Abstract
Background
The optimal treatment of anastomotic leak after rectal cancer resection is unclear. This worldwide cohort study aimed to provide an overview of four treatment strategies applied.
Methods
Patients from 216 centres and 45 countries with anastomotic leak after rectal cancer resection between 2014 and 2018 were included. Treatment was categorized as salvage surgery, faecal diversion with passive or active (vacuum) drainage, and no primary/secondary faecal diversion. The primary outcome was 1-year stoma-free survival. In addition, passive and active drainage were compared using propensity score matching (2 : 1).
Results
Of 2470 evaluable patients, 388 (16.0 per cent) underwent salvage surgery, 1524 (62.0 per cent) passive drainage, 278 (11.0 per cent) active drainage, and 280 (11.0 per cent) had no faecal diversion. One-year stoma-free survival rates were 13.7, 48.3, 48.2, and 65.4 per cent respectively. Propensity score matching resulted in 556 patients with passive and 278 with active drainage. There was no statistically significant difference between these groups in 1-year stoma-free survival (OR 0.95, 95 per cent c.i. 0.66 to 1.33), with a risk difference of −1.1 (95 per cent c.i. −9.0 to 7.0) per cent. After active drainage, more patients required secondary salvage surgery (OR 2.32, 1.49 to 3.59), prolonged hospital admission (an additional 6 (95 per cent c.i. 2 to 10) days), and ICU admission (OR 1.41, 1.02 to 1.94). Mean duration of leak healing did not differ significantly (an additional 12 (−28 to 52) days).
Conclusion
Primary salvage surgery or omission of faecal diversion likely correspond to the most severe and least severe leaks respectively. In patients with diverted leaks, stoma-free survival did not differ statistically between passive and active drainage, although the increased risk of secondary salvage surgery and ICU admission suggests residual confounding.
The optimal treatment strategy for anastomotic leakage after restorative rectal cancer surgery remains unknown. This large, international collaborative study investigated various outcomes after four predefined treatment strategies for anastomotic leakage. Substantial differences were observed in patient and leakage characteristics, as well as outcomes following the four treatment strategies. However, no statistically significant differences were reported in stoma-free survival rates between active (vacuum) drainage and passive drainage.
Introduction
A feared complication after restorative rectal cancer resection is anastomotic leak (AL), owing to its significant impact on morbidity1–4. The incidence of AL remains high, with rates of up to 20 per cent5, despite developments in surgical technique and perioperative care. AL is associated with prolonged hospital stay, reintervention, a stage-dependent decrease in survival, bowel dysfunction, and a high risk of a permanent stoma6–10. Although the consequences of AL are evident, international consensus and standardization of treatment strategies is lacking11, possibly because of heterogeneity of both the AL as well as the patients it affects.
Treatment of AL mostly depends on the patient’s clinical symptoms and severity of AL12,13, which varies from occult leaks in patients with a diverting stoma, to faecal peritonitis with multiple organ failure14,15. Traditionally, patients with AL have been treated with faecal diversion, either with or without abscess drainage, and in a minority of patients the anastomosis is dismantled with creation of an end-colostomy16–18. These treatment strategies are associated with high rates of non-healing, particularly in irradiated patients16. Moreover, persistent leakage might give rise to a chronic presacral sinus that can cause long-term problems, such as fistula formation, fasciitis, and bleeding. Pelvic sepsis originating from a non-healed leak is a serious condition, contributing to hospital admission and multiple interventions, often requiring extensive salvage surgery19,20.
In the past decade, active treatment strategies have emerged starting with the introduction of endoscopic vacuum therapy (EVT)21. This strategy was subsequently modified by closing the anastomotic defect as soon as appropriate granulation of the cavity was observed. This so-called endoscopic vacuum-assisted transanal closure (EVAC) can yield high success rates in experienced hands16,21,22. The main objective of EVAC is more effective and faster healing of the anastomosis with preservation of bowel continuity23–25. However, substantial heterogeneity exists among ALs, and clinical decision-making is also dependent on various patient, clinical, and surgical characteristics (for example, co-morbidity, time from diagnosis, defect circumference)12,26,27. No large studies have evaluated different AL treatment strategies, simultaneously considering all these various characteristics.
This worldwide cohort study aimed to evaluate four different treatment strategies, with 1-year stoma-free survival as the main outcome. In the TENTACLE-Rectum study, detailed data were collected from a large number of patients who developed AL after rectal cancer resection with at least 1 year of follow-up17. In the present explorative study, outcomes after AL were analysed according to the following predefined primary treatment strategies: salvage surgery, faecal diversion with passive drainage, faecal diversion with active drainage, and no faecal diversion. The outcomes encompassed the need for secondary salvage surgery, total duration of hospital stay, ICU admission, time to healing of the leak, and 1-year stoma-free survival. Moreover, as robust comparative studies are scarce, the aforementioned outcomes were additionally compared among patients with a diverted leak who underwent either active (EVT) or passive drainage of the perianastomotic abscess.
Methods
Study design
This was an international retrospective cohort study encompassing 216 centres from 45 countries. The TENTACLE-Rectum study protocol was approved by the institutional board at Radboud University Medical Centre On 17 October 201917. All collaborating centres adhered to the regulations of their own ethical committees. The study was registered at ClinicalTrials.gov (NCT04127734), and was conducted in agreement with the STROBE guidelines for reporting of observational studies28.
Patient selection
Patients with rectal cancer who underwent surgery between 1 January 2014 and 31 December 2018 in the participating centres were included if they were diagnosed with AL within 1 year after index surgery. AL was defined as ‘a defect of the intestinal wall at the anastomotic site (including suture and staple lines of neorectal reservoirs) leading to a communication between the intra- and extraluminal compartments’29. Included patients were: aged 18 years or older; diagnosed with rectal cancer, defined as an adenocarcinoma with its lower border below the level of the sigmoid take-off30; and underwent surgical resection with creation of a primary anastomosis for either primary cancer, salvage resection for regrowth, or completion total mesorectal excision (TME) after local excision. Patients were excluded if they underwent surgery for benign or recurrent disease, or had an emergency resection. Patients with missing data regarding treatment of AL were also excluded.
Treatment strategies for anastomotic leak after rectal cancer resection
Four main treatment strategies for AL were defined based on a case-vignette study among international experts, in which the use of basic treatment principles was evaluated for different leak scenarios27. These four strategies comprised salvage surgery, faecal diversion with passive drainage, faecal diversion with active drainage, and no faecal diversion. Salvage surgery included dismantling of the anastomosis and formation of an end-colostomy, or immediate or delayed (Turnbull–Cutait) redo anastomosis. Faecal diversion could be accomplished using a primary diverting stoma that was constructed during index surgery, or a secondary diverting stoma after diagnosis of AL. Passive drainage comprised solely faecal diversion, or a combination of faecal diversion and transabdominal or transgluteal drain placement, endoscopic or surgical washout of the abscess cavity, or abdominal lavage. Active drainage comprised faecal diversion and EVT (Endo-SPONGE®) with or without transanal closure. In the fourth strategy (no faecal diversion), no primary or secondary diverting stoma was created, and treatment could consist of any of the following modalities alone or in combination: antibiotics, drainage (transabdominal or transgluteal drain placement, endoscopic or surgical abscess drainage, abdominal or colonic lavage), endoscopic clipping or stenting, or transanal surgical closure. Primary treatment was defined as the first treatment strategy after diagnosis of AL, and was considered to have failed if another secondary treatment strategy was used afterwards. All patients were categorized and subsequently analysed based on the primary treatment modality, according to the intention-to-treat principle.
Definitions
Healing of the leak was confirmed by CT, MRI, endoscopy or contrast enema, and time to healing calculated in patients with an anastomosis in situ. The healed anastomosis could either be a primary or secondary anastomosis, the latter being created by excision of the leaking primary anastomosis by either an immediate or delayed (Turnbull–Cutait) redo procedure. Presacral abscess present more than 1 year after index surgery was defined as a chronic sinus. A chronic sinus was considered a non-healed anastomosis.
Outcomes
The primary outcome of this study was 1-year stoma-free survival, defined as being alive without a temporary or permanent ileostomy or colostomy 1 year after index surgery. Secondary outcomes were: failure of first treatment necessitating salvage surgery, number of secondary anastomoses, total duration of hospital stay, ICU admission, total duration of ICU stay, and time to leak healing.
Statistical analysis
Patient, tumour, index treatment, and leak characteristics were evaluated for the four treatment strategies using descriptive statistics. Baseline characteristics are presented as numbers with percentages, and continuous data according to their distribution as mean(s.d.) or median (i.q.r.). All missing data were considered to be missing at random, and multiple imputation using chained equations was performed31,32. Additional information about handling of missing data and multiple imputation can be found in the supplementary material. Owing to the explorative nature of this study, a sample size calculation was not performed.
A comparative analysis was undertaken among patients who underwent primary or secondary faecal diversion, with either primary passive or primary active drainage of the perianastomotic abscess, and propensity score matching (PSM) was used to minimize confounding bias (supplementary material and Table S1). Multivariable logistic regression modelling was used to calculate propensity scores, including the following known confounders: age, sex, BMI, ASA fitness grade, clinical metastatic disease category, neoadjuvant therapy, abdominal approach, transanal approach (transanal TME), multivisceral resection, presence of a primary diverting stoma, clinical setting of AL diagnosis, postoperative day of AL diagnosis, presence of severe clinical symptoms, anastomotic defect circumference, ischaemic afferent colon, anastomotic fistula, retraction of the afferent colon, abdominal contamination, and reactivation leakage33. Cases were matched using the nearest-neighbour method, with a caliper of 0.2 and a 2 : 1 ratio34,35. To assess the covariate balance between the two treatment strategies before and after PSM, standardized mean differences (SMDs) were calculated. There was considered to be sufficient balance between cohorts when the SMD was below 0.1.
