Abstract
Gestational diabetes mellitus (GDM) is a pathological condition in which the placenta releases a hormone called human placental lactogen that prevents maternal insulin uptake. GDM is characterised by varying degrees of carbohydrate intolerance and is first identified during pregnancy. Around 5-17% of pregnancies are GDM pregnancies. Older or obese women have a higher risk of developing GDM during gestation. Hyperglycemia is a classic manifestation of GDM and leads to alterations in eNOS and iNOS expression and subsequently causes ROS and RNS overproduction. ROS and RNS play an important role in maintaining normal physiology, when present in low concentrations. Increased concentrations of ROS is harmful and can cause cellular and tissue damage. Oxidative stress is defined as an imbalance between pro-oxidant and antioxidant molecules that manifests due to hyperglycemia. miRNAs are short, non-coding RNAs that play a critical role in regulating gene expression. Studies have shown that the placenta expresses more than 500 miRNAs, which play a crucial role in trophoblast division, movement, and apoptosis. Latest research has revealed that hyperglycemic conditions and increased oxidative stress, characteristic of GDM, can lead to the dysregulation of miRNAs. The placenta also releases miRNAs into the maternal circulation. The secreted miRNAs are encapsulated in exosomes or vesicles. These exosomes interact with tissues and organs at distant sites, releasing their cargo intracellularly. This crosstalk between hyperglycemia, ROS and miRNA expression in GDM has detrimental effects on both foetal and maternal health. One of the complications of GDM is preterm labour. GDM induced iNOS expression has been implicated in cervical ripening, which in turn causes preterm birth. This article focuses on the speculations of oxidative and nitrative stress markers that lead to detrimental effects in GDM. We have also envisaged the role of non-coding miRNA interactions in regulating gene expression for oxidative damage.
Graphical Abstract
Holistic view of miRNA in GDM.
-
I)
(A) Placenta as a metabolic organ that provides the foetus with nutrients, oxygen and hormones to maintain pregnancy. Human placental lactogen (hPL) is one such hormone that is released into maternal circulation. hPL is known to induce insulin resistance. (B) ß-cell dysfunction leads to reduced glucose sensing and insulin production. Insulin resistance, a characteristic of GDM, exacerbates insulin ß cell dysfunction leading to maternal hyperglycemia. Hyperglycemia leads to increased ROS and RNS production through several mechanisms. Consequently, GDM is characterised by increased oxidative and nitrative stress.
-
II)
Exposure to maternal hyperglycemia causes increased ROS and RNS production in trophoblast cells. Oxidative stress caused by hyperglycemia may lead to eNOS uncoupling, causing eNOS to behave as a superoxide producing enzyme. iNOS expression in trophoblast cells leads to increased NO production. iNOS-derived NO reacts with ROS to produce RNS, thereby increasing nitrosative stress. Expression of antioxidant defences are reduced. Hyperglycemia and oxidative stress may alter the expression of some miRNAs. Some miRNAs are upregulated while others are downregulated. Some miRNAs are secreted into maternal circulation in the form of exosomes. Oxidative stress markers, nitrative stress markers and circulating miRNAs are found to be increased in maternal circulation.
Keywords: Gestational diabetes Mellitus, ROS, miRNA, iNOS, Preterm labour
Introduction
The placenta is an endocrine organ that functions as a barrier between foetal and maternal circulation and aids in foetal growth and development [1]. It is composed of syncytiotrophoblasts, cytotrophoblasts, stromal cells, and fibroblasts, among other cells [2]. The placenta supports the survival of the foetus by the transport of gases, nutrients and by the synthesis of placental hormones. Human placental lactogen (hPL) is one such hormone that is involved in the pathogenesis of GDM [3]. GDM is a metabolic disorder that causes insulin resistance (IR) and hyperglycemia in pregnant mothers. It occurs for the first time during pregnancy and may occur during subsequent pregnancies. This disorder is caused by human placental lactogen. Insulin uptake is impaired and causes glucose to build up in the maternal circulation [4]. Hyperglycemia can affect the pregnancy as well as the health of the offspring. The offspring and mother may develop some health complications, including T2DM, metabolic syndrome, and cardiovascular disorders later in life. A variety of factors cause GDM, some of which may include genetic factors [5], maternal age [6], obesity [7, 8], polycystic ovarian syndrome (PCOS), ethnicity, and exposure to toxicity [9].
Pregnancy is a state of enhanced oxidative stress. However, pathological pregnancies such as GDM can exacerbate pre-existing oxidative stress, which has detrimental effects on maternal and foetal health [10]. Hyperglycemia during GDM is known to enhance ROS production [11] and RNS [10] leading to elevated oxidative stress. There are a variety of mechanisms by which hyperglycemia increases ROS production. Hyperglycemia leads to the activation of NADPH oxidases [10], Xanthine oxidases [12], PKC pathway [13], the polyol pathway [11] and mitochondrial ROS production [14]. This leads to an imbalance between oxidants and antioxidants, a condition called oxidative stress [15]. Recent studies have revealed that NADPH oxidases are significant sources of ROS in the placenta. NOX1 is found to be expressed in placental trophoblast cells. Studies have also suggested that NADPH oxidases may be activated in a PKC dependent manner which could account for increased oxidative stress during diabetes [16].
RNS are reactive nitrogen-containing molecules which include Nitric oxide [NO] and peroxynitrite, a derivative of NO and is a powerful oxidant [17]. NO is the smallest gaseous signalling molecule that has been implicated in maintaining vascular homeostasis [18, 19]. NOS exists in 3 isoforms namely iNOS, eNOS and nNOS. Hyperglycemia causes alteration in eNOS and iNOS expression. Oxidative stress, characteristic of GDM, is known to cause eNOS uncoupling. This causes eNOS to become a superoxide generating enzyme [20]. iNOS expression is regulated transcriptionally and is activated by pro-inflammatory cytokines. iNOS mediates inflammation and produces a large amount of NO [21]. ROS molecules react with NO resulting in the formation of peroxynitrites that damage feto-placental circulation and vasculature [10]. iNOS is known to play a crucial role in cervical ripening. ROS and RNS contribute to oxidative stress that causes cervical ripening which in turn causes preterm labour.
miRNAs are short non-coding RNA that regulate the expression of genes. miRNAs bind to the 3’UTR region of the mRNA of a target gene. They are implicated in causing translational repression and mRNA deadenylation [22]. The placenta expresses around 500 miRNAs that are involved in gene regulation [23]. Dysregulation of these miRNAs take place in GDM which has harmful effects on both foetal and maternal health. The major factors that characterise GDM, namely, hyperglycemia and oxidative stress have been associated with the deregulation of miRNAs [24]. Recent literature has revealed that some miRNAs are upregulated while others are downregulated which in turn alter the expression of those genes that regulate maternal metabolic adaptations. Modification in the expression of genes that are required for normal maternal metabolism causes further insulin resistance, impaired glucose and lipid metabolism. This further exacerbates GDM conditions [24].
Figure 1 is a schematic representation that shows the interaction between various factors such as hyperglycemia, oxidative stress, nitrosative stress and miRNAs during GDM.
Fig. 1.
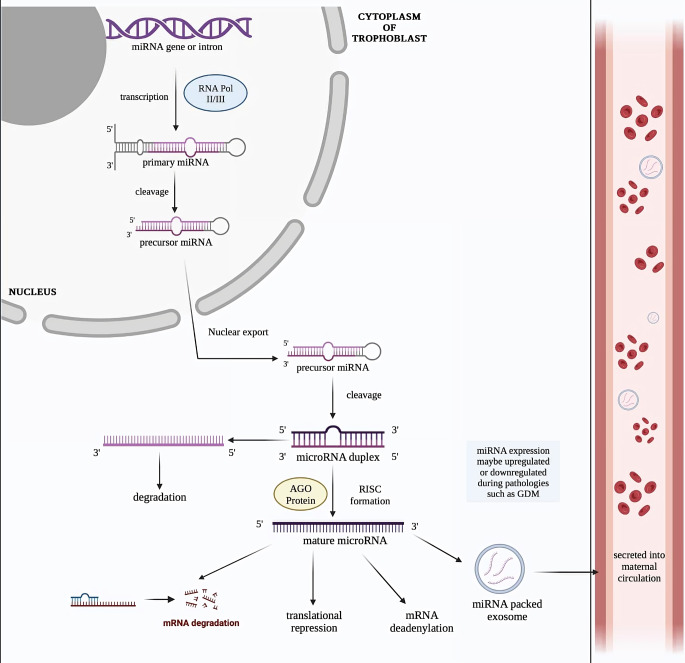
Precursor and Mature miRNA biogenesis
miRNAs are encoded by the introns of a gene. These genes are transcribed into primary RNA (pri-miRNA) by RNA polymerase II/III. Pri-miRNAs are further processed into precursor miRNA (pre-miRNA) and are transported to the cytoplasm of the cell where they are further processed to form the miRNA duplex. The AGO protein binds to one of the strands of the miRNA duplex, referred to as the guide strand. The strand complementary to the guide strand undergoes degradation. AGO protein and the guide strand together form the RNA-inducing silencing (RISC) complex. The mature miRNA involved in mRNA degradation, translational repression and mRNA deadenylation. Some miRNAs are packed into exosomes and secreted into maternal circulation in the form of circulating miRNAs. The expression of miRNAs may be upregulated or down regulated by placental pathologies such as GDM. Circulating miRNAs may be used as potential diagnostic biomarkers for screening of GDM.
Gestational diabetes mellitus(GDM)
GDM is glucose intolerance of any magnitude that is first recognised during pregnancy. GDM is of 2 main types namely : A1GDM and A2GDM. Adequate glycemic control can be achieved by nutritional therapy and dietary control in the case of A1GDM whereas A2GDM can only be managed by medication [25].
Etiology
GDM has been extensively studied over the past decade and it has been postulated that gestational diabetes is a disorder that develops in response to hormonal release from the placenta. The placenta is a functional metabolic organ that acts as a natural selective barrier between foetal and maternal circulation [1, 3]. It plays a special role in supporting foetal growth and development [1] by providing the foetus with nutrients, oxygen and hormones to maintain the pregnancy [4]. The human placenta produces lactogen, a hormone that has been chiefly associated with insulin resistance in GDM [25]. Human placental lactogen is known to have a contra-insulin effect leading to increased maternal glucose. This effect usually begins 20 to 24 weeks into the pregnancy and may aid in foetal growth. This pregnancy induced insulin resistance is overcome by the ability of the mother’s beta islet cells to produce additional insulin. However, as the foetus develops, the placenta grows, resulting in increased production of pregnancy hormones which subsequently lead to higher insulin resistance. GDM manifests when maternal insulin production is unable to overcome the effects of the placental hormones [4].
Beta cell dysfunction is also one of the two major contributors to the pathogenesis of GDM [11]. Storage and secretion of insulin in response to a glucose spike is the main function of β-cells. β-cell dysfunction refers to the condition in which β-cells can no longer sense the concentration of blood glucose or release sufficient insulin in response. Insulin resistance, characteristic of GDM, exacerbates β-cell dysfunction. The insulin-mediated uptake of glucose is reduced which further contributes to hyperglycemia. The resulting hyperglycemia overburdens the β-cells to produce additional insulin. β-cell dysfunction leads to the onset of a vicious cycle of insulin resistance, hyperglycemia and further β-cell dysfunction [26].
