Abstract
Objectives
Diabetes and its complications, as a major health concern, are associated with morbidity and mortality around the world. One of these complications is diabetic foot ulcer. Factors such as hyperglycemia, neuropathy, vascular damage and impaired immune system can cause foot ulcers. The present review aims to study the potential effects of melatonin, the main product of pineal glands, on diabetic foot ulcers.
Methods
A narrative review was performed using present literature in an attempt to identify the different aspects of melatonin’s impact on diabetic foot ulcers by searching related keywords in electronic databases without any restriction.
Results
This review shows that, melatonin has anti-diabetic effects. It is effective in reducing the risk of hyperglycemia, neuropathy, vascular damage and immune system impairment in diabetic patients. By reducing these complications with melatonin, correspondingly, the incidence of diabetic foot ulcers may also decrease in these patients.
Conclusions
The results of this study indicate promising properties of melatonin while dealing with diabetic foot ulcers and their common underlying conditions, but still, it needs to be investigated more in future studies.
Keywords: Melatonin, Diabetic foot ulcers, Hyperglycemia, Neuropathy, Vascular damage, Impaired immune system
Background
Diabetes is an illness caused by lack of insulin secretion or insulin resistance that will show itself through hyperglycemia [1]. Every year, the number of diabetic patients increases worldwide. Estimations done by International Diabetes Federation (IDF) shows that by the year 2045, 783 million of world population will be diabetic [2].
Diabetes is associated with various complications, and one such complication is diabetic foot ulcers. Diabetic neuropathy (DN) [3–5], hyperglycemia [4] and peripheral arterial disease [5] are the primary causes of diabetic foot ulcers. Other factors, including delayed wound healing and immune suppression, also contribute to the development of diabetic foot ulcers [6]. If foot ulcers left untreated, these may progress to blackening of foot and amputation [5]. Therefore, effective treatment and prevention of complications such as hyperglycemia, neuropathy and vascular damage, can help reduce the risk of foot ulcers and future amputations.
Recently, a relation between melatonin and blood glucose and insulin level has been found. Melatonin is a circadian endocrine hormone, secreted by the pineal glands in the dark at night [7, 8] (Fig. 1). Melatonin is an antioxidant and has a free radical scavenger effect [9–11]. Thus, melatonin has shown promising effect on diabetic neuropathy, retinopathy, nephropathy, immunity and cardiovascular disease [8–10]. It also effects insulin production [8] and insulin resistance [10]. In this review we focus on the potential effects of melatonin on reducing the risk of diabetic foot ulcers.
Fig. 1.
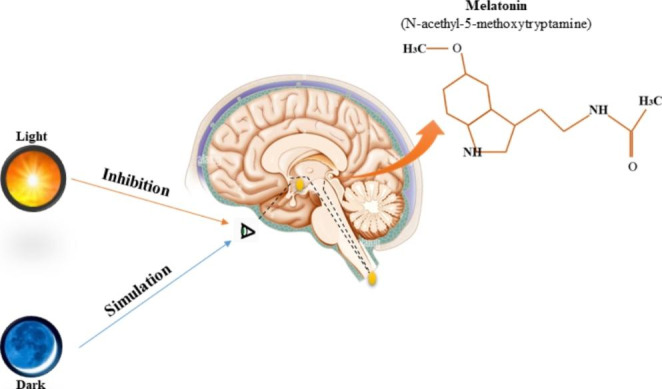
Schematic representation of the melatonin secretion in the pineal gland (major pineal gland hormone). (Original figure by the authors)
Melatonin
The pineal gland secretes melatonin in response to the onset of darkness at night [7–9]. Factors such as light exposure [9], obesity and type 2 diabetes [7, 9] have a decreasing effect on melatonin. Melatonin is an antioxidant and has a free radical scavenger effect and ROS scavenger by itself [9, 10]. Pancreatic beta cells, produce mass amounts of ROS which will end in oxidative stress. Oxidative stress is the reason of many diabetic complications [9]. Hyperglycemia, for instance induces oxidative stress and injures ganglion neurons [12]. Aside from being an antioxidant and ROS scavenger, melatonin has direct effect on other diabetes related complication, such as cardiovascular disease, diabetic neuropathy and retinopathy.
Here’s the revised paragraph with corrected grammar:
Melatonin and hyperglycemia
As previous mentioned, hyperglycemia increases the risk of developing diabetic foot ulcers. Studies have indicated a correlation between high insulin resistance and low levels of melatonin in the body. Melatonin has two main receptors in the human body, melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2). These two receptors are also expressed in the pancreatic tissue and islet cells. These receptors raise the possibility of an increase in insulin production with higher levels of melatonin in type 2 diabetic patients. Melatonin also reduces the overstraining aspect of high insulin production in beta cells. This path results in a lower risk of hyperglycemia in these patients (Fig. 2) [9, 12, 13]. Melatonin also has a role in glucose intolerance. Study on pinealectomized rats, has shown an increased glucose intolerance and insulin resistance. The rats daily blood glucose levels were altered as well as a great shift of glucose levels at night. But, treating these rats with melatonin, showed a significant decrease in glucose intolerance [9, 12]. In a study published in 2011, 36 type 2 diabetic patients were divided into two groups, placebo and receiving 2 mg of melatonin for a period of 5 months. This study shows that after 3 weeks of melatonin intake, before going to bed, didn’t show any difference in blood sugar or glycosylated hemoglobin (HbA1c) levels. However, after 5 months of melatonin treatment, a beneficial effect on HbA1c level was seen in these patients, a significant decrease from 9.13% ± 1.55–8.47% ± 1.67%. Also, the mean serum glucose level was significantly lower after 5 months than after the third week tests [14]. In another study, 64 type 2 diabetic patients received placebo for 12 weeks and then received 6 mg of melatonin for another 12 weeks. They measured these patients fasting blood sugar (FBS), total triglyceride (TG), total cholesterol (CHOL), high-density (HDL) and low-density lipoprotein (LDL) cholesterol and HbA1c at baseline, 12 and 24 weeks. After 3 months of melatonin intake, a significant lower level of Hb1c was seen compared to the baseline figures, 7.65% ± 0.0686% versus 7.1% ± 0.111%. The mean FBS had also decreased significantly after 12 weeks of intake. HDL levels were increased at the end of the study, but no change in TG, CHOL and LDL levels were seen [15]. Membrane signaling by melatonin receptors is presented in Fig. 3 [16, 17].
