Abstract
A fast, sensitive, and target contaminant-modulable method was developed to detect viable bacteria, molds, and yeasts after heat treatment. By reverse transcriptase PCR with elongation factor gene (EF-Tu or EF-1α)-specific primers, the detection level was 10 cells ml of milk−1. The simplicity and rapidity (4 h) of the procedure suggests that this method may be easily transposable to other foods and other contaminants.
Methods to estimate the total number of living contaminants in food samples accurately and reliably have not yet been encountered and therefore remain a public health concern. The definition of viability of microorganisms being neither simple nor straightforward (14), in this study viable cells are considered to be those capable of mRNA production of a protein synthesis elongation factor. The translation elongation factor EF-Tu (for eubacteria) (reviewed in reference 21) or EF-1α (for archaebacteria and eukaryotes) (reviewed in reference 11) plays a basic role in protein synthesis, guiding the aminoacylated tRNAs to the acceptor site in ribosomes under conditions of GTP consumption (19). Currently employed standard methods such as plate counting, microscopic enumeration, and indirect activity measurements are usually time-consuming and inadequate to protect human health (1). New methods using fluorescent nucleic acid stains seem efficient for measuring bacterial viability but are nonspecific (15). A technique that offers the greatest potential for the detection and/or discrimination of live and dead cells is reverse transcriptase PCR (RT-PCR) (5, 9, 13). In this study, our aim was to find a simple and fast procedure by means of the RT-PCR technique enabling us to detect specifically various viable bacteria, molds, and yeasts in milk after heat treatment and easily transposable to other foods.
Primer pairs were designed after sequence comparison, using Lasergene Software (Dnastar, Madison, Wis.), of the coding regions of the elongation factors of different species and resulted in detection of different fragment lengths (Table 1).
TABLE 1.
Oligonucleotide primers used in this study
| Primer pair sequencesa | Specificity | GenBank or EMBL accession no. | Microorganism(s) tested | Fragment length (bp) |
|---|---|---|---|---|
| 5′CGCTGGAAGGCGACGMRRAG3′, 5′CGGAAGTAGAACTGCGGACGGTAG3′ | Salmonella typhimurium, Mycobacterium tuberculosis, Mycobacterium leprae, Escherichia coli, Brevibacterium linens, Streptomyces ramocissimus | X55116, X55117, X63539, Z14314, X57091, X76863, X67057 | E. coli, Salmonella enteritidis | 462 |
| 5′TCCATGGTACAAGGGTTGGGAA3′, 5′GCGAATCTACCTAATGGTGGGT3′ | Saccharomyces cerevisiae, Candida albicans | X01638, M29934, M29935 | S. cerevisiae, C. albicans | 657 |
| 5′GCTGGTATCTCCAAGGATGG3′, 5′CGACGGACTTGACTTCRGTGG3′ | Mucor racemosus, Neurospora crassa, Trichoderma reesei, Absidia glauca, Aureobasidium pullulans, Histoplasma capsulatum, Puccinia graminis | J02605, M16352, X17475, X17476, D45837, Z23012, X54730, U19723, U14100, X73529 | M. racemosus, A. pullulans | 498 |
| 5′GCCAAGCCTGCAAAGAAGAAAGAA3′, 5′CCAGGGCATCACTCAAAAACAAAA3′ | Triticum aestivum | D13147 | T. aestivum | 431 |
R represents G or A.
The strains used in this study were Escherichia coli (ATCC 19110T), Salmonella enteritidis (IP 8297T), Saccharomyces cerevisiae S288C (12), Candida albicans (DSM 70014), Mucor racemosus (CBS 11308T), and Aureobasidium pullulans (CBS 14630T). E. coli and S. enteritidis were grown at 37°C in Luria broth (18); S. cerevisiae, C. albicans, M. racemosus, and A. pullulans were grown at 30°C in YPD (2% Bacto Peptone, 1% yeast extract [both from Difco Laboratories], and 2% glucose). Solid media were prepared by the addition of agar (2%; Difco Laboratories). Part of the cells were collected during the exponential growth phase in 2-ml samples and exposed to lethal conditions by incubation at 65°C for 15 min (E. coli, S. enteritidis, S. cerevisiae, and C. albicans) or at 120°C for 30 min (M. racemosus and A. pullulans). Serial dilutions of samples were plated on agar media to check the viability and numerate cells. For molds, spores were plated on OGY Agar medium (Merck). On the other hand, samples were used to inoculate milk samples.