Placental pathophysiology during GDM
Insulin resistance during pregnancy is attributed to the secretion of various cytokines and hormones from the placenta. The placenta functions as a barrier between foetal and maternal circulation, is therefore exposed to hyperglycemic environments and therefore the consequences of GDM. Transport of amino acids, glucose and lipids across the placenta is impaired due to GDM [27].
Glucose is the key source of energy for the placenta and the foetus and must therefore be available at all times. Glucose is transported across the placenta via GLUT1, by carrier-mediated sodium-independent diffusion [28]. Although insulin is not needed for glucose transport across the placenta, insulin receptors are expressed on the placenta and insulin signalling can alter the metabolism of glucose in the placenta [29]. The placenta is quite receptive to the uptake of glucose, implying that it is responsive to maternal hyperglycemia. This is directly attributable to foetal macrosomia. Altered transport of amino acids across the placenta may also be one of the mechanisms by which excessive intake of proteins lead to GDM. Pro-inflammatory cytokines such as IL-6 and TNF-alpha are responsible for the modulation of placental uptake of amino acids [30, 31]. Alterations in placental gene expression occur mostly in the lipid pathways during GDM. Some placental lipid genes are also activated during GDM [32]. GDM affects glucose, lipid and amino acid transport across the placenta and impacts foetal growth and placental function.
GDM is characterised by increased maternal blood glucose (i.e. hyperglycemia). A direct link has been established between hyperglycemia and oxidative stress. There are various processes by which hyperglycemia induces oxidative stress. However, enhanced mitochondrial ROS production is the most common source of ROS. Hyperglycemic conditions disrupt the mitochondrial electron transport chain (ETC) resulting in overproduction of superoxide ion (O2−). This results in oxidative stress. Excessive RNS and ROS cause damage to the structure of cells and tissues. They function via the oxidation of bio-macromolecules such as proteins, DNA and lipids among others. Studies have discovered elevated levels of ROS and reduced antioxidant defences in the placenta of GDM patients. The levels of ROS were also found to be higher in high glucose treated JEG-3 trophoblast cells in comparison to the ones treated in low glucose conditions. Excessive ROS can further exacerbate hyperglycemia and insulin resistance [33]. This mutually enhancing relationship between hyperglycemia and ROS may account for the pathophysiology of GDM.
Recent studies have taken an active interest in the short, non-coding strands of RNA referred to as miRNAs. Placental trophoblasts express miRNAs which play an essential role in regulating various cellular processes. Recent evidence suggests that the placenta sheds miRNA containing exosomes into maternal circulation during gestation. These miRNAs can affect cell function and may have a role to play in the pathogenesis of GDM [34, 35].
A study of literature has revealed that there are various factors that may be responsible for the pathogenesis of GDM, including miRNAs, hyperglycemia, oxidative stress and alterations in expressions of genes that mediate placental uptake of glucose, lipids and amino acids.
Risk factors for GDM
Effective prevention and control strategies are required to manage serious health conditions such as GDM. There are many risk factors that contribute to the development of this disease. Diagnosis and prevention of this condition requires knowledge of the rudimentary causes of gestational diabetes. Maternal age and obesity are the two paramount factors independently affecting the risk of GDM [6]. Other risk factors involved are physical inactivity, family history of GDM, certain ethnicities and polycystic ovarian syndrome (PCOS), among others. Some medications can also trigger GDM [26, 36].
PCOS
Polycystic ovarian syndrome (PCOS) is a metabolic and endocrine disorder that develops in females. Most PCOS patients present with dysfunction in ovulation which subsequently leads to infertility. Studies have elucidated that PCOS patients are more likely to develop GDM. It has been hypothesised that the mechanism of development may be associated with IR. Patients with PCOS are also known to be at a higher risk for developing preeclampsia during pregnancy, preterm birth and neonatal hypoglycemia. Identification of GDM may be achieved at an earlier stage using PCOS as a factor in its early diagnosis. This can aid in preventing various short term as well as long term consequences of GDM which may affect the health of the foetus and the mother. A review of literature has revealed that after PCOS manifests, insulin produced during hyperinsulinemia interacts with IGFs present in the ovary. This causes failure in the mechanism that converts androstenedione into oestrogen in the cells of the follicular membrane. This in turn leads to an increased androgen concentration and ultimately leads to hyperandrogenemia formations. Research has established a link between androgens and ß-cell apoptosis. Yet another mechanism has been proposed that leaves PCOS patients at an increased risk of developing GDM. The liver produces a glycoprotein called SHBG which regulates the concentration of sex hormones in the blood and is responsible for the transport of sex hormones. SHBG mostly binds testosterone and insulin is a significant regulator of the metabolism of SHBG. IR leads to decreased insulin sensitivity which in turn causes an increase in the production of insulin. Increased levels of insulin inhibits SHBG production and synthesis in the liver. Reduced SHBG in the circulatory system causes sex hormone disorders which in turn causes disorders associated with the metabolism of glucose and fat. This exacerbates IR culminating in the manifestation of GDM [37]. The prevalence of the occurrence of PCOS prior to GDM was found to be 10.4% in comparison to 7.4% of PCOS cases that occurred in non-diabetic women [38].
Race and ethnicity
The association between the country of birth of the mother and GDM risk remains unexplored. A cohort study performed on women who delivered an infant between the years 1995 and 2004 and whose plasma glucose levels were measured using the screening 50-g, diagnostic 100-g glucose and the 3 h oral glucose tolerance test. Results from this study concluded that black and non-hispanic white women have the lowest risk of developing GDM. Mexican, Japanese and pacific islanders have an intermediate chance of developing GDM. Filipina, Chinese, Korean, South east Asian and Asian Indian women have higher chances of developing GDM, with Asian Indian women having the highest chance of developing GDM. A potential explanation for these findings could be due to the different types of environments that these women are exposed to in their country of origin. Serum levels of organic pollutants have been associated with the occurrence of GDM and T2DM and are known to have a role to play in developing IR. India, Mexico and China are the 3 largest producers of persistent chemical pollutants in the world. Consequently, women born in these countries are prone to exposure to higher levels of diabetogenic environments which in turn increases their risk of GDM. Rapid lifestyle transitions and various other environmental factors may interact with the genetic predisposition and thereby lead to the development of GDM [39].
Lifestyle factors
Obesity
Abnormal or excessive fat accumulation is a risk to health. The metric currently used for defining height/weight characteristics in adults is Body Mass Index (BMI) and is used for the classification of individuals into groups. BMI is generally categorised into classes : 15-19.9 Underweight ; 20-24.9 - Normal ; 25-29.9 - Overweight ; 30 < = Obesity of varying classes [7]. Gestational obesity in the mother is quantified by maternal BMI. Maternal obesity is a significant risk factor for GDM. Obese women are at a high risk of spontaneous miscarriage and is similar to observations recorded in women with pre-existing glucose intolerance. Avoiding excessive gestational weight gain may decrease maternal weight retention as well as a variety of other perinatal morbidities in future pregnancies [8].
Advanced maternal age
Yet another risk factor for GDM is advancing maternal age. Women above the age of 35 years are at an increased risk of developing GDM according to latest studies. High incidences of GDM have been associated with advancing maternal age. It is therefore important to plan pregnancies before the age of 35 years and older pregnant women must be provided with premium screening strategies [6].
Genetic factors
A good amount of evidence suggests that GDM is inherited. Research has revealed that increased risk of GDM is associated with a family history of diabetes. Insulin resistance and insulin deficiency, both being hallmarks of GDM, are heritable. Despite dietary modifications, GDM manifests in subsequent pregnancies. This evidence supports the concept of a genetic component to the disease. It is suspected that some genes such as Human Leukocyte Antigen (HLA) gene, Insulin (INS) gene, Insulin-Like Growth factor 2 gene (IGF-2) etc., can cause an individual to be susceptible to GDM [5].
Environmental factors
The link between GDM and environmental factors have been investigated. Studies revealed that environmental health influences such as exposure to cadmium, arsenic, phthalate,ambient temperature, air pollution, and season corresponds to GDM. The health of the offspring and mother are affected by environmental factors. Environmental factors and the mechanism by which it affects GDM is still uncertain and requires further investigation [40]. Bisphenol A (BPA) is an endocrine disrupting chemical (EDC), found in food packaging material and consumer based products. Exposure to BPA has been associated with GDM. It has been hypothesised that EDCs modulate exosomal signalling in the placenta [41].
The placenta : changes during GDM
The placenta is a transient, endocrine organ that is essential for foetal growth and development [1]. During the entire tenure of gestation, the foetus is entirely dependent on the placenta for its survival [42]. The placenta supports the growth of the foetus via transport of gases, nutrients and it synthesises and secretes a variety of hormones [43]. ATP derived from the mitochondria is required for active transport of nutrients across the placenta as well as for the synthesis of placental hormones [1]. The first type of cell that develops is the trophectoderm(TE). TE later differentiates into trophoblast cells to form the placenta [44]. The placenta is composed of syncytiotrophoblasts, cytotrophoblasts, mesenchymal cells, endothelial cells, stromal cells and fibroblasts, among others [2].
Various ROS molecules are produced by the placenta. Some of these molecules include superoxide, peroxynitrite and nitric oxide [43]. Escape of electrons from the mitochondria leads to local superoxide formation in the human placenta. Studies have revealed that ROS-producing NOXs are expressed in the placental cells, especially in cytotrophoblasts. ROS play a significant role in maintaining normal physiological function. Studies suggest that NADPH oxidases may function as oxygen sensors. As oxygen tension increases, NOX enzymes regulate the differentiation of cytotrophoblasts to syncytiotrophoblasts. Therefore, NOX-derived superoxide is hypothesised to have a regulatory role in the growth of the placenta [45] and ROS producing NOX enzymes have a role in trophoblast cell differentiation and proliferation [10].
However, mitochondrial function of the placental cells is impaired by the hyperglycemic environment during GDM. This leads to atypical oxidative phosphorylation and increased oxidative stress. This can affect foetal development as well as the long term health of the offspring [1]. The pathophysiology of GDM has been associated with aberrant production of free radicals as well as mitochondrial dysfunction in placental cells. The human placental mitochondria may also synthesise hormones such as hPL and progesterone which may cause insulin resistance. Oxidative stress is observed in GDM and excessive ROS is connected with insulin resistance and hyperglycemia [46]. Various studies have been conducted to understand the impact of hyperglycemia on ROS production. These studies revealed that the exposure of mouse trophoblast cells to high concentrations of glucose cause aberrant production of ROS. It is concluded that some of this ROS is, at least in part, contributed by NOX enzymes [47]. Studies have shown that trophoblasts can be a significant source of ROS which may be responsible for the various diseases that manifest during pregnancy [48]. However, further investigations are required to determine the role of mitochondria in the production of ROS.
miRNA in GDM : a new dimension to explore ?
miRNAs are short, endogenous, non-coding RNA that play a crucial role in regulating gene expression and are essential for normal development. Majority of miRNAs are transcribed from nuclear DNA into primary miRNA (pri-miRNA) which are reprocessed into precursor miRNA (pre-miRNA) and finally into mature miRNAs. These miRNAs are usually found in the cytoplasm. miRNAs function by binding mainly to the 3’UTR region of the mRNA of a specific target gene. Evidence also suggests that miRNA binding sites are present in the coding region, gene promoter and 5’ UTR regions of the target mRNA. Latest literature has revealed that miRNAs activate the expression of target genes [22]. miRNAs that are secreted into circulation and are exceptionally stable are called circulating miRNAs [49]. Abnormal miRNA expression has been linked to numerous human diseases. Furthermore, miRNAs are discharged into circulation in vesicles or exosomes [50] and may be used as potential biomarkers of disease conditions [51].
