Abstract
Background
Loss to follow-up (LTFU) and death are unfavorable outcomes of human immunodeficiency virus (HIV) treatment. This study aimed to identify the predictors of LTFU and death among individuals with newly diagnosed HIV receiving dolutegravir (DTG)–based first-line antiretroviral treatment (ART) in eastern Ethiopia.
Methods
A multisite prospective cohort study was carried out between October 2020 and July 2022. New case patients who started ART were enrolled consecutively and then followed up for the next 6 months. A structured questionnaire and checklists were used to collect data. HIV viral load was determined using the Abbott RealTime HIV-1 assay. Bivariable and multivariable logistic regression models were used to identify baseline factors associated with the outcomes.
Results
A total of 235 people with newly diagnosed HIV were enrolled; 16.6% (95% confidence interval, 12.3%–21.9%) were lost to follow-up, and 5.9% (3.5%–9.8%) died within 6 months of follow-up. Baseline World Health Organization clinical stage I (adjusted odds ratio, 3.93 [95% confidence interval, 1.34–11.57]), low viral load (3.67 [1.09–12.36]), and body weight (1.04 [1.01–1.07]) were predictors of LTFU, whereas nonfunctional status (10.02 [1.9–51.3]) was the only factor associated with death.
Conclusions
LTFU and death rates among patients with DTG were relatively high, accounting for roughly a quarter of the attrition of people with newly diagnosed HIV from ART care and services. Thus, targeted interventions are required to reduce LTFU and death among individuals with HIV on ART. Further investigation is necessary to evaluate the long-term effects of DTG-based regimens on LTFU and its impact on HIV mortality rates, and qualitative research, specifically tracing LTFU, is recommended.
Keywords: death, dolutegravir, eastern Ethiopia, loss to follow-up, newly diagnosed people with HIV
To address the 2030 human immunodeficiency virus (HIV) global goals and provide a full package of amenities, optimal retention in care is crucial in HIV treatment and care programs [1]. Retention in care is also a paramount component for achieving the second and third 95% Joint United Nations (UN) Programme on HIV/AIDS (UNAIDS) targets [2], improving quality of life, decreasing HIV/AIDS-related morbidity and mortality rates, reducing resistance to antiretrovirals, and lowering the costs of treating opportunistic infections [3–5].
However, both loss to follow-up (LTFU) and death are unfavorable outcomes of HIV care and treatment, which are serious public health issues [4]. LTFU increases the risks of drug resistance, HIV transmission, disease, early death, and treatment failure [6]. The burden of LTFU among patients with HIV is predicted to be significant in low- and middle-income countries, posing a serious risk to coordinated efforts to end the HIV epidemic by achieving near-universal antiretroviral treatment (ART) [7]. In Africa, the proportion of LTFU ranged from 8.9% to 24.6% in Uganda [8, 9], 17.3% in Debre Markos [10], and 40.8% in the Bichena, Amhara region, Northwest Ethiopia [11].
There are several definitions of LTFU. While some studies define LTFU as a discontinuation of ART for ≥3 months for various reasons [12, 13], others define it as 1 month [14], 2 months [15], or 12 months of discontinuation [16]. In our study, LTFU was defined as not receiving ART for >1 month after a previous missed drug collection session. It is unknown whether these patients had transferred their care services elsewhere, died, or had other causes of LTFU.
On the other hand, death from HIV/AIDS-related illness is common and mortality rates are still high, especially in sub-Saharan Africa [2, 17, 18]. This is true even though highly active ART significantly increases the survival rate of PWH. Sociodemographic, behavioral, baseline clinical, immunological, virological, and institutional factors have all been identified as having an impact on the incidence of death and LTFU in sub-Saharan African countries, including Ethiopia [6, 17–19].
The magnitude of LTFU and deaths as well as the contributing factors vary across countries, regions, populations, and health facilities. Ethiopia is currently aiming to achieve the three 95% UN targets for ending the AIDS epidemic. Hence, estimating the magnitude and identification of predictors of LTFU and death is essential to pinpoint key intervention areas and enhance viral suppression to improve the quality of life of people with HIV (PWH). To the best of our knowledge, data derived from a prospective cohort study on dolutegravir (DTG) treatment are lacking. The current study aimed to identify the predictors of LTFU and death among individuals with newly diagnosed HIV receiving DTG-based first-line ART in eastern Ethiopia.
METHODS
Study Design, Setting, and Period
A multisite health facility-based prospective cohort study was conducted among individuals with newly diagnosed HIV receiving DTG-based first-line ART at selected public health facilities in eastern Ethiopia. The study was carried out between October 2020 and July 2022. The study included 15 health facilities from Harari and Somali regional states, Dire Dawa city, and the East and West Hararghe zones of the Oromia region. Treatment and follow-up care were based on the national ART treatment guidelines for HIV infections in Ethiopia.