Fig. 2.
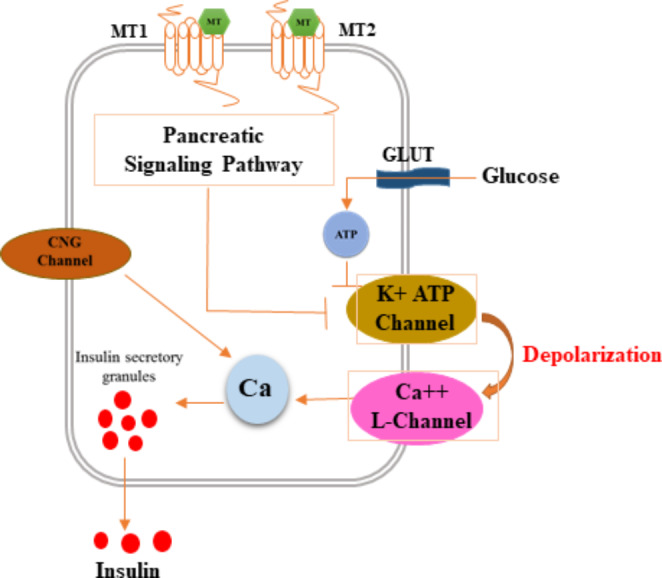
Schematic representation of the mechanism by which melatonin protects hyperglycemia. Melatonin attaches to MT1 and MT2 receptors, which effect K-Ca channels and increase Ca levels. Increase in Ca levels increase insulin secretory granules which result in higher levels of insulin. (Original figure by the authors)
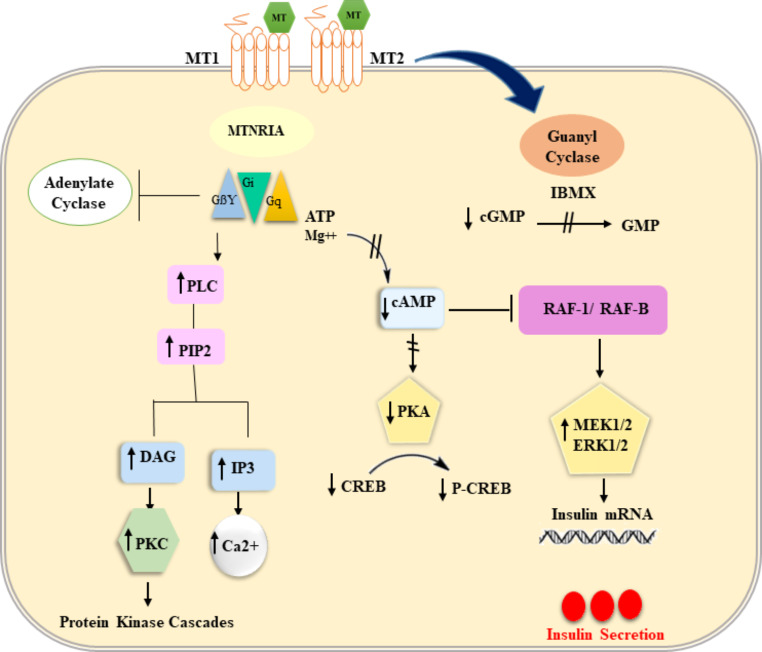
Fig. 3.
Schematic representation of the membrane signaling by melatonin receptors MT 1 and MT 2. In part of the figure is shown the interaction of Gi with adenyl cyclase (AC) to decrease cAMP levels and therefore cyclic AMP-dependent protein kinase activity (PKA) and interaction of G q with phospholipase C (PLC) leading to the cleavage of phosphatidyl inositol diphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG). These second messengers stimulate increased intracellular Ca2 + and protein kinase C (PKC), respectively. In another part of the figure is shown the melatonin/MTNR/Raf-1/ERK signaling pathway. Melatonin binds to the MT2 receptor followed by reduced cAMP production and PKA inhibition. In turn, this inactivates the Ras/Raf-1/ERK pathway, eventually decreasing insulin gene transcription. (Original figure by the authors)
Melatonin and diabetic neuropathy
Diabetic Neuropathy (DN) is a highly seen neurological complication in patients with diabetes [18]. DN affects near 50% of this population [19]. This complication includes peripheral, autonomic, proximal and focal neuropathy. Peripheral neuropathy is the most common form of DN and serves as the main cause of foot ulceration, neuropathic pain and amputation [18–20]. Some peripheral nervous system damage mechanisms are observed in foot nerve damage. The interaction of oxidative stress and inflammation caused by hyperglycemia, hyperlipidemia and insulin resistance play an important role in DN pathogenesis [7, 9, 21]. The mentioned interaction results in an increase in polyol, advanced glycation end products (AGEs), protein kinase C (PKC), Poly ADP ribose polymerase (PARP) and hexosamine [22, 23]. Aside from the mentioned metabolites, insulin signaling is also lost. They all lead to mitochondrial dysfunction, altered gene expression and diminished K-channel activity and augmented Na-channel activity. All of these, along with oxidative stress and inflammation, result into nerve damage and cell death [22].
