Abstract
Rheumatoid arthritis is a chronic, systemic autoimmune disease predominantly based on joint lesions with an extremely high disability and deformity rate. Several drugs have been used for the treatment of rheumatoid arthritis, but their use is limited by suboptimal bioavailability, serious adverse effects, and nonnegligible first-pass effects. In contrast, transdermal drug delivery systems (TDDSs) can avoid these drawbacks and improve patient compliance, making them a promising option for the treatment of rheumatoid arthritis (RA). Of course, TDDSs also face unique challenges, as the physiological barrier of the skin makes drug delivery somewhat limited. To overcome this barrier and maximize drug delivery efficiency, TDDSs have evolved in terms of the principle of transdermal facilitation and transdermal facilitation technology, and different generations of TDDSs have been derived, which have significantly improved transdermal efficiency and even achieved individualized controlled drug delivery. In this review, we summarize the different generations of transdermal drug delivery systems, the corresponding transdermal strategies, and their applications in the treatment of RA.
Key words: Rheumatoid arthritis, Transdermal drug delivery systems, Different generations, Enhancement strategies, Drug therapy, Transdermal delivery mechanism, Advanced devices, Microneedles
Graphical abstract
Penetration strategies of different transdermal drug delivery systems and their applications in rheumatoid arthritis.
1. Introduction
Rheumatoid arthritis (RA) is a congestive and systemic autoimmune disease mainly based on partial joint lesions. In the early stage, synovial joints exhibit symptoms of chronic inflammation, and articular cartilage and bone tissue are gradually destroyed, resulting in joint dysfunction or loss, with a higher disability and teratogenicity rate1. Although the morbidity of rheumatoid arthritis in China is lower than that in some European and American countries in recent years, most middle-aged and elderly patients still suffer from it. Some standard treatment choices for RA, such as methotrexate, are effective, but they are also associated with adverse effects caused by the dosage form2. When administered orally, bioavailability is low and nearly 70% of patients experience gastrointestinal side effects such as nausea, vomiting, and diarrhea. When administered by injection, methotrexate is rapidly eliminated in the body, and patient compliance is poor, making self-administration difficult. Due to these side effects, nearly 30% of patients have to give up methotrexate (MTX) treatment. Therefore, an appropriate drug delivery system is essential for developing improved treatments and ultimately for increasing the patient recovery rate in RA. Transdermal drug delivery systems (TDDSs) can decrease systemic adverse effects, improve bioavailability, facilitate patient self-administration, and have high compliance; therefore, their application in the treatment of RA is very promising3,4.
As an autoimmune disease, the cause of RA has not been completely defined, and it is confirmed that RA is not caused by unilateral factors but is closely related to genetics, gender, and environmental factors. For example, studies have shown that in terms of genetic aspects, class I (HLA-A, B, C), class II (HLA-DR, DP, DQ), and class III subregions of major histocompatibility complex (MHC) genes are associated with RA susceptibility5. In addition, RA affects approximately three times as many women as men and is linked to sex hormones, which have an immune regulatory effect. Emerging studies illuminate that decreased estrogen levels during menopause and postpartum are related to an increased risk and severity of RA6. Although the potential of regulating sex hormones to treat RA has not yet been successfully tested in clinical trials, it is undeniable that sex hormones are an important factor affecting the pathogenesis of RA. In addition, microbial infection is also considered to be an important cause of RA. Changes in the intestinal bacterial composition in preclinical and diagnosed patients indicate the vital influence of the intestinal microbiota on the characteristics of RA immune dysfunction. Although the mechanism by which dysregulation of the intestinal flora leads to RA is not fully understood, currently, we still know some mechanisms, mainly including the production of intestinal mucosal barrier damage, proinflammatory metabolites, and molecular mimicry of self-antigens7.
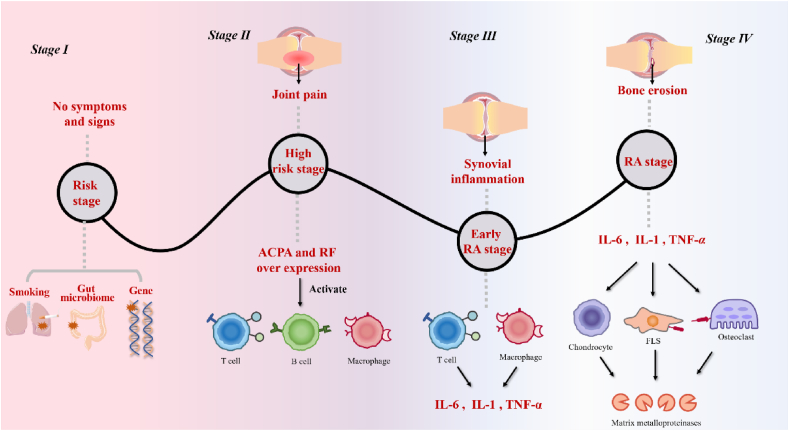
The pathogenesis of RA is very complicated, and we have been able to identify only a few potential mechanisms, namely the aberrant activation of congenital and adaptive immune responses and the breakdown of self-tolerance in genetically predisposed individuals, as shown in Fig. 18. In brief, our joint tissue is regarded as an intruder by the immune system to assault. The occurrence of rheumatoid arthritis is related to many cytokines, with tumor necrosis factor (TNF)-α and interleukin (IL)-6 as the core. Other cytokines, such as IL-7, IL-21 granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, and IL-18, also play a role9. The first stage in the course of the onset of RA is the risk stage: genes, the environment, and other factors increase the risk of patients suffering from RA. A study in the association between single nucleotide polymorphisms (SNPs) and minor allele frequency (MAF) and articular damage was analyzed has shown that two genes are involved in autoimmune disease risk: the rs451066 gene on chromosome 14 and the rs11908352 gene on chromosome 20 affect the progression of RA, as reviewed by de Rooy et al10. Among them, near the gene encoding matrix metalloproteinase-9 (MMP-9), rs11908352 significantly affects the level of serum MMP-9, whose overexpression causes the decomposition of collagen and proteoglycan, and eventually induces cartilage and bone destruction11. However, there may be no symptoms or signs at this stage. The second stage is the high-risk stage of RA: various inducements cause overexpression of anti-citrullinated protein antibodies (ACPA) and rheumatoid factor (RF), promoting the humoral immune response and activating T cells, B cells, and macrophages12. Pain and other symptoms occur at this stage. The third stage is the early RA stage, where synovial inflammation may occur. The synovium is loose connective tissue around joints, composed of fibroblasts, whose pathology is mainly related to immune cell subsets in synovial tissue, including macrophage subsets and T-cell subsets, and nonimmune cell subsets, including fibroblast subsets. When T cells and macrophages in synovial tissue are activated, inflammatory factors, such as IL-1 and TNF-α, are released13; meanwhile, FAPα+THY1− fibroblasts advance inflammation by producing chemokines to promote the transport of immune cells to inflamed joints. At this stage, the patient has arthritis. The fourth stage is the RA stage. Inflammatory factors produced in the third stage activate fibroblast-like synovial cells (FLSs), which produce a large amount of CSF, such as GM-CSF and M-CSF. These factors induce macrophages to differentiate into osteoclasts, generate MMPs, destroy joints, and cause dysfunction11.
Figure 1.
Pathogenesis and pathways of rheumatoid arthritis.
2. Drug therapies for RA
The main clinical treatments for RA are surgery and drug therapy. In the presence of joint dysfunction and joint deformity, or after conservative treatment of symptoms without significant relief, surgical treatment is usually used. For patients in the early and middle stages, drug therapy is usually used14. The drugs for RA are nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), traditional Chinese medicine (TCM), and disease-modifying antirheumatic drugs (DMARDs). DMARDs include traditional conventional synthetic DMARDs (csDMARDs), targeted synthetic DMARDs (tsDMARDs), and biological agent DMARDs (bDMARDs)15, as shown in Table 116, 17, 18, 19, 20, 21, 22, 23, 24, 25.
Table 1.
Mechanism of action and adverse effects of common RA drugs.
| Category | Common drug | Dosage form | Mechanism | Adverse reaction | Ref. |
|---|---|---|---|---|---|
| NSAIDs | Acetylsalicylic acid | Tablet | Bind to COX-1 and COX-2, inhibit the conversion of AA | Gastrointestinal reactions include peptic ulcer, esophagitis, first-pass effect | 16 |
| Phenylbutazone | Tablet | Compete with AA for the active site of COX and inhibit the production of PG | |||
| Celecoxib | Capsule | ||||
| GCs | Hydrocortisone | Tablet | Binds to glucocorticoid receptor GR to enhance transcription of anti-inflammatory genes or inhibit inflammatory genes | Gastrointestinal side effects such as gastrointestinal bleeding | 17 |
| Methylprednisolone | Tablet | ||||
| csDMARDs | Methotrexate | Tablet | The folic acid antagonistic pathway inhibits the proliferation of RA-associated inflammatory cells | Gastrointestinal side effects include vomiting, nausea | 18 |
| Leflunomide | Tablet | Inhibit DHODH, inhibit joint oxidative damage | Diarrhea, nausea | 19 | |
| Iguratimod | Tablet | Inhibition of secretion of IL-1 and IL-6 by macrophages | Gastrointestinal discomfort | 20 | |
| tsDMARDs | Baricitinib | Tablet | Inhibition of JAK kinase to inhibit immune and inflammatory responses | First-pass metabolism, gastrointestinal, and kidney damage | 21 |
| Tofacitinib | Tablet | ||||
| bDMARDs | Infliximab | Injection | Blocking TNF-α, the core molecule of the inflammatory response, through TNF-receptor 1 and TNF-receptor 2 | Poor patient compliance, pain, difficulty in self-administration | 22,23 |
| Adalimumab | Injection | ||||
| Anakinra | Injection | Inhibit IL-1/IL-6 receptors | |||
| Traditional Chinese medicine | Sinomenine | Tablet | Reduce JAK/STAT phosphorylation and regulate cytokine levels IL-17 and TNF-α levels | Poor water solubility, low bioavailability, and first-pass effect | 24 |
| Tripterygium glycosides | Tablet | Inhibit IL-1β, IL-6, IL-8, TNF-α, IFN-γ, IL-17 and other inflammatory factors through p53 and RANKL pathway | 25 |
NSAIDs, nonsteroidal anti-inflammatory drugs; GCs, glucocorticoids; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs; bDMARDs, biological agent disease-modifying antirheumatic drugs; COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; AA, arachidonic acid; COX, cyclooxygenase; PG, prostaglandin; GR, glucocorticoid receptor; RA, rheumatoid arthritis; DHODH, dihydroorotate dehydrogenase; IL-1, interleukin-1; IL-6, interleukin-6; JAK, Janus kinase; TNF-α, tumor necrosis factor-α;JAK/STAT, Janus kinase/signal transducer and activator of transcription; IL-17, interleukin-17; IL-1β, interleukin-1β; IL-8, interleukin-8; IFN-γ, interferon-γ; RANKL, receptor activator of nuclear factor-B ligand.
2.1. NSAIDs
NSAIDs are the most common drugs used in the therapy of RA, with significant anti-inflammatory and analgesic effects. Although the chemical structures of NSAIDs differ from each other, they all achieve anti-inflammatory effects by inhibiting the conversion of arachidonic acid (AA) to prostaglandin (PG)26. All NSAIDs can inhibit cyclooxygenase (COX), but the different effects and binding modes of COX active sites are related to the chemical structures of NSAIDs. Although NSAIDs are widely used, there are some adverse reactions in the gastrointestinal tract and kidney related to NSAIDs inhibiting cyclooxygenase-2 (COX-2) and preventing PG production in inflamed or other tissue damage sites.
2.2. GCs
GC, a steroid hormone that is also applied widely in the treatment of RA, can be divided into endogenous glucocorticoids and exogenous glucocorticoids. Endogenous glucocorticoids refer to a class of steroid hormones excreted by the adrenal cortex under physiological conditions; conversely, exogenous glucocorticoids refer to synthetic glucocorticoids. The anti-inflammatory mechanisms of GC are as follows. From the genetic perspective, GC enhances or inhibits the transcription of anti-inflammatory genes by binding to glucocorticoid receptor (GR), thereby affecting the production of inflammatory cells and molecules27. From the cellular perspective, GCs can inhibit cellular or humoral immunity, decrease the number of mononuclear macrophages, and impede the synthesis of inflammatory factors, prostaglandins, and the expression of Fc receptors28. Although GCs are widely used, long-term use may lead to osteoporosis, diabetes, adrenal axis inhibition, and other adverse reactions29.
2.3. DMARDs
As a class of drugs that work slowly, csDMARDs reduce or prevent joint destruction and deformity, effectively relieve the disease symptoms of RA patients, and thus interfere with the whole disease process30. The chemical structure and anti-inflammatory mechanism of csDMARDs vary, among which methotrexate and leflunomide are the most common. Methotrexate (MTX) has been internationally considered a preferred drug for the treatment of RA recommended by both the European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR), which regards low-dose MTX monotherapy as the primary treatment for RA patients. In addition, MTX has also become a standard treatment choice for medium-to long-term, moderate to severe RA31. The known mechanisms are complex and commonly associated with the folic acid antagonistic pathway, adenosine signaling pathway, and p38 mitogen-activated protein kinase (p38MAPK) signaling pathways. Leflunomide (LEF) exerts immunoregulatory properties and has been included in the commonly used csDMARDs. LEF functions by inhibiting the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH) to restrain de novo synthesis of pyrimidine ribonucleotide uridine monophosphate (rUMP) and thus has anti-inflammatory effects. In addition, one study showed that A77 1726, the active metabolite of leflunomide, induces HO-1 activity in CD4+ T cells and regulates Th17-Treg cell homeostasis, thereby inhibiting oxidative damage in joints and reducing the expression of proinflammatory cytokines32. Long-term use of anti-rheumatic drugs to improve the condition may lead to gastrointestinal reactions, liver damage, and other side effects. Therefore, patients with liver disease should go to the hospital for regular examination during medication.
TsDMARDs are multitarget Janus kinase (JAK) inhibitors that suppress immune and inflammatory responses by inhibiting JAK kinase. The JAK/signal transducer and activator of transcription (STAT) pathway plays a significant role in mediating multiple proinflammatory and anti-inflammatory cytokines in the treatment of RA33. Compared with several previous drugs, tsDMARDs are more direct and targeted and can effectively delay the progression of RA34. BDMARDs are biological products, mainly including TNF-α blockers, IL-1/IL-6 receptor blockers, T-cell costimulation blockers, etc. Safety studies have suggested a modestly increased risk of severe infection in patients using bDMARDs compared with those using csDMARDs, while no difference was found in patients using bDMARDs. Regarding other safety risks, such as cancer risk and herpes zoster infection risk, there was no increased risk in patients using bDMARDs35.
2.4. Traditional Chinese medicine
Traditional Chinese medicine (TCM) has been proven to be effective in the treatment of RA. Compared with chemical drugs, traditional Chinese medicine has the advantages of good safety and fewer adverse reactions and can be used for an extended period. Therefore, it has been used alone or in concert with chemical drugs in the research and treatment of clinical RA. At present, several TCMs are effective in alleviating RA, including sinomenine, curcumin, tripterygium glycosides, and total glucosides of Paeonia, which regulate the levels of inflammatory factors through the JAK/STAT pathway, NF-κB pathway, MAPK, and other pathways, respectively, and exert anti-inflammatory effects. These TCMs generally suffer from poor water solubility and low oral bioavailability36.
Oral administration and injection are common modes of drug delivery for RA. Oral administration often has a first-pass effect, which reduces the number of drugs that are absorbed into the blood. In addition, oral administration is prone to result in gastrointestinal reactions, nausea, upper abdominal discomfort, and other adverse reactions. The poor compliance of patients administered by injection is not conducive to self-management and brings many inconveniences to patients' lives. Therefore, it is essential to find a way to avoid the above problems for the treatment of RA. It is for this reason that TDDSs have come to our attention37.
3. Transdermal drug delivery systems
With the launch of the first transdermal patch “Scopolamine Hydrobromide Adhesive Patches” in 1979, TDDSs came to the attention of the public38. TDDSs are a noninvasive type of preparation in which drugs enter the skin at a certain speed, pass through the epidermis, dermis, and subcutaneous tissues of the skin in turn, and finally absorb effective drugs through the skin microcirculation. Compared with oral drug delivery, TDDSs have the main advantages, including avoiding first-pass effects, improving bioavailability, and relieving gastrointestinal reactions, epigastric discomfort, and other adverse reactions. Patients with TDDSs are more likely to self-administer than those treated by injection, improving patients' convenience and avoiding the risk of infection associated with the injection. In addition, TDDSs provide drugs with a more stable rate of absorption and maintain a more stable blood drug concentration39. Thus, for the treatment of many diseases, TDDSs provide a safer and more effective platform than oral and injectable drug delivery.
3.1. Transdermal routes of drug absorption
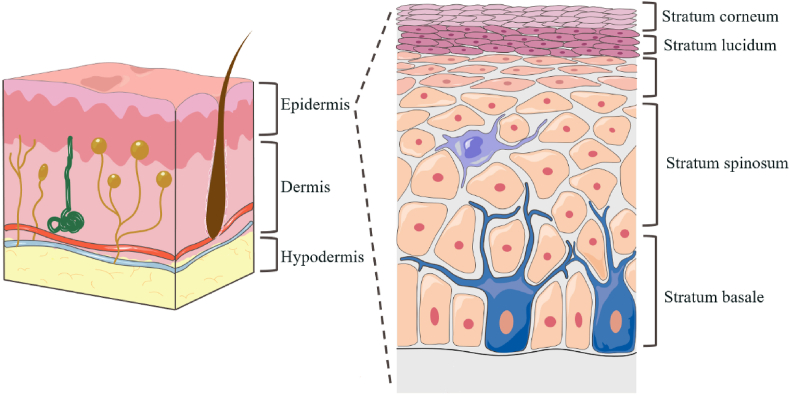
As the largest organ of the human body, the surface area of the skin is 1.5–2 square meters, accounting for approximately 15% of the total mass of an average person40, which can cause the body to be exempt from external interference as a physical barrier, including damage from microorganisms, allergens, ultraviolet radiation, and chemicals. In addition, the skin plays a vital role in preventing water loss and regulating body temperature. Human skin is multilayered, with the epidermis, dermis, and subcutaneous tissue from outside to inside, as shown in Fig. 241.