In patients with a diverted leak who underwent passive or active drainage as initial treatment for the perianastomotic abscess, the primary outcome was assessed using logistic regression by estimating an OR with 95 per cent confidence interval and a risk difference (RD) between treatment strategies. Secondary outcomes were evaluated using logistic regression (OR with 95 per cent c.i.) and RD, or linear regression (mean difference with 95 per cent c.i.). To assess the effect of annual case volume on outcomes, a sensitivity analysis was performed including annual case volume (low, below 20; middle, 20–49; high, 50 or more) in the multivariable logistic regression model. PSM and the subsequent comparative analyses were performed in each data set, and these results were pooled according to Rubin’s rule. All analyses were carried out in R version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria) using packages mice and MatchIt.
Results
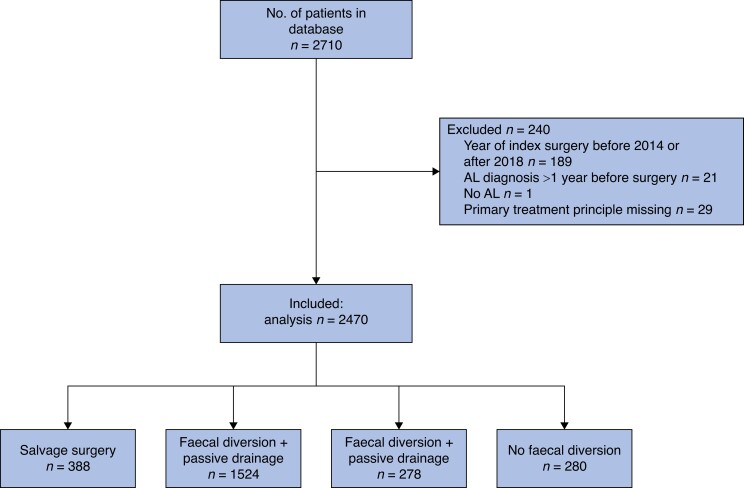
In total, 2710 patients were included in the database. After exclusion of 240 patients because of incorrect year of index surgery (189), AL diagnosis beyond 1 year (21), absence of AL (1), and missing primary treatment modality (29), 2470 patients remained for analysis. Based on the primary treatment strategy for AL, these patients were categorized into salvage surgery (388 patients, 16.0 per cent), faecal diversion with passive drainage (1524 patients, 62.0 per cent), faecal diversion with active drainage (278 patients, 11.0 per cent) and no faecal diversion (280 patients, 11.0 per cent) cohorts (Fig. 1). Baseline characteristics of the participating centres are summarized in Tables S2 and S3.
Fig. 1.
Study flow chart
AL, anastomotic leak.
Characteristics and outcomes of the four treatment strategies
Table 1 shows the patient, tumour, surgical, and leak characteristics; several proportional differences were observed between cohorts. In the primary salvage cohort, fewer patients received a primary diverting stoma at index surgery than in the passive and active drainage cohorts (47.4, 79.2, and 76.6 per cent respectively). Patients who required salvage surgery had proportionally more severe clinical symptoms at the time of AL diagnosis (40.9, 26.2, 17.4, and 26.5 per cent in salvage surgery, faecal diversion with passive drainage, faecal diversion with active drainage, and no faecal diversion cohorts), and differences in leak characteristics including abdominal contamination (68.4, 30.7, 20.7, and 32.4 per cent), and ischaemic afferent colon (39.9, 7.4, 8.5, and 6.1 per cent). More patients had moderate-to-severe anastomotic dehiscence (more than 25 to 100 per cent) in the salvage surgery and faecal diversion with active drainage cohorts (76.2, 37.2, 51.6, and 29.4 per cent). In the faecal diversion with active drainage cohort, proportionally more patients originated from a high-volume hospital (55.2, 53.3, 62.2, and 41.1 per cent).
Table 1.
Baseline characteristics for the four predefined treatment strategies
| Salvage surgery (n = 388) |
Faecal diversion + passive drainage (n = 1524) |
Faecal diversion + active drainage (n = 278) |
No faecal diversion (n = 280) |
SMD | |
|---|---|---|---|---|---|
| Age (years), median (i.q.r.) | 65 (59–73) | 66 (57–71) | 64 (56–72) | 65 (57–74) | 0.099 |
| Sex ratio (M : F) | 256 : 132 | 1104 : 420 | 229 : 49 | 192 : 88 | 0.199 |
| ASA fitness grade | 0.172 | ||||
| I | 48 (12.7) | 246 (16.4) | 47 (17.3) | 36 (13.5) | |
| II | 196 (51.9) | 863 (57.7) | 165 (60.7) | 150 (56.2) | |
| III–IV | 134 (35.4) | 387 (25.9) | 60 (22.1) | 81 (30.3) | |
| Missing | 10 | 28 | 6 | 13 | |
| BMI (kg/m²) | 0.157 | ||||
| Underweight (< 18.5) | 26 (7.4) | 68 (4.8) | 8 (3.0) | 16 (6.6) | |
| Normal (18.5–24.9) | 120 (34.0) | 449 (31.9) | 88 (32.8) | 83 (34.0) | |
| Overweight (25.0–29.9) | 124 (35.1) | 599 (42.6) | 103 (38.4) | 96 (39.3) | |
| Obese (≥ 30.0) | 83 (23.5) | 291 (20.7) | 69 (25.7) | 49 (20.1) | |
| Missing | 35 | 117 | 10 | 36 | |
| Clinical metastasis category | 0.036 | ||||
| cM0 | 303 (89.4) | 1202 (91.4) | 234 (91.1) | 201 (91.4) | |
| cM1 | 36 (10.6) | 113 (8.6) | 23 (8.9) | 19 (8.6) | |
| Missing | 49 | 209 | 21 | 60 | |
| Neoadjuvant therapy | 0.323 | ||||
| None | 209 (53.9) | 575 (37.7) | 111 (39.9) | 176 (62.9) | |
| Radiotherapy | 34 (8.8) | 202 (13.3) | 37 (13.3) | 18 (6.4) | |
| Chemotherapy | 10 (2.6) | 23 (1.5) | 6 (2.2) | 7 (2.5) | |
| Chemoradiation | 135 (34.8) | 724 (47.5) | 124 (44.6) | 79 (28.2) | |
| Clinical setting of AL diagnosis | 0.281 | ||||
| Surgical ward | 296 (76.3) | 1030 (67.6) | 182 (65.5) | 182 (65.5) | |
| ICU/HC | 35 (9.0) | 50 (3.3) | 11 (4.0) | 11 (4.0) | |
| Emergency department | 28 (7.2) | 149 (9.8) | 28 (10.1) | 41 (14.7) | |
| Outpatient clinic | 29 (7.5) | 294 (19.3) | 57 (20.5) | 44 (15.8) | |
| Missing | 0 | 1 | 0 | 2 | |
| POD of AL diagnosis, median (i.q.r.) | 6 (4–10) | 8 (5–20) | 10 (5–19) | 8 (5–17) | 0.098 |
| Abdominal approach | 0.283 | ||||
| Laparoscopic | 223 (57.5) | 962 (63.1) | 170 (61.2) | 159 (57.0) | |
| Robot-assisted | 26 (6.7) | 134 (8.8) | 53 (19.1) | 20 (7.2) | |
| Laparotomy | 139 (35.8) | 428 (28.1) | 55 (19.8) | 100 (35.8) | |
| Missing | 0 | 0 | 0 | 1 | |
| Transanal TME | 0.179 | ||||
| No | 331 (85.3) | 1240 (81.4) | 201 (72.3) | 239 (85.4) | |
| Yes | 57 (14.7) | 284 (18.6) | 77 (27.7) | 41 (14.6) | |
| Multivisceral resection | 0.080 | ||||
| No | 352 (92.4) | 1393 (93.6) | 255 (94.1) | 249 (90.2) | |
| Yes | 29 (7.6) | 96 (6.4) | 16 (5.9) | 27 (9.8) | |
| Missing | 7 | 35 | 7 | 4 | |
| Primary defunctioning stoma | 1.353 | ||||
| No | 204 (52.6) | 310 (20.8) | 65 (23.4) | 280 (100) | |
| Yes | 184 (47.4) | 1214 (79.2) | 213 (76.6) | 0 (0) | |
| Fistula | 0.154 | ||||
| No | 332 (90.0) | 1351 (93.0) | 262 (96.7) | 226 (90.0) | |
| Yes | 37 (10.0) | 101 (7.0) | 9 (3.3) | 25 (10.0) | |
| Missing | 19 | 72 | 7 | 29 | |
| Retraction of afferent colon | 0.268 | ||||
| No | 245 (86.9) | 1161 (96.8) | 212 (92.2) | 191 (98.5) | |
| Yes | 37 (13.1) | 39 (3.2) | 18 (7.8) | 3 (1.5) | |
| Missing | 106 | 324 | 48 | 86 | |
| Abdominal contamination | 0.535 | ||||
| No | 117 (31.6) | 965 (69.3) | 191 (79.3) | 167 (67.6) | |
| Yes | 253 (68.4) | 428 (30.7) | 50 (20.7) | 80 (32.4) | |
| Missing | 18 | 131 | 37 | 33 | |
| Bowel wall ischaemia | 0.447 | ||||
| No | 197 (60.1) | 1141 (92.6) | 226 (91.5) | 200 (93.9) | |
| Yes | 131 (39.9) | 91 (7.4) | 21 (8.5) | 13 (6.1) | |
| Missing | 60 | 292 | 31 | 67 | |
| Anastomotic defect circumference (%) | 0.629 | ||||
| 0–24.9 | 54 (23.8) | 368 (62.8) | 80 (48.5) | 60 (70.6) | |
| 25–49.9 | 81 (35.7) | 145 (24.7) | 60 (36.4) | 20 (23.5) | |
| 50–100 | 92 (40.5) | 73 (12.5) | 25 (15.2) | 5 (5.9) | |
| Missing | 161 | 938 | 113 | 195 | |
| Reactivation leakage | 0.129 | ||||
| No | 155 (91.7) | 1071 (89.9) | 178 (91.8) | 184 (96.3) | |
| Yes | 14 (8.3) | 120 (10.1) | 16 (8.2) | 7 (3.7) | |
| Missing | 219 | 333 | 84 | 89 | |
| Severe clinical symptoms | 0.267 | ||||
| No | 185 (47.7) | 878 (73.8) | 185 (82.6) | 158 (73.5) | |
| Yes | 128 (40.9) | 312 (26.2) | 39 (17.4) | 57 (26.5) | |
| Missing | 75 | 334 | 54 | 65 | |
| Annual procedure volume of hospital | 0.263 | ||||
| Low (<20) | 24 (6.2) | 120 (7.9) | 13 (4.7) | 44 (15.7) | |
| Middle (20–49) | 150 (38.7) | 592 (38.8) | 92 (33.1) | 121 (43.2) | |
| High (>50) | 214 (55.2) | 812 (53.3) | 173 (62.2) | 115 (41.1) |
Values are n (%) unless otherwise indicated. SMD, standardized mean difference; AL, anastomotic leak; HC, high care; POD, postoperative day; TME, total mesorectal excision.
