Abstract
Owing to the inherent shortcomings of traditional therapeutic drugs in terms of inadequate therapeutic efficacy and toxicity in clinical treatment, nanomedicine designs have received widespread attention with significantly improved efficacy and reduced non-target side effects. Nanomedicines hold tremendous theranostic potential for treating, monitoring, diagnosing, and controlling various diseases and are attracting an unfathomable amount of input of research resources. Against the backdrop of an exponentially growing number of publications, it is imperative to help the audience get a panorama image of the research activities in the field of nanomedicines. Herein, this review elaborates on the development trends of nanomedicines, emerging nanocarriers, in vivo fate and safety of nanomedicines, and their extensive applications. Moreover, the potential challenges and the obstacles hindering the clinical translation of nanomedicines are also discussed. The elaboration on various aspects of the research trends of nanomedicines may help enlighten the readers and set the route for future endeavors.
Key words: Nanomedicine, Drug delivery, Nanoparticles, Nanocarriers, Trigger-responsive, In vivo fate, Protein corona, Clinical translation
Graphical abstract
This review elaborates on the development trends of nanomedicines, emerging nanocarriers, in vivo fate and safety of nanomedicines, and their extensive applications.
1. Introduction
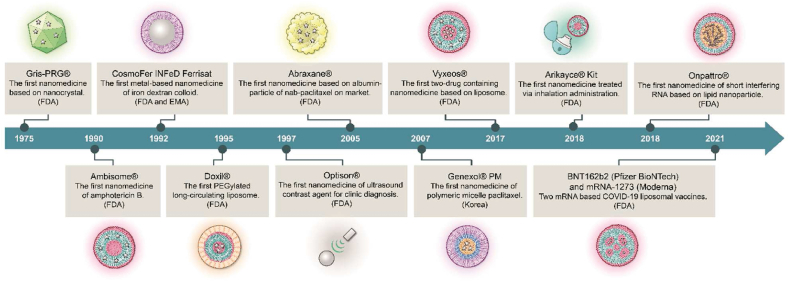
Nanomedicines are generally defined as medicines that apply nanotechnology and are intended for therapeutic or diagnostic applications with dimensions controlled within the nanoscale range (1–1000 nm)1. In pharmaceutical sciences, nanomedicines refer to the use of nanotechnologies in producing active pharmaceutical ingredients (APIs) as nanoscale particles or combining APIs with suitable nanomaterials to produce nanoscale particles further formulated into versatile dosage forms. The nanomedicine market is dominated by applications based on drug delivery, surpassing regenerative medicine, diagnosis both in vitro and in vivo, and vaccine-oriented applications. For cancers and infectious, cardiac, and orthopedic disorders, almost 40% of phase II clinical trials are based on nanomedicines2. Searching the database of ClinicalTrials.gov using the keyword “nanomedicine”, over 500 active clinical trials involving nanoparticles are found by the end of 2022. So far, the US Food and Drug Administration (FDA) of the United States has approved more than 60 nanomedicines3. Fig. 1 illustrates the milestones in the development of nanomedicines.
Figure 1.
Historical timeline of major nanomedicine development.
Against the backdrop of tremendous input into the development of nanomedicine products, basic, translational, and product-oriented research is flourishing too. A huge number of nanocarrier drug delivery systems (NDDSs) prepared from various materials, including lipids, polymers, proteins, metals, and inorganic materials, have been reported over the past decades4, 5, 6, 7, 8, 9, 10. NDDSs work through versatile mechanisms in effectuating transporting across biobarriers; however, targeted delivery is always the top quest. Passive targeting is frequently combined with active targeting to optimize drug delivery to diseased sites such as tumors and minimize non-target drug distribution. Active targeting is commonly attained by surface modification of nanocarriers with ligands recognizing and binding with receptors expressed on membranes of target cells11, 12, 13, 14, 15, 16. Actively and passively targeted nanomedicines have been exploited as imaging tools, in addition to therapeutics, in recent years with impressive preclinical and clinical potentials14,17, 18, 19, 20, 21, 22.
Albeit prosperous in research, most NDDSs under investigation get lost in translation owing to one or more issues of polymer/carrier toxicity, poor manufacturability, instability, and difficulties in quality control. Even though problems have been actively addressed, little is known about the in vivo fate and underlying mechanisms, which hampers the successful translation of NDDSs into commercial products. It is crucial to unveil the biological (in vivo and subcellular) fate by exploring where, when, and how the fundamental components of NDDSs interact with the body and with each other23, 24, 25, 26, 27.
This review aims to update the research trends of nanomedicines. In addition to pinpointing the development trends by data analysis and introducing emerging nanocarriers and trigger-responsive mechanisms, we also discuss the in vivo fate and safety issues of nanomedicines, as well as their potential in treating various diseases. This review aims to provoke discussion which may benefit the translation of notions into clinical products.
2. Bibliographic analysis
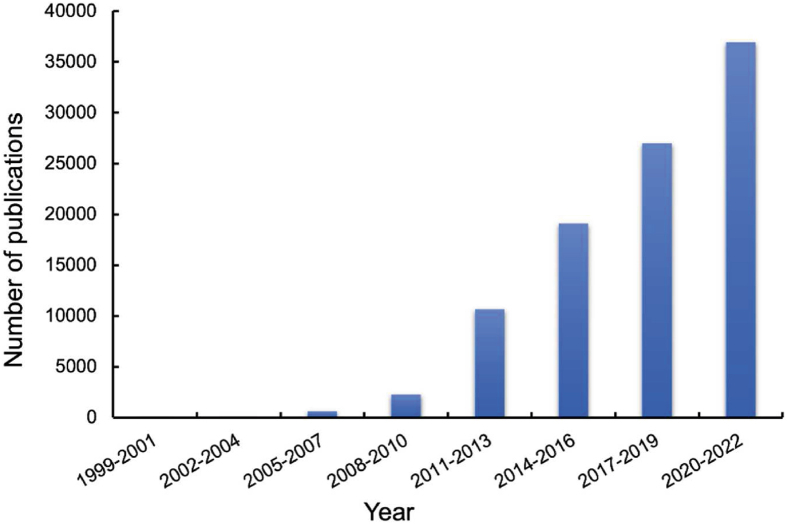
Increasingly attention has been paid to nanomedicines, and the number of products under clinical investigations is growing rapidly. A search of the database "Web of Science" using the keyword “nanomedicine∗” from Jan 1999 to Dec 2022 gives a total number of 100,192 publications with 80% published from 2015 to April 13, 2023 (Fig. 2). Meanwhile, more and more efforts in developing advanced nanomedicines to further enhance therapeutic efficacy and reduce side effects is still in full swing.
Figure 2.
The trend of publications related to nanomedicines over the past decades. Data are derived from the Web of Science utilizing the keyword “nanomedicine∗”.
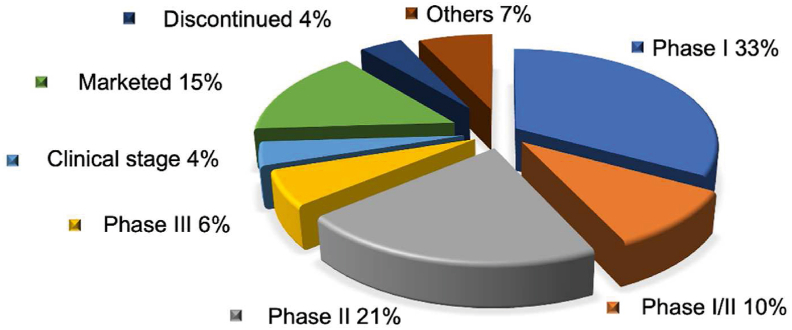
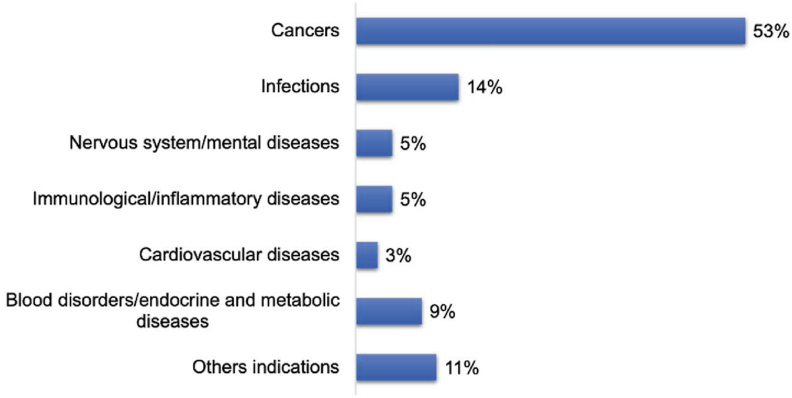
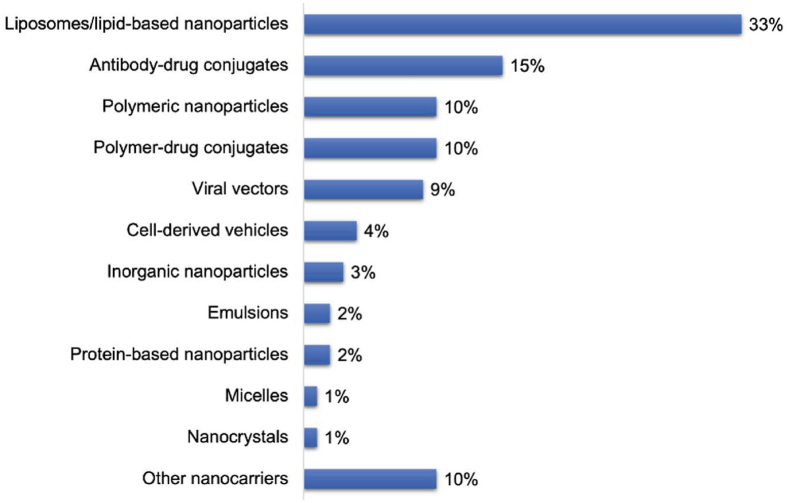
To date, more than 60 nanomedicines have been marketed. Over 500 nanomedicine-related products are in the stages of clinical trials (Data records are retrieved from ClinicalTrials.gov and the Cortellis Drug Discovery Intelligence database). Most of them are in phase I (33%) or II (21%) clinical trials for the treatment of cancer (53%), infectious diseases (14%) (Fig. 3), and other diseases (Fig. 4), such as inflammation and those inflicting the circulatory, immunological, nervous, cardiovascular, mental, endocrine and metabolic systems. Liposomes/lipid nanoparticles comprise the biggest fraction of carriers (33%), antibody-drug conjugates the second with 15%, polymeric nanoparticles, polymer-drug/protein conjugates, and viral vectors the third with 9%‒10% each, and each of other carriers with less than 5% (Fig. 5). The success of lipid-based ones, such as liposomes and lipid nanoparticles, is mainly attributed to the superior biocompatibility and biodegradability conferred by their endogenous compositions or derivatives. Other drug carriers, such as polymeric micelles, are hot in earlier research but have a low translation rate, owing to issues of safety, stability, mass production, and quality control. The most successful nanomedicines target cancer and infectious disease treatment. For the treatment of the COVID-19 pandemic, nanomedicines were considered promising strategies for developing new vaccines against coronavirus. For instance, to meet the urgent need in 2020, two types of lipid-based messenger RNA (mRNA) vaccines (Pfizer BioNTech Vaccine and Moderna COVID-19 Vaccine) were launched because they could effectively deliver antigens, mimic the structure of the coronavirus, and serve as adjuvant ideally28,29.
Figure 3.
Statistics of nanomedicines marketed and in different stages of clinical trials.
Figure 4.
Indications of nanomedicines on the market or in clinical trials.
Figure 5.
The percent (%) of nanomedicine types on the market or in clinical trials.
3. Emerging nanocarriers
Nanomedicines with special biological effects have been widely applied in various diseases for treatment30, monitoring31, diagnosis32, and controlled drug release33, as conventional chemical drugs present prevalent defects, including insufficient accumulation in specific lesion sites,34 rapid metabolic clearance in circulation35, and extensive systemic toxicity36 Owing to the unique properties of a specific nanostructure or nanomaterial37, such as nanoscale particle size38 and particular surface modifications39, nanocarriers have been endowed with a legion of advantages, for example, increasing the selectivity of drug delivery40, improving drug solubility and stability41, synergizing delivery of multiple drugs42, reforming bioavailability,43 mediating sustained or controlled drug release44, enhancing therapeutic efficiency, and reducing side effects45
Over the past decades, nanocarriers of diverse structures and constituents such as liposomes46,47, nanocrystals,48, 49, 50, 51, 52 carrier-free nanoassemblies,53 albumin nanoparticles54, polymeric micelles55, and nanoemulsions56 have been intensively investigated both in basic research and commercial translation57 Liposomes (Doxil®, 1995) are the first nanocarriers approved by FDA. The treatment spectra of liposomes have expanded into various diseases with drug payloads covering a range of small molecules and biomacromolecules (e.g., peptides, genes, monoclonal antibodies, etc.)58, 59, 60.
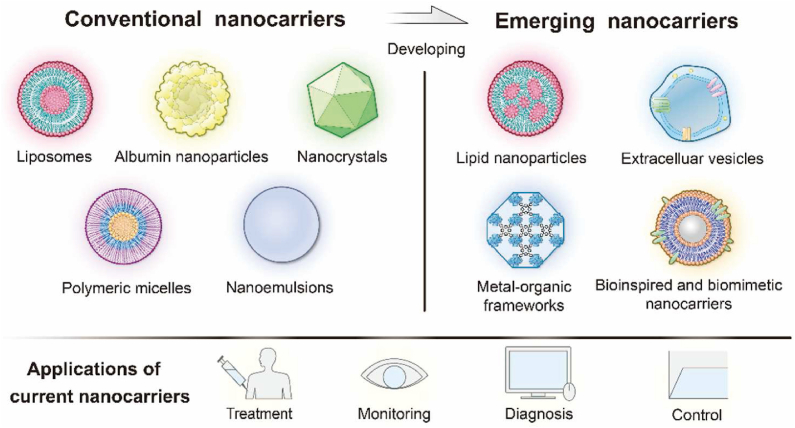
In company with continuing advances in nanotechnology and a rapidly growing nanomedicine market, more and more nanomedicines with enhanced therapeutic efficacy have been approved57 In addition, the emergence of advanced natural/synthetic materials61 and novel nanocarriers such as lipid-based nanoparticles62, extracellular vesicles63, bioinspired and biomimetic nanocarriers64, metal–organic framework (MOF)65 and so on broaden the horizon of nanomedicine research and development. Herein, examples are given to illustrate the current applications of emerging nanocarriers (Fig. 6).
Figure 6.
Overview of development from conventional nanocarriers to new nanocarriers and their applications.
3.1. Lipid nanoparticles
In recent years, lipid nanoparticles (LNPs) have reinvigorated interests and redeveloped to tackle unmet needs in the delivery of more challengeable entities, notably mRNA and deoxyribonucleic acid (DNA) antigens66, oligonucleotides67, and CRISPR68 LNPs integrate the advantages of conventional colloidal nanocarriers while negating some related drawbacks. Conventional LNPs are generally suitable for encapsulating and delivering lipophilic drugs and well adapted for administration via different routes, including transdermal, dermal, intramuscular, mucosal, and ocular routes. Through optimization of constituting lipids and preparative techniques, as well as functional surface modifications, LNPs could be engineered with appealing characteristics69: 1) improved biocompatibility; 2) optimized drug encapsulation capacity; 3) harmonized co-encapsulation of both hydrophilic and hydrophobic drugs; 4) reduced toxicity; 5) controlled or targeted delivery of therapeutic agents.
LNPs-based drug delivery has been used to treat various diseases, including cancer70, infections71, and those inflicting the cardiovascular72, neural73, and dermal systems74 via different routes (e.g., oral, parenteral, ocular, topical, brain, and pulmonary routes). Some of them, exemplified by Onpattro®, have been approved for clinical use as systemic delivery platforms75 A recent study reported a novel LNP-based mRNA delivery system rationally constructed by mitigating surface charge from neutral to anionic, illustrating the potential to selectively target hepatic reticuloendothelial system (RES) via the comprehensive in vivo biodistribution and clearance mechanism of LNPs76 Also, LNPs can target the spleen and lungs by replacing standard helper lipids with anionic (phosphatidylserine, phosphatidylglycerol, and phosphatidic acid) or cationic (1,2-dioleoyl-3-trimethylammonium propane (DOTAP) and ethyl phosphatidylcholine) lipids77
Furthermore, LNPs played a vital role in developing vaccines against COVID-19, highlighting the powerful potential of delivering nucleic acid-based drugs and vacciness78 The keys to this success rest with the tremendous impact of LNP components, including structure, size, apparent pKa, stability, encapsulation efficiency, cellular uptake, and endosomal escape79 Despite the numerous advantages of LNPs in drug delivery, the translation from preclinical concepts to industrial scale-up production still lags behind80 Stable, reliable, and reproducible scale-up methods are in urgent need for LNP production.
3.2. Extracellular vesicles
Extracellular vesicles (EVs) are heterogeneous submicron cell-derived nanoparticles that function as critical mediators in intercellular communication and many (patho)physiological processes81,82 EVs are generally nanosized (40‒1000 nm) and comprised primarily of lipid membranes, with various specific molecules embedded, including proteins, nucleic acids, metabolites, and enzymes83 Engineered EVs function to deliver their payloads to nearby and distant recipient cells84 Defined by their endosomal origin and size, EVs are typically categorized into three subpopulations: 1) apoptotic bodies (50‒5000 nm), which are generated by fragmentation from cells undergoing programmed death85, 2) microvesicles (100‒1000 nm) which are derived from specific types of endosomes and can fuse with the plasma membranes to release their content in the extracellular spaces85, and 3) exosomes (40‒150 nm) which are derived from multivesicular bodies86. As phospholipid membrane vesicles derived from various cells that play a key role in antigen presentation and cell–cell communication, EVs are often utilized to encapsulate and deliver biopharmaceuticals, such as proteins, antigens, microRNAs (miRNAs) and mRNAs8.
EVs have drawn considerable attention and emerged as potential drug delivery carriers, owing to their bestowed biocompatibility and ability to circumvent biobarriers8. For instance, EVs show the potential to cross the blood‒brain barrier (BBB) and blood‒brain tumor barrier (BBTB), one of the most intriguing and challenging obstacles in brain delivery. Niu et al.86 fabricated natural EVs-based nanoparticles decorated with doxorubicin (DOX)-loaded heparin-based nanoparticles (EV-DNs). Through EVs-mediated receptor-dependent transcytosis and membrane fusion, EV-DNs could bypass BBB/BBTB and penetrate tumor tissues, significantly promoting tumor accumulation, cellular internalization, and antiproliferation efficiency86. In addition, various functionalized modifications have been engineered on EVs to reinforce their natural features87.
So far, several clinical trials of EVs-based therapeutic agents have been completed or are underway to investigate their in vivo therapeutic efficacy and safety88. Some exosomes loaded with tumor peptides/drugs were involved in Phase I clinical trials to treat advanced non-small cell lung cancer, metastatic melanoma, colorectal cancer, etc89. However, even though extensive studies have demonstrated the promise of EVs-based drug delivery systems, clinical translation of EVs-based therapeutics remains challenging, owing to obstacles such as low drug loading, low isolation yield, potential safety concerns, and considerable complexity90. Meanwhile, insights into the mechanisms that endow EVs with effective immune escape, intracellular uptake, and targeted delivery are needed to unlock their full potential63,91.
3.3. Bioinspired and biomimetic nanocarriers
Biomimetic drug delivery systems (BDDSs), inspired by “natural camouflage" strategies, recently emerged to overcome defects of conventional synthetic nanocarriers, such as insufficient targeting selectivity92, easy recognition and clearance by the RES93, inadequate biocompatibility94, poor tumor penetration and intracellular internalization6, and excessive systemic toxicity95. BDDSs can mimic natural cells and viruses by regulating size, surface charge, shape, and material consistency64. Various mammalian cell-, virus- and bacteria/fungus-inspired drug delivery systems are increasingly employed in biomedicine and targeted drug delivery96. To date, bioinspired and biomimetic carriers have been developed as promising tools for drug delivery because they can mimic features of biological constituents and systems97. Through the novel strategy of “articles used as homing systems” that takes inspiration from biological molecules, organisms, and cells, these carriers can deliver the therapeutic agents to the targeted site more specifically than conventional nanocarriers98.
Biomimetic or bioinspired nanocarriers are constructed by integrating biomimetic surface functionalization of synthetic nanocarriers and natural cell-derived or bioinspired artificial membranes. They inherit the plasticity and flexibility of synthetic materials and the diverse biological functionalities of biomimetic materials99. Many endogenous materials, including erythrocytes100, leukocytes, tumor cells, macrophages, and neutrophils, are used as “self-camouflage" modifications in constructing biomimetic nanocarriers. Biomimicking endows conventional nanocarriers with significantly enhanced biocompatibility, prolonged in vivo circulation, and the ability to target specific pathological sites101, 102, 103. Song et al.103 fused platelet membranes onto poly(lactic-co-glycolic acid) (PLGA) nanoparticles to treat atherosclerosis. The membrane-coated nanoparticles demonstrated higher affinity to the plaques and significantly enhanced targeting efficiency in lesions than the naked nanoparticles. Given the simplified endogenous functions of vectors limited by a single cell type, multiple cell-membrane fusing is always used. Bu et al.104 modified iron oxide magnetic nanoparticles using hybridized cancer stem cell and platelet membranes for dual-targeting tumors highly expressing CD44.
Increasing BDDSs focus on targeted drug delivery, aiming at transporting drugs into the cytoplasm and reducing toxicity to circulation, organs, and tissues105. In particular, the emergence of biomimetic or bioinspired nanocarriers promotes the application of drug delivery because they are promising to solve the problems regarding biological drugs, insoluble drugs, and those with high toxicity106. However, their translation remains challenging107. For instance, the clinical trial of biomimetic nanocarriers always suffers from tough checks compared to traditional small molecular drugs108. Furthermore, continuous scale-up production presents another obstacle to translation109.
3.4. Metal-organic framework
MOFs, advanced hybrid porous structures constructed by metal ions/clusters and organic linkers, have received broad attention as emerging nanocarriers in the past decade65,110. MOFs have become promising drug delivery platforms owing to their advantages, including well-defined composition and structure, ultra-high surface area and porosity, adjustable pore size, biocompatibility, low toxicity, and versatile functionality111. MOF has flexible variable and adjustable properties and thus can be used as nanocarriers to load various types of drugs112. The MOFs with hydrophobic pores, such as the first MOF carrier-MIL (Materials of Institut Lavoisier), are suitable for encapsulating drug molecules with poor aqueous solubility113. Conversely, MOFs with hydrophilic pores can carry either positive or negative charges and can be applied to load the charged-active compounds114. For instance, Zhao et al.115 constructed MOF nanoparticles (ZIF-8) for miRNA delivery. The nanoparticles significantly enhanced miRNA stability, released miRNA responsively in lysosomes, and induced apoptosis of tumor cells via the generation of reactive oxygen species triggered by Zn2+ 115. Hitherto, the porous structures impart MOF-based nanocarriers a superior capacity to load small molecular, macromolecular, or biomolecular cargoes and realize controlled drug release.