Figure 2.
Schematic diagram of skin structure.
The epidermis is the outer layer of the skin, approximately 50–100 μm in thickness depending on its location on the body, and is mainly composed of corneocytes. It is classified into five parts, namely, the stratum corneum (SC), stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale42. The SC is mainly composed of dead corneocytes and is filled with dense keratin. In addition, the SC contains a lipid layer, including ceramides, fatty acids, cholesterol, squalene, and triglycerides. Corneocytes are indirectly connected through interconnected lipid layers in the form of multilayers, forming a dense structure of the SC. In addition, corneocytes are closely connected by desmosomes and tight junctions, providing stability and an additional barrier to the SC43. Just below the stratum corneum is a transparent and thin structure called the hyaline layer, consisting of 2–3 layers of keratin-forming cells and only existing in the very thick epidermis of the palms of the hands and feet44. The lower layer of the hyaline layer is the granular layer, where cells have thicker membranes, and there are two types of granules at the granular layer: hyaline keratin granules and lamellipodia. The lower layer of the granular layer is the spiny layer, which contains lymphatic and tissue fluids that provide nutrients to epidermal cells. The deepest layer of the epidermis is the basal layer, in which cells proliferate and provide the basis for keratin-forming cells that differentiate into keratinocytes in the stratum corneum45.
A basement membrane zone exists between the dermis and the epidermis, which consists of proteins and glycoproteins whose main function is to provide adhesion and connect the epidermis to the dermis46. The dermis is approximately 2–5 mm thick, depending on the different body parts. It mainly consists of mesenchyme (collagen, elastin fibers, etc.), cells (fibroblasts, mast cells, adipocytes, phagocytes, histiocytes, etc.), lymphatic vessels, blood vessels, sweat glands, nerve endings, etc.47. The capillary network constitutes the skin microcirculation system, which is responsible for drug absorption into the systemic circulation48. The reticular dermis is the main part of the dermis and contains a large number of collagen fibers that make it denser and elastic49. In addition, the dermis plays an important role in immunity and sebum secretion due to the presence of histiocytes, sweat glands, and hair follicles47. The hypodermis tissue is the innermost zone of the skin, consisting of adipocytes, lymph, and blood vessels, and its main function is to protect the body from physical damage, store heat, and control metabolism50.
The transdermal routes include the paracellular pathway, intracellular pathway, and transfollicularr pathway. Paracellular transport refers to the entry of drugs into the dermis through the spaces between corneocytes and is generally associated with itsmall-moleculele heterophilic drugs. Intracellular transport refers to the passage of the drug through the cells of the SC, a process that involves the partitioning of the hydrophilic and heterophilic environment several times and is demanding in terms of drug properties; therefore, most drugs do not go this route51. Transfollicular transport, also known as the trans adnexal route, refers to the absorption of drugs by sweat glands, hair follicles, or sebaceous glands. This approach bypasses the stratum corneum with a higher rate of drug absorption than the first two approaches. However, the total area of the skin appendages is too small, limiting the total amount of drug absorption; therefore, this approach is not the major method of transdermal drug absorption41.
3.2. Difficulties in transdermal drug delivery
One of the main and most difficult barriers the skin uses to protect itself and enable it to prevent external substances from entering is the stratum corneum42. It has a dense “mortar and brick” structure; the intercellular lipid layer is considered “mortar”, and corneocytes with highly insoluble keratinized envelopes and keratin filaments are often considered “bricks”52, closely connected through desmosomes and tight junction-related structures. First, the compact structure formed by the tight junction-related structure leads to a small intercellular space between the SC, which limits the permeability of macromolecular drugs and proteins53. In addition, both the keratinized envelope of corneocytes and the intercellular lipid layer are lipid soluble, thus greatly limiting the penetration of water-soluble drugs into the skin. In contrast, lipid-soluble drugs bypass corneocytes to achieve diffusion through the lipid layer. Of course, this does not mean that as long as the drug is fat-soluble, it can pass through the SC because the SC is not a completely fat-soluble environment. The keratin in the SC contains a natural moisturizing factor (NMF), which provides approximately 20% hydration for the SC and maintains proper hydration of the SC54. Therefore, TDDSs have high requirements for the drug, including the appropriate log P value, molecular weight, and several hydrogen-bonded donor acceptors. With the continuous development and innovation of transdermal strategies, four generations of TDDSs have been developed to gradually overcome the dilemma of transdermal drug delivery and achieve maximum efficiency or even intelligent individualized drug delivery.
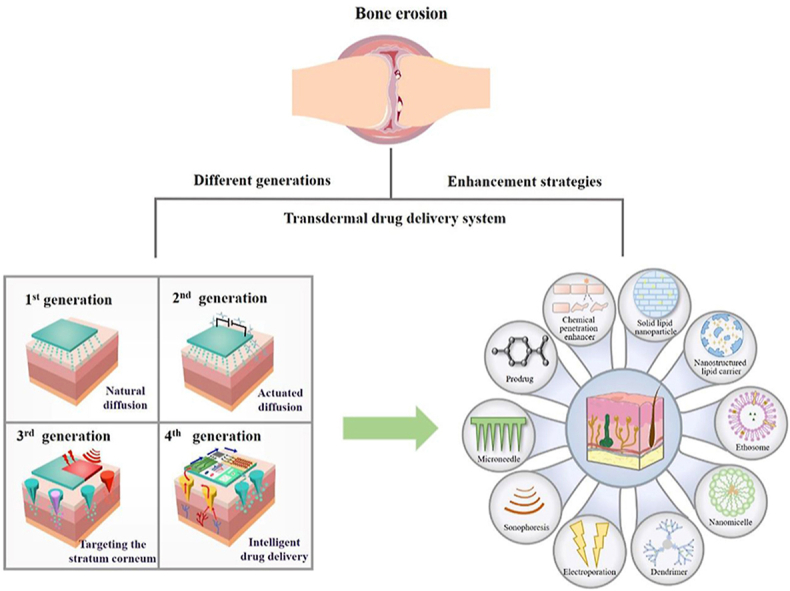
3.3. Different generations of TDDSs
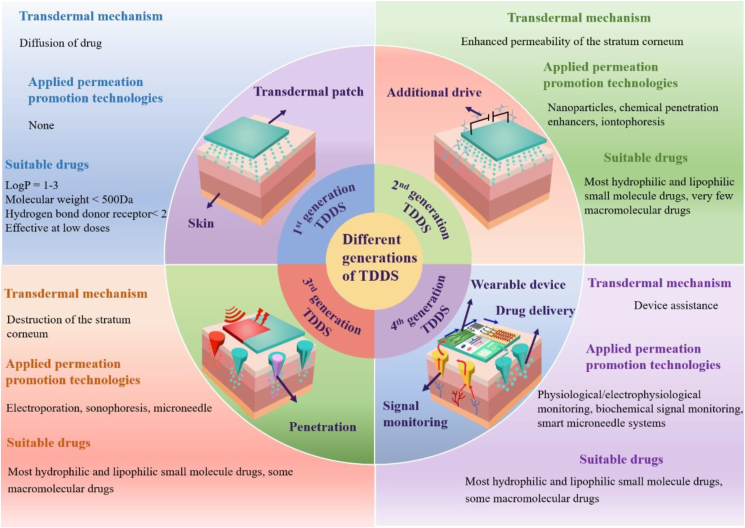
Currently, TDDSs are divided into four generations, as illustrated in Fig. 3. The first generation of TDDSs is designed based on the principle of natural diffusion of drugs. Drugs diffuse from TDDSs to the skin through natural diffusion and are absorbed and take effect. The first generation of TDDSs focused on the properties of the drug itself, only for lipophilic, low molecular weight, effective drugs at low doses, so they are limited55.
Figure 3.
Classification of transdermal generations based on mechanism, applicable drugs, and applied technologies.
To expand the transdermal delivery capacity of other small molecule drugs, the second generation of TDDSs came into being, which is designed based on the reversible destruction of the SC structure or providing an additional driving force for drug entry into the skin to improve the drug delivery efficiency from both drug and SC aspects. On the one hand, the drug is modified to have a suitable log P value to facilitate drug transfer through the skin; on the other hand, the SC structure is disrupted or pore channels are formed in the SC by some physicochemical means to achieve greater transdermal drug delivery efficiency. Second-generation TDDSs have laid the foundation for maximizing drug delivery efficiency, but limitations still exist. How to balance maximizing the passage through the SC and protecting deep tissues from damage was a problem faced and urgently needed to be solved with second-generation TDDSs, which led to the emergence of third-generation TDDSs56.
Third-generation TDDSs are a generation of TDDSs specifically for SC, and they can target SC by physicochemical or other means, which can achieve the destruction of SC structure and improve the efficiency of drug transdermal delivery without affecting the deeper tissues. At the same time, the range of drugs that can be delivered has also become wider. In addition to small molecules, third-generation TDDSs can also achieve transdermal delivery of macromolecular drugs and vaccines, further improving the shortcomings of TDDSs in drug delivery. Fourth-generation TDDSs, the most advanced TDDSs at present, are intelligent wearable devices combined with bioelectronic technology57, which are no different from the second and third generations in terms of maximizing transdermal drug delivery, especially in that they can intelligently adjust drug delivery by monitoring the signal level of the body in real-time. Specifically, the physiological, electrophysiological, and biochemical signals are accurately measured by sensors and transmitted to the actuator, which transfers energy to the drug-loaded patch in a controlled manner to release the drug into the skin for precise, personalized treatment55.
From the first generation of TDDSs to the fourth generation of TDDSs, the skin barrier is gradually overcome, the applicable drug range is expanded, the drug delivery efficiency is maximized, the safety of transdermal drug delivery is enhanced, and skin irritation is reduced. Even fourth-generation TDDSs can realize real-time monitoring of drug delivery while possessing the above advantages. This fine and controllable intelligent drug delivery method is undoubtedly a milestone in TDDSs.
4. Enhancement strategies for TDDSs
Second-generation TDDSs, with their chemical penetration enhancers and nanocarriers, increase the transdermal delivery of small molecules, and third-generation TDDSs, with their enhancement strategies, enable the successful transdermal delivery of various large molecules. In this section, we specifically describe the permeation enhancement strategies of second-and third-generation TDDSs58, and we summarize their advantages, limitations, and transdermal delivery mechanisms, as indicated in Table 259, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78.
Table 2.
Advantages, limitations, and transdermal delivery mechanism of different enhancement strategies.
| Different generations of TDDSs | Enhancement strategy | Specific measure | Advantage | Limitation | Transdermal delivery mechanism | Transdermal drug delivery system | Ref. |
|---|---|---|---|---|---|---|---|
| Second-generation TDDS | Stratum corneum modification | Chemical penetration enhancer | Wide application and low cost | Excessive concentration can be irritating to the skin | Alters the structure of the SC, providing a concentration gradient | Drug-loaded patch, hydrogel patch, gel, ointment | 59 |
| Drug modification | Prodrug | The hydrophilic and hydrophobicity of drugs can be regulated | Prodrugs may cause toxicity and may lead to the production of toxic metabolites | Modification of drugs to alter their physicochemical properties so that they conform to the requirements for transdermal drug delivery | Direct drug-loaded patch, microneedle, gel, alcohol hydrogel, lipid nanoparticles, nanoemulsion | 60 | |
| Ion pair | Simple preparation and low cost | The hydrogen bonds forming ion pairs are unstable and break easily under conditions such as ultrasound | Transdermal patch | 61 | |||
| Electricity-driven methods | Iontophoresis | Rapid drug release into the skin, ability to deliver large molecules | Difficult autonomous drug delivery, unknown long-term safety, and compliance | Provide driving force to form pores in SC | Usually used in combination with semi-solid formulations | 62 | |
| Nanocarriers | Nanoemulsion | Good stability and compliance, low toxicity and irritation | Disruption of the integrity of the SC due to CPEs | Reduce the interfacial tension, dissolve the lipid layer | Hydrogel patch, gel, nanoemulsion, film | 63,64 | |
| Solid lipid nanoparticle | Good biocompatibility and skin tolerance | Poor physical stability, prone to sudden drug release | Form a lipid membrane on the skin surface, loosen SC cells, and interact with SC lipids | Hydrogel patch, gel, transdermal patch, microemulsion, drug-loaded nanostructured lipid carriers | 65 | ||
| Nanostructured lipid carrier | High drug loading capacity, good physical stability | Short skin retention time | 66 | ||||
| Nanomicelle | Capable of loading both hydrophilic and heterophilic drugs | Low stability, susceptible to the influence of pH | Be blocked by the SC, creating a reservoir of the drug on the skin surface and providing the additional driving force for the drug | Hydrogel patch, gel, microneedle, organized | 67 | ||
| Dendrimer | Dermal penetration of drugs can be guided by dendrimer | Less stable and prone to leakage | Transdermal patch, cream | 67 | |||
| Liposome | Biodegradable, non-toxic, able to encapsulate hydrophilic and lipophilic substances | Large volume, and lack of elasticity, to some extent, hinder effective skin penetration | Interact with the lipid structure of the SC, disrupt the lipid layer | Hydrogel patch, gel, microneedle, the liposome formulation | 68 | ||
| Ethosome | Both hydrophilic and lipophilic drugs can be carried | Long-term skin irritation experiments to be verified | Ethanol in ethanol bodies fluidizes the SC lipid layer and forms a pathway in the SC | Hydrogel patch, gel, transdermal patch, that formulation | 69 | ||
| Transfersome | Highly elastic, super deformable | Cannot encapsulate hydrophobic drugs | Pass through the narrow gaps of the SC cells | Hydrogel patch, gel, microneedle | 70 | ||
| Plant exosome-like nanovesicle | Low immunogenicity toxicity, uniform size good stability | Unknown factors such as interactions with exogenous drugs | Interfuse with the lipid layer, cross the SC, and enhance penetration | Hydrogel patch, gel | 71,72 | ||
| Third-generation TDDS | Electricity-driven methods | Sonophoresis | Less skin damage, simple operation, high universality | Time-consuming, requires sophisticated instruments | Bubbles and collapses are formed in the SC, destroying the SC | Gel, microneedle | 73,74 |
| Electroporation | controllable, wide applications range, especially for biomacromolecule | Complex and costly equipment, the potential for cell damage | Disturbing the structure of the SC lipid layer, forming pores in the lipid layer | Gel, microneedle | 75 | ||
| Cold atmospheric plasma | Stabilization of drug blood levels, avoidance of drug metabolism | Larger clinical trials are still needed to determine the safety of CAP therapy in human | Free radicals in CAP interact with stratum corneum lipids, causing lipid peroxidation and the formation of pores between keratinocytes | N/A | 76 | ||
| Microneedle | N/A | Bypass the SC, lifting the restriction of the SC on drugs | Industrialization difficulties and regulatory issues | Penetrate the epidermis to directly reach the dermal tissue. | Microneedle | 77,78 |
N/A, not applicable. SC, stratum corneum; CPEs, chemical penetration enhancers; CAP, cold atmospheric plasma.
4.1. Strategies of second-generation TDDSs
4.1.1. Stratum corneum modification
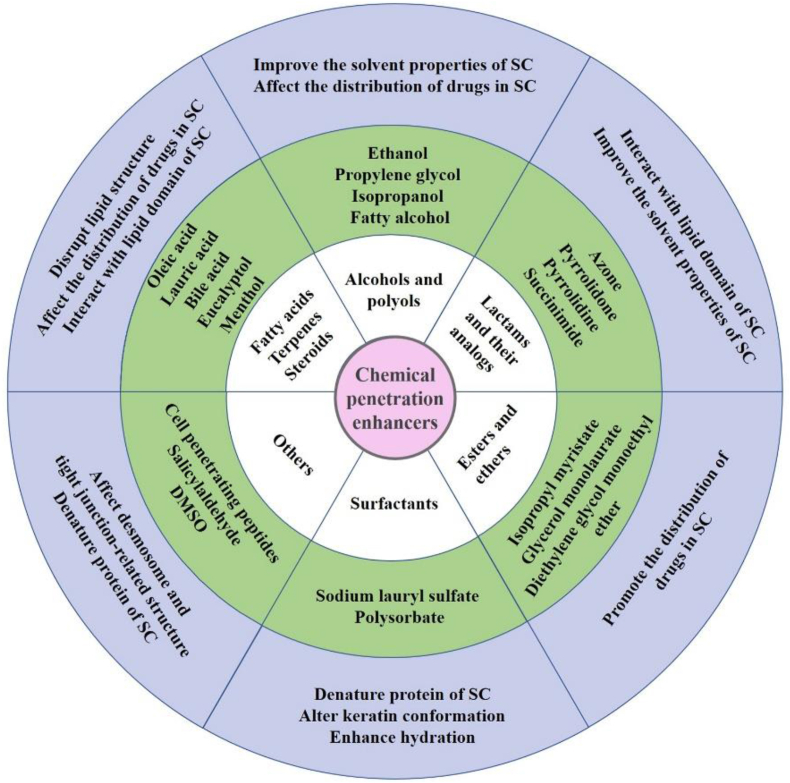
The main obstacles to transdermal delivery stem from the protective layer structure of the skin42. The permeability of the drug in the skin may be improved by modification of the SC, and chemical penetration enhancers (CPEs) have faded in our view. The modification principles of SC by CPEs are classified into four groups: 1. denaturing protein of the SC, altering keratin conformation, and enhancing hydration79. 2. Improving the solvent properties of SC and affecting the distribution of drugs in the SC80. 3. Affecting desmosome and tight junction-related structure. 4. Changing lipid fluidity, interacting with the lipid domain of the SC, and disrupting the lipid structure. According to the database of CPEs established by Vasyuchenko et al.81, CPEs are divided into six categories by molecular structure, as detailed in Fig. 4. The mechanisms of common CPEs are also shown in Fig. 4. CPEs have been used as a means of permeation promotion in TDDSs with a wide application range and longer use time for the treatment of inflammation82, Alzheimer's disease83, migraine59, and other diseases.
Figure 4.
Summary of chemical penetration enhancers according to molecular structure. SC, stratum corneum.