Study Population and Sampling
The study population consisted of all individuals with newly diagnosed HIV who started DTG-based first-line ART per the current national test and treat guidelines in the selected health facilities. This study was nested within a larger study that aimed to determine HIV pretreatment drug resistance among individuals with newly diagnosed HIV who were initiating ART. Therefore, the World Health Organization (WHO) sample size determination concept for HIV pretreatment drug resistance was used to calculate the sample size [20]. Accordingly, the cohort included 235 individuals with newly diagnosed HIV starting DTG-based first-line ART. Consecutive sampling was applied to enroll clients with HIV in the selected health facilities. The cohort had 2 data collection phases, at baseline and during follow-up. During the baseline data collection phase, study participants were enrolled consecutively, and in the follow-up phase, the cohort was followed up during ART for the next 6 months. Eligible individuals who had already started ART before blood sample collection and those who were critically ill at enrollment were excluded from the study.
Data Collection, Procedures, and Outcomes
At baseline, a structured questionnaire and checklists were used to collect sociodemographic factors, clinical data, laboratory parameters, and information on the first-line ART regimen. Individual interviews were conducted in person and privately with study participants or caregivers/guardians of children/adolescents <18 years old by trained nurses, after informed consent. Participants provided approximately 5 mL of blood samples for the baseline viral load (VL) test at the start of ART and again at 6 months, as recommended by the national guidelines. Plasma was separated from whole blood according to standard operating procedures and transported to the Harari Health Research and Regional Laboratory in Harar, Ethiopia, for HIV-1 RNA VL testing. To extract HIV-1 RNA and determine plasma VL, we used the Abbott m2000sp automated sample preparation system and the Abbott m2000rt system with the quantitative Abbott RealTime HIV-1 assay (Abbott Molecular), respectively.
The main outcome factors in this study were LTFU and death within 6 months. Here, the LTFU category includes any patient who was either not known to have died or had been receiving treatment elsewhere but was absent at the official treatment facility during the regular refilling time where the patient had been registered. After the last missed appointment, the patient had not been seen at the ART clinic for ≥30 days, and healthcare providers were unable to reach them by phone (contact address). Death was determined using patients’ medical records and registrations at the ART clinic reported by the case manager during case tracing period.
Data Analysis
The data were coded and entered using EpiData Manager software (version 4.6.0.4). For data analysis, STATA/SE software (version 14.0; StataCorp) was used. Descriptive statistics were used to summarize the sociodemographic and clinical characteristics of the study participants. Bivariable and multivariable logistic regression models were used to identify the baseline factors associated with LTFU and death at 6 months. All variables with a P value ≤.25 from bivariable analyses were included in the multivariable logistic regression for further analysis. In multivariable logistic regression analysis, a variable with a P value <.05 was considered a significant factor associated with the outcomes at a 95% confidence level.
Ethical Clearance
Ethical clearance was obtained from the Institutional Health Research and Ethics Review Committee of the College of Health and Medical Sciences, Haramaya University (reference no. IHRERC/202/2020) and the Armauer Hansen Research Institute/ALERT Ethics Research Committee (AAERC PO/22/20). Each participant signed a written consent form. To maintain confidentiality, participants’ records and information were deidentified and anonymized before analysis.
RESULTS
Baseline Characteristics and Follow-Up Status of Participants
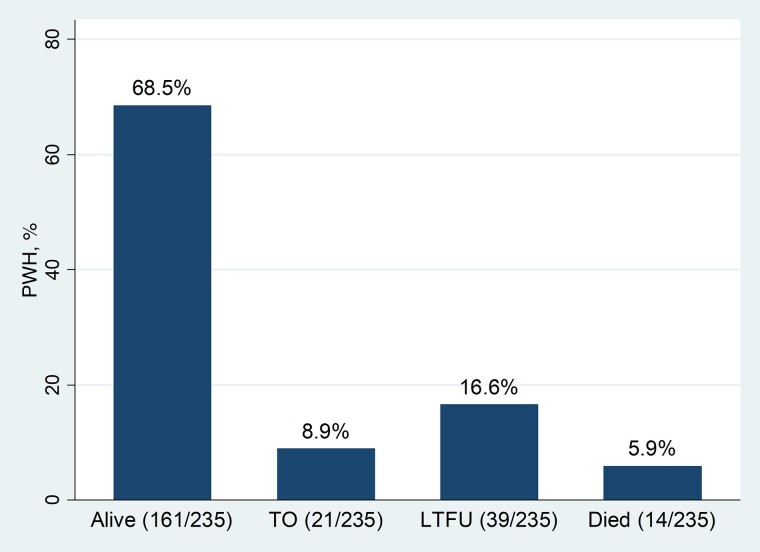
A total of 235 patients with newly diagnosed HIV were enrolled in this cohort, of whom 70.6% were female. The mean age of the participants was 33.9 years (standard deviation, 12.1 years), with a range of 2–70 years. Details of the study participants’ characteristics have been reported elsewhere [21]. Figure 1 shows that 16.6% (95% confidence interval [CI], 12.3%–21.9%) of the patients had LTFU and 5.9% (3.5%–9.8%) had died at the end of the study period.
Figure 1.
Treatment outcomes among people with newly diagnosed human immunodeficiency virus (PWH) after 6 months of follow-up in Eastern Ethiopia. Abbreviations: LTFU, loss to follow-up; TO, transferred out.