This path shows melatonin contributes in neurological complications of diabetes. So, it can be used as a treatment of DN. Due to melatonin’s antioxidant activity, it prevents neuron apoptosis in diabetic patients. It reduces factors such as ethanol-induced ROS, NF-κB activation and increases factors such as Nrf2 (an antioxidant) and heme oxygenase-1 [24]. Melatonin also has an increasing effect on the formation and release of neurotransmitters, which shows a better neuronal function in these patients [25]. The melatonin beneficial effect is shown in Fig. 4 [26–28]. In a study conducted by Metwally et al. on diabetic male Wistar rats induced by streptozotocin (STZ), the rats were treated with 50 mg/kg of melatonin per day for 45 days, starting 72 h before STZ injection. Metwally et al.’s findings show an improvement in diabetes-induced oxidative stress and neurodegeneration. This shows that melatonin has great effect on neurological complications of hyperglycemia [29]. In a study on diabetes induced by STZ on female Wistar albino rats shows the effect of melatonin on diabetic neuropathy. These rats received 10 mg/kg melatonin per day for 14 days. After 2 weeks, fresh samples of hippocampal and dorsal root ganglion were taken. The results show that melatonin treatment has a beneficial role in improving oxidative stress and reducing neuron death which results in improved DN [30]. In 2013, STZ-diabetic Sprague-Dawley rats received 3 and 10 mg/kg melatonin for periods of 2 weeks. Melatonin intake in these rats, shows improved levels of neurotransmitters, glutamate and gamma-aminobutyric acid (GABA). Jangra et al. suggest that melatonin has a beneficial effect on central nervous system (CNS) damage caused by diabetes [25]. Ali et al. gave diabetic-induced female Sprague-Dawley rats a single dose of 20 mg/kg of melatonin. After four hours, samples of brain were taken to observe the results. The acute levels of melatonin showed a reduction in NF-κB active levels. Also, this treatment showed a decrease ethanol-induced apoptosis, neurodegeneration and neuroinflammatory levels. The presence of melatonin had also a great effect on ROS levels due to its antioxidant activity. The results of this study demonstrate that acute melatonin treatment, has a preventive effect on DN [31]. These pathways, reduction of neurotransmitters, GABA and glutamate, increased levels of ROS, increased in polyol, AGEs production, protein kinase C (PKC), Poly (ADP-ribose) polymerase (PARP) and hexosamine result in an impaired neuronal function. Studies indicate that the malfunction of neurons plays a major part in the occurrence of diabetic foot ulcers. [18–20].
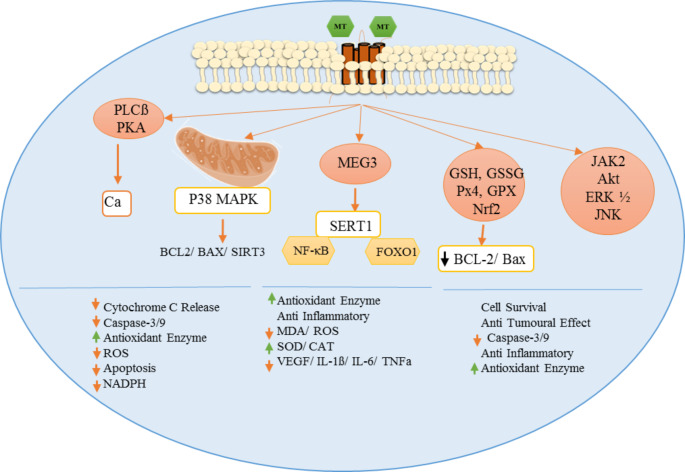
Fig. 4.