A short while ago, it was discovered that miRNAs are important factors that aid in metabolic adaptation during pregnancy. Studies have elucidated that there exists a connection between maternal miRNAs and pregnancy complications such as GDM [51]. Hyperglycemia is a classic manifestation of GDM, caused by the accumulation of glucose in maternal blood. Glucose intolerance induces anomalous expression of two circulating miRNAs, namely; miR-192 and miR-193b [50]. GDM-induced hyperglycemia induces the upregulation of miR − 221 and miR-222 which in turn reduces the expression of ICAM-1 by post-transcriptional modification [52].
The placenta is a metabolic organ [3] that is composed of trophoblasts, endothelial cells and stromal cells among others [53]. Insights from the latest studies give us reason to believe that the placenta plays an indispensable role in the development of GDM. The placenta is known to express more than 500 miRNAs. miRNAs that are predominantly expressed in the placenta are encoded by 3 clusters of miRNA genes. These include chromosome 14 cluster (C14MC), chromosome 19 cluster (C19MC) and miR-371-373 cluster. The 58 miRNAs encoded by C19MC and 63 miRNA encoded by C14MC are expressed in trophoblasts. Expression of all these miRNAs are essential for cell migration, proliferation and apoptosis [23]. Dysregulation of some placenta-specific miRNAs may lead to the pathogenesis of GDM.
Continual growth of trophoblast cells requires 2 rudimentary cellular processes: proliferation and migration. In vitro studies have demonstrated that high glucose conditions lead to elevated expression of miR-134-5p and subsequently the expression of FOXP2 gene transcription. This in turn is responsible for decreased cell migration, proliferation, viability, inflammation and apoptosis of trophoblast cells [54]. One of the C19MC miRNAs, miR-518d, is found to be hyper expressed in the placenta of GDM women in comparison to the placentae of normal pregnant women. The same group of researchers discovered that miR-518d modulated the expression of the PPARα gene. PPARα is associated with maternal adaptations in metabolism during pregnancy. It is known to regulate glucose and lipid metabolism. Studies showed an inverse relationship between PPARα and miR-518d [55].
Downregulation of certain miRNAs have also been associated with the pathophysiology of GDM. Diminished expression of miR-29b has been negatively associated with HIF3A gene expression. Decreased levels of miR-29b have been associated with increased activity of trophoblast cells. It has therefore been hypothesised that miR-29b may be responsible for the pathogenesis of GDM [56]. Reports show that placenta-derived circulating microRNAs may be secreted into maternal circulation, in exosomes, as early as 6 weeks into pregnancy. Syncytiotrophoblasts are held mostly responsible for the release of these miRNAs into the plasma [23].
Exosomes are small, stable, bilayered nanovesicles that deliver cargo to tissues at distant sites. They fuse with the target cells and release their molecular cargo intracellularly. Exosomes contain a variety of molecules including miRNAs and proteins [57]. The placenta regulates maternal function and communicates with other organs and tissues via the release of exosomes. The secretion of placental exosomes can be modulated by external stimuli such as hyperglycemia [23]. Studies, for the first time, have explored the role of exosomes in the pathophysiology of GDM. In vitro studies showed increased release of exosomes from first trimester trophoblasts that were cultured in high glucose conditions. The plasma of pregnant GDM women have high concentrations of exosomes [58].
GDM is a disease in which oxidative stress is increased due to aberrant ROS production. Hyperglycemia induces anomalous ROS production by a variety of mechanisms. Oxidative stress alters the expression of miRNAs [24]. Upregulation of miR-210, under increased oxidative stress conditions, inhibits trophoblast mitochondrial respiration, migration and metabolism of steroids [59]. Trophoblast proliferation and migration are downregulated by expression of miR-155. miR-16 inhibits trophoblast proliferation, invasion, and angiogenesis. Circulating miR-132, miR-29a and miR-222 are shown to be downregulated in GDM and have been proposed as potential biomarkers of the disease as their levels can be determined from circulation [24].
Some other circulating miRNA have been linked to the development of GDM. Some of these include :-.
miR-29a-3p and miR-29b-3p: These miRNAs are found to be upregulated by three folds in GDM pregnancies. Their upregulation was particularly observed in the second trimester of pregnancy. Inhibition of miR-39a-3p yielded improved insulin resistance and better glycaemic control. The exact mechanism of regulation is not known. However, they possess the potential to be used as diagnostic biomarkers.
miR-16-5p: miR-16-5p affects insulin sensitivity in human tissues. Elevated expression was identified in the first trimester, following a 2.5 fold increase in the second and third trimester of pregnancy in GDM patients. It can be used as a prognostic marker of GDM.
miR-17-5p: Increased expression was observed throughout gestation. Insulin resistance is pronounced in GDM pregnancies. It was found that a positive correlation exists between insulin resistance and miRNA expression. Studies also observed that the downregulation of miR-17-5p is linked to reduction in the size of pancreatic islet cells. This leads to increased blood glucose and loss of glucose tolerance. Therefore, downregulation of miR-17-5p in ß-cells is associated with the pathogenesis of diabetes.
miR-132-3p: Decreased levels of this miRNA were observed in the second trimester of pregnancy in GDM patients. All studies concluded that miR-132-3p is downregulated in GDM circulation. Silencing of miR-132-3p results in increased insulin secretion and lower blood glucose levels.
Expression of miR-19a-3p, miR-19b-3p, miR-223-3p, miR-125a-5p and miR-125b-5p are upregulated in GDM pregnancies. However, inconsistency in studies and paucity of data about regulation of these miRNAs, limits them from being used as diagnostic biomarkers in the screening for GDM [60].
The miRNAs that are upregulated and down regulated during GDM are shown in Table 1. Therefore, it can be concluded that miRNAs are good candidates to serve as biomarkers for the examination of gestational diabetes mellitus and may aid in achieving a deeper understanding of the pathophysiology of this malady. Further studies are required to elucidate the link between miRNAs and the pathophysiology of GDM. Figure 2 summarises the mechanism of miRNA biogenesis.
Table 1.
Differential expression of various circulating and tissue miRNAs in GDM
| Study | Source | Type of miRNA | Upregulated microRNA in GDM | Downregulated microRNA in GDM |
|---|---|---|---|---|
|
Zhao et al. [34] |
Serum | circulating-miRNA | / |
miR-29a,miR-123, miR-222 |
|
Zhu et al. [61] |
Plasma | circulating-miRNA |
miR-16-5p, miR-17-5p, miR-19a-3p,miR-19b-3p, miR-20a-5p |
/ |
|
Cao et al. [62] |
Plasma | circulating-miRNA |
miR-16-5p, miR-17-5p, miR-20a-5p |
/ |
|
Wander et al. [63] |
Plasma | circulating-miRNA |
miR-155-5p, miR-21-3p, miR-210-3p, miR-155-5p, miR-146b-5p, miR-223-3p, miR-517-5p, miR-29a-3p |
/ |
|
Lamadrid-Romero et al. [64] |
Serum | circulating-miRNA |
miR-183-5p, miR-200b-3p, miR-125a-5p, miR-1290 |
/ |
|
Sebastiani et al. [65] |
Plasma | circulating-miRNA | miR-330-3p | / |
|
Plows t et al. [54] |
Placenta | Tissue-miRNA | miR-134-5p | / |
|
Catalano et al. [55] |
Placenta | Tissue-miRNA | miR-518d | / |
|
Sun, D. G et al. [56] |
Trophoblast | Cellular-miRNA | / | miR-29b |
|
Rudov et al. [24] |
Trophoblast | Cellular-miRNA | miR-210 | - |
|
S.Muralimanhar et al. [59] |
Serum | Circulating miRNA | - | miR-132 |
Fig. 2.
Oxidative and Nitrosative implications during GDM
There are a variety of mechanisms by which hyperglycemia induces ROS and RNS production in trophoblast cells
A) iNOS expression is increased in trophoblasts, resulting in aberrant NO production
B) eNOS expression is upregulated by H 2 O 2 derived from NOX-derived superoxide. eNOS-
derived NO reacts with NOX-derived superoxide resulting in the formation of
peroxynitrite which causes BH 4 oxidation. Decrease in BH 4 causes eNOS uncoupling,
causing eNOS to function as a superoxide producing enzyme
C) The PKC pathway is activated by maternal hyperglycemia. This in turn activates the
NOX enzyme. NOX catalyses the conversion of molecular oxygen into superoxide in an
NADPH-dependent manner
D) Xanthine oxidase catalyses the conversion of molecular oxygen to superoxide, alongside
the conversion of hypoxanthine to xanthine
E) Mitochondrial ROS is yet another source of ROS in the trophoblast cells. It is produced
by incorrectly coupled electron transport during oxidative phosphorylation
Superoxide and hydrogen peroxide contribute to increased oxidative stress while peroxynitrite
and NO causes an increase in nitrosative stress in the placental trophoblast cells
The role of miR-375 as a biomarker
MiR-375 is a non-coding RNA that is expressed specifically in pancreatic islet cells. It is required for the normal genesis of the pancreas and mediates ß-cell mass as well as alpha cell mass. It regulates genes associated with pancreatic development such as cell proliferation, growth and development as well as genes associated with the secretion of insulin. Additionally, it has been found that miR-375 is highly expressed in the fully formed endoderm of the developing embryo. This indicates that miR-375 has an important role in the regulation of pancreatic development [66]. The islet cells of the pancreas are involved in regulating glucose homeostasis and an impairment in the function of islets leads to dysregulated blood glucose levels. Islet compensation is a process by which the ß-cell mass expands in response to IR in order to maintain normoglycemia. A lack of islet compensation leads to GDM. Studies suggest that reduced expression of miR-375 was concomitant with the states of pre-diabetes mellitus and T2DM. Elevated levels of the expression of miR-375 is found to be associated with the decrease in IR. miR-375 has been established as a marker of ß-cell death and was found to be downregulated in patients with Type 1 diabetes mellitus [67]. The levels of circulating miR-375 was found to be elevated in pre-diabetic mice. The elevation in the levels of miR-375 occurred prior to an increase in blood glucose levels, 2 weeks before diabetes onset. It was also observed that the levels of miR-375 gradually decreased post diabetes onset. Additionally, islet cells isolated from prediabetic samples of C57BL/6 mice were treated with inducers of ß-cell death in the presence and absence of miRNA inhibitors. This was done in an effort to assess the mechanism by which levels of miR-375 increase in the pre-diabetic condition. Cytokine mediated miR-375 release could be inhibited by treatment with suitable inhibitors, indicating that miR-375 levels are elevated before cell death occurs [68]. Recent studies have identified increased expression of miR-375 concomitant with increase in gestational age. miR-375 was found to be upregulated in the case of foetal macrosomia [69]. miR-375 might have a clinical utility as it may be used to screen individuals prone to T2DM in attempts to prevent or treat diabetes, to detect ongoing death of ß-cells and to provide treatments that aid in preventing the death of ß-cells [68]. Further studies are required to confirm if miR-375 may be used as a biomarker in human beings similar to observations in mouse models. miR-375 has been found to be 100% conserved between mice and human beings. miR-375 may be used as a biomarker alongside other markers.
