Abstract
Objectives
Extensive application of stevia in the treatment of type 2 diabetes mellitus (DM) has been proven by a large number of previous studies. We prepared stevia loaded in nanoniosomes (nanostevia) to improve its bioavailability, functionality, and stability and explore its protective effects and underlying mechanisms in the liver of STZ-induced diabetic rats.
Methods
Single-dose intraperitoneal injection of STZ (50 mg/kg body weight) was used to establish diabetic model. The mRNA levels of PEPCK and GCK genes and the protein level of INSR were evaluated by Real time-PCR and Western blot assays, respectively. TUNEL assay was used to detect apoptotic cell death in the liver tissue.
Results
Diabetic rats exhibited significantly reduced levels of INSR (*** P < 0.001) as well as elevated levels of PEPCK (*** P < 0.001). Both stevia and nano-stevia were capable of increasing levels of GCK and INSR and reducing levels of PEPCK (## P < 0.01 and ### P < 0.001, respectively). In addition, significantly increased number of apoptotic cell death was seen in the liver tissue of diabetic rats (*** P < 0.001) which was markedly mitigated by treatment with both Stevia and nano-Stevia (#P < 0.05 and ## P < 0.01, respectively).
Conclusion
Both stevia and nano-stevia demonstrates potent anti-apoptotic activity in the liver tissue of diabetic rats by targeting PEPCK/GCK genes and INSR pathway. These finding show that nano-stevia has more potential to reduce the liver injury caused by STZ-induced diabetes in rats and hence can be considered a valid agent and alternative therapy for attenuating complications of type 2 DM.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-023-01278-2.
Keywords: Diabetes mellitus, Streptozotocin, Liver injury, PEPCK/GCK genes, Apoptosis
Introduction
Diabetes mellitus (DM) is a lifelong progressive disease that is characterized by elevated blood glucose levels [1]. High blood glucose level in DM patients can be attributed to body’s inability for producing or using insulin [2]. The prevalence of DM around the world has been estimated to be 4.4% which in turn give rise to increasing the number of DM patients to 439–552 million by 2030 [3, 4]. The most prevalent form of DM is type 2 DM which includes 90–95% of cases [5]. The main symptoms of type 2 DM are loss of pancreatic β-cell mass, insulin resistance, derangements in lipid and carbohydrate metabolism, and accumulating human islet amyloid peptide deposits [6, 7]. Elevated blood glucose levels within cellar vicinity enhance generation of free radicles and weaken natural antioxidant defenses which in turn leads to triggering oxidative stress and diabetes complications such as liver damage, blindness [8], renal failure [9], and neurological and cerebrovascular disorders [10]. Owing to the key role of liver tissue in glycogen synthesis, gluconeogenesis, and the homeostasis of insulin and plasma glucose, measuring serum liver enzymes like gamma-glutamyltransferase, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) can be considered a valid method to predict type 2 DM [11]. Hosphoenolpyruvate carboxylase (PEPCK) was known to be one of the key enzymes in the liver regulating the conversion of non-sugar substances into glucose during gluconeogenesis process [12]. Increasing the expression of PEPCK in the liver of type 2 DM rats and subsequently enhancing gluconeogenesis has been reported by previous studies [13]. Glucokinase (GCK) or hexokinase IV (EC 2.7.1.2) is another important enzyme which plays an important role in enhancing glucose uptake for glycogenesis and energy storage in hepatocytes and promoting the insulin release by pancreatic β cells [14, 15]. It has been reported that gene variations in GCK increase the risk of type 2 DM [16]. In addition, a previous study has shown that therapeutic agents could ameliorate T2D in high-fat diet (HFD) + streptozotocin (STZ) treated mice by reducing apoptotic markers and targeting PEPCK and GCK [17]. Furthermore, the insulin receptor (INSR) is highly expressed in the liver tissue and displays a powerful role in normalizing in vivo response to insulin [18, 19]. Unwanted side effects of available drugs for type 2 DM has limited their extensive use and hence medicinal plants can be considered alternative agents for them [20]. Stevia (Stevia rebaudiana Bertoni), sweet herb of Paraguay, is a natural and the non-caloric sugar compound belonging to the family Asteraceae [21]. Stevia was known to be a potent antioxidant and anti-inflammatory agent against various diseases such as cancer [22], and diabetes [23]. It has been well-known that nanocarriers have ability of improving stability, functionality, and bioavailability of phenolics in the treatment of different diseases [24]. Here, the aim of the study was to reduce the dose of stevia and keep its prolonged activity by loading into nanoniosomes. We have investigated the protective effects of stevia loaded in nanoniosomes (nanostevia) in STZ treated rats. In order to assess the effects of stevia on molecular mechanisms, we evaluated the mRNA levels of PEPCK and GCK and apoptotic cell death.