In addition, various surface chemical modifications have been utilized to enhance suspending stability, protect against degradation, and improve the delivery efficiency of MOF-based nanocarriers116. Zhuang et al.117 coated MOFs with the platelet membranes by physical co-extrusion to develop platelet membrane-cloaked porous MOFs, aiming to load a small-interfering RNA (siRNA) (P-MOF-siRNA) for specific gene slicing therapy. The results indicated that P-MOF-siRNA had natural tumor-homing characteristics and biocompatibility and significantly enhanced the delivery efficiency and siRNA stability117.
Collectively, MOFs exhibit the huge potential to act as drug carriers and MOF–synergistic drug systems118. The porous structure makes MOFs candidates for drug loading and flexible modification due to the multi-selectivity of metal ions and ligands119. However, several challenges remain for their clinical translation. For instance, drug molecules incorporated by ordinary pore encapsulation or surface adsorption suffer from gradual leakage due to insufficient interaction forces. The covalent binding provides stronger interactions; however, the complex synthetical procedures may compromise the efficacy of functional molecules120. Moreover, their fundamental understanding is yet to be discovered, e.g., metabolism, excretion, and toxicity121.
4. Trigger-responsive designs in nanomedicines
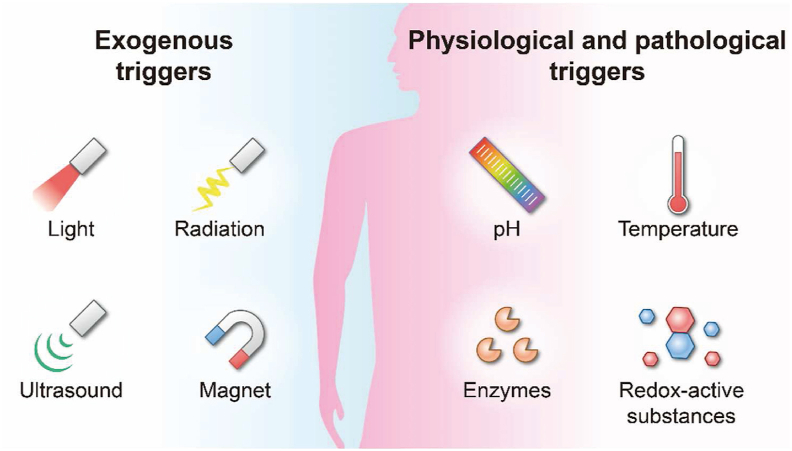
Nanomedicines hold tremendous potential for the selective delivery of therapeutic agents and efficient treatment of various diseases2,122. Surface modification is widely used to elevate nanoparticle selectivity. Incorporating trigger-responsive materials is always utilized to improve delivery and tackle systemic and intracellular drug delivery barriers123. The triggers can be classified as exogenous (e.g., light, radiation, ultrasound, and magnet)124 and physiological/pathological triggers (e.g., pH, temperature, enzyme, redox, and hypoxia)125, as summarized in Fig. 7.
Figure 7.
Mechanisms involved in responsive nanomedicines. Physiological and pathological triggers include pH, temperature, enzymes, redox, etc. Exogenous triggers include light, radiation, ultrasound, magnet, etc.
4.1. Exogenous triggers
4.1.1. Light
Light has been extensively investigated as an attractive trigger for responsive nanomedicines, whose strength could be adjusted by modulating specific areas, wavelengths, and irradiation power. Various nanocarriers (e.g., liposomes126, polymeric micelles127, poly-ion complex vesicles128, and nanorods129) have been designed with light- or photo-responsiveness to simultaneously improve the treatment efficiency and minimize the potential damage to normal tissues. In general, nanomedicines could respond to light triggers via several mechanisms: 1) disassemble nanocarriers or reversibly assemble nanoparticles by breaking light-sensitive chemical bonds130; 2) induce responsive drug release at target sites131; 3) activate temperature changes in photothermal therapy (PTT)132; 4) generate reactive oxygen species (ROS) in photodynamic therapy (PDT)133; 5) mediate light-activated imaging for multi-mode theranostics134.
Light-triggering is promising in controlling drug delivery and precise treatment135. In a recent study, a near-infrared (NIR) photo-controlled spherical nucleic acid (PSNA) was reported for delivery of siRNA and antisense oligonucleotide (ASO)136. Upon irradiation by NIR light, singlet oxygen (1O2) was produced by the inner photosensitizer in tumor cells, disassembling PSNA by breaking the 1O2-cleavable linker between siRNA and ASO136. In addition, light can also promote drug delivery by intervening in primitive biological functions. For example, Vickerman et al.137 developed light-triggered-engineered red blood cells (RBC) for targeted delivery of proteins, using light-induced RBC hemolysis and subsequent drug release. The surface modification with a dormant hemolytic peptide enabled the RBCs to have prolonged circulation time and selectively deliver coagulating enzymes under spatiotemporal control137. However, using light-responsive nanomedicines is always limited by insufficient sensitivity and poor tissue penetration of common light138.
4.1.2. Magnet
Nanocarriers constructed by magnet-responsive materials can respond to magnetic triggers non-invasively under an external magnetic field139. Magnet-triggering could mediate targeted drug delivery and generate local hyperthermia for responsive drug release or PTT140. So far, various magnet-responsive NDDSs have been investigated, such as liposomes141, polymeric micelles142, polymer nanoparticles143, superparamagnetic iron-oxide nanoparticles (SPIO)144, magnetic nanogels145, and magnetic nanoclusters146. Recently, Chung et al.147 developed magnet-responsive nitrogen oxide (NO)-releasing materials conjugated with MOF-derived porous Fe3O4 nanocomposites for selective activation of NO and treatment of bacteria-infected cutaneous wounds. The results demonstrated burst or intermittent NO-releasing using continuous or pulsatile magnet stimulation147.
Magnet-responsive nanomedicines are often designed for local therapy. Conventional magnet-responsive nanoparticles could be endowed with drug delivery by surface functional modifications141. For example, Schleich et al.148 developed a dual tumor targeting (active/passive) PLGA nanomedicine loading paclitaxel and SPIO. The integrated nanoparticles exhibited enhanced tumor targeting and effective tumor ablation, owing to magnet-mediated guidance and bifunctional arginine-glycine-aspartic acid (RGD) modification.148
4.1.3. Ultrasound
Ultrasound by frequency-switchable sound waves is a widely used exogenous trigger149. Ultrasound-responsive nanomedicines are always used in bioimaging150, diagnosis151, triggered drug delivery, and therapy152. An ultrasound-responsive nanoemulsion was developed for intratracheal and intravenous delivery for lung cancer therapy, using low-intensity focused ultrasound (LIFU) guided by 19F magnetic resonance imaging153. The nanoemulsion could rapidly distribute in the lungs and tumor tissues after intravenous injection. In particular, LIFU triggered burst drug release and increased the permeability of tumor tissues153. In another study, air-trapped poly(butyl cyanoacrylate) nanocarriers loaded with cyclodextrin were reported for multimodal imaging and therapy of atherosclerosis154. The results indicated that the ultrasound treatment allowed increased uptake and elevated anti-atherosclerotic efficacy in an ApoE−/− mouse model.
In addition, ultrasound can provide some special biological effects for developing the desirable ultrasound-responsive nanomedicine155. Lammers et al.156 used poly(butyl cyanoacrylate)-based microbubbles (MB) to load ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles on the shell for mediating and monitoring BBB permeation. The USPIO-MB can be destroyed by exposure to transcranial ultrasound pulses, resulting in acoustic forces inducing vessel permeability and promoting the accumulation of USPIOs in extravascular brain tissue. However, the use of ultrasound-sensitive nanomedicines is limited to untoward biodistribution157 and trans-vascular transport due to large size158.
4.2. Physiological and pathological triggers
4.2.1. pH
The pH conditions in vivo differ significantly because of the differences in compositions and microenvironment of organs and tissues. Generally, the pH values are around 7.0–7.4 in normal blood and tissues159, 4–6 in intracellular lysosomal/endosomal organelles160, and 6–8 in the lesions (e.g., tumor microenvironment)161. The pH-responsive approach can be used for precise delivery, using the pH gradients between normal and diseased tissues162. Pujara et al.163 prepared pH-responsive nanoparticles using bovine β-lactoglobulin (BLG) and succinylated BLG crosslinked with epsilon poly-l-lysine to improve oral delivery of curcumin. As succinylated BLG dissolved readily at pH 7.4, the constructed nano colloids respond to the pH trigger for sustained release163. Conversely, curcumin was not released in acidic conditions and was protected from degradation163. In addition, the pH-responsive mechanism can be applied to regulate cellular ion homeostasis. Ploetz et al.164 reported lipid-coated iron-based MOF nanocarriers (Lipid@MOF) for delivering iron ions into cells. The constructed Lipid@MOF nanoparticles were absorbed into cells, followed by the release of iron ions due to degradation in response to acidic pHs in the lysosomes164. The authors concluded that Lipid@MOF could control the release of iron ions into cells to promote distinct ferroptosis- and pyroptosis-mediated programmed cell death and reduce off-target side effects164.
4.2.2. Temperature
Temperature is another trigger attracting extensive attention in drug delivery. Various smart temperature-responsive NDDSs, initiated by external temperature and stimulating the body temperature, have been designed for precision medicine165, 166, 167. Prawatborisut et al.168 developed a nanoparticle coated with poly(n-isopropyl acrylamide). The results indicated that the hydrophobicity and protein binding on nanoparticles could be altered by adjusting the temperature during incubation with human plasma168. The temperature-dependent protein binding significantly affected the nanoparticle uptake by RAW264.7 and HeLa cells, and nanoparticles primarily associated with apolipoprotein A1 and E at 37 °C, while bonded with apolipoprotein J at 25 °C168. Inoue et al.169 synthesized amphiphilic side-chain liquid crystalline polymers (LCPs) having nematic–isotropic phase transition at physiological temperature and size of ∼130 nm in aqueous media. The formed LCPs demonstrated enhanced drug release at temperatures higher than the nematic-isotropic phase transition temperature (TNI) but reduced drug release at temperatures lower than TNI169.
4.2.3. Enzymes
Enzymes, such as lipases, matrix metalloproteinases (MMPs), cathepsins, glycosidases, and oxidoreductase enzymes, play vital roles in biological reactions and homeostasis. They act as plausible triggers upon abnormal expression in specific tissues/organs for enzyme-responsive drug delivery. The enzyme-triggered mechanisms applied to accelerate chemical reactions include: 1) directly breaking the bond/conjugation between drugs and carriers for enhanced drug release170, 2) disassociating or degrading the carrier structures via enzyme-induced hydrolysis171, and 3) cleaving the enzyme-sensitive chemical bonds for activating prodrugs, biological probes, ligands, and so on172. Enzymes-sensitive nanomedicines provide great opportunities in diagnosis, monitoring, tissue prevention, and disease treatment173.
For instance, an enzyme-sensitive paclitaxel-loaded hyaluronic acid nanogel modified with CD44 and biotin was constructed for targeted delivery to breast cancers174. Rapid release of paclitaxel was demonstrated, caused by the catalysis of hyaluronidases and/or lipases after dual bio-specific receptor-mediated tumor-targeting174. To improve the sensitivity, multiple responsive mechanisms could be involved175. He et al.176 developed enzyme-responsive nanoparticles, using mitochondria-targeting triphenylphosphonium derivative as cores loaded with lonidamine dimers and CD44-targeting hyaluronic acid (HA) as shells. The results demonstrated that the system could sensitively respond to hyaluronidases for degradation of HA-DOX shells and accelerate DOX release in cascade176.
4.2.4. Redox
Inspired by different reduction capacities in specific pathophysiological conditions, redox-sensitive nanomedicines have been extensively studied177. Many engineered nanocarriers, such as liposomes, polymersomes, polymeric micelles, gold nanoparticles, and polymer–drug conjugates, are integrated with a redox-responsive strategy for targeted therapy178. Lou et al.179 reported redox-responsive paclitaxel-maleimide prodrug nanoassemblies. By conjugating with paclitaxel and albumin-binding maleimide, the paclitaxel prodrugs could self-assemble into nanoparticles without excipients and immediately bind to albumin in circulation following intravenous administration and readily disintegrate into prodrug/albumin nanoaggregates, facilitating targeted accumulation in tumors via albumin receptor-mediated active targeting179. Furthermore, through controllable redox-responsive reactions, the in vivo fate of nanocarriers can be effectively regulated to improve delivery efficiency while reducing unwanted side effects. For instance, redox-responsive hyperbranched polyprodrug micelles by Zhong et al.180 displayed on-demand and accurate control of gemcitabine release in the tumor acidic microenvironment with minimized systemic toxicity.
Abundant trigger-responsive nanomedicines have been extensively investigated in drug delivery; however, few formulations have been translated181. The following challenges often limit the translation: 1) the differences between human and animal models; 2) inadequacy in vivo validation of the triggering mechanisms; 3) systemic toxicity, side effects, biodegradability, biocompatibility, and biosafety pending improvement.
5. In vivo behaviors of nanomedicines
The leading trait that endows nanomedicine with promising drug delivery capabilities is its small size which generally falls within the nanoscale range with greatly reduced size-dependent untoward interactions. Nevertheless, the extraordinarily small size poses a big challenge in tracking and unraveling the in vivo behaviors of nanomedicines. Ignorance or negligence of the in vivo fate presents as one of the bottlenecks to translation of preclinical nano-delivery designs to clinical therapeutic products25,26. Unraveling the in vivo and subcellular fate of nanomedicines is gaining interest from various disciplines recently23,25, 26, 27,182. It is believed that a good understanding of the in vivo behaviors of nanomedicines helps optimize the designs of delivery systems and accelerate the process of product development.
5.1. The general consideration on in vivo fate of nanomedicines
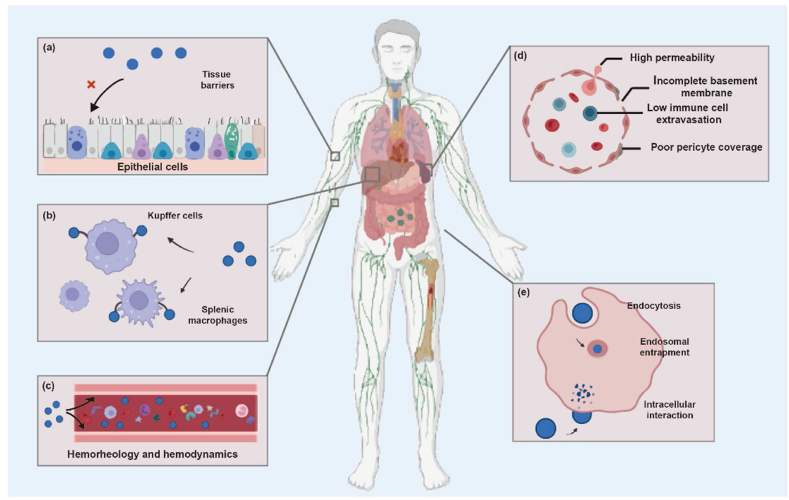
The in vivo process of nanomedicines begins when they make a contact with the body. Fig. 8 describes the sequential steps that may take place to nanomedicines after entry into the body183.
Figure 8.
Sequential in vivo processes that nanomedicines may go through: (a) interactions with the tissue barriers that prevent nanomedicines from entering into the body; (b) uptake of nanomedicines by the mononuclear phagocyte system (MPS); (c) hemorheological and hemodynamic disposition of nanomedicines which alters the integrity, kinetics, and interaction with hematic components; (d) post-MPS distribution and disposition of nanomedicines by specialized physiological mechanisms such as passive tumor targeting owing to the EPR effect; (e) endosomal compartmentalization and cell membrane trafficking of nanomedicines. Adapted with permission from Ref. 183. Copyright©2022 Elsevier.
5.1.1. Biobarriers restraining the migration of nanocarriers
The biological barriers present as the first and outmost impediment to the entry of nanomedicines into the body. These physiological barriers made up of densely packed cells prevent both paracellular and trans-cellular transport of nanocarriers, hence restricting the gross circulatory exposure of nanomedicines184. Tight junctions formed from multiprotein complexes serve as gatekeepers for paracellular trafficking across closely packed epithelial cells. Nanocarriers with sizes bigger than a few tens of nanometers (e.g., micelles) cannot go across membrane pores less than one nanometer in diameter. However, nanocarriers could be taken up by epithelial cells via endocytosis pathways, whereas the "easy endocytosis, hard exocytosis" conundrum prevents nanocarriers from crossing multilayers of cellular barriers185, 186, 187. Except for the intravenous route, nanomedicines administered via different routes (e.g., ocular, transdermal, oral, pulmonary, nasal, etc.) are hampered by the epithelial barriers and tight junctions184,188. In addition to the epithelial barriers, the endothelial barriers block the back door to the blood circulation184,189. Even for the intramuscular and subdermal routes, nanomedicines are unable to be delivered to the blood circulation directly, and there are complicated biobarriers that restrain the migration of nanocarriers.
5.1.2. Uptake by the mononuclear phagocyte system
Nanocarriers are generally recognized as foreign objects with nanoscale sizes. The first thing the body will do is to "fight" and clear them out. The immune systems keep alert at the forefront of the battlefield, ready to tame the nanovehicles. When nanomedicines enter the body, the sentinels, especially the MPS, proactively interact with the nanoparticles by a mechanism of phagocytosis which dramatically clears the particles from the blood circulation and accumulates them in the MPS. The eventual fate of the captured particles involves intracellular trafficking, lysosomal disintegration, polymer degradation, drug release, and sometimes translocation of non-degraded particle remnants. The MPS is comprised primarily of blood monocytes, tissue macrophages, dendritic cells, and bone marrow progenitor cells which reside mainly in the vital organs (e.g., lungs, liver, kidneys, and spleen). The design of long circulation evades opsonization and diverts a fraction of nanocarriers to organs and tissues beyond the MPS93,190.
The sequestration of nanocarriers from blood involves sequential steps, including blood circulation, marginalization, firm attachment, and cell internalization191. Serum albumin complements, immunoglobulins, and apolipoproteins, among other plasma proteins, bind to nanocarrier surfaces and form “protein corona (PC)”. Depending on surface hydrophobicity/hydrophilicity and functionalization (e.g., with ligands), as well as size, shape, and zeta potential, the “PC” defines the physiological features of nanomedicines. Following internalization and binding to specific phagocyte receptors, protein-coated nanocarriers are delivered to the phagosomes before lysosomal fusion. The spleen, liver, and lymph nodes contain macrophages responsible for this process. Opsonization is a crucial step that determines the pharmacological and toxicological effects of nanomedicines. As reported, opsonization can also influence the functionality of targeting ligands, resulting in a lack of selectivity. Salvati et al.192 found that transferrin (Tf)-decorated silica nanoparticles did not bind to Tf receptors (TfR) on A549 cells or soluble TfR, demonstrating how opsonization prevents ligand/receptor binding and results in a loss of specificity. In-depth analysis has connected the physicochemical properties of particles such as size and surface charge with PC formation193,194. Smaller particles favor protein attachment due to a higher surface area-to-volume ratio195,196. Regarding surface charge, positively charged nanocarriers are rapidly cleared from the blood, whereas neutral and negatively charged ones circulate longer in the blood and could attain enhanced accumulation in peripheral tissues such as tumors197,198.
On the other hand, the sequestration mechanisms of the MPS can be targeted as well when diseases inflict the MPS themselves. For instance, macrophages play a significant role in inflammation, resulting in diseases like atherosclerosis and cancers. As a result, macrophages may be regarded as prospective therapeutic targets199. For example, aquaporin 3, a membrane protein channel highly expressed by monocytes/macrophages, can be utilized for macrophage targeting.200 Hara-Chikuma et al.201 developed an anti-aquaporin 3 monoclonal antibody that successfully prevented liver fibrosis in a carbon tetrachloride (CCl4)-induced mouse model.
5.1.3. Impact of hemorheology and hemodynamics
Site-specific distribution can be achieved by long-circulating engineered nanocarriers. To engage target cells, nanocarriers must maintain prolonged circulation and drift laterally through the core layer of blood cells before adhering to the vessel walls. The size and position of the vasculature affect the hemodynamics and flow of circulatory blood cells202,203. As reported, hemodynamic factors (e.g., velocity, rheology, flow pattern, and shear stress) effectuate in starting and strengthening platelet adhesion to arterial walls204. Platelet or leukocyte adhesion is higher in steady-state conditions with high shear stress than in pulsatile conditions204, 205, 206. To adjust the physicochemical properties of nanocarriers and achieve better adhesion to the endothelial walls, it is necessary to understand the vasculatures and the hemodynamics related to disease pathology. Nanocarriers with spherical geometry have less lateral drift, a lower propensity to marginalize towards the arterial walls, and a lower affinity for endothelial cells, and therefore do not frequently contact or attach to the endothelial walls. They stay in the cell-free layers between the RBC core and artery walls, inhibiting site-specific delivery207,208. To reach the endothelial cells and relevant receptors, optimum nanocarriers are required to dissociate from the erythrocyte cores, aggregate in the cell-free layers, and travel toward the vessel walls. The non-spherical analogs can make highly complex motions like rolling and tossing. Without assistance from outside factors, they are capable of marginalization. Furthermore, it has been established that the aspect ratio of the nanocarriers directly influences the lateral drift velocity. Rod-shaped particles are more straightforward to the edge than spherical particles because of their rotating and oscillating motion from one wall to the next191.
Another factor affecting adhering to vessel walls is the equilibrium among the ligand density on carrier surfaces, the number of receptors on cell surfaces, and the shear stress at the vessel walls. Particle adhesion is also influenced by particle size and shape. Submicron particles have weak hydrodynamic forces. Few ligand/receptor interactions can survive the shear stress because of the limited particle/cell contact area. Although the hydrodynamic force also increases with larger particles, the number of ligand/acceptor links increases too209. Utilizing parallel plate flow experiments, Chareonphol et al210. revealed that spherical particles with a size of 2–5 μm demonstrated better wall edges than submicron particles at a relatively high shear rate and channel height.
5.1.4. Site-specific extravasation and tissue accumulation
Although site-specific extravasation and accumulation of nanocarriers are seen in the treatment of many diseases, the notion is best understood in the context of cancer. Nanocarriers are discovered to accumulate in various tumor regions following continuous circulation and selective yet robust adherence to the vessel walls. Before being absorbed by the cancer cells, they cross the endothelia and travel further into the interstitial spaces of tumors. The tumor vascular system is distinguished from normal blood vessels by complicated, heterogeneous leaky vessels with heterogeneous blood flow211,212, which can promote passive tumor targeting and accumulation of nanocarriers based on the EPR effect213,214.
Receptors like VEGF, αvβ3, and so on are overexpressed on the endothelial surfaces of the neointimal tumor systems. For targeted adhesion and internalization, ligands unique to these receptors are typically used to functionalize nanocarriers215. It should be aware that the physiological characteristics of tumor vasculatures can affect the penetration ability of nanoparticles. The tumor heterogeneity impacts nanocarriers' vascular permeability, which in turn impacts the accumulation rate in tumors. Studies also reveal that the tumor microenvironment impacts nanocarriers' tumor accumulation and vascular permeability. According to a study, EPR is influenced by the tumor's type and the tissue where it is located. The accumulation of liposomes was evaluated in multiple tumor xenografts at various original and metastatic sites, including breast cancer (T1), lung cancer (LL), and colon cancer (CT26) cells216. This is correlated with the ratio of tissue inhibitor of metalloproteinase one to matrix metalloproteinase 9 (MMP-9), indicating that elevated MMP-9 levels are indicative of higher vascular permeability217,218.