Table 2 shows outcomes for the four primary treatment strategies. The 1-year stoma free survival rate was 13.7 per cent after salvage surgery, 48.3 per cent after faecal diversion with passive drainage, 48.2 per cent after faecal diversion with active drainage, and 65.4 per cent with no faecal diversion group. The percentage of patients requiring secondary salvage surgery was 5.2 per cent after faecal diversion with passive drainage, 10.4 per cent after faecal diversion with active drainage, and 2.9 per cent after no faecal diversion. The proportion of secondary anastomoses created was 12.4, 1.8, 2.2, and 0.4 per cent in the salvage surgery, faecal diversion with passive drainage, faecal diversion with active drainage, and no faecal diversion groups. The median total duration of hospital stay was 29 (i.q.r. 20–43), 22 (15–35), 30 (20–45), and 20 (12–30) days respectively. Some 61.6, 34.9, 42.8, and 36.1 per cent of patients respectively were admitted to ICU within 1 year of index surgery, for a median of 4 (2–10), 3 (1–6), 3 (1–6), and 3 (1–7) days. If an anastomosis was present, median time to healing of the leak in the four groups was 148 (77–260), 154 (83–252), 155 (92–224), and 125 ( 38–251) days.
Table 2.
Outcomes for the four predefined treatment strategies
| Salvage surgery (n = 388) |
Faecal diversion + passive drainage (n = 1524) |
Faecal diversion + active drainage (n = 278) |
No faecal diversion (n = 280) |
|
|---|---|---|---|---|
| 1-year stoma-free survival | 53 (13.7) | 736 (48.3) | 134 (48.2) | 183 (65.4) |
| Patients requiring secondary salvage surgery | – | 79 (5.2) | 29 (10.4) | 8 (2.9) |
| No. of secondary anastomoses created | 48 (12.4) | 28 (1.8) | 6 (2.2) | 1 (0.4) |
| Total duration of hospital stay within 1 year (days), median (i.q.r.) | 29 (20–43) | 22 (15–35) | 30 (20–45) | 20 (12–30) |
| Patients admitted to ICU within 1 year | 239 (61.6) | 532 (34.9) | 119 (42.8) | 101 (36.1) |
| Duration of ICU stay within 1 year (days), median (i.q.r) | 4 (2–10) | 3 (1–6) | 2 (1–4) | 3 (1–7) |
| Time to healing AL within 1 year (days), median (i.q.r.) | 148 (77–260) | 154 (83–252) | 155 (92–224) | 125 (38–251) |
Values are n (%) unless otherwise indicated. AL, anastomotic leak.
Outcomes after passive versus active drainage
Baseline characteristics of patients with a diverted leak who were treated with either primary passive drainage or primary active drainage, before and after PSM, are presented in Table 3. Several proportional differences with an SMD exceeding 0.1 were observed between the two groups before matching. After PSM, all co-variates had an SMD below 0.1, and were considered to be sufficiently balanced between the two cohorts (Fig. S1).
Table 3.
Baseline characteristics for groups with passive and active drainage, before and after propensity score matching
| Before PSM | After PSM* | |||||
|---|---|---|---|---|---|---|
| Faecal diversion + passive drainage (n = 1524) |
Faecal diversion + active drainage (n = 278) |
SMD | Faecal diversion + passive drainage (n = 556) |
Faecal diversion + active drainage (n = 278) |
SMD | |
| Age (years), median (i.q.r) | 66 (57–71) | 64 (56–72)) | 0.068 | 65 (57–73) | 64 (56–72) | 0.018 |
| Sex ratio (M : F) | 1104 : 420 | 229 : 49 | 0.239 | 455 : 101 | 229 : 49 | 0.014 |
| ASA fitness grade | 0.089 | 0.035 | ||||
| I | 246 (16.4) | 47 (17.3) | 100 (18.0) | 47 (16.9) | ||
| II | 863 (57.7) | 165 (60.7) | 331 (59.5) | 170 (61.2) | ||
| III–IV | 387 (25.9) | 60 (22.1) | 125 (22.5) | 61 (21.9) | ||
| Missing | 28 | 6 | 0 | 0 | ||
| BMI (kg/m²) | 0.156 | 0.052 | ||||
| Underweight (< 18.5) | 68 (4.8) | 8 (3.0) | 17 (3.1) | 8 (2.9) | ||
| Normal (18.5–24.9) | 449 (31.9) | 88 (32.8) | 178 (32.0) | 92 (33.1) | ||
| Overweight (25.0–29.9) | 599 (42.6) | 103 (38.4) | 221 (39.7) | 104 (37.4) | ||
| Obese (≥ 30.0) | 291 (20.7) | 69 (25.7) | 140 (25.2) | 74 (26.6) | ||
| Missing | 117 | 10 | 0 | 0 | ||
| Neoadjuvant therapy | 0.073 | 0.037 | ||||
| None | 575 (37.7) | 111 (39.9) | 215 (38.7) | 111 (39.9) | ||
| Radiotherapy only | 202 (13.3) | 37 (13.3) | 78 (14.0) | 37 (13.3) | ||
| Chemotherapy | 23 (1.5) | 6 (2.2) | 14 (2.5) | 6 (2.2) | ||
| Chemoradiation | 724 (47.5) | 124 (44.6) | 249 (44.8) | 124 (44.6) | ||
| Clinical metastasis category | 0.036 | 0.029 | ||||
| cM0 | 1202 (91.4) | 234 (91.1) | 493 (88.7) | 252 (89.6) | ||
| cM1 | 113 (8.6) | 23 (8.9) | 63 (11.3) | 29 (10.4) | ||
| Missing | 209 | 21 | 0 | 0 | ||
| Clinical setting of AL diagnosis | 0.281 | 0.062 | ||||
| Surgical ward | 1030 (67.6) | 182 (65.5) | 376 (67.6) | 182 (65.5) | ||
| ICU/HC | 50 (3.3) | 11 (4.0) | 20 (3.6) | 11 (4.0) | ||
| Emergency department | 149 (9.8) | 28 (10.1) | 47 (7.5) | 28 (10.1) | ||
| Outpatient clinic | 294 (19.3) | 57 (20.5) | 113 (20.3) | 57 (20.5) | ||
| Missing | 1 | 0 | 0 | 0 | ||
| POD of AL diagnosis, median (i.q.r) | 8 (5–20) | 10 (5–19) | 0.181 | 9 (5–19) | 10 (5–19) | 0.040 |
| Abdominal approach | 0.283 | 0.033 | ||||
| Laparoscopic | 962 (63.1) | 170 (61.2) | 347 (62.4) | 170 (61.2) | ||
| Robot-assisted | 134 (8.8) | 53 (19.1) | 103 (18.5) | 55 (19.8) | ||
| Laparotomy | 428 (28.1) | 55 (19.8) | 106 (19.1) | 53 (19.1) | ||
| Transanal TME | 284 (18.6) | 77 (27.7) | 0.179 | 151 (27.2) | 77 (27.7) | 0.012 |
| Multivisceral resection | 0.080 | 0.049 | ||||
| Yes | 96 (6.3) | 16 (5.8) | 26 (4.7) | 16 (5.8) | ||
| Missing | 35 | 7 | 0 | 0 | ||
| Primary defunctioning stoma | 1214 (79.2) | 213 (76.6) | 0.074 | 439 (79.0) | 213 (76.6) | 0.056 |
| Fistula | 0.154 | 0.039 | ||||
| Yes | 101 (3.2) | 9 (3.3) | 22 (4.0) | 9 (3.2) | ||
| Missing | 72 | 7 | 0 | 0 | ||
| Retraction of afferent colon | 0.268 | 0.059 | ||||
| Yes | 39 (3.2) | 18 (7.8) | 41 (7.4) | 25 (9.0) | ||
| Missing | 324 | 48 | 0 | 0 | ||
| Abdominal contamination | 0.535 | 0.004 | ||||
| Yes | 428 (30.7) | 50 (20.7) | 121 (21.8) | 60 (21.6) | ||
| Missing | 131 | 37 | 0 | 0 | ||
| Bowel wall ischaemia | 0.041 | 0.039 | ||||
| Yes | 91 (7.4) | 21 (8.5) | 44 (7.9) | 25 (9.0) | ||
| Missing | 292 | 31 | 0 | 0 | ||
| Anastomotic defect circumference (%) | 0.629 | 0.059 | ||||
| 0–25 | 368 (62.8) | 80 (48.5) | 292 (52.5) | 138 (49.6) | ||
| 25–50 | 145 (24.7) | 60 (36.4) | 179 (32.2) | 96 (34.5) | ||
| 50–100 | 73 (12.5) | 25 (15.2) | 85 (15.3) | 44 (15.8) | ||
| Missing | 938 | 113 | 0 | 0 | ||
| Reactivation leakage | 0.129 | 0.068 | ||||
| Yes | 120 (10.1) | 16 (8.2) | 57 (10.3) | 23 (8.3) | ||
| Missing | 333 | 84 | 0 | 0 | ||
| Severe clinical symptoms | 0.214 | 0.033 | ||||
| Yes | 312 (26.2) | 39 (17.4) | 93 (16.7) | 50 (18.0) | ||
| Missing | 334 | 54 | 0 | 0 | ||
Values are n (%) unless otherwise indicated. *Data shown after propensity score matching (PSM) originate from 1 randomly selected data set out of 100 imputation sets. SMD, standardized mean difference; AL, anastomotic leak; HC, high care; POD, postoperative day; TME, total mesorectal excision.