Methods and materials
Haigen Bio-tech Company (China) was producer of Stevia rebaudiana Bertoni powder. According to the manufacturer’s instructions, it has been extracted and crystalized as TSG (total steviol glycosides). The powder includes 97% Rebaudioside A and 3% of other glycosides like stevioside. More information about ingredients of stevia rebaudiana Bertoni powder can be observed in COA[https://www.steviahaigen.com/steviol-glycosides/stevia-extract-powder-natural sweeteners.html#].Solarbio Science & Technology Co. (Beijing, china CAT No. S8050) supplied Streptozotocin. Other chemicals such as chloroform, cholesterol, sorbitan monostearate (Span 60), and polyoxyethylene sorbitan monostearate (Tween 60) were obtained from Merck, Germany.
Animals and Ethics statement
Male Wistar rats (total number: 40, weight: 200–270 g) were procured from the experimental and comparative studies center of the Iran University of Medical Sciences (Tehran, Iran). Wistar rats were kept at 22 ± 2 °C with a 12-h light/dark schedule. The animals had free access to standard chow and water. Ethical approval was obtained from Islamic Azad University ethical committee for animal research.
Preparation of nano‑ niosomes
Nano‑ niosomes were prepared according to thin layer hydration method [25]. In brief, after dissolving cholesterol and surfactants (span60 and tween 60) with 1:1 molar ratio in 10 ml chloroform, the rotary evaporator was used for 1 hat 60 °C and 120 rpm to evaporate chloroform. Afterwards, the dried thin films were hydrated using stevia solution in PBS (10 mL, 60 mg/mL, at 30 °C for 1 h and 120 rpm). Eventually, the resulting solution was sonicated for 5 min and stored at 4 °C in a refrigerator.
Characterization of Nano‑niosomes
The hydrodynamic size of nano-niosomes containing drug or without drug was measured using dynamic light scattering (DLS) using He–Ne laser at a wavelength of 632.8 nm (Malvern, Zetasizer Nano ZSE, Worcestershire, UK).
Entrapment efficiency
Entrapment efficiency was measured according to a previous study [26]. Briefly, the formulations were ultra-filtered at 4000×g for 30 min. D using a exerting an Amicon.
Ultra-15-membrane (MWCO 30,000 Da). Filtration resulted in passing free drugs through a filter membrane while the Stevia carrying niosomes remained in the top chamber. In order to evaluate drug concentration, UV–visible spectroscopy (JASCO, V-530, Tokyo, Japan) at a wavelength in the range 420 nm was utilized. For each formulation, the drug concentration was measured to its standard curve. Finally, we used the following formula to calculate encapsulation efficiency:
Encapsulation Efficiency (%) = [ (A − B)∕A ] × 100.
A is defined as the initial proportion of drug-loaded niosomal formulations, and B is defined as the amount of free drug transmitted through the membrane.
Drug release study
In order to evaluate drug release, a dialysis bag (MWCO = 12 kDa) was used to compare drug release of free drug and drug-loaded niosomal formulations. The bag was put in PBS solution (50 mL, 1X, pH = 3, 5, 7.4) under gradual stirring condition (50 rpm) at 37 °C. The aliquots were taken and replaced by fresh PBS solution at particular time intervals [26].
Physical stability assessment
In order to determine physical stability, we measured nanoparticle size and EE of niosomal solutions during the refrigerated storage at interval times of 0, 14, 30, and 60 days based on a previous study [27].
Induction of diabetes Mellitus
The animals have fasted overnight and type 2 DM was induced by intraperitoneal injection of a single dose of STZ (50 mg/kg body weight i.p.) dissolved in PBS buffer solution. The rats had a limited access to sucrose solution for one night (20% sucrose solution) to prevent hypoglycemic mortality by STZ. A glucometer (Accu-Chek, Roche Diagnostics, Penzberg, Germany) was used to measure fasting blood glucose after 72 h of STZ injection and evaluate the progression of DM in the animals. The animals with a 200 mg/dl glucose level or more on the third day after STZ injection were accepted to be diabetic. The normal control animals only received distilled water and standard diet.
Experimental design
This study includes five groups and eight rats were designated in each group and categorized as explained below:
Normal control group. This group includes normal healthy rats (control, n = 8).
Diabetic group: All the rats received only a single dose of STZ (50 mg/kg) dissolved in PBS buffer solution (diabetic, n = 8).
Nano‑ niosome group. STZ-induced diabetic rats were intra-gastrically treated with 1ml of only nano-niosome for 30 days daily (Nano‑ niosomes, n = 8).
Stevia group. STZ-induced diabetic rats were intra-gastrically treated with 1ml of stevia (20 mg/dl) for 30 days daily (Stevia, n = 8).
Nano-stevia group: STZ-induced diabetic rats were intra-gastrically treated with 1ml of nano-stevia (20 mg/dl) for 30 days daily.