The interstitial compartment of tumor tissues has a very different makeup from normal tissues218. Due to unregulated cancer cell development, extensive fibrosis, dense extracellular matrix, and a lack of anatomically well-defined working lymphatic networks, interstitial fluid pressure (IFP) is raised in tumors219. While vascular permeability and tumoral accumulation could be enhanced owing to EPR, raising IFP makes it harder for extravasation to reach the area of interest. This could have a meaningful impact on how nanocarriers are used to diagnose and treat tumor development and metastasis219.
5.1.5. Subcellular fate of nanocarriers
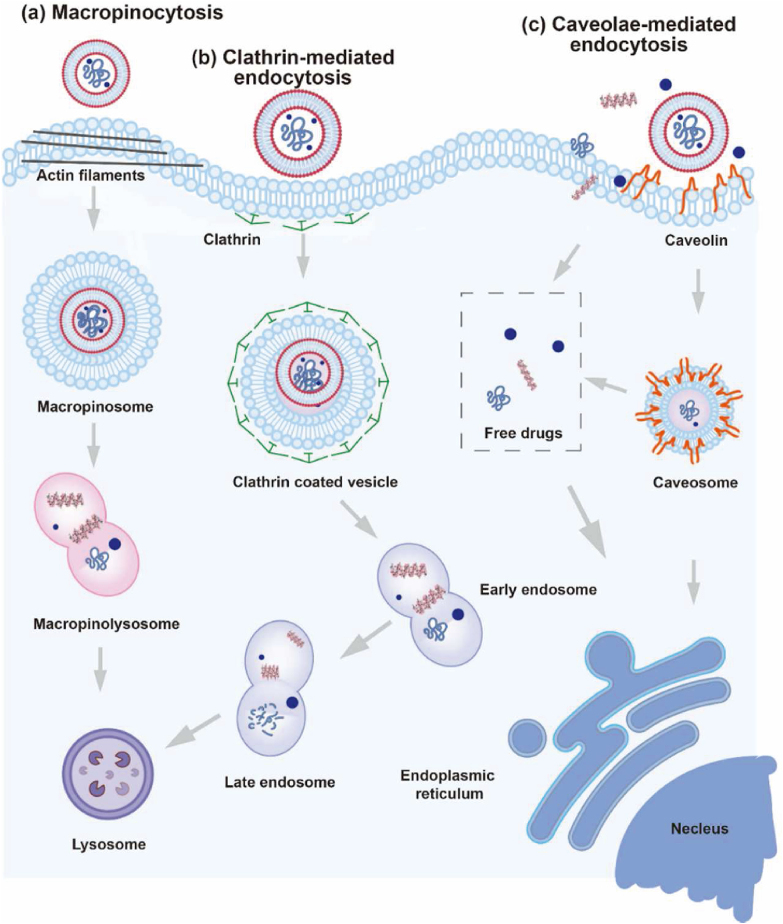
NDDS should be delivered directly to subcellular organelles when intracellular drug release is demanded. Understanding the processes of internalization and the subcellular fate of nanocarriers conveys important information to unravel the underlying mechanisms. “Big” as their size is, nanocarriers could not permeate across cell membranes through passive diffusion as small molecules do. Drug nanocarriers are generally endocytosed and processed within cells before breaking down to release drugs at target organelles. The physicochemical and surface properties such as size, shape, surface charge, surface morphology, elasticity, surface chemical decorations, and so on affect the subcellular behaviors of nanocarriers220, 221, 222, 223, 224, 225. Fig. 9 delineates the common pathways for endocytosis. During endocytosis, nanocarriers will form membrane invagination and fuse with highly acidic lysosomes to create intracellular vesicles (phagosomes or endosomes)226. Although phagocytosis-mediated endocytosis is frequently seen in MPS cells, clathrin-mediated endocytosis is the conventional mechanism for nearly all nanocarriers. When endocytosis is mediated by clathrin or receptors, ligand-functionalized nanocarriers are attached to cell surface receptors. Following the creation of clathrin-coated pits, the ligand–receptor complexes separate from the membrane to create clathrin-coated vesicles, which thereafter combine with the early endosomes and loop back to the plasma membrane or enter the lysosomes. Many nanocarriers, together with drug payloads, end up in lysosomes, raising concerns about the functionality and activity of the delivery systems. Caveolae-mediated endocytosis is an alternative pathway. Membrane invaginations with a size of 50–60 nm, known as caveolae, are created in this instance by intact membrane proteins called caveolins and peripheral membrane proteins called cavins. The caveolae separate from the membrane and combine with caveolae having a neutral pH. Caveolae, unlike phagosomes or endosomes, occasionally skip the lysosomes (Fig. 9)220.
Figure 9.
Summary of cellular endocytosis routes for nanomedicines. (a) Micropinocytosis. (b) Clathrin-mediated endocytosis. (c) Caveolae-mediated endocytosis. (d) Phagocytosis. Adapted with permission from Ref. 220. Copyright©2019 Elsevier.
5.2. Pharmacokinetics of drugs vs. nanomedicines
Contrary to conventional medications, the pharmacokinetics (PK) of nanomedicines involves a complicated web of interactions between free drugs, encapsulated drugs, and empty nanocarriers. The equilibrium dynamics in between are crucial for proper PK modeling. To acquire a pertinent PK model, it is important to mind that conventional PK models cannot be directly applied to describe the PK of particles. Conventional PK is primarily based on the equilibrium between blood and target drug levels, which in turn depends on the distribution of drug molecules between blood and tissues by a mechanism of diffusion. Nevertheless, nanomedicines present as particles during the in vivo disposition processes and there are no such mechanisms as those of small molecules. The level of nanomedicines in tissues may be more informative than that in the blood. Currently, multiple (two- or three-) compartment models have been attempted in PK modeling of nanoparticles190,227, but the prospect of application is yet to be assessed. One thing is for sure, the non-compartmental models such as statistical moment is a practical approach to obtaining fundamental PK parameters of particulates190,227.
The area under the curve (AUC) following oral administration is a crucial parameter in conventional PK models to calculate the absorption rate. However, in the case of nanomedicines, calculating plasma drug concentration by time alone may be irrelevant because of ignorance of the stability of nanomedicines. Groo et al.228 found in a subcutaneous tumor model that the most potent paclitaxel nanoformulation was not the one with the largest AUC following oral delivery. Notably, the tumors are not resistant to encapsulated but free paclitaxel. The unstable formulation produced the maximum AUC during absorption; however, the released paclitaxel was ineffective against the tumor. Despite less paclitaxel being absorbed (smaller AUC), it worked better when enclosed in stable nanocarriers228.
The goal of the development of nanomedicines is to optimize their biodistribution to increase activity and/or decrease toxicity. Due to the interaction of particles with tissues and body fluids throughout the distribution process, conventional PK models cannot accurately describe the dynamics of distribution. The physicochemical properties of nanomedicines determine the in vivo fate of nanocarriers. Environment-responsive fluorophores have been employed to label and track nanocarriers in vivo based on rationales of Förster resonance energy transfer (FRET) and aggregation-caused quenching (ACQ)229, 230, 231, 232, 233. FRET is currently one of the primary methods for assessing the biodistribution of nanomedicines. Laine et al.234 employed FRET to assess the behavior of several lipid nanocarriers, including lipid nanocrystals (LNCs), polyethylene glycosylated LNCs, and lipid nanoemulsions (LNEs). The findings revealed various release kinetic patterns, with extended circulation duration and strong structural stability for several hours following intravenous injection for PEGylated LNCs and LNEs234. Gravier et al.235 utilized macroscopic and microscopic bioimaging to assess the stability of LNEs and LNCs in the biological environment. According to the findings, LNCs were more stable than LNEs in the presence of serum or cancer cells235. These investigations deepen our understanding of what happens to nanocarriers in vivo, but FRET fails to accommodate precise quantification of nanocarriers.
5.3. The influence of protein corona on nanomedicines in vivo
After entering the bloodstream, nanomedicines quickly bind with different plasma proteins and generate a protein coat known as PC123,236,237. PC often arises from a complicated and dynamic mechanism. Typically, “hard corona” is formed by proteins with a greater affinity and a longer residence time, whereas “soft corona” is made up of proteins with a lower affinity and a shorter residence time238, 239, 240. The features of the nanomedicines, such as size, shape, and surface qualities significantly impact the compositions of PC241,242. The larger ones with more surface area and less surface curvature often result in better protein coverage243, 244, 245. Additionally, more plasma proteins are drawn to charged or hydrophobic interfaces246, 247, 248. The physicochemical properties of nanomedicines are altered as a result of the dynamic interactions between nanocarriers and proteins, endowing them with new biological features and altering their in vivo fate.
5.3.1. Stability
Nanoformulations are typically more stable in vitro than in vivo owing to the adsorption of body proteins, which may alter the in vivo behaviors of nanocarriers before reaching their targets249. Ho et al.250 investigated the effect of PC [IgG, fibrinogen, ApoA1, and human serum albumin (HSA)] on the aggregation of gold nanoparticles with low protein/nanoparticle ratio and physiological pH associated with increased aggregation. On the other hand, thorough coating with PC at a higher ratio decreases the attraction between nearby nanoparticles and limits aggregation. PCs are more effective in preventing nanoparticles from aggregation than bare nanoparticles250. Similarly, despite the medium being supplemented with NaCl, which has been demonstrated to enhance silver nanoparticle aggregation in a concentration-dependent manner251, PC formation in vitro after incubation of silver nanoparticles with Fetal Bovine Serum (FBS)+/Dulbecco's modified Eagle's medium (DMEM) decreased their aggregation. Additionally, PC development in the gastrointestinal tract may severely impact the stability of nanoparticles taken orally. Cao et al.252. examined the relationship between casein and titanium dioxide nanoparticles. Notably, the electrostatic attraction between the particles diminished by neutralizing the positive charge of the nanoparticles, increasing the aggregation of protein complexes252. Additionally, PC may change the release of nanoparticles. The release of DOX from magnetic mesoporous silica nanoparticles was retarded by PC, as reported by Pourjavadi et al.253. Coating the nanoparticles with polyethylene glycol (PEG) decreases PC formation253.
5.3.2. Cellular uptake
PC alters the way that nanomedicines interact with non-phagocytic cells, changing how they are taken up by cells and endocytic pathways. Cheng et al.254 examined the impact of PC on the cellular entrance of gold nanoparticles of three distinct sizes (5, 20, and 50 nm) and found that the cellular absorption of gold nanoparticles decreased significantly with the formation of PC. Aliyandi et al.255 demonstrated that cellular uptake of PC-precoated silica nanoparticles into endothelial cells was affected by the size and compositions of PC. Notably, pre-coating with histidine-rich glycoprotein decreased the endothelial absorption of nanoparticles255. As a result of the selective binding of ApoE to low-density lipoprotein (LDL) receptors, PC was discovered to increase endothelial internalization of magnetosomes via LDL receptor-mediated endocytosis. Additionally, nanoparticles can alter the integrity of the endothelium layer by interacting with intercellular linker proteins such as vascular endothelial calmodulin256.
5.3.3. Targeting capability
PC may reduce the effectiveness of targeting, whether actively or passively257, 258, 259. It has been commonly observed that PC interferes with ligand/receptor interactions, impairing the targeting efficiency of many targeted nanomedicines. For instance, the active targeting ability of polystyrene nanoparticles modified with transferrin is reconciled by cerebrospinal fluid proteins in a way similar to the uptake of polyethylene glycolyzed polystyrene nanoparticles with the same size and surface charge260. Similar findings were made by Varnamkhasti et al.261 who discovered that mucin receptors loaded on chitosan nanoparticles for targeting colon cancer cells are missing after protein adsorption. Nemati et al.262 reported that PC significantly reduced the targeting abilities of folate-modified chitosan nanoparticles and hindered uptake by tumor cells. In vivo adsorption of proteins, particularly IgM, conceals the specific identification of folate receptors on tumor cells263.
The targeting ability of nanomedicines does not always suffer from PC formation. Suchanova et al.264 reported that the targeting capacity of Tf-modified virus-like nanoparticles was not impacted by serum proteins. PC can also direct nanocarriers to intended therapeutic targets. For instance, PC coating does not influence the targeting specificity of Tf-modified PEGylated nanoparticles to brain malignant cells258. However, proteins that adsorbed on nanoparticles, primarily ApoA-I, made it easier for them to cross the BBB258. Therefore, while constructing targeted nanoparticles based on disease type, nanoparticle system, and targeting fraction, it is crucial to thoroughly investigate the effect of PC on targeting efficiency.
5.3.4. Pharmacokinetics
The plasma proteins encapsulated on nanomedicines' surfaces give them a “new face” which alters their pharmacokinetics. Firstly, the nanomedicines' surface-bound biomolecules impact the circulation time and interaction with the body. Complement proteins can attach to nanomedicines' PC and be exchanged in vivo when nanomedicines are introduced into the blood265. Thus, PC improves cellular absorption and phagocytosis of nanomedicines by the MPS, decreases cell membrane adherence, and attenuates cell membrane disruption induced by naked nanomedicines266, 267, 268, 269. Particularly, nanomedicines' internalization and biodistribution can be significantly influenced by binding proteins270. IgG adsorption, complement factors, and fibrinogen, for instance, facilitate the phagocytosis of nanomedicines, while troponins like albumin are hypothesized to increase the circulation of nanomedicines in the blood271. Spherical nucleic acids are internalized by macrophage complement receptors, where they are then distributed throughout the liver and spleen272. By tuning the corona compositions (e.g., Apos, protein C, and hemoglobin subunits), nano-polyelectrolyte complexes could be imparted with extended circulation duration273. When PC was enriched with HSA, dendritic cells’ ability to phagocytose nanomedicines was decreased274. The prevalence of clusters in PC was shown to limit macrophage absorption of non-specific cells275. Complements, immunoglobulins, and immunological antibodies are modulators that cause macrophage absorption and hasten nanomedicine clearance276. Therefore, the lifespan of nanoparticles and the mechanisms and effects of PC production must be considered when designing drug delivery systems.
Nanomedicines must be delivered to specific cell targets to work effectively. Combined passive and active targeting strategies are used to improve the accumulation of nanomedicines in disease sites. However, PCs may misguide nanocarriers to off-track destinations. For instance, when loaded with biological substances such as human albumins277, PC formation dramatically lowers the hepatic retention of gold nanoparticles. The plasma protein ApoE can bind preferentially to nanoparticles coated with polysorbate 80, promoting nanoparticle accumulation in the brain199. Bionic liposomes with a peptide tag are associated with exchangeable apolipoproteins and absorb significant amounts of ApoA1, E, and J, improving the bionic liposomes’ ability to target the brain278. Therefore, understanding the relationship between PC and nanomedicine biodistribution may help work more effectively in therapeutic applications.
In addition to cellular uptake and disposition, PC affects the biodegradation of nanomedicines as well. For instance, induced myeloperoxidase release and hypochlorite production in neutrophils cause single-walled carbon nanotubes (SWCNT) precoated with HSA to degrade at a greater rate279.
5.3.5. Cytotoxicity
PC can increase the safety of nanomedicines against normal cells280, but it can also cause immunotoxicity. Cationic nanocarriers may associate with negatively charged cell surfaces, disrupting the plasma membrane and ultimately causing cellular damage281. However, the presence of PC protects the positive charges, raising the nanomedicine level of safety. For instance, compared to uncoated carbon nanotubes, bioprotein-coated ones considerably reduced the cytotoxicity of human monocytes and endothelial cells281. Additionally, the hemolysis of nanomedicines can be successfully avoided by the quick formation of human PC282.
5.3.6. Immunotoxicity
Ingested nanocarriers are recognized as exogenous particles that could cause immunological reactions. PC coatings on the surface of nanomedicines may provide an efficient biological interface for nanomedicines engaging with the immune system. PC increases adaptive immune responses, the complement system, and innate immune cell (e.g., monocyte) differentiation.
PC mediates the recognition and differentiation of immune cells (e.g., monocytes, macrophages, and neutrophils). For example, Mo et al.283 found that PC coating of black phosphorus nanocarriers elicits immunotoxicity and activates macrophages. Yan et al.284 discovered that in immune cells like monocytes and other macrophage-like cells, PC formation is necessary for nanomedicine internalization. Additionally, it has been demonstrated that poly(acrylic acid)-modified gold nanocarriers promote the unfolding of ingested fibrinogen, resulting in inflammatory responses285.
It is believed that activation of the complement system by nanomedicine-PC complexes initiates the opsonization processes286. Evidence shows nanomedicines bind quickly to a third complement component after exposure to human serum or blood265. Adhesion of IgM to liposome surfaces, whether naked or surface-modified, negatively impacts complement activation263,286,287. Vu et al.288 noted that PC accelerates the binding of immunoglobulin with all nanocarriers. Pattern-recognition molecules such as collection, mannose-binding lectin, and ficolin recognize structural fingerprints on nanocarrier surfaces to activate the complement system289. PCs on nanomedicines also cause both adaptive and innate immune reactions. Conformational changes in corona proteins adsorbed on nanocarriers exposed immunogenic epitopes, resulting in opsonization and adaptive immune responses290.
It is concluded that PC has a two-sided effect on both in vitro and in vivo performance of nanomedicines. The topic of PC emerges as a critical issue in nanomedicine research. Stealthy coating with hydrophilic polymers such as PEGs repels the adsorption of proteins and therefore mitigates the effect of PC. A fresh perspective on PC is that targeting of nanocarriers is somehow mediated by the proteomes of PC and the protein compositions of PC could be actively modulated for specific targeting purposes. Overall, PC exhibits considerable promise in offering fresh design concepts and thought-out viewpoints for drug delivery involving nanocarrier systems291.
5.4. Nanotoxicity
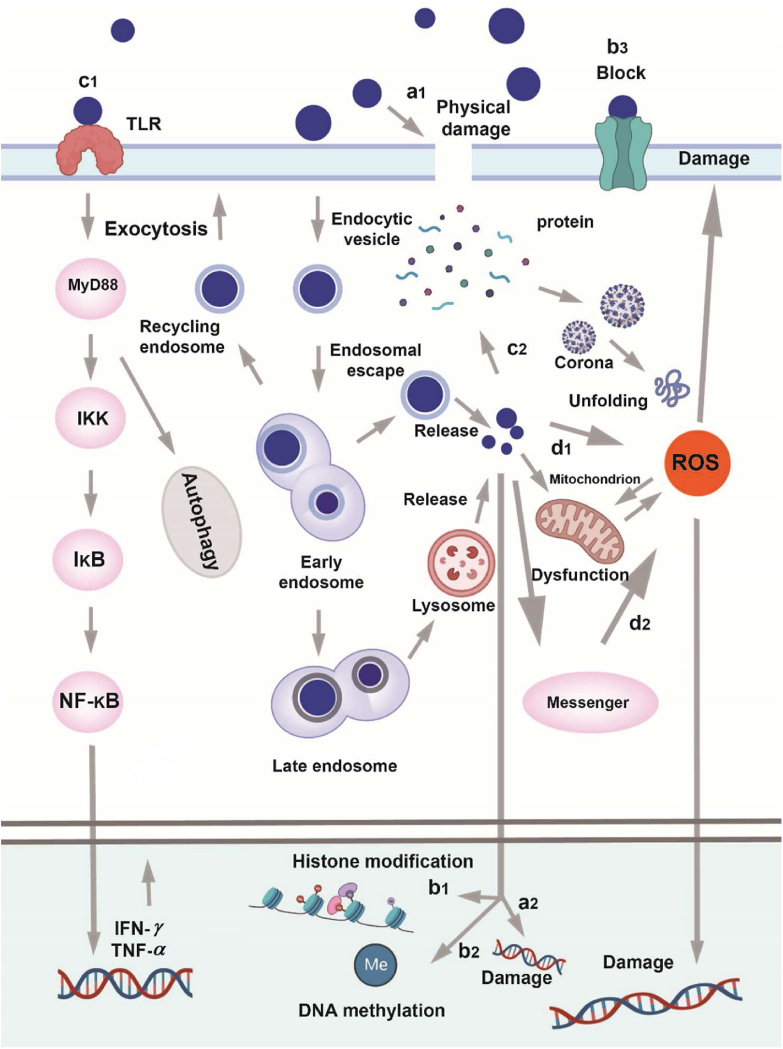
Although various nanomaterials have been tested in preclinical and clinical trials, we still do not fully understand the safety hazards of new nanomedicines292, 293, 294, especially inorganic ones. Due to structural instability and subsequent cadmium ion leakage, CdSe quantum dots, a bioimaging agent, demonstrated considerable hepatotoxicity in earlier in vitro investigations295. Studies also demonstrated that carbon nanotubes could cause adverse physiological reactions such as inflammation, genetic damage in different cells, and even synergistic toxicity when combined with other pollutants like zinc ions. Despite therapeutic efficacy, the unfavorable toxicity of nanomedicines to normal tissues may have undesirable biological repercussions. It is crucial to develop nanomedicines with maximized efficacy but minimized toxicity (Fig. 10).
Figure 10.
The summary of possible toxigenic pathways for nanomaterials (a1) Physical injury to biological membranes directly (a2) Direct deterioration of DNA (b3) Nanomaterials blocking ion channels (c1) The activation of autophagy and inflammatory bodily reactions by interactions between nanomaterials and biomolecules (c2) Interactions with proteins that impact the way they operate (d1) Directly generated ROS by catalytic reaction (d2) The activation of ROS-related signaling pathways results in the indirect production of ROS.
5.4.1. Direct physical deterioration of DNA and biological membranes
Nanostructures with rigid surfaces and sharp edges, particularly inorganic and carbon-based nanomaterials, can be physically associated with biological systems and damage biological activities296 in an irreversible and/or concentration-dependent manner. Carbon nanotubes (CNTs) that are thin, stiff, and have needle-like features can pierce the plasma and nuclear membranes of mesothelial cells, resulting in pathogenicity similar to asbestos297. Recent studies have proven that inorganic nanoparticles can rupture the membranes of Escherichia coli, causing macropores298. Similarly, Gram-positive Staphylococcus aureus and Gram-negative E. coli can interact with the highly sharp edges of graphene oxide, directly damaging their membranes298. The findings suggest that strong hydrophobic interactions between graphene and lipid molecules allow graphene nanosheets to penetrate the membrane and absorb significant amounts of phospholipids, affecting the integrity and stability of the membrane and even creating nanoscale gaps299, 300, 301. Additionally, nanomaterials smaller than 10 nm in one dimension have the potential to directly enter the nucleus, damage DNA structure, and have genotoxic consequences such as chromosome breakage. This would result in DNA strand breaks302, 303, 304. It has been documented that centrosomes can incorporate SWCNTs with diameters of 1‒4 nm, disrupting the mitotic spindle, breaking DNA, and resulting in cellular aneuploidy305.
5.4.2. Physical obstruction
SiO2 and quantum dot-induced epigenetic effects and CNTs’ ability to inhibit ion channels306 are examples of physical disturbances that may be brought by nanomaterials whose dimensions and shapes are similar to the essential cellular machinery304,307. Physical disturbance typically results in functional rather than structural damage, in contrast to the direct physical damages mentioned above. Both iron oxide nanoparticles308 and CNTs309 can associate with actin myofilaments and induce aberrant cellular activity, including unchecked growth linked to cancer. Another study demonstrated that CNTs310 could interfere spatially with DNA, causing substantial chromatin reconfiguration or heritable gene changes. Notably, physical interference rather than direct DNA damage produces altered gene expression predominantly through point mutations or translational suppression284.