Table 4 shows the raw outcomes for passive and active drainage of the perianastomotic abscess in patients with faecal diversion, before and after matching. The results of the matched comparative analysis between passive and active drainage in patients with faecal diversion are summarized in Table 5. There was no statistically significant difference in 1-year stoma-free survival (RD −1.1 (95 per cent c.i. −9 to 7) per cent; OR 0.95, 95 per cent c.i. 0.69 to 1.33). After faecal diversion with active drainage, significantly more patients required secondary salvage surgery (RD 5.0 (0.8 to 9) per cent; OR 2.32, 1.49 to 3.59) and ICU admission (RD 8.0 (0.4 to 16) per cent; OR 1.41, 1.02 to 1.94). The mean total duration of hospital stay within 1 year was 36 days following faecal diversion with active drainage, and 30 days after faecal diversion with passive drainage, which differed significantly by 6 (95 per cent c.i. 2 to 10) days. There was no statistically significant difference in the number of secondary anastomoses created (RD 0.05 (−2 to 2) per cent; OR 1.04, 0.36 to 3.02). The mean total duration of ICU stay was 6 days after faecal diversion with active drainage and 5 days after faecal diversion with passive drainage, with no significant difference (1 (−2 to 3) days). The mean time to healing of the leak was 234 days after faecal diversion with active drainage, and 222 days after faecal diversion with passive drainage, again with no significant difference (12 (−28 to 52) days).
Table 4.
Outcomes for treatment strategies before and after multiple imputation
| Before PSM | After PSM* | |||
|---|---|---|---|---|
| Faecal diversion + passive drainage (n = 1524) |
Faecal diversion + active drainage (n = 278) |
Faecal diversion + passive drainage (n = 556) |
Faecal diversion + active drainage (n = 278) |
|
| 1-year stoma-free survival | 736 (48.3) | 134 (48.2) | 280 (50.4) | 135 (48.6) |
| Patients requiring secondary salvage surgery | 79 (5.2) | 29 (10.4) | 24 (4.3) | 29 (10.4) |
| No. of secondary anastomoses created | 28 (1.8) | 6 (2.2) | 12 (2.2) | 6 (2.2) |
| Total duration of hospital stay within 1 year (days), median (i.q.r.) | 22 (15–35) | 30 (20–45) | 22 (16–36) | 30 (21–45) |
| Patients admitted to ICU within 1 year | 532 (34.9) | 119 (42.8) | 193 (34.7) | 119 (42.8) |
| Duration of ICU stay within 1 year (days), median (i.q.r.) | 3 (1–6) | 2 (1–4) | 0 (0–1) | 0 (0–2) |
| Time to healing AL within 1 year (days), median (i.q.r.) | 154 (83–252) | 155 (92–224) | 145 (90–219) | 152 (90–217) |
Values are n (%), unless otherwise indicated. *Data shown after propensity score matching (PSM) originate from 1 randomly selected data set out of 100 imputation sets. AL, anastomotic leak.
Table 5.
Outcomes after faecal diversion and active drainage compared with faecal diversion and passive drainage, after propensity score matching
| Faecal diversion + active drainage versus faecal diversion + passive drainage (reference)* | |
|---|---|
| 1-year stoma-free survival | |
| Risk difference (%) | −1.1 (−9, 7) |
| OR | 0.95 (0.69, 1.33) |
| Secondary salvage surgery | |
| Risk difference (%) | 5.0 (0.8, 9) |
| OR | 2.32 (1.49, 3.59) |
| Secondary anastomosis | |
| Risk difference (%) | 0.05 (−2, 2) |
| OR | 1.04 (0.36, 3.02) |
| Total duration of hospital stay within 1 year (days) | 6 (2, 10) |
| ICU admission within 1 year | |
| Risk difference (%) | 8.0 (0.4, 16) |
| OR | 1.41 (1.02, 1.94) |
| Duration of ICU stay within 1 year (days) | 1 (−2, 3) |
| Time to healing AL within 1 year (days) | 12 (−28, 52) |
Values in parentheses are 95% confidence intervals. *Derived from logistic and linear regression; results from linear regression are differences in means. AL, anastomotic leak.
Sensitivity analysis assessing the effect of annual case volume
The effect of annual procedure volume was assessed in a sensitivity analysis (Tables S2 and S3). After including annual procedure volume in the multivariable logistic regression modelling, no statistically significant differences were observed in 1-year stoma-free survival between treatment strategies (RD −1.6 (95 per cent c.i. −10 to 6) per cent; OR 0.94, 95 per cent c.i. 0.68 to 1.29). In addition, all secondary outcomes remained comparable to those in the initial analysis without inclusion of annual procedure volume, confirming the robustness of the analysis (Tables S4 and S5).
Discussion
This large retrospective international multicentre study explored four predefined treatment strategies for AL after restorative rectal cancer resection. Substantial differences were found in several leak characteristics between the four treatment strategies. Primary salvage surgery resulted in a 1-year stoma-free survival rate of 14 per cent, reflecting the severity of these leaks, whereas non-diverted leaks had a rate of 65 per cent. Patients with a diverted leak who underwent active drainage (EVT) had worse leak characteristics than those who had passive drainage of the perianastomotic abscess. After matching, there was no statistically significant difference in 1-year stoma-free survival, but patients treated with EVT more frequently underwent secondary salvage surgery, were more often admitted to the ICU, and had a longer total hospital stay.
Primary salvage surgery was performed in 16 per cent of the total cohort. Although the proportion of redo anastomoses was highest in this group (12 per cent), salvage surgery mostly consisted of dismantling the anastomosis, which explains the low 1-year stoma-free survival rate. Salvage surgery is sometimes the only option (for example, in the event of ischaemic afferent colon). In other instances, immediately deciding that preservation of bowel continuity is no longer the ultimate goal can be a wise decision. This might prevent a patient having a long period of treatment that ultimately ends in a permanent stoma anyway. Conversely, there might have been a subgroup of anastomoses that could have been preserved using an alternative strategy. Further studies are necessary to identify these specific patients.
The group of patients who did not undergo primary or secondary faecal diversion had the highest stoma-free survival rate. Surgeons likely decided this most conservative strategy based on favourable patient and leak characteristics. Remarkably, this group also included several patients with adverse leak characteristics. The abdominal cavity was contaminated in 32 per cent, and an ischaemic afferent colon was identified in 6 per cent. There is inherent heterogeneity within this group, which includes patients with only purulent fluid and an early sealed leak that can be effectively treated with laparoscopic lavage, as well as those with sepsis and substantial anastomotic dehiscence and four-quadrant peritonitis that require more aggressive management.
Over the past few decades, minimally invasive active treatment strategies, such as EVT, have emerged as an alternative to major surgery21,22,36. EVT is indicated for extraperitoneal AL, and consists of endoscopic placement of open-cell polyurethane sponges connected to a continuous negative pressure system for drainage and debridement of the perianastomotic abscess. EVT can be used either as a single modality or combined with transanal closure of the defect21,37,38. The present study failed to show differences between EVT and passive drainage in patients with diverted leaks in terms of 1-year stoma-free survival after AL, which could be explained by several factors. First, early diagnosis of AL and initiation of EVT is crucial for its success, as the neorectum is still pliant and not impaired by chronic inflammation16,22. Borstlap et al.16 showed that, if EVT is initiated within 3 weeks of index surgery, it can lead to acceptable anastomotic healing rates of 73.0 per cent, with corresponding rates of restoration of continuity. A similar trend was observed by the GRECCAR group39, which showed significantly improved anastomotic healing when EVT was initiated within 15 days of AL compared with after 15 days (72.4 versus 27.8 per cent). In the present study, no differentiation was made between early and late initiation of EVT. These results reflect a non-trial setting with application of EVT in non-specialized centres as well. Optimizing EVT treatment with early commencement and combining it with surgical closure could potentially have yielded better outcomes; however, this remains to be proven. Furthermore, the GRECCAR group39 identified predictive factors for success of EVT, and showed significantly lower success rates in patients who underwent percutaneous transgluteal drainage before initiating EVT. As a result of the present retrospective study design, patients who were referred for EVT after a failed primary passive drainage strategy would have been registered as having primary active drainage.
No RCTs have yet been performed to establish robust evidence for the effectiveness of EVT36, and comparative studies are scarce. A study by Kühn et al.25 compared patients who underwent EVT with a conventionally treated historical cohort, showing advantageous outcomes for EVT in terms of restoration of continuity (86.7 versus 37.5 per cent). Another comparative study was undertaken by Eriksen et al.40, who reported significantly higher stoma rates 1 year after EVT compared with conventional management (33.9 versus 13.5 per cent). This could also explain the higher rates of secondary salvage surgery and ICU admission in the present study, as patients treated with EVT had larger anastomotic defects and retraction at baseline compared with those treated conventionally. This hypothesis implies that patients who underwent conventional treatment might have had more favourable baseline leak characteristics. The high rates of secondary salvage surgery could therefore potentially be attributed to the use of EVT to control pelvic sepsis in patients with a large anastomotic dehiscence or significant retraction of the afferent colon. Subsequently, these patients undergo complete dismantling of the anastomosis with creation of a permanent end-colostomy or redo surgery. This course of action may be chosen owing to the unsuitability of EVT as a treatment strategy in these specific situations41. Furthermore, salvage surgery can be complex and might necessitate ICU admission or additional readmissions to hospital. Another plausible explanation for these findings could be attributed to matching, a process carried out using previously identified confounding factors for stoma-free survival33. Although matching was undertaken to create a homogeneous population with comparable characteristics, the multifaceted nature of stoma-free survival as an outcome introduces complexity, and residual bias may have remained.