Sample collection
A mixture of ketamine (50 mg/kg) and xylazine (10 mg/kg) was used to anesthetize the animals which were fasted overnight at the end of the experiment. Liver tissue samples were collected from all the rats from different groups and washed in an ice-cold saline solution. Some liver tissues were stored at -80 ○C for assessing expression of genes and some of them were fixed in 10% formalin solution and processed for histological evaluations using paraffin technique.
Tunnel assay
Tunnel assay was performed based on Roche kit protocol. To This end, the samples were deparaffied and placed in xylene solutions. Afterwards, the sections were rehydrated using a graded series of ethanol (90%, 80%, and 70%) and rinsed with PBS buffer. After incubation with proteinase K for 20 min at 37 º C, 50 µL Tunnel dye was added to each sample and samples were incubated at 37 º C for 1 h again. Finally, samples were rinsed with PBS buffer and detecting TUNEL-positive cells (green) was carried out using Zeiss LSM 5 fluorescent microscope. Only TUNNEL label solution was utilized for negative control samples whereas both TUNEL-enzyme solution and TUNEL-label solution (1:9) were used for positive control samples. The nuclei were stained by incubating the tissue by wit DAPI dye (1 mg/ml) (blue dots).
Western blot assay
The RIPA lysis buffer was used to obtain tissue lysis. The BCA Protein Assay kit (The Thermo Scientific Pierce BCA Protein Assay Kit, Therfmofisher Scientific, US) was used to detect protein concentrations. In order to separate soluble protein, 4–20% gradient SDS/PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) was used. Afterwards, separate soluble protein were transferred to the nitrocellulose membrane and nonspecific reaction sites of PVDF (Immobilon®-FL PVDF membrane pore size 0.45 μm-Sigma-Aldrich) membranes were blocked with TBS buffer. The membranes were incubated with primary antibody (Dilution 1:1000) at 4 °C overnight and then the appropriate horseradish peroxidase-conjugated secondary antibody (Dilution 1:3000) at room temperature for 2 h. The antibody incubations were followed by rinsing using TBS buffer (three times). Enhanced chemiluminescence (ECL) was used to detect the protein bands. Finally, a film camera was used to visualize the bands. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was served as loading control and for normalization. Ratio of expression of each protein to the expression of GAPDH in the same sample was measured using Alpha Ease ® FC Imaging System according to their optical densities.
Real-time PCR assay
A mortar and pestle was utilized to powder frozen liver tissues in liquid nitrogen. Total RNA was extracted by homogenization of samples in TRIzol (Invitrogen). A cDNA synthesis kit Fermentas was used to reverse RNA into cDNA based on manufacturer’s instructions. Using Rotor-Gene Q 5plex System using SYBR® Premix Ex Taq ™ II (TliRNaseH Plus, RR820Q), a triplicate run of quantitative PCR was performed. The internal control in this study was GAPDH. Calculating relative changes in gene expression was performed using the 2− ΔΔCT method. Primers used in this study are listed in Table 1.
Table 1.
Primers used in this study
| Primer | Forward | Reverse | Size |
|---|---|---|---|
| PEPCK | TTGAGATTTTGTAGAGCACA | TCTATCACAGCTCTGGAAAT | 290 bp |
| GCK | ATATTTTTTTAGGATTTGCC | ATATCACTCTCTTCGCGAAT | 350 bp |
| GAPDH | ATTCTCTGATTTGGTCGTATT | TTTCATGGTGGAATCATATT | 130 bp |
Statistical analysis
Results were presented as means ± standard error (S.E.M) for all statistical tests using GraphPad Prism software version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by Tukey post hoc test was used to evaluate multiple group differences. In order to determine normal distribution of all data, shapiro–Wilk and Kolmogorov–Smirnov tests were used. Differences were considered to be statistically significant at p < 0.05.
Results
Characterization of nano‑niosomes containing Nano-Stevia
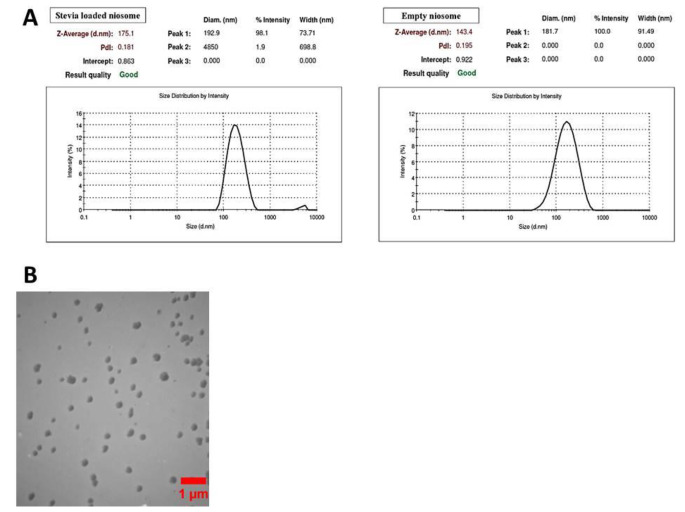
As shown in Fig. 1A, DLS results revealed that the hydrodynamic size of only nano-niosomes was about 143.4 while a shift to 175.1 nm was seen in nano-niosomes containing stevia. Compared to placebo niosomes, increased hydrodynamic size of nano-niosomes was observed after loading of nano-stevia. As shown in Fig. 1B and according to TEM image, only nano-niosomes were spherical in shape and had good stability. In addition, TEM image showed that size of only nano-niosomes was about 140 ± 3.35.