4.1.2. Drug modifications
Drug modifications are mainly divided into prodrug, ion-pair, and eutectic systems84. The special structure of the SC makes it highly demanding for the properties of the drug, including moderate molecular weight, appropriate oil-water partition coefficient (log P) value, suitable melting point, solubility, etc.56. Therefore, nonconforming drugs need to be modified to allow them to better penetrate the skin. Prodrug strategy refers to modifying the structure of the parent drug to change its solubility and log P value85. After administration, the prodrug is metabolized into the parent drug. An ion pair is a pair of two ions with opposite charges that do not bond covalently but rely on Coulombic gravity86. Ionic drugs, do not easily penetrate the cell membrane, so they form ion pairs to neutralize electricity and allow the drug to enter the skin more effectively. Eutectic systems refer to the formation of supramolecular compounds by the interaction of active drug molecules (APIs) and cocrystal forms (CCFs) under noncovalent and nonionic bonds and have already been widely utilized in transdermal delivery for the treatment of Parkinson's disease87, inflammatory diseases88 and psychiatric disorders89.
4.1.3. Electricity-driven methods
The electricity-driven method refers to a method that utilizes the energy generated by an electric device to promote drug transdermal delivery. Iontophoresis is a method to stimulate drug entry into the skin using an electric current as a driving force. The device is composed of a power supply, control circuit, and electrodes. The earliest research on iontophoresis dates back to 1908, when Leduc first used an electric current to drive molecules through the skin90, laying the foundation for the later application of iontophoresis in TDDS. With continued research, iontophoresis has been transformed from a primitive physical technology to a controlled and potential drug delivery method. The iontophoresis method has two transmission mechanisms: electromigration and electroosmosis91. Electromigration is defined as the forced entry of charged drugs into the skin using electrostatic repulsion and is suitable for enhanced transdermal penetration of ionized drugs. During treatment, the cationic drug is placed under the anode, the anionic drug is placed under the cathode, and the anionic and cationic drugs are moved to opposite electrodes under the repulsion pulse of the same-sex electrode to induce drug penetration through the skin. Electroosmosis is caused by using a voltage difference across a charged membrane to cause fluid flow in the same direction as that of the counteracting ions and is suitable for the skin penetration of neutral drugs. In addition, electroosmosis affects the permeation of ionized drugs. At physiological pH, the skin is electronegative, so the antagonistic ions are cations, resulting in electroosmosis from the anode to the cathode; therefore, electroosmosis promotes the transport of cationic drugs and negatively affects the transport of anionic drugs. Usually, in the presence of electric current, ions tend to select the path of minimum resistance through the skin, that is, through the skin appendages that bypass the cuticle into the skin. Of course, nonvesicular pathways also assume an important role in drug transport, and researchers have shown that iontophoresis technology leads to flipping movements of peptide helices in the SC, which in turn form voltage-dependent pore channels92. In addition, iontophoresis leads to an increasing skin water content, enhancing hydration and perturbing the lipid layer, which in turn increases drug penetration. Along with the development of iontophoresis technology, matching dosage forms also need to be developed simultaneously. Semisolid preparations, especially gels, are the most suitable choices and are compatible with both skin and equipment. Currently, iontophoresis has been used in neurodegenerative diseases93, hypertension94, inflammation95, and other diseases.
4.1.4. Nanocarriers
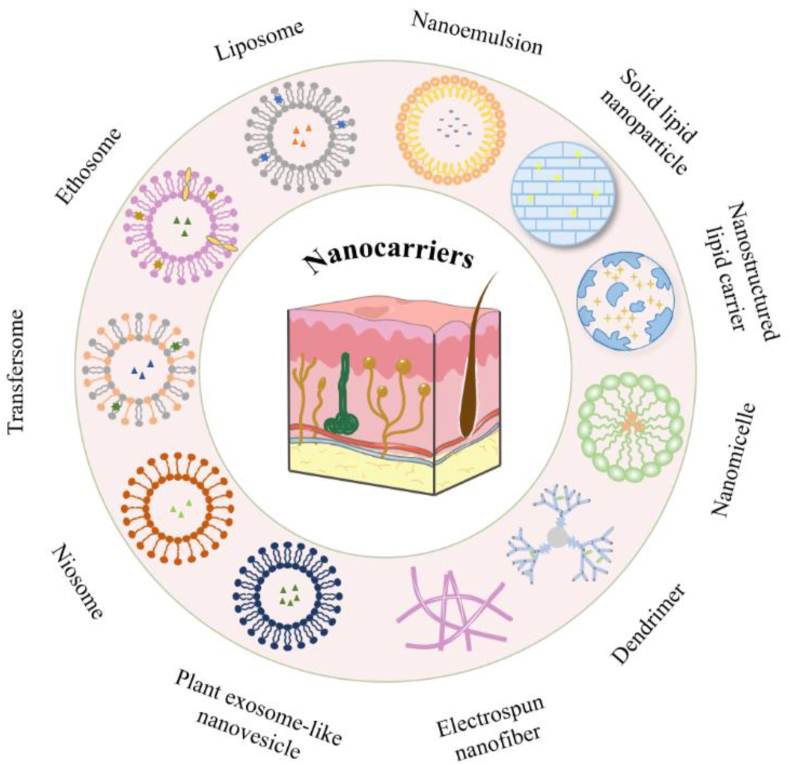
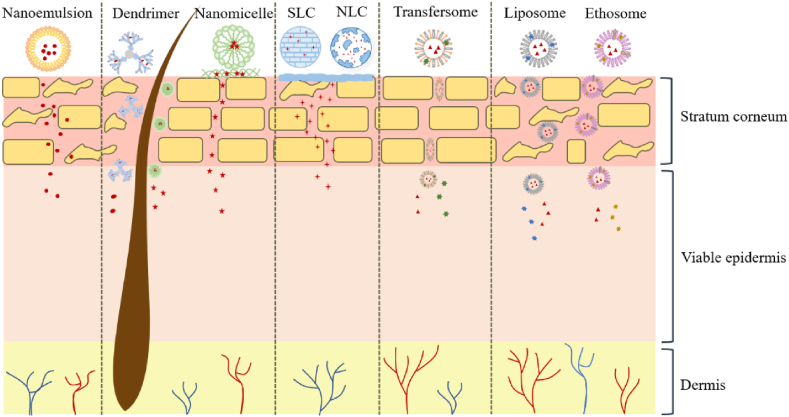
Nanocarriers are commonly used in TDDSs as a means of facilitating permeation, such as solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), micelles, and liposomes, as shown in Fig. 5. These nanoparticles are loaded into hydrogels or microneedles for transdermal administration. The nanoparticles that have been studied to improve the transdermal absorption of RA drugs and their penetration mechanism are shown in Fig. 6.
Figure 5.
Nanocarriers and vesicular systems for enhancing drug penetration.
Figure 6.
The mechanism of different nanocarriers.
4.1.4.1. SLNs and NLCs
SLNs are drug delivery systems that are composed of solid lipids as carriers and encapsulate or embed drugs in the lipid membrane96; by contrast, NLCs are drug delivery systems that are composed of a mixture of liquid and solid lipids as carriers and encapsulate or embed drugs in the lipid membrane97, which together constitute lipid nanoparticles98. SLNs, considered a promising drug carrier system, especially for applications requiring sustained release, were created in the early 1990s99 and consist of water, surfactants, and solid lipids including fatty acids, steroids, triglycerides, waxes, etc.98. Solid lipids are dispersed in the aqueous phase, and surfactants are added as stabilizers for preparation. Common preparation methods include high-shear homogenization, ultrasonic treatment, solvent emulsification, and microemulsion. At room temperature, SLNs are solid particles, and drugs are loaded into solid lipids. Compared with hydrophilic drugs, lipophilic drugs, and lipids have similar properties, resulting in more drugs loaded into lipids. NLCs consist of a mixture of liquid and solid lipids, water, and surfactants100, and studies have shown that the weight ratio of solid to liquid lipids in NLCs is usually between 70:30 and 90:1097. Compared to SLNs, NLCs have a higher drug loading capacity and are also a drug delivery system with broad prospects92. Showing skin adhesion, lipid nanoparticles exhibit adequate contact with SC with a small size, and the lipids in the nanoparticles form a lipid membrane that produces a blocking effect on the skin surface101, reducing water evaporation and loosening SC cells, thereby increasing drug penetration. In addition, the surfactants in lipid nanoparticles interact with skin lipids, destroying lipid structures, increasing intercellular space, and enhancing drug penetration65.
4.1.4.2. Nanomicelles
Nanomicelles consist of amphiphilic polymers or surfactants agglomerated in an aqueous medium; lipophilic drugs are loaded in the inner core, while hydrophilic drugs are located in the outer shell, which increases skin permeability by increasing the water solubility of drugs65. Their hydrophilic structure can counteract the recognition of the reticuloendothelial system (RES) to prolong the action time of drugs in vivo and improve the bioavailability of drugs12. The micelles usually enter the skin via the hair follicle route, where the polymeric component is blocked by the SC, creating a drug reservoir on the skin surface and increasing drug penetration through the SC102. Nanomicelles and CPEs are usually used in combination to enhance the transdermal effect of drugs103. Currently, the materials used to synthesize micelles are not only organic polymers but also novel biomaterials that can themselves be recognized and interact with immune cells such as macrophages and T cells and in combination with encapsulated drugs to achieve co-administration and effectively improve the therapeutic effect, especially in immune-related diseases such as RA104. At present, no micelle technology has achieved success in TDDSs for wound repair105, skin diseases106, cancer107, bacterial infection108, and other diseases.
4.1.4.3. Dendrimers
Dendrimers are high-branched polymeric nanocarriers used for transporting hydrophobic agents. Poly(amidoamine) (PAMAM) has the widest range of applications and permeabilizes the lipid layer by positively charged dendrimers, contributing to skin penetration109. Dendrimers are blocked by the SC of the skin, but they can interact with skin lipids and destroy the lipid layer structure, thereby enhancing drug penetration. They can interact with lipids present in the cell membrane to improve its permeability. Therefore, they increase the solubility and permeability of lipophilic drugs but cannot be used as carriers for hydrophilic drugs65. At present, dendritic polymers have been successfully used in the treatment of tumors110, HIV111, antiviral103, and other immune diseases112,113.
4.1.4.4. Liposomes
In addition to the abovementioned nanoparticles, vesicle systems are also commonly used as a proven means of promoting permeation. Nanovesicles are defined as a double-layer spherical vesicle structure composed of amphiphilic lipids or other analogs114, where hydrophilic drugs are encapsulated in the vesicle core and hydrophobic drugs are encapsulated in a hydrophobic lipid layer115. Liposomes are the earliest and most classical nanovesicles and are spherical vesicles with a phospholipid bilayer structure. The amphiphilic character of liposomes makes them ideal drug carriers for molecules of different polarities. The phospholipids in liposomes interact with the lipid structure of the SC, thereby destroying the lipid layer and enlarging the transdermal amount of the drug116. Common preparation methods include thin-film hydration, the reverse-phase evaporation method117, the pH-gradient method, the solvent injection method118, the dehydration-rehydration method, and the novel combined method119. Liposomes have been applied for transdermal drug delivery to treat a variety of diseases, such as tumors120, rheumatoid arthritis121, fungal infections, and other diseases.
4.1.4.5. Ethosomes
Ethosomes are composed of a bilayer spherical structure consisting of phosphatidylcholine, cholesterol, ethanol, and water. Compared with conventional liposomes, the addition of alcohol in ethosomes makes them more flexible, while alcohol also acts as a CPE to extract SC lipids, destroy the SC lipid layer structure, and improve drug distribution122. In addition, ethanol also provides a negative charge on the surface of the vesicle structure to prevent the aggregation of vesicles due to static electricity92. The preparation methods of ethosomes include the mechanical dispersion method123, pH-gradient method, reversed-phase evaporation method124, ethanol injection-sonication method, and membrane hydration method125. Ethosomes have been successfully used in the treatment of cerebral infarction126, gout127, melanoma128, myocardial ischemia129, and other diseases.
4.1.4.6. Transfersomes
Transfersomes are composed of phospholipids, surfactants, ethanol, and water92 and are defined as a highly elastic-deformable aqueous core with a lipid bilayer possessing an edge activator surrounding130. The surfactant acts as an edge activator, offering a high curvature radius and increasing membrane deformability by destabilizing lipid bilayers, conferring higher elasticity to liposome vesicles and good deformability92. The favorable deformability of the transfersome enables it to pass through the narrow gap of the skin SC cells, thereby enhancing the absorption of drugs. Common preparation methods include the reverse-phase evaporation method131 and ethanol injection method132. At present, transfersomes have been used as TDDSs to treat different kinds of diseases, such as tumors133,134, inflammation135, and dermatosis70.
4.1.4.7. Niosomes
Niosomes are single or multilayer orbicular structures consisting of cholesterol and nonionic surfactants. They may improve the fluidity of skin membranes by interacting with the lipophilic layer136. Meanwhile, by reducing the loss of water through the epidermis, niosomes cause changes in SC characteristics, resulting in SC hydration increasing and its tightly packed cell structure loosening137. Niosomes are appropriate to load both hydrophilic and hydrophobic drugs, whereas large monolayer niosomes have larger water cores and are more suitable for the encapsulation of hydrophilic drug molecules, and multilayer niosomes have multiple bilayers and are more suitable for the loading of heterophilic drugs. The commonly used preparation methods include thin film hydration138, the ether injection method139, the reversed-phase evaporation method140, and supercritical reversed-phase evaporation141. Niosomes have been successfully applied in the treatment of diabetes142, Alzheimer's disease143, wound healing144, actinic keratosis145, and other diseases.
4.1.4.8. Plant exosome-like nanovesicle
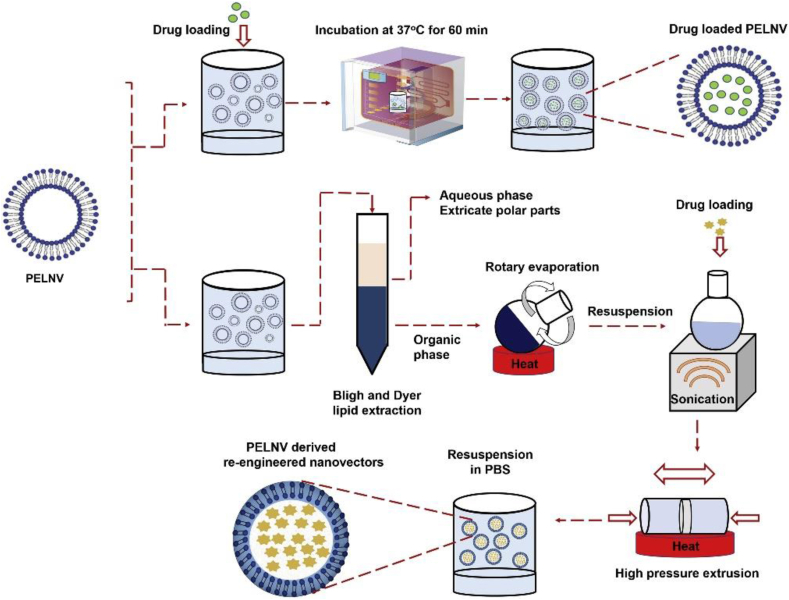
The last vesicle structure is planted exosome-like nanovesicles (PELNVs), a novel drug delivery platform that is a penetration promotion method with great potential as a TDDS. PELNVs mainly consist of active lipids, proteins, nucleic acids, and other pharmacologically active molecules146. As an emerging means of delivery, how to stably obtain high-quality nanoplatforms is an urgent problem to be solved. Currently, there are two types of nanoplatforms71. One is to obtain PELNVs directly from plants, and the supernatant composed of extracellular vesicle subpopulations is continuously isolated by differential centrifugation and purified by sucrose gradient centrifugation. Second, nanolipids can be extracted from PELNVs and used as carriers for drug delivery147. The concept of exosome-like nanovesicles (ELNVs) seems to be more familiar than PELNVs, which can be secreted by most types of mammalian cells and are present in large quantities in blood, saliva, and urine. As a central mediator of intercellular communication, ELNVs play a vital role in the pathogenesis of various diseases in vivo148. PELNVs were discovered earlier than animal ELNVs, but PELNVs have not been extensively studied. Similar to the role of ELNVs, PELNVs play an important physiological role in plants. In addition to being responsible for communication between plant cells, they also act as a defense barrier against plant infection and participate in the formation of cell wall remodeling enzymes (CWREs) and defense proteins149. To enhance the therapeutic effect of PELNVs, exogenous therapeutic molecules such as proteins, siRNA, and DNA are usually contained in PELNVs150. The drug loading methods of PELNVs are similar to those of other vesicular structures and are divided into the passive drug loading method, active drug loading method, and charge adsorption method. The first two methods are shown in Fig. 7, and in the latter, because the surface of exosomes is electronegative they can therefore adsorb drugs with opposite charges and neutral drugs by electrostatic force151,152. As a biocompatible vesicular structure, the potential of PELNVs in TDDSs cannot be underestimated. The bilayer lipid structure allows PELNVs to interfuse with the lipid layer in the SC, thereby penetrating the SC and enhancing penetration71. At present, studies on PELNVs in transdermal drug delivery for disease treatment are not yet mature; however, based on the properties of PELNVs and existing research, we believe that PELNVs have promising prospects as an emerging nano platform for drug delivery.
Figure 7.
Drug loading methods of PELNV: passive drug loading method and active drug loading method. Reprinted with the permission from Ref. 188. Copyright © 2021 Elsevier.
In addition to the abovementioned nanocarriers, there are some other biological nanoparticles that can be used as a new type of nanoplatform for transdermal drug delivery, such as peptide nanoparticles, siRNA-loaded, miRNA nucleic acid nanoassemblies, etc. They have good biosafety and diverse modifications153. Existing studies have shown that their combination with microneedles can successfully achieve transdermal delivery of biological macromolecules such as proteins and nucleic acids154. Although the application of nanocarriers for transdermal drug delivery is relatively mature, it still has its own problems. Difficulties in clinical translation due to species differences between experimental animals and humans, difficulties in production and high prices due to complex processes, interactions between nanomedicines and in vivo organelles, and the safety of the nanomaterials themselves are all issues that we need to address in the process of successfully bringing nanomedicines to the clinic. We believe that with the full-scale evaluation of experiments and the continuous development of advanced preparation techniques, we can achieve a balance between the excellent efficacy of nanomedicines and their problems155, 156, 157.