In order to detect contaminants in milk, serial concentrations ranging from 105 to 1 cell ml−1 were performed in commercially available cow milk pasteurized at an ultrahigh temperature. PCR interference by the milk was eliminated by four cycles of washing with phosphate-buffered saline (PBS) as mentioned by Cooray et al. (3). A filtration step was added. The final pellet of washed milk was resuspended with PBS to the original volume of milk and filtered through a PBS-presoaked 25-mm-diameter polycarbonate membrane (0.8-μm pore size; Millipore). The filter was transferred to a 15-ml Falcon tube (Becton Dickinson). One milliliter of PBS was added and vortexed vigorously for 30 s. After recovery, 0.5 ml more of PBS was used and the resulting suspensions were collected and then centrifuged for 5 min at 5,000 × g. To extract RNA, 40 U of RNase inhibitor (Boehringer) and H2O adjusted with 0.05% diethylpyrocarbonate to inactivate nucleases (14) were added to a final volume of 50 μl. Cells were then disrupted by adding 50 mg of glass beads (0.5-mm diameter; Biospec Products, Bartlesville, Okla.). The tubes were shaken twice for 20 s at 900 × g, at 10-s intervals, and left on ice between periods of shaking. After decanting, 10 μl of the lysate was transferred to a new tube. A solution containing 1× EZ buffer (Perkin-Elmer), 2.5 mM manganese acetate, and 10 U of DNase I (RNase free; Boehringer) was added to a final volume of 20 μl. This DNase step was included in order to prevent amplification of contaminating genomic sequences (20). After incubation for 10 min at 37°C, DNase was inactivated by raising the temperature to 95°C for 5 min. Both steps, reverse transcription and PCR, were performed successively in the same tube. For the reverse transcription step, 15 μl of the lysate digested with DNase I, 1× EZ buffer, 1.4 mM manganese acetate, 0.3 mM deoxyribonucleoside triphosphate (Perkin-Elmer), 0.4 μM each primer (upper and lower strands), and 5 U of rTth DNA polymerase (Perkin-Elmer) were added to a final volume of 50 μl. The mixture was incubated for 2.5 min at 95°C, 20 min at 60°C, and 1 min at 95°C. For each step, 40 cycles were directly performed, each consisting of 15 s at 95°C and 30 s at 60°C. A final extension was performed for 10 min at 60°C. False-positive detection due to DNA contamination was verified by adding 5 μl of the lysate digested with DNase I, 1× Taq buffer (Boehringer), 0.2 mM deoxyribonucleoside triphosphate, 0.4 μM each primer, and 2.5 U of Taq DNA polymerase (Boehringer) to a final volume of 50 μl. The amplification products (10 μl) were separated by horizontal gel electrophoresis (1 to 2% agarose in Tris-acetate-EDTA) and visualized by ethidium bromide staining and UV illumination. As a positive RT-PCR control, 0.1 μg of total RNA was analyzed and extracted by using the RNAgent Total RNA Isolation kit (Promega). The amount of RT-PCR products was normalized with respect to a reference template corresponding to the reverse transcription and amplification of EF-1α from 0.1 μg of total RNA of Triticum aestivum added in samples. To confirm the identity of amplified products, the bands of expected size were gel purified (Gel Extract kit; Qiagen), and then 1 μl of the 50 μl of DNA recovery solution was used as a template in a second PCR using internal primers (data not shown).
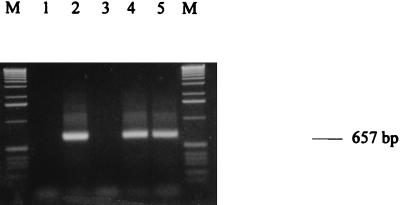
Using the above-mentioned RT-PCR setting, several controls were routinely carried out: negative control without cells (Fig. 1, lane 1), RT-PCR control with pure extracted total RNA (Fig. 1, lane 2), and DNase I control with single PCR on lysate digested with DNase I (Fig. 1, lane 3). No detectable PCR products occurred; thus, inclusion of the DNase I step appears to prevent amplification of contaminating genomic sequences. Due to the fast RNA extraction procedure we used, we systematically coextracted DNA that gave false positives (data not shown). Other authors previously mentioned this problem (9, 20), even though some others did not observe the same phenomenon (18). The importance of the RT step was highlighted by performing RT-PCR on lysate without DNase I and RT-PCR on lysate without DNase I but with RNase (Fig. 1, lanes 4 and 5, respectively). In the second case, DNA only is responsible for the PCR product, giving a weaker signal.
FIG. 1.