Baseline Characteristics and Predictors of LTFU
Overall, the characteristics of participants with LTFU were similar to those of participants still under care. However, as depicted in Table 1, the following variables showed variation in clients with LTFU compared with those retained and active in care. This included the absence of baseline comorbid conditions (20.9% vs 79.0%), WHO clinical stage I (25.2% vs 74.8%), baseline VL ≤1000 copies/mL (30.6% vs 69.4), and baseline mean weight of (58.5 vs 52.6 kg) at ART initiation. Of those with LTFU, approximately three-fourths (76.9%), 84.6%, and 56.4% of the participants were female, WHO clinical stage I, and baseline VL ≤1000 copies/mL, respectively.
Table 1.
Baseline Characteristics and Predictors of Loss to Follow-up at 6 Months Among People with Newly Diagnosed Human Immunodeficiency Virus in Eastern Ethiopia, 2020–2021 (N = 235)
| Characteristic | Total No. (%)a | LTFU at 6 mo, No. (%)a | Bivariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|---|
| Yes | No | COR (95% CI) | P Value | AOR (95% CI) | P Value | ||
| Sex | |||||||
| Male | 69 (29.4) | 9 (13.1) | 60 (86.9) | 1.00 | … | … | … |
| Female | 166 (70.6) | 30 (18.1) | 136 (81.9) | 1.47 (.65–3.28) | .35 | … | … |
| Age group, y | |||||||
| ≤24 | 50 (21.3) | 11 (22.0) | 39 (78.0) | 1.58 (.72–3.45) | .25 | 1.87 (.77–4.51) | .16 |
| >24 | 185 (78.7) | 28 (15.1) | 157 (84.9) | 1.00 | … | … | >.99 |
| Health facility type | |||||||
| Hospital | 176 (74.9) | 28 (15.9) | 148 (84.1) | 1.00 | … | … | … |
| Health center | 59 (25.1) | 11 (18.6) | 48 (81.4) | 1.21 (.56–2.61) | .62 | … | … |
| Any comorbid condition at baseline | |||||||
| Yes | 92 (39.2) | 9 (9.8) | 83 (90.2) | 1.00 | … | … | 1.00 |
| No | 143 (60.8) | 30 (20.9) | 113 (79.0) | 2.45 (1.10–5.43) | .03b | 1.46 (.53–3.97) | .46 |
| Functionality status | |||||||
| Functional | 187 (79.6) | 32 (17.1) | 155 (82.9) | 1.21 (.49–2.94) | .68 | … | … |
| Nonfunctional | 48 (20.4) | 7 (14.6) | 41 (85.4) | 1.00 | … | … | … |
| Tuberculosis history | |||||||
| Yes | 40 (17.0) | 7 (17.5) | 33 (82.5) | 1.00 | … | … | … |
| No | 195 (82.9) | 32 (16.4) | 163 (83.6) | 0 .9 (.37–2.27) | .87 | … | … |
| INH eligibility | |||||||
| Yes | 170 (87.2) | 28 (16.5) | 142 (83.5) | 1.04 (.33–3.24) | .95 | … | … |
| No | 25 (12.8) | 4 (16.0) | 21 (84.0) | 1.00 | … | … | … |
| Cotrimoxazole prophylaxis | |||||||
| Yes | 148 (62.9) | 27 (18.2) | 121 (81.8) | 1.39 (.67–2.92) | .38 | … | … |
| No | 87 (37.0) | 12 (13.8) | 75 (86.2) | 1.00 | … | … | … |
| WHO clinical stage | |||||||
| I | 131 (55.7) | 33 (25.2) | 98 (74.8) | 5.5 (2.21–13.72) | <.001b | 3.93 (1.34–11.57) | .01b |
| II–IV | 104 (44.3) | 6 (5.8) | 98 (94.2) | 1.00 | … | … | >.99 |
| Same-day ART initiation | |||||||
| Yes | 171 (72.8) | 32 (18.7) | 139 (81.3) | 1.87 (.78–4.49) | .16 | 1.37 (.52–3.58) | .51 |
| No | 64 (27.2) | 7 (10.9) | 57 (89.1) | 1.00 | … | … | >.99 |
| History of substance use | |||||||
| Yes | 83 (35.3) | 16 (19.3) | 67 (80.7) | 1.34 (.66–2.70) | .42 | … | … |
| No | 152 (64.7) | 23 (15.1) | 129 (84.9) | 1.00 | … | … | … |
| Baseline VL category, copies/mL | |||||||
| ≤1000 | 72 (30.6) | 22 (30.6) | 50 (69.4) | 5.61 (1.8–17.4) | .003b | 3.67 (1.09–12.36) | .04b |
| 1001–10 000 | 51 (21.7) | 7 (13.7) | 44 (86.3) | 2.03 (.56–7.39) | .28 | 1.37 (.34–5.38) | .65 |
| 10 001–100 000 | 57 (24.3) | 6 (10.5) | 51 (89.5) | 1.5 (.39–5.63) | .55 | 1.38 (.35–5.44) | .65 |
| >100 000 | 55 (23.4) | 4 (7.3) | 51 (92.7) | 1.00 | … | … | >.99 |
| Baseline body weight, mean (SD), kg | 53.6 (11.9) | 58.5 (9.0) | 52.6 (12.2) | 1.05 (1.01–1.08) | .006b | 1.04 (1.01–1.07) | .02b |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; INH, isoniazid; LTFU, loss to follow-up; SD, standard deviation; VL, viral load; WHO, World Health Organization.
aData represent no. (%) of participants unless otherwise identified as mean (SD).
bSignificant at P < .05.