5.4.3. Physical contact with cells
Nanomaterials interact with cells and may activate specific physiological processes that can be harmful311,312. For instance, immune cells like platelets, neutrophils, and macrophages interact with nanomaterials such as CNTs. A large number of cytokines like interleukins and tumor necrosis factor may be produced as a result, potentiating a chain of immunological reactions that lead to fibrosis and bronchial granulomas313, 314, 315, 316. Instead, nanoparticles like SiO2, TiO2, and hydroxyapatite increase cellular contractility by severely disrupting intracellular microtubule assembly without appearing toxic, which eventually facilitates strong substrate adherence and limits cell mobility317,318. Nanomaterials may be broken down in an acidic environment and/or by hydrolases in lysosomes when endocytosed and subsequently transferred to endosomes by fusion of lysosomes with late endosomes, releasing toxic components and resulting in lysosomal dysfunction319,320. The released nanomaterials progressively induce inflammation and ROS or associate with cytoplasmic biomolecules such as glutathione and organelles (e.g., mitochondria and endoplasmic reticulum), causing additional malfunction and even cell death321,322. Additionally, autophagic malfunction brought by nanomaterials may result in apoptosis or autophagic cell death323,324.
5.4.4. Production of ROS
Oxidative stress and subsequent mitochondrial damage, lipid peroxidation, and DNA damage may result from excessive ROS production by a metal oxide or carbon-based nanomaterials325. Numerous studies have demonstrated that iron oxide nanoparticles promote the conversion of intracellular hydrogen peroxide (H2O2) to hydroxyl radical (HO⋅) via Fenton, Fenton-like, or Haber–Weiss cycle reactions, resulting in oxidative damage and even cell death326. It is also demonstrated that graphene oxide nanosheets could lead to oxidative stress-induced cytotoxicity by catalyzing the conversion of H2O2 to HO· and NDUFB9-mediated biological pathways to generate ROS327,328. Setyawati et al.329,330 discovered that raising intracellular ROS and Ca2+, nanodiamonds, and gold nanoparticles could cause loss of inter-endothelial cell connections and quasi-stable cytoskeletal remodeling of the vascular barriers.
To date, much work has gone into identifying the causes of nanotoxicity and using that information to create safe nanomedicines. Various nanomaterials, including liposomes, polymers, mesoporous silica, carbon nanotubes, and graphene, have been investigated to build efficient and secure nanosystems for drug delivery, bioimaging, tissue engineering, and biosensing. It is crucial to comprehend the connection between the design of nanomaterials and their biological effects to extract information that can be utilized to develop safer medical nanosystems.
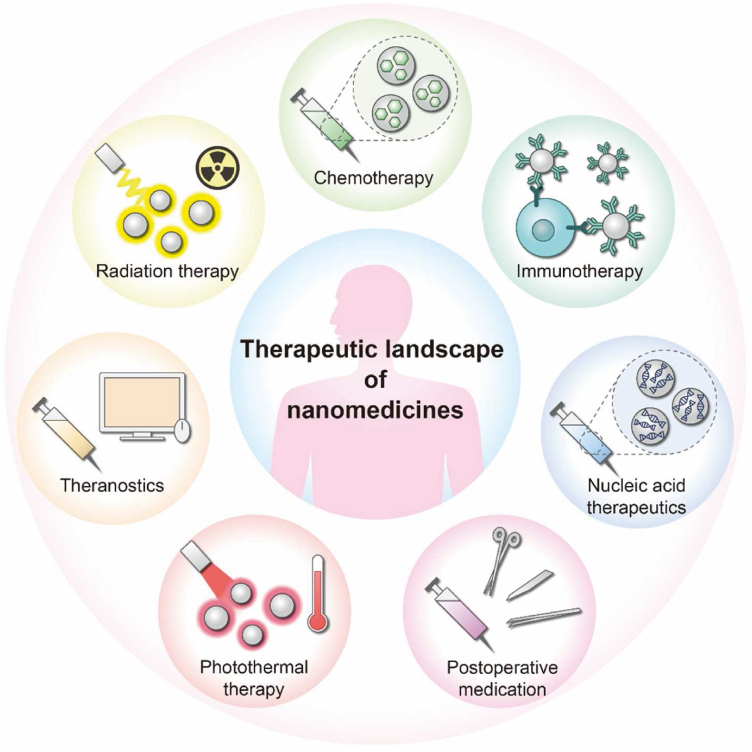
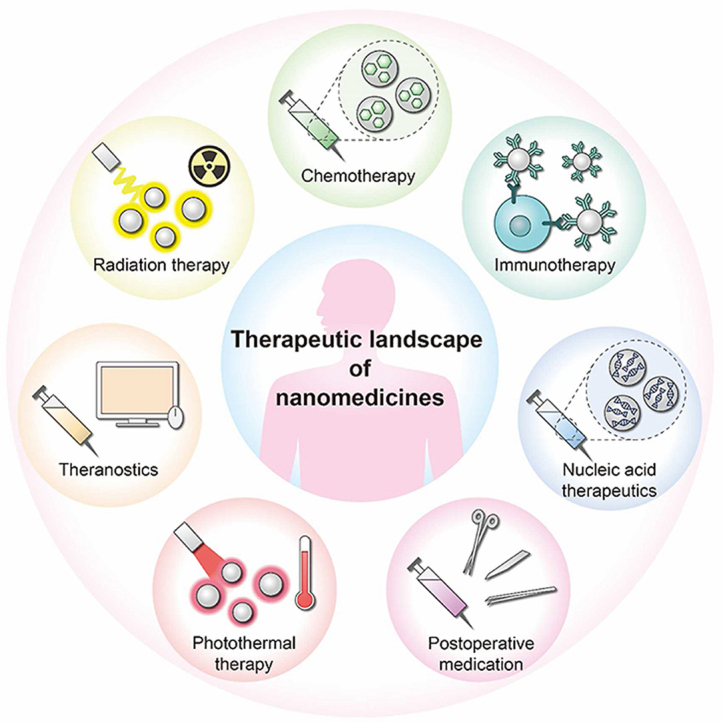
6. Therapeutic landscape of nanomedicines
6.1. Chemotherapy
Emerging intelligent nanomedicines offer great promise for effective cancer chemotherapy (Fig. 11)331, 332, 333. Cancer has developed into a leading cause of human death due to its high malignancy, rapid progression, and difficulty in effective eradication334. However, conventional chemotherapy often fails to treat cancer due to severe systemic side effects335. Increasing nanomedicines are utilized for targeted cancer therapy via the EPR effect336. Various nanocarriers, including inorganic, organic, or bionic carriers, have been employed to load chemotherapeutic drugs. The liposomal doxorubicin (Doxil®) is the first approved nanomedicine for cancer treatment. Following their footsteps, various nanomedicines have entered different stages of translation3 by exploiting various delivery strategies337 and a better understanding of disease mechanisms and biological barriers338. Ren et al.339 reported a nanoparticle consisting of epigallocatechin-3-gallate (EGCG), phenolic platinum (IV) prodrug (Pt–OH), and polyphenol-modified block copolymer (PEG-b-PPOH). This intelligent drug delivery platform demonstrated excellent anticancer efficacy via a combination of chemotherapy and chromodynamic therapy, as well as avoidance of systemic toxicity caused by platinum339.
Figure 11.
Therapeutic landscape of nanomedicines. Nanomedicines have tremendous potential in several therapeutic fields, such as chemotherapy, immunotherapy, nucleic acid therapy, radiation therapy, photothermal therapy, postoperative medication, etc.
Furthermore, to adapt to clinical precision treatment and reduce the damage to normal tissues, many nanomedicines are further furnished with complex integrated multifunctionalities340. A “Jekyll and Hyde” nanoparticle-hydrogel hybrid delivery system reported by Wu et al.341 could keep dormant in normal tissues and be activated in the acidic or hyaluronidase-rich tumor microenvironment. The biodegradation of nanoparticle-gel could lead to a unidirectional tumor-targeted release of DOX-loaded nanoparticles for enhanced tumor tissue penetration341. In addition, the nanoparticle-hydrogel demonstrated other advantages, such as biodegradability, tissue adhesiveness for continuous on-site activation, and shear-thinning behavior for injection341. This preparation demonstrated promising long-acting efficacy and precise anticancer treatment against locoregional treatment341. In addition, some stimuli-responsive drug delivery approaches have also been used to enhance the targeting of nanomedicines to cancer cells342,343, utilizing the active triggers at an amplifying level in the cancer microenvironment.
6.2. Nucleic acid therapy
Unlike conventional medications, nucleic acid therapy produces long-lasting therapeutic effects via gene addition, replacement, inhibition, and editing, which holds great promise by targeting therapeutic nucleic acids to their genetic blueprints. However, many challenges remain due to degradation by nucleases344, difficulty in encapsulation345, poor membrane penetration346, and off-target effects347. Nanocarriers are considered a viable strategy to facilitate the delivery of nucleic acids, i.e., ASO348, siRNA349,350, miRNA351, and mRNA352. Especially, the FDA recently approved two nucleic acid-based therapeutics to combat the COVID-19 pandemic353, 354, 355.
An octopus-like multivalent penetratin system, using multi-arm PEG as a core and conjugating with penetratin, was developed to promote compression and delivery of nucleic acids356. The multivalent penetratins improved cellular uptake (∼100%) and transfection rate (>75%) and efficiently inhibited target protein expression without toxicity356. Moreover, combined use with other carriers or agents could improve the targeting therapy of nucleic acid nanomedicines. For instance, dosing a nanoprimer before administering nucleic acids-loaded LNPs could transiently occupy hepatocytes and, as a result, evade MPS uptake and improve systemic delivery of nucleic acid357,358.
6.3. Immunotherapy
Immunotherapy acts by enhancing or suppressing the innate immune responses to the diseased cells359. Nanomedicines against inflammation360, cancer, and autoimmune disorders122 have been discovered with great promise for innate immune regulation in conjunction with the adaptive immune system, such as directed immunotherapeutic approaches (e.g., checkpoint blockade), chimeric antigen receptor T cells (e.g., T cell co-stimulation) or by modifying the tumor microenvironment361. Especially with the clinical translation of the COVID-19 mRNA vaccines, nanomedicine immunotherapy is attracting tremendous attention362.
In a recent study, a macrophage-biomimetic nanomedicine was developed by macrophage membrane-coating ROS-sensitive rosiglitazone-loaded β-CD for targeted treatment of ulcerative colitis363. The preparation effectively polarized macrophages to M2, regulating the immune response and protecting epithelial cells from oxidative stresses363. The macrophage membrane guided the nanomedicine to the inflammatory colon and helped suppress inflammation by sequestering proinflammatory cytokines, exhibiting synergistic therapeutic effects against ulcerative colitis363. Wei et al.364 developed a self-assembled selenopeptide nanoparticle to strengthen tumor chemoimmunotherapy. The constructed nanoparticles delivered DOX to solid tumors and activated the natural killer cells in a programmed manner through enzyme-induced size-reduction and reactive-oxygen-species-driven desalinization364. The results indicated that the formulation significantly elevated antitumor efficacy by the chemotherapy and immunotherapy synergy induced by DOX and selenopeptide364. Nevertheless, despite many innovative features integrated into laboratory studies365, nanomedicine immunotherapy must be simple and affordable for success in the clinic366.
6.4. Photothermal therapy
PTT is a promising non-invasive method mediated by photothermal agents to generate hyperthermia under specific laser irradiation to cause local thermal damage at the target site367. The photothermal agents could be transformed from the singlet state to the excited singlet state by absorbing the photons' energy upon laser irradiation, leading to collisions between the excited photothermal agents and surrounding molecules368. Thus, the increased kinetic energy could generate heating in the confined microenvironment132. It is reported that tissues or cells exposed to temperatures up to 45 °C will initiate a heat shock response and causes irreversible damage369. Using this warming-killing mechanism, photothermal therapy has been applied in treating bacterial infection370, rheumatoid arthritis371, and cancer372, among others.
PTT shows promise in fighting bacteria with reduced resistance and toxicity. A pH-sensitive nanoparticle grafted by glycol chitosan (GCS) and polyaniline was developed for luminescence imaging-guided precise PTT373. The delivery system could respond to the bacterial acidic environment and adhere to bacteria, attaining spatial accuracy of NIR irradiation and specific photothermal effect directed to bacteria373. Another study combined PTT with chemotherapy for synergistic treatment of rheumatoid arthritis374. The methotrexate-loaded gold nanorods coated with mesoporous silica shells could target activated macrophages via surface modification of folate374. Under laser irradiation at 808 nm, the delivery system enabled local hyperthermia induced by gold nanorods' photothermal conversion and rapid release of methotrexate via internal heating-mediated hydrogen bond cleavage, leading to efficient macrophage-killing374. In recent years, PTT is gaining interest in synergistic cancer therapy by promoting the release of tumor-related antigens and triggering immune responses by inducting immunogenic cell death375, 376, 377.
6.5. Other therapeutic applications of nanomedicines
Nanotechnology demonstrates great potential to integrate multiple functions, such as imaging and diagnostic chemiluminescence, for medical use378. Accordingly, nanomedicines are also applied in radiation therapy, theranostics, postoperative medication, etc.379.
Radiotherapy (RT) is one of the first-line therapies and is always combined with chemotherapy/surgery in oncology380. Since the first recorded use to treat lesions via X-ray, RT has been increasingly utilized in curative and adjuvant treatment381. Furthermore, by employing nanomedicines as radiosensitizers, RT is conferred with improved effectiveness, targeting ability, and reduced toxicity382. Always, RT enables the production of intracellular oxygen-centric free radicals and efficient killing effect by directly damaging nuclear or consistently destructing DNA. Recently, Cheng et al.383 developed a radiosensitizer, a hybrid anisotropic nanostructure constructed by Au and titanium dioxide (TiO2), for RT against triple-negative breast cancer (TNBC). The designed Au-TiO2 nanoparticles displayed a significantly enhanced radiation effect, owing to the strong asymmetric electric coupling at their interfaces of the high atomic number metals and dielectric oxides.
Nanotechnology is also used to integrate diagnostics and therapeutics by involving multiple materials384. To obtain a chelating magnetic resonance imaging and targeting αvβ3-integrin over-expressed on tumor angiogenic endothelium simultaneously, Chen et al.385 constructed a core–shell structured nanoparticles (HSA-Ce6@HSA-RGD) by inducing the self-assembling of HSA modified with either a photosensitizer chlorine e6 or RGD. This nanotheranostic combined photodynamic and chemotherapy and offered precisely in vivo tumor imaging and remarkably improved therapeutic efficacy compared to the monotherapies385. Nanotheranostics hold potential in treating complicated diseases386.
Postoperative patients often encounter wound healing difficulties, infection, or surgical site pain; however, traditional treatment is modest to alleviate the symptoms387. The nanomedicines provide a new alternative opportunity for postoperative therapy388. For instance, Lu et al.389 constructed a wound nano-silver/chitosan dressing through nanometer and self-assembly technology. The wound dressing significantly increased the wound healing efficacy and improved the assessed biosafety389. Another study reported a nanoemulsion complex constructed using propolis and vitamin C, significantly promoting wound healing in the oral mucosa390. In addition, to reduce the pain of postoperative patients, Rose et al.391 explored liposomes as a parenteral controlled release system to prolong postoperative analgesia. The results indicated that the liposome treatment could significantly alleviate the sustained bout of pain and improve the life quality391.
7. Challenges and perspectives
Numerous issues, including low stability, nanocarrier toxicity, scaling-up difficulties, and lack of efficient quality control measures, have come to light in company with the development of nanomedicine products. Although these issues are actively addressed, the in vivo fate and acting mechanisms of nanomedicine designs remain poorly studied: (a) when and where is the drug released from nanocarriers? (b) Which substances in the body interact with nanomedicines and how? (c) Whether nanomedicines retain structural integrity following a series of in vivo disposition processes? (d) How could intact nanomedicines, free drugs, and nanocarrier materials be accurately tracked and distinguished in real time? Translating nanomedicine concepts into clinical therapeutics is constrained by an inadequate understanding of the in vivo processes.
A critical barrier to the understanding of the biodistribution, clearance, and long-term fate of nanomedicines is the lack of specialized and reliable analytical methods for quantifying drugs in tissues, organs, and cellular architectures392. Fluorescence bioimaging based on FRET393,394 has been successfully utilized to study nano-bio interactions to direct nanomedicine development393,395, as well as to visualize molecular signatures of biological systems396. Other NIR fluorescent probes based on rationales of ACQ397, 398, 399 and aggregation-induced emission (AIE)400,401 and a combination of different rationales402 have been developed to shed light on the integrity and biological fate of nanomedicines in the body. However, there are so far no perfect analytical tools for accurate real-time monitoring of nanocarriers. Dissociation of ACQ fluorophores after aggregation can lead to fluorescence re-illumination227, and AIE-based methods can also result in fluorescence re-illumination in vivo due to re-aggregation and poor linearity between fluorescence and probe concentration403, 404, 405. Differences in light penetration depth at different wavelengths can affect the accuracy of quantitative analysis by FRET imaging. The development of more reliable and specialized tools in the future may help advance our knowledge of the in vivo fate and potential acting mechanisms of nanomedicines and accelerate the translation of nano-delivery concepts from the lab to the clinic.
Acknowledgments
This work was financially supported by the Science and Technology Commission of Shanghai Municipality (No. 21430760800, China) and the National Natural Science Foundation of China (Nos. 82273867, 82030107, 82241002, and 82073782).
Author contributions
Wei Wu, Wei He, and Zhongjian Chen proposed and outlined this review. Qiuyue Liu and Jiahui Zou drafted the manuscript. Wei Wu, Wei He, and Zhongjian Chen revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Zhongjian Chen, Email: aajian818@163.com.
Wei He, Email: weihe@cpu.edu.cn.
Wei Wu, Email: wuwei@shm.edu.cn.