Gel electrophoresis of RT-PCR products of the EF-1α gene of S. cerevisiae suspended in milk at 103 cells ml−1. Lane 1, negative control without cells; lane 2, RT-PCR control with pure extracted total RNA; lane 3, DNase I control (PCR on lysate digested with DNase I); lane 4, RT-PCR on lysate without DNase I; lane 5, RT-PCR on lysate without DNase I and with RNase; lanes M, 1-kb ladder (Gibco BRL).
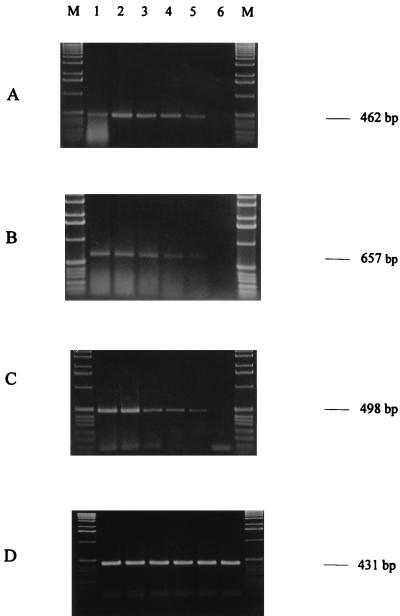
We then tried to detect E. coli, S. cerevisiae, and M. racemosus suspended at serial concentrations ranging from 105 to 1 cell ml−1 in milk. With all the strains, it was possible to detect the elongation factor at contaminant concentrations as low as 10 cells ml−1, corresponding to 5 to 10 cells per reaction tube in our procedure (Fig. 2A to C, lanes 5). No transcripts were detected at lower contaminant concentrations and in noncontaminated milk (Fig. 2A to C, lanes 6 and 7, respectively). The same intensity of specific band from T. aestivum total RNA suggests the level of RT-PCR is roughly the same in each dilution (Fig. 2D). We used the same method on contaminated beer and yogurt, and microorganisms were detected with the same level of sensitivity.
FIG. 2.
Gel electrophoresis of RT-PCR products of the EF-Tu gene of E. coli (A), of the EF-1α gene of S. cerevisiae (B) or M. racemosus (C), or of 0.1 μg of total RNA of T. aestivum with S. cerevisiae (D) suspended in milk at serial 10-fold dilutions from 105 cells ml−1 (lane 1) to 1 cell ml−1 (lane 6). Lanes M, 1-kb ladder (Gibco BRL).
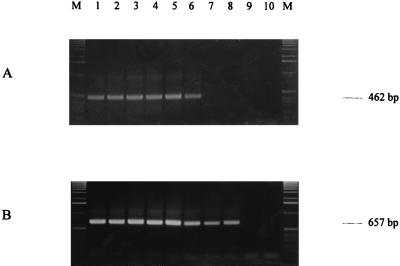
The correlation between appearance of RT-PCR products and cell viability after heat treatment was verified by performing a time course experiment during heat treatment at 60°C. One half of the cells was used for our RT-PCR procedure and the other half was used for plating. For E. coli, no amplified products of EF-Tu mRNA were detected 6 min after heat treatment (Fig. 3A, lane 7), and no colonies were detected on plates after 4 min. For S. cerevisiae, no amplified products of EF-1α mRNA were detected 8 min after heat treatment (Fig. 3B, lane 9) and no colonies were detected on plates after 2 min. We thus conclude that detection of mRNA for EF-Tu or EF-1α is appropriate for measuring cell viability and that the half-lives of these mRNAs are less than 6 min for EF-Tu and less than 8 min for EF-1α after heat treatment.
FIG. 3.
Gel electrophoresis of RT-PCR products of the EF-Tu gene of E. coli (A) or of the EF-1α gene of S. cerevisiae (B) suspended in milk at 104 to 105 cells ml−1 after 0 min (lane 1) to 9 min (lane 10) at 60°C; lanes M, 1-kb ladder (Gibco BRL).