We have learned about the penetration mechanism of nanocarriers, such as the above and Fig. 6, so how to concretize nanocarriers and how to build a transdermal delivery platform for nanocarriers are the next problems to be solved. In recent years, with the rapid development of the material field, more hydrophilic polymers have attracted attention, and hydrogels have gradually been valued by scientists and applied to transdermal drug delivery. Hydrogels are macromolecular networks composed of hydrophilic polymers, and nanocarriers are dispersed in this network or adsorbed on the surface of macromolecules158,159. These macromolecules can absorb water and expand into hydrogels with high water content, micro elasticity, and certain adhesion to be easily applied to the skin. Compared with traditional patches, creams, or gels, hydrogels have better comfort and controllability, which can not only improve the comfort of patient administration but also enhance the hydration of the SC and promote the transdermal penetration of drugs160. At the same time, the unique macromolecular network structure of hydrogels enables them to control the slow release of nanocarriers, thereby achieving long-term administration. Some specific examples of using hydrogels as nanocarrier delivery platforms are covered in the next section161. At present, with the vigorous development of the field of biomaterials, research on hydrogels is also in the hot stage. Researchers have continuously developed hydrogels with better performance that are more suitable for transdermal administration and have been applied to rheumatoid arthritis162, wound healing163, and other diseases.
4.2. Strategies of third-generation TDDSs
4.2.1. Electricity-driven methods
Sonophoresis is a method of using ultrasonic waves of different frequencies to get drugs into the skin164 and can be applied to deliver small and large-molecule drugs, including insulin. It can be allocated to low-frequency sonophoresis (20–100 kHz) and high-frequency sonophoresis (2–6 MHz), and studies have shown that low-frequency sonophoresis is better than high-frequency sonophoresis in terms of permeation promotion and safety165. Therefore, low-frequency sonophoresis is preferred for clinical applications. Sonophoresis has two mechanisms in permeation promotion: the thermal effect and the cavitation effect. The thermal effect refers to ultrasound waves that increase the temperature of the skin, leading to an increase in the drug transmission rate84,166. The cavitation effect is defined as the formation and collapse of bubbles in the SC caused by ultrasound, and the resulting shock wave or microjet disrupts the lipid bilayer structure of the SC, enhancing drug penetration167. In addition, the drug penetration enhancement effect is also related to ultrasound intensity, frequency, and duration of use. Sonophoresis has been successfully applied in the treatment of Alzheimer's disease168, inflammation169, rheumatoid arthritis73, and other diseases.
Electroporation is a method of forming microscopic holes in the skin by applying a high voltage for a very short period of time to penetrate the drug deep into the skin, which can be applied to deliver various macromolecular and small molecule drugs. Studies have shown that electroporation-enhanced skin permeation is related to a variety of factors, including solubility, log P, dissociation constant (pKa), and penetration time of the drug, while we can optimize these factors to achieve higher cumulative drug permeation170. Electroporation forms pores in the lipid layer by disrupting the structure of the SC lipid layer, thereby allowing drugs to enter the skin and enhancing drug penetration. Electroporation has been successfully used in the treatment of analgesic171, glAUComa75, rheumatoid arthritis172, and other diseases.
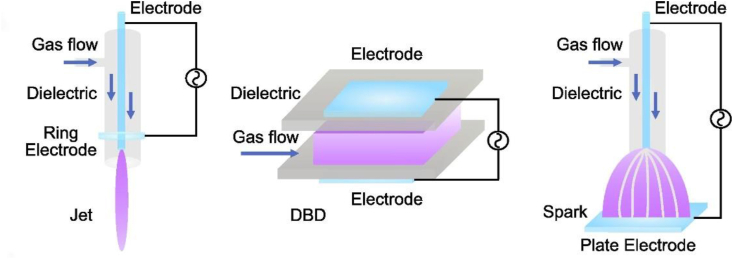
Cold atmospheric plasma (CAP) is an ionized gas consisting of charged particles, neutral particles, and photons produced under atmospheric pressure conditions173. CAP has been extensively researched over the past decade because of free production in open spaces at atmospheric pressure and room temperature. These features allow it to directly come in contact with the body without causing any thermal injury to the tissue174. CAP has three generation devices, dielectric barrier discharge (DBD), plasma jet, and corona discharge, as detailed in Fig. 8175. The mechanism of CAP promotion of permeation is not wholly clarified, but some studies have shown that it may be related to free radical-cuticle lipid interactions, lipid peroxidation, and the generation of intercellular pores in CAP166,173. CAP contains a variety of free radicals such as O, OH, and nitrogen species (RONS), which interact with the skin and change its properties. Van et al.176 have shown that O and OH radicals can interact with α-linolenic acid to produce oxidized fatty acid derivatives, thereby increasing the hydrophilicity of fatty acids in the lipid matrix and improving the transdermal penetration of drugs. In addition, Marschewski et al.177 utilized electron spectroscopy to explore the effect of CAP on changes in skin lipid composition using electron energy spectroscopy and concluded that CAP administration caused obvious changes in the human skin lipid barrier. These studies provide evidence and a theoretical basis for the application of CAP in TDDSs. At present, CAP has been studied in the transdermal delivery of cyclosporine A178 and Alzheimer's disease173. It is believed that with the maturation of technology and the depth of research, CAP will gradually be widely applied to TDDSs.
Figure 8.
Generating devices of cold atmospheric plasma generating device. Reprinted with the permission from Ref. 175. Copyright © 2022 Elsevier.
4.2.2. Microneedles
Microneedles (MNs) are array patches consisting of micrometer-sized needles with a height of 10–2000 μm and a width of 10–50 μm179 that avoid nociceptive receptors to form micron-sized channels in the skin, capable of penetrating the epidermis to deliver small molecule drugs, proteins or vaccines directly to the dermal tissue180. MNs are widely used in TDDSs due to their safety, high efficiency, convenience, and painlessness179. Good microneedles should have good biocompatibility, high mechanical strength, and low production costs. Currently, microneedles can be mainly classified as solid microneedles, coated microneedles, hollow microneedles, dissolving microneedles, and hydrogel microneedles181,182.
Solid MNs are made of hard materials such as silicon and metal. When solid microneedles are used for administration, microneedles are first pressed into the skin to form pores, and then transdermal patches are attached to make the drug enter the skin microcirculation through the pores92. These microneedles are often combined with an electric field to achieve better results. However, there is a risk that the generated micropores may be infected by microorganisms or penetrated by other harmful substances. Therefore, how to form pores to achieve drug delivery while ensuring safety is something we need to constantly explore180.
Coated microneedles mean that the surface of the needle tip is coated with a drug, and as the microneedle is inserted, the coating is gradually dissolved by the interstitial fluid and releases the drug. The limitation of the surface area of the tip of a coated microneedle results in a small drug loading capacity of the coated microneedle, while increasing the thickness of the coating affects the mechanical strength of the tip, which is currently the main problem facing coated microneedles183,184.
Hollow microneedles have a cavity within the tip that acts as an orifice for the drug to enter the skin. After insertion of the microneedle into the skin, the drug is injected into the skin through holes in the central part of the microneedle, and these orifices ensure that the drug is administered at a sufficient and constant rate. When the tip of a hollow microneedle is pierced into the skin, the orifice is filled with skin tissue, which affects the entry of the drug into the skin. This problem can be solved by placing the orifice on the side of the tip, but this certainly adds to the difficulty of the manufacturing process185,186.
The tips of hydrogel microneedles are made of swelling materials such as poly(lactic-co-glycolic acid) (PLGA), poly(lactic-co-glycolic acid) (PLA), and poly(vinyl alcohol) (PVA). As the tip penetrates the skin, the swelling material absorbs the interstitial fluid (ISF) and expands, thereby controlling the release of drugs. Since the materials used to prepare hydrogels are mostly biodegradable polymers that can slowly release drugs, microneedles are mostly used for long-term administration180,187.
Dissolving microneedles are mainly composed of drugs and biocompatible polymers. Drugs are encapsulated in polymer materials188. As the material degrades or dissolves in body fluids, the tip and the base are separated, and drug release is realized56,184. A new idea for designing microneedles was derived from soluble microneedles, that is, rapid separation microneedles. We know that conventional dissolving microneedles usually take tens of minutes or even hours to achieve separation after pressing into the skin189,190, which is very inconvenient for patients, so it is necessary to develop microneedles that can separate quickly. Li et al.191 developed an effervescent MN for rapid separation by adding citric acid and sodium bicarbonate to the base of the MN, and when the MN was pressed into the skin, the base came into contact with the tissue fluid of the spinous layer, and an acid–base reaction occurred, producing bubbles and leading to a rapid separation. The results show that this MN can be completely separated within 1 min, achieving the predetermined effect and providing a new idea for innovative microneedles191.
5. TDDSs in RA drug therapy
In existing studies, different enhancement strategies have been explored to facilitate the transdermal delivery of drugs approved for RA treatment, and in this section, the applications of different enhancement strategies for TDDSs in RA therapeutics are specifically described.
5.1. TDDSs for NSAIDs
Diflunisal (DIF) is a salicylic acid derivative with an analgesic and anti-inflammatory activity that can be applied in the therapy of RA. The current problem with the transdermal administration of DIF is poor water solubility; therefore, certain transdermal strategies are needed to improve the log P value of DIF for better skin penetration. Kaur et al.192 developed a supramolecular nanoengineered lipid carrier (SNLC) loaded with DIF-phospholipid 90G. First, equal molar amounts of DIF and phospholipids were formed into the DIF-PL compound, and then the compound was incorporated into SNLC, and Carbopol was added to construct the SNLC gel. The drug content of the formed DIF-PL compound was as high as 97.29% ± 0.54% w/w and significantly improved the solubility of DIF in water and PBS pH 7.4 solution. The design method of QbD was used to optimize the prescribing of SNLC, and the optimized SNLC contained up to 99.75% w/w. The results showed that the cumulative permeation of the compound SNLC gel group was better than that of the other formulation groups. Synovial fluid, serum parameters, and histopathological results of rat joints showed that the complex SNLC gel group significantly reduced the level of TNF-α and effectively inhibited palm swelling, and the anti-RA activity was better than that of the monotherapy group. Bashir et al.193 developed DIF-IC nanoemulsions with improved solubility formed by drugs with Tween 80, eucalyptus oil, and Transcutol P and then dispersed them into the gelling agent XG to prepare drug-loaded nanoemulsions. In vitro, skin permeation experiments indicated that the cumulative release of drug-loaded XG nanoemulsion gel was much higher than that of other gels. The results of foot swelling showed that DIF-IC nanoemulgel significantly reduced the edema volume of the foot swelling model, and the histopathological results of rat foot suggested that the number of inflammatory cells in the DIF-IC treatment group was evidently reduced.
Aceclofenac (ACE) is a derivative of diclofenac applied extensively in the treatment of RA, which can conquer the side effects of diclofenac and has an anti-inflammatory effect by inhibiting the activity of cyclooxygenase 2. Sharma et al.194 designed a dual-carrier system of ACE cocrystals. ACE and l-lysine (LYS) formed a cocrystal complex, which was encapsulated in liposomes, and then liposomes were added to Carbomer 940 to form a gel to complete the design of dual carriers. Radiant heat tail-flick experiments showed that the analgesic rate of cocrystal-loaded liposomal gel was better than that of MKT gel (marketed gel formulation of ACE). The results of ear histopathology in mice indicated that the ears of the cocrystal-loaded liposomal gel treatment group were completely healed without edema formation, and the therapeutic effect was decidedly better than that of the MKT gel treatment group without complete recovery of the ears. Histopathological results of rat paw and ankle joints showed that in the cocrystal-loaded liposomal gel treatment group, periarticular inflammation and fibrosis were significantly reduced, joint histopathology was improved, and RA was effectively treated compared with the MKT gel group. Garg et al.195 developed an ACE hydrogel based on a nanostructured lipid carrier (NLC). The NLC microemulsion was optimized by a pseudo-ternary phase diagram, and the optimized NLC was added to Carbopol 940 to form the final preparation gel. The results of the ex vivo skin permeation study showed that the developed NLC gel had a larger cumulative skin penetration than the MKT-gel preparation. Additionally, the results of drug distribution across skin layers showed that the concentration of the drug in the dermis after administration of the NLC gel formulation was superior to that of the ordinary MKT-gel formulations. On the downside, pharmacodynamic studies of NLC gel formulations and ordinary MKT-gel formulations have not been conducted, which is slightly lacking in terms of RA therapeutic efficacy.
Lornoxicam is a potent nonsteroidal anti-inflammatory drug pertaining to the BCSⅡ class of drugs that can be used to reduce inflammation and pain in RA. He et al.196 prepared a cellulose microsponge containing lornoxicam using ethylcellulose (EC) and hydroxypropyl methylcellulose (HPMC) and dispersed it in a carbomer matrix to make a gel formulation for continuous treatment of RA and relief of nocturnal pain. The results of in vitro release experiments indicated that lornoxicam, as a spongy gel, was able to release the drug continuously for up to 12 h, achieving long-lasting release. The results of in vivo anti-inflammatory experiments showed that the sponge gel inhibited rat paw edema at a higher rate than that of the pure drug, while the combination of sponge gel and microneedle treatments resulted in a 72% reduction in inflammation, providing sustained and effective relief of RA symptoms.
5.2. TDDSs for DMARDs
In this section, we focus on the application of methotrexate (MTX) as an example of different augmentation strategies in the treatment of RA.
Shen et al.197 prepared an MTX-loaded thermal-responsible flexible liposome (MTFL), which was then incorporated within an incorporated carbomer matrix to form a gel (MTFL/Gel) for augmentation of RA treatment. Thermosensitive liposomes can release drugs rapidly at a specific temperature compared with conventional liposomes, while microwave hyperthermia (MH), an adjunct to RA treatment, also has certain anti-inflammatory effects, so the dual action can significantly improve the therapeutic effect of RA. The results of the hind paw swelling experiment showed that the combination group of thermosensitive liposome gel and microwave hyperthermia (MTFL/Gel + MH) had the strongest anti-foot swelling degree and could significantly improve the thickness of the hind paw in mice, which was significantly better than the MTX single drug gel group and other treatment groups. In addition, the results of histological analysis of mouse joints showed that in the MTFL/Gel + MH group, the serious degree of cartilage injury and erosion were significantly reduced, and the most efficient therapeutic effect was found in the MTFL/Gel + MH group compared with the MH, free MTX/Gel, MFL/Gel, and MTFL/Gel treatment groups. Meanwhile, the results of cytokine levels in mouse hindlimb tissues showed that the MTFL/Gel + MH group had the lowest expression levels of CD68 and receptor activator of nuclear factor-B ligand (RANKL), and MTFL/Gel + MH was better at reducing cytokine production and was the best treatment group for RA. Overall, the combination of MTFL/Gel and MH has the potential to become a novel TDDS to enhance the therapeutic effect of RA.
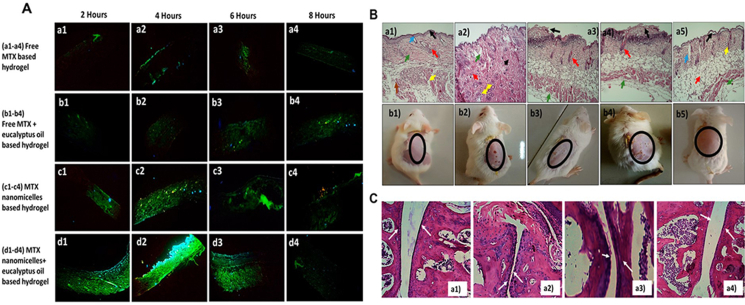
Qindeel et al.2 designed a nanomicelle gel containing MTX by loading MTX into polycaprolactone-polyethylene glycol-polycaprolactone (PCL-PEG-PCL) to form nanomicelles and then loading the micelles into a Carbomer 934 gel matrix and adding the permeation promoter eucalyptus oil to form the final gel formulation to achieve low toxicity of MTX targeted delivery. The skin irritation, degree of foot swelling, biochemical assays, and joint histopathology studies of different treatment groups was explored, and the results are depicted in Fig. 9. The results in Fig. 9A–C showed the following skin penetration: MTX-NMs + EO group > MTX-NMs group > Free MTX + EO group > Free MTX group. The MTX-NMs + EO group had the lowest skin irritation with only some gaps and exhibited the lowest degree of bone erosion, comparable to the normal saline group. Furthermore, biodistribution studies of MTX were conducted, and fluorescence results showed that the free MTX hydrogel group exhibited elevated fluorescence across all major organ tissues, while the MTX-NMs + EO hydrogel group showed the highest fluorescence only in mouse paw tissue, indicating that the hydrogel formed by encapsulating MTX in micelles can indeed achieve targeted drug delivery and reduce MTX systemic adverse reactions while achieving better RA therapeutic effects.
Figure 9.
(A) Fluorescence images of different hydrogel groups containing green fluorescein at 2, 4, 6, and 8 h of in vitro skin permeation. (a1–a4) Free MTX group, (b1–b4) Free MTX + EO group, (c1–c4) MTX-NMs group, (d1–d4) MTX-NMs + EO group. (B) Histopathological images (a1–a5) and visual representation (b1–b5) of skin irritability experiments treated with (a1,b1) normal saline, (a2,b2) formalin, (a3,b3) Free MTX, (a4,b4) Free MTX + EO, (a5,b5) MTX-NMs + EO. (C) Histopathological sections (a1–a4) of the ankle joint of mice in different treatment groups. (a1) Normal saline, (a2) CFA, (a3) free MTX-based hydrogel, (a4) MTX-NMs + EO-based hydrogel. Reprinted with the permission from Ref. 3. Copyright © 2020 Elsevier.