References
- 1.Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules. 2019;25:112. doi: 10.3390/molecules25010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Germain M., Caputo F., Metcalfe S., Tosi G., Spring K., Åslund A.K.O., et al. Delivering the power of nanomedicine to patients today. J Contr Release. 2020;326:164–171. doi: 10.1016/j.jconrel.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shan X., Gong X., Li J., Wen J., Li Y., Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12:3028–3048. doi: 10.1016/j.apsb.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heshmati Aghda N., Dabbaghianamiri M., Tunnell J.W., Betancourt T. Design of smart nanomedicines for effective cancer treatment. Int J Pharm. 2022;621:121791. doi: 10.1016/j.ijpharm.2022.121791. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen P.V., Hervé-Aubert K., Chourpa I., Allard-Vannier E. Active targeting strategy in nanomedicines using anti-EGFR ligands—a promising approach for cancer therapy and diagnosis. Int J Pharm. 2021;609:121134. doi: 10.1016/j.ijpharm.2021.121134. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y., Yu X., Thamphiwatana S.D., Zheng Y., Pang Z. Nanomedicines modulating tumor immunosuppressive cells to enhance cancer immunotherapy. Acta Pharm Sin B. 2020;10:2054–2074. doi: 10.1016/j.apsb.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z., Lu Y., Qi J., Wu W. An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm Sin B. 2021;11:2449–2468. doi: 10.1016/j.apsb.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y., Li X., Zhang W., Yu L., Wang Y., Deng Z., et al. Trends in the biological functions and medical applications of extracellular vesicles and analogues. Acta Pharm Sin B. 2021;11:2114–2135. doi: 10.1016/j.apsb.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Wang X., Zhang J., Wang L., Ou C., Su Y., et al. Gambogic acid-encapsulated polymeric micelles improved therapeutic effects on pancreatic cancer. Chin Chem Lett. 2019;30:885–888. [Google Scholar]
- 10.Yadav R., Pradhan M., Yadav K., Mahalvar A., Yadav H. Present scenarios and future prospects of herbal nanomedicine for antifungal therapy. J Drug Deliv Sci Technol. 2022;74:103430. doi: 10.1016/j.jddst.2022.103430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong N., Liu Z., He H., Lu Y., Qi J., Wu W. "Hook&Loop" multivalent interactions based on disk-shaped nanoparticles strengthen active targeting. J Contr Release. 2023;354:279–293. doi: 10.1016/j.jconrel.2023.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Huda S., Alam M.A., Sharma P.K. Smart nanocarriers-based drug delivery for cancer therapy: an innovative and developing strategy. J Drug Deliv Sci Technol. 2020;60:102018. [Google Scholar]
- 13.Alkilany A.M., Zhu L., Weller H., Mews A., Parak W.J., Barz M., et al. Ligand density on nanoparticles: a parameter with critical impact on nanomedicine. Adv Drug Deliv Rev. 2019;143:22–36. doi: 10.1016/j.addr.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Cao X., Hu Y., Luo S., Wang Y., Gong T., Sun X., et al. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B. 2019;9:575–589. doi: 10.1016/j.apsb.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Qian Y., Liu T., Zhang G., Liu S. Light-triggered concomitant enhancement of magnetic resonance imaging contrast performance and drug release rate of functionalized amphiphilic diblock copolymer micelles. Biomacromolecules. 2012;13:3877–3886. doi: 10.1021/bm301425j. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Xu C.F., Iqbal S., Yang X.Z., Wang J. Responsive nanocarriers as an emerging platform for cascaded delivery of nucleic acids to cancer. Adv Drug Deliv Rev. 2017;115:98–114. doi: 10.1016/j.addr.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Stoia D., Nistor M., Suciu M., Borlan R., Campu A., Rugina D., et al. NIR photothermal-activable drug-conjugated microcapsules for in vitro targeted delivery and release: an alternative treatment of diabetic retinopathy. Int J Pharm. 2023;635:122700. doi: 10.1016/j.ijpharm.2023.122700. [DOI] [PubMed] [Google Scholar]
- 18.Lu X., Zhang Z., Xia Q., Hou M., Yan C., Chen Z., et al. Glucose functionalized carbon quantum dot containing organic radical for optical/MR dual-modality bioimaging. Mater Sci Eng C Mater Biol Appl. 2018;82:190–196. doi: 10.1016/j.msec.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Yin D., Liao J., Wang Y., Gou R., Tang C., et al. Regulation of protein corona on liposomes using albumin-binding peptide for targeted tumor therapy. J Contr Release. 2023;355:593–603. doi: 10.1016/j.jconrel.2023.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Q., Zoulikha M., Qiu M., Teng C., Lin C., Li X., et al. The effects of protein corona on in vivo fate of nanocarriers. Adv Drug Deliv Rev. 2022;186:114356. doi: 10.1016/j.addr.2022.114356. [DOI] [PubMed] [Google Scholar]
- 21.Rosenblum D., Joshi N., Tao W., Karp J.M., Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun. 2018;9:1410. doi: 10.1038/s41467-018-03705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X.C., Zhang T., Gao J.Q. The in vivo fate and targeting engineering of crossover vesicle-based gene delivery system. Adv Drug Deliv Rev. 2022;187:114324. doi: 10.1016/j.addr.2022.114324. [DOI] [PubMed] [Google Scholar]
- 23.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 25.Wu W., Li T. Unraveling the in vivo fate and cellular pharmacokinetics of drug nanocarriers. Adv Drug Deliv Rev. 2019;143:1–2. doi: 10.1016/j.addr.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Wu W., Li T. Deepening the understanding of the in vivo and cellular fate of nanocarriers. Adv Drug Deliv Rev. 2022;189:114529. doi: 10.1016/j.addr.2022.114529. [DOI] [PubMed] [Google Scholar]
- 27.Wu W., Li T., Zheng Y. Editorial of special issue "the biological fate of drug nanocarriers". Acta Pharm Sin B. 2021;11:850–851. doi: 10.1016/j.apsb.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung J.Y., Thone M.N., Kwon Y.J. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain A., Yang H., Zhang M., Liu Q., Alotaibi G., Irfan M., et al. mRNA vaccines for COVID-19 and diverse diseases. J Contr Release. 2022;345:314–333. doi: 10.1016/j.jconrel.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taliyan R., Kakoty V., Sarathlal K.C., Kharavtekar S.S., Karennanavar C.R., Choudhary Y.K., et al. Nanocarrier mediated drug delivery as an impeccable therapeutic approach against Alzheimer's disease. J Contr Release. 2022;343:528–550. doi: 10.1016/j.jconrel.2022.01.044. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Orr A.A., Tao K., Wang Z., Ruggiero A., Shimon L.J.W., et al. High-efficiency fluorescence through bioinspired supramolecular self-assembly. ACS Nano. 2020;14:2798–2807. doi: 10.1021/acsnano.9b10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bariwal J., Ma H., Altenberg G.A., Liang H. Nanodiscs: a versatile nanocarrier platform for cancer diagnosis and treatment. Chem Soc Rev. 2022;51:1702–1728. doi: 10.1039/d1cs01074c. [DOI] [PubMed] [Google Scholar]
- 33.Kass L.E., Nguyen J. Nanocarrier–hydrogel composite delivery systems for precision drug release. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14 doi: 10.1002/wnan.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Z., Chen Q., Rong S., Dai R., Jia Z., Peng X., et al. Two-stage activated nano-truck enhanced specific aggregation and deep delivery for synergistic tumor ablation. Nanoscale. 2020;12:15845–15856. doi: 10.1039/d0nr03661g. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Zhong X., Li J., Liu Z., Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem Soc Rev. 2021;50:8669–8742. doi: 10.1039/d0cs00461h. [DOI] [PubMed] [Google Scholar]
- 36.Batty C.J., Bachelder E.M., Ainslie K.M. Historical perspective of clinical nano and microparticle formulations for delivery of therapeutics. Trends Mol Med. 2021;27:516–519. doi: 10.1016/j.molmed.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su C., Liu Y., Li R., Wu W., Fawcett J.P., Gu J. Absorption, distribution, metabolism and excretion of the biomaterials used in nanocarrier drug delivery systems. Adv Drug Deliv Rev. 2019;143:97–114. doi: 10.1016/j.addr.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Kamanzi A., Gu Y., Tahvildari R., Friedenberger Z., Zhu X., Berti R., et al. Simultaneous, single-particle measurements of size and loading give insights into the structure of drug-delivery nanoparticles. ACS Nano. 2021;15:19244–19255. doi: 10.1021/acsnano.1c04862. [DOI] [PubMed] [Google Scholar]
- 39.Escriche-Navarro B., Escudero A., Lucena-Sánchez E., Sancenón F., García-Fernández A., Martínez-Máñez R. Mesoporous silica materials as an emerging tool for cancer immunotherapy. Adv Sci. 2022;9 doi: 10.1002/advs.202200756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumbhar P., Kole K., Khadake V., Marale P., Manjappa A., Nadaf S., et al. Nanoparticulate drugs and vaccines: breakthroughs and bottlenecks of repurposing in breast cancer. J Contr Release. 2022;349:812–830. doi: 10.1016/j.jconrel.2022.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Gupta R., Chen Y., Xie H. In vitro dissolution considerations associated with nano drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13 doi: 10.1002/wnan.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Q., Feng J., Liu W., Wen C., Wu Y., Liao Q., et al. Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv Drug Deliv Rev. 2022;188:114445. doi: 10.1016/j.addr.2022.114445. [DOI] [PubMed] [Google Scholar]
- 43.Jain S., Dongare K., Nallamothu B., Parkash Dora C., Kushwah V., Katiyar S.S., et al. Enhanced stability and oral bioavailability of erlotinib by solid self nano emulsifying drug delivery systems. Int J Pharm. 2022;622:121852. doi: 10.1016/j.ijpharm.2022.121852. [DOI] [PubMed] [Google Scholar]
- 44.Davoodi P., Lee L.Y., Xu Q., Sunil V., Sun Y., Soh S., et al. Drug delivery systems for programmed and on-demand release. Adv Drug Deliv Rev. 2018;132:104–138. doi: 10.1016/j.addr.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Stater E.P., Sonay A.Y., Hart C., Grimm J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat Nanotechnol. 2021;16:1180–1194. doi: 10.1038/s41565-021-01017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magar K.T., Boafo G.F., Li X., Chen Z., He W. Liposome-based delivery of biological drugs. Chin Chem Lett. 2022;33:587–596. [Google Scholar]
- 47.Li X., Gu J., Xiao Q., Liu Y., Zhou P., Fan L., et al. Liposomal codelivery of inflammation inhibitor and collagen protector to the plaque for effective anti-atherosclerosis. Chin Chem Lett. 2023;34:107483. [Google Scholar]
- 48.Zhang J., Corpstein C.D., Li T. Intracellular uptake of nanocrystals: probing with aggregation-induced emission of fluorescence and kinetic modeling. Acta Pharm Sin B. 2021;11:1021–1029. doi: 10.1016/j.apsb.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren S., Liu M., Hong C., Li G., Sun J., Wang J., et al. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystals. Acta Pharm Sin B. 2019;9:59–73. doi: 10.1016/j.apsb.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang W., Fang Z., Zheng X., Qi J., Wu W., Lu Y. Green and controllable fabrication of nanocrystals from ionic liquids. Chin Chem Lett. 2022;33:4079–4083. [Google Scholar]
- 51.Patel D., Zode S.S., Bansal A.K. Formulation aspects of intravenous nanosuspensions. Int J Pharm. 2020;586:119555. doi: 10.1016/j.ijpharm.2020.119555. [DOI] [PubMed] [Google Scholar]
- 52.Lu Y., Li Y., Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6:106–113. doi: 10.1016/j.apsb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li G., Sun B., Li Y., Luo C., He Z., Sun J. Small-molecule prodrug nanoassemblies: an emerging nanoplatform for anticancer drug delivery. Small. 2021;17 doi: 10.1002/smll.202101460. [DOI] [PubMed] [Google Scholar]
- 54.Tan Y.L., Ho H.K. Navigating albumin-based nanoparticles through various drug delivery routes. Drug Discov Today. 2018;23:1108–1114. doi: 10.1016/j.drudis.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 55.Hwang D., Ramsey J.D., Kabanov A.V. Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020;156:80–118. doi: 10.1016/j.addr.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rai V.K., Mishra N., Yadav K.S., Yadav N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Contr Release. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 57.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4 doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen G.M., Hodgson D.F. Opportunities and challenges in commercial pharmaceutical liposome applications. Adv Drug Deliv Rev. 2020;154–155:2–12. doi: 10.1016/j.addr.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 59.Boafo G.F., Shi Y., Xiao Q., Magar K.T., Zoulikha M., Xing X., et al. Targeted co-delivery of daunorubicin and cytarabine based on the hyaluronic acid prodrug modified liposomes. Chin Chem Lett. 2022;33:4600–4604. [Google Scholar]
- 60.El Moukhtari S.H., Rodríguez-Nogales C., Blanco-Prieto M.J. Oral lipid nanomedicines: current status and future perspectives in cancer treatment. Adv Drug Deliv Rev. 2021;173:238–251. doi: 10.1016/j.addr.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Younis M.A., Tawfeek H.M., Abdellatif A.A.H., Abdel-Aleem J.A., Harashima H. Clinical translation of nanomedicines: challenges, opportunities, and keys. Adv Drug Deliv Rev. 2022;181:114083. doi: 10.1016/j.addr.2021.114083. [DOI] [PubMed] [Google Scholar]
- 62.Huang Y., Yu Q., Chen Z., Wu W., Zhu Q., Lu Y. In vitro and in vivo correlation for lipid-based formulations: current status and future perspectives. Acta Pharm Sin B. 2021;11:2469–2487. doi: 10.1016/j.apsb.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrmann I.K., Wood M.J.A., Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 64.Parodi A., Molinaro R., Sushnitha M., Evangelopoulos M., Martinez J.O., Arrighetti N., et al. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–168. doi: 10.1016/j.biomaterials.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 65.He S., Wu L., Li X., Sun H., Xiong T., Liu J., et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm Sin B. 2021;11:2362–2395. doi: 10.1016/j.apsb.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura T., Sato Y., Yamada Y., Abd Elwakil M.M., Kimura S., Younis M.A., et al. Extrahepatic targeting of lipid nanoparticles in vivo with intracellular targeting for future nanomedicines. Adv Drug Deliv Rev. 2022;188:114417. doi: 10.1016/j.addr.2022.114417. [DOI] [PubMed] [Google Scholar]
- 67.Bost J.P., Barriga H., Holme M.N., Gallud A., Maugeri M., Gupta D., et al. Delivery of oligonucleotide therapeutics: chemical modifications, lipid nanoparticles, and extracellular vesicles. ACS Nano. 2021;15:13993–14021. doi: 10.1021/acsnano.1c05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Behr M., Zhou J., Xu B., Zhang H. In vivo delivery of CRISPR-Cas9 therapeutics: progress and challenges. Acta Pharm Sin B. 2021;11:2150–2171. doi: 10.1016/j.apsb.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bottger R., Pauli G., Chao P.H., Al Fayez N., Hohenwarter L., Li S.D. Lipid-based nanoparticle technologies for liver targeting. Adv Drug Deliv Rev. 2020;154–155:79–101. doi: 10.1016/j.addr.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 70.Moro M., Di Paolo D., Milione M., Centonze G., Bornaghi V., Borzi C., et al. Coated cationic lipid-nanoparticles entrapping miR-660 inhibit tumor growth in patient-derived xenografts lung cancer models. J Contr Release. 2019;308:44–56. doi: 10.1016/j.jconrel.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Hou X., Zhang X., Zhao W., Zeng C., Deng B., McComb D.W., et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat Nanotechnol. 2020;15:41–46. doi: 10.1038/s41565-019-0600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Evers M.J.W., Du W., Yang Q., Kooijmans S.A.A., Vink A., van Steenbergen M., et al. Delivery of modified mRNA to damaged myocardium by systemic administration of lipid nanoparticles. J Contr Release. 2022;343:207–216. doi: 10.1016/j.jconrel.2022.01.027. [DOI] [PubMed] [Google Scholar]
- 73.Patel R.B., Rao H.R., Thakkar D.V., Patel M.R. Comprehending the potential of metallic, lipid, and polymer-based nanocarriers for treatment and management of depression. Neurochem Int. 2022;153:105259. doi: 10.1016/j.neuint.2021.105259. [DOI] [PubMed] [Google Scholar]
- 74.Araujo V.H.S., Delello Di Filippo L., Duarte J.L., Spósito L., Camargo B.A.F., da Silva P.B., et al. Exploiting solid lipid nanoparticles and nanostructured lipid carriers for drug delivery against cutaneous fungal infections. Crit Rev Microbiol. 2021;47:79–90. doi: 10.1080/1040841X.2020.1843399. [DOI] [PubMed] [Google Scholar]
- 75.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 76.Pattipeiluhu R., Arias-Alpizar G., Basha G., Chan K.Y.T., Bussmann J., Sharp T.H., et al. Anionic lipid nanoparticles preferentially deliver mRNA to the hepatic reticuloendothelial system. Adv Mater. 2022;34 doi: 10.1002/adma.202201095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LoPresti S.T., Arral M.L., Chaudhary N., Whitehead K.A. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J Contr Release. 2022;345:819–831. doi: 10.1016/j.jconrel.2022.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zoulikha M., Huang F., Wu Z., He W. COVID-19 inflammation and implications in drug delivery. J Contr Release. 2022;346:260–274. doi: 10.1016/j.jconrel.2022.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hald Albertsen C., Kulkarni J., Witzigmann D., Lind M., Petersson K., Simonsen J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022;188:114416. doi: 10.1016/j.addr.2022.114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y., Sun C., Wang C., Jankovic K.E., Dong Y. Lipids and lipid derivatives for RNA delivery. Chem Rev. 2021;121:12181–12277. doi: 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y., Xia Y., Smollar J., Mao W., Wan Y. The roles of small extracellular vesicles in lung cancer: molecular pathology, mechanisms, diagnostics, and therapeutics. Biochim Biophys Acta Rev Cancer. 2021;1876:188539. doi: 10.1016/j.bbcan.2021.188539. [DOI] [PubMed] [Google Scholar]
- 82.Rankin-Turner S., Vader P., O'Driscoll L., Giebel B., Heaney L.M., Davies O.G. A call for the standardised reporting of factors affecting the exogenous loading of extracellular vesicles with therapeutic cargos. Adv Drug Deliv Rev. 2021;173:479–491. doi: 10.1016/j.addr.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pirisinu M., Pham T.C., Zhang D.X., Hong T.N., Nguyen L.T., Le M.T. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: recent advances, current obstacles, and challenges for clinical translation. Semin Cancer Biol. 2022;80:340–355. doi: 10.1016/j.semcancer.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 84.Thakur A., Ke X., Chen Y.W., Motallebnejad P., Zhang K., Lian Q., et al. The mini player with diverse functions: extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell. 2022;13:631–654. doi: 10.1007/s13238-021-00863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H., Chengalvala V., Hu H., Sun D. Tumor-derived exosomes: nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B. 2021;11:2136–2149. doi: 10.1016/j.apsb.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Niu W., Xiao Q., Wang X., Zhu J., Li J., Liang X., et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21:1484–1492. doi: 10.1021/acs.nanolett.0c04753. [DOI] [PubMed] [Google Scholar]
- 87.Richter M., Vader P., Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv Drug Deliv Rev. 2021;173:416–426. doi: 10.1016/j.addr.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 88.Gao J., Wang S., Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kooijmans S.A.A., de Jong O.G., Schiffelers R.M. Exploring interactions between extracellular vesicles and cells for innovative drug delivery system design. Adv Drug Deliv Rev. 2021;173:252–278. doi: 10.1016/j.addr.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 90.Cheng L., Hill A.F. Therapeutically harnessing extracellular vesicles. Nat Rev Drug Discov. 2022;21:379–399. doi: 10.1038/s41573-022-00410-w. [DOI] [PubMed] [Google Scholar]
- 91.Draguet F., Bouland C., Dubois N., Bron D., Meuleman N., Stamatopoulos B., et al. Potential of mesenchymal stromal cell-derived extracellular vesicles as natural nanocarriers: concise review. Pharmaceutics. 2023;15:558. doi: 10.3390/pharmaceutics15020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cong V.T., Houng J.L., Kavallaris M., Chen X., Tilley R.D., Gooding J.J. How can we use the endocytosis pathways to design nanoparticle drug-delivery vehicles to target cancer cells over healthy cells? Chem Soc Rev. 2022;51:7531–7559. doi: 10.1039/d1cs00707f. [DOI] [PubMed] [Google Scholar]
- 93.Haroon H.B., Hunter A.C., Farhangrazi Z.S., Moghimi S.M. A brief history of long circulating nanoparticles. Adv Drug Deliv Rev. 2022;188:114396. doi: 10.1016/j.addr.2022.114396. [DOI] [PubMed] [Google Scholar]
- 94.Lee G., Choi H.E., Hong S.H., Choi M., Han D.W., Lee J., et al. Upconversion nanomaterials and delivery systems for smart photonic medicines and healthcare devices. Adv Drug Deliv Rev. 2022;188:114419. doi: 10.1016/j.addr.2022.114419. [DOI] [PubMed] [Google Scholar]
- 95.Karimi M., Kamali H., Fakhrmohammadi S., Khezri E., Malaekeh-Nikouei B., Mohammadi M. Prolonged local delivery of doxorubicin to cancer cells using lipid liquid crystalline system. Int J Pharm. 2023;639:122947. doi: 10.1016/j.ijpharm.2023.122947. [DOI] [PubMed] [Google Scholar]
- 96.Sabu C., Rejo C., Kotta S., Pramod K. Bioinspired and biomimetic systems for advanced drug and gene delivery. J Contr Release. 2018;287:142–155. doi: 10.1016/j.jconrel.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 97.Johnson A.P., Sabu C., Nivitha K.P., Sankar R., Ameena Shirin V.K., Henna T.K., et al. Bioinspired and biomimetic micro- and nanostructures in biomedicine. J Contr Release. 2022;343:724–754. doi: 10.1016/j.jconrel.2022.02.013. [DOI] [PubMed] [Google Scholar]
- 98.Gagliardi M. Biomimetic and bioinspired nanoparticles for targeted drug delivery. Ther Deliv. 2017;8:289–299. doi: 10.4155/tde-2017-0013. [DOI] [PubMed] [Google Scholar]
- 99.Le Q.V., Lee J., Lee H., Shim G., Oh Y.K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm Sin B. 2021;11:2096–2113. doi: 10.1016/j.apsb.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mao Y., Zou C., Jiang Y., Fu D. Erythrocyte-derived drug delivery systems in cancer therapy. Chin Chem Lett. 2021;32:990–998. [Google Scholar]
- 101.Dhas N., García M.C., Kudarha R., Pandey A., Nikam A.N., Gopalan D., et al. Advancements in cell membrane camouflaged nanoparticles: a bioinspired platform for cancer therapy. J Contr Release. 2022;346:71–97. doi: 10.1016/j.jconrel.2022.04.019. [DOI] [PubMed] [Google Scholar]
- 102.Liu X., Zhong X., Li C. Challenges in cell membrane-camouflaged drug delivery systems: development strategies and future prospects. Chin Chem Lett. 2021;32:2347–2358. [Google Scholar]
- 103.Song Y., Huang Z., Liu X., Pang Z., Chen J., Yang H., et al. Platelet membrane-coated nanoparticle-mediated targeting delivery of Rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE−/−) mice. Nanomedicine. 2019;15:13–24. doi: 10.1016/j.nano.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 104.Bu L.L., Rao L., Yu G.T., Chen L., Deng W.W., Liu J.F., et al. Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv Funct Mater. 2019;29:1807733. [Google Scholar]