Bivariable logistic regression analysis revealed that the following baseline factors increased the likelihood of LTFU: absence of comorbid conditions (crude odds ratio [COR], 2.45 [95% CI, 1.10–5.43]), WHO stage I (5.5 [2.21–13.7]), low baseline VL (≤1000 copies/mL) (5.61 [1.8–117.4]), and high baseline body weight (higher than sample mean) (1.05 [1.10–5.43]) (Table 1).
Similarly, multivariable logistic regression analysis showed that WHO stage I (adjusted odds ratio [AOR], 3.93 [95% CI, 1.34–11.57]), low baseline VL (≤1000 copies/mL) (3.67 [1.09–12.36]), and baseline body weight (1.04 [1.01–1.07]) were independently associated with LTFU at 6 months. The odds of LTFU at the end of the study were about 4 times higher among HIV-positive participants presenting with WHO clinical stage I during ART initiation compared with the other WHO stages. Likewise, PWH with low baseline VL had approximately 4-fold higher odds of LTFU than those who had high baseline VL copies/mL. Moreover, the odds of LTFU within 6 months of the study period also increased by 4% as the average baseline body weight increased by 1 kg (compared with mean baseline body weights of the participants and those retained in the care) (Table 1).
Baseline Characteristics and Predictors of Death
Among participants who died, there were higher proportions of comorbid conditions (11.9%), nonfunctional status (20.8%), and advanced WHO clinical stage (9.6%) during ART initiation (Table 2). According to the bivariable logistic regression, the following were significantly associated with mortality rate: the presence of a comorbid condition (COR, 6.3 [95% CI, 1.72–23.4]), functional status (ambulatory or bedridden) (12.04 [3.6–40.4]), advanced WHO stages (3.37 [1.03–11.1]), and baseline VL (>100 000 copies/mL) (10.4 [1.23–86.8]). However, after adjustment, only clients with HIV who were nonfunctional status at baseline had higher odds of death than those in the working functionality group (AOR, 10.02 [95% CI, 1.9–51.3]) (Table 2).
Table 2.
Baseline Characteristics and Predictors of Death within 6 Months Among People With Newly Diagnosed Human Immunodeficiency Virus in Eastern Ethiopia, 2020–2021 (N = 235)
| Characteristic | Total No. (%)a | Death at 6 mo, No. (%)a | Bivariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|---|---|---|
| Yes | No | COR (95% CI) | P Value | AOR (95% CI) | P Value | ||
| Sex | |||||||
| Male | 69 (29.4) | 7 (10.1) | 62 (89.9) | 2.56 (.86–7.61) | .09 | 3.56 (.97–13.0) | .054 |
| Female | 166 (70.6) | 7 (4.2) | 159 (95.8) | 1.00 | … | … | >.99 |
| Age group, y | |||||||
| ≤ 30 | 102 (43.3) | 3 (2.9) | 99 (97.1) | 1.00 | … | … | >.99 |
| >30 | 133 (56.6) | 11 (8.3) | 122 (91.7) | 2.97 (.81–10.9) | .10 | 2.00 (.48–8.23) | .34 |
| Health facility type | |||||||
| Hospital | 176 (74.9) | 13 (7.4) | 163 (92.6) | 4.6 (.59–36.14) | .14 | 1.67 (.16–17.3) | .67 |
| Health center | 59 (25.1) | 1 (1.7) | 58 (98.3) | 1.00 | … | … | >.99 |
| Any comorbid condition at baseline | |||||||
| Yes | 92 (39.2) | 11 (11.9) | 81 (88.1) | 6.3 (1.72–23.38) | .006b | 1.67 (.22–12.7) | .62 |
| No | 143 (60.8) | 3 (2.1) | 140 (97.9) | 1.00 | … | … | >.99 |
| Functionality status | |||||||
| Functional | 187 (79.6) | 4 (2.1) | 183 (97.9) | 1.00 | … | … | >.99 |
| Nonfunctional | 48 (20.4) | 10 (20.8) | 38 (79.2) | 12.04 (3.6–40.4) | >.001b | 10.02 (1.9–51.3) | .01b |
| Tuberculosis history | |||||||
| Yes | 40 (17.0) | 3 (7.5) | 37 (92.5) | 1.35 (.36–5.10) | .65 | … | … |
| No | 195 (82.9) | 11 (5.6) | 184 (94.4) | 1.00 | … | … | … |
| Cotrimoxazole prophylaxis | |||||||
| Yes | 148 (62.9) | 9 (6.1) | 139 (93.9) | 1.00 | … | … | … |
| No | 87 (37.0) | 5 (5.8) | 82 (94.2) | 0.94 (.31–2.91) | .92 | … | … |
| WHO clinical stage | |||||||
| Stage I | 131 (55.7) | 4 (3.1) | 127 (96.9) | 1.00 | … | … | >.99 |
| Stage II–IV | 104 (44.3) | 10 (9.6) | 94 (90.4) | 3.37 (1.03–11.1) | .045b | 0.27 (.03–2.72) | .27 |
| Same-day ART initiation | |||||||
| Yes | 171 (72.8) | 10 (5.8) | 161 (94.2) | 1.00 | … | … | … |
| No | 64 (27.2) | 4 (6.2) | 60 (93.8) | 1.07 (.32–3.55) | .91 | … | … |
| History of substance use | |||||||
| Yes | 83 (35.3) | 6 (7.2) | 77 (92.8) | 1.40 (.47–4.18) | .54 | … | … |
| No | 152 (64.7) | 8 (5.3) | 144 (94.7) | 1.00 | … | … | … |
| Baseline VL category, copies/mL | |||||||
| ≤1000 | 72 (30.6) | 1 (1.4) | 71 (98.4) | 1.00 | … | … | >.99 |
| 1001–10 000 | 51 (21.7) | 3 (5.9) | 48 (94.1) | 4.4 (.45–43.9) | .20 | 5.5 (.42–72.2) | .19 |
| 10 001–100 000 | 57 (24.3) | 3 (5.3) | 54 (94.7) | 3.9 (.39–38.9) | .24 | 2.2 (.17–27.4) | .55 |
| >100 000 | 55 (23.4) | 7 (12.7) | 48 (87.3) | 10.4 (1.23–86.8) | .03b | 5.5 (.46–65.6) | .18 |
| Baseline body weight, mean (SD), kg | 53.6 (11.9) | 51.8 (13.9) | 53.7 (11.8) | 0.98 (.94–1.03) | .57 | … | … |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; COR, crude odds ratio; SD, standard deviation; VL, viral load; WHO, World Health Organization.