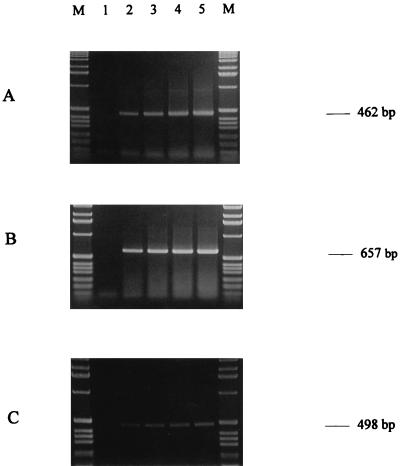
The RT-PCR procedure was then applied to a mix of living and dead cells after heat treatment of E. coli, S. cerevisiae, and M. racemosus in contaminated milk. When all cells had died (verified by plating), RT-PCR detection was negative (Fig. 4, lanes 1), indicating that the RT-PCR method developed in this study appears to detect only living cells. Although production of quantitative results is quite difficult by conventional RT-PCR, since the yield of both amplification (7) and reverse transcription (6) steps can be grossly variable in different reactions, the gel electrophoresis analysis showed a quantitative increase in the intensity of the specific band as the number of viable cells increased (Fig. 4, lanes 2 to 5), indicating the apparent increase in RT-PCR target DNA as the number of viable cells increased.
FIG. 4.
Gel electrophoresis of RT-PCR products of the EF-Tu gene of E. coli (A) or of the EF-1α gene of S. cerevisiae (B) or M. racemosus (C) suspended in milk at 104 to 105 cells ml−1. Lane 1, 100% dead cells; lane 2, 25% dead cells and 75% live cells; lane 3, 50% (each) live and dead cells; lane 4, 75% live cells and 25% dead cells; lane 5, 100% live cells; lanes M, 1-kb ladder (Gibco BRL).
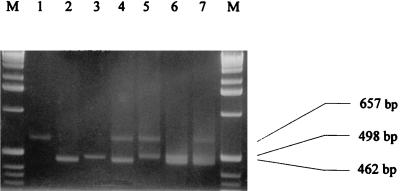
In some case, foods are contaminated by several microorganisms of different families and different kingdoms. Therefore, it seemed helpful to develop a procedure in which various contaminants could be detected simultaneously in a simple step. In this way, we performed a simultaneous RT-PCR detection of the EF-Tu gene of E. coli and the EF-1α gene of S. cerevisiae and M. racemosus in one reaction. Using milk contaminated with E. coli, S. cerevisiae, and M. racemosus, the RT-PCR-amplified products of expected size (Table 1) were simultaneously detected when two (Fig. 5, lanes 4 to 6) or three (Fig. 5, lane 7) pairs of primers were placed in the same reaction mixture without modification of RT-PCR conditions.
FIG. 5.
Gel electrophoresis of RT-PCR products of the EF-Tu gene of E. coli and of the EF-1α gene of S. cerevisiae and M. racemosus obtained in a single RT-PCR. All cells were suspended in milk at 102 cells ml−1. Lane 1, yeast primers; lane 2, bacterial primers; lane 3, mold primers; lane 4, bacterial and yeast primers; lane 5, yeast and mold primers; lane 6, bacterial and mold primers; lane 7, bacterial, mold, and yeast primers; lanes M, 1-kb ladder (Gibco BRL).
RT-PCR is a powerful method in the detection of viable contaminants due to its high potential to increase detection sensitivities and its speed. The detection level was 10 cells ml of milk−1, and the described RT-PCR setting, including washing and filtering of the milk sample, could be carried out in 4 h without need of a preenrichment step. The choice of the target gene is of importance. Unlike other genes (9), the use of primers specific for the coding region of the elongation factor gene seems to be reasonable for several reasons. First, the elongation factor gene may be considered as an appropriate viability marker since inactivation of both the prokaryotic and eukaryotic corresponding genes is a lethal event (2, 4). Second, the elongation factor gene encodes one of the most abundant prokaryotic and eukaryotic proteins (8, 17), allowing a considerable increase in the sensitivity level. Third, the function conserved throughout prokaryotes and eukaryotes as well as the similarity of primary structures (10) of the elongation factor allows the modulation of the specificity of detection. As far as we know this study is the first description of simultaneous detection of viable organisms belonging to two kingdoms. Fourth, due to the short mRNA half-lives, detecting elongation factor mRNA would indicate the presence of living cells that had been present within about the last 6 to 8 min after heat treatment, depending on the microorganism studied.
We successfully transposed the procedure described in this study to use with yogurt and beer. This method can be used for the detection of viability after heat treatment of other contaminants in other foods, provided appropriate primers and food pretreatment are available. We are currently investigating more precise quantitative analysis by real-time quantitative RT-PCR.
Acknowledgments
We thank R. Leitz for providing microorganisms and E. Perrin and J.-M. Simonet for critically reading the manuscript.