- 105.Yoo J.W., Irvine D.J., Discher D.E., Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 106.Sheikhpour M., Barani L., Kasaeian A. Biomimetics in drug delivery systems: a critical review. J Contr Release. 2017;253:97–109. doi: 10.1016/j.jconrel.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 107.Pelaz B., Alexiou C., Alvarez-Puebla R.A., Alves F., Andrews A.M., Ashraf S., et al. Diverse applications of nanomedicine. ACS Nano. 2017;11:2313–2381. doi: 10.1021/acsnano.6b06040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harris J.C., Scully M.A., Day E.S. Cancer cell membrane-coated nanoparticles for cancer management. Cancers. 2019;11:1836. doi: 10.3390/cancers11121836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gareev K.G., Grouzdev D.S., Koziaeva V.V., Sitkov N.O., Gao H., Zimina T.M., et al. Biomimetic nanomaterials: diversity, technology, and biomedical applications. Nanomaterials. 2022;12:2485. doi: 10.3390/nano12142485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun Y., Zheng L., Yang Y., Qian X., Fu T., Li X., et al. Metal-organic framework nanocarriers for drug delivery in biomedical applications. Nano-Micro Lett. 2020;12:103. doi: 10.1007/s40820-020-00423-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chu C., Su M., Zhu J., Li D., Cheng H., Chen X., et al. Metal-organic framework nanoparticle-based biomineralization: a new strategy toward cancer treatment. Theranostics. 2019;9:3134–3149. doi: 10.7150/thno.33539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McKinlay A.C., Morris R.E., Horcajada P., Férey G., Gref R., Couvreur P., et al. BioMOFs: metal-organic frameworks for biological and medical applications. Angew Chem Int Ed Engl. 2010;49:6260–6266. doi: 10.1002/anie.201000048. [DOI] [PubMed] [Google Scholar]
- 113.Huxford R.C., Della Rocca J., Lin W. Metal-organic frameworks as potential drug carriers. Curr Opin Chem Biol. 2010;14:262–268. doi: 10.1016/j.cbpa.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gangu K.K., Maddila S., Mukkamala S.B., Jonnalagadda S.B. A review on contemporary metal-organic framework materials. Inorg Chim Acta. 2016;446:61–74. [Google Scholar]
- 115.Zhao H., Li T., Yao C., Gu Z., Liu C., Li J., et al. Dual roles of metal-organic frameworks as nanocarriers for miRNA delivery and adjuvants for chemodynamic therapy. ACS Appl Mater Interfaces. 2021;13:6034–6042. doi: 10.1021/acsami.0c21006. [DOI] [PubMed] [Google Scholar]
- 116.Meng J., Liu X., Niu C., Pang Q., Li J., Liu F., et al. Advances in metal-organic framework coatings: versatile synthesis and broad applications. Chem Soc Rev. 2020;49:3142–3186. doi: 10.1039/c9cs00806c. [DOI] [PubMed] [Google Scholar]
- 117.Zhuang J., Gong H., Zhou J., Zhang Q., Gao W., Fang R.H., et al. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci Adv. 2020;6 doi: 10.1126/sciadv.aaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang S., McGuirk C.M. d'Aquino A, Mason JA, Mirkin CA. Metal-organic framework nanoparticles. Adv Mater. 2018;30:1800202. doi: 10.1002/adma.201800202. [DOI] [PubMed] [Google Scholar]
- 119.Cai X., Bao X., Wu Y. Metal-organic frameworks as intelligent drug nanocarriers for cancer therapy. Pharmaceutics. 2022;14:2641. doi: 10.3390/pharmaceutics14122641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guo L., Chen Y., Wang T., Yuan Y., Yang Y., Luo X., et al. Rational design of metal-organic frameworks to deliver methotrexate for targeted rheumatoid arthritis therapy. J Contr Release. 2021;330:119–131. doi: 10.1016/j.jconrel.2020.10.069. [DOI] [PubMed] [Google Scholar]
- 121.Ding M., Liu W., Gref R. Nanoscale MOFs: from synthesis to drug delivery and theranostics applications. Adv Drug Deliv Rev. 2022;190:114496. doi: 10.1016/j.addr.2022.114496. [DOI] [PubMed] [Google Scholar]
- 122.Bae Y.H., Park K. Advanced drug delivery 2020 and beyond: perspectives on the future. Adv Drug Deliv Rev. 2020;158:4–16. doi: 10.1016/j.addr.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hong T., Shen X., Syeda M.Z., Zhang Y., Sheng H., Zhou Y., et al. Recent advances of bioresponsive polymeric nanomedicine for cancer therapy. Nano Res. 2023;16:2660–2671. doi: 10.1007/s12274-022-5002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karimi M., Ghasemi A., Sahandi Zangabad P., Rahighi R., Moosavi Basri S.M., Mirshekari H., et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457–1501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Devnarain N., Osman N., Fasiku V.O., Makhathini S., Salih M., Ibrahim U.H., et al. Intrinsic stimuli-responsive nanocarriers for smart drug delivery of antibacterial agents—an in-depth review of the last two decades. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13 doi: 10.1002/wnan.1664. [DOI] [PubMed] [Google Scholar]
- 126.Yuan Z., Gottsacker C., He X., Waterkotte T., Park Y.C. Repetitive drug delivery using light-activated liposomes for potential antimicrobial therapies. Adv Drug Deliv Rev. 2022;187:114395. doi: 10.1016/j.addr.2022.114395. [DOI] [PubMed] [Google Scholar]
- 127.Yang L., Hou X., Zhang Y., Wang D., Liu J., Huang F., et al. NIR-activated self-sensitized polymeric micelles for enhanced cancer chemo-photothermal therapy. J Contr Release. 2021;339:114–129. doi: 10.1016/j.jconrel.2021.09.017. [DOI] [PubMed] [Google Scholar]
- 128.Jin Q., Cai T., Wang Y., Wang H., Ji J. Light-responsive polyion complex micelles with switchable surface charge for efficient protein delivery. ACS Macro Lett. 2014;3:679–683. doi: 10.1021/mz500290s. [DOI] [PubMed] [Google Scholar]
- 129.Li C., Zhang Y., Li Z., Mei E., Lin J., Li F., et al. Light-responsive biodegradable nanorattles for cancer theranostics. Adv Mater. 2018;30:1706150. doi: 10.1002/adma.201706150. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y., Chen J., Hua X., Meng X., Cai H., Wang R., et al. Photocaging of activity-based ubiquitin probes via a C-terminal backbone modification strategy. Angew Chem Int Ed Engl. 2022;61 doi: 10.1002/anie.202203792. [DOI] [PubMed] [Google Scholar]
- 131.Huang R., Lan R., Shen C., Zhang Z., Wang Z., Bao J., et al. Remotely controlling drug release by light-responsive cholesteric liquid crystal microcapsules triggered by molecular motors. ACS Appl Mater Interfaces. 2021;13:59221–59230. doi: 10.1021/acsami.1c16367. [DOI] [PubMed] [Google Scholar]
- 132.Gupta N., Malviya R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188532. doi: 10.1016/j.bbcan.2021.188532. [DOI] [PubMed] [Google Scholar]
- 133.Zha M., Yang G., Li Y., Zhang C., Li B., Li K. Recent advances in AIEgen-based photodynamic therapy and immunotherapy. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202101066. [DOI] [PubMed] [Google Scholar]
- 134.Cai Y., Wei Z., Song C., Tang C., Han W., Dong X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem Soc Rev. 2019;48:22–37. doi: 10.1039/c8cs00494c. [DOI] [PubMed] [Google Scholar]
- 135.Arun Kumar S., Good J., Hendrix D., Yoo E., Kim D., Deo K.A., et al. Nanoengineered light-activatable polybubbles for on-demand therapeutic delivery. Adv Funct Mater. 2020;30:2003579. doi: 10.1002/adfm.202003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen L., Li G., Wang X., Li J., Zhang Y. Spherical nucleic acids for near-infrared light-responsive self-delivery of small-interfering RNA and antisense oligonucleotide. ACS Nano. 2021;15:11929–11939. doi: 10.1021/acsnano.1c03072. [DOI] [PubMed] [Google Scholar]
- 137.Vickerman B.M., O'Banion C.P., Tan X., Lawrence D.S. Light-controlled release of therapeutic proteins from red blood cells. ACS Cent Sci. 2021;7:93–103. doi: 10.1021/acscentsci.0c01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Long K., Yang Y., Lv W., Jiang K., Li Y., Lo A.C.Y., et al. Green light-triggered intraocular drug release for intravenous chemotherapy of retinoblastoma. Adv Sci. 2021;8 doi: 10.1002/advs.202101754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Day N.B., Wixson W.C., Shields C.W. 4th. Magnetic systems for cancer immunotherapy. Acta Pharm Sin B. 2021;11:2172–2196. doi: 10.1016/j.apsb.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Domagalski J.T., Xifre-Perez E., Tabrizi M.A., Ferre-Borrull J., Marsal L.F. Magnetic nanoparticle decorated anodic alumina nanotubes for fluorescent detection of cathepsin B. J Colloid Interface Sci. 2021;584:236–245. doi: 10.1016/j.jcis.2020.09.109. [DOI] [PubMed] [Google Scholar]
- 141.Kono Y., Nakai T., Taguchi H., Fujita T. Development of magnetic anionic liposome/atelocollagen complexes for efficient magnetic drug targeting. Drug Deliv. 2017;24:1740–1749. doi: 10.1080/10717544.2017.1402219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y., Fens M.H., van Kronenburg N.C.H., Shi Y., Lammers T., Heger M., et al. Magnetic beads for the evaluation of drug release from biotinylated polymeric micelles in biological media. J Contr Release. 2022;349:954–962. doi: 10.1016/j.jconrel.2022.07.044. [DOI] [PubMed] [Google Scholar]
- 143.Song G., Chen M., Zhang Y., Cui L., Qu H., Zheng X., et al. Janus iron oxides @ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett. 2018;18:182–189. doi: 10.1021/acs.nanolett.7b03829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chan N., Laprise-Pelletier M., Chevallier P., Bianchi A., Fortin M.A., Oh J.K. Multidentate block-copolymer-stabilized ultrasmall superparamagnetic iron oxide nanoparticles with enhanced colloidal stability for magnetic resonance imaging. Biomacromolecules. 2014;15:2146–2156. doi: 10.1021/bm500311k. [DOI] [PubMed] [Google Scholar]
- 145.Yang X., Zhang C., Deng D., Gu Y., Wang H., Zhong Q. Multiple stimuli-responsive MXene-based hydrogel as intelligent drug delivery carriers for deep chronic wound healing. Small. 2022;18 doi: 10.1002/smll.202104368. [DOI] [PubMed] [Google Scholar]
- 146.Yin B., Zhang Q., Xia X., Li C., Ho W., Yan J., et al. A CRISPR-Cas12a integrated SERS nanoplatform with chimeric DNA/RNA hairpin guide for ultrasensitive nucleic acid detection. Theranostics. 2022;12:5914–5930. doi: 10.7150/thno.75816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chung C.W., Liao B.W., Huang S.W., Chiou S.J., Chang C.H., Lin S.J., et al. Magnetic responsive release of nitric oxide from an MOF-derived Fe3O4@PLGA microsphere for the treatment of bacteria-infected cutaneous wound. ACS Appl Mater Interfaces. 2022;14:6343–6357. doi: 10.1021/acsami.1c20802. [DOI] [PubMed] [Google Scholar]
- 148.Schleich N., Po C., Jacobs D., Ucakar B., Gallez B., Danhier F., et al. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J Contr Release. 2014;194:82–91. doi: 10.1016/j.jconrel.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 149.Athanassiadis A.G., Ma Z., Moreno-Gomez N., Melde K., Choi E., Goyal R., et al. Ultrasound-responsive systems as components for smart materials. Chem Rev. 2022;122:5165–5208. doi: 10.1021/acs.chemrev.1c00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fan K., Zeng L., Guo J., Xie S., Yu Y., Chen J., et al. Visualized podocyte-targeting and focused ultrasound responsive glucocorticoid nano-delivery system against immune-associated nephropathy without glucocorticoid side effect. Theranostics. 2021;11:2670–2690. doi: 10.7150/thno.53083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang L., Lu H., Gao Q., Yuan C., Ding F., Li J., et al. A multifunctional theranostic contrast agent for ultrasound/near infrared fluorescence imaging-based tumor diagnosis and ultrasound-triggered combined photothermal and gene therapy. Acta Biomater. 2019;99:373–386. doi: 10.1016/j.actbio.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 152.Huang L., Quesada C., Aliabouzar M., Fowlkes J.B., Franceschi R.T., Liu Z., et al. Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds. Acta Biomater. 2021;129:73–83. doi: 10.1016/j.actbio.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang J., Li Y., Sun J., Zou H., Sun Y., Luo J., et al. An osimertinib-perfluorocarbon nanoemulsion with excellent targeted therapeutic efficacy in non-small cell lung cancer: achieving intratracheal and intravenous administration. ACS Nano. 2022;16:12590–12605. doi: 10.1021/acsnano.2c04159. [DOI] [PubMed] [Google Scholar]
- 154.Mehta S., Bongcaron V., Nguyen T.K., Jirwanka Y., Maluenda A., Walsh A.P.G., et al. An ultrasound-responsive theranostic cyclodextrin-loaded nanoparticle for multimodal imaging and therapy for atherosclerosis. Small. 2022;18 doi: 10.1002/smll.202200967. [DOI] [PubMed] [Google Scholar]
- 155.Cui X., Han X., Yu L., Zhang B., Chen Y. Intrinsic chemistry and design principle of ultrasound-responsive nanomedicine. Nano Today. 2019;28:100773. [Google Scholar]
- 156.Lammers T., Koczera P., Fokong S., Gremse F., Ehling J., Vogt M., et al. Theranostic USPIO-loaded microbubbles for mediating and monitoring blood‒brain barrier permeation. Adv Funct Mater. 2015;25:36–43. doi: 10.1002/adfm.201401199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cai X., Jiang Y., Lin M., Zhang J., Guo H., Yang F., et al. Ultrasound-responsive materials for drug/gene delivery. Front Pharmacol. 2020;10:1650. doi: 10.3389/fphar.2019.01650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Entzian K., Aigner A. Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics. 2021;13:1135. doi: 10.3390/pharmaceutics13081135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Batool N., Sarfraz R.M., Mahmood A., Zafar N., Minhas M.U., Hussain Z., et al. Biocompatible polymeric blend for pH driven delivery of cytarabine: effect of feed contents on swelling and release kinetics. J Biomed Mater Res B Appl Biomater. 2022;110:1545–1562. doi: 10.1002/jbm.b.35016. [DOI] [PubMed] [Google Scholar]
- 160.Kim J.S., Choi D.K., Shin J.Y., Shin S.M., Park S.W., Cho H.S., et al. Endosomal acidic pH-induced conformational changes of a cytosol-penetrating antibody mediate endosomal escape. J Contr Release. 2016;235:165–175. doi: 10.1016/j.jconrel.2016.05.066. [DOI] [PubMed] [Google Scholar]
- 161.Boedtkjer E., Pedersen S.F. The acidic tumor microenvironment as a driver of cancer. Annu Rev Physiol. 2020;82:103–126. doi: 10.1146/annurev-physiol-021119-034627. [DOI] [PubMed] [Google Scholar]
- 162.Ding H., Tan P., Fu S., Tian X., Zhang H., Ma X., et al. Preparation and application of pH-responsive drug delivery systems. J Contr Release. 2022;348:206–238. doi: 10.1016/j.jconrel.2022.05.056. [DOI] [PubMed] [Google Scholar]
- 163.Pujara N., Giri R., Wong K.Y., Qu Z., Rewatkar P., Moniruzzaman M., et al. pH-Responsive colloidal carriers assembled from β-lactoglobulin and Epsilon poly-L-lysine for oral drug delivery. J Colloid Interface Sci. 2021;589:45–55. doi: 10.1016/j.jcis.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 164.Ploetz E., Zimpel A., Cauda V., Bauer D., Lamb D.C., Haisch C., et al. Metal-organic framework nanoparticles induce pyroptosis in cells controlled by the extracellular pH. Adv Mater. 2020;32 doi: 10.1002/adma.201907267. [DOI] [PubMed] [Google Scholar]
- 165.Jensen M.M., Jia W., Schults A.J., Isaacson K.J., Steinhauff D., Green B., et al. Temperature-responsive silk-elastinlike protein polymer enhancement of intravesical drug delivery of a therapeutic glycosaminoglycan for treatment of interstitial cystitis/painful bladder syndrome. Biomaterials. 2019;217:119293. doi: 10.1016/j.biomaterials.2019.119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Karimi M., Sahandi Zangabad P., Ghasemi A., Amiri M., Bahrami M., Malekzad H., et al. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS Appl Mater Interfaces. 2016;8:21107–21133. doi: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mansfield E.D.H., Filippov S.K., de la Rosa V.R., Cook M.T., Grillo I., Hoogenboom R., et al. Understanding the temperature induced aggregation of silica nanoparticles decorated with temperature-responsive polymers: can a small step in the chemical structure make a giant leap for a phase transition? J Colloid Interface Sci. 2021;590:249–259. doi: 10.1016/j.jcis.2021.01.044. [DOI] [PubMed] [Google Scholar]
- 168.Prawatborisut M., Oberlander J., Jiang S., Graf R., Avlasevich Y., Morsbach S., et al. Temperature-responsive nanoparticles enable specific binding of apolipoproteins from human plasma. Small. 2022;18 doi: 10.1002/smll.202103138. [DOI] [PubMed] [Google Scholar]
- 169.Inoue Y., Takada K., Kawamura A., Miyata T. Amphiphilic liquid crystalline polymer micelles that exhibit a phase transition at body temperature. ACS Appl Mater Interfaces. 2022;14:31513–31524. doi: 10.1021/acsami.2c00592. [DOI] [PubMed] [Google Scholar]
- 170.Wang M., Gao B., Wang X., Li W., Feng Y. Enzyme-responsive strategy as a prospective cue to construct intelligent biomaterials for disease diagnosis and therapy. Biomater Sci. 2022;10:1883–1903. doi: 10.1039/d2bm00067a. [DOI] [PubMed] [Google Scholar]
- 171.Alkekhia D., LaRose C., Shukla A. β-Lactamase-responsive hydrogel drug delivery platform for bacteria-triggered cargo release. ACS Appl Mater Interfaces. 2022;14:27538–27550. doi: 10.1021/acsami.2c02614. [DOI] [PubMed] [Google Scholar]
- 172.Liang B., Jiang D., Pan L., Xiong F., Feng S., Wu S., et al. Lipase-triggered drug release from BCL2 inhibitor ABT-199-loaded nanoparticles to elevate anti-leukemic activity through enhanced drug targeting on the mitochondrial membrane. Acta Biomater. 2022;145:246–259. doi: 10.1016/j.actbio.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 173.Shahriari M., Zahiri M., Abnous K., Taghdisi S.M., Ramezani M., Alibolandi M. Enzyme responsive drug delivery systems in cancer treatment. J Contr Release. 2019;308:172–189. doi: 10.1016/j.jconrel.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 174.Gao D., Asghar S., Ye J., Zhang M., Hu R., Wang Y., et al. Dual-targeted enzyme-sensitive hyaluronic acid nanogels loading paclitaxel for the therapy of breast cancer. Carbohydr Polym. 2022;294:119785. doi: 10.1016/j.carbpol.2022.119785. [DOI] [PubMed] [Google Scholar]
- 175.Lu G., Gao X., Zhang H., Zhang Y., Yu Y., Sun Z., et al. Near-infrared light(NIR)-responsive nanoliposomes combining photodynamic therapy and chemotherapy for breast tumor control. Chin Chem Lett. 2022;33:1923–1926. [Google Scholar]
- 176.He Y., Lei L., Cao J., Yang X., Cai S., Tong F., et al. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci Adv. 2021;7 doi: 10.1126/sciadv.aba0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen M., Liu D., Liu F., Wu Y., Peng X., Song F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J Contr Release. 2021;332:269–284. doi: 10.1016/j.jconrel.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 178.Li D., Zhang R., Liu G., Kang Y., Wu J. Redox-responsive self-assembled nanoparticles for cancer therapy. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.202000605. [DOI] [PubMed] [Google Scholar]
- 179.Lou X., Zhang D., Ling H., He Z., Sun J., Sun M., et al. Pure redox-sensitive paclitaxel-maleimide prodrug nanoparticles: endogenous albumin-induced size switching and improved antitumor efficiency. Acta Pharm Sin B. 2021;11:2048–2058. doi: 10.1016/j.apsb.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhong W., Zhang X., Duan X., Liu H., Fang Y., Luo M., et al. Redox-responsive self-assembled polymeric nanoprodrug for delivery of gemcitabine in B-cell lymphoma therapy. Acta Biomater. 2022;144:67–80. doi: 10.1016/j.actbio.2022.03.035. [DOI] [PubMed] [Google Scholar]
- 181.Banstola A., Poudel K., Kim J.O., Jeong J.H., Yook S. Recent progress in stimuli-responsive nanosystems for inducing immunogenic cell death. J Contr Release. 2021;337:505–520. doi: 10.1016/j.jconrel.2021.07.038. [DOI] [PubMed] [Google Scholar]
- 182.Hang L., Shen C., Shen B., Yuan H. Insight into the in vivo fate of intravenous herpetrione amorphous nanosuspensions by aggregation-caused quenching probes. Chin Chem Lett. 2022;33:4948–4951. [Google Scholar]
- 183.Cai Y., Qi J., Lu Y., He H., Wu W. The in vivo fate of polymeric micelles. Adv Drug Deliv Rev. 2022;188:114463. doi: 10.1016/j.addr.2022.114463. [DOI] [PubMed] [Google Scholar]
- 184.González-Mariscal L., Nava P., Hernández S. Critical role of tight junctions in drug delivery across epithelial and endothelial cell layers. J Membr Biol. 2005;207:55–68. doi: 10.1007/s00232-005-0807-y. [DOI] [PubMed] [Google Scholar]
- 185.He B., Lin P., Jia Z., Du W., Qu W., Yuan L., et al. The transport mechanisms of polymer nanoparticles in Caco-2 epithelial cells. Biomaterials. 2013;34:6082–6098. doi: 10.1016/j.biomaterials.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 186.He H., Xie Y., Lv Y., Qi J., Dong X., Zhao W., et al. Bioimaging of intact polycaprolactone nanoparticles using aggregation-caused quenching probes: size-dependent translocation via oral delivery. Adv Healthc Mater. 2018;7 doi: 10.1002/adhm.201800711. [DOI] [PubMed] [Google Scholar]
- 187.Wu L., Bai Y., Liu M., Li L., Shan W., Zhang Z., et al. Transport mechanisms of butyrate modified nanoparticles: insight into "easy entry, hard transcytosis" of active targeting system in oral administration. Mol Pharm. 2018;15:4273–4283. doi: 10.1021/acs.molpharmaceut.8b00713. [DOI] [PubMed] [Google Scholar]
- 188.Zoya I., He H., Wang L., Qi J., Lu Y., Wu W. The intragastrointestinal fate of paclitaxel-loaded micelles: implications on oral drug delivery. Chin Chem Lett. 2021;32:1545–1549. [Google Scholar]
- 189.Bisht R., Mandal A., Jaiswal J.K., Rupenthal I.D. Nanocarrier mediated retinal drug delivery: overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10 doi: 10.1002/wnan.1473. [DOI] [PubMed] [Google Scholar]
- 190.Fan W., Peng H., Yu Z., Wang L., He H., Ma Y., et al. The long-circulating effect of pegylated nanoparticles revisited via simultaneous monitoring of both the drug payloads and nanocarriers. Acta Pharm Sin B. 2022;12:2479–2493. doi: 10.1016/j.apsb.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Decuzzi P., Pasqualini R., Arap W., Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res (N Y) 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 192.Salvati A., Pitek A.S., Monopoli M.P., Prapainop K., Bombelli F.B., Hristov D.R., et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 193.Docter D., Distler U., Storck W., Kuharev J., Wünsch D., Hahlbrock A., et al. Quantitative profiling of the protein coronas that form around nanoparticles. Nat Protoc. 2014;9:2030–2044. doi: 10.1038/nprot.2014.139. [DOI] [PubMed] [Google Scholar]
- 194.Röcker C., Pötzl M., Zhang F., Parak W.J., Nienhaus G.U. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat Nanotechnol. 2009;4:577–580. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 195.Champion J.A., Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Champion J.A., Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res (N Y) 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Arvizo R.R., Miranda O.R., Moyano D.F., Walden C.A., Giri K., Bhattacharya R., et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yao C., Wang P., Zhou L., Wang R., Li X., Zhao D., et al. Highly biocompatible zwitterionic phospholipids coated upconversion nanoparticles for efficient bioimaging. Anal Chem. 2014;86:9749–9757. doi: 10.1021/ac5023259. [DOI] [PubMed] [Google Scholar]
- 199.Kreuter J., Shamenkov D., Petrov V., Ramge P., Cychutek K., Koch-Brandt C., et al. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood‒brain barrier. J Drug Target. 2002;10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 200.da Silva I.V., Cardoso C., Martinez-Banaclocha H., Casini A., Pelegrin P., Soveral G. Aquaporin-3 is involved in NLRP3-inflammasome activation contributing to the setting of inflammatory response. Cell Mol Life Sci. 2021;78:3073–3085. doi: 10.1007/s00018-020-03708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hara-Chikuma M., Tanaka M., Verkman A.S., Yasui M. Inhibition of aquaporin-3 in macrophages by a monoclonal antibody as potential therapy for liver injury. Nat Commun. 2020;11:5666. doi: 10.1038/s41467-020-19491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gazit Y., Berk D.A., Leunig M., Baxter L.T., Jain R.K. Scale-invariant behavior and vascular network formation in normal and tumor tissue. Phys Rev Lett. 1995;75:2428–2431. doi: 10.1103/PhysRevLett.75.2428. [DOI] [PubMed] [Google Scholar]