Examining the link between hyperglycemia and production of reactive oxygen species (ROS)
Reactive oxygen species (ROS) are referred to as reactive species that contain oxygen. superoxide (O2▪−), hydrogen peroxide (H2O2), singlet oxygen (1O2), hydroxyl radical (OH▪),lipid hydroperoxide (LOOH) peroxyl radical (LOO▪), peroxynitrite (ONOO−), alkoxyl radical (LO▪),, hypochlorous acid (HOCl), and ozone (O3). are some of the molecules classified as ROS [70].
Antioxidants are substances that fight free radicals. An equilibrium between production of antioxidant and ROS defences are requisite for normal physiological function [71]. Imbalance between levels of ROS production and antioxidant defences is referred to as oxidative stress. Enhanced oxidative stress usually leads to cellular and tissue damage. Increased oxidative stress has been correlated with the onset of a variety of diseases including cancer, diabetes and atherosclerosis [15]. GDM is characterised by maternal hyperglycemia. Research shows a connection between hyperglycemia and oxidative stress. Evidence suggests an overabundance of free radicals and impaired antioxidant defences in women with GDM [10].
Studies have suggested several mechanisms by which hyperglycemia may exacerbate ROS production in pathological conditions [11]. These include.
NADPH oxidase.
Xanthine Oxidase.
Protein Kinase C(PKC).
Mitochondrial ROS production.
NADPH oxidase
NADPH Oxidases (NOX) is accountable for the production of ROS. They are responsible for catalysing the transfer of one electron to molecular oxygen. This results in the production of superoxide anion [72]. NOX exists in 7 isoforms namely NOX1, NOX2, NOX3,NOX4,NOX5,Duox1and Duox2, all of which are involved in ROS production [73]. Hypoxia, AGEs and insulin are some of the factors responsible for the activation of NADPH oxidases. Once activated in response to hyperglycemia, NADPH oxidases catalyse the transfer of electrons from NADPH to molecular oxygen resulting in the production of superoxide and hydrogen peroxide. Therefore high glucose levels lead to the generation of NOX-derived ROS. NOX1 is expressed in the stromal cells and syncytiotrophoblasts of the placenta. Therefore NADPH oxidase is a chief source of superoxide in the placenta [10].
Xanthine oxidase
Xanthine Oxidoreductase (XOR) exists in two forms namely : XO and Xanthine dehydrogenase(XDH). Formation of xanthine from hypoxanthine is catalysed by XDH and xanthine to uric acid is catalysed by XO [10, 12]. When XO is reoxidised, the 6 free electrons in the enzyme are transferred to molecular oxygen. This results in the formation of H2O2 and O2−. Studies have revealed that XO is expressed in the human placenta. Umbilical cord blood obtained from foetuses from GDM pregnancies showed increased levels of XO. This suggests that foetal ROS is formed in GDM conditions [10].
Protein kinase C
PKC is a group of kinases having many isoforms. Some of the enzymes belonging to this family are activated by diacylglycerol(DAG). PKC mediates a range of biological processes including cell differentiation and proliferation, glucose metabolism and gene expression, among other functions [10]. Studies have also revealed that hyperglycemic conditions can cause the activation of the PKCß2 isoform in the cardiomyocytes of diabetic mice [13]. Mitochondrial NADPH oxidases are activated by PKC which leads to increased oxidative stress [74]. Findings have also suggested NADPH oxidase is activated in a PKC-dependent manner and may be considered responsible for increased oxidative stress in diabetes [16]. It can, therefore, be hypothesised that hyperglycemia leads to the activation of the PKC pathway successively resulting in enhanced ROS production.
Mitochondrial ROS production
It is known that the mitochondria is one of the preeminent sources of ROS production in almost all eukaryotic cells. The metabolic activity of the mitochondria is increased during pregnancy. Therefore, normal pregnancy is a state of elevated oxidative stress. The mitochondria is also responsible for NOX-mediated superoxide production during gestation [10]. Oxidative phosphorylation is the mechanism by which oxygen reduction is harnessed to generate chemical energy in the form of ATP [75]. Incorrectly coupled electron transport during oxidative phosphorylation leads to aberrant ROS production. ROS generation is known to cause damage to the mitochondria [76]. Hyperglycemia induces aberrant ROS in extra-placental tissues and that ROS production can be reduced by the use of inhibitors of the ETC [14]. Data suggests that hyperglycemia-induced ROS induces morphological changes in the mitochondria [77]. The exact mechanism by which hyperglycemia induces mitochondrial ROS production is yet to be elucidated and requires further experimentation.
The production of ROS via the polyol pathway
The polyol pathway is a two step metabolic pathway which occurs in the cell cytosol and is responsible for the conversion of glucose to sorbitol. This pathway is based on two enzymes namely; aldolase reductase and sorbitol dehydrogenase. Aldolase reductase catalyses the reduction of glucose to sorbitol using NADPH as a cofactor. NADPH is essential for the generation of intracellular antioxidants and is later reduced to GSSG. Sorbitol dehydrogenase (SDH) catalyses the conversion of sorbitol to fructose using NAD + as cofactor and produces NADH. Only 3% of cellular glucose is converted into sorbitol under normal conditions whereas in hyperglycemia around 30% of the glucose is metabolised through this pathway. There are 3 mechanisms by which the polyol pathway participates in oxidative stress induced by hyperglycemia [11].
In hyperglycemic conditions, aldolase reductase reduces the additional glucose to sorbitol Sorbitol is a hydrophilic alcohol and cannot diffuse through the cell membrane with ease. It is difficult to metabolise sorbitol. Intracellular accumulation of the additional sorbitol produced in hyperglycemia is damaging to the cell. Under hyperglycemic conditions, aldolase uses additional NADPH to convert the glucose to sorbitol which in turn reduces the availability of NADPH for the synthesis of intracellular antioxidants. The results in the cells losing its ability to respond to oxidative stress which results in oxidative stress [78].
The enhanced SDH activity leads to increased production of NADH alongside the conversion of sorbitol to fructose. NADH is the substrate for NOX. Increased concentrations of NADH leads to increased NOX activity. This leads to overproduction of the superoxide anion thereby increasing the oxidative stress within the cell [11].
Fructose, which is the final product of the polyol pathway, can be further converted to 3-deoxyglucosone and fructose-3-phosphate, both of which are potent nonenzymatic glycation agents. These can form advanced glycation end-products (AGEs). AGEs have been found in the serum of GDM patients and have been associated with malformations of the foetus and abnormal foetal outcomes, thereby posing as a risk factor for foetal and maternal health in GDM patients [11].
It can therefore be inferred that the polyol pathway is a significant pathway of ROS production in pathological conditions such as GDM and preeclampsia. It plays an important role in adding to the oxidative stress environment caused by hyperglycemia.
GDM and production of reactive nitrogen species
RNS are reactive nitrogen-containing molecules which include nitric oxide (NO.) and its derivative, peroxynitrite (ONOO−), which is a powerful oxidant, capable of causing damage to a variety of biomolecules. Similar to ROS, RNS can be harmful as well as beneficial to living organisms [79]. Both ROS and RNS are required for maintenance of normal physiological functions at low concentrations. Higher concentrations of RNS, called nitrosative stress, inactivate important biomolecules and are harmful to living organisms. The exact mechanism by which the concentration of RNS shifts from physiological levels to higher concentrations are not known [17]. NO and ROS interact to form peroxynitrites, thereby reducing the bioavailability of NO. NO is produced by enzymes referred to as nitric oxide synthases (NOS). They exist in 3 isoforms, namely: endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS). While eNOS and nNOS are expressed in neuronal and endothelial cells respectively, iNOS is transcriptionally regulated and only expressed during pathological conditions. It is known to mediate septic shock and inflammation. iNOS produces a large amount of NO which can be damaging to cells [21]. NO is the smallest signalling molecule which plays an important role in maintaining vascular homeostasis [18, 19], averting apoptosis and maintaining endothelial barrier function [19].
Hyperglycemia induced dysregulation of eNOS
eNOS is one of the 3 isoforms of nitric oxide synthases and is referred to as constitutive NOS and is found on chromosome 7. All NOS isoforms use L-arginine as a substrate, NADPH and molecular oxygen as co-substrates to produce L-citrulline and NO. A functional NOS enzyme is composed of homodimers. The reductase domain of 1 monomer interacts with the oxygenase domain of the other monomer. The C-terminal reductase domain binds NADPH, FMN and FAD and the N-terminal oxygenase domain carries a heme group and also binds to BH4 [20]. A functional unit of eNOS transfers electrons from NADPH, via FAD and FMN in the reductase domain, to the heme group in the oxygenase domain. L-arginine is oxidised to L-citrulline and NO in the oxygenase domain [80]. eNOS-derived NO plays an important role in vasodilation. It stimulates soluble guanylyl cyclase (sGC) which in turn increases the amount of cyclic guanosine monophosphate (cGMP). Nitric oxide secreted into the vascular lumen is a strong inhibitor of adhesion and aggregation of platelets [81]. The expression of eNOS has been characterised in human placentas as well as in rat placentas, under physiological conditions. eNOS was found to be localised to the syncytiotrophoblasts and to the endothelium of the stem villous vessels [18]. Electron flow within the NOS enzyme is well-regulated and any disruption in this process will cause uncoupling of the O2 reduction and NO formation. Consequently, superoxide is generated from the amino-terminal oxygenase domain [80].