aData represent no. (%) of participants unless otherwise identified as mean (SD).
bSignificant at P < .05.
DISCUSSION
In this cohort, the LTFU proportion was 16.6%. Low baseline VL, WHO stage I disease, and higher body weight were significantly associated with LTFU. During the 6-month follow-up period, 5.9% of the patients died. The only predictor associated with death was the baseline nonfunctional status of the patients.
The LTFU in the first 6 months of ART initiation was high in our study, consistent with findings of retrospective studies undertaken in various parts of Ethiopia: Debre Markos (17.6%) [10], Jigjiga (14.8%) [22], Hadiya zone (20.8%) [23], and Tanzania (11.2%–17%) [24]. This figure, however, is lower than that in previous studies conducted in different corners of Ethiopia and African countries. Two studies from Ethiopia found greater rates of LTFU from HIV care services, 40.8% [11] and 22.6% [25]. Similarly, significant LTFU was reported from Tanzania (26.4%–42.2%) [24, 26], Burundi (25.3%) [27], South Africa (28%) [28], and the Democratic Republic of Congo (26.5%–28.8%) [6]. On the other hand, LTFU in the current study was higher than that reported in previous studies conducted elsewhere in Ethiopia, including 9.0% in Woldia [29] and 9.1% in Arba Minch [30].
The variation In LTFU could be attributed to the population's heterogeneity, lack of a standard definition of LTFU, duration of the follow-up period included in the study, sample size, situation of the study settings, disparity in strategies used to track patients, and baseline sociodemographic and clinical factors [6, 31]. Moreover, the variation might be due to the differences in advancement of ART, improvement in comprehensive care, and performance of healthcare services [32, 33]. Furthermore, the studies found that the likelihood of LTFU was high in the first 6 months after ART initiation. This could be explained by the fact that the likelihood of interrupting antiretroviral drugs and developing drug resistance will increase owing to drug adverse effects and noncompliance [34]. This, in turn, increases the liability to LTFU, making it a persistent challenge for ART programs in sub-Saharan Africa [31]. In our study, the coronavirus disease 2019 (COVID-19) pandemic may have contributed to the increased LTFU because the study period coincided with the introduction of COVID-19 in Ethiopia, which disrupted all HIV services [35].
In the current study, those with newly diagnosed HIV who were WHO clinical stage I at enrollment were more prone to LTFU. This finding was concordant with that of a report from Miza-Aman, Ethiopia [36], which found that advanced WHO clinical stages were associated with lower LTFU. A possible reason for this is that more focused good adherence and palliative care might be given to patients with advanced WHO stages, which might help them to be retained in care and services. Those with WHO stage I may feel as if they are in good health and, as a result, may drop out of care or be silently transferred to another facility. This finding contrasts with those of previous studies from Pawi, Ethiopia [25], and Burundi [27], which found advanced WHO stages to be predictors of LTFU. This might be because patients at WHO stages 3 or 4 are less likely to visit the ART center owing to their poor health, and they are also more likely to die at home and remain unreported, increasing LTFU during the follow-up period.
In the current study, a low baseline VL was a predictor of LTFU. The odds were 3.6 times higher compared with high baseline VLs. This finding, however, is in contradiction with previous findings from other studies in Ethiopia [29]. The discrepancy may be due to differences in study design and the time when the VL was measured. In the previous study, the study design was retrospective, using available data from the registration logbook, including routine VLs performed at 6 months or annually according to the guidelines. Thus, PWH are aware of their VL status and appreciate the value of good adherence to lowering VL and reducing the risk of attrition.