Potential mechanism(s) of action of melatonin in diabetes. PLC: phospholipase C, PKA: protein kinase A, GSH: reduced glutathione, GSSG: oxidized glutathione, GPx: glutathione peroxidase, Nrf2: nuclear factor erythroid 2-related factor 2, AKT: protein kinase B, ERK: extracellular signal-regulated kinase, MAPK: mitogen-activated protein kinase, SIRT1: silent information regulator 1, FoXO1: fork head box protein O1, AMPK: AMP-activated protein kinase,, ROS: reactive oxygen species, NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells. (Original figure by the authors)
Melatonin and vascular damage
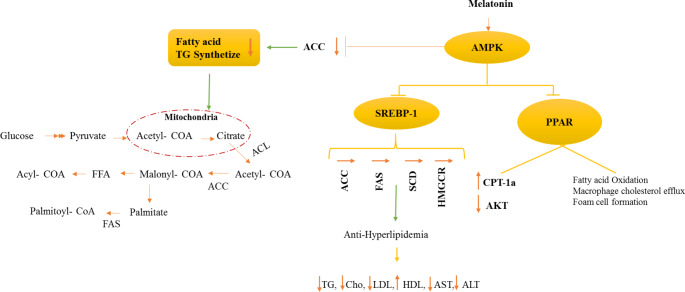
Diabetes vascular disease has a wide range of atherosclerosis related disease. Coronary artery disease, peripheral vascular disease and stroke are examples of diabetes induced vascular complications. Long-term follow-up of blood glucose control has shown a decrease in atherosclerosis related complications in diabetic patients [32]. Insulin resistance and hyperglycemia are the main factors of diabetic vascular disease [7, 8, 10, 32]. There are multiple molecular mechanisms that explains hyperglycemic induced complications. Increase in polyol pathway, activation of diacylglycerol (DAG)/protein kinase C (PKC) pathway, increased oxidative stress, increase in AGE (advanced glycation end) products formation and activation also increased hexosamine pathway are some of these most studied pathways. [10, 32]. Increase in polyol pathway activation will cause vascular pathologies through osmotic damage and reduction in Na+-K+-ATPase activity. This pathway will result in decreased cellular levels of NADPH and increased NADH/NAD+ ratio which will result in an imbalance of intercellular redox. This changed balanced results in oxidative stress. Increased oxidative stress leads to endothelial cells dysfunction [33]. Increased levels of glucose will end up in high concentration of DAG in many cells such as aortic endothelial cells [32]. Chronic elevated levels of DAG activate PKC pathways. Other methods of PKC activation are ROS species and free fatty acids (FFA) [34, 35]. Elevated PKC activation has been related to alteration in blood flow, basement membrane thickening, increase in vascular permeability, abnormal angiogenesis, excessive apoptosis, increased leukocyte adhesion and change in enzyme activity such as Na+-K+-ATPase, cPLA2, PI3K and mitogen activated protein kinase (MAPK) [36]. Furthermore, PKC activation results in overexpression of plasminogen activatior-1 (PAI-1) [37], NF-κB activation [38] and the activation of NADPH oxidase [39] in many vascular cells such as endothelial cells, smooth muscle cells, pericytes and mesangial cells [38]. The PKC activation is an underlying factor in the pathology of cardiovascular disease in patients with chronic hyperglycemia [39]. Also, extra glucose in the cell ends into the production of pyruvate in the cytoplasm by glycolysis pathway. Pyruvate is turned into acetyl-CoA in the mitochondria. Then it is transported to the cytoplasm as in the form of citrate, which is reconverted by ATP citrate lyase (ACL). Acetyl-CoA is then carboxylated into malonyl-CoA in the presence of acetyl-CoA carboxylase (ACC) as catalyzer. Malonyl-CoA and acetyl-CoA are used as substances to produce palmitate in the presence of fatty acid synthase (FAS). Afterwards, malonyl-CoA is converted back into acetyl-CoA via malynol-CoA decarboxylase (MCD). Long-chain fatty acyl-CoA synthetase (LCFACS) catalyzes the esterification of fatty acids. Long-chain fatty acyl-CoA, such as palmitoyl-CoA are imported to the mitochondria for fatty acid oxidation by the enzyme named carnitine palmitoyltransferase 1 (CPT1). The later enzyme is inhibited allosterically by malonyl-CoA. Depending on requirements, the saturated fatty acids created by FAS can be metabolized, desaturated, derived to triglyceride or channeled to phospholipids and derivates in charge of signaling and membrane functions. ACC, MCD and FAS activities are controlled by AMP-activated protein kinase (AMPK) which is composed of two regulatory subunits (β1 or β2 and γ1 or γ2 or γ3) and catalytic subunit (α1 or α2). Melatonin has an impact on AMPK. It increases the enzymes activity which will inhibit lipid production. It also decreases the activity of ACC. Thus, fatty acid TG synthetize activity is reduced and it results in the reduction of products such as palmioyl-CoA and acyl-CoA. These effects of melatonin have a great role in decreasing the risk of hyperlipidemia (Fig. 5) [40]. Previously mentioned, high levels of ROS result in oxidative stress which has a role in forming diabetic complications [21]. ROS production is increased by abnormal metabolism of glucose, FFA and other reactive metabolites in diabetes [41]. ROS byproducts also elevate PKC which will end in activating NADPH oxidase that increases ROS production [21]. Also increased levels of FFA result in higher levels of ROS [42]. These pathways will result in higher oxidative stress in patients with diabetes and cardiovascular damage. Hyperlipidemia is one of the most common reasons of atherosclerosis. Hyperlipidemia is highly seen in diabetic patients [9]. Melatonin intake of 10 mg/kg per day for 6 weeks in Zucker diabetic