GDM is one among several diseases that is associated with increased ROS production. NOX, XO and mitochondrial ROS are a few sources by which ROS is produced. Enhanced oxidative stress has been known to cause eNOS uncoupling [20]. This is the process by which the eNOS enzyme undergoes monomerization which causes it to produce superoxide instead of NO [82]. Additionally BH4 is a critical cofactor that is required for eNOS functioning. Electron transfer required for the oxidation of L-arginine is facilitated by the binding of two BH4 molecules to each eNOS subunit. Increase in BH4 oxidation or decrease in its synthesis, limits BH4 which in turn causes eNOS uncoupling and superoxide production. A slew of evidence suggests that eNOS uncoupling may be attributed to NOX-derived ROS [83]. Various disorders including T2DM cause PKC activation. NOX is activated by the PKC pathway. Activated NOX produces superoxide ions, some of which are converted to H2O2 by SOD. This causes an increase in eNOS expression. eNOS-derived NO reacts with NOX-derived superoxide resulting in the formation of peroxynitrite. The peroxynitrite so formed oxidises BH4 to BH3 which can further be oxidised to BH2. Consequently, eNOS driven reduction of oxygen is uncoupled from NO production. This phenomenon is responsible for converting functional eNOS into a superoxide generating enzyme [20]. Studies have elucidated that ROS may cause eNOS uncoupling which in turn may exacerbate oxidative stress. In vitro studies have demonstrated eNOS uncoupling in peroxynitrite-treated endothelial cells [84] as well as in blood vessels isolated from streptozotocin-induced diabetes rats [85]. Diabetes induced endothelial dysfunction in diabetes patients is attributed to eNOS uncoupling [86]. It may therefore be hypothesised that eNOS uncoupling in the placenta may also contribute to oxidative stress in GDM.
Hyperglycemia induced upregulation of iNOS
iNOS also referred to as NOS2, isoform of NO that is produced in response to an inflammatory environment [87]. Various physiological roles are fulfilled by iNOS-derived NO some of which include, wound repair, host defences and in pathophysiological conditions including diabetes [88]. Studies have reported the absence of iNOS in normal human placenta. Current literature suggests that iNOS is expressed in the placenta of women with GDM and is associated with increased NO production [18]. Increase in iNOS expression has been observed in the GDM placenta. Maternal hyperglycemia and a proinflammatory milieu in GDM has been associated with the induction of iNOS and the subsequent generation of nitrosative stress. The placenta is the prime target of these alterations. iNOS expression has also been observed in syncytiotrophoblasts and cytotrophoblasts and may induce apoptosis. An increase in NO levels has been associated with activation of caspase and apoptosis of cytotrophoblasts in GDM. Additionally, increased protein nitration has been observed in the placenta of obese women who are at a higher risk of developing GDM. Investigations have hypothesised that iNOS derived NO may have a pathological influence on cellular defence, blood flow and regulation against chorangiosis. Immature villous structure can also be attributed to immature structure of placental villi. Further studies are required to understand the role of iNOS-derived NO in GDM [89].
This can bring about alterations in the feto-placental circulation during disease conditions. Excessive ROS and NO stimulate the formation of peroxynitrites which cause nitrative stress. An increase in peroxynitrite concentrations can damage the placenta, cause endothelial dysfunction in the mother, cause damage to umbilical vessels and may predispose the offspring to metabolic and vascular disorders [10]. Therefore, GDM conditions lead to alterations in eNOS and iNOS expression. This is linked to enhanced ROS and NO, which produce RNS.
Figure 3 represents the various mechanisms by which oxidative stress is produced in the placental trophoblast cells on being exposed to maternal hyperglycemia during GDM.
Fig. 3.
Pro-Inflammatory mediators during GDM
GDM is associated with a pro-inflammatory environment. Cytokines IL-6 and TNF-alpha induce the production of iNOS. Additionally, trophoblasts exposed to hyperglycemia contribute to an increase in iNOS-derived NO as well as ROS. NO induces apoptosis and causes softening of the cervix. NO is also known to induce the expression of MMP-1 in human cervical fibroblasts. MMP-1 degrades collagen that aids in cervical ripening. ROS produced by trophoblast cells Cervical ripening in GDM leads to preterm labour
Markers of oxidative stress and nitrative stress in GDM
The imbalance between the inactivation and formation of free radicals causes oxidative damage. This is characterised by the destruction of membrane lipids, DNA, and proteins. Hyperglycemia is linked with oxidative stress. The hypothesis that is currently being followed is that various mechanisms are responsible for the production of ROS thereby increasing oxidative stress. This is a pathogenic factor that produces a conducive environment for ß-cell dysfunction, glucose intolerance and insulin resistance. ROS has also been implicated in the activation of NF-kB. Articulation of immunological and inflammatory genes are regulated by NF-kB. Some of these genes include those of pro-inflammatory cytokines, enzymes and proteins that are involved in ROS production. Reduced availability of NO is an injurious effect of oxidative stress in diabetes. A slew of evidence suggests that oxidative stress plays a role in the pathogenesis of GDM [90]. It has been observed that GDM patients have impaired free radical scavenging systems and overproduce free radicals. Research demonstrated a link between oxidative stress and pregnant women who are complicated by gestational diabetes mellitus (GDM) and it also illustrates a connection between GDM and oxidative indicators like xanthine oxidase (XO), malondialdehyde (MDA) lipid hydroperoxide (LHP), and 8-isoprostane (8-Isop) [91]. Free radicals are described as radical and non-radical derivatives of oxygen. Direct in vivo measurement of ROS is remarkably difficult as ROS molecules are present in low concentrations and have extremely short half-life. Products of oxidative damage are used as biomarkers of oxidative stress [90]. Some of these biomarkers include.
Lipid peroxidation
Lipid peroxidation is the process by which pro-oxidant molecules attack the carbon-carbon double bonds in lipids, especially polyunsaturated fatty acids (PUFAs) [92]. Conjugated dienes and lipid hydroperoxides are formed as secondary metabolic products during lipid peroxidation. Intermediates of lipid oxidation can damage cell membranes and are toxic to cells. Some of the end products of lipid peroxidation include malondialdehyde (MDA) which is stable but toxic. It is a commonly used biomarker of oxidative stress. MDA interacts with thiobarbituric acid (TBA) in a 1:2 ratio, thereby forming thiobarbituric acid reactive substances (TBARS). However, MDA may also be produced by cyclooxygenase and therefore cannot qualify as a diagnostic biomarker of GDM. Measurement of TBARS is also notoriously difficult [90]. Free radicals catalyse the oxidation of arachidonic acid in a cyclooxygenase independent, non-enzymatic manner. This results in the formation of 8-isoprostane [93]. This is an accurate, sensitive and stable indicator of oxidative stress. Plasma and urine are non-invasive sample sources whereas the placenta and other tissues are more commonly used samples [90].
Markers of NO mediated DNA damage
RNS produced under hyperglycemic conditions, interacts with DNA bases thereby producing the mutagenic product 8-NitroG which is a potent biomarker of nitrosative stress. RNS are also responsible for post-translational modifications in proteins. Metabolism of nitric oxide in the presence of oxidants yields peroxynitrites and NO2. Pro-inflammatory conditions seen during GDM can be attributed to RNS production. The NO2 produced is added to the tyrosine residues of proteins. This generates 3-nitrotyrosine residues which function as biomarkers [90]. Recent studies have revealed that lipids are reacted upon by NO to form trans- or cis- nitroalkanes. The lipid bilayer is the usual location of this reaction. It has been associated with the regulation of inflammation [94].
Markers of antioxidant defences
Antioxidants are either water soluble or lipid soluble. Antioxidants that are water soluble react with pro- oxidants in the plasma as well as the cellular cytosol. These include lipoic acid, vitamin C, glutathione and uric acid. The cell membrane is protected from lipid peroxidation by lipid soluble antioxidants. These include vitamin E, carotene and ubiquinol [95].
Superoxide detoxification is carried out by enzymatic antioxidants. Superoxide dismutase (SOD) is an enzyme that catalyses the conversion of O2− to H2O2. Subsequently, catalases (CAT) catalyse the conversion of H2O2 to H2O and remove the superoxide radicals. The glutathione system of enzymes include GSH, GPx, GST and glutathione. The measurement of antioxidant activity is done using total antioxidant capacity (TAC) rather than measuring the activity of individual antioxidants [96]. Studies revealed that SOD and TAC levels were decreased in GDM. Reduced antioxidants and increased ROS have been reported into the umbilical cord blood of GDM women.
Additionally, the inflammatory markers TNF-alpha, galanin, and IL-6 rose during GDM pregnancies and were associated with the severity of the hyperglycemic condition. Studies showing the levels of oxidative stress also looked at antioxidant mechanisms. The increase of TNF-alpha shows inflammatory process activation in placental cells [91].
Therefore, hyperglycemia induced ROS and RNS can be clinically identified using biomarkers. These are shown in Table 2.
Table 2.
Comparison of levels of oxidative stress markers and antioxidants in GDM women with control individuals
| Markers of oxidative stress and antioxidants | GDM/controls | Weeks of gestation | Results: GDM vs. control |
|---|---|---|---|
|
MDA [97] |
16/27 | Third-trimester | ↑ GDM |
|
TBARS [98] |
3/16 | Third-trimester | - |
|
LPO [99] |
53/25 | Second and third-trimester | ↑ GDM |
|
TAC [100] |
20/20 | Third-trimester | ↓ GDM |
|
SOD [100] |
20/20 | Third-trimester | ↓ GDM |
|
GPX [97] |
16/27 | Third-trimester | ↓ GDM |
|
GSH [99] |
53/25 | Second and third-trimester | - |
|
CAT [97] |
16/27 | Third-trimester | ↓ GDM |
Cervical ripening : a risk of GDM
The cervix is a structure that provides a physical as well as an immunological barrier for foetal development over the course of pregnancy. The cervix consists of smooth muscles, connective tissues as well as ground substance consisting of blood vessels. Additionally, the cervix consists of fibroblasts and mast cells. However, the cervix undergoes extensive changes during the final stages of gestation. These changes are characterised by the transformation of tough and firm cervical tissue into soft tissue that is prone to dilate. These changes are mediated by a series of biochemical pathways that cause the rearrangement of the cervical ECM. These complex changes in the cervix can be termed as cervical ripening. This aids in the passing of the foetus via the birth canal. ROS are produced at low concentrations during normal conditions and they function as secondary messengers [101].
Excessive ROS production may however impair the antioxidant defence system and contribute to tissue dysfunction. H2O2, superoxide anion and peroxynitrite are some of the main free radicals produced. Superoxide anions are the most reactive oxygen species and may be produced by electron leakage from the mitochondria, during the process of oxidative phosphorylation. NOX enzymes, endoplasmic reticulum and cytochrome P450 are some of the other physiological sources of superoxide ions. RNS including NO play a central role in the process of cervical ripening. ROS and RNS together contribute to oxidative stress [101]. Cervical cell apoptosis is significantly increased at term pregnancy and is speculated to be caused by oxidative stress [102]. Three major pathways regulate apoptosis and each of these pathways may be directly or indirectly stimulated by ROS. ROS may cause fragmentation of mitochondrial DNA, may activate the p53 N-terminal kinase / c-Jun (JNK) pathway which in turn activates the pro-apoptotic Bcl-2 proteins or it may cause mitochondrial membrane depolarization which disrupts oxidative phosphorylation [103, 104]. It may therefore be speculated that excessive ROS has been associated with increased cervical cell apoptosis and may be associated with premature cervical ripening during GDM.
Studies have shown evidence that all the 3 isoforms of NOS; nNOS, eNOS and iNOS are expressed in the cervix of a pregnant woman. However, iNOS is considered to be the main source of nitric oxide (NO) in the cervix and is considered responsible for the acute surge in NO concentrations during parturition. This has been observed in the cervix of pregnant rats. The cervix remains firm and closed during gestation in order to protect the intrauterine environment of the foetus. As the end of gestation approaches, the cervix gradually becomes softer due to palpitation during a process called cervical ripening. NO is a messenger molecule that plays a significant role in softening the cervix prior to labour. Onset of parturition is characterised by cervical softening, myometrial and abdominal contraction and cervical dilation which subsequently allows the foetus to pass through the birth canal [105].