Wu et al.198 developed a biodegradable PVP polymer microneedle for the delivery of MTX with the addition of reactive oxygen species (ROS) scavenger polydopamine/manganese dioxide nanoparticles (PDA@MnO2) to the microneedle, expecting to enhance the anti-inflammatory effect by the dual action of MTX and PDA@MnO2. First, the anti-inflammatory effect of PDA@MnO2 was confirmed. Immunofluorescence results of cyclooxygenase 2 and protein blot analysis showed that synthetic PDA@MnO2 effectively inhibited the expression of cyclooxygenase 2 and reduced the level of inflammatory factors, which had a reducing effect on inflammation. Next, the therapeutic effects of the oral MTX group, MTX MN group, PDA@MnO2 MN group, and MTX/PDA@MnO2 group on RA model mice were investigated, and the results suggested that the anti-inflammatory effects of the PDA@MnO2 MN group and MTX/PDA@MnO2 group were comparable, and both evidently reduced the levels of inflammatory factors, but the MTX/PDA@MnO2 group had an evidently lower rate of foot swelling than the PDA@MnO2 MN group. Therefore, based on the results of these two experiments, it was determined that MTX combined with ROS inhibitors could achieve better therapeutic effects and create new avenues for RA treatment.
Wei et al.199 prepared a dissolving microneedle array (DMNA) including MTX nanocrystals (MTX-NCs) (MTX-NC@DMNA) with the expectation of improving the solubility and bioavailability issues of MTX through nanocrystals and delivering the drug through microneedles to achieve better therapeutic effects. The results of solubility experiments showed that MTX-NCs increased the saturated solubility of MTX in water and PBS by approximately 240 and 5 times, respectively. Next, studies on the effects of different administration groups on foot swelling, rat synovial membrane, and ankle joint histology were performed. The results suggested that in terms of foot swelling rate, MTX-NC@DMNA group < oral MTX group < MTX-NC@Cream group, and MTX-NC@DMNA notably relieved foot swelling in rats. The histological results of the synovium and ankle joint showed that the joint tissue of rats in the MTX-NC@Cream group and the oral MTX group was improved compared to the model group, but there were still uneven joint surfaces, obvious vascular opacification and inflammatory cell infiltration, whereas the morbid condition in the MTX-NC@DMNA group was similar to that in the normal group, with flat joint surfaces and no inflammatory cell infiltration, and the treatment effect of this group was considerably superior to that of the remaining two groups. Meanwhile, the production of anti-inflammatory factors and pro-inflammatory factors (IL-1β, IL-6, and TNF-α) was also studied. The levels of anti-inflammatory factors in the MTX-NC@DMNA group were comparable to those in the normal group and were tremendously higher than those in the MTX-NC@Cream group and the oral MTX group, while the levels of pro-inflammatory factors were lower than those in the other two groups, suggesting that the designed MTX-NC@DMNA regulates the equilibrium between anti-inflammatory and pro-inflammatory factors, alleviates synovial and joint damage, and effectively treats RA.
5.3. TDDSs for GCs
GC is a commonly used drug for RA that achieves anti-inflammatory effects by blocking the formation of AA and PG and is widely used in clinical practice. Zhao et al.121 designed a dexamethasone (DEX) flexible liposome hydrogel (DS-FLs/DEX) capable of targeting macrophages to address the low efficacy and systemic side effects of DEX. The results of in vitro release experiments indicated that the DS-FLs/DEX was able to release approximately 90% in inflammatory pH solutions. In addition, compared to conventional liposomal hydrogels, DS-FLs/DEX hydrogels possess better skin retention and skin penetration due to their good deformability, with DEX retention rates at least twice as high as those of DS-RLs/DEX hydrogels. Permeation studies indicated that the DEX penetration of the DS-FLs/DEX hydrogel was particularly increased compared with that of the DS-RLs/DEX hydrogel. In vivo, DS-FLs/DEX hydrogel can indeed penetrate and accumulate in inflamed joints, dramatically improving joint engorgement and reducing the damaging effects of RA on bone in rats. It also reduced the levels of proinflammatory factors, effectively treating RA.
5.4. TDDSs for traditional Chinese medicines
In this section, we introduce different enhancement strategies in the treatment of RA, using tetrandrine, sinomenine hydrochloride, and triptolide as examples.
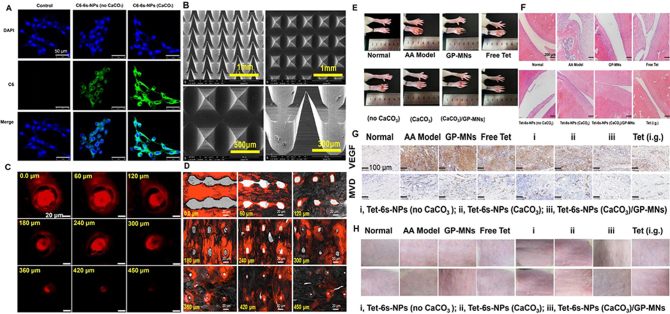
Hu et al.200 designed a soluble microneedle containing acid-responsive PEGylated branching PLGA nanoparticles of tetrandrine (Tet) to achieve high drug loading and controlled release of the microneedle, while PEGylated multiarmed PLGA provided an immune escape, and the combination of this nanoparticle and microneedle enhanced the transdermal delivery of Tet. First, amphiphilic multiarmed PLGA with PEGylation was compounded as a transporter for Tet, and functionalized PLGA was crossbred with CaCO3 to institute acid-responsive carriers 6 s-NPs (CaCO3). Next, drug-loaded multifunctional PLGA nanoparticles were loaded into soluble microneedles made of peach gum polysaccharides to form Tet-6 s-NPs (CaCO3)/GP-MNs for topical treatment of RA. The experimental results are depicted in Fig. 10. Fig. 10A–D showed that the Tet-6 s-NPs (CaCO3)/GP-MNs were greater than 450 μm, and the drug could cross the SC to reach the deep skin, inhibiting the greatest degree of foot swelling in rats compared with the commercial Tet patch group and other administrative groups. Furthermore, in vitro release experiments as depicted in Fig. 10E–H showed that the transdermal throughput and accumulative concentration of Tet in the MN and nanocarrier combined group were higher than those in the passive spreading group, and the transdermal penetration of Tet enhanced by nanocarriers was superior to that of the free Tet group. In total, the binding of microneedles and nanocarriers greatly enhanced the therapeutic effect on RA.
Figure 10.
(A) CLSM images of FLS uptake of DAPI-labeled and C6-labeled nanoparticles. DAPI stained the nucleus and C6 stained the cytoplasm. (B) SEM images of Tet-6 s-NPs (CaCO3)/PG-MNs. (C) CLSM images of penetration of sulforhodamine B-labeled 6 s-NPs (CaCO3)/PG-MNs into the skin. (D) Sulforhodamine B-labeled 6 s-NPs (CaCO3)/PG-MNs-treated skin, OCT embedded and liquid nitrogen freezer, sliced horizontally with the skin surface, supporting the results of the puncture performance study. (E) The appearance of paw swelling. (F) Micrographs of H&E-stained pathological sections of ankle joints from AA-bearing rats treated with the test preparations. (G) Micrographs of immunohistochemical sections of VEGF and MVD in synovial tissues from AA-bearing rats. (H) Irritation response of the skin in AA-bearing rats before and after treatment with the test preparations. Reprinted with the permission from Ref. 200. Copyright © 2022 Elsevier.
Song et al.201 developed a sinomenine hydrochloride(SH)-loaded transethosome modified with ascorbic acid to form an antioxidant surface transethosome (AS-TE) to improve the transdermal permeability and the accumulation of the drug. The prepared AS-TE was a multilayer spherical structure with good deformability and suitable encapsulation efficiency. The results of in vitro permeation experiments suggested that the transdermal permeation efficiency of AS-TE was better than that of ordinary ethosomes within 48 h. The results of knee cavity microdialysis in a rabbit model of RA showed that API from the AS-TE group was more easily deposited in the RA ankle joint site. Meanwhile, the results of foot swelling assays and inflammatory factor levels in rats showed that the prepared AS-TE had similar therapeutic effects as gastric administration. Zheng et al.202 designed mixed monoterpenes edge-activated PEGylated transfersomes (MMPTs) to enhance the delivery of sinomenine (SIN), using limonene–citral combination as edge activators and modifying TFSs with DSPE-PEG 2000 to obtain MMPTs. The enhancement effect was demonstrated by CLSM and double-site microdialysis. CLSM results showed that MMPTs were distributed in the deeper layer of the skin, while LPS was confined to the SC, which may be attributed to the proper transformability of MMPTs. The results of double-site microdialysis pharmacokinetics indicated that the intra-articular steady-state concentration (Css) and AUC0‒t of SIN in MMPTs were approximately double those in LPS, indicating that MMPTs could indeed enhance the transdermal delivery of SIN.
Gu et al.100 designed triptolide-loaded nanostructured lipid carriers (TPL-NLCs) to enhance the transdermal delivery of TLP. First, TPL-NLC was fabricated by the emulsification method and optimized by response surface methodology. The prepared TPL-NLCs were stable with an encapsulation rate of 97.15 ± 9.46%. The results of in vitro infiltration experiments indicated that the 12 h permeation amount of the drug in TPL-NLCs was approximately twice that of TPL in the TPL-nanoemulsion, and in vivo pharmacokinetic studies showed that the Cmax of TPL-NLCs was better than that of the TPL-nanoemulsion in both skin and blood, indicating that TPL-NLCs can efficiently infiltrate the skin. Meanwhile, the effects of TPL-NLCs on the swelling of the knee joint and the production of inflammatory factors in rats were investigated. The results indicated that TPL-NLCs could significantly reduce the swelling of the knee joint and inhibit the levels of inflammatory factors, as well as improve synovial hyperplasia, joint cavity gap, fibrosis, and abrasion, which suggested that TPL-NLCs could enhance the transdermal delivery of TPL and were a promising topical administration in therapy for RA. Shan et al.203 fabricated cubic (V2) and hexagonal liquid crystals (H2) loaded with TPL as drug carriers to enhance the transdermal infiltration of the drug; the CPLM results showed a dark field of view and fan-shaped pattern, respectively, indicating successful preparation of cubic and hexagonal liquid crystals. The results of in vitro skin permeation experiments indicated that the V2 and H2 phases remarkably improved the in vitro transdermal permeation of TPL compared to the gel group, and the cumulative permeation of TPL from the V2 and H2 phases was 1.65 and 1.76 times better than that of the gel. In addition, in vivo, pharmacokinetics results showed that the AUC and MRT of cubic and hexagonal liquid crystals were higher than those of the gel group, and the TPL in V2 and H2 stayed longer in vivo. V2 and H2 had good anti-inflammatory effects, inhibiting foot swelling and reducing TNF-α and IL-1β levels in rats, and there was no synovial hyperplasia, neutrophil infiltration, pannus formation, or synovial inner cell proliferation at the joints. Meanwhile, cubic and hexagonal liquid crystals loaded with ryanodine had no significant toxicity and represented a promising method for RA therapy. Liu et al.204 prepared a triptolide phospholipid complex (TPCX) and prepared it into a cream to improve the skin permeability of triptolide. The results showed that compared to TPL cream, TPCX increased the solubility of TPL, the in vitro transdermal amount of TPCX cream was significantly increased, and skin permeability was improved. The pharmacokinetic results showed that TPCX cream had a longer biological half-life and mean residence time. Furthermore, TPCX cream downregulated the production of inflammatory factors, reduced rat foot swelling, inhibited the proliferation of synovial cells, vascular opacification, and inflammatory cell infiltration, and had good anti-RA effects.
5.5. TDDSs for other drugs
In addition to the common clinical classes of drugs, some other types of drugs also have certain anti-inflammatory effects and can be used to treat RA. Melittin, a cationic peptide containing 26 nucleic acids, is the main component of bee venom with an anti-RA effect205. Du et al.206 fabricated melittin-loaded hyaluronic acid (HA) microneedles to reinforce the transdermal infiltration of drugs while achieving a certain sustained release effect. First, the sustained-release material methacrylate-modified hyaluronic acid (MeHA) was synthesized and loaded with melittin to obtain melittin-loaded MeHA microneedles (Mel-MeHA-MN) and melittin-loaded HA microneedles (Mel-HA-MN). The results indicated that the two kinds of MNs had desirable mechanical properties, rapid release in vitro in general, and release times up to 400 min for sustained-release microneedles. At the same time, the therapeutic effects of the two microneedles on RA were also researched, and the results suggested that compared with the Mel-HA-MN group and melittin injection group, the degree of foot swelling in mice was dramatically inhibited in the Mel-MeHA-MN group, the infiltration of inflammatory cells was decreased, the completeness of cartilage was maintained, better joint protection was exhibited, and the levels of proinflammatory factors in the blood and claws of mice were considerably decreased. Interestingly, the percentage of Treg cells in popliteal lymph nodes of the Mel-MeHA-MN group was lower than that in the three groups, and there was no promoting effect on Treg cells in popliteal lymph nodes. In addition, hematological parameters showed that melittin treatment did not cause blood cell toxicity. In conclusion, this microneedle is a promising means of long-lasting treatment for RA206.
Elkomy et al.207 designed chitosan-coated bilosomes containing berberine (BER-CTS-BLS) to facilitate transdermal absorption of BER. First, BER-CTS-BLS was fabricated by the thin film dispersion method and loaded into hydrogels. The results of in vitro skin penetration experiments indicated that the permeation rate of BER in BER-CTS-BLS was much higher than that of the normal BER solution. In addition, the percentage of rat paw edema and swelling was significantly decreased in the BER-CTS-BLS treatment group, and the prepared BER-CTS-BLS had a significant anti-inflammatory effect. Other TDDSs of the abovementioned classes of drugs in the treatment of RA are given in Table 3208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219.
Table 3.
Application of other TDDS in RA treatment drugs.
| Type of drugs | Drug | Type of transdermal strategy | Method of preparation | Final dosage form | Ref. |
|---|---|---|---|---|---|
| NSAIDs | Ibuprofen | Microemulsion | Self-emulsified method | Gel | 208 |
| Naproxen sodium | Nanoethosomal | Cold method | Gel | 209 | |
| Nabumetone | Microemulsion | Titration method | Microemulgel | 210 | |
| Piroxicam | Transethosomal | Thin film hydration method | Gel | 211 | |
| Diclofenac sodium | Electroporation | Mezoforte Duo Mez 120905-D instrument | N/A | 212 | |
| Bilosomes | Thin film hydration method | Hydrogel | 213 | ||
| DMARDs | Methotrexate | Aspasome | Thin film hydration method | Gel | 214 |
| Sonophoresis | N/A | Patch | |||
| Tofacitinib | Propose | Cold mixing method | N/A | 215 | |
| Microneedle | Solvent casting method | Microneedle | 216 | ||
| Traditional Chinese medicines | Sinomenine | N/A | Casting method | Dissolving microneedle | 217 |
| Electroporation | N/A | N/A | |||
| Triptolide | Glycerosome | Injection method | Nanoformulation | 218 | |
| Thymoquinone | Oil-stabilized nanostructured lipid carrier | Hot melt-emulsification method followed by sonication | Gel | 219 |
N/A, not applicable.
6. Conclusions and outlook
RA is a systemic immune disease with lesions commonly found in the joints, which can be teratogenic in severe cases and affect daily life. Therefore, intervening with drugs in the initial stage of the disease is vital. Currently, several drugs used clinically for the treatment of RA, although effective, also have many side effects, which indicates that it is essential to develop a drug delivery system that can exert drug efficacy while reducing side effects; thus, transdermal drug delivery systems (TDDSs) caught our attention. While TDDSs have the advantages of reducing systemic adverse effects and improving bioavailability, they also have their own limitations: selectivity regarding the drugs to be administered transdermally. Not all drugs are suitable for transdermal administration. Therefore, various penetration enhancement methods have been generated and successfully applied to RA-related drugs. Overall, from first-generation TDDSs to fourth-generation TDDSs, the scope of drug use has been gradually expanded so that drug delivery is no longer limited by the physical and chemical properties of the drug. In addition, different nanocarriers and electrochemical methods have been developed to achieve the delivery of macromolecular drugs. At present, these strategies have shown good transdermal effects in research, but few products have been approved for marketing, which is a major challenge for the application of TDDSs, that is, how to transform from research to clinical products. The second, third, and fourth generations of TDDSs have better drug delivery effects but are also accompanied by more complex preparation processes, more difficulty evaluating safety, and more expensive prices. This requires cross-disciplinary integration. While we have a more comprehensive and in-depth understanding of the molecular mechanisms of different transdermal strategies, we continue to explore more advanced and more easily industrialized manufacturing methods to minimize costs and achieve a balance between efficacy and price. At present, there are a few TDDSs in clinical trials, and we believe that the feedback results will be positive because TDDSs have great potential in the delivery of small molecule drugs and biomacromolecule drugs, which is expected to have a significant impact on the drug delivery system and become an effective means of treating RA and even more diseases.
Acknowledgments
This work was supported by the Scientific Research Project of Liaoning Province Education Department (2020LJC16), China.
Author contributions
Yuyi Xu: Writing — original draft, Visualization. Ming Zhao: Writing — original draft. Jinxue Cao: Data curation, Visualization. Ting Fang: Resources, Writing — review & editing. Jian Zhang: Resources. Yanli Zhen: Visualization, Writing — review & editing. Fangling Wu: Writing — review & editing. Xiaohui Yu: Supervision. Yaming Liu: Visualization. Ji Li: Conceptualization, Supervision, Writing — review & editing. Dongkai Wang: Conceptualization, Supervision, Writing — review & editing.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Ji Li, Email: syphuliji@163.com.
Dongkai Wang, Email: wangycsyphu@126.com.