rats, shows a reduction in LDL-cholesterol as well as an increase in HDL-cholesterol [43]. In another study on diabetic male Wistar rats, a significant reduction in serum TG levels was seen after melatonin treatment for 6 weeks [12]. In 2018, Hadjzadeh et al. studied the effect of melatonin on serum concentrations of TG, LDL-chol, HDL-chol and total cholesterol (TC). They divided 40 male Wistar rats into 5 groups: control, diabetic and three groups of STZ-induced diabetics, each group receiving different levels of melatonin, 5 mg/kg, 10 mg/kg and 20 mg/kg for 14 days. The results indicate lower levels of LDL, TG and TC in diabetic rats treated with melatonin [44]. Studies show that exogenous melatonin intake acts as antioxidant. This is how melatonin has a beneficial effect on hyperlipidemia. It increases antioxidant enzymes and glutathione levels, removes free radicals and decreases lipid peroxidation [16]. Along with endogenous melatonin, it has a beneficial effect on reducing oxidative stress [45]. Melatonin also increases antioxidant enzymes such as superoxide dismutase (SOD), catalase and glutathione peroxidase (GPX). A study on STZ-induced diabetic Wistar rats with melatonin intake of 50 mg/kg/day, shows that these rats have a higher levels of antioxidant enzymes. Therefore, better vascular function has been seen among them [8, 46]. In 2000, the effect of melatonin intake was assessed in alloxan induced diabetes male Wistar rats. This study shows a reduction in antioxidant enzymes in these rats after becoming diabetic comparing to the control group. Diabetic rats were treated with melatonin for overall 7 weeks. After this period, SOD and glutathione peroxidase concentrations were restored significantly in these rats [47]. Increase in ROS, hyperlipidemia induced by high glucose level, PKC activation and increase in ACC, MCD and FAS activity are the underlying factors of vascular complication seen in patients with diabetes. Studies show that vascular complications in diabetic patients have a great role in the incidence of diabetic foot ulcers [3, 7]. Also, the anti-inflammatory and antioxidative effects of Melatonin have been seen in several other studies [48, 49].
Fig. 5.
prevented hyperlipidemia by up-regulating the AMPK pathway and regulation of lipid metabolism. AMPK: AMP-activated protein kinase, SREBP1: sterol regulatory element binding protein-1, ACC: Acetyl-CoA carboxylase, ACL: ATP citrate lyase, FAS: fatty acid synthase, HMGCR: 3-hydroxy-3-methylglutaryl-CoA reductase, SCD: stearoyl-CoA desaturase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, TG: triglyceride, Cho: cholesterol, LDL: low-density lipoprotein cholesterol, HDL: high-density lipoprotein cholesterol, CPT-1: Carnitine palmitoyl transferase 1, AKT: protein kinase B. (Original figure by the authors)
Melatonin and immune function
Melatonin plays a significant role in the immune system by regulating immune functions, enhancing immune cell production and activity, reducing oxidative stress and inflammation, and maintaining immune system balance. Its antioxidant and anti-inflammatory properties contribute to a healthier immune system and optimal immune responses (Fig. 6) [50]. Melatonin receptors are expressed on some T cells and B cells [51]. Studies on mice treated with melatonin show that it may also increase the proliferation of T cells and the production of natural killer cells and monocytes in their bone marrow [52, 53]. Some studies show that melatonin intake in mice has decreased the expression of interleukin-2 (IL-2) and interferon-γ (IFN-γ) and the expression of T helper 2 cell cytokines like IL-4 and IL-10 has increased [54, 55]. Ozkanlar et al. assessed the effect of melatonin on diabetic rats’ immune system. In this study, they had four groups of male Smrague-Dawley rats: control (C) group, a melatonin (Mel) group receiving 10 mg/kg/per day of melatonin for 15 days, diabetic (DM) group who were diabetes induced with alloxan which received placebo and the diabetic-melatonin (DM-Mel) group who after developing diabetes with alloxan received 10 mg/kg per day of melatonin for 15 days. The results show a significant increase in WBC, neutrophil count of DM group compared to the control rats. Compared to DM group, WBC, monocyte and neutrophil count were significantly lower in the DM-Mel group. Furthermore, WBC, monocyte and neutrophil count were significantly lower in Mel group compared to the C group. As for cytokine figures, IL-1β was slightly lower in the DM-Mel group comparing to the DM group. But TNF-α did not change in DM-Mel rats. A significant increase in both cytokines was seen in DM rats compared to the control group. Thus, study shows that melatonin can be used as an immune-modulatory agent in diabetes [56]. Apoptosis and inflammation are two mechanisms that cause this damage. In these patients, increase in inflammatory cytokines, such as IL-8, IL-6 and TNF-α, decrease in anti-inflammatory cytokines, IL-10 and IL-2, and decrease in antioxidant barrier is seen. These three pathways result in increased inflammation and apoptosis. Thus, wound healing process is impaired and will eventually result into diabetic foot ulcers [57].
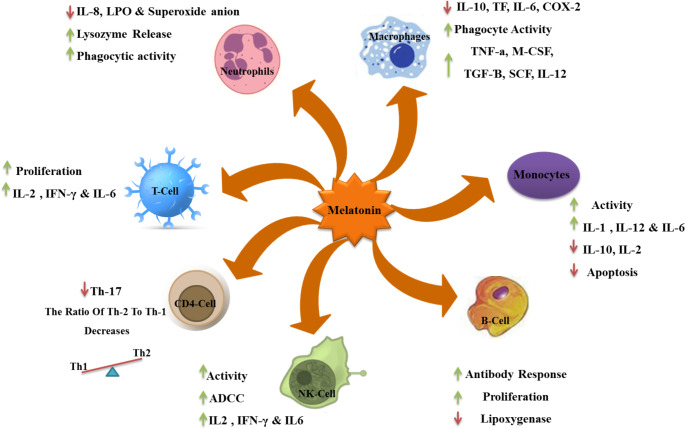
Fig. 6.