Previous studies were impeded by a heterogeneous patient population and small sample sizes23, whereas the present study encompassed a large sample with robust and detailed data. However, several limitations should be discussed. First, the retrospective study design could have contributed to inconsistencies and missing data. To overcome this issue, data verification and validation was performed. Some missing data remained, but statistical power was preserved by imputing the missing data through multiple imputation31,32. Second, participating centres had to include their own patients retrospectively and it is anticipated that not all primary treatment strategies were reported correctly. Third, not all centres from a defined geographical region participated in this study, potentially introducing selection bias from differing referral patterns as it is expected that participating centres are more likely to consist of academic centres, potentially impairing external validity.
In conclusion, this large worldwide cohort study has provided detailed insights into patient and leak characteristics of four predefined treatment strategies for AL after restorative rectal cancer surgery. The 1-year stoma-free survival rate was low for patients undergoing primary salvage surgery and high in those with non-diverted anastomoses, and did not differ significantly between matched patients treated with faecal diversion and either EVT or passive drainage. Nonetheless, significantly more patients required secondary salvage surgery and ICU admission within 1 year of EVT, indicating a potential allocation bias.
Supplementary Material
Acknowledgements
The authors thank all collaborators in the TENTACLE-Rectum Collaborative Group who were involved in the study for their efforts and contribution to this large, international collaborative study.
Contributor Information
Nynke G Greijdanus, Department of Surgery, Radboud University Medical Centre, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
Kiedo Wienholts, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Treatment and Quality of Life, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Imaging and Biomarkers, Amsterdam, the Netherlands.
Sander Ubels, Department of Surgery, Radboud University Medical Centre, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
Kevin Talboom, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Treatment and Quality of Life, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Imaging and Biomarkers, Amsterdam, the Netherlands.
Gerjon Hannink, Department of Medical Imaging, Radboud University Medical Centre, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
Albert Wolthuis, Department of Surgery, UZ Leuven, Leuven, Belgium.
F Borja de Lacy, Gastrointestinal Surgery Department, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Jérémie H Lefevre, Department of Digestive Surgery, Sorbonne Université, AP-HP, Hôpital Saint Antoine, Paris, France.
Michael Solomon, Department of Surgery, University of Sydney Central Clinical School, Camperdown, New South Wales, Australia.
Matteo Frasson, Department of Surgery, Valencia University Hospital La Fe, Valencia, Spain.
Nicolas Rotholtz, Department of Surgery, Hospital Alemán, Buenos Aires, Argentina.
Quentin Denost, Bordeaux Colorectal Institute, Clinique Tivoli, Bordeaux, France.
Rodrigo O Perez, Colorectal Surgery, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil.
Tsuyoshi Konishi, Department of Colon and Rectal Surgery, University of Texas MD Anderson Cancer Center, Anderson, Texas, USA.
Yves Panis, Colorectal Surgery Centre, Groupe Hospitalier Privé Ambroise Paré-Hartmann, Neuilly Seine, France.
Martin Rutegård, Surgical and Perioperative Sciences, Surgery, Umeå University, Umeå, Sweden; Wallenberg Centre for Molecular Medicine, Umeå University, Umeå, Sweden.
Roel Hompes, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Treatment and Quality of Life, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Imaging and Biomarkers, Amsterdam, the Netherlands.
Camiel Rosman, Department of Surgery, Radboud University Medical Centre, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
Frans van Workum, Department of Surgery, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands.
Pieter J Tanis, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Treatment and Quality of Life, Amsterdam, the Netherlands; Cancer Centre Amsterdam, Imaging and Biomarkers, Amsterdam, the Netherlands; Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus Medical Centre, Rotterdam, the Netherlands.
Johannes H W de Wilt, Department of Surgery, Radboud University Medical Centre, Radboud Institute for Health Sciences, Nijmegen, the Netherlands.
TENTACLE-Rectum Collaborative Group:
Andreas J A Bremers, Floris T Ferenschild, Stefanie de Vriendt, André D’Hoore, Gabriele Bislenghi, Jordi Farguell, Antonio M Lacy, Paula González Atienza, Charlotte S van Kessel, Yann Parc, Thibault Voron, Maxime K Collard, Jorge Sancho Muriel, Hannia Cholewa, Laura A Mattioni, Alice Frontali, Sebastiaan W Polle, Fatih Polat, Ndidi J Obihara, Bruna B Vailati, Miranda Kusters, Jurriaan B Tuynmann, Sanne J A Hazen, Alexander A J Grüter, Takahiro Amano, Hajime Fujiwara, Mario Salomon, Hernán Ruiz, Ricardo Gonzalez, Diego Estefanía, Nicolas Avellaneda, Augusto Carrie, Mateo Santillan, Diana A Pantoja Pachajoa, Matias Parodi, Manuel Gielis, Alf-Dorian Binder, Thomas Gürtler, Peter Riedl, Sarit Badiani, Christophe Berney, Matthew Morgan, Paul Hollington, Nigel da Silva, Gavin Nair, Yiu M Ho, Michael Lamparelli, Raj Kapadia, Hidde M Kroon, Nagendra N Dudi-Venkata, Jianliang Liu, Tarik Sammour, Nicolas Flamey, Paul Pattyn, Ahmed Chaoui, Louis Vansteenbrugge, Nathalie E J van den Broek, Patrick Vanclooster, Charles de Gheldere, Pieter Pletinckx, Barbara Defoort, Maxime Dewulf, Mihail Slavchev, Nikolay Belev, Boyko Atanasov, Panche Krastev, Manol Sokolov, Svilen Maslyankov, Petar Gribnev, Vasil Pavlov, Tsvetomir Ivanov, Martin Karamanliev, Emil Filipov, Pencho Tonchev, Felix Aigner, Martin Mitteregger, Caterina Allmer, Gerald Seitinger, Nicola Colucci, Nicolas Buchs, Frédéric Ris, Christian Toso, Eleftherios Gialamas, Aurélie Vuagniaux, Roland Chautems, Marc-Olivier Sauvain, Silvio Daester, Markus von Flüe, Marc-Olivier Guenin, Stephanie Taha-Mehlitz, Gabriel F Hess, Lubomír Martínek, Matej Skrovina, Maria Machackova, Vladimir Benčurik, Deniz Uluk, Johann Pratschke, Luca S Dittrich, Safak Guel-Klein, Daniel Perez, Julia-Kristin Grass, Nathaniel Melling, Simone Mueller, Lene H Iversen, Jacob D Eriksen, Gunnar Baatrup, Issam Al-Najami, Thomas Bjørsum-Meyer, Jüri Teras, Roland M Teras, Fatma A Monib, Nagm Eldin Abu Elnga Ahmed, Eithar Alkady, Ahmed K Ali, Gehan Abd Elatti Khedr, Ahmed Samir Abdelaal, Fouad M Bassyouni Ashoush, Moataz Ewedah, Eslam M Elshennawy, Mohamed Hussein, Daniel Fernández-Martínez, Luis J García-Flórez, María Fernández-Hevia, Aida Suárez-Sánchez, Izaskun del Hoyo Aretxabala, Iria Losada Docampo, Jesús Gómez Zabala, Patricia Tejedor, Javier T Morales Bernaldo de Quirós, Ignacio Bodega Quiroga, Antonio Navarro-Sánchez, Iván Soto Darias, Cristina López Fernández, Cristina de La Cruz Cuadrado, Luis Sánchez-Guillén, Francisco López-Rodríguez-Arias, Álvaro Soler-Silva, Antonio Arroyo, Juan C Bernal-Sprekelsen, Segundo Á Gómez-Abril, Paula Gonzálvez, María T Torres, Teresa Rubio Sánchez, Francisco Blanco Antona, Juan E Sánchez Lara, José A Alcázar Montero, Fernando Mendoza-Moreno, Manuel Díez-Alonso, Belén Matías-García, Ana Quiroga-Valcárcel, Enrique Colás-Ruiz, Marta M Tasende-Presedo, Ignacio Fernández-Hurtado, José A Cifuentes-Ródenas, Marta Castro Suárez, Manuel Losada, Miguel Hernández, Alfredo Alonso, Beatriz Diéguez, Daniel Serralta, Rita E