There are 2 plausible explanations for this discrepancy. First, in the current study, the VL test was done during ART initiation, and the reference category for VL was different (current vs previous study; 100 000 vs 1000 copies/mL). Second, in our study approximately 31% of the individuals with newly diagnosed HIV had low baseline VL at ART inception. These people might not actually be newly infected but rather dropouts from another facility who rejoined the current health facility as a new HIV case patient. Consequently, the chances of their revisiting the facility might be low. This falsely increases the number of new HIV infections and, in parallel, increases LTFU. These factors have their own effects on national planning, policy, and HIV prevention and control programs. Thus, a feasible strategy should be in place during the enrollment of PWH into ART care to overcome this challenge.
Furthermore, it was found that for every kilogram that the average baseline body weight increased in the current study, the odds of LTFU within the first 6 months of ART initiation increased by 4%. This finding is congruent with that of a study conducted in Arba Minch [30]. A possible reason could be that patients with a higher body weight may be more susceptible to noncommunicable diseases. This could affect their retention in care and adherence, and because of these chronic noncommunicable diseases they might have a suboptimal adherence level, which is a predictor of LTFU [26]. Our findings from the univariate analysis suggest that the presence of baseline comorbid conditions is associated with decreased LTFU (Table 1), signifying that individuals with comorbid conditions are more likely to stay in care. In other words, individuals with comorbid conditions were more likely to die, which is why they were not categorized as LTFU (COR, 6.3; P = .006) (Table 2). Either way, the increased LTFU at higher weights may reflect a healthier set of individuals who do not need close follow-up (as with individuals presenting with WHO clinical stage I). Therefore, further data are required to reach a consensus on this argument.
The overall mortality rate of PWH, in the current study is in concordance with findings of studies conducted in Addis Ababa (8.9%) [37], Gondar, Northwest (5.93%) [38], and the Debre Markos Referral Hospital (4.8%–8.9%) [39] in Ethiopia. However, it is lower than that reported in Afar (11.7%) [40], Debre-Berhan (12.1%) [41], and Addis Ababa, Ethiopia (20%–46%) [37]. This could be because the current study had a higher proportion of patients in the early stages (WHO clinical stage I; 55.7%). Moreover, the difference is also due to variations in the sample size, length of the study period, initiation of the ART regimen, and time.
According to the findings of this study, individuals with nonfunctional status had a higher likelihood of death than those who were in functional status group at treatment initiation (AOR, 10.02 [95% CI, 1.9–51.3]]. Many studies done in Ethiopia, including study conducted in Debre Markos [adjusted hazard ratio, 6.11 [95% CI, 2.42–15.41]) [39], Debre-Berhan (3.069 [1.111–8.480]) [41], and Afar (5.91 [2.71–12.88), [40], , have found a strong association between ambulatory/bedridden functional status and the survival of patients on ART. This could be because of poor immunological response and an advanced clinical stage of the disease associated with the worst health conditions and complications. This, in turn, increases the likelihood of death compared with that in patients with a working functional status [42].
In sum, based on our findings and the three 95% targets of UNAIDS and Ethiopia, the 2 adverse outcomes of HIV treatment and care —LTFU and death —were both relatively high. Thus, in order to meet the defined goals in Ethiopia, the implementation of effective, feasible, and sound strategies to reduce the high risk of LTFU and early death is warranted. One option would be to introduce and strengthen baseline VL tests for all HIV-positive people linked to ART. Furthermore, targeted counseling and follow-up, efforts to increase the availability of low-cost assays for baseline VL measurement, optimizing ART and care to limit premature deaths, and making extra efforts to reengage patients with LTFU to prevent health impairments and reduce HIV transmission in the community are recommended.
This study had its own strengths and drawbacks. The strengths of this study include its focus on newly diagnosed clients with HIV and the use of patient-level primary data collected in real time. Unlike many previous retrospective studies, this analysis included the baseline VL to assess its association with LTFU and early death. Moreover, the study findings shed light on the magnitude and factors related to LTFU and early death among patients on DTG-based first-line ART. One of the study's shortcomings was that the follow-up period was relatively short. LTFU could be overestimated by patients who enrolled in other health facilities following self-referral or unreported deaths. On the other hand, the mortality rate might be underestimated by the fact that patients with LTFU and dropout may have died at home without being reported. The study also did not examine the potential predictors of death, such as immunological profiles, malnutrition, and adherence to ART. However, these constraints had no significant impact on this study's findings. The findings emanating from this study are practical and may help to develop strategies to reduce LTFU and mortality risk. Thus, these issues should be addressed to meet the UNAIDS targets.
In conclusion, LTFU and death rates among patients receiving DTG-based ART in this study were relatively high, accounting for roughly a quarter of the attrition of PWH from ART care and services. WHO clinical stage I, low baseline VL, and higher body weight are baseline predictors of LTFU, whereas the baseline nonfunctional status of the patients is the only factor associated with death after 6 months of follow-up. Thus, focused interventions are required to reduce early LTFU and death among individuals with HIV on ART, as well as to meet the UNAIDS targets of 95–95-95 and the 2030 aim of ending the HIV epidemic as a public health problem. Further investigation is necessary to evaluate the long-term effect of DTG-based regimens on LTFU and its impact on HIV mortality rates, and qualitative research, specifically tracing patients with LTFU, is recommended.