The figure showing actions of the melatonin on cells involved in the innate immune response. Melatonin can inhibit neutrophil function, increase the levels of NK cells and monocytes; regulate CD4+, CD8 + cell differentiation, Monocytes and Bcell activation. In addition, MLT can influence the NO/NOS pathway, improve mitochondrial function. (Original figure by the authors)
The aforementioned complications, including hyperglycemia, diabetic neuropathy, vascular problems, and impaired immunity, are underlying causes of diabetic foot ulcers [3–6]. By effectively managing or preventing these complications, the risk of diabetic foot ulcers can be reduced. Recent studies on the effects of melatonin have shown its potential in lowering the risk of diabetic neuropathy [24, 25, 29–31], hyperglycemia [9, 12, 14, 15], vascular complications [8, 9, 12, 16, 43–47] and improving immunity [50] in diabetic patients. It also helps the body have stronger immunity [56]. By reducing these complications, the incidence of diabetic foot ulcer may also decrease in affected individuals. It is worth mentioning that factors related to melatonin cycle, such as lifestyle [58], climate [59], and ethnic differences [60] could be targeted in personalized medicine research focusing on diabetic foot ulcers and their underlying conditions. Moreover, future research efforts may aim to determine the optimal dosage of melatonin, as a metabolite involved in circadian rhythm, for the control and prevention of diabetic foot ulcers.
Conclusion
Studies show that melatonin is effective in the underlying conditions of diabetic foot ulcers. Therefore, conducting additional observational and clinical studies involving individuals with diabetes could enhance our understanding of the impact of melatonin on the prevention and treatment of diabetic foot ulcers. Overall, considering the impact of melatonin on hyperglycemia, neuropathy, vascular damage and immune system this study suggests that melatonin with its potential therapeutic and preventive properties may contribute to reducing the risk of diabetic foot ulcers.
Acknowledgements
Not applicable.
Authors’ contributions
Zahra Sajjadpour and Zahra Hoseini Tavassol: Investigation, designing, writing – original draft, visualization. Hamid Reza Aghaei Meybodi: Conceptualization, designing, writing – review & editing. Maryam Eskandarynasab: Investigation, visualization, writing – review & editing. Mahnaz Pejman Sani: Conceptualization, designing, project administration, supervision, writing – review & editing. Shirin Hasani-Ranjbar and Bagher Larijani: Conceptualization, writing – review & editing. All authors reviewed and approved the final manuscript.
Funding
Not applicable.
Data Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zahra Sajjadpour and Zahra Hoseini Tavassol contributed equally to this work.
References
- 1.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, 2022. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. [DOI] [PMC free article] [PubMed]
- 3.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet (London England) 2003;361(9368):1545–51. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 4.Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am J Surg. 1998;176(2A Suppl):5S–10S. doi: 10.1016/s0002-9610(98)00181-0. [DOI] [PubMed] [Google Scholar]
- 5.Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes therapy : research, treatment and education of diabetes and related disorders. 2012;3(1):4. 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed]
- 6.Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 7.Pourhanifeh MH, Hosseinzadeh A, Dehdashtian E, Hemati K, Mehrzadi S. Melatonin: new insights on its therapeutic properties in diabetic complications. Diabetol Metab Syndr. 2020;12:30. doi: 10.1186/s13098-020-00537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behram Kandemir Y, Guntekin U, Tosun V, Korucuk N, Bozdemir MN. Melatonin protects against streptozotocin-induced diabetic cardiomyopathy by the phosphorylation of vascular endothelial growth factor-A (VEGF-A). Cell Mol Biol (Noisy-le-grand). 2018;64(14):47–52. 10.14715/cmb/2018.64.14.8. [PubMed]
- 9.Mok JX, Ooi JH, Ng KY, Koh RY, Chye SM. A new prospective on the role of melatonin in diabetes and its complications. Horm Mol Biol Clin Investig. 2019;40(1). 10.1515/hmbci-2019-0036. [DOI] [PubMed]
- 10.Otamas A, Grant PJ, Ajjan RA. Diabetes and atherothrombosis: the circadian rhythm and role of melatonin in vascular protection. Diabetes Vasc Dis Res. 2020;17(3):1479164120920582. doi: 10.1177/1479164120920582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wajid F, Poolacherla R, Mim FK, Bangash A, Rutkofsky IH. Therapeutic potential of melatonin as a chronobiotic and cytoprotective agent in diabetes mellitus. J Diabetes Metabolic Disorders. 2020;19(2):1797–825. doi: 10.1007/s40200-020-00585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bibak B, Khalili M, Rajaei Z, Soukhtanloo M, Hadjzadeh MA, Hayatdavoudi P. Effects of melatonin on biochemical factors and food and water consumption in diabetic rats. Adv Biomedical Res. 2014;3:173. doi: 10.4103/2277-9175.139191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghav A, Mishra BK, Tomar R, Raghav S, Jeong G-B. Prospective role of N-Acetyl-5-Methoxytryptamine and 5-Hydroxytryptophan in β-Cell health and improved insulin sensitivity in hyperglycemia. Chronobiology in Medicine. 2021;3(1):4–7. doi: 10.33069/cim.2021.000. [DOI] [Google Scholar]