Medina Quintana, Jose M Gil Lopez, Francisca Lima Pinto, Elena Nieto-Moreno, Alba Correa Bonito, Carlos Cerdán Santacruz, Elena Bermejo Marcos, Javier García Septiem, Aránzazu Calero-Lillo, Javier Alanez-Saavedra, Salvador Muñoz-Collado, Manuel López-Lara, María Labalde Martínez, Eduardo Ferrero Herrero, Francisco Javier García Borda, Óscar García Villar, Jorge Escartín, Juan L Blas, Rocío Ferrer, Jorge García Egea, Antonio Rodríguez-Infante, Germán Mínguez-Ruiz, Guillermo Carreño-Villarreal, Gerardo Pire-Abaitua, Jana Dziakova, Carlos Sáez-Cazallas Rodríguez, María J Pizarro Aranda, José M Muguerza Huguet, Nerea Borda-Arrizabalaga, José M Enriquez-Navascués, Garazi Elorza Echaniz, Yolanda Saralegui Ansorena, Mercedes Estaire-Gómez, Carlos Martínez-Pinedo, Alejandro Barbero-Valenzuela, Pablo Ruíz-García, Miquel Kraft, María J Gómez-Jurado, Gianluca Pellino, Eloy Espín-Basany, Eddy Cotte, Nathalie Panel, Claire-Angéline Goutard, Nicola deÁngelis, Lelde Lauka, Shafaque Shaikh, Laura Osborne, George Ramsay, Vladimir-Ion Nichita, Santosh Bhandari, Panchali Sarmah, Rob M Bethune, Heather C M Pringle, Lisa Massey, George E Fowler, Hytham K S Hamid, Belinda D de Simone, James Kynaston, Nicholas Bradley, Roxane M Stienstra, Shashank Gurjar, Tanmoy Mukherjee, Ashfaq Chandio, Safia Ahmed, Baljit Singh, Francois Runau, Sanjay Chaudhri, Oliver Siaw, Janahan Sarveswaran, Victor Miu, Daniel Ashmore, Haitham Darwich, Deepak Singh-Ranger, Nirbhaibir Singh, Mohamed Shaban, Fahed Gareb, Thalia Petropolou, Adreas Polydorou, Mit Dattani, Asma Afzal, Akshay Bavikatte, Boby Sebastian, Nicholas Ward, Amitabh Mishra, Dimitrios Manatakis, Christos Agalianos,Nikolaos Tasis, Maria-Ioanna Antonopoulou, Ioannis Karavokyros, Alexandros Charalabopoulos, Dimitrios Schizas, Efstratia Baili, Athanasios Syllaios, Lysandros Karydakis, Michail Vailas, Dimitrios Balalis, Dimitrios Korkolis, Aris Plastiras, Aliki Rompou, Sofia Xenaki, Evangelos Xynos, Emmanuel Chrysos, Maria Venianaki, Grigorios Christodoulidis, Konstantinos Perivoliotis, George Tzovaras, Ioannis Baloyiannis, Man-Fung Ho, Simon Siu-man Ng, Tony Wing-chung Mak, Kaori Futaba, Goran Šantak, Damir Šimleša, Jurica Ćosić, Goran Zukanović, Michael E Kelly, John O Larkin, Paul H McCormick, Brian J Mehigan, Tara M Connelly, Peter Neary, Jessica Ryan, Peter McCullough, Maytham A Al-Juaifari, Hayder Hammoodi, Ali Hashim Abbood, Marcello Calabrò, Andrea Muratore, Antonio La Terra, Francesca Farnesi, Carlo V Feo, Nicolò Fabbri, Antonio Pesce, Marta Fazzin, Francesco Roscio, Federico Clerici, Andrea Lucchi, Laura Vittori, Laura Agostinelli, Maria Cristina Ripoli, Daniele Sambucci, Andrea Porta, Giovanni Sinibaldi, Giacomo Crescentini, Antonella larcinese, Emanuele Picone, Roberto Persiani, Alberto Biondi, Roberto Pezzuto, Laura Lorenzon, Gianluca Rizzo, Claudio Coco, Luca D’Agostino, Antonino Spinelli, Matteo M Sacchi, Michele Carvello, Caterina Foppa, Antonino Spinelli, Matteo M Sacchi, Michele Carvello, Caterina Foppa, Annalisa Maroli, Gian M Palini, Gianluca Garulli, Nicola Zanini, Paolo Delrio, Daniela Rega, Fabio Carbone, Alessia Aversano, Giovanni Pirozzolo, Alfonso Recordare, Lucrezia D’Alimonte, Chiara Vignotto, Carlo Corbellini, Gianluca M Sampietro, Leonardo Lorusso, Carlo A Manzo, Federico Ghignone, Giampaolo Ugolini, Isacco Montroni, Franceso Pasini, Francesco Pasini, Michele Ballabio, Pietro Bisagni, Francesca T Armao, Marco Longhi, Omar Ghazouani, Raffaele Galleano, Nicolò Tamini, Massimo Oldani, Luca Nespoli, Arcangelo Picciariello, Donato F Altomare, Giovanni Tomasicchio, Giuliano Lantone, Fausto Catena, Mario Giuffrida, Alfredo Annicchiarico, Gennaro Perrone, Ugo Grossi, Giulio A Santoro, Giacomo Zanus ,Alessandro Iacomino, Simone Novello, Nicola Passuello, Martino Zucchella, Lucia Puca, Maurizio deGiuli, Rossella Reddavid, Stefano Scabini, Alessandra Aprile, Domenico Soriero, Emanuela Fioravanti, Matteo Rottoli, Angela Romano, Marta Tanzanu, Angela Belvedere, Nicolò M Mariani, Andrea P Ceretti, Enrico Opocher, Gaetano Gallo, Giuseppe Sammarco, Gilda de Paola, Salvatore Pucciarelli, Francesco Marchegiani, Gaya Spolverato, Gianluca Buzzi, Salomone Di Saverio, Paola Meroni, Cristiano Parise, Elisa I Bottazzoli, Pierfrancesco Lapolla, Gioia Brachini, Bruno Cirillo, Andrea Mingoli, Giuseppe Sica, Leandro Siragusa, Vittoria Bellato, Daniele Cerbo, Carlo A de Pasqual, Giovanni de Manzoni, Maria A di Cosmo, Bourhan M H Alrayes, Mahmoud W M Qandeel, Mohammad Bani Hani, Alexander Rabadi, Mohammad S el Muhtaseb, Basel Abdeen, Fahed Karmi, Justas Žilinskas, Tadas Latkauskas, Algimantas Tamelis, Ingrida Pikūnienė, Vygintas Šlenfuktas, Tomas Poskus, Marius Kryzauskas, Matas Jakubauskas, Saulius Mikalauskas, Lina Jakubauskiene, Soha Y Hassan, Amani Altrabulsi, Eman Abdulwahed, Reem Ghmagh, Abdulqudus Deeknah, Entisar Alshareea, Muhammed Elhadi, Saleh Abujamra, Ahmed A Msherghi, Osama W E Tababa, Mohammed A Majbar, Amine Souadka, Amine Benkabbou, Raouf Mohsine, Sabrillah Echiguer, Paulina Moctezuma-Velázquez, Noel Salgado-Nesme, Omar Vergara-Fernández, Juan C Sainz-Hernández, Francisco E Alvarez-Bautista, Andee D Zakaria, Zaidi Zakaria, Michael P K Wong, Razif Ismail, Aini F Ibrahim, Nik A N Abdullah, Rokayah Julaihi, Sameer Bhat, Greg O’Grady, Ian Bissett, Bas Lamme, Gijsbert D Musters, Anne M Dinaux, Brechtje A Grotenhuis, Ernst J Steller Arend G J Aalbers, Marjolein M Leeuwenburgh, Harm J T Rutten, Jacobus W A Burger, Johanne G Bloemen, Stijn H J Ketelaers, Usama Waqar, Tabish Chawla, Hareem Rauf, Pallavi Rani, Aaldert K Talsma, Lieke Scheurink, Jasper B van Praagh, Josefin Segelman, Jonas Nygren, Kajsa Anderin, Marit Tiefenthal, Beatriz de Andrés, Juan P Beltrán de Heredia, Andrea Vázquez, Tania Gómez, Parisa Golshani, Rawaz Kader, Abudi Mohamed, Marinke Westerterp, Andreas Marinelli, Quirine Niemer, Pascal G Doornebosch, Joël Shapiro, Maarten Vermaas, Eelco J R de Graaf, Hendrik L van Westreenen, Marije Zwakman, Annette D van Dalsen, Wouter J Vles, Joost Nonner, Boudewijn R Toorenvliet, Paul T J Janssen, Emiel G G Verdaasdonk, Femke J Amelung, Koen C M J Peeters Renu R Bahadoer, Fabian A Holman, Jeroen Heemskerk, Noortje Vosbeek, Jeroen W A Leijtens, Sophie B M Taverne, Bob H M Heijnen, Youssef El-Massoudi, Irene de Groot-van Veen, Christiaan Hoff, Daniela Jou-Valencia, Esther C J Consten Thijs A Burghgraef, Ritch Geitenbeek, Lorenzo G W L Hulshof, Gerrit D Slooter, Muriël Reudink, Nicole D Bouvy, Aurelia C L Wildeboer, Sonja Verstappen, Alexander J Pennings, Berber van den Hengel, Allard G Wijma, Jael de Haan, Lindsey C F de Nes, Vera Heesink, Tom Karsten, Charlotte M Heidsma, Willem J Koemans, Jan-Willem T Dekker, Charlène J van der Zijden, Daphne Roos, Ahmet Demirkiran, Sjirk van der Burg, Steven J Oosterling, Tijs J Hoogteijling, Bastiaan Wiering, Diederik P J Smeeing, Klaas Havenga, Hamid Lutfi, Esther C J Consten, Konstantinos Tsimogiannis, Filip Sköldberg, Joakim Folkesson, Frank den Boer, Ted G van Schaik, Pieter van Gerven, Colin Sietses, Jeroen C Hol, Evert-Jan G Boerma, Davy M J Creemers, Johannes K Schultz, Tone Frivold, Rolf Riis, Hilde Gregussen, Sondre Busund, Ole H Sjo, Maria Gaard, Nina Krohn, Amanda L Ersryd, Edmund Leung, Usama Waqar, Tabish Chawla, Hareem Rauf, Pallavi Rani, Hytham Sultan, Baraa Nabil Hajjaj, Ahmed Jehad Alhisi, Ahmed A E Khader, Ana Filipa Dias Mendes, Miguel Semião, Luis Queiroz Faria, Constança Azevedo, Helena M da Costa Devesa, Sónia Fortuna Martins, Aldo M Rodrigues Jarimba, Sónia M Ribeiro Marques, Rita Marques Ferreira, António Oliveira, Cátia Ferreira, Ricardo Pereira, Valeriu M Surlin, Giorgiana M Graure, Stefan Patrascu Sandu D Ramboiu, Ionut Negoi, Cezar Ciubotaru, Bogdan Stoica, Ioan Tanase, Bogdan Stoica, Cezar Ciubotaru, Valentina M Negoita, Sabrina