Acknowledgments
The authors are grateful to Haramaya University and the Armauer Hansen Research Institute for their financial support during data collection. They thank the Harari Health Research and Regional Laboratory and staff for supporting the viral load tests and acknowledge the support of the data collectors from each health facility and study participants for their participation. They also thank Hussein Mohammed for his technical and professional support with the data analysis.
Contributor Information
Abdella Gemechu, School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Adane Mihret, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Abraham Aseffa, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Rawleigh Howe, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Berhanu Seyoum, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Andargachew Mulu, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Author contributions. Study conception and data collection: A. G. Manuscript design and interpretation of results: All authors. All authors have seen and approved the final version of the manuscript.
Disclaimer. The funding sources had no role in study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit it for publication.
Financial support. This work was supported by the Haramaya University postgraduate directorate and the Armauer Hansen Research Institute (support for data collection).
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS) . Global AIDS update 2016. Geneva, Switzerland: UNAIDS; 2019. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS) . Global HIV & AIDS statistics—2020 fact sheet. Available at: https://www.unaids.org/en/resources/fact-sheet. Accessed 10 July, 2023. 2020.
- 3. Koole O, et al. Reasons for missing antiretroviral therapy: results from a multi-country study in Tanzania, Uganda, and Zambia. PLoS One 2016; 11:e0147309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, July 2017. Geneva: World Health Organization; 2017. [PubMed] [Google Scholar]
- 5. Bain LE, Nkoke C, Noubiap JJN. UNAIDS 90–90–90 targets to end the AIDS epidemic by 2020 are not realistic: comment on “Can the UNAIDS 90–90–90 target be achieved? A systematic analysis of national HIV treatment cascades.” BMJ Global Health 2017; 2:e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buju RT, Akilimali PZ, Kamangu EN, et al. Incidence and predictors of loss to follow up among patients living with HIV under dolutegravir in Bunia, Democratic Republic of Congo: a prospective cohort study. Int J Environ Res Pub Health 2022; 19:4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frijters EM, Hermans LE, Wensing AMJ, et al. Risk factors for loss to follow-up from antiretroviral therapy programmes in low-income and middle-income countries. AIDS 2020; 34:1261–88. [DOI] [PubMed] [Google Scholar]
- 8. Asiimwe SB, Kanyesigye M, Bwana B, et al. Predictors of dropout from care among HIV-infected patients initiating antiretroviral therapy at a public sector HIV treatment clinic in sub-Saharan Africa. BMC Infect Dis 2015; 16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiwanuka J, Mukulu Waila J, Muhindo Kahungu M, et al. Determinants of loss to follow-up among HIV positive patients receiving antiretroviral therapy in a test and treat setting: a retrospective cohort study in Masaka, Uganda. PLoS One 2020; 15:e0217606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birhanu MY, Leshargie CT, Alebel A, et al. Incidence and predictors of loss to follow-up among HIV-positive adults in northwest Ethiopia: a retrospective cohort study. Trop Med Health 2020; 48:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Telayneh AT, Tesfa M, Woyraw W, et al. Time to lost to follow-up and its predictors among adult patients receiving antiretroviral therapy retrospective follow-up study Amhara Northwest Ethiopia. Sci Rep 2022; 12:2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Federal Ministry of Health . National guidelines for comprehensive HIV prevention, care and treatment. Addis Ababa, Ethiopia: Federal Ministry of Health; 2017. [Google Scholar]
- 13. Teshome W, Belayneh M, Moges M, et al. Do loss to follow-up and death rates from ART care vary across primary health care facilities and hospitals in south Ethiopia? a retrospective follow-up study. HIV AIDS (Auckl) 2015; 7:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tadesse K, Fisiha H. Predictors of loss to follow up of patients enrolled on antiretroviral therapy: a retrospective cohort study. J AIDS Clin Res 2014; 5:2. [Google Scholar]
- 15. Mehari D, Mache T, Hailemariam L. Predictors of lost to follow up to antiretroviral therapy in primary public hospital of Wukro, Tigray, Ethiopia: a case control study. J AIDS HIV Res 2015; 7:1–9. [Google Scholar]
- 16. Melaku Z, Lamb MR, Wang C, et al. Characteristics and outcomes of adult Ethiopian patients enrolled in HIV care and treatment: a multi-clinic observational study. BMC Pub Health 2015; 15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aemro A, Wassie M, Chekol B. Incidence and predictors of mortality within the first year of antiretroviral therapy initiation at Debre-Markos Referral Hospital, Northwest Ethiopia: a retrospective follow up study. PLoS One 2021; 16:e0251648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buju RT, Akilimali PZ, Tran N-T, et al. Determinants of survival of HIV patients receiving dolutegravir: a prospective cohort study in conflict-affected Bunia, Democratic Republic of Congo. Int J Environ Res Pub Health 2022; 19:10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kebede HK, Mwanri L, Ward P, et al. Predictors of lost to follow up from antiretroviral therapy among adults in sub-Saharan Africa: a systematic review and meta-analysis. Infect Dis Poverty 2021; 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Surveillance of HIV drug resistance in populations initiating antiretroviral therapy (pre-treatment HIV drug resistance): concept note. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 21. Gemechu A, Mihret A, Atire FA, et al. Virological non-suppression among newly diagnosed HIV-positive individuals on dolutegravir-based antiretroviral treatment in eastern Ethiopia: follow-up study. Trop Med Infect Dis 2023; 8:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seifu W, Ali W, Meresa B. Predictors of loss to follow up among adult clients attending antiretroviral treatment at Karamara General Hospital, Jigjiga town, Eastern Ethiopia, 2015: a retrospective cohort study. BMC Infect Dis 2018; 18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bikoro B, Oljira L, Gobena T, et al. Incidence and predictors of loss to follow-up among human immunodeficiency virus-infected adult patients on anti-retroviral therapy at Hadiya zone public hospitals, southern Ethiopia: a retrospective cohort study. J Pub Health 2022; 30:229–40. [Google Scholar]