Fig. 1.
Nano-niosome characterization. (A) DLS results show hydrodynamic size distribution for nano-niosomes and nano‑niosomes containing Nano-Stevia. (B) Results of TEM image demonstrated that nano-niosomes were spherical in shape and had a good stability
Optimization of nano‑niosomes containing Nano-Stevia
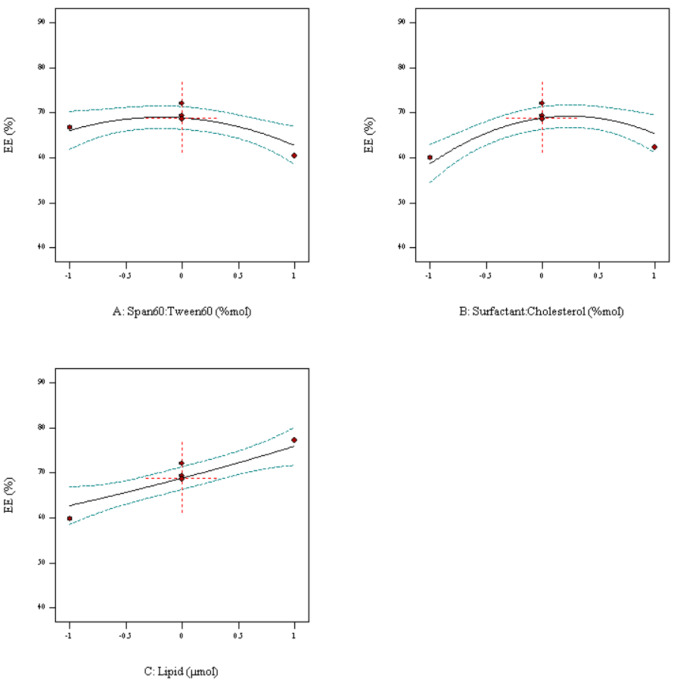
Entrapment efficiency (EE) and release rate were measured to evaluate the optimization process. The outcomes of the Box–Behnken trials revealed that EE% in nanoniosomes containing drug was about 68.89. A direct association between EE% and increasing drug content, as well as the surfactant was seen (Fig. 2).
Fig. 2.
Box-Behnken method for encapsulation efficiency (EE) as a function of the parameters
As depicted in Figs. 3, 91% of drug released in 6 h and a constant release process was seen after 6 h.
Fig. 3.
Release profile of stevia and nano-stevia during 72 h
Physical stability
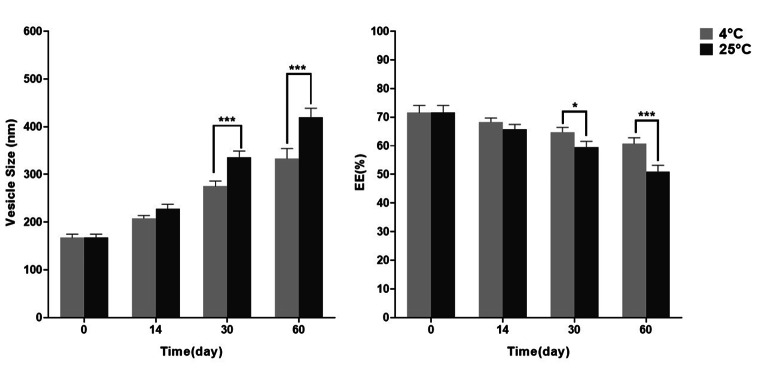
The particle size and EE of nano-Stevia Stevia loaded niosomes during 2 months of storage at 4 ± 2 °C and 25 ± 2 °C showed that there were no significant differences in the particle size and EE of particles at 4 ± 2 °C and 25 ± 2 °C on Day 0 and 14 (Fig 4). There was no sediment from the water-insoluble contents of the layers of niosomes over this time.
Fig. 4.
Stability of optimum stevia loaded niosomes during 2 months of storage at 4 ± 2 °C and 25 ± 2 °C, (* p-value < 0.05, ** p-value < 0.01, and *** p-value < 0.001)
Significant differences in the particle size and EE of particles at 4 ± 2 °C and 25 ± 2 °C were observed on Day 30 and 60.