Cervical ripening is accompanied by infiltration of neutrophils, monocytes and a slew of inflammatory cells. It is said to resemble an inflammatory process. Cytokines are responsible for the recruitment of the inflammatory cells and are responsible for ECM remodelling [105, 106]. The exact mechanism by which NO initiates cervical ripening continues to remain elusive. Animal studies have been conducted to elucidate this mechanism. Speculations suggest that abundant iNOS activity in the epithelial and stromal cells of the cervix is owing to cytokine-induced inflammatory cells. iNOS derived NO causes the production of Matrix Metalloproteinases-1 (MMP-1) is human cervical fibroblasts. MMP-1 is involved in cervical ripening as it degrades collagen. NO has also been implicated as a potent agonist of apoptosis, a process central to cervical ripening. Additionally, leukocyte influx in the cervical stroma is promoted by NO-stimulated vasodilation [101]. Studies have stated that the local application of NO donors has effectively induced cervical ripening. It can therefore be concluded that iNOS mediates cervical softening and plays a remarkable role in prelabor ripening [106].
GDM is a state of enhanced oxidative stress with a significant increase in iNOS expression and ROS production in the placenta. This in turn may cause aberrant NO production which is responsible for cervical ripening. It can therefore be hypothesised that GDM may contribute to premature cervical ripening, which in turn may be the causative factor for preterm delivery.
Preterm labour and GDM
Preterm labour is typically characterised by consistent contractions and cervical changes that start at a gestational age of fewer than 37 weeks. A preterm birth is one that occurs after 37 weeks of pregnancy [107]. The main cause of neonatal death and a significant portion of all birth-related short- and long-term morbidity is preterm birth. More than half of preterm deliveries result from spontaneous preterm labour [108]. The myometrium contracts during labour, whether it is mature or preterm. Myosin that has been phosphorylated interacts with actin to produce actomyosin, which causes myometrial contraction. The energy for contraction is provided by the phosphorylated protein-protein complexes formed following hydrolysis. Myometrial contraction results from an increase in intracellular Ca2 + concentration and/or a decline in cyclic nucleotide concentration, whereas myometrial relaxation results from a decrease in intracellular calcium concentration and/or an increase in cyclic nucleotide concentration [109].
Cervical ripening is strongly associated with preterm labour. Pro-inflammatory cytokines, induction of MMPs and prostaglandins have been identified as factors that cause membranes to rupture and cause cervical changes. These factors ultimately cause preterm labour [110]. Foetal and maternal factors correlate with preterm delivery in GDM mothers. Stringent glycemic control during pregnancy has been associated with differences in the outcome and duration of gestation. Intensive insulin treatment in GDM patients has been associated with reduced instances of preterm delivery [111]. Additional literature is required to elucidate the effect of glucose intolerance in inducing preterm labour.
In the HAPO study, roughly 1608 of the 23,316 participants 6.9% endured an induced or spontaneous preterm delivery, as opposed to 9.6% of LGA infants and 8.0% of infants admitted for intensive neonatal care. The coexistence of additional conditions with GDM that may result in indicated or induced preterm delivery may help to partly explain the link between GDM and preterm birth. Pre-eclampsia and conditions linked to high blood pressure, like intrauterine growth limitation and placental abruption, are examples of such conditions. Although a danger of GDM, spontaneous preterm birth is less frequent than other undesirable outcomes. Though it accounts for about three-quarters of preterm births and is unrelated to GDM, spontaneous preterm delivery, also known as birth without conditions necessitating medical intervention, is common [108].
Conclusion
A number of maternal and foetal co-morbidities were connected to GDM. GDM increases the likelihood that both the mother and the child will eventually acquire T2DM. GDM may therefore be regarded as a serious health concern. A variety of factors cause GDM, some of which may include genetic factors, maternal age, obesity, polycystic ovarian syndrome (PCOS), ethnicity, and exposure to toxicity. The pathophysiology of GDM has remained elusive. The placenta has been implicated to play a crucial role in the development of this disorder. miRNAs are a new avenue that is being explored to explain the pathophysiology of this condition. Literature has revealed that the human placenta expresses more than 500 microRNAs, and only a portion of them get expressed in other tissues and organs. The downregulation of certain miRNAs has also been associated with the pathophysiology of GDM. Reports show that placenta-derived circulating microRNAs may be secreted into maternal circulation, in exosomes ,as early as 6 weeks into pregnancy. Syncytiotrophoblasts are mostly responsible for the release of these miRNAs into the plasma. Some other circulating miRNA have been linked to the development of GDM and can potentially be used as diagnostic biomarkers in the screening for GDM. The miRNAs include miR-29a-3p, miR-29b-3p, miR-16-5p, miR-17-5p, miR-132-3p, miR-19a-3p, and miR-19b-3p. The study suggests that miRNAs have the potential to serve as biomarkers for the diagnosis of gestational diabetes mellitus and may aid in achieving a deeper understanding of the pathophysiology of this indisposition.GDM is characterised by maternal hyperglycemia. A review of literature has revealed a strong interlink between hyperglycemia, ROS and RNS production and cervical ripening. Evidence suggests an overproduction of free radicals and impaired antioxidant defences in women with GDM. Oxidative stress is characterised by ROS and RNS production. Hyperglycemia causes PKC dependent NOX activation. NOX-derived ROS has been shown to interact with eNOS derived ROS to produce peroxynitrite. ONOO- so formed causes eNOS uncoupling through BH4 oxidation. iNOS upregulation has been observed in placental trophoblast cells. ROS and RNS have incredibly short half lives and cannot be directly measured. Markers of oxidative stress and nitrative stress may be used to measure the damage invoked by ROS and RNS. iNOS-derived NO is implicated in apoptosis and cervical ripening. This in turn may cause aberrant NO production, which is responsible for cervical ripening. It can therefore be hypothesised that GDM may contribute to premature cervical ripening, which in turn may be the causative factor for preterm delivery. This study needs to be further investigated in order to be used in future clinical therapies.
Acknowledgements
SRMIST’s Department of Biotechnology provided the chemicals and other study supplies, and we are grateful for their generosity. We also want to thank the biotechnology students who assisted us in this research by working in the animal cell and tissue culture lab.
Abbreviations
- GDM
Gestational Diabetes Mellitus
- ROS
Reactive Oxygen Species
- RNS
Reactive Nitrogen Species
- miRNA
microRNA
- RNA
Ribonucleic Acid
- iNOS
Inducible Nitric Oxide Synthase
- eNOS
Endothelial Nitric Oxide Synthase
- nNOS
Neuronal Nitric Oxide Synthase
- T2DM
Type 2 Diabetes Mellitus
- PCOS
Polycystic Ovarian Syndrome
- PKC
Protein Kinase C
- NOX
NADPH oxidase
- NADPH
Nicotinamide-adenine-dinucleotide phosphate
- XO
Xanthine Oxidase
- NO
Nitric Oxide
- DNA
Deoxyribonucleic Acid
- ETC
Electron Transport Chain
- BMI
Body Mass Index
- HLA
Human Leukocyte Antigen
- INS
Insulin
- IGF
Insulin like growth factor
- IGF-2
Insulin-like Growth Factor 2
- Duox
Dual oxidases
- XOR
Xanthine Oxidoreductases
- XDH
Xanthine Dehydrogenases
- DAG
Diacylglycerol
- ONOO
Peroxynitrite
- NF-kB
Nuclear-Factor Kappa B
- MDA
Malondialdehyde
- 8-Isop
8-Isoprostane
- LHP
Lipid Hydroperoxide
- PUFA
Polyunsaturated Fatty acids
- TBA
Thiobarbituric acid
- TBARS
Thiobarbituric acid reactive species
- SOD
Superoxide Dismutase
- CAT
Catalase
- GPX
Glutathione peroxidase
- TAC
Total antioxidant capacity
- NO2
Nitrogen dioxide
- TNF-alpha
Tumour Necrosis Factor alpha
- IL-6
Interleukin 6
- pri-miRNA
Primary-miRNAs
- pre-miRNA
Precursor-miRNAs
- ICAM-1
Intercellular Adhesion Molecule 1
- PPAR-alpha
Peroxisome proliferator-activated receptor alpha
- C19MC
Chromosome 19 miRNA cluster
- C14MC
Chromosome 14 miRNA cluster
- TE
Trophectoderm
- ECM
Extracellular matrix
- MMP-1
Matrix Metalloproteinase 1
- HAPO
Hyperglycemia and Adverse Pregnancy Outcomes
- LGA
Large for Gestational Age
- FOXP2
Forkhead box protein P2
- HPL
Human Placental Lactogen
- GLUT1
Glucose transporter 1
- BPA
Bisphenol A
- EDC
Endocrine disrupting chemicals
- sGC
Soluble guanylyl cyclase
- cGMP
Cyclic guanosine monophosphate
- FMN
Flavin mononucleotide
- FAD
Flavin adenine dinucleotide
- BH4
(6R-)5,6,7,8-tetrahydrobiopterin
- PTB
Preterm birth
- A1GDM
Gestational diabetes mellitus type 1
- A2GDM
Gestational diabetes mellitus type 2
- SHBG
Sex hormone binding globulin
- GSSG
Glutathione
- SDH
Sorbitol dehydrogenase
- AGEs
Advanced glycation end-products
Funding
This research did not receive any grants from funding agencies.
Declarations
Ethical statement
Not applicable.
Competing interests
No potential conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jarmuzek P, Wielgos M, Bomba-Opon D. (2015). Placental pathologic changes in gestational diabetes mellitus. Neuroendocrinology letters, 36(2), 101–105. Retrieved from https://www.nel.edu/. [PubMed]
- 2.Wang Y, Zhao S. Vascular Biology of the Placenta. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 4, Cell Types of the Placenta. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53245/. [PubMed]
- 3.Jiang S, Teague AM, Tryggestad JB, Chernausek SD. Role of microRNA-130b in the placental PGC-1α/TFAM mitochondrial biogenesis pathway. Biochem Biophys Res Commun. 2017;487(3):607–12. doi: 10.1016/j.bbrc.2017.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean L, McEntyre J. The Genetic Landscape of Diabetes [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004. Chapter 5, Gestational Diabetes. 2004 Jul 7. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1668/.