References
- 1.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Qindeel M., Khan D., Ahmed N., Khan S., Rehman A.U. Surfactant-free, self-assembled nanomicelles-based transdermal hydrogel for safe and targeted delivery of methotrexate against rheumatoid arthritis. ACS Nano. 2020;14:4662–4681. doi: 10.1021/acsnano.0c00364. [DOI] [PubMed] [Google Scholar]
- 3.Wang W., Zhou H., Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi: 10.1016/j.ejmech.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y.J., Anzaghe M., Schuelke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9:880. doi: 10.3390/cells9040880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrelli F., Mariani F.M., Alunno A., Puxeddu I. Pathogenesis of rheumatoid arthritis: one year in review 2022. Clin Exp Rheumatol. 2022;40:475–482. doi: 10.55563/clinexprheumatol/l9lyen. [DOI] [PubMed] [Google Scholar]
- 6.Raine C., Giles I. What is the impact of sex hormones on the pathogenesis of rheumatoid arthritis?. Front Med. 2022;9 doi: 10.3389/fmed.2022.909879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao T., Wei Y., Zhu Y., Xie Z., Hai Q., Li Z., et al. Gut microbiota and rheumatoid arthritis: from pathogenesis to novel therapeutic opportunities. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picerno V., Ferro F., Adinolfi A., Valentini E., Tani C., Alunno A. One year in review: the pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2015;33:551–558. [PubMed] [Google Scholar]
- 9.Kondo N., Kuroda T., Kobayashi D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222010922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rooy D.P., Zhernakova A., Tsonaka R., Willemze A., Kurreeman B.A., Trynka G., et al. A genetic variant in the region of MMP-9 is associated with serum levels and progression of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2014;73:1163–1169. doi: 10.1136/annrheumdis-2013-203375. [DOI] [PubMed] [Google Scholar]
- 11.Yoshitomi H. Regulation of immune responses and chronic inflammation by fibroblast-like synoviocytes. Front Immunol. 2019;10:1395. doi: 10.3389/fimmu.2019.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Gao Z., Chao S., Lu W., Zhang P. Transdermal delivery of inflammatory factors regulated drugs for rheumatoid arthritis. Drug Deliv. 2022;29:1934–1950. doi: 10.1080/10717544.2022.2089295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floudas A., Smith C.M., Tynan O., Neto N., Krishna V., Wade S.M., et al. Distinct stromal and immune cell interactions shape the pathogenesis of rheumatoid and psoriatic arthritis. Ann Rheum Dis. 2022;81:1224–1242. doi: 10.1136/annrheumdis-2021-221761. [DOI] [PubMed] [Google Scholar]
- 14.Louwerens J.W., Schrier J.C. Rheumatoid forefoot deformity: pathophysiology, evaluation and operative treatment options. Int Orthop. 2013;37:1719–1729. doi: 10.1007/s00264-013-2014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richette P., Doherty M., Pascual E., Barskova V., Becce F., Castañeda-Sanabria J., et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 16.Crofford L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15:S2. doi: 10.1186/ar4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orange D.E., Mehta B. Rethinking the balance of risks and rewards of chronic low-dose glucocorticoids in rheumatoid arthritis. Ann Intern Med. 2020;173:933–934. doi: 10.7326/M20-6010. [DOI] [PubMed] [Google Scholar]
- 18.Gao J., Xiao N., Wang Q., Xu Z., Xiao F., Yang Z., et al. OAT3 mediates methotrexate resistance in the treatment of rheumatoid arthritis. Biomed Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113558. [DOI] [PubMed] [Google Scholar]
- 19.Koller G., Cusnir I., Hall J., Ye C. Reversible alopecia areata: a little known side effect of leflunomide. Clin Rheumatol. 2019;38:2015–2016. doi: 10.1007/s10067-019-04577-3. [DOI] [PubMed] [Google Scholar]
- 20.Mimori T., Harigai M., Atsumi T., Fujii T., Kuwana M., Matsuno H., et al. Safety and effectiveness of iguratimod in patients with rheumatoid arthritis: final report of a 52-week, multicenter postmarketing surveillance study. Mod Rheumatol. 2019;29:314–323. doi: 10.1080/14397595.2018.1460230. [DOI] [PubMed] [Google Scholar]
- 21.Angelini J., Talotta R., Roncato R., Fornasier G., Barbiero G., Dal Cin L., et al. JAK-inhibitors for the treatment of rheumatoid arthritis: a focus on the present and an outlook on the future. Biomolecules. 2020;10:1002. doi: 10.3390/biom10071002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song H., Liu C., Ruan J., Yang D., Zhong T., Liu Y., et al. Effect of the combination of permeation enhancer and ion-pairs strategies on transdermal delivery of tofacitinib. Int J Pharm. 2022;611 doi: 10.1016/j.ijpharm.2021.121190. [DOI] [PubMed] [Google Scholar]
- 23.Ogata A., Kato Y., Higa S., Yoshizaki K. IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod Rheumatol. 2019;29:258–267. doi: 10.1080/14397595.2018.1546357. [DOI] [PubMed] [Google Scholar]
- 24.Jakobsson P.J., Robertson L., Welzel J., Zhang M., Zhihua Y., Kaixin G., et al. Where traditional Chinese medicine meets Western medicine in the prevention of rheumatoid arthritis. J Intern Med. 2022;292:745–763. doi: 10.1111/joim.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X., Xie D., Huang J., Lu A., Wang R., Jin Y., et al. Therapeutic effects of (5R)-5-hydroxytriptolide on fibroblast-like synoviocytes in rheumatoid arthritis via lncRNA WAKMAR2/miR-4478/E2F1/p53 axis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.605616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B., Wu L., Chen J., Dong L., Chen C., Wen Z., et al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther. 2021;6:94. doi: 10.1038/s41392-020-00443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stahn C., Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 28.Ehrchen J.M., Roth J., Barczyk-Kahlert K. More than suppression: glucocorticoid action on monocytes and macrophages. Front Immunol. 2019;10:2028. doi: 10.3389/fimmu.2019.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oray M., Samra K.A., Ebrahimiadib N., Meese H., Foster C.S. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457–465. doi: 10.1517/14740338.2016.1140743. [DOI] [PubMed] [Google Scholar]
- 30.Smolen J.S., Aletaha D., Koeller M., Weisman M.H., Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese L.H., Calabrese C., Kirchner E. The 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis should include new standards for hepatitis B screening: comment on the article by Singh et al. Arthritis Care Res. 2016;68:723–724. doi: 10.1002/acr.22865. [DOI] [PubMed] [Google Scholar]
- 32.Moon S.J., Kim E.K., Jhun J.Y., Lee H.J., Lee W.S., Park S.H., et al. The active metabolite of leflunomide, A77 1726, attenuates inflammatory arthritis in mice with spontaneous arthritis via induction of heme oxygenase-1. J Transl Med. 2017;15:31. doi: 10.1186/s12967-017-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon L.S., Taylor P.C., Choy E.H., Sebba A., Quebe A., Knopp K.L., et al. The Jak/STAT pathway: a focus on pain in rheumatoid arthritis. Semin Arthritis Rheum. 2021;51:278–284. doi: 10.1016/j.semarthrit.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Burmester G.R., Pope J.E. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389:2338–2348. doi: 10.1016/S0140-6736(17)31491-5. [DOI] [PubMed] [Google Scholar]
- 35.Sepriano A., Kerschbaumer A., Smolen J.S., van der Heijde D., Dougados M., van Vollenhoven R., et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2020;79:760–770. doi: 10.1136/annrheumdis-2019-216653. [DOI] [PubMed] [Google Scholar]
- 36.Sana E., Zeeshan M., Ain Q.U., Khan A.U., Hussain I., Khan S., et al. Topical delivery of curcumin-loaded transfersomes gel ameliorated rheumatoid arthritis by inhibiting NF-kappa beta pathway. Nanomedicine. 2021;16:819–837. doi: 10.2217/nnm-2020-0316. [DOI] [PubMed] [Google Scholar]
- 37.Mounach A., Rezqi A., Nouijai A., Ghozlani I., Achemlal L., Maghraoui A.E., et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351–1353. doi: 10.1007/s00296-011-2212-4. [DOI] [PubMed] [Google Scholar]
- 38.Sharma G., Alle M., Chakraborty C., Kim J.C. Strategies for transdermal drug delivery against bone disorders: a preclinical and clinical update. J Control Release. 2021;336:375–395. doi: 10.1016/j.jconrel.2021.06.035. [DOI] [PubMed] [Google Scholar]
- 39.Qindeel M., Ullah M.H., Fakhar-Ud-Din, Ahmed N., Rehman A.U. Recent trends, challenges and future outlook of transdermal drug delivery systems for rheumatoid arthritis therapy. J Control Release. 2020;327:595–615. doi: 10.1016/j.jconrel.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Liu X., Kruger P., Maibach H., Colditz P.B., Roberts M.S. Using skin for drug delivery and diagnosis in the critically ill. Adv Drug Deliv Rev. 2014;77:40–49. doi: 10.1016/j.addr.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Alkilani A.Z., McCrudden M.T., Donnelly R.F. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7:438–470. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan Q.F., Yu Y.L., Chen D., Jiao G.L., Liu X.W. Enhanced penetration strategies for transdermal delivery. Front Chem Sci Eng. 2020;14:378–388. [Google Scholar]
- 43.Ishida-Yamamoto A., Igawa S. The biology and regulation of corneodesmosomes. Cell Tissue Res. 2015;360:477–482. doi: 10.1007/s00441-014-2037-z. [DOI] [PubMed] [Google Scholar]
- 44.Volk N., Lacy B. Anatomy and physiology of the small bowel. Gastrointest Endosc Clin N Am. 2017;27:1–13. doi: 10.1016/j.giec.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Matsui T., Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol. 2015;27:269–280. doi: 10.1093/intimm/dxv013. [DOI] [PubMed] [Google Scholar]
- 46.Cheong J.E.L., McGrath J.A. Structure and function of skin, hair and nails. Medicine. 2021;49:337–342. [Google Scholar]
- 47.Nguyen A.V., Soulika A.M. The dynamics of the skin's immune system. Int J Mol Sci. 2019;20:1811. doi: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rippa A.L., Kalabusheva E.P., Vorotelyak E.A. Regeneration of dermis: scarring and cells involved. Cells. 2019;8:607. doi: 10.3390/cells8060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benson H.A.E., Grice J.E., Mohammed Y., Namjoshi S., Roberts M.S. Topical and transdermal drug delivery: from simple potions to smart technologies. Curr Drug Deliv. 2019;16:444–460. doi: 10.2174/1567201816666190201143457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arda O., Göksügür N., Tüzün Y. Basic histological structure and functions of facial skin. Clin Dermatol. 2014;32:3–13. doi: 10.1016/j.clindermatol.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 51.Subongkot T., Pamornpathomkul B., Rojanarata T., Opanasopit P., Ngawhirunpat T. Investigation of the mechanism of enhanced skin penetration by ultradeformable liposomes. Int J Nanomed. 2014;9:3539–3550. doi: 10.2147/IJN.S65287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLafferty E., Hendry C., Alistair F. The integumentary system: anatomy, physiology and function of skin. Nurs Stand. 2012;27:35–42. doi: 10.7748/ns2012.10.27.7.35.c9358. [DOI] [PubMed] [Google Scholar]
- 53.Elias P.M. Structure and function of the stratum corneum extracellular matrix. J Investig Dermatol. 2012;132:2131–2133. doi: 10.1038/jid.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallinger J., Kuhn A., Wessel S., Behm P., Heinecke S., Filbry A., et al. Depth-dependent hydration dynamics in human skin: vehicle-controlled efficacy assessment of a functional 10% urea plus NMF moisturizer by near-infrared confocal spectroscopic imaging (KOSIM IR) and capacitance method complemented by volunteer perception. Skin Res Technol. 2022;28:342–349. doi: 10.1111/srt.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H., Song C., Baik S., Kim D., Hyeon T., Kim D.H. Device-assisted transdermal drug delivery. Adv Drug Deliv Rev. 2018;127:35–45. doi: 10.1016/j.addr.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Villanueva-Martinez A., Merino V., Ganem-Rondero A. Transdermal formulations and strategies for the treatment of osteoporosis. J Drug Delivery Sci Technol. 2022;69 [Google Scholar]
- 57.Dixon R.V., Skaria E., Lau W.M., Manning P., Birch-Machin M.A., Moghimi S.M., et al. Microneedle-based devices for point-of-care infectious disease diagnostics. Acta Pharm Sin B. 2021;11:2344–2361. doi: 10.1016/j.apsb.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan T., Pan Q., Ping Y. Microneedle-assisted genome editing: a transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders. Sci Adv. 2021;7 doi: 10.1126/sciadv.abe2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ameen D., Michniak-Kohn B. Transdermal delivery of dimethyl fumarate for Alzheimer's disease: effect of penetration enhancers. Int J Pharm. 2017;529:465–473. doi: 10.1016/j.ijpharm.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Devarajan-Ketha H., Sloan K.B.N. Nʹ-Dialkylaminoalkylcarbonyl (DAAC) prodrugs and aminoalkylcarbonyl (AAC) prodrugs of 4-hydroxyacetanilide and naltrexone with improved skin permeation properties. Bioorg Med Chem Lett. 2011;21:4078–4082. doi: 10.1016/j.bmcl.2011.04.118. [DOI] [PubMed] [Google Scholar]
- 61.Huo S., Zhao P., Shi Z., Zou M., Yang X., Warszawik E., et al. Mechanochemical bond scission for the activation of drugs. Nat Chem. 2022;13:131–139. doi: 10.1038/s41557-020-00624-8. [DOI] [PubMed] [Google Scholar]
- 62.Roustit M., Blaise S., Cracowski J.L. Trials and tribulations of skin iontophoresis in therapeutics. Br J Clin Pharmacol. 2014;77:63–71. doi: 10.1111/bcp.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abd E., Namjoshi S., Mohammed Y.H., Roberts M.S., Grice J.E. Synergistic skin penetration enhancer and nanoemulsion formulations promote the human epidermal permeation of caffeine and naproxen. J Pharm Sci. 2016;105:212–220. doi: 10.1002/jps.24699. [DOI] [PubMed] [Google Scholar]
- 64.Shukla T., Upmanyu N., Agrawal M., Saraf S., Saraf S., Alexander A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed Pharmacother. 2018;108:1477–1494. doi: 10.1016/j.biopha.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Yu Z., Meng X., Zhang S., Chen Y., Zhang Z., Zhang Y. Recent progress in transdermal nanocarriers and their surface modifications. Molecules. 2021;26:3093. doi: 10.3390/molecules26113093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chauhan M.K., Sharma P.K. Optimization and characterization of rivastigmine nanolipid carrier loaded transdermal patches for the treatment of dementia. Chem Phys Lipids. 2019;224 doi: 10.1016/j.chemphyslip.2019.104794. [DOI] [PubMed] [Google Scholar]
- 67.Parat A., Bordeianu C., Dib H., Garofalo A., Walter A., Bégin-Colin S., et al. Dendrimer-nanoparticle conjugates in nanomedicine. Nanomedicine. 2015;10:977–992. doi: 10.2217/nnm.14.196. [DOI] [PubMed] [Google Scholar]
- 68.Xu Y., Cai Y., Meng Y., Wu L., Chen J., Cao W., et al. Liposome and microemulsion loaded with ibuprofen: from preparation to mechanism of drug transport. J Microencapsul. 2022;39:539–551. doi: 10.1080/02652048.2022.2131920. [DOI] [PubMed] [Google Scholar]
- 69.Salem H.F., Nafady M.M., Kharshoum R.M., Abd el-Ghafar O.A., Farouk H.O. Novel enhanced therapeutic efficacy of dapoxetine HCl by nano-vesicle transdermal gel for treatment of carrageenan-induced rat paw edema. AAPS PharmSciTech. 2020;21:113. doi: 10.1208/s12249-020-01656-6. [DOI] [PubMed] [Google Scholar]
- 70.Jiang T., Wang T., Li T., Ma Y., Shen S., He B., et al. Enhanced transdermal drug delivery by transfersome-embedded oligopeptide hydrogel for topical chemotherapy of melanoma. ACS Nano. 2018;12:9693–9701. doi: 10.1021/acsnano.8b03800. [DOI] [PubMed] [Google Scholar]
- 71.Dad H.A., Gu T.W., Zhu A.Q., Huang L.Q., Peng L.H. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29:13–31. doi: 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Avadhani K.S., Manikkath J., Tiwari M., Chandrasekhar M., Godavarthi A., Vidya S.M., et al. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017;24:61–74. doi: 10.1080/10717544.2016.1228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaidya J., Shende P. Potential of sonophoresis as a skin penetration technique in the treatment of rheumatoid arthritis with transdermal patch. AAPS PharmSciTech. 2020;21:180. doi: 10.1208/s12249-020-01725-w. [DOI] [PubMed] [Google Scholar]
- 74.Gao Y., Du L., Li Q., Li Q., Zhu L., Yang M., et al. How physical techniques improve the transdermal permeation of therapeutics: a review. Medicine. 2022;101 doi: 10.1097/MD.0000000000029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denet A.R., Préat V. Transdermal delivery of timolol by electroporation through human skin. J Control Release. 2003;88:253–262. doi: 10.1016/s0168-3659(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 76.Shimizu K., Hayashida K., Blajan M. Novel method to improve transdermal drug delivery by atmospheric microplasma irradiation. Biointerphases. 2015;10 doi: 10.1116/1.4919708. [DOI] [PubMed] [Google Scholar]
- 77.Nagarkar R., Singh M., Nguyen H.X., Jonnalagadda S. A review of recent advances in microneedle technology for transdermal drug delivery. J Drug Delivery Sci Technol. 2020;59 [Google Scholar]