Nano-Stevia exerts protective effects in the liver tissue of STZ-induced diabetic rats by changing the mRNA levels of PEPCK and GCK genes
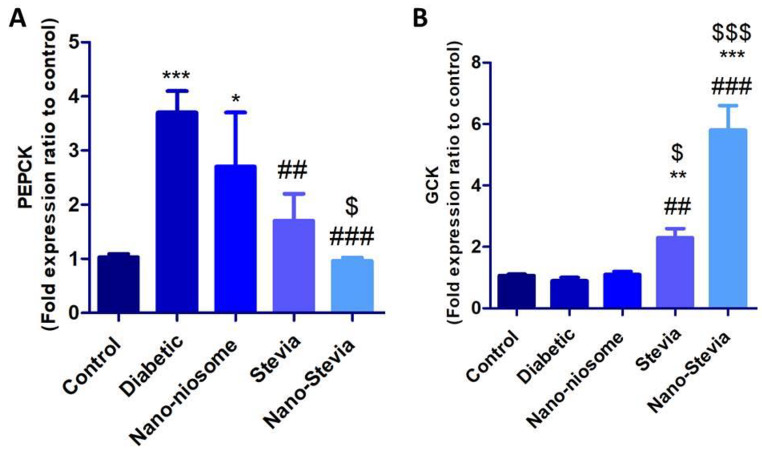
As shown in Fig. 5A, the mRNA levels of PEPCK were significantly higher in diabetic rats than in non-diabetic control group. Compared with the diabetic group, STZ-induced diabetic rats treated by both stevia and nano-stevia exhibited significantly reduced levels of PEPCK. Nano-stevia displayed more powerful effects on reducing the mRNA levels of PEPCK compared to only stevia. Significantly decreased expression levels of GCK were seen in the liver of STZ-induced diabetic rats compared to non-diabetic control group. Interestingly, treatment with both stevia and nano-stevia markedly increased the expression levels of GCK compared to diabetic control group (Fig. 5B). On the contrary, treatment with only nanoniosomes failed to create noticeable changes in the mRNA levels of GCK in the liver of STZ-induced diabetic rats compared to diabetic control group.
Fig. 5.
The effects of intra-gastrically administration with saline, nano-niosome, Stevia, and Nano-Stevia on mRNA levels of (A) PEPCK and (B) GCK. Significant differences: * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to control group. ## P < 0.01 and ### P < 0.001 compared with diabetic control group. $ P < 0.05 and $$$ P < 0.001 compared with nano-niosome (n = 8)
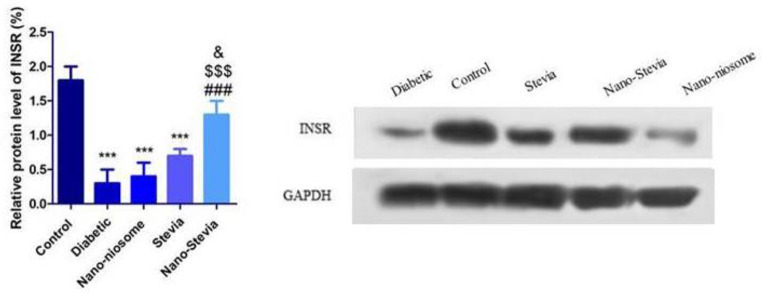
Nano-Stevia exerts protective effects in the liver tissue of STZ-induced diabetic rats by increasing the protein level of INSR.
Significantly reduced protein levels of INSR were observed in the liver of STZ-induced diabetic rats compared to non-diabetic control group (Fig. 6). Either stevia or nano-stevia treatment markedly increased protein levels of INSR, with nano-stevia exerting a more powerful effect than stevia. There were no significant differences between only nanoniosomes and diabetic control groups.
Fig. 6.
The effects of intra-gastrically administration with saline, nano-niosome, Stevia, and Nano-Stevia on protein levels of INSR. Significant differences: *** P < 0.001 compared to control group. ### P < 0.001 compared with diabetic control group. $$$ P < 0.001 compared with nano-niosome. & P < 0.05 compared to Stevia (n = 4)
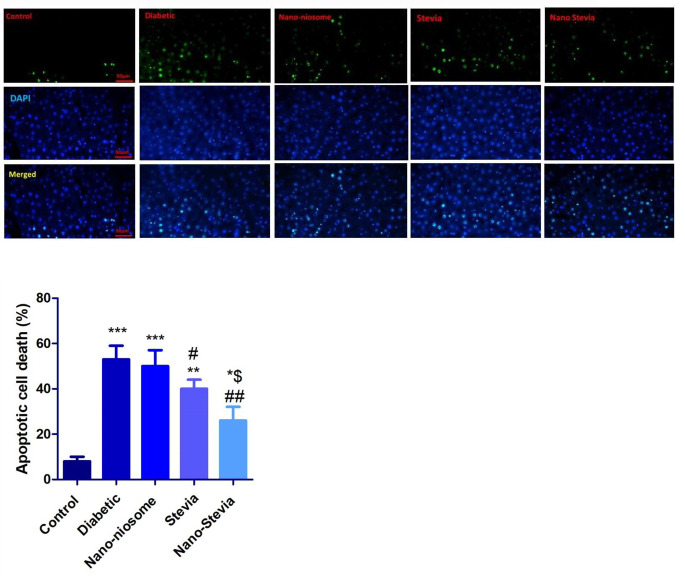
Nano-Stevia exerts protective effects in the liver tissue of STZ-induced diabetic rats by reducing apoptotic cell death
The number of apoptotic cells significantly increased in the liver of STZ-induced diabetic rats compared to non-diabetic control group. Reducing the number of apoptotic cells was more pronounced in the liver of STZ-induced diabetic rats treated by both stevia and nano-stevia (Fig. 7). No significant differences were seen between only nanoniosomes and diabetic control groups.