- 24. Tesha E-D, Kishimba R, Njau P, et al. Predictors of loss to follow up from antiretroviral therapy among adolescents with HIV/AIDS in Tanzania. PLoS One 2022; 17:e0268825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Assemie MA, Muchie KF, Ayele TA. Incidence and predictors of loss to follow up among HIV-infected adults at Pawi General Hospital, northwest Ethiopia: competing risk regression model. BMC Res Notes 2018; 11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mushy SE, Mtisi E, Mboggo E, et al. Predictors of the observed high prevalence of loss to follow-up in ART-experienced adult PLHIV: a retrospective longitudinal cohort study in the Tanga region, Tanzania. BMC Infect Dis 2023; 23:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nshimirimana C, Ndayizeye A, Smekens T, et al. Loss to follow-up of patients in HIV care in Burundi: a retrospective cohort study. Trop Med Int Health 2022; 27:574–82. [DOI] [PubMed] [Google Scholar]
- 28. Chauke P, Huma M, Madiba S. Lost to follow up rate in the first year of ART in adults initiated in a universal test and treat programme: a retrospective cohort study in Ekurhuleni district, South Africa. Pan Afr Med J 2020; 37:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dejen D, Jara D, Yeshanew F, et al. Attrition and its predictors among adults receiving first-line antiretroviral therapy in Woldia town public health facilities, northeast Ethiopia: a retrospective cohort study. HIV AIDS (Auckl) 2021; 13:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gebremichael MA, Gurara MK, Weldehawaryat HN, et al. Predictors of loss to follow-up among HIV-infected adults after initiation of the first-line antiretroviral therapy at Arba Minch General Hospital, Southern Ethiopia: a 5-year retrospective cohort study. Biomed Res Int 2021; 2021:8659372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aliyu A, Adelekan B, Andrew N, et al. Predictors of loss to follow-up in art experienced patients in Nigeria: a 13 year review (2004–2017). AIDS Res Ther 2019; 16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bantie B, Abate MW, Nigat AB, et al. Attrition rate and its predictors among adults receiving anti-retroviral therapy following the implementation of the “universal test and treat strategy” at public health institutions in northern Ethiopia: a retrospective follow-up study. Heliyon 2022; 8:e11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gumede SB, Venter F, de Wit J, et al. Antiretroviral therapy uptake and predictors of virological failure in patients with HIV receiving first-line and second-line regimens in Johannesburg, South Africa: a retrospective cohort data analysis. BMJ Open 2022; 12:e054019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fisiha Kassa S, Zemene Worku W, Atalell KA, et al. Incidence of loss to follow-up and its predictors among children with HIV on antiretroviral therapy at the University of Gondar comprehensive specialized referral hospital: a retrospective data analysis. HIV AIDS (Ackl) 2020; 12:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chilot D, Woldeamanuel Y, Manyazewal T. COVID-19 burden on HIV patients attending antiretroviral therapy in Addis Ababa, Ethiopia: a multicenter cross-sectional study. Front Med 2022; 9:741862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berheto TM, Haile DB, Mohammed S. Predictors of loss to follow-up in patients living with HIV/AIDS after initiation of antiretroviral therapy. N Am J Med Sci 2014; 6:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tesfayohannes S, et al. Mortality and its predictors among adult human immune-deficiency virus-infected patients attending their antiretroviral treatment at health centers, Addis Ababa, Ethiopia: multicenter retrospective cohort study. AIDS Res Treat 2022; 2022:6128718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teka Z, Mohammed K, Workneh G, et al. Survival of HIV/AIDS patients treated under ART follow-up at the University hospital, northwest Ethiopia. Environ Health Prev Med 2021; 26:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Birhanu H, Alle A, Birhanu MY. Rate and predictors of mortality among adults on antiretroviral therapy at Debre Markos Referral Hospital, north west Ethiopia. HIV AIDS (Ackl) 2021; 13:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salih AM, Yazie TS, Gulente TM. Survival analysis and predictors of mortality among adult HIV/AIDS patients initiated antiretroviral therapy from 2010 to 2015 in Dubti General Hospital, Afar, Ethiopia: A retrospective cohort study. Heliyon 2023; 9:e12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nigussie F, Alamer A, Mengistu Z, et al. Survival and predictors of mortality among adult HIV/AIDS patients initiating highly active antiretroviral therapy in Debre-Berhan referral hospital, Amhara, Ethiopia: a retrospective study. HIV AIDS (Ackl) 2020; 12:757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chernet AG, Biru MD. Survival analysis of HIV/AIDS patients under ART follow up in Attat Referral Hospital. Sci J Appl Math Stat 2020; 8:42–6. [Google Scholar]