- 78.Zhao X., Li X., Zhang P., Du J., Wang Y. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor. J Control Release. 2018;286:201–209. doi: 10.1016/j.jconrel.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 79.Mueller J., Trapp M., Neubert R.H.H. The effect of hydrophilic penetration/diffusion enhancer on stratum corneum lipid models: part II∗: DMSO. Chem Phys Lipids. 2019;225 doi: 10.1016/j.chemphyslip.2019.104816. [DOI] [PubMed] [Google Scholar]
- 80.Zeng L., Huang F., Zhang Q., Liu J., Quan D., Song W. Molecular perspective of efficiency and safety problems of chemical enhancers: bottlenecks and recent advances. Drug Deliv Transl Res. 2022;12:1376–1394. doi: 10.1007/s13346-021-01044-y. [DOI] [PubMed] [Google Scholar]
- 81.Vasyuchenko E.P., Orekhov P.S., Armeev G.A., Bozdaganyan M.E. CPE-DB: an open database of chemical penetration enhancers. Pharmaceutics. 2021;13:66. doi: 10.3390/pharmaceutics13010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu C., Guan Y., Tian Q., Shi X., Fang L. Transdermal enhancement strategy of ketoprofen and teriflunomide: the effect of enhanced drug‒drug intermolecular interaction by permeation enhancer on drug release of compound transdermal patch. Int J Pharm. 2019;572 doi: 10.1016/j.ijpharm.2019.118800. [DOI] [PubMed] [Google Scholar]
- 83.Serna-Jiménez C.E., del Rio-Sancho S., Calatayud-Pascual M.A., Balaguer-Fernández C., Femenía-Font A., López-Castellano A., et al. Development of antimigraine transdermal delivery systems of pizotifen malate. Int J Pharm. 2015;492:223–232. doi: 10.1016/j.ijpharm.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 84.Ramadon D., McCrudden M.T.C., Courtenay A.J., Donnelly R.F. Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Deliv Transl Res. 2022;12:758–791. doi: 10.1007/s13346-021-00909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rautio J., Meanwell N.A., Di L., Hageman M.J. The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov. 2018;17:559–587. doi: 10.1038/nrd.2018.46. [DOI] [PubMed] [Google Scholar]
- 86.Song T., Quan P., Xiang R., Fang L. Regulating the skin permeation rate of escitalopram by ion-pair formation with organic acids. AAPS PharmSciTech. 2016;17:1267–1273. doi: 10.1208/s12249-015-0474-y. [DOI] [PubMed] [Google Scholar]
- 87.Liu C., Qu X., Song L., Shang R., Wan X., Fang L. Investigation on the effect of deep eutectic formation on drug‒polymer miscibility and skin permeability of rotigotine drug-in-adhesive patch. Int J Pharm. 2020;574 doi: 10.1016/j.ijpharm.2019.118852. [DOI] [PubMed] [Google Scholar]
- 88.Marei H.F., Arafa M.F., Essa E.A., El Maghraby G.M. Lidocaine as eutectic forming drug for enhanced transdermal delivery of nonsteroidal anti-inflammatory drugs. J Drug Delivery Sci Technol. 2021;61 [Google Scholar]
- 89.Al-Akayleh F., Adwan S., Khanfar M., Idkaidek N., Al-Remawi M. A novel eutectic-based transdermal delivery system for risperidone. AAPS PharmSciTech. 2021;22:4. doi: 10.1208/s12249-020-01844-4. [DOI] [PubMed] [Google Scholar]
- 90.Banga A.K., Chien Y.W. Iontophoretic delivery of drugs: fundamentals, developments and biomedical applications. J Control Release. 1988;7:1–24. [Google Scholar]
- 91.Wang Y., Zeng L., Song W., Liu J. Influencing factors and drug application of iontophoresis in transdermal drug delivery: an overview of recent progress. Drug Deliv Transl Res. 2022;12:15–26. doi: 10.1007/s13346-021-00898-6. [DOI] [PubMed] [Google Scholar]
- 92.Phatale V., Vaiphei K.K., Jha S., Patil D., Agrawal M., Alexander A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J Control Release. 2022;351:361–380. doi: 10.1016/j.jconrel.2022.09.025. [DOI] [PubMed] [Google Scholar]
- 93.Junaid M.S.A., Banga A.K. Transdermal delivery of baclofen using iontophoresis and microneedles. AAPS PharmSciTech. 2022;23:84. doi: 10.1208/s12249-022-02232-w. [DOI] [PubMed] [Google Scholar]
- 94.Teaima M.H., Mohamed M.A.A., Abd El Rehem R.T., Tayel S.A., El-Nabarawi M.A., Fouad S.A. Enhanced transdermal delivery of bisoprolol hemifumarate via combined effect of iontophoresis and chemical enhancers: ex vivo permeation/in vivo pharmacokinetic studies. Pharmaceutics. 2021;13:682. doi: 10.3390/pharmaceutics13050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kazemi M., Mombeiny R., Tavakol S., Keyhanyar P., Mousavizadeh K. A combination therapy of nanoethosomal piroxicam formulation along with iontophoresis as an anti-inflammatory transdermal delivery system for wound healing. Int Wound J. 2019;16:1144–1152. doi: 10.1111/iwj.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcê A., Amaral M.H., Sousa Lobo J.M., Silva A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: a review. Eur J Pharm Sci. 2018;112:159–167. doi: 10.1016/j.ejps.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 97.Jain S., Patel N., Shah M.K., Khatri P., Vora N. Recent advances in lipid-based vesicles and particulate carriers for topical and transdermal application. J Pharm Sci. 2017;106:423–445. doi: 10.1016/j.xphs.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 98.Carter P., Narasimhan B., Wang Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int J Pharm. 2019;555:49–62. doi: 10.1016/j.ijpharm.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 99.Goyal R., Macri L.K., Kaplan H.M., Kohn J. Nanoparticles and nanofibers for topical drug delivery. J Control Release. 2016;240:77–92. doi: 10.1016/j.jconrel.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu Y., Tang X., Yang M., Yang D., Liu J. Transdermal drug delivery of triptolide-loaded nanostructured lipid carriers: preparation, pharmacokinetic, and evaluation for rheumatoid arthritis. Int J Pharm. 2019;554:235–244. doi: 10.1016/j.ijpharm.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 101.Sithole M.N., Marais S., Maree S.M., Plessis L.H., Du Plessis J., Gerber M. Development and characterization of nano-emulsions and nano-emulgels for transdermal delivery of statins. Expert Opin Drug Deliv. 2021;18:789–801. doi: 10.1080/17425247.2021.1867533. [DOI] [PubMed] [Google Scholar]
- 102.Yotsumoto K., Ishii K., Kokubo M., Yasuoka S. Improvement of the skin penetration of hydrophobic drugs by polymeric micelles. Int J Pharm. 2018;553:132–140. doi: 10.1016/j.ijpharm.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 103.Pitorre M., Gondé H., Haury C., Messous M., Poilane J., Boudaud D., et al. Recent advances in nanocarrier-loaded gels: which drug delivery technologies against which diseases? J Control Release. 2017;266:140–155. doi: 10.1016/j.jconrel.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 104.Wei Q., Su Y., Xin H., Zhang L., Ding J., Chen X. Immunologically effective biomaterials. ACS Appl Mater Interfaces. 2021;13:56719–56724. doi: 10.1021/acsami.1c14781. [DOI] [PubMed] [Google Scholar]
- 105.Gong C., Wu Q., Wang Y., Zhang D., Luo F., Zhao X., et al. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials. 2013;34:6377–6387. doi: 10.1016/j.biomaterials.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 106.de Graaf A.J., Azevedo Próspero dos Santos I.I., Pieters E.H.E., Rijkers D.T., van Nostrum C.F., Vermonden T., et al. A micelle-shedding thermosensitive hydrogel as sustained release formulation. J Control Release. 2012;162:582–590. doi: 10.1016/j.jconrel.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 107.Kim Y.-M., Song S.-C. Targetable micelleplex hydrogel for long-term, effective, and systemic siRNA delivery. Biomaterials. 2014;35:7970–7977. doi: 10.1016/j.biomaterials.2014.05.070. [DOI] [PubMed] [Google Scholar]
- 108.Feng X., Xu W., Li Z., Song W., Ding J., Chen X. Immunomodulatory nanosystems. Adv Sci. 2019;6 doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y., Sunoqrot S., Stowell C., Ji J., Lee C.W., Kim J.W., et al. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules. 2012;13:2154–2162. doi: 10.1021/bm300545b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kojima C., Nishisaka E., Suehiro T., Watanabe K., Harada A., Goto T., et al. The synthesis and evaluation of polymer prodrug/collagen hybrid gels for delivery into metastatic cancer cells. Nanomedicine. 2013;9:767–775. doi: 10.1016/j.nano.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 111.Rupp R., Rosenthal S.L., Stanberry L.R. VivaGel (TM) (SPL7013 Gel): a candidate dendrimer microbicide for the prevention of HIV and HSV infection. Int J Nanomed. 2007;2:561–566. [PMC free article] [PubMed] [Google Scholar]
- 112.Feng X., Liu J., Xu W., Li G., Ding J. Tackling autoimmunity with nanomedicines. Nanomedicine. 2020;15:1585–1597. doi: 10.2217/nnm-2020-0102. [DOI] [PubMed] [Google Scholar]
- 113.Su T., Feng X., Yang J., Xu W., Liu T., Zhang M., et al. Polymer nanotherapeutics to correct autoimmunity. J Control Release. 2022;343:152–174. doi: 10.1016/j.jconrel.2021.12.036. [DOI] [PubMed] [Google Scholar]
- 114.Wadhwa S., Garg V., Gulati M., Kapoor B., Singh S.K., Mittal N. Nanovesicles for nanomedicine: theory and practices. Methods Mol Biol. 2019;2000:1–17. doi: 10.1007/978-1-4939-9516-5_1. [DOI] [PubMed] [Google Scholar]
- 115.Safinya C.R., Ewert K.K. Materials chemistry: liposomes derived from molecular vases. Nature. 2012;489:372–374. doi: 10.1038/489372b. [DOI] [PubMed] [Google Scholar]
- 116.Large D.E., Abdelmessih R.G., Fink E.A., Auguste D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176 doi: 10.1016/j.addr.2021.113851. [DOI] [PubMed] [Google Scholar]
- 117.Ebrahimifar M., Nili-Ahmadabadi A., Akbarzadeh A., Shahemabadi H.E., Hasanzadegan M., Moradi-Sardareh H., et al. Preparation, characterization and cytotoxic effects of pegylated nanoliposomal containing carboplatin on ovarian cancer cell lines. Indian J Clin Biochem. 2017;32:230–234. doi: 10.1007/s12291-016-0596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rajput A., Osmani R.A.M., Khire A., Jaiswal S., Banerjee R. Levonorgestrel microneedle array patch for sustained release contraception: formulation, optimization and in vivo characterization. Molecules. 2022;27:2349. doi: 10.3390/molecules27072349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li L.L., An X.Q. A novel combined method of thin-film evaporation and a supercritical carbon dioxide technique to prepare a fluorescent siRNA-liposome. RSC Adv. 2016;6:92115–92119. [Google Scholar]
- 120.Haider M., Abdin S.M., Kamal L., Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: a review. Pharmaceutics. 2020;12:288. doi: 10.3390/pharmaceutics12030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao Y.P., Han J.F., Zhang F.Y., Liao T.T., Na R., Yuan X.F., et al. Flexible nano-liposomes-based transdermal hydrogel for targeted delivery of dexamethasone for rheumatoid arthritis therapy. Drug Deliv. 2022;29:2269–2282. doi: 10.1080/10717544.2022.2096718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y.T., Shen L.N., Wu Z.H., Zhao J.H., Feng N.P. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int J Pharm. 2014;471:449–452. doi: 10.1016/j.ijpharm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 123.Khan N.R., Wong T.W. 5-Fluorouracil ethosomes‒skin deposition and melanoma permeation synergism with microwave. Artif Cells Nanomed Biotechnol. 2018;46:568–577. doi: 10.1080/21691401.2018.1431650. [DOI] [PubMed] [Google Scholar]
- 124.Eskolaky E.B., Ardjmand M., Akbarzadeh A. Evaluation of anti-cancer properties of pegylated ethosomal paclitaxel on human melanoma cell line SK-MEL-3. Trop J Pharm Res. 2015;14:1421–1425. [Google Scholar]
- 125.Sudhakar K., Mishra V., Jain S., Rompicherla N.C., Malviya N., Tambuwala M.M. Development and evaluation of the effect of ethanol and surfactant in vesicular carriers on Lamivudine permeation through the skin. Int J Pharm. 2021;610 doi: 10.1016/j.ijpharm.2021.121226. [DOI] [PubMed] [Google Scholar]
- 126.Mao Y.T., Hua H.Y., Zhang X.G., Zhu D.X., Li F., Gui Z.H., et al. Ethosomes as delivery system for transdermal administration of vinpocetine. Pharmazie. 2013;68:381–382. [PubMed] [Google Scholar]
- 127.Zhang Y., Zhang N., Song H., Li H., Wen J., Tan X., et al. Design, characterization and comparison of transdermal delivery of colchicine via borneol-chemically-modified and borneol-physically-modified ethosome. Drug Deliv. 2019;26:70–77. doi: 10.1080/10717544.2018.1559258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu X., Du L., Li Y., Fu G., Jin Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed Pharmacother. 2015;73:6–11. doi: 10.1016/j.biopha.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 129.Liu X., Liu H., Zeng Z., Zhou W., Liu J., He Z. Pharmacokinetics of ligustrazine ethosome patch in rats and anti-myocardial ischemia and anti-ischemic reperfusion injury effect. Int J Nanomed. 2011;6:1391–1398. doi: 10.2147/IJN.S20263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fernández-García R., Lalatsa A., Statts L., Bolás-Fernández F., Paloma Ballesteros M., Serrano D.R. Transferosomes as nanocarriers for drugs across the skin: quality by design from lab to industrial scale. Int J Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118817. [DOI] [PubMed] [Google Scholar]
- 131.Vasanth S., Dubey A., Ravi G.S., Lewis S.A., Ghate V.M., El-Zahaby S.A., et al. Development and investigation of vitamin c-enriched adapalene-loaded transfersome gel: a collegial approach for the treatment of acne vulgaris. AAPS PharmSciTech. 2020;21:61. doi: 10.1208/s12249-019-1518-5. [DOI] [PubMed] [Google Scholar]
- 132.Hasibi F., Nasirpour A., Varshosaz J., Garcia-Manrique P., Carmen Blanco-Lopez M., Gutierrez G., et al. Formulation and characterization of taxifolin-loaded lipid nanovesicles (liposomes, niosomes, and transfersomes) for beverage fortification. Eur J Lipid Sci Technol. 2020;122 [Google Scholar]
- 133.Hou L., Kong M. Enhanced transdermal lymphatic drug delivery of hyaluronic acid modified transfersome for tumor metastasis therapy. J Control Release. 2015;213:e77. doi: 10.1016/j.jconrel.2015.05.128. [DOI] [PubMed] [Google Scholar]
- 134.Yang H., Wu X., Zhou Z., Chen X., Kong M. Enhanced transdermal lymphatic delivery of doxorubicin via hyaluronic acid based transfersomes/microneedle complex for tumor metastasis therapy. Int J Biol Macromol. 2019;125:9–16. doi: 10.1016/j.ijbiomac.2018.11.230. [DOI] [PubMed] [Google Scholar]
- 135.Zhang J., Froelich A., Michniak-Kohn B. Topical delivery of meloxicam using liposome and microemulsion formulation approaches. Pharmaceutics. 2020;12:282. doi: 10.3390/pharmaceutics12030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aziz D.E., Abdelbary A.A., Elassasy A.I. Implementing central composite design for developing transdermal diacerein-loaded niosomes: ex vivo permeation and in vivo deposition. Curr Drug Deliv. 2018;15:1330–1342. doi: 10.2174/1567201815666180619105419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kassem A.A., Abd El-Alim S.H., Asfour M.H. Enhancement of 8-methoxypsoralen topical delivery via nanosized niosomal vesicles: formulation development, in vitro and in vivo evaluation of skin deposition. Int J Pharm. 2017;517:256–268. doi: 10.1016/j.ijpharm.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 138.Yeo L.K., Chaw C.S., Elkordy A.A. The effects of hydration parameters and co-surfactants on methylene blue-loaded niosomes prepared by the thin film hydration method. Pharmaceuticals. 2019;12:46. doi: 10.3390/ph12020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ilhan-Ayisigi E., Ulucan F., Saygili E., Saglam-Metiner P., Gulce-Iz S., Yesil-Celiktas O. Nano-vesicular formulation of propolis and cytotoxic effects in a 3D spheroid model of lung cancer. J Sci Food Agric. 2020;100:3525–3535. doi: 10.1002/jsfa.10400. [DOI] [PubMed] [Google Scholar]
- 140.Kanaani L., Javadi I., Ebrahimifar M., Ebrahimi Shahmabadi H., Akbarzadeh Khiyav A., Mehrdiba T. Effects of cisplatin-loaded niosomal nanoparticleson BT-20 human breast carcinoma cells. Asian Pac J Cancer Prev. 2017;18:365–368. doi: 10.22034/APJCP.2017.18.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamaguchi S., Tsuchiya K., Sakai K., Abe M., Sakai H. Preparation of nonionic vesicles using the supercritical carbon dioxide reverse phase evaporation method and analysis of their solution properties. J Oleo Sci. 2016;65:21–26. doi: 10.5650/jos.ess15192. [DOI] [PubMed] [Google Scholar]
- 142.Shehata T.M., Nair A.B., Al-Dhubiab B.E., Shah J., Jacob S., Alhaider I.A., et al. Vesicular emulgel based system for transdermal delivery of insulin: factorial design and in vivo evaluation. Appl Sci. 2020;10:5341. [Google Scholar]
- 143.Nayak A.S., Chodisetti S., Gadag S., Nayak U.Y., Govindan S., Raval K. Tailoring solulan C24 based niosomes for transdermal delivery of donepezil: in vitro characterization, evaluation of pH sensitivity, and microneedle-assisted ex vivo permeation studies. J Drug Delivery Sci Technol. 2020;60 [Google Scholar]
- 144.Jamaludin R., Daud N.M., Sulong R.S.R., Yaakob H., Aziz A.A., Khamis S., et al. Andrographis paniculata-loaded niosome for wound healing application: characterisation and in vivo analyses. J Drug Delivery Sci Technol. 2021;63 [Google Scholar]
