. [DOI] [PubMed] [Google Scholar]
- Jena KK, Mackill DJ. Molecular markers and their use in marker-assisted selection in rice. Crop Sci. 2008;48:1266–1276. doi: 10.2135/cropsci2008.02.0082. [DOI] [Google Scholar]
- Joseph M, Gopalakrishnan S, Sharma RK, Singh AK, Singh VP, Singh NK, Mohapatra T. Combining bacterial blight resistance and Basmati quality characteristics by phenotypic and molecular marker assisted selection in rice. Mol Breed. 2004;13:377–387. doi: 10.1023/B:MOLB.0000034093.63593.4c. [DOI] [Google Scholar]
- Kauffman HE, Reddya PK, Hiesh SPY, Merca SD. An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis Rep. 1973;57:537–541. [Google Scholar]
- Kennedy G, Burlingame B, Nguyen VN (2002) Nutritional contribution of rice and impact of biotechnology and biodiversity in rice-consuming countries. Proceeding of the 20th session of the international rice commission. Bangkok, Thailand: FAO
- Khush GS, Mackill DJ, Sidhu GS (1989) Breeding rice for resistance to bacterial blight. Proc Int. Workshop Bacterial Blight Rice. IRRI, Manila, Philippines 14–18:207–217
- Lalitha DG, Pranitha K, Vinay S, Lalitha SM. Improvement of resistance to bacterial blight through marker assisted backcross breeding and field validation in rice (Oryza sativa) Res J Biol. 2013;1:52–66. [Google Scholar]
- Mew TW, Vera Cruz CM, Medalla ES. Changes in race frequency of Xanthomonas oryzae pv. oryzae in response to rice cultivars planted in the Philippines. Plant Dis. 1992;76(10):1029–1032. doi: 10.1094/PD-76-1029. [DOI] [Google Scholar]
- Miah G, Rafii MY, Ismail MR, Puteh AB, Rahim HA, Latif MA. Recurrent parent genome recovery analysis in a marker-assisted backcrossing program of rice (Oryza sativa L.) C R Biol. 2015;338:83–94. doi: 10.1016/j.crvi.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Neelam K, Mahajan R, Gupta V, Bhatia D, Gill BK, Komal R, Lore JS, Mangat GS, Singh K. High-resolution genetic mapping of a novel bacterial blight resistance gene xa-45(t) identified from Oryza glaberrima and transferred to Oryza sativa. Theor Appl Genet. 2020;133(3):689–705. doi: 10.1007/s00122-019-03501-2. [DOI] [PubMed] [Google Scholar]
- Ou SH (1985) Common wealth Mycological Institute, Kew. Rice dis 242
- Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, Centeno GS, Khush GS, Cassman KG. Rice yields decline with higher night temperature from global warming. Proc Natl Acad Sci USA. 2004;101:9971–9975. doi: 10.1073/pnas.0403720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan SK, Nayak DK, Mohanty S, Behera L, Barik SR, Pandit E, Lenka S, Anandan A. Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deep water rice variety. Jalmagna Rice. 2015;8(19):1–14. doi: 10.1186/s12284-015-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam J, Savitha P, Ganesh A, Saraswathi R, Chandrababu R. Functional marker assisted improvement of stable cytoplasmic male sterile lines of rice for bacterial blight resistance. Front Plant Sci. 2017;8:1131. doi: 10.3389/fpls.2017.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KK, Lakshminarasu M, Jena KK. DNA markers and marker-assisted breeding for durable resistance to bacterial blight disease in rice. Biotechnol Adv. 2002;20:33–47. doi: 10.1016/S0734-9750(02)00002-2. [DOI] [PubMed] [Google Scholar]
- Rekha G, Abhilash Kumar V, Viraktamath BC, Pranathi K, Kousik MBVN, Laxmi Prasanna B, et al. Marker-assisted improvement of blast resistance of the popular, high yielding, fine-grain type, bacterial blight resistant rice variety, improved Samba Mahsuri. J Plant Biochem Biot. 2018;27(4):463–472. doi: 10.1007/s13562-018-0455-9. [DOI] [Google Scholar]
- Ronald PC, Albano B, Tabien R, Abenes L, Wu K, McCouch SR, Tanksley SD. Genetic and physical analysis of the rice bacterial blight disease resistance locus, Xa21. Mol Genet Genomics. 1992;236:113–120. doi: 10.1007/BF00279649. [DOI] [PubMed] [Google Scholar]