REFERENCES
- 1.Blackburn C D W. A review: rapid and alternative methods for the detection of salmonellas in food. J Appl Bacteriol. 1993;75:199–214. doi: 10.1111/j.1365-2672.1993.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 2.Bosh L, Kraal B, Van der Meide P H, Duistervinkel F J, van Noort J M. The elongation factor EF-Tu and its two encoding genes. Prog Nucleic Acid Res Mol Biol. 1983;30:91–126. doi: 10.1016/s0079-6603(08)60684-4. [DOI] [PubMed] [Google Scholar]
- 3.Cooray K J, Nishibori T, Xiong H, Matsuyama T, Fujita M, Mitsuyama M. Detection of multiple virulence-associated genes of Listeria monocytogenes by PCR in artificially contaminated milk samples. Appl Environ Microbiol. 1994;60:3023–3026. doi: 10.1128/aem.60.8.3023-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotrelle P, Cool M, Thuriaux P, Price V L, Thiele D, Buhler J M, Fromageot P. Either one of the two yeast EF-1α genes is required for cell viability. Curr Genet. 1985;9:693–697. doi: 10.1007/BF00449823. [DOI] [PubMed] [Google Scholar]
- 5.Dubois E, Le Guyader F, Haugarreau L, Kopecka H, Cormier M, Pommepuy M. Molecular epidemiological survey of rotaviruses in sewage by reverse transcriptase seminested PCR and restriction fragment length polymorphism assay. Appl Environ Microbiol. 1997;63:1794–1800. doi: 10.1128/aem.63.5.1794-1800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferre F. Quantitative or semi-quantitative PCR: reality versus myth. PCR Methods Appl. 1992;2:1–9. doi: 10.1101/gr.2.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Frye R A, Benz C C, Liu E. Detection of amplified oncogenes by differential polymerase chain reaction. Oncogene. 1989;4:1153–1157. [PubMed] [Google Scholar]
- 8.Gordon J. Regulation of the in vivo synthesis of the polypeptide chain elongation factors in Escherichia coli. Biochemistry. 1970;9:912–917. doi: 10.1021/bi00806a028. [DOI] [PubMed] [Google Scholar]
- 9.Herman L. Detection of viable and dead Listeria monocytogenes by PCR. Food Microbiol. 1997;14:103–110. [Google Scholar]
- 10.Ludwig W, Weizenegger M, Betzl D, Leidl E, Lenz T, Ludvigsen A, Möllenhoff D, Wenzig P, Schleifer K H. Complete nucleotide sequences of seven eubacterial genes coding for the elongation factor Tu: functional, structural and phylogenetic evaluations. Arch Microbiol. 1990;153:241–247. doi: 10.1007/BF00249075. [DOI] [PubMed] [Google Scholar]
- 11.Merrick W C. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortimer R K, Johnston J R. Genealogy of principle strains of the yeast genetic stock center. Genetics. 1986;113:35–45. doi: 10.1093/genetics/113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochelle P A, Ferguson D M, Handojo T J, De Leon R, Stewart M H, Wolfe R L. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of waterborne Cryptosporidium parvum. Appl Environ Microbiol. 1997;63:2029–2037. doi: 10.1128/aem.63.5.2029-2037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roszak D B, Colwell R R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth B L, Martin P, Yue S T, Millard P J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–2431. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Schirmaier F, Philippsen P. Identification of two genes coding for the translation elongation factor EF-1α of S. cerevisiae. EMBO J. 1984;3:3311–3315. doi: 10.1002/j.1460-2075.1984.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tebbe C C, Wenderoth D F, Vahjen W, Lübke K, Munch J C. Direct detection of recombinant gene expression by two genetically engineered yeasts in soil on the transcriptional and translational levels. Appl Environ Microbiol. 1995;61:4296–4303. doi: 10.1128/aem.61.12.4296-4303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson R C. EF-Tu provides an internal kinetic standard for translational accuracy. Trends Biochem Sci. 1988;13:91–93. doi: 10.1016/0968-0004(88)90047-3. [DOI] [PubMed] [Google Scholar]
- 20.Tong J, Bendahhar S, Chen H, Agnew W S. A simplified method for single RT-PCR that can detect and distinguish genomic DNA and mRNA transcripts. Nucleic Acids Res. 1994;22:3253–3254. doi: 10.1093/nar/22.15.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weijland A, Harmark K, Cool R H, Anbagh P H, Parmeggiani A. Elongation factor Tu: a molecular switch in protein synthesis. Mol Microbiol. 1992;6:683–688. doi: 10.1111/j.1365-2958.1992.tb01516.x. [DOI] [PubMed] [Google Scholar]