- 145.Shubhra R., Vikas P., Gopal R. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: the state of the art. Nano Rev Exp. 2017;8 doi: 10.1080/20022727.2017.1325708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., et al. Reassessment of exosome composition. Cell. 2019;177:428–445. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Loureiro J.A., Andrade S., Duarte A., Neves A.R., Queiroz J.F., Nunes C., et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer's disease. Molecules. 2017;22:277. doi: 10.3390/molecules22020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.You J.Y., Kang S.J., Rhee W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6:4321–4332. doi: 10.1016/j.bioactmat.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Regente M., Pinedo M., San Clemente H., Balliau T., Jamet E., de la Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- 150.Deng Z., Rong Y., Teng Y., Mu J., Zhuang X., Tseng M., et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol Ther. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.György B., Hung M.E., Breakefield X.O., Leonard J.N. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fuhrmann G., Serio A., Mazo M., Nair R., Stevens M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 153.Chen J., Yang J., Ding J. Rational construction of polycystine-based nanoparticles for biomedical applications. J Mater Chem B. 2022;10:7173–7182. doi: 10.1039/d2tb00581f. [DOI] [PubMed] [Google Scholar]
- 154.Mengnan Z., Rujing W., Kunmeng Y., Yuhong J., Yachen P., Yuke L., et al. Nucleic acid nanoassembly-enhanced RNA therapeutics and diagnosis. Acta Pharm Sin B. 2023;13:916–941. doi: 10.1016/j.apsb.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng C.X., Li M.Q., Ding J.X. Challenges and opportunities of nanomedicines in clinical translation. BIO Integr. 2021;2:57–60. [Google Scholar]
- 156.Hua S., de Matos M.B.C., Metselaar J.M., Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wicki A., Witzigmann D., Balasubramanian V., Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 158.Kamoun E.A., Kenawy E.R.S., Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8:217–233. doi: 10.1016/j.jare.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jacob S., Nair A.B., Shah J., Sreeharsha N., Gupta S., Shinu P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics. 2021;13:357. doi: 10.3390/pharmaceutics13030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhu J., Tang X., Jia Y., Ho C.T., Huang Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermal delivery—a review. Int J Pharm. 2020;578 doi: 10.1016/j.ijpharm.2020.119127. [DOI] [PubMed] [Google Scholar]
- 161.Liang Y., He J., Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15:12687–12722. doi: 10.1021/acsnano.1c04206. [DOI] [PubMed] [Google Scholar]
- 162.Chen L., Zhao X., Cai J., Guan Y., Wang S., Liu H., et al. Triptolide-loaded microemulsion-based hydrogels: physical properties and percutaneous permeability. Acta Pharm Sin B. 2013;3:185–192. [Google Scholar]
- 163.Tsegaye M.M., Chouhan G., Fentie M., Tyagi P., Nand P. Therapeutic potential of green synthesized metallic nanoparticles against staphylococcus aureus. Curr Drug Res Rev. 2019;13:172–183. doi: 10.2174/2589977513666210226123920. [DOI] [PubMed] [Google Scholar]
- 164.Dragicevic N., Maibach H. Combined use of nanocarriers and physical methods for percutaneous penetration enhancement. Adv Drug Deliv Rev. 2018;127:58–84. doi: 10.1016/j.addr.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 165.Park J., Lee H., Lim G.S., Kim N., Kim D., Kim Y.C. Enhanced transdermal drug delivery by sonophoresis and simultaneous application of aonophoresis and iontophoresis. AAPS PharmSciTech. 2019;20:96. doi: 10.1208/s12249-019-1309-z. [DOI] [PubMed] [Google Scholar]
- 166.Azagury A., Khoury L., Enden G., Kost J. Ultrasound mediated transdermal drug delivery. Adv Drug Deliv Rev. 2014;72:127–143. doi: 10.1016/j.addr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 167.Park D., Park H., Seo J., Lee S. Sonophoresis in transdermal drug deliverys. Ultrasonics. 2014;54:56–65. doi: 10.1016/j.ultras.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 168.Yu Z.W., Liang Y., Liang W.Q. Low-frequency sonophoresis enhances rivastigmine permeation in vitro and in vivo. Pharmazie. 2015;70:379–380. [PubMed] [Google Scholar]
- 169.Masterson J., Kluge B., Burdette A., Sr G.L. Sustained acousticmedicine; sonophoresis for nonsteroidal anti-inflammatory drug delivery in arthritis. Ther Deliv. 2020;11:363–372. doi: 10.4155/tde-2020-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen X., Zhu L., Li R., Pang L., Zhu S., Ma J., et al. Electroporation-enhanced transdermal drug delivery: effects of logP, pKa, solubility and penetration time. Eur J Pharm Sci. 2020;151 doi: 10.1016/j.ejps.2020.105410. [DOI] [PubMed] [Google Scholar]
- 171.Sung K.C., Fang J.Y., Wang J.J., Hu O.Y. Transdermal delivery of nalbuphine and its prodrugs by electroporation. Eur J Pharm Sci. 2003;18:63–70. doi: 10.1016/s0928-0987(02)00244-0. [DOI] [PubMed] [Google Scholar]
- 172.Feng S., Zhu L., Huang Z., Wang H., Li H., Zhou H., et al. Controlled release of optimized electroporation enhances the transdermal efficiency of sinomenine hydrochloride for treating arthritis in vitro and in clinic. Drug Des Devel Ther. 2017;11:1737–1752. doi: 10.2147/DDDT.S136313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wen X., Xin Y., Hamblin M.R., Jiang X. Applications of cold atmospheric plasma for transdermal drug delivery: a review. Drug Deliv Transl Res. 2021;11:741–747. doi: 10.1007/s13346-020-00808-2. [DOI] [PubMed] [Google Scholar]
- 174.Weltmann K.D., von Woedtke T. Plasma medicine-current state of research and medical application. Plasma Phys Controlled Fusion. 2017;59 [Google Scholar]
- 175.Tan F., Fang Y., Zhu L., Al-Rubeai M. Cold atmospheric plasma as an interface biotechnology for enhancing surgical implants. Crit Rev Biotechnol. 2022;41:425–440. doi: 10.1080/07388551.2020.1853671. [DOI] [PubMed] [Google Scholar]
- 176.Van der Paal J., Aernouts S., van Duin A.C.T., Neyts E.C., Bogaerts A. Interaction of O and OH radicals with a simple model system for lipids in the skin barrier: a reactive molecular dynamics investigation for plasma medicine. J Phys D Appl Phys. 2013;46 [Google Scholar]
- 177.Marschewski M., Hirschberg J., Omairi T., Höfft O., Viöl W., Emmert S., et al. Electron spectroscopic analysis of the human lipid skin barrier: cold atmospheric plasma-induced changes in lipid composition. Exp Dermatol. 2012;21:921–925. doi: 10.1111/exd.12043. [DOI] [PubMed] [Google Scholar]
- 178.Kristof J., Miyamoto H., Tran A.N., Blajan M., Shimizu K. Feasibility of transdermal delivery of cyclosporine A using plasma discharges. Biointerphases. 2017;12 doi: 10.1116/1.4982826. [DOI] [PubMed] [Google Scholar]
- 179.Swain S., Singh A.P., Rajesh K.Y. A review on polymer hydrogel and polymer microneedle based transdermal drug delivery system. Mater Today Proc. 2022;61:1061–1066. [Google Scholar]
- 180.Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G., Singh M., et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 181.Jung J.H., Jin S.G. Microneedle for transdermal drug delivery: current trends and fabrication. J Pharm Investig. 2021;51:503–517. doi: 10.1007/s40005-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vora L.K., Moffatt K., Tekko I.A., Paredes A.J., Volpe-Zanutto F., Mishra D., et al. Microneedle array systems for long-acting drug delivery. Eur J Pharm Biopharm. 2021;159:44–76. doi: 10.1016/j.ejpb.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 183.van der Maaden K., Jiskoot W., Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161:645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 184.Yang J., Liu X., Fu Y., Song Y. Recent advances of microneedles for biomedical applications: drug delivery and beyond. Acta Pharm Sin B. 2019;9:469–483. doi: 10.1016/j.apsb.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Larrañeta E., McCrudden M.T.C., Courtenay A.J., Donnelly R.F. Microneedles: a new frontier in nanomedicine delivery. Pharm Res. 2016;33:1055–1073. doi: 10.1007/s11095-016-1885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cárcamo-Martínez Á., Mallon B., Domínguez-Robles J., Vora L.K., Anjani Q.K., Donnelly R.F. Hollow microneedles: a perspective in biomedical applications. Int J Pharm. 2021;599 doi: 10.1016/j.ijpharm.2021.120455. [DOI] [PubMed] [Google Scholar]
- 187.Ahmed Saeed Al-Japairai K., Mahmood S., Hamed Almurisi S., Reddy Venugopal J., Rebhi Hilles A., Azmana M., et al. Current trends in polymer microneedle for transdermal drug delivery. Int J Pharm. 2020;587 doi: 10.1016/j.ijpharm.2020.119673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ning X., Wiraja C., Chew W.T.S., Fan C., Xu C. Transdermal delivery of Chinese herbal medicine extract using dissolvable microneedles for hypertrophic scar treatment. Acta Pharm Sin B. 2021;11:2937–2944. doi: 10.1016/j.apsb.2021.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shim W.S., Hwang Y.M., Park S.G., Lee C.K., Kang N.G. Role of polyvinylpyrrolidone in dissolving microneedle for efficient transdermal drug delivery: in vitro and clinical studies. Bull Korean Chem Soc. 2018;39:789–793. [Google Scholar]
- 190.Xing M., Wang X., Zhao L., Zhou Z., Liu H., Wang B., et al. Novel dissolving microneedles preparation for synergistic melasma therapy: combined effects of tranexamic acid and licorice extract. Int J Pharm. 2021;600 doi: 10.1016/j.ijpharm.2021.120406. [DOI] [PubMed] [Google Scholar]
- 191.Li W., Tang J., Terry R.N., Li S., Brunie A., Callahan R.L., et al. Long-acting reversible contraception by effervescent microneedle patch. Sci Adv. 2019;5 doi: 10.1126/sciadv.aaw8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaur A., Bhoop B.S., Chhibber S., Sharma G., Gondil V.S., Katare O.P. Supramolecular nano-engineered lipidic carriers based on diflunisal-phospholipid complex for transdermal delivery: QbD based optimization, characterization and preclinical investigations for management of rheumatoid arthritis. Int J Pharm. 2017;533:206–224. doi: 10.1016/j.ijpharm.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 193.Bashir M., Ahmad J., Asif M., Khan S.-U.-D., Irfan M., Ibrahim A.Y., et al. Nanoemulgel, an innovative carrier for diflunisal topical delivery with profound anti-inflammatory effect: in vitro and in vivo evaluation. Int J Nanomed. 2021;16:1457–1472. doi: 10.2147/IJN.S294653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sharma G., Saini M.K., Thakur K., Kapil N., Garg N.K., Raza K., et al. Aceclofenac cocrystal nanoliposomes for rheumatoid arthritis with better dermatokinetic attributes: a preclinical study. Nanomedicine. 2017;12:615–638. doi: 10.2217/nnm-2016-0405. [DOI] [PubMed] [Google Scholar]
- 195.Garg N.K., Tandel N., Bhadada S.K., Tyagi R.K. Nanostructured lipid carrier-mediated transdermal delivery of aceclofenac hydrogel present an effective therapeutic approach for inflammatory diseases. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.713616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.He Y., Majid K., Maqbool M., Hussain T., Yousaf A.M., Khan I.U., et al. Formulation and characterization of lornoxicam-loaded cellulosic-microsponge gel for possible applications in arthritis. Saudi Pharm J. 2020;28:994–1003. doi: 10.1016/j.jsps.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shen Q., Tang T., Hu Q., Ying X., Shu G., Teng C., et al. Microwave hyperthermia-responsible flexible liposomal gel as a novel transdermal delivery of methotrexate for enhanced rheumatoid arthritis therapy. Biomater Sci. 2021;9:8386–8395. doi: 10.1039/d1bm01438b. [DOI] [PubMed] [Google Scholar]
- 198.Wu C., Cheng J., Li W., Yang L., Dong H., Zhang X. Programmable polymeric microneedles for combined chemotherapy and antioxidative treatment of rheumatoid arthritis. ACS Appl Mater Interfaces. 2021;13:55559–55568. doi: 10.1021/acsami.1c17375. [DOI] [PubMed] [Google Scholar]
- 199.Wei F., Wang Q., Liu H., Yang X., Cao W., Zhao W., et al. High efficacy combined microneedles array with methotrexate nanocrystals for effective anti-rheumatoid arthritis. Int J Nanomed. 2022;17:2397–2412. doi: 10.2147/IJN.S365523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hu H., Ruan H., Ruan S., Pei L., Jing Q., Wu T., et al. Acid-responsive PEGylated branching PLGA nanoparticles integrated into dissolving microneedles enhance local treatment of arthritis. Chem Eng J. 2022;431 [Google Scholar]
- 201.Song H., Wen J., Li H., Meng Y., Zhang Y., Zhang N., et al. Enhanced transdermal permeability and drug deposition of rheumatoid arthritis via sinomenine hydrochloride-loaded antioxidant surface transethosome. Int J Nanomed. 2019;14:3177–3188. doi: 10.2147/IJN.S188842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zheng H., Xu C., Fei Y., Wang J., Yang M., Fang L., et al. Monoterpenes-containing PEGylated transfersomes for enhancing joint cavity drug delivery evidenced by CLSM and double-sited microdialysis. Mater Sci Eng C Mater Biol Appl. 2020;113 doi: 10.1016/j.msec.2020.110929. [DOI] [PubMed] [Google Scholar]
- 203.Shan Q.Q., Jiang X.J., Wang F.Y., Shu Z.X., Gui S.Y. Cubic and hexagonal liquid crystals as drug carriers for the transdermal delivery of triptolide. Drug Deliv. 2019;26:490–498. doi: 10.1080/10717544.2019.1602796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu X.Y., Pei W.J., Wu Y.Z., Ren F.L., Yang S.Y., Wang X. Transdermal delivery of triptolide-phospholipid complex to treat rheumatoid arthritis. Drug Deliv. 2021;28:2127–2136. doi: 10.1080/10717544.2021.1986603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee G., Bae H. Anti-Inflammatory applications of melittin, a major component of bee venom: detailed mechanism of action and adverse effects. Molecules. 2016;21:616. doi: 10.3390/molecules21050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Du G., He P., Zhao J., He C., Jiang M., Zhang Z., et al. Polymeric microneedle-mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J Control Release. 2021;336:537–548. doi: 10.1016/j.jconrel.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 207.Elkomy M.H., Alruwaili N.K., Elmowafy M., Shalaby K., Zafar A., Ahmad N., et al. Surface-modified bilosomes nanogel bearing a natural plant alkaloid for safe management of rheumatoid arthritis inflammation. Pharmaceutics. 2022;14:563. doi: 10.3390/pharmaceutics14030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Naeem M., Iqbal T., Nawaz Z., Hussain S., Ahmer A., Kumar H., et al. Optimization of ibuprofen therapeutic system for enhanced transdermal delivery: a quality by design approach. Lat Am J Pharm. 2020;39:2241–2249. [Google Scholar]
- 209.Anjum F., Zakir F., Verma D., Aqil M., Singh M., Jain P., et al. Exploration of nanoethosomal transgel of naproxen sodium for the treatment of arthritis. Curr Drug Deliv. 2020;17:885–897. doi: 10.2174/1567201817666200724170203. [DOI] [PubMed] [Google Scholar]
- 210.Jagdale S.C., Deore G.K., Chabukswar A.R. Development of microemulsion based nabumetone transdermal delivery for treatment of arthritis. Recent Pat Drug Deliv Formul. 2018;12:130–149. doi: 10.2174/1872211312666180227091059. [DOI] [PubMed] [Google Scholar]
- 211.Garg V., Singh H., Bhatia A., Raza K., Singh S.K., Singh B., et al. Systematic development of transethosomal gel system of piroxicam: formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech. 2017;18:58–71. doi: 10.1208/s12249-016-0489-z. [DOI] [PubMed] [Google Scholar]
- 212.Hartmann P., Butt E., Fehér Á., Szilágyi Á.L., Jász K.D., Balázs B., et al. Electroporation-enhanced transdermal diclofenac sodium delivery into the knee joint in a rat model of acute arthritis. Drug Des Devel Ther. 2018;12:1917–1930. doi: 10.2147/DDDT.S161703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mahmoud T.M., Nafady M.M., Farouk H.O., Mahmoud D.M., Ahmed Y.M., Zaki R.M., et al. Novel bile salt stabilized vesicles-mediated effective topical delivery of diclofenac sodium: a new therapeutic approach for pain and inflammation. Pharmaceuticals. 2022;15:1106. doi: 10.3390/ph15091106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ghosh S., Mukherjee B., Chaudhuri S., Roy T., Mukherjee A., Sengupta S. Methotrexate aspasomes against rheumatoid arthritis: optimized hydrogel loaded liposomal formulation with in vivo evaluation in wistar rats. AAPS PharmSciTech. 2018;19:1320–1336. doi: 10.1208/s12249-017-0939-2. [DOI] [PubMed] [Google Scholar]
- 215.Kathuria H., Nguyen D.T.P., Handral H.K., Cai J., Cao T., Kang L. Proposome for transdermal delivery of tofacitinib. Int J Pharm. 2020;585 doi: 10.1016/j.ijpharm.2020.119558. [DOI] [PubMed] [Google Scholar]
- 216.Li Y., Sun Y., Wei S., Zhang L., Zong S. Development and evaluation of tofacitinib transdermal system for the treatment of rheumatoid arthritis in rats. Drug Dev Ind Pharm. 2021;47:878–886. doi: 10.1080/03639045.2021.1916521. [DOI] [PubMed] [Google Scholar]
- 217.Shu Z., Cao Y., Tao Y., Liang X., Wang F., Li Z., et al. Polyvinylpyrrolidone microneedles for localized delivery of sinomenine hydrochloride: preparation, release behavior of in vitro & in vivo, and penetration mechanism. Drug Deliv. 2020;27:642–651. doi: 10.1080/10717544.2020.1754524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu C., Zhang Y., Wu T., He Z., Guo T., Feng N. Optimizing glycerosome formulations via an orthogonal experimental design to enhance transdermal triptolide delivery. Acta Pharm. 2022;72:135–146. doi: 10.2478/acph-2022-0006. [DOI] [PubMed] [Google Scholar]
- 219.Pal R.R., Rajpal V., Singh N., Singh S., Mishra N., Singh P., et al. Downregulation of pro-inflammatory markers IL-6 and TNF-α in rheumatoid arthritis using nano-lipidic carriers of a quinone-based phenolic: an in vitro and in vivo study. Drug Deliv Transl Res. 2022;13:627–641. doi: 10.1007/s13346-022-01221-7. [DOI] [PubMed] [Google Scholar]