- Sanchez AC, Brar DS, Huang N, Li Z, Khush GS. Sequence tagged site marker-assisted selection for three bacterial blight resistance genes in rice. Crop Sci. 2000;40:792–797. doi: 10.2135/cropsci2000.403792x. [DOI] [Google Scholar]
- Sere Y, Onasanya A, Verdier V, Akator K, Ouedraogo LS, Segda Z, Mbare MM, Sido AY, Basso A. Rice bacterial leaf blight in West Africa: preliminary studies on disease in farmers’ fields and screening released varieties for resistance to the bacteria. Asian J Plant Sci. 2005;4:577–579. doi: 10.3923/ajps.2005.577.579. [DOI] [Google Scholar]
- Shivalingaiah US. Characterisation of Xanthomonas oryzae pv. oryzae from major rice growing regions of Karnataka. Bioscan. 2011;6(1):5–10. [Google Scholar]
- Singh S, Sidhu JS, Huang N, Vikal Y, Li Z, Brar DS, Dhaliwal HS, Khush GS. Pyramiding three bacterial blight resistance genes (xa5, xa13 and Xa21) using marker-assisted selection into indica rice cultivar PR106. Theor Appl Genet. 2001;102:1011–1015. doi: 10.1007/s001220000495. [DOI] [Google Scholar]
- Singh VK, Singh A, Singh SP, Ellur RK, Singh D, Bhowmick PK, Gopalakrishnan S, Nagarajan M, Vinod KK, Mohapatra T, Prabhu KV, Singh AK (2012) Effect of residual parent segment of the performance of improved hybrids developed through MAS for resistance to bacterial blight and blast. Presented in the International Symposium on Plant Biotechnology for Food Security, Feb 21–24, NASC Complex, New Delhi, India
- Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, Fauquet C, Ronald PC. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Sci. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Sonti RV. Bacterial leaf blight of rice: new insights from molecular genetics. Curr Sci. 1998;74:206–212. [Google Scholar]
- Sundaram RM, Vishnupriya MR, Biradar SK, Laha GS, Reddy GA, ShobaRani N, Sarma NP, Sonti RV. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica. 2008;160:411–422. doi: 10.1007/s10681-007-9564-6. [DOI] [Google Scholar]
- Sundaram RM, Priya MRV, Laha GS, Rani NS, Rao PS, Balachandran SM, Reddy GA, Sarma NP, Sonti RV. Introduction of bacterial blight resistance into Triguna, a high yielding, mid-early duration rice variety by molecular marker assisted breeding. Biotechnol J. 2009;4:400–407. doi: 10.1002/biot.200800310. [DOI] [PubMed] [Google Scholar]
- Sundaram RM, Subhadeep C, Ricardo O, Laha GS, Jan EL, Ramesh VS, Casiana VC (2014) Update on bacterial blight of rice: 4th Int. Conf. Bacterial Blight. Rice 7:12
- Swathi G, Durga Rani CV, Jamaloddin M, Madhav MS, Vanisree S, Anuradha CN, Jagadeeswar R. Marker-assisted introgression of the major bacterial blight resistance genes, Xa21 and xa13, and blast resistance gene, Pi54, into the popular rice variety, JGL1798. J Plant Mol Breed. 2019;39(4):1–12. [Google Scholar]
- Swings J, Mooter MV, Vauterin L, Hoste B, Gillis M, Mew TW, Kersters K. Reclassification of the causal agents of bacterial blight of rice (Xanthomonas campestris pv. oryzae) and bacterial leaf streak (Xanthomonas campestris pv. oryzicola) of rice as pathovars of Xanthomonas oryzae. Int J Syst Bacteriol. 1990;40:309–311. doi: 10.1099/00207713-40-3-309. [DOI] [Google Scholar]
- Tanksley SD, Young ND, Paterson AH, Bonierbale MW. RFLP mapping in plant breeding: new tools for an old science. Biotechnol. 1989;7:257. [Google Scholar]
- Van Berloo R. GGT: software for the display of graphical genotypes. J Hered. 1999;90:328–329. doi: 10.1093/jhered/90.2.328. [DOI] [Google Scholar]
- Yoshimura S, Yoshimura A, Iwata N, McCouch SR, Abenes ML, Baraoidian MR, et al. Tagging and combining bacterial blight resistance genes in rice using RAPD and RFLP markers. Mol Breed. 1995;1:375–387. doi: 10.1007/BF01248415. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data has been provided in the manuscript.