Fig. 7.
The effects of intra-gastrically administration with saline, nano-niosome, stevia, and nano-stevia on apoptotic cell death in the liver of STZ-induced diabetic rats. Tunel assay 50 μm (apoptotic cells = green dot, DAPI stained nucleus: blue dots). Significant differences: * P < 0.05, ** P < 0.01, and *** P < 0.001 compared to control group. # P < 0.05 and ## P < 0.01 compared with diabetic control group. $ P < 0.05 compared with nano-niosome (n = 4)
Discussion
One of the most commonly used compounds to establish T2DM in animal models is STZ [28]. Upon STZ treatment, excessive reactive oxygen species (ROS) production leads to DNA alkylation and fragmentation, increasing lipid peroxidation, reduced synthesis and release of insulin, and organ dysfunction [29, 30]. In this study, T2DM was induced by STZ to establish an accepted model for recognizing the pathophysiology of T2DM and evaluating protective effects of therapeutic agents. Intraperitoneal injection of STZ (50 mg/kg) could give rise to changing the expression of the mRNA levels of PEPCK and GCK genes, the protein level of INSR, and enhancing apoptotic cell death in the liver of STZ-induced diabetic rats. Both stevia and nano-stevia were capable of reducing apoptotic cell death in the liver of STZ-induced diabetic rats by targeting PEPCK, GCK, and INSR. It has been reported that diabetic condition is associated with increasing rate of hepatic gluconeogenesis. Hence, targeting the gluconeogenic pathway by therapeutic agents can be considered an efficient mechanism to attenuate liver damage in STZ-induced diabetic rats and control blood glucose levels [31, 32]. PEPCK was known to play a key role in the first step of gluconeogenesis in which oxaloacetate is converted into phosphor–pyruvate [33]. A large number of previous studies have shown an increased expression of PEPCK in the liver of STZ-induced diabetic rats [34–36]. In keeping with these previous studies, our findings showed that the mRNA levels of PEPCK markedly increased in the liver of STZ-induced diabetic rats that reversed by both stevia and nano-stevia. Likewise, a previous study by our group showed that STZ-induced diabetic rats treated by stevia and nano-stevia could exhibit noticeable reduction in blood glucose levels [37]. This can be contributed to regulation of the gluconeogenic pathway by stevia and nano-stevia. It seems that there is a close relationship between increasing PEPCK expression and apoptotic cell death in diabetic conditions [38]. In addition, it has been reported that decreasing cellular ATP production, reducing insulin secretion and glucokinase (GCK) expression occurs in high-glucose–treated cells during apoptosis. In fact, exposure to a chronically high dose of glucose give rise to reducing interactions between GCK and mitochondria which in turn results in enhancing Bax binding to mitochondria and cytochrome C release [39]. GCK overexpression has demonstrated abilities for reducing apoptotic cell death during diabetic conditions. A previous study has shown that hepatic GCK expression was suppressed more than 60% in diabetic subjects with HbA1c > 7.0 [40]. In agreement with these previous studies, our findings demonstrated that reducing GCK expression in the liver of STZ-induced diabetic rats enhanced apoptotic cell death that reversed by both stevia and nano-stevia. In the line with present study, several previous reports show that protective effects of stevia against degenerative disorders are associated with reducing apoptotic cell death [41, 42].
Reducing total INSR abundance in liver tissue during different diseases is associated with increasing apoptotic cell death [43]. In the line with this previous study, our findings indicate that the protein levels of INSR markedly decreased in the liver of STZ-induced diabetic rats that reversed by both stevia and nano-stevia. The present study might have some limitations. One of the major limitations of this study is that some parameters are measured by RT-PCR only which cannot confirm the protein level.
Conclusion
In conclusion, the findings of this study suggested that both stevia and nano-stevia are capable of reducing apoptotic cell death in the liver of STZ-induced diabetic rats by regulation of the gluconeogenic pathway and the insulin receptor (INSR). Our findings showed that nano-stevia could attenuate the liver injury caused by STZ-induced diabetes in rats by targeting PEPCK/GCK genes, INSR pathway and apoptosis. Our results showed that nano-stevia has more powerful effects in reducing diabetes related liver injury in rats. Increasing stability of stevia in a nanocarier might explain these differences. This study has some limitations. One of the limitations is that more evaluations using Western blot are required to approve our results since there is the frequent mismatch between mRNA transcription and protein translation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the IAUTMU’s Herbal pharmacology research center and animal room for their support and equipment. As well specially thank Tame Tabiat pharmaceutical drugs company for purveying Stevia powder for free to this study.