- 203.Hussain S.T. Blood flow measurements in lower limb arteries using duplex ultrasound. Ann R Coll Surg Engl. 1997;79:323–330. [PMC free article] [PubMed] [Google Scholar]
- 204.Sakariassen K.S., Bolhuis P.A., Sixma J.J. Platelet adherence to subendothelium of human arteries in pulsatile and steady flow. Thromb Res. 1980;19:547–559. doi: 10.1016/0049-3848(80)90027-4. [DOI] [PubMed] [Google Scholar]
- 205.Lowe G.D. Virchow's triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–457. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 206.Ruggeri Z.M. Platelet adhesion under flow. Microcirculation. 2009;16:58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Decuzzi P., Lee S., Bhushan B., Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng. 2005;33:179–190. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 208.Tan J., Shah S., Thomas A., Ou-Yang H.D., Liu Y. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid Nanofluidics. 2013;14:77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Decuzzi P., Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 210.Charoenphol P., Huang R.B., Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31:1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 211.Jain R.K. Barriers to drug delivery in solid tumors. Sci Am. 1994;271:58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 212.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 213.Maeda H., Nakamura H., Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 214.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 215.Murphy E.A., Majeti B.K., Barnes L.A., Makale M., Weis S.M., Lutu-Fuga K., et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yokoi K., Tanei T., Godin B., van de Ven A.L., Hanibuchi M., Matsunoki A., et al. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer Lett. 2014;345:48–55. doi: 10.1016/j.canlet.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yokoi K., Kojic M., Milosevic M., Tanei T., Ferrari M., Ziemys A. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. 2014;74:4239–4246. doi: 10.1158/0008-5472.CAN-13-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Hu J., Yuan X., Wang F., Gao H., Liu X., Zhang W. The progress and perspective of strategies to improve tumor penetration of nanomedicines. Chin Chem Lett. 2021;32:1341–1347. [Google Scholar]
- 219.Heldin C.H., Rubin K., Pietras K., Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 220.Donahue N.D., Acar H., Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. doi: 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 221.Mazumdar S., Chitkara D., Mittal A. Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers. Acta Pharm Sin B. 2021;11:903–924. doi: 10.1016/j.apsb.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hadji H., Bouchemal K. Effect of micro- and nanoparticle shape on biological processes. J Contr Release. 2022;342:93–110. doi: 10.1016/j.jconrel.2021.12.032. [DOI] [PubMed] [Google Scholar]
- 223.Wang Y., Wang J., Zhu D., Wang Y., Qing G., Zhang Y., et al. Effect of physicochemical properties on in vivo fate of nanoparticle-based cancer immunotherapies. Acta Pharm Sin B. 2021;11:886–902. doi: 10.1016/j.apsb.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhao Z., Ukidve A., Krishnan V., Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv Drug Deliv Rev. 2019;143:3–21. doi: 10.1016/j.addr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 225.Zhou Q., Li J., Xiang J., Shao S., Zhou Z., Tang J., et al. Transcytosis-enabled active extravasation of tumor nanomedicine. Adv Drug Deliv Rev. 2022;189:114480. doi: 10.1016/j.addr.2022.114480. [DOI] [PubMed] [Google Scholar]
- 226.Wang X., Qiu Y., Wang M., Zhang C., Zhang T., Zhou H., et al. Endocytosis and organelle targeting of nanomedicines in cancer therapy. Int J Nanomed. 2020;15:9447–9467. doi: 10.2147/IJN.S274289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.He H., Zhang J., Xie Y., Lu Y., Qi J., Ahmad E., et al. Bioimaging of intravenous polymeric micelles based on discrimination of integral particles using an environment-responsive probe. Mol Pharm. 2016;13:4013–4019. doi: 10.1021/acs.molpharmaceut.6b00705. [DOI] [PubMed] [Google Scholar]
- 228.Groo A.C., Bossiere M., Trichard L., Legras P., Benoit J.P., Lagarce F. In vivo evaluation of paclitaxel-loaded lipid nanocapsules after intravenous and oral administration on resistant tumor. Nanomedicine. 2015;10:589–601. doi: 10.2217/nnm.14.124. [DOI] [PubMed] [Google Scholar]
- 229.Chen T., He B., Tao J., He Y., Deng H., Wang X., et al. Application of forster resonance energy transfer (FRET) technique to elucidate intracellular and in vivo biofate of nanomedicines. Adv Drug Deliv Rev. 2019;143:177–205. doi: 10.1016/j.addr.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 230.Lebreton V., Kaeokhamloed N., Vasylaki A., Hilairet G., Mellinger A., Bejaud J., et al. Pharmacokinetics of intact lipid nanocapsules using new quantitative FRET technique. J Contr Release. 2022;351:681–691. doi: 10.1016/j.jconrel.2022.09.057. [DOI] [PubMed] [Google Scholar]
- 231.Qi J., Hu X., Dong X., Lu Y., Lu H., Zhao W., et al. Towards more accurate bioimaging of drug nanocarriers: turning aggregation-caused quenching into a useful tool. Adv Drug Deliv Rev. 2019;143:206–225. doi: 10.1016/j.addr.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 232.Yang Y., Lv Y., Shen C., Shi T., He H., Qi J., et al. In vivo dissolution of poorly water-soluble drugs: proof of concept based on fluorescence bioimaging. Acta Pharm Sin B. 2021;11:1056–1068. doi: 10.1016/j.apsb.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Weng C., Fan N., Xu T., Chen H., Li Z., Li Y., et al. FRET-based polymer materials for detection of cellular microenvironments. Chin Chem Lett. 2020;31:1490–1498. [Google Scholar]
- 234.Lainé A.L., Gravier J., Henry M., Sancey L., Béjaud J., Pancani E., et al. Conventional versus stealth lipid nanoparticles: formulation and in vivo fate prediction through FRET monitoring. J Contr Release. 2014;188:1–8. doi: 10.1016/j.jconrel.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 235.Gravier J., Sancey L., Hirsjärvi S., Rustique E., Passirani C., Benoît J.P., et al. FRET imaging approaches for in vitro and in vivo characterization of synthetic lipid nanoparticles. Mol Pharm. 2014;11:3133–3144. doi: 10.1021/mp500329z. [DOI] [PubMed] [Google Scholar]
- 236.Khan S., Sharifi M., Gleghorn J.P., Babadaei M.M.N., Bloukh S.H., Edis Z., et al. Artificial engineering of the protein corona at bio-nano interfaces for improved cancer-targeted nanotherapy. J Contr Release. 2022;348:127–147. doi: 10.1016/j.jconrel.2022.05.055. [DOI] [PubMed] [Google Scholar]
- 237.Wang W., Liu H., Huang Z., Fu F., Wang W., Wu L., et al. The effect of organic ligand modification on protein corona formation of nanoscale metal organic frameworks. Chin Chem Lett. 2022;33:4185–4190. [Google Scholar]
- 238.García-Álvarez R., Vallet-Regí M. Hard and soft protein corona of nanomaterials: analysis and relevance. Nanomaterials. 2021;11:888. doi: 10.3390/nano11040888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kihara S., Ghosh S., McDougall D.R., Whitten A.E., Mata J.P., Köper I., et al. Structure of soft and hard protein corona around polystyrene nanoplastics—particle size and protein types. Biointerphases. 2020;15 doi: 10.1116/6.0000404. [DOI] [PubMed] [Google Scholar]
- 240.Tenzer S., Docter D., Kuharev J., Musyanovych A., Fetz V., Hecht R., et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 241.Huang W., Xiao G., Zhang Y., Min W. Research progress and application opportunities of nanoparticle-protein corona complexes. Biomed Pharmacother. 2021;139:111541. doi: 10.1016/j.biopha.2021.111541. [DOI] [PubMed] [Google Scholar]
- 242.Yang M., Wu E., Tang W., Qian J., Zhan C. Interplay between nanomedicine and protein corona. J Mater Chem B. 2021;9:6713–6727. doi: 10.1039/d1tb01063h. [DOI] [PubMed] [Google Scholar]
- 243.Goy-López S., Juárez J., Alatorre-Meda M., Casals E., Puntes V.F., Taboada P., et al. Physicochemical characteristics of protein-NP bioconjugates: the role of particle curvature and solution conditions on human serum albumin conformation and fibrillogenesis inhibition. Langmuir. 2012;28:9113–9126. doi: 10.1021/la300402w. [DOI] [PubMed] [Google Scholar]
- 244.Hühn D., Kantner K., Geidel C., Brandholt S., De Cock I., Soenen S.J., et al. Polymer-coated nanoparticles interacting with proteins and cells: focusing on the sign of the net charge. ACS Nano. 2013;7:3253–3263. doi: 10.1021/nn3059295. [DOI] [PubMed] [Google Scholar]
- 245.Xu F., Reiser M., Yu X., Gummuluru S., Wetzler L., Reinhard B.M. Lipid-mediated targeting with membrane-wrapped nanoparticles in the presence of corona formation. ACS Nano. 2016;10:1189–1200. doi: 10.1021/acsnano.5b06501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Almalik A., Benabdelkamel H., Masood A., Alanazi I.O., Alradwan I., Majrashi M.A., et al. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci Rep. 2017;7:10542. doi: 10.1038/s41598-017-10836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Caracciolo G., Caputo D., Pozzi D., Colapicchioni V., Coppola R. Size and charge of nanoparticles following incubation with human plasma of healthy and pancreatic cancer patients. Colloids Surf B Biointerfaces. 2014;123:673–678. doi: 10.1016/j.colsurfb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 248.Walkey C.D., Olsen J.B., Guo H., Emili A., Chan W.C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 249.Zeng L., Gao J., Liu Y., Gao J., Yao L., Yang X., et al. Role of protein corona in the biological effect of nanomaterials: investigating methods. Trends Anal Chem. 2019;118:303–314. [Google Scholar]
- 250.Ho Y.T., N Azman, Loh F.W.Y., Ong G.K.T., Engudar G., Kriz S.A., et al. Protein corona formed from different blood plasma proteins affects the colloidal stability of nanoparticles differently. Bioconjugate Chem. 2018;29:3923–3934. doi: 10.1021/acs.bioconjchem.8b00743. [DOI] [PubMed] [Google Scholar]
- 251.Bélteky P., Rónavári A., Zakupszky D., Boka E., Igaz N., Szerencsés B., et al. Are smaller nanoparticles always better? Understanding the biological effect of size-dependent silver nanoparticle aggregation under biorelevant conditions. Int J Nanomed. 2021;16:3021–3040. doi: 10.2147/IJN.S304138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cao X., Han Y., Li F., Li Z., McClements D.J., He L., et al. Impact of protein–nanoparticle interactions on gastrointestinal fate of ingested nanoparticles: not just simple protein corona effects. NanoImpact. 2019;13:37–43. [Google Scholar]
- 253.Pourjavadi A., Tehrani Z.M., Mahmoudi N. The effect of protein corona on doxorubicin release from the magnetic mesoporous silica nanoparticles with polyethylene glycol coating. J Nano Res. 2015;17:197. [Google Scholar]
- 254.Cheng X., Tian X., Wu A., Li J., Tian J., Chong Y., et al. Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner. ACS Appl Mater Interfaces. 2015;7:20568–20575. doi: 10.1021/acsami.5b04290. [DOI] [PubMed] [Google Scholar]
- 255.Aliyandi A., Reker-Smit C., Bron R., Zuhorn I.S., Salvati A. Correlating corona composition and cell uptake to identify proteins affecting nanoparticle entry into endothelial cells. ACS Biomater Sci Eng. 2021;7:5573–5584. doi: 10.1021/acsbiomaterials.1c00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Huang Y., Wang S., Zhang J., Wang H., Zou Q., Wu L. Stealthy nanoparticles protect endothelial barrier from leakiness by resisting the absorption of VE-cadherin. Nanoscale. 2021;13:12577–12586. doi: 10.1039/d1nr03155d. [DOI] [PubMed] [Google Scholar]
- 257.Oh J.Y., Kim H.S., Palanikumar L., Go E.M., Jana B., Park S.A., et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat Commun. 2018;9:4548. doi: 10.1038/s41467-018-06979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Xiao W., Wang Y., Zhang H., Liu Y., Xie R., He X., et al. The protein corona hampers the transcytosis of transferrin-modified nanoparticles through blood‒brain barrier and attenuates their targeting ability to brain tumor. Biomaterials. 2021;274:120888. doi: 10.1016/j.biomaterials.2021.120888. [DOI] [PubMed] [Google Scholar]
- 259.Yu L., Xu M., Xu W., Xiao W., Jiang X.H., Wang L., et al. Enhanced cancer-targeted drug delivery using precoated nanoparticles. Nano Lett. 2020;20:8903–8911. doi: 10.1021/acs.nanolett.0c03982. [DOI] [PubMed] [Google Scholar]
- 260.Wang Y., Zhang H., Xiao W., Liu Y., Zhou Y., He X., et al. Unmasking CSF protein corona: effect on targeting capacity of nanoparticles. J Contr Release. 2021;333:352–361. doi: 10.1016/j.jconrel.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 261.Varnamkhasti B.S., Hosseinzadeh H., Azhdarzadeh M., Vafaei S.Y., Esfandyari-Manesh M., Mirzaie Z.H., et al. Protein corona hampers targeting potential of MUC1 aptamer functionalized SN-38 core-shell nanoparticles. Int J Pharm. 2015;494:430–444. doi: 10.1016/j.ijpharm.2015.08.060. [DOI] [PubMed] [Google Scholar]
- 262.Nemati M., Bani F., Sepasi T., Zamiri R.E., Rasmi Y., Kahroba H., et al. Unraveling the effect of breast cancer patients' plasma on the targeting ability of folic acid-modified chitosan nanoparticles. Mol Pharm. 2021;18:4341–4353. doi: 10.1021/acs.molpharmaceut.1c00525. [DOI] [PubMed] [Google Scholar]
- 263.Wang H., Ding T., Guan J., Liu X., Wang J., Jin P., et al. Interrogation of folic acid-functionalized nanomedicines: the regulatory roles of plasma proteins reexamined. ACS Nano. 2020;14:14779–14789. doi: 10.1021/acsnano.0c02821. [DOI] [PubMed] [Google Scholar]
- 264.Suchanova J.Z., Hejtmankova A., Neburkova J., Cigler P., Forstova J., Spanielova H. The protein corona does not influence receptor-mediated targeting of virus-like particles. Bioconjugate Chem. 2020;31:1575–1585. doi: 10.1021/acs.bioconjchem.0c00240. [DOI] [PubMed] [Google Scholar]
- 265.Chen F., Wang G., Griffin J.I., Brenneman B., Banda N.K., Holers V.M., et al. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12:387–393. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Salatin S., Maleki Dizaj S., Yari Khosroushahi A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int. 2015;39:881–890. doi: 10.1002/cbin.10459. [DOI] [PubMed] [Google Scholar]
- 267.Lesniak A., Fenaroli F., Monopoli M.P., Aberg C., Dawson K.A., Salvati A. Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano. 2012;6:5845–5857. doi: 10.1021/nn300223w. [DOI] [PubMed] [Google Scholar]
- 268.Monopoli M.P., Aberg C., Salvati A., Dawson K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 269.Treuel L., Brandholt S., Maffre P., Wiegele S., Shang L., Nienhaus G.U. Impact of protein modification on the protein corona on nanoparticles and nanoparticle–cell interactions. ACS Nano. 2014;8:503–513. doi: 10.1021/nn405019v. [DOI] [PubMed] [Google Scholar]
- 270.Owens D.E., 3rd, Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 271.Aggarwal P., Hall J.B., McLeland C.B., Dobrovolskaia M.A., McNeil S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chinen A.B., Guan C.M., Ko C.H., Mirkin C.A. The impact of protein corona formation on the macrophage cellular uptake and biodistribution of spherical nucleic acids. Small. 2017;13:1603847. doi: 10.1002/smll.201603847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Tekie F.S.M., Hajiramezanali M., Geramifar P., Raoufi M., Dinarvand R., Soleimani M., et al. Controlling evolution of protein corona: a prosperous approach to improve chitosan-based nanoparticle biodistribution and half-life. Sci Rep. 2020;10:9664. doi: 10.1038/s41598-020-66572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Thiele L., Diederichs J.E., Reszka R., Merkle H.P., Walter E. Competitive adsorption of serum proteins at microparticles affects phagocytosis by dendritic cells. Biomaterials. 2003;24:1409–1418. doi: 10.1016/s0142-9612(02)00525-2. [DOI] [PubMed] [Google Scholar]
- 275.Schöttler S., Becker G., Winzen S., Steinbach T., Mohr K., Landfester K., et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11:372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 276.Tay M.Z., Wiehe K., Pollara J. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front Immunol. 2019;10:332. doi: 10.3389/fimmu.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Schäffler M., Sousa F., Wenk A., Sitia L., Hirn S., Schleh C., et al. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials. 2014;35:3455–3466. doi: 10.1016/j.biomaterials.2013.12.100. [DOI] [PubMed] [Google Scholar]
- 278.Zhang Z., Guan J., Jiang Z., Yang Y., Liu J., Hua W., et al. Brain-targeted drug delivery by manipulating protein corona functions. Nat Commun. 2019;10:3561. doi: 10.1038/s41467-019-11593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lu N., Li J., Tian R., Peng Y.Y. Binding of human serum albumin to single-walled carbon nanotubes activated neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Chem Res Toxicol. 2014;27:1070–1077. doi: 10.1021/tx5001317. [DOI] [PubMed] [Google Scholar]
- 280.Jafari S., Izadi Z., Alaei L., Jaymand M., Samadian H., Kashani V.O., et al. Human plasma protein corona decreases the toxicity of pillar-layer metal organic framework. Sci Rep. 2020;10:14569. doi: 10.1038/s41598-020-71170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Li X., Sun H., Li H., Hu C., Luo Y., Shi X., et al. Multi-responsive biodegradable cationic nanogels for highly efficient treatment of tumors. Adv Funct Mater. 2021;31:2100227. [Google Scholar]
- 282.Ge C., Du J., Zhao L., Wang L., Liu Y., Li D., et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc Natl Acad Sci U S A. 2011;108:16968–16973. doi: 10.1073/pnas.1105270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mo J., Xie Q., Wei W., Zhao J. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona. Nat Commun. 2018;9:2480. doi: 10.1038/s41467-018-04873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yan Y., Gause K.T., Kamphuis M.M., Ang C.S., O'Brien-Simpson N.M., Lenzo J.C., et al. Differential roles of the protein corona in the cellular uptake of nanoporous polymer particles by monocyte and macrophage cell lines. ACS Nano. 2013;7:10960–10970. doi: 10.1021/nn404481f. [DOI] [PubMed] [Google Scholar]
- 285.Deng Z.J., Liang M., Monteiro M., Toth I., Minchin R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6:39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 286.Ding T., Guan J., Wang M., Long Q., Liu X., Qian J., et al. Natural IgM dominates in vivo performance of liposomes. J Contr Release. 2020;319:371–381. doi: 10.1016/j.jconrel.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 287.Guan J., Shen Q., Zhang Z., Jiang Z., Yang Y., Lou M., et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9:2982. doi: 10.1038/s41467-018-05384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vu V.P., Gifford G.B., Chen F., Benasutti H., Wang G., Groman E.V., et al. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat Nanotechnol. 2019;14:260–268. doi: 10.1038/s41565-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Moghimi S.M., Simberg D., Skotland T., Yaghmur A., Hunter A.C. The interplay between blood proteins, complement, and macrophages on nanomedicine performance and responses. J Pharmacol Exp Therapeut. 2019;370:581–592. doi: 10.1124/jpet.119.258012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bogart L.K., Pourroy G., Murphy C.J., Puntes V., Pellegrino T., Rosenblum D., et al. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano. 2014;8:3107–3122. doi: 10.1021/nn500962q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Jiang Z., Chu Y., Zhan C. Protein corona: challenges and opportunities for targeted delivery of nanomedicines. Expet Opin Drug Deliv. 2022;19:833–846. doi: 10.1080/17425247.2022.2093854. [DOI] [PubMed] [Google Scholar]
- 292.Domingues C., Santos A., Alvarez-Lorenzo C., Concheiro A., Jarak I., Veiga F., et al. Where is nano today and where is it headed? A review of nanomedicine and the dilemma of nanotoxicology. ACS Nano. 2022;16:9994–10041. doi: 10.1021/acsnano.2c00128. [DOI] [PubMed] [Google Scholar]
- 293.Sharifi S., Behzadi S., Laurent S., Forrest M.L., Stroeve P., Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang Y., Santos A., Evdokiou A., Losic D. An overview of nanotoxicity and nanomedicine research: principles, progress and implications for cancer therapy. J Mater Chem B. 2015;3:7153–7172. doi: 10.1039/c5tb00956a. [DOI] [PubMed] [Google Scholar]
- 295.Derfus A.M., Chan W.C.W., Bhatia S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Øvrevik J., Låg M., Schwarze P., Refsnes M. p38 and Src-ERK1/2 pathways regulate crystalline silica-induced chemokine release in pulmonary epithelial cells. Toxicol Sci. 2004;81:480–490. doi: 10.1093/toxsci/kfh214. [DOI] [PubMed] [Google Scholar]
- 297.Nagai H., Okazaki Y., Chew S.H., Misawa N., Yamashita Y., Akatsuka S., et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:E1330–E1338. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Akhavan O., Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–5736. doi: 10.1021/nn101390x. [DOI] [PubMed] [Google Scholar]
- 299.Duan G., Zhang Y., Luan B., Weber J.K., Zhou R.W., Yang Z., et al. Graphene-induced pore formation on cell membranes. Sci Rep. 2017;7:42767. doi: 10.1038/srep42767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu X., Chen K.L. Interactions of graphene oxide with model cell membranes: probing nanoparticle attachment and lipid bilayer disruption. Langmuir. 2015;31:12076–12086. doi: 10.1021/acs.langmuir.5b02414. [DOI] [PubMed] [Google Scholar]
- 301.Tu Y., Lv M., Xiu P., Huynh T., Zhang M., Castelli M., et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat Nanotechnol. 2013;8:594–601. doi: 10.1038/nnano.2013.125. [DOI] [PubMed] [Google Scholar]
- 302.Bakina O., Glazkova E., Pervikov A., Lozhkomoev A., Rodkevich N., Svarovskaya N., et al. Design and preparation of silver–copper nanoalloys for antibacterial applications. J Cluster Sci. 2021;32:779–786. [Google Scholar]
- 303.Wu K., Zhou Q., Ouyang S. Direct and indirect genotoxicity of graphene family nanomaterials on DNA—a review. Nanomaterials. 2021;11:2889. doi: 10.3390/nano11112889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Winnik F.M., Maysinger D. Quantum dot cytotoxicity and ways to reduce it. Acc Chem Res. 2013;46:672–680. doi: 10.1021/ar3000585. [DOI] [PubMed] [Google Scholar]
- 305.Sargent L.M., Hubbs A.F., Young S.H., Kashon M.L., Dinu C.Z., Salisbury J.L., et al. Single-walled carbon nanotube-induced mitotic disruption. Mutat Res. 2012;745:28–37. doi: 10.1016/j.mrgentox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Park K.H., Chhowalla M., Iqbal Z., Sesti F. Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem. 2003;278:50212–50216. doi: 10.1074/jbc.M310216200. [DOI] [PubMed] [Google Scholar]
- 307.Gong C., Tao G., Yang L., Liu J., Liu Q., Zhuang Z. SiO2 nanoparticles induce global genomic hypomethylation in HaCaT cells. Biochem Biophys Res Commun. 2010;397:397–400. doi: 10.1016/j.bbrc.2010.05.076. [DOI] [PubMed] [Google Scholar]
- 308.Soenen S.J., Nuytten N., De Meyer S.F., De Smedt S.C., De Cuyper M. High intracellular iron oxide nanoparticle concentrations affect cellular cytoskeleton and focal adhesion kinase-mediated signaling. Small. 2010;6:832–842. doi: 10.1002/smll.200902084. [DOI] [PubMed] [Google Scholar]