Florea, Florin Macau, Mihai Vasile, Victor Stefanescu, Gabriel-Mihail Dimofte, Sorinel Luncă, Cristian-Ene Roată, Ana-Maria Mușină, Tatiana Garmanova, Mikhail N Agapov, Daniil G Markaryan, Galliamov Eduard, Alexey Yanishev, Alexander Abelevich, Andrey Bazaev, Sergey V Rodimov, Victor B Filimonov, Andrey A Melnikov, Igor A Suchkov, Evgeniy S Drozdov, Dmitriy N Kostromitskiy, Olle Sjöström, Peter Matthiessen, Bayar Baban, Soran Gadan, Kaveh Dehlaghi Jadid, Maria Staffan, Jennifer M Park, Daniel Rydbeck, Marie-Louise Lydrup, Pamela Buchwald, Henrik Jutesten, Lotten Darlin, Ebba Lindqvist, Karl Nilsson, Per-Anders Larsson, Staffan Jangmalm, Jurij A Košir, Aleš Tomažič, Jan Grosek, Tajda Košir Božič, Aya Zazo, Rama Zazo, Hala Fares, Kusay Ayoub, Ammar Niazi, Ali Mansour, Ayman Abbas, Mohammad Tantoura, Alaa Hamdan, Naya Hassan, Bassam Hasan, Ahmad Saad, Amine Sebai, Anis Haddad, Houcine Maghrebi, Montasser Kacem, Ömer Yalkın, Mehmet Veysi Samsa, İbrahim Atak, Bengi Balci, Elifcan Haberal, Lütfi Dogan, Ibrahim E Gecim, Cihangir Akyol, Mehmet A Koc, Emre Sivrikoz, Deniz Piyadeoğlu, John O Larkin, Dara O avanagh, Selman Sökmen, Tayfun Bişgin, Erşan Günenç, Melek Güzel, Sezai Leventoğlu, Osman Yüksel, Ramazan Kozan, Hüseyin Göbüt, Fevzi Cengiz, Kemal Erdinc, Nihan Coşgun Acar, Erdinc Kamer, İlker Özgür, Oguzhan Aydın, Metin Keskin, Mehmet Türker Bulut, Cemil B Kulle, Yasin Kara, Osman Sıbıç, İbrahim H Özata, Dursun Buğra, Emre Balık, Cemil B Kulle, Murat Çakır, Anas Alhardan, Elif Colak, Ahmet B CiftciEngin Aybar, Ahmet Can Sari, Semra Demirli Atici, Tayfun Kaya, Ayberk Dursun, Bulent Calik, Ömer Faruk Özkan, Hanife Şeyda Ülgür, Özgül Düzgün, John Monson, Sarah George, Kayla Woods, Fatima Al-Eryani, Rudaina Albakry, Emile Coetzee, Adam Boutall, Ayesiga Herman, Claire Warden, Naser Mugla, Tim Forgan, Imraan Mia, and Anton Lambrechts
Collaborators
TENTACLE – Rectum Collaborators: Andreas J.A. Bremers, Floris T. Ferenschild (Radboud University Medical Centre, Radboud Institute for Health Sciences, Nijmegen, The Netherlands) Stefanie de Vriendt, André D’Hoore, Gabriele Bislenghi (University Hospitals Leuven, Leuven, Belgium); Jordi Farguell, Antonio M. Lacy, Paula González Atienza (Hospital Clínic de Barcelona, Barcelona, Spain); Charlotte S. van Kessel (Royal Prince Albert Hospital, Sydney, Australia); Yann Parc, Thibault Voron, Maxime K. Collard (Sorbonne Université, AP-HP, Hôpital Saint Antoine, Paris, France); Jorge Sancho Muriel, Hannia Cholewa (Valencia University Hospital La Fe, Valencia, Spain); Laura A. Mattioni (Hospital Alemán, Buenos Aires, Argentina); Alice Frontali (Beaujon Hospital, Clichy, and University of Paris, Clichy, France); Sebastiaan W. Polle, Fatih Polat, Ndidi J. Obihara (Canisius Wilhelmina Hospital, Nijmegen, the Netherlands); Bruna B. Vailati (Hospital Alemão Oswaldo Cruz, São Paulo, Brazil); Miranda Kusters, Jurriaan B. Tuynmann, Sanne J.A. Hazen, Alexander A.J. Grüter (Amsterdam University Medical Centers, location VUmc, Amsterdam, The Netherlands; Cancer Center Amsterdam, Treatment and Quality of Life, Amsterdam, The Netherlands; Cancer Center Amsterdam, Imaging and Biomarkers, Amsterdam, The Netherlands); Takahiro Amano, Hajime Fujiwara (Cancer Institute Hospital of the Japanese Foundation for Cancer Research, Tokyo, Japan); Mario Salomon, Hernán Ruiz, Ricardo Gonzalez, Diego Estefanía (Buenos Aires British Hospital, Buenos Aires, Argentina); Nicolas Avellaneda, Augusto Carrie, Mateo Santillan (CEMIC University Hospital, Buenos Aires, Argentina); Diana A. Pantoja Pachajoa, Matias Parodi, Manuel Gielis (Clínica Universitaria Reina Fabiola, Córdoba, Argentina); Alf-Dorian Binder, Thomas Gürtler, Peter Riedl (Universitätsklinikum Tulln, Tulln an der Donau, Austria); Sarit Badiani, Christophe Berney, Matthew Morgan (Bankstown-Lidcombe Hospital, Sydney, New South Wales, Australia); Paul Hollington, Nigel da Silva, Gavin Nair (Flinders Medical Centre, Adelaide, South Australia, Australia); Yiu M. Ho, Michael Lamparelli, Raj Kapadia (Rockhampton Hospital, Queensland, Australia); 19 Hidde M. Kroon, Nagendra N. Dudi-Venkata, Jianliang Liu, Tarik Sammour (Royal Adelaide Hospital, Adelaide, South Australia, Australia); Nicolas Flamey, Paul Pattyn, Ahmed Chaoui, Louis Vansteenbrugge (AZ Delta, Roeselare, Belgium); Nathalie E.J. van den Broek, Patrick Vanclooster, Charles de Gheldere (Heilig-Hartziekenhuis, Lier, Belgium); Pieter Pletinckx, Barbara Defoort, Maxime Dewulf (Maria Middelares Ghent, Belgium); Mihail Slavchev, Nikolay Belev, Boyko Atanasov, Panche Krastev (University Hospital Eurohospital - Medical University Plovdiv, Plovdiv, Bulgaria); Manol Sokolov, Svilen Maslyankov, Petar Gribnev, Vasil Pavlov (Aleksandrovska University Hospital, Sofia, Bulgaria); Tsvetomir Ivanov, Martin Karamanliev, Emil Filipov, Pencho Tonchev (Medical University Pleven, Pleven, Bulgaria); Felix Aigner, Martin Mitteregger, Caterina Allmer, Gerald Seitinger (St. John of God Hospital Graz, Graz, Austria); Nicola Colucci, Nicolas Buchs, Frédéric Ris, Christian Toso (Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland); Eleftherios Gialamas, Aurélie Vuagniaux, Roland Chautems, Marc-Olivier Sauvain (Neuchâtel Hospital, Neuchâtel, Switzerlandl); Silvio Daester, Markus von Flüe, Marc-Olivier Guenin, Stephanie Taha-Mehlitz, Gabriel F. Hess (St. Clara Hospital and University Hospital Basel, Basel, Switzerland); Lubomír Martínek, Matej Skrovina, Maria Machackova, Vladimir Benčurik (Hospital Nový Jičín, Nový Jičín, Czech Republic); Deniz Uluk, Johann Pratschke, Luca S. Dittrich, Safak Guel-Klein (Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin and Berlin Institut of Health, Berlin, Germany); Daniel Perez (Asclepios Clinic Altona, Hamburg, Germany); Julia-Kristin Grass, Nathaniel Melling, Simone Mueller (University Medical Centre of Hamburg-Eppendorf, Hamburg, Germany); Lene H. Iversen, Jacob D. Eriksen (Aarhus University Hospital, Aarhus, Denmark); Gunnar Baatrup, Issam Al-Najami, Thomas Bjørsum-Meyer (Odense University Hospital, Svendborg Sygehus, Denmark); Jüri Teras, Roland M. Teras (North Estonia Medical Centre Foundation, Tallinn, Estonia); Fatma A. Monib, Nagm Eldin Abu Elnga Ahmed, Eithar Alkady, Ahmed K. Ali (Assiut University Hospital, Assiut, Egypt); Gehan Abd Elatti Khedr, Ahmed Samir Abdelaal, Fouad M. Bassyouni Ashoush, Moataz Ewedah (Alexandria Main University Hospital, Alexandria Governorate, Egypt); Eslam M. Elshennawy, Mohamed Hussein (Kafr Elshikh University Hospital, Kafr el-Sheikh, Egypt); Daniel Fernández-Martínez, Luis J. García-Flórez, María Fernández-Hevia, Aida Suárez-Sánchez (Central University Hospital of Asturias, Asturias, Spain); Izaskun del Hoyo Aretxabala, Iria Losada Docampo, Jesús Gómez Zabala (Basurto University Hospital, Bilbao, Spain); Patricia Tejedor, Javier T. Morales Bernaldo de Quirós, Ignacio Bodega Quiroga (Hospital Universitario Gómez Ulla, Spain); Antonio Navarro-Sánchez, Iván Soto Darias, Cristina López Fernández, Cristina de La Cruz Cuadrado (Hospital Materno Infantil de Gran Canaria, Las Palmas, Spain); Luis Sánchez-Guillén, Francisco López-Rodríguez-Arias, Álvaro Soler-Silva, Antonio Arroyo (University Hospital of Elche, Elche, Spain); Juan C. Bernal-Sprekelsen, Segundo Á. Gómez-Abril, Paula Gonzálvez, María T. Torres (Hospital Universitario Dr. Peset, Valencia, Spain); Teresa Rubio Sánchez, Francisco Blanco Antona, Juan E. Sánchez Lara, José A. Alcázar Montero (University Hospital of Salamanca, Salamanca, Spain); Fernando Mendoza-Moreno, Manuel Díez-Alonso, Belén Matías-García, Ana Quiroga-Valcárcel (Hospital Universitario Príncipe de Asturias, Spain); Enrique Colás-Ruiz, Marta M. Tasende-Presedo, Ignacio Fernández-Hurtado, José A. Cifuentes-Ródenas, Marta Castro