Authors’ contribution
M.N: Conceptualization, Supervision, Visualization, Validation, Writing - review & editing, M.M-A, E.GH, F.A, and F.KH: Writing - original draft, Investigation, Formal analysis, Resources, and N.M: review & editing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Declarations
The Ethics Committee of the Islamic Azad University approved all the experiments and protocols. All the efforts were carried out to minimize animals suffering.
Competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dehghan M et al. Progress toward molecular therapy for diabetes mellitus: a focus on targeting inflammatory factors. Diabetes Res Clin Pract, 2022: p. 109945. [DOI] [PubMed]
- 2.Rom S, et al. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol. 2019;56:1883–96. doi: 10.1007/s12035-018-1195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Whiting DR, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Velandia M, et al. Healthy lifestyle, metabolomics and incident type 2 diabetes in a population-based cohort from Spain. Int J Behav Nutr Phys Activity. 2022;19(1):8. doi: 10.1186/s12966-021-01219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Reviews Endocrinol. 2020;16(7):349–62. doi: 10.1038/s41574-020-0355-7. [DOI] [PubMed] [Google Scholar]
- 7.Malik A, et al. Oxidative stress and inflammatory markers in type 2 diabetic patients. Eur J Clin Invest. 2020;50(6):e13238. doi: 10.1111/eci.13238. [DOI] [PubMed] [Google Scholar]
- 8.Wykoff CC, et al. Risk of blindness among patients with diabetes and newly diagnosed diabetic retinopathy. Diabetes Care. 2021;44(3):748–56. doi: 10.2337/dc20-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sh DA, Badridinova B. Methods for preventing the development of terminal renal failure in patients with type 2 diabetes mellitus. BMJ, 2022. 2(1).
- 10.van Sloten TT, et al. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Volume 8. The lancet Diabetes & endocrinology; 2020. pp. 325–36. 4. [DOI] [PMC free article] [PubMed]
- 11.Ahn H-R, et al. The association between liver enzymes and risk of type 2 diabetes: the Namwon study. Diabetol Metab Syndr. 2014;6(1):1–8. doi: 10.1186/1758-5996-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y-X, et al. Effect of oxymatrine on liver gluconeogenesis is associated with the regulation of PEPCK and G6Pase expression and AKT phosphorylation. Biomedical Rep. 2021;15(1):1–10. doi: 10.3892/br.2021.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Backer I, et al. Insights into the role of neuronal glucokinase. Am J Physiology-Endocrinology Metabolism. 2016;311(1):E42–E55. doi: 10.1152/ajpendo.00034.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iynedjian P. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mir MM, et al. Potential impact of GCK, MIR-196A-2 and MIR-423 gene abnormalities on the development and progression of type 2 diabetes mellitus in Asir and Tabuk regions of Saudi Arabia. Mol Med Rep. 2022;25(5):1–14. doi: 10.3892/mmr.2022.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathwa N, et al. Calorie restriction potentiates the therapeutic potential of GABA in managing type 2 diabetes in a mouse model. Life Sci. 2022;295:120382. doi: 10.1016/j.lfs.2022.120382. [DOI] [PubMed] [Google Scholar]
- 18.Zhu A, et al. Associations between INSR and MTOR polymorphisms in type 2 diabetes mellitus and diabetic nephropathy in a Northeast Chinese Han population. Genet Mol Res. 2015;14(1):1808–18. doi: 10.4238/2015.March.13.9. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto H, et al. Restoration of liver insulin signaling in insr knockout mice fails to normalize hepatic insulin action. J Clin Investig. 2005;115(5):1314–22. doi: 10.1172/JCI200523096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villegas Vílchez LF, Ascencios JH, Dooley TP. GlucoMedix®, an extract of Stevia rebaudiana and Uncaria tomentosa, reduces hyperglycemia, hyperlipidemia, and hypertension in rat models without toxicity: a treatment for metabolic syndrome. BMC Complement Med Ther. 2022;22(1):62. doi: 10.1186/s12906-022-03538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobus-Moryson M, Gramza-Michałowska A. Directions on the use of stevia leaves (Stevia rebauidana) as an additive in food products. Acta Scientiarum Polonorum Technologia Alimentaria. 2015;14(1):5–13. doi: 10.17306/J.AFS.2015.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Iatridis N, et al. Anti-cancer properties of Stevia rebaudiana; more than a sweetener. Molecules. 2022;27(4):1362. doi: 10.3390/molecules27041362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dooley TP, Pérez JMP, Rodriquez CR. Stevia and Uncaria extract (GlucoMedix®) reduces glucose levels and the need for medications in type 2 diabetes: an open label case series of six patients. Clin Phytoscience. 2022;8:1–7. doi: 10.1186/s40816-021-00332-x. [DOI] [Google Scholar]