- 14.Garfinkel D, Zorin M, Wainstein J, Matas Z, Laudon M, Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metabolic Syndrome and Obesity : Targets and Therapy. 2011;4:307–13. doi: 10.2147/DMSO.S23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezvanfar MR, Heshmati G, Chehrei A, Haghverdi F, Rafiee F, Rezvanfar F. Effect of bedtime melatonin consumption on diabetes control and lipid profile. Int J Diabetes Developing Ctries. 2016;37(1):74–7. doi: 10.1007/s13410-016-0497-2. [DOI] [Google Scholar]
- 16.Imenshahidi M, Karimi G, Hosseinzadeh H. Effects of melatonin on cardiovascular risk factors and metabolic syndrome: a comprehensive review. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(4):521–36. doi: 10.1007/s00210-020-01822-4. [DOI] [PubMed] [Google Scholar]
- 17.Masana MI, Dubocovich ML. Melatonin receptor signaling: finding the path through the dark. Sci STKE. 2001;2001(107):pe39. doi: 10.1126/stke.2001.107.pe39. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diab/Metab Res Rev. 2012;28(Suppl 1):8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 20.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiology Pharmacol. 2019;11(3):45–63. [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New Horizons in Diabetic Neuropathy: mechanisms, Bioenergetics, and Pain. Neuron. 2017;93(6):1296–313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11(6):521–34. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negi G, Kumar A, Sharma SS. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: effects on NF-kappaB and Nrf2 cascades. J Pineal Res. 2011;50(2):124–31. doi: 10.1111/j.1600-079X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 25.Jangra A, Datusalia AK, Khandwe S, Sharma SS. Amelioration of diabetes-induced neurobehavioral and neurochemical changes by melatonin and nicotinamide: implication of oxidative stress-PARP pathway. Pharmacology, biochemistry, and behavior. 2013;114–115:43–51. 10.1016/j.pbb.2013.10.021. [DOI] [PubMed]
- 26.Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Reviews Endocrinol. 2019;15(2):105–25. doi: 10.1038/s41574-018-0130-1. [DOI] [PubMed] [Google Scholar]
- 27.Jin H, Zhang Z, Wang C, Tang Q, Wang J, Bai X, et al. Melatonin protects endothelial progenitor cells against AGE-induced apoptosis via autophagy flux stimulation and promotes wound healing in diabetic mice. Exp Mol Med. 2018;50(11):1–15. doi: 10.1038/s12276-018-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witt-Enderby PA, Bennett J, Jarzynka MJ, Firestine S, Melan MA. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 2003;72(20):2183–98. doi: 10.1016/s0024-3205(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 29.Metwally MMM, Ebraheim LLM, Galal AAA. Potential therapeutic role of melatonin on STZ-induced diabetic central neuropathy: a biochemical, histopathological, immunohistochemical and ultrastructural study. Acta Histochem. 2018;120(8):828–36. doi: 10.1016/j.acthis.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Kahya MC, Naziroglu M, Ovey IS. Modulation of Diabetes-Induced oxidative stress, apoptosis, and ca(2+) entry through TRPM2 and TRPV1 channels in dorsal Root Ganglion and Hippocampus of Diabetic rats by melatonin and selenium. Mol Neurobiol. 2017;54(3):2345–60. doi: 10.1007/s12035-016-9727-3. [DOI] [PubMed] [Google Scholar]
- 31.Ali T, Rehman SU, Shah FA, Kim MO. Acute dose of melatonin via Nrf2 dependently prevents acute ethanol-induced neurotoxicity in the developing rodent brain. J Neuroinflamm. 2018;15(1):119. doi: 10.1186/s12974-018-1157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Invest. 2010;1(3):77–89. doi: 10.1111/j.2040-1124.2010.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesfamariam B. Free radicals in diabetic endothelial cell dysfunction. Free Radic Biol Med. 1994;16(3):383–91. doi: 10.1016/0891-5849(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 35.Kuroki T, Inoguchi T, Umeda F, Nawata H. Effect of eicosapentaenoic acid on glucose-induced diacylglycerol synthesis in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1998;247(2):473–7. doi: 10.1006/bbrc.1998.8814. [DOI] [PubMed] [Google Scholar]
- 36.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55(6):498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 37.Ahn JD, Morishita R, Kaneda Y, Lee KU, Park JY, Jeon YJ, et al. Transcription factor decoy for activator protein-1 (AP-1) inhibits high glucose- and angiotensin II-induced type 1 plasminogen activator inhibitor (PAI-1) gene expression in cultured human vascular smooth muscle cells. Diabetologia. 2001;44(6):713–20. doi: 10.1007/s001250051680. [DOI] [PubMed] [Google Scholar]