- 309.Holt B.D., Short P.A., Rape A.D., Wang Y.L., Islam M.F., Dahl K.N. Carbon nanotubes reorganize actin structures in cells and ex vivo. ACS Nano. 2010;4:4872–4878. doi: 10.1021/nn101151x. [DOI] [PubMed] [Google Scholar]
- 310.Öner D., Moisse M., Ghosh M., Duca R.C., Poels K., Luyts K., et al. Epigenetic effects of carbon nanotubes in human monocytic cells. Mutagenesis. 2017;32:181–191. doi: 10.1093/mutage/gew053. [DOI] [PubMed] [Google Scholar]
- 311.Mailänder V., Landfester K. Interaction of nanoparticles with cells. Biomacromolecules. 2009;10:2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- 312.Wang P., Nie X., Wang Y., Li Y., Ge C., Zhang L., et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-beta/Smad signaling pathway. Small. 2013;9:3799–3811. doi: 10.1002/smll.201300607. [DOI] [PubMed] [Google Scholar]
- 313.Barna B.P., Judson M.A., Thomassen M.J. Carbon nanotubes and chronic granulomatous disease. Nanomaterials. 2014;4:508–521. doi: 10.3390/nano4020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Dobrovolskaia M.A., Germolec D.R., Weaver J.L. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol. 2009;4:411–414. doi: 10.1038/nnano.2009.175. [DOI] [PubMed] [Google Scholar]
- 315.Ryman-Rasmussen J.P., Cesta M.F., Brody A.R., Shipley-Phillips J.K., Everitt J.I., Tewksbury E.W., et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sun B., Wang X., Ji Z., Li R., Xia T. NLRP3 inflammasome activation induced by engineered nanomaterials. Small. 2013;9:1595–1607. doi: 10.1002/smll.201201962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Setyawati M.I., Sevencan C., Bay B.H., Xie J., Zhang Y., Demokritou P., et al. Nano-TiO2 drives epithelial-mesenchymal transition in intestinal epithelial cancer cells. Small. 2018;14 doi: 10.1002/smll.201800922. [DOI] [PubMed] [Google Scholar]
- 318.Tay C.Y., Cai P., Setyawati M.I., Fang W., Tan L.P., Hong C.H., et al. Nanoparticles strengthen intracellular tension and retard cellular migration. Nano Lett. 2014;14:83–88. doi: 10.1021/nl4032549. [DOI] [PubMed] [Google Scholar]
- 319.Wang L., Zhang T., Li P., Huang W., Tang J., Wang P., et al. Use of synchrotron radiation-analytical techniques to reveal chemical origin of silver-nanoparticle cytotoxicity. ACS Nano. 2015;9:6532–6547. doi: 10.1021/acsnano.5b02483. [DOI] [PubMed] [Google Scholar]
- 320.Xia L., Gu W., Zhang M., Chang Y.N., Chen K., Bai X., et al. Endocytosed nanoparticles hold endosomes and stimulate binucleated cells formation. Part Fibre Toxicol. 2016;13:63. doi: 10.1186/s12989-016-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Huo L., Chen R., Zhao L., Shi X., Bai R., Long D., et al. Silver nanoparticles activate endoplasmic reticulum stress signaling pathway in cell and mouse models: the role in toxicity evaluation. Biomaterials. 2015;61:307–315. doi: 10.1016/j.biomaterials.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 322.Tsai Y.Y., Huang Y.H., Chao Y.L., Hu K.Y., Chin L.T., Chou S.H., et al. Identification of the nanogold particle-induced endoplasmic reticulum stress by omic techniques and systems biology analysis. ACS Nano. 2011;5:9354–9369. doi: 10.1021/nn2027775. [DOI] [PubMed] [Google Scholar]
- 323.Cohignac V., Landry M.J., Boczkowski J., Lanone S. Autophagy as a possible underlying mechanism of nanomaterial toxicity. Nanomaterials. 2014;4:548–582. doi: 10.3390/nano4030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Stern S.T., Adiseshaiah P.P., Crist R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. doi: 10.1186/1743-8977-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Fu P.P., Xia Q., Hwang H.M., Ray P.C., Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Abdal Dayem A., Hossain M.K., Lee S.B., Kim K., Saha S.K., Yang G.M., et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci. 2017;18:120. doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wu W., Yan L., Chen S., Li Q., Gu Z., Xu H., et al. Investigating oxidation state-induced toxicity of PEGylated graphene oxide in ocular tissue using gene expression profiles. Nanotoxicology. 2018;12:819–835. doi: 10.1080/17435390.2018.1480813. [DOI] [PubMed] [Google Scholar]
- 328.Zhang W., Yan L., Li M., Zhao R., Yang X., Ji T., et al. Deciphering the underlying mechanisms of oxidation-state dependent cytotoxicity of graphene oxide on mammalian cells. Toxicol Lett. 2015;237:61–71. doi: 10.1016/j.toxlet.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 329.Setyawati M.I., Mochalin V.N., Leong D.T. Tuning endothelial permeability with functionalized nanodiamonds. ACS Nano. 2016;10:1170–1181. doi: 10.1021/acsnano.5b06487. [DOI] [PubMed] [Google Scholar]
- 330.Setyawati M.I., Tay C.Y., Bay B.H., Leong D.T. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano. 2017;11:5020–5030. doi: 10.1021/acsnano.7b01744. [DOI] [PubMed] [Google Scholar]
- 331.Xu Y., Xiong J., Sun X., Gao H. Targeted nanomedicines remodeling immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Acta Pharm Sin B. 2022;12:4327–4347. doi: 10.1016/j.apsb.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Xiao Q., Li X., Liu C., Jiang Y., He Y., Zhang W., et al. Improving cancer immunotherapy via co-delivering checkpoint blockade and thrombospondin-1 downregulator. Acta Pharm Sin B. 2022 doi: 10.1016/j.apsb.2022.07.012. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zhang Y., Ding S., Li J., Peng X., Li J., Chang J., et al. In vivo formation of Cu(DDC)2 complex induced by nanomedicine for mesothelioma chemotherapy. Chin Chem Lett. 2020;31:3168–3172. [Google Scholar]
- 334.Byrd D.R., Brierley J.D., Baker T.P., Sullivan D.C., Gress D.M. Current and future cancer staging after neoadjuvant treatment for solid tumors. Ca - Cancer J Clin. 2021;71:140–148. doi: 10.3322/caac.21640. [DOI] [PubMed] [Google Scholar]
- 335.Wei G., Wang Y., Yang G., Wang Y., Ju R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics. 2021;11:6370–6392. doi: 10.7150/thno.57828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Fang J., Islam W., Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020;157:142–160. doi: 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 337.Shi Y., van der Meel R., Chen X., Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10:7921–7924. doi: 10.7150/thno.49577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Singh S., Drude N., Blank L., Desai P.B., Königs H., Rütten S., et al. Protease responsive nanogels for transcytosis across the blood‒brain barrier and intracellular delivery of radiopharmaceuticals to brain tumor cells. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ren Z., Sun S., Sun R., Cui G., Hong L., Rao B., et al. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy. Adv Mater. 2020;32 doi: 10.1002/adma.201906024. [DOI] [PubMed] [Google Scholar]
- 340.Sanità G., Carrese B., Lamberti A. Nanoparticle surface functionalization: how to improve biocompatibility and cellular internalization. Front Mol Biosci. 2020;7:587012. doi: 10.3389/fmolb.2020.587012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wu D., Shi X., Zhao F., Chilengue S.T.F., Deng L., Dong A., et al. An injectable and tumor-specific responsive hydrogel with tissue-adhesive and nanomedicine-releasing abilities for precise locoregional chemotherapy. Acta Biomater. 2019;96:123–136. doi: 10.1016/j.actbio.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 342.Li J., Wang Y., Xu C., Yu Q., Wang X., Xie H., et al. Rapid pH-responsive self-disintegrating nanoassemblies balance tumor accumulation and penetration for enhanced anti-breast cancer therapy. Acta Biomater. 2021;134:546–558. doi: 10.1016/j.actbio.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 343.Gao Y., Qiu W., Liang M., Ma X., Ye M., Xue P., et al. Active targeting redox-responsive mannosylated prodrug nanocolloids promote tumor recognition and cell internalization for enhanced colon cancer chemotherapy. Acta Biomater. 2022;147:299–313. doi: 10.1016/j.actbio.2022.05.046. [DOI] [PubMed] [Google Scholar]
- 344.Kulkarni J., Witzigmann D., Thomson S., Chen S., Leavitt B., Cullis P., et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16:630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 345.Xu C.F., Iqbal S., Shen S., Luo Y.L., Yang X., Wang J. Development of "CLAN" nanomedicine for nucleic acid therapeutics. Small. 2019;15 doi: 10.1002/smll.201900055. [DOI] [PubMed] [Google Scholar]
- 346.Li D., Mastaglia F.L., Fletcher S., Wilton S.D. Progress in the molecular pathogenesis and nucleic acid therapeutics for Parkinson's disease in the precision medicine era. Med Res Rev. 2020;40:2650–2681. doi: 10.1002/med.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.van den Berg A.I.S., Yun C.O., Schiffelers R.M., Hennink W.E. Polymeric delivery systems for nucleic acid therapeutics: approaching the clinic. J Contr Release. 2021;331:121–141. doi: 10.1016/j.jconrel.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 348.Desjardins C., Yao M., Hall J., O'Donnell E., Venkatesan R., Spring S., et al. Enhanced exon skipping and prolonged dystrophin restoration achieved by TfR1-targeted delivery of antisense oligonucleotide using FORCE conjugation in mdx mice. Nucleic Acids Res. 2022;50:11401–11414. doi: 10.1093/nar/gkac641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Guan X., Meng F., Tan H., Wang X., Li J., Wei J., et al. Modular and hierarchical self-assembly of siRNAs into supramolecular nanomaterials for soft and homogeneous siRNA loading and precise and visualized intracellular delivery. Chem Sci. 2022;13:8657–8666. doi: 10.1039/d2sc02488h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zoulikha M., Xiao Q.Q., Boafo G.F., Sallam M.A., Chen Z.J., He W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm Sin B. 2022;12:600–620. doi: 10.1016/j.apsb.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Holjencin C., Jakymiw A. MicroRNAs and their big therapeutic impacts: delivery strategies for cancer intervention. Cells. 2022;11:2332. doi: 10.3390/cells11152332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ni H., Hatit M.Z.C., Zhao K., Loughrey D., Lokugamage M.P., Peck H.E., et al. Piperazine-derived lipid nanoparticles deliver mRNA to immune cells in vivo. Nat Commun. 2022;13:4766. doi: 10.1038/s41467-022-32281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Covid-19 update Pfizer/BioNTech and Moderna vaccines authorized for children ≥6 months old. Med Lett Drugs Ther. 2022;64:110–112. [PubMed] [Google Scholar]
- 354.Alesci A., Gitto M., Kotańska M., Lo Cascio P., Miller A., Nicosia N., et al. Immunogenicity, effectiveness, safety and psychological impact of COVID-19 mRNA vaccines. Hum Immunol. 2022;83:755–767. doi: 10.1016/j.humimm.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J Contr Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Jiang K., Hu Y., Gao X., Zhan C., Zhang Y., Yao S., et al. Octopus-like flexible vector for noninvasive intraocular delivery of short interfering nucleic acids. Nano Lett. 2019;19:6410–6417. doi: 10.1021/acs.nanolett.9b02596. [DOI] [PubMed] [Google Scholar]
- 357.Saunders N.R.M., Paolini M.S., Fenton O.S., Poul L., Devalliere J., Mpambani F., et al. A nanoprimer to improve the systemic delivery of siRNA and mRNA. Nano Lett. 2020;20:4264–4269. doi: 10.1021/acs.nanolett.0c00752. [DOI] [PubMed] [Google Scholar]
- 358.Van de Vyver T., Bogaert B., De Backer L., Joris F., Guagliardo R., Van Hoeck J., et al. Cationic amphiphilic drugs boost the lysosomal escape of small nucleic acid therapeutics in a nanocarrier-dependent manner. ACS Nano. 2020;14:4774–4791. doi: 10.1021/acsnano.0c00666. [DOI] [PubMed] [Google Scholar]
- 359.Cabral H., Kinoh H., Kataoka K. Tumor-targeted nanomedicine for immunotherapy. Acc Chem Res. 2020;53:2765–2776. doi: 10.1021/acs.accounts.0c00518. [DOI] [PubMed] [Google Scholar]
- 360.Magar K.T., Boafo G.F., Zoulikha M., Jiang X., Li X., Xiao Q., et al. Metal phenolic network-stabilized nanocrystals of andrographolide to alleviate macrophage-mediated inflammation in-vitro. Chin Chem Lett. 2023;34:107453. [Google Scholar]
- 361.Osipov A., Murphy A., Zheng L. From immune checkpoints to vaccines: the past, present and future of cancer immunotherapy. Adv Cancer Res. 2019;143:63–144. doi: 10.1016/bs.acr.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 362.Mrak D., Simader E., Sieghart D., Mandl P., Radner H., Perkmann T., et al. Immunogenicity and safety of a fourth COVID-19 vaccination in rituximab-treated patients: an open-label extension study. Ann Rheum Dis. 2022;81:1750–1756. doi: 10.1136/ard-2022-222579. [DOI] [PubMed] [Google Scholar]
- 363.Sun T., Kwong C., Gao C., Wei J., Yue L., Zhang J., et al. Amelioration of ulcerative colitis inflammatory regulation by macrophage-biomimetic nanomedicine. Theranostics. 2020;10:10106–10119. doi: 10.7150/thno.48448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wei Z., Yi Y., Luo Z., Gong X., Jiang Y., Hou D., et al. Selenopeptide nanomedicine activates natural killer cells for enhanced tumor chemoimmunotherapy. Adv Mater. 2022;34 doi: 10.1002/adma.202108167. [DOI] [PubMed] [Google Scholar]
- 365.Park K.S., Nam J., Son S., Moon J.J. Personalized combination nano-immunotherapy for robust induction and tumor infiltration of CD8+ T cells. Biomaterials. 2021;274:120844. doi: 10.1016/j.biomaterials.2021.120844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Doshi A.S., Cantin S., Prickett L.B., Mele D.A., Amiji M. Systemic nano-delivery of low-dose STING agonist targeted to CD103+ dendritic cells for cancer immunotherapy. J Contr Release. 2022;345:721–733. doi: 10.1016/j.jconrel.2022.03.054. [DOI] [PubMed] [Google Scholar]
- 367.Chen Y., Chen S., Yu H., Wang Y., Cui M., Wang P., et al. D-A type NIR-II organic molecules: strategies for the enhancement fluorescence brightness and applications in NIR-II fluorescence imaging-navigated photothermal therapy. Adv Healthc Mater. 2022;11 doi: 10.1002/adhm.202201158. [DOI] [PubMed] [Google Scholar]
- 368.Zhao L., Zhang X., Wang X., Guan X., Zhang W., Ma J. Recent advances in selective photothermal therapy of tumor. J Nanobiotechnol. 2021;19:335. doi: 10.1186/s12951-021-01080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Zhang T., Wu B., Akakuru O.U., Yao C., Sun S., Chen L., et al. Hsp90 inhibitor-loaded IR780 micelles for mitochondria-targeted mild-temperature photothermal therapy in xenograft models of human breast cancer. Cancer Lett. 2021;500:41–50. doi: 10.1016/j.canlet.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 370.Zhang Y., Wang Y., Li Z., Yang D., Qiu X. Engineering of near-infrared-activated lignin-polydopamine-nanosilver composites for highly efficient sterilization. ACS Appl Bio Mater. 2022;5:4256–4263. doi: 10.1021/acsabm.2c00474. [DOI] [PubMed] [Google Scholar]
- 371.Chen M.W., Lu Q.J., Chen Y.J., Hou Y.K., Zou Y.M., Zhou Q., et al. NIR-PTT/ROS-scavenging/oxygen-enriched synergetic therapy for rheumatoid arthritis by a pH-responsive hybrid CeO-ZIF-8 coated with polydopamine. ACS Biomater Sci Eng. 2022;8:3361–3376. doi: 10.1021/acsbiomaterials.2c00592. [DOI] [PubMed] [Google Scholar]
- 372.Jiang G., Fan D., Tian J., Xiang Z., Fang Q. Self-confirming magnetosomes for tumor-targeted T/T dual-mode MRI and MRI-guided photothermal therapy. Adv Healthc Mater. 2022;11 doi: 10.1002/adhm.202200841. [DOI] [PubMed] [Google Scholar]
- 373.Yan L.X., Chen L.J., Zhao X., Yan X.P. pH switchable nanoplatform for in vivo persistent luminescence imaging and precise photothermal therapy of bacterial infection. Adv Funct Mater. 2020;30:1909042. [Google Scholar]
- 374.Li X., Hou Y., Meng X., Li G., Xu F., Teng L., et al. Folate receptor-targeting mesoporous silica-coated gold nanorod nanoparticles for the synergistic photothermal therapy and chemotherapy of rheumatoid arthritis. RSC Adv. 2021;11:3567–3574. doi: 10.1039/d0ra08689d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Xiang Q., Yang C., Luo Y., Liu F., Zheng J., Liu W., et al. Near-infrared II nanoadjuvant-mediated chemodynamic, photodynamic, and photothermal therapy combines immunogenic cell death with PD-L1 blockade to enhance antitumor immunity. Small. 2022;18 doi: 10.1002/smll.202107809. [DOI] [PubMed] [Google Scholar]
- 376.Yu J., He X., Wang Z., Wang Y., Liu S., Li X., et al. Combining PD-L1 inhibitors with immunogenic cell death triggered by chemo-photothermal therapy via a thermosensitive liposome system to stimulate tumor-specific immunological response. Nanoscale. 2021;13:12966–12978. doi: 10.1039/d1nr03288g. [DOI] [PubMed] [Google Scholar]
- 377.Sun Z., Deng G., Peng X., Xu X., Liu L., Peng J., et al. Intelligent photothermal dendritic cells restart the cancer immunity cycle through enhanced immunogenic cell death. Biomaterials. 2021;279:121228. doi: 10.1016/j.biomaterials.2021.121228. [DOI] [PubMed] [Google Scholar]
- 378.Gharagozloo M., Majewski S., Foldvari M. Therapeutic applications of nanomedicine in autoimmune diseases: from immunosuppression to tolerance induction. Nanomedicine. 2015;11:1003–1018. doi: 10.1016/j.nano.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 379.Song X., Sun Z., Li L., Zhou L., Yuan S. Application of nanomedicine in radiotherapy sensitization. Front Oncol. 2023;13:1088878. doi: 10.3389/fonc.2023.1088878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Goel S., Ni D., Cai W. Harnessing the power of nanotechnology for enhanced radiation therapy. ACS Nano. 2017;11:5233–5237. doi: 10.1021/acsnano.7b03675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.DuRoss A.N., Phan J., Lazar A.J., Walker J.M., Guimaraes A.R., Baas C., et al. Radiotherapy reimagined: integrating nanomedicines into radiotherapy clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 doi: 10.1002/wnan.1867. Available from: [DOI] [PubMed] [Google Scholar]
- 382.Hagan C.T. 4th, Mi Y, Knape NM, Wang AZ. Enhancing combined immunotherapy and radiotherapy through nanomedicine. Bioconjugate Chem. 2020;31:2668–2678. doi: 10.1021/acs.bioconjchem.0c00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Cheng K., Sano M., Jenkins C.H., Zhang G., Vernekohl D., Zhao W., et al. Synergistically enhancing the therapeutic effect of radiation therapy with radiation activatable and reactive oxygen species-releasing nanostructures. ACS Nano. 2018;12:4946–4958. doi: 10.1021/acsnano.8b02038. [DOI] [PubMed] [Google Scholar]
- 384.Lammers T., Aime S., Hennink W.E., Storm G., Kiessling F. Theranostic nanomedicine. Acc Chem Res. 2011;44:1029–1038. doi: 10.1021/ar200019c. [DOI] [PubMed] [Google Scholar]
- 385.Chen Q., Wang X., Wang C., Feng L., Li Y., Liu Z. Drug-induced self-assembly of modified albumins as nano-theranostics for tumor-targeted combination therapy. ACS Nano. 2015;9:5223–5233. doi: 10.1021/acsnano.5b00640. [DOI] [PubMed] [Google Scholar]
- 386.Prabhu P., Patravale V. The upcoming field of theranostic nanomedicine: an overview. J Biomed Nanotechnol. 2012;8:859–882. doi: 10.1166/jbn.2012.1459. [DOI] [PubMed] [Google Scholar]
- 387.Beiranvand S., Sorori M.M. Pain management using nanotechnology approaches. Artif Cells, Nanomed Biotechnol. 2019;47:462–468. doi: 10.1080/21691401.2018.1553885. [DOI] [PubMed] [Google Scholar]
- 388.Chakravarthy K.V., Boehm F.J., Christo P.J. Nanotechnology: a promising new paradigm for the control of pain. Pain Med. 2018;19:232–243. doi: 10.1093/pm/pnx131. [DOI] [PubMed] [Google Scholar]
- 389.Lu S., Gao W., Gu H.Y. Construction, application and biosafety of silver nanocrystalline chitosan wound dressing. Burns. 2008;34:623–628. doi: 10.1016/j.burns.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 390.Fidoski J., Benedetti A., Kirkov A., Iliev A., Stamatoski A., Baftijari D. Nano-emulsion complex (propolis and vitamin C) promotes wound healing in the oral mucosa. Oral Maxillofac Pathol J. 2020;11:1–5. [Google Scholar]
- 391.Rose J.S., Neal J.M., Kopacz D.J. Extended-duration analgesia: update on microspheres and liposomes. Reg Anesth Pain Med. 2005;30:275–285. doi: 10.1016/j.rapm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 392.Bourquin J., Milosevic A., Hauser D., Lehner R., Blank F., Petri-Fink A., et al. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv Mater. 2018;30 doi: 10.1002/adma.201704307. [DOI] [PubMed] [Google Scholar]
- 393.Charron D.M., Zheng G. Nanomedicine development guided by FRET imaging. Nano Today. 2018;18:124–136. [Google Scholar]
- 394.Chen N.T., Cheng S.H., Liu C.P., Souris J.S., Chen C.T., Mou C.Y., et al. Recent advances in nanoparticle-based Forster resonance energy transfer for biosensing, molecular imaging and drug release profiling. Int J Mol Sci. 2012;13:16598–16623. doi: 10.3390/ijms131216598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Chen T., Li C., Li Y., Yi X., Lee S.M., Zheng Y. Oral delivery of a nanocrystal formulation of schisantherin a with improved bioavailability and brain delivery for the treatment of Parkinson's disease. Mol Pharm. 2016;13:3864–3875. doi: 10.1021/acs.molpharmaceut.6b00644. [DOI] [PubMed] [Google Scholar]
- 396.Bunt G., Wouters F.S. FRET from single to multiplexed signaling events. Biophys Rev. 2017;9:119–129. doi: 10.1007/s12551-017-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Hu X., Dong X., Lu Y., Qi J., Zhao W., Wu W. Bioimaging of nanoparticles: the crucial role of discriminating nanoparticles from free probes. Drug Discov Today. 2017;22:382–387. doi: 10.1016/j.drudis.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 398.Hu X., Zhang J., Yu Z., Xie Y., He H., Qi J., et al. Environment-responsive aza-BODIPY dyes quenching in water as potential probes to visualize the in vivo fate of lipid-based nanocarriers. Nanomedicine. 2015;11:1939–1948. doi: 10.1016/j.nano.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 399.Xie Y., Shi B., Xia F., Qi J., Dong X., Zhao W., et al. Epithelia transmembrane transport of orally administered ultrafine drug particles evidenced by environment sensitive fluorophores in cellular and animal studies. J Contr Release. 2018;270:65–75. doi: 10.1016/j.jconrel.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 400.Wang Y.F., Zhang T., Liang X.J. Aggregation-induced emission: lighting up cells, revealing life! Small. 2016;12:6451–6477. doi: 10.1002/smll.201601468. [DOI] [PubMed] [Google Scholar]
- 401.Xue X., Zhao Y., Dai L., Zhang X., Hao X., Zhang C., et al. Spatiotemporal drug release visualized through a drug delivery system with tunable aggregation-induced emission. Adv Mater. 2014;26:712–717. doi: 10.1002/adma.201302365. [DOI] [PubMed] [Google Scholar]
- 402.Wang C., Wang Z., Zhao X., Yu F., Quan Y., Cheng Y., et al. DOX loaded aggregation-induced emission active polymeric nanoparticles as a fluorescence resonance energy transfer traceable drug delivery system for self-indicating cancer therapy. Acta Biomater. 2019;85:218–228. doi: 10.1016/j.actbio.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 403.Tong H., Hong Y., Dong Y., Häussler M., Li Z., Lam J.W., et al. Protein detection and quantitation by tetraphenylethene-based fluorescent probes with aggregation-induced emission characteristics. J Phys Chem B. 2007;111:11817–11823. doi: 10.1021/jp073147m. [DOI] [PubMed] [Google Scholar]
- 404.Wang F., Wen J., Huang L., Huang J., Ouyang J. A highly sensitive "switch-on" fluorescent probe for protein quantification and visualization based on aggregation-induced emission. Chem Commun. 2012;48:7395–7397. doi: 10.1039/c2cc33172a. [DOI] [PubMed] [Google Scholar]
- 405.Zhu Q., Huang L., Su J., Liu S. A sensitive and visible fluorescence-turn-on probe for the CMC determination of ionic surfactants. Chem Commun. 2014;50:1107–1109. doi: 10.1039/c3cc45244a. [DOI] [PubMed] [Google Scholar]