- 5.Shaat N, Groop L. Genetics of gestational diabetes mellitus. Curr Med Chem. 2007;14(5):569–83. doi: 10.2174/092986707780059643. [DOI] [PubMed] [Google Scholar]
- 6.Wen L, Ge H, Qiao J, et al. Maternal dietary patterns and risk of gestational diabetes mellitus in twin pregnancies: a longitudinal twin pregnancies birth cohort study. Nutr J. 2020;19:13. doi: 10.1186/s12937-020-00529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuttall FQ. Body Mass Index: obesity, BMI, and Health: a critical review. Nutr Today. 2015;50(3):117–28. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano PM. The impact of gestational diabetes and maternal obesity on the mother and her offspring. J Dev origins health disease. 2010;1(4):208–15. doi: 10.1017/S2040174410000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burzynska-Pedziwiatr I, Jankowski A, Kowalski K, Sendys P, Zieleniak A, Cypryk K, Zurawska-Klis M, Wozniak LA, Bukowiecka-Matusiak M. Associations of Arginine with Gestational Diabetes Mellitus in a Follow-Up study. Int J Mol Sci. 2020;21(21):7811. doi: 10.3390/ijms21217811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. 2011;15(12):3061–100. doi: 10.1089/ars.2010.3765. [DOI] [PubMed] [Google Scholar]
- 11.Turek I, Wozniak L, Cypryk K, Wojcik M. Hyperglycemia-induced oxidative stress in gestational diabetes mellitus (GDM) Diabetologia Kliniczna. 2015;4:189–98. doi: 10.5603/DK.2015.0022. [DOI] [Google Scholar]
- 12.Danijela A, Kostić, Danica S, Dimitrijević, Gordana S, Stojanović, Ivan R, Palić, Aleksandra S, Đorđević, Jovana D, Ickovski. “Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition”, Journal of Chemistry, vol. 2015, Article ID 294858, 8 pages, 2015. 10.1155/2015/294858.
- 13.Nagareddy PR, Soliman H, Lin G, Rajput PS, Kumar U, McNeill JH, MacLeod KM. Selective inhibition of protein kinase C beta(2) attenuates inducible nitric oxide synthase-mediated cardiovascular abnormalities in streptozotocin-induced diabetic rats. Diabetes. 2009;58(10):2355–64. doi: 10.2337/db09-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 15.Betteridge DJ. What is oxidative stress? Metab Clin Exp. 2000;49(2 Suppl 1):3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 16.Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrology: JASN. 2003;14(8 Suppl 3):227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 17.Di Meo S, Reed TT, Venditti P, Victor VM. (2016). Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative medicine and cellular longevity, 2016, 1245049. 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed]
- 18.Schönfelder G, John M, Hopp H, Fuhr N, van Der Giet M, Paul M. Expression of inducible nitric oxide synthase in placenta of women with gestational diabetes. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1996;10(7):777–84. doi: 10.1096/fasebj.10.7.8635695. [DOI] [PubMed] [Google Scholar]
- 19.Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4(1):3–24. doi: 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2011;164(2):213–23. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Förstermann U, Sessa WC. (2012). Nitric oxide synthases: regulation and function. European heart journal, 33(7), 829–837d. 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed]
- 22.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guarino E, Delli Poggi C, Grieco GE, Cenci V, Ceccarelli E, Crisci I, Sebastiani G, Dotta F. (2018). Circulating MicroRNAs as Biomarkers of Gestational Diabetes Mellitus: Updates and Perspectives. International journal of endocrinology, 2018, 6380463. 10.1155/2018/6380463. [DOI] [PMC free article] [PubMed]
- 24.Rudov A, Balduini W, Carloni S, Perrone S, Buonocore G, Albertini MC. (2014). Involvement of miRNAs in placental alterations mediated by oxidative stress. Oxidative medicine and cellular longevity, 2014, 103068. 10.1155/2014/103068. [DOI] [PMC free article] [PubMed]
- 25.Quintanilla Rodriguez BS, Mahdy H, Gestational Diabetes. [Updated 2022 Sep 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545196/. [PubMed]
- 26.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes Mellitus. Int J Mol Sci. 2018;19(11):3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes Mellitus. Int J Mol Sci. 2018;19(11):3342. doi: 10.3390/ijms19113342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Augustin R. The protein family of glucose transport facilitators: it’s not only about glucose after all. IUBMB Life. 2010;62(5):315–33. doi: 10.1002/iub.315. [DOI] [PubMed] [Google Scholar]
- 29.Hiden U, Maier A, Bilban M, Ghaffari-Tabrizi N, Wadsack C, Lang I, Dohr G, Desoye G. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia. 2006;49(1):123–31. doi: 10.1007/s00125-005-0054-x. [DOI] [PubMed] [Google Scholar]
- 30.Jansson T, Powell TL. (2007). Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clinical science (London, England: 1979), 113(1), 1–13. 10.1042/CS20060339. [DOI] [PubMed]
- 31.Jones HN, Jansson T, Powell TL. IL-6 stimulates system a amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. American journal of physiology. Cell Physiol. 2009;297(5):C1228–35. doi: 10.1152/ajpcell.00195.2009. [DOI] [PubMed] [Google Scholar]
- 32.Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM, Mouzon H-D. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am J Obstet Gynecol. 2009;201(2):209e1–10. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang TT, Sun WJ, Liu HY, Ma HL, Cui BX. p66Shc-mediated oxidative stress is involved in gestational diabetes mellitus. World J diabetes. 2021;12(11):1894–907. doi: 10.4239/wjd.v12.i11.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, Dong J, Jiang T et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. (2011) PLoS One 2011;6(8, article e23925) 10.1371/journal.pone.0023925. [DOI] [PMC free article] [PubMed]
- 35.Li J, Song L, Zhou L, Wu J, Sheng C, Chen H, Liu Y, Gao S, Huang W. (2015). A MicroRNA Signature in Gestational Diabetes Mellitus Associated with Risk of Macrosomia. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology, 37(1), 243–52. 10.1159/000430349. [DOI] [PubMed]
- 36.Amiri FN, Faramarzi M, Bakhtiari A, Omidvar S. Risk factors for gestational diabetes Mellitus: a case-control study. Am J Lifestyle Med. 2018;15(2):184–90. doi: 10.1177/1559827618791980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Liu X, Zuo Y, Gao J, Liu Y, Zheng W. The risk factors of gestational diabetes mellitus in patients with polycystic ovary syndrome: what should we care. Medicine. 2021;100(31):e26521. doi: 10.1097/MD.0000000000026521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mustaniemi S, Vääräsmäki M, Eriksson JG, Gissler M, Laivuori H, Ijäs H, Bloigu A, Kajantie E, Morin-Papunen L. Polycystic ovary syndrome and risk factors for gestational diabetes. Endocr connections. 2018;7(7):859–69. doi: 10.1530/EC-18-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24(5):441–8. doi: 10.1111/j.1365-3016.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eberle C, Stichling S. Environmental health influences in pregnancy and risk of gestational diabetes mellitus: a systematic review. BMC Public Health. 2022;22:1572. doi: 10.1186/s12889-022-13965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, Ho SM. Endocrine disruptors: a potential risk factor for gestational diabetes Mellitus. Am J Perinatol. 2016;33(13):1313–8. doi: 10.1055/s-0036-1586500. [DOI] [PubMed] [Google Scholar]
- 42.Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140066. doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myatt L. (2010). Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta, 31 Suppl(Suppl), S66–S69. 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed]
- 44.Marikawa Y, Alarcon VB. Creation of trophectoderm, the first epithelium, in mouse preimplantation development. Result Probl Cell Differ. 2012;55:165–84. doi: 10.1007/978-3-642-30406-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poston L, Raijmakers MT, Suppl A. 72–S78. 10.1016/j.placenta.2004.01.003.
- 46.Fisher JJ, Bartho LA, Perkins AV, Holland OJ. Placental mitochondria and reactive oxygen species in the physiology and pathophysiology of pregnancy. Clin Exp Pharmacol Physiol. 2020;47(1):176–84. doi: 10.1111/1440-1681.13172. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Santos A, Martínez-Hernández MG, Contreras-Ramos A, Ortega-Camarillo C, Baiza-Gutman LA. Hyperglycemia-induced mouse trophoblast spreading is mediated by reactive oxygen species. Mol Reprod Dev. 2018;85(4):303–15. doi: 10.1002/mrd.22965. [DOI] [PubMed] [Google Scholar]
- 48.Manes C. Human placental NAD(P)H oxidase: solubilization and properties. Placenta. 2001;22(1):58–63. doi: 10.1053/plac.2000.0589. [DOI] [PubMed] [Google Scholar]
- 49.Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R, Zhang X. Circulating MicroRNAs in Cancer: potential and challenge. Front Genet. 2019;10:626. doi: 10.3389/fgene.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Párrizas M, Brugnara L, Esteban Y, González-Franquesa A, Canivell S, Murillo S, Gordillo-Bastidas E, Cussó R, Cadefau JA, García-Roves PM, Servitja JM, Novials A. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J Clin Endocrinol Metab. 2015;100(3):E407–15. doi: 10.1210/jc.2014-2574. [DOI] [PubMed] [Google Scholar]
- 51.Masete M, Dias S, Malaza N, Adam S, Pheiffer C. A big role for microRNAs in gestational diabetes Mellitus. Front Endocrinol. 2022;13:892587. doi: 10.3389/fendo.2022.892587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Díaz-Pérez FI, Hiden U, Gauster M, Lang I, Konya V, Heinemann A, Lögl J, Saffery R, Desoye G, Cvitic S. Post-transcriptional down regulation of ICAM-1 in feto-placental endothelium in GDM. Cell Adhes Migr. 2016;10(1–2):18–27. doi: 10.1080/19336918.2015.1127467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Zhao S. Vascular Biology of the Placenta. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Chapter 4, Cell Types of the Placenta. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53245/. [PubMed]
- 54.Ke W, Chen Y, Zheng L, Zhang Y, Wu Y, Li L. Mir-134-5p promotes inflammation and apoptosis of trophoblast cells via regulating FOXP2 transcription in gestational diabetes mellitus. Bioengineered. 2022;13(1):319–30. doi: 10.1080/21655979.2021.2001219. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Zhao C, Zhang T, Shi Z, Ding H, Ling X. MicroRNA-518d regulates PPARα protein expression in the placentas of females with gestational diabetes mellitus. Mol Med Rep. 2014;9(6):2085–90. doi: 10.3892/mmr.2014.2058. [DOI] [PubMed] [Google Scholar]
- 56.Sun DG, Tian S, Zhang L, Hu Y, Guan CY, Ma X, Xia HF. The miRNA-29b is downregulated in Placenta during Gestational Diabetes Mellitus and May alter Placenta Development by regulating trophoblast Migration and Invasion through a HIF3A-Dependent mechanism. Front Endocrinol. 2020;11:169. doi: 10.3389/fendo.2020.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rice GE, Scholz-Romero K, Sweeney E, Peiris H, Kobayashi M, Duncombe G, Mitchell MD, Salomon C. The Effect of glucose on the release and bioactivity of Exosomes from First Trimester Trophoblast cells. J Clin Endocrinol Metab. 2015;100(10):E1280–8. doi: 10.1210/jc.2015-2270. [DOI] [PubMed] [Google Scholar]