- 38.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48(4):855–64. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 39.Shao B, Bayraktutan U. Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C-ssI and prooxidant enzyme NADPH oxidase. Redox Biol. 2014;2:694–701. doi: 10.1016/j.redox.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ou TH, Tung YT, Yang TH, Chien YW. Melatonin improves fatty liver syndrome by inhibiting the Lipogenesis Pathway in Hamsters with High-Fat Diet-Induced Hyperlipidemia. Nutrients. 2019;11(4):748. doi: 10.3390/nu11040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Jin X, Kei Lam CW, Yan SK. Oxidative stress and diabetes mellitus. Clin Chem Lab Med. 2011;49(11):1773–82. doi: 10.1515/CCLM.2011.250. [DOI] [PubMed] [Google Scholar]
- 42.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88(10):4673–6. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 43.Agil A, Navarro-Alarcon M, Ruiz R, Abuhamadah S, El-Mir MY, Vazquez GF. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res. 2011;50(2):207–12. doi: 10.1111/j.1600-079X.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- 44.Hadjzadeh MAR, Alikhani V, Hosseinian S, Zarei B, Keshavarzi Z. The effect of melatonin against gastric oxidative stress and Dyslipidemia in Streptozotocin-Induced Diabetic rats. Acta endocrinologica (Bucharest, Romania : 2005). 2018;14(4):453–8. 10.4183/aeb.2018.453. [DOI] [PMC free article] [PubMed]
- 45.Espino J, Rodriguez AB, Pariente JA. Melatonin and oxidative stress in the Diabetic State: clinical implications and potential therapeutic applications. Curr Med Chem. 2019;26(22):4178–90. doi: 10.2174/0929867325666180410094149. [DOI] [PubMed] [Google Scholar]
- 46.Kandemir YB, Tosun V, Guntekin U. Melatonin protects against streptozotocin-induced diabetic cardiomyopathy through the mammalian target of rapamycin (mTOR) signaling pathway. Advances in clinical and experimental medicine. Official Organ Wroclaw Medical University. 2019;28(9):1171–7. doi: 10.17219/acem/103799. [DOI] [PubMed] [Google Scholar]
- 47.Sailaja Devi MM, Suresh Y, Das Preservation of the antioxidant status in chemically-induced diabetes mellitus by melatonin. J Pineal Res. 2000;29(2):108–15. doi: 10.1034/j.1600-079x.2000.290207.x. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffari S, Hasani-Ranjbar S, Abdollahi M. The mechanisms of positive effects of melatonin in dyslipidemia: a systematic review of animal and human studies. Int J Pharmacol. 2012;8(6):496–509. doi: 10.3923/ijp.2012.496.509. [DOI] [Google Scholar]
- 49.Mohammadi-Sartang M, Ghorbani M, Mazloom Z. Effects of melatonin supplementation on blood lipid concentrations: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;37(6 Pt A):1943-54. 10.1016/j.clnu.2017.11.003. [DOI] [PubMed]
- 50.Calvo JR, Gonzalez-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J Pineal Res. 2013;55(2):103–20. doi: 10.1111/jpi.12075. [DOI] [PubMed] [Google Scholar]
- 51.Pozo D, Delgado M, Fernandez-Santos JM, Calvo JR, Gomariz RP, Martin-Lacave I, et al. Expression of the Mel1a-melatonin receptor mRNA in T and B subsets of lymphocytes from rat thymus and spleen. FASEB J. 1997;11(6):466–73. doi: 10.1096/fasebj.11.6.9194527. [DOI] [PubMed] [Google Scholar]
- 52.Currier NL, Sun LZ, Miller SC. Exogenous melatonin: quantitative enhancement in vivo of cells mediating non-specific immunity. J Neuroimmunol. 2000;104(2):101–8. doi: 10.1016/s0165-5728(99)00271-4. [DOI] [PubMed] [Google Scholar]
- 53.Pioli C, Caroleo MC, Nistico G, Doria G. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int J Immunopharmacol. 1993;15(4):463–8. doi: 10.1016/0192-0561(93)90060-c. [DOI] [PubMed] [Google Scholar]
- 54.Raghavendra V, Singh V, Kulkarni SK, Agrewala JN. Melatonin enhances Th2 cell mediated immune responses: lack of sensitivity to reversal by naltrexone or benzodiazepine receptor antagonists. Mol Cell Biochem. 2001;221(1–2):57–62. doi: 10.1023/a:1010968611716. [DOI] [PubMed] [Google Scholar]
- 55.Raghavendra V, Singh V, Shaji AV, Vohra H, Kulkarni SK, Agrewala JN. Melatonin provides signal 3 to unprimed CD4(+) T cells but failed to stimulate LPS primed B cells. Clin Exp Immunol. 2001;124(3):414–22. doi: 10.1046/j.1365-2249.2001.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozkanlar S, Kara A, Sengul E, Simsek N, Karadeniz A, Kurt N. Melatonin Modulates the Immune System Response and Inflammation in Diabetic Rats Experimentally-Induced by Alloxan. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2016;48(2):137 – 44. 10.1055/s-0035-1548937. [DOI] [PubMed]
- 57.Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chemico-Biol Interact. 2014;219:101–12. doi: 10.1016/j.cbi.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS ONE. 2008;3(8):e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maroszek J, Morita T, Błazejczyk K. Melatonin secretion in various climate zones. Berichte des Meteorologischen Instituts der Albert-Ludwigs-Universität Freiburg. 2010:273.
- 60.Jeong J, Zhu H, Harris RA, Dong Y, Su S, Tingen MS, et al. Ethnic differences in Nighttime Melatonin and Nighttime Blood pressure: a study in European Americans and African Americans. Am J Hypertens. 2019;32(10):968–74. doi: 10.1093/ajh/hpz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, 2022. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. [DOI] [PMC free article] [PubMed]
Data Availability Statement
Not applicable.