Suárez (Son Llàtzer Hospital, Illes Balears, Spain); Manuel Losada, Miguel Hernández, Alfredo Alonso, Beatriz Diéguez (Hospital Universitario del Sureste, Madrid, Spain); Daniel Serralta, Rita E. Medina Quintana, Jose M. Gil Lopez, Francisca Lima Pinto, Elena Nieto-Moreno (Hospital Infanta Leonor, San Sebastián de los Reyes, Madrid, Spain); Alba Correa Bonito, Carlos Cerdán Santacruz, Elena Bermejo Marcos, Javier García Septiem (University Hospital de La Princesa, Madrid, Spain); Aránzazu Calero-Lillo, Javier Alanez-Saavedra, Salvador Muñoz-Collado,, Manuel López-Lara (Fundación Hospital del Espíritu Santo, Santa Coloma de Gramenet, Barcelona, Spain); María Labalde Martínez, Eduardo Ferrero Herrero, Francisco Javier García Borda, Óscar García Villar (12 de Octubre University Hospital, Madrid, Spain); Jorge Escartín, Juan L. Blas, Rocío Ferrer, Jorge García Egea (Hospital Royo Villanova, Zaragoza, Spain); Antonio Rodríguez-Infante, Germán Mínguez-Ruiz, Guillermo Carreño-Villarreal, Gerardo Pire-Abaitua (Hospital Universitario San Agustín, Avilés, Spain); Jana Dziakova, Carlos Sáez-Cazallas Rodríguez, María J. Pizarro Aranda, José M. Muguerza Huguet (Hospital Universitario Clínico San Carlos, Madrid, Spain); Nerea Borda-Arrizabalaga, José M. Enriquez-Navascués, Garazi Elorza Echaniz, Yolanda Saralegui Ansorena (Donostia University Hospital, Donostia, Spain); Mercedes Estaire-Gómez, Carlos Martínez-Pinedo, Alejandro Barbero-Valenzuela, Pablo Ruíz-García (Hospital General Universitario de Ciudad Real, Ciudad Real, Spain); Miquel Kraft, María J. Gómez-Jurado, Gianluca Pellino, Eloy Espín-Basany (Vall d’Hebron University Hospital, Universitat Autonoma de Barcelona, Barcelona, Spain); Eddy Cotte, Nathalie Panel, Claire-Angéline Goutard (Hospices Civils de Lyon, Lyon Sud University Hospital, Pierre Bénite, France); Nicola deÁngelis, Lelde Lauka (Henri Mondor Hospital, AP-HP, Créteil, France); Shafaque Shaikh, Laura Osborne, George Ramsay (Aberdeen Royal Infirmary, NHS Grampian, Aberdeen, United Kingdom); Vladimir-Ion Nichita, Santosh Bhandari, Panchali Sarmah (Cambridgeshire in Peterborough City Hospital, Peterborough, United Kingdom); Rob M. Bethune, Heather C.M. Pringle, Lisa Massey, George E. Fowler (Royal Devon and Exeter Hospital, Exeter, United Kingdom); Hytham K.S. Hamid, Belinda D. de Simone (East Kent Hospitals University NHS Foundation Trust, Ashford, United Kingdom); James Kynaston, Nicholas Bradley, Roxane M. Stienstra (Forth Valley Royal Hospital, Larbert, Scotland); Shashank Gurjar, Tanmoy Mukherjee, Ashfaq Chandio, Safia Ahmed (Bedfordshire Hospitals NHS Foundation Trust, Luton, United Kingdom); Baljit Singh, Francois Runau, Sanjay Chaudhri, Oliver Siaw (Leicester General Hospital, Leicester, United Kingdom); Janahan Sarveswaran, Victor Miu, Daniel Ashmore, Haitham Darwich (Pinderfields Hospital, Wakefield, United Kingdom); Deepak Singh-Ranger, Nirbhaibir Singh (The Royal Wolverhampton NHS Trust, Wolverhampton, West Midlands, United Kingdom); Mohamed Shaban (Newcastle upon Tyne NHS Foundation Trust, Newcastle upon Tyne, United Kingdom); Fahed Gareb (Queen Elizabeth The Queen Mother Hospital, Margate, United Kingdom); Thalia Petropolou, Adreas Polydorou (Euroclinic Athens, Athens, Greece); Mit Dattani, Asma Afzal (University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom); Akshay Bavikatte, Boby Sebastian, Nicholas Ward, Amitabh Mishra (West Suffolk Hospital, Suffolk, United Kingdom); Dimitrios Manatakis, Christos Agalianos,Nikolaos Tasis, Maria-Ioanna Antonopoulou (Athens Naval and Veterans Hospital, Athens, Greece); Ioannis Karavokyros, Alexandros Charalabopoulos, Dimitrios Schizas, Efstratia Baili, Athanasios Syllaios, Lysandros Karydakis, Michail Vailas (Laikon General Hospital- National and Kapodistrian University of Athens, Greece); Dimitrios Balalis, Dimitrios Korkolis, Aris Plastiras, Aliki Rompou (Saint Savvas Anti-Cancer Hospital, Athens Greece); Sofia Xenaki, Evangelos Xynos, Emmanuel Chrysos, Maria Venianaki (University Hospital of Heraklion Crete, Greece); Grigorios Christodoulidis, Konstantinos Perivoliotis, George Tzovaras, Ioannis Baloyiannis (University Hospital of Larissa, Larissa Greece); Man-Fung Ho, Simon Siu-man Ng, Tony Wing-chung Mak, Kaori Futaba (Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong); Goran Šantak, Damir Šimleša, Jurica Ćosić, Goran Zukanović (General County Hospital Požega, Požega, Croatia); Michael E. Kelly, John O. Larkin, Paul H. McCormick, Brian J. Mehigan (The Trinity St. James’s Cancer Institute, Dublin+School of Medicine, Trinity College Dublin, Ireland); Tara M. Connelly, Peter Neary, Jessica Ryan, Peter McCullough (University Hospital Waterford, Waterford, Ireland); Maytham A. Al-Juaifari, Hayder Hammoodi, Ali Hashim Abbood (Al-Sadder Teaching Hospital, Najaf, Iraq); Marcello Calabrò, Andrea Muratore, Antonio La Terra, Francesca Farnesi (Edoardo Agnelli Hospital, Pinerolo, Italy); Carlo V. Feo, Nicolò Fabbri, Antonio Pesce, Marta Fazzin (Azienda Unità Sanitaria Locale di Ferrara, Università di Ferrara, Ferrara, Italy); Francesco Roscio, Federico Clerici (ASST Valle Olona Busto Arsizio Italy, Busto Arsizio VA, Italy); Andrea Lucchi, Laura Vittori, Laura Agostinelli, Maria Cristina Ripoli (AUSL Romagna Ceccarini Hospital, Riccione, Italy); Daniele Sambucci, Andrea Porta (Fatebenefratelli Hospital “Holy Family”, Erba, Italy); Giovanni Sinibaldi, Giacomo Crescentini, Antonella larcinese, Emanuele Picone (Fatebbenefratelli Hospital, Isola Tiberina, Rome, Italy); Roberto Persiani, Alberto Biondi, Roberto Pezzuto, Laura Lorenzon, Gianluca Rizzo, Claudio Coco, Luca D’Agostino (“A. Gemelli” University Hospital, Catholic University of Rome, Rome, Italy); Antonino Spinelli, Matteo M. Sacchi, Michele Carvello, Caterina Foppa (Humanitas University, Milan, Italy); Antonino Spinelli, Matteo M. Sacchi, Michele Carvello, Caterina Foppa, Annalisa Maroli (IRCCS Humanitas Research Hospital, Milan, Italy); Gian M. Palini, Gianluca Garulli, Nicola Zanini (Infermi Hospital of Rimini, AUSL Della Romagna, Rimini, Italy); Paolo Delrio, Daniela Rega, Fabio Carbone, Alessia Aversano (Fondazione Giovanni Pascale - IRCCS, Naples, Italy); Giovanni Pirozzolo, Alfonso Recordare, Lucrezia D’Alimonte, Chiara Vignotto (Dell’Angelo Hospital, Venice, Italy); Carlo Corbellini, Gianluca M. Sampietro, Leonardo Lorusso, Carlo A. Manzo (ASST Rhodense, Rho Memorial Hospital, Milano, Italy); Federico Ghignone, Giampaolo Ugolini, Isacco Montroni, Franceso Pasini (Ospedale Santa Maria delle Croci, Ravenna, Italy); Francesco Pasini (Ospedale per gli Infermi, Faenza, Italy); Michele Ballabio, Pietro Bisagni, Francesca T. Armao, Marco Longhi (Maggiore Hospital in Lodi, Lodi, Italy); Omar Ghazouani, Raffaele Galleano (Santa Corona Hospital, Pietra Ligure, Italy); Nicolò Tamini, Massimo Oldani, Luca Nespoli (San Gerardo Hospital, Monza, Italy); Arcangelo Picciariello, Donato F. Altomare, Giovanni Tomasicchio, Giuliano Lantone (University of Bari Aldo Moro, Bari, Italy); Fausto Catena, Mario Giuffrida, Alfredo Annicchiarico, Gennaro Perrone (Parma University Hospital, Parma, Italy); Ugo Grossi, Giulio A. Santoro, Giacomo Zanus ,Alessandro Iacomino, Simone Novello, Nicola Passuello, Martino Zucchella (Regional Hospital Treviso, Treviso, Italy); Lucia Puca, Maurizio deGiuli, Rossella Reddavid (San Luigi University Hospital, Orbassano, Torino, Italy); Stefano Scabini, Alessandra Aprile, Domenico Soriero, Emanuela Fioravanti (AOU San Martino Hospital, Genoa, Italy); Matteo Rottoli, Angela Romano, Marta Tanzanu, Angela Belvedere (IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy); Nicolò M. Mariani, Andrea P. Ceretti, Enrico Opocher (ASST Santi Paolo e Carlo, Milan, Italy); Gaetano Gallo, Giuseppe