- 58.Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, Longo S, Duncombe G, Mitchell MD, Rice GE, Illanes SE. Gestational diabetes Mellitus is Associated with Changes in the concentration and Bioactivity of Placenta-Derived Exosomes in maternal circulation across Gestation. Diabetes. 2016;65(3):598–609. doi: 10.2337/db15-0966. [DOI] [PubMed] [Google Scholar]
- 59.Muralimanoharan S, Maloyan A, Mele J, Guo C, Myatt LG, Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia, Placenta, Volume 33, Issue 10 2012, Pages 816–823,ISSN 0143–4004 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed]
- 60.Dinesen S, El-Faitarouni A, Dalgaard LT. (2023). Circulating microRNAs associated with gestational diabetes mellitus: useful biomarkers?, J Endocrinol, 256(1), e220170. Retrieved Feb 23, 2023, from https://joe.bioscientifica.com/view/journals/joe/256/1/JOE-22-0170.xml. [DOI] [PubMed]
- 61.Zhu Y, Tian F, Li H, Zhou Y, Lu J, Ge Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. Int J Gynecol Obstet. 2015;130(1):49–53. doi: 10.1016/j.ijgo.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Cao Y-L, Jia Y-J, Xing B-H, Shi DD, Dong XJ, Plasma microRNA-16-5p, -17-5p and – 20a-5p: novel diagnostic biomarkers for gestational diabetes mellitus. J Obstet Gynaecol Res. 2017;43(6):974–81. doi: 10.1111/jog.13317. [DOI] [PubMed] [Google Scholar]
- 63.Wander PL, Boyko EJ, Hevner K, et al. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res Clin Pract. 2017;132:1–9. doi: 10.1016/j.diabres.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lamadrid-Romero M, Solís KH, Cruz-Reséndiz MS et al. Central nervous system development-related microRNAs levels increase in the serum of gestational diabetic women during the first trimester of pregnancy. Neuroscience Research 2017 10.1016/j.neures.2017.08.003. In the press. [DOI] [PubMed]
- 65.Sebastiani G, Nigi L, Grieco GE, Mancarella F, Ventriglia G, Dotta F. Circulating microRNAs and diabetes mellitus: a novel tool for disease prediction, diagnosis, and staging? J Endocrinol Investig. 2017;40(6):591–610. doi: 10.1007/s40618-017-0611-4. [DOI] [PubMed] [Google Scholar]
- 66.104) Li X. MiR-375, a microRNA related to diabetes. Gene. 2014;533(1):1–4. doi: 10.1016/j.gene.2013.09.105. [DOI] [PubMed] [Google Scholar]
- 67.Vasu S, Kumano K, Darden CM, Rahman I, Lawrence MC, Naziruddin B. MicroRNA signatures as future biomarkers for diagnosis of Diabetes States. Cells. 2019;8(12):1533. doi: 10.3390/cells8121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ. Circulating miR-375 as a biomarker of β-cell death and diabetes in mice. Endocrinology. 2013;154(2):603–8. doi: 10.1210/en.2012-1744. [DOI] [PubMed] [Google Scholar]
- 69.Ibarra A, Vega-Guedes B, Brito-Casillas Y, Wägner AM. Diabetes in pregnancy and MicroRNAs: promises and Limitations in their clinical application. Non-coding RNA. 2018;4(4):32. doi: 10.3390/ncrna4040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li R, Jia Z, Trush MA. Defining ROS in Biology and Medicine. Reactive oxygen species (Apex NC) 2016;1(1):9–21. doi: 10.20455/ros.2016.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vermot A, Petit-Härtlein I, Smith SME, Fieschi F. NADPH oxidases (NOX): an overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxid (Basel Switzerland) 2021;10(6):890. doi: 10.3390/antiox10060890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sedeek M, Nasrallah R, Touyz RM, Hébert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrology: JASN. 2013;24(10):1512–8. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122(4):333–8. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 75.Deshpande OA, Mohiuddin SS. (2022). Biochemistry, Oxidative Phosphorylation. In StatPearls. (2022) StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553192/. [PubMed]
- 76.Zhang DX, Gutterman DD. (2007). Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. American journal of physiology. Heart and circulatory physiology, 292(5), H2023–H2031. 10.1152/ajpheart.01283.2006. [DOI] [PubMed]
- 77.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103(8):2653–8. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathebula SD. Polyol pathway: A possible mechanism of diabetes complications in the eye. Afr. Vision Eye Health. (2015); 74(1), Art. #13, 5 pages. 10.4102/aveh.v74i1.13.
- 79.Suresh V, Reddy A. Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impedement. J Diabetes Metab Disord. 2021;20(1):989–1002. doi: 10.1007/s40200-021-00799-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stuehr D, Pou S, Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001;276(18):14533–6. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 81.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 82.Yang YM, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297(5):H1829–36. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Investig. 2003;111(8):1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoe-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103(9):1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 85.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circul Res. 2001;88(2):E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 86.Heitzer T, Krohn K, Albers S, Meinertz T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type II diabetes mellitus. Diabetologia. 2000;43(11):1435–8. doi: 10.1007/s001250051551. [DOI] [PubMed] [Google Scholar]
- 87.Suresh V, Reddy A. Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impediment. J Diabetes Metab Disord. 2021;20(1):989–1002. doi: 10.1007/s40200-021-00799-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Sem Cancer Biol. 2005;15(4):277–89. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Visiedo F, Santos-Rosendo C, Mateos-Bernal RM, Gil-Sánchez MD, Bugatto F, Aguilar-Diosdado M, Segundo C, López-Tinoco C. (2017). Characterization of NO-Induced Nitrosative Status in Human Placenta from Pregnant Women with Gestational Diabetes Mellitus. Oxidative medicine and cellular longevity, 2017, 5629341. 10.1155/2017/5629341. [DOI] [PMC free article] [PubMed]
- 90.Cristina Lopez-Tinoco, Carmen Segundo and Manuel Aguilar-Diosdado. (2014) Gestational diabetes and oxidative stress. Current trends in Endocrinology. Volume 7 45–55. Retrieved from http://www.researchtrends.net/tia/title.asp?id=59.
- 91.Jatavan P. (2020). Oxidative stress in gestational diabetes mellitus. In Diabetes (pp. 79–85). Academic Press. 10.1016/B978-0-12-815776-3.00008-5.
- 92.Ayala A, Muñoz MF, Argüelles S. (2014). Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative medicine and cellular longevity, 2014, 360438. 10.1155/2014/360438. [DOI] [PMC free article] [PubMed]
- 93.Haleagrahara N, Julian V, Chakravarthi S. N-acetylcysteine offers cardioprotection by decreasing cardiac lipid hydroperoxides and 8-isoprostane level in isoproterenol-induced cardiotoxicity in rats. Cardiovasc Toxicol. 2011;11(4):373–81. doi: 10.1007/s12012-011-9132-0. [DOI] [PubMed] [Google Scholar]
- 94.Delmastro-Greenwood M, Freeman BA, Wendell SG. Redox-dependent anti-inflammatory signaling actions of unsaturated fatty acids. Annu Rev Physiol. 2014;76:79–105. doi: 10.1146/annurev-physiol-021113-170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J Gastroenterol. 2013;19(39):6540–7. doi: 10.3748/wjg.v19.i39.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harris ED. (1992). Regulation of antioxidant enzymes. FASEB journal: official publication of the Federation of American Societies for Experimental Biology, 6(9), 2675–83. 10.1096/fasebj.6.9.1612291. [DOI] [PubMed]
- 97.Suhail M, Patil S, Khan S, Siddiqui S. Antioxidant vitamins and lipoperoxidation in non-pregnant, pregnant, and gestational diabetic women: erythrocytes osmotic fragility profiles. J Clin Med Res. 2010;2(6):266. doi: 10.4021/jocmr454w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uzun H, Benian A, Madazlı R, Topçuoğlu MA, Aydın S, Albayrak M. Circulating oxidised low-density lipoprotein and paraoxonase activity in preeclampsia. Gynecol Obstet Invest. 2005;60(4):195–200. doi: 10.1159/000087205. [DOI] [PubMed] [Google Scholar]
- 99.Urbaniak SK, Boguszewska K, Szewczuk M, Kaźmierczak-Barańska J, Karwowski BT. 8-Oxo-7, 8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) as a potential biomarker for gestational diabetes mellitus (GDM) development. Molecules. 2020;25(1):202. doi: 10.3390/molecules25010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chaudhary L, Tandon OP, Vaney N, Agarwal N. (2003). Lipid peroxidation and antioxidant enzymes in gestational diabetics. Indian journal of physiology and pharmacology, 47, 441–446. Retrieved from https://ijpp.com/. [PubMed]
- 101.Socha MW, Flis W, Wardęga M, Stankiewicz M. Impact of oxidative stress on Molecular Mechanisms of Cervical ripening in pregnant women. Int J Mol Sci. 2022;23(21):12780. doi: 10.3390/ijms232112780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leppert PC, Yu SY. Apoptosis in the cervix of pregnant rats in association with cervical softening. Gynecol Obstet Invest. 1994;37(3):150–4. doi: 10.1159/000292546. [DOI] [PubMed] [Google Scholar]
- 103.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–92. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 104.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 105.Aalberts M, van Dissel-Emiliani FM, van Tol HT, Taverne MA, Breeveld-Dwarkasing VN. High iNOS mRNA and protein levels during early third trimester suggest a role for NO in prelabor cervical ripening in the bovine. Mol Reprod Dev. 2007;74(3):378–85. doi: 10.1002/mrd.20546. [DOI] [PubMed] [Google Scholar]
- 106.Tschugguel W, Schneeberger C, Lass H, Stonek F, Zaghlula MB, Czerwenka K, Schatten C, Kaider A, Husslein P, Huber JC. Human Cervical Ripening Is Associated with an Increase in Cervical Inducible Nitric Oxide Synthase Expression, Biology of Reproduction, Volume 60, Issue 6, 1 June 1999, Pages 1367–72, 10.1095/biolreprod60.6.1367. [DOI] [PubMed]
- 107.American College of Obstetricians and Gynecologists, & Committee on Practice Bulletins—Obstetrics ACOG practice bulletin no. 127: management of preterm labor. Obstet Gynecol. 2012;119(6):1308–17. doi: 10.1097/AOG.0b013e31825af2f0. [DOI] [PubMed] [Google Scholar]
- 108.HAPO Study Cooperative Research Group. (2002). The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 78(1), 69–77. 10.1016/s0020-7292(02)00092-9. [DOI] [PubMed]
- 109.Gibb W, Challis JR. Mechanisms of term and preterm birth. Journal of obstetrics and gynaecology Canada: JOGC = Journal d’obstetrique. et gynecologie du Canada : JOGC. 2002;24(11):874–83. doi: 10.1016/s1701-2163(16)31044-1. [DOI] [PubMed] [Google Scholar]
- 110.Ekman-Ordeberg G, Dubicke A. (2012). Preterm Cervical Ripening in humans. Facts, views & vision in ObGyn, 4(4), 245–253. Retrieved from https://fvvo.eu/. [PMC free article] [PubMed]
- 111.Bar-Hava I, Barnhard Y, Scarpelli SA, Orvieto R, Ben-Rafael, Divon MY. Gestational diabetes and preterm labour: is glycaemic control a contributing factor? Eur J Obstet Gynecol Reprod Biol. 1997;73(2):111–4. doi: 10.1016/s0301-2115(97)02707-3. [DOI] [PubMed] [Google Scholar]