- 24.Delfanian M, Sahari MA. Improving functionality, bioavailability, nutraceutical and sensory attributes of fortified foods using phenolics-loaded nanocarriers as natural ingredients. Food Res Int. 2020;137:109555. doi: 10.1016/j.foodres.2020.109555. [DOI] [PubMed] [Google Scholar]
- 25.Jafari-Rastegar N et al. Oral administration of nano-tyrosol reversed the diabetes-induced liver damage in streptozotocin-induced diabetic rats. J Diabetes Metabolic Disorders, 2022: p. 1–9. [DOI] [PMC free article] [PubMed]
- 26.Akbarzadeh I, et al. Preparation, optimization and in-vitro evaluation of curcumin-loaded niosome@ calcium alginate nanocarrier as a new approach for breast cancer treatment. Biology. 2021;10(3):173. doi: 10.3390/biology10030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javani R, et al. Quercetin-loaded niosomal nanoparticles prepared by the thin-layer hydration method: Formulation development, colloidal stability, and structural properties. LWT. 2021;141:110865. doi: 10.1016/j.lwt.2021.110865. [DOI] [Google Scholar]
- 28.Karganov MY, et al. Streptozotocin (STZ)-induced diabetes affects tissue trace element content in rats in a dose-dependent manner. Biol Trace Elem Res. 2020;198:567–74. doi: 10.1007/s12011-020-02090-2. [DOI] [PubMed] [Google Scholar]
- 29.Agbonifo-Chijiokwu E et al. Underlying biochemical effects of intermittent fasting, exercise and honey on streptozotocin-induced liver damage in rats. J Diabetes Metabolic Disorders, 2023: p. 1–13. [DOI] [PMC free article] [PubMed]
- 30.Karayakali M, et al. Crocin treatment exerts anti-inflammatory and anti-oxidative effects in liver tissue damage of pinealectomized diabetic rats. Environmental Science and Pollution Research; 2023. pp. 1–15. [DOI] [PubMed]
- 31.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel-Rahman RF, et al. Ficus deltoidea extract down-regulates protein tyrosine phosphatase 1B expression in a rat model of type 2 diabetes mellitus: a new insight into its antidiabetic mechanism. J nutritional Sci. 2020;9:e2. doi: 10.1017/jns.2019.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez-Valadés AG, et al. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Mol Ther. 2006;13(2):401–10. doi: 10.1016/j.ymthe.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Farsi E, et al. Standardized extract of Ficus deltoidea stimulates insulin secretion and blocks hepatic glucose production by regulating the expression of glucose-metabolic genes in streptozitocin-induced diabetic rats. BMC Complement Altern Med. 2014;14(1):1–13. doi: 10.1186/1472-6882-14-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia X, et al. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE. 2011;6(2):e16556. doi: 10.1371/journal.pone.0016556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Althurwi HN, et al. Vulgarin, a Sesquiterpene Lactone from Artemisia judaica, improves the antidiabetic effectiveness of Glibenclamide in Streptozotocin-Induced Diabetic rats via modulation of PEPCK and G6Pase genes expression. Int J Mol Sci. 2022;23(24):15856. doi: 10.3390/ijms232415856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khakpai F, et al. Intra-gastrically administration of Stevia and particularly Nano-Stevia reversed the hyperglycemia, anxiety, and memory impairment in streptozotocin-induced diabetic rats. Physiology & Behavior; 2023. p. 114100. [DOI] [PubMed]
- 38.Chen SH, Liu XN, Peng Y. MicroRNA-351 eases insulin resistance and liver gluconeogenesis via the PI3K/AKT pathway by inhibiting FLOT2 in mice of gestational diabetes mellitus. J Cell Mol Med. 2019;23(9):5895–906. doi: 10.1111/jcmm.14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim W-H, et al. Exposure to chronic high glucose induces β-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic β-cells. Diabetes. 2005;54(9):2602–11. doi: 10.2337/diabetes.54.9.2602. [DOI] [PubMed] [Google Scholar]
- 40.Haeusler RA, et al. Decreased expression of hepatic glucokinase in type 2 diabetes. Mol metabolism. 2015;4(3):222–6. doi: 10.1016/j.molmet.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Nashar EM, et al. Effects of Stevia rebaudiana Bertoni extracts in the rat model of epilepsy induced by pentylenetetrazol: Sirt-1, at the crossroads between inflammation and apoptosis. J Integr Neurosci. 2022;21(1):21. doi: 10.31083/j.jin2101021. [DOI] [PubMed] [Google Scholar]
- 42.Potočnjak I, et al. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of ERK1/2, STAT3, and NF-κB activation. Food Chem Toxicol. 2017;107:215–25. doi: 10.1016/j.fct.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira D, et al. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia. 2011;54:1788–98. doi: 10.1007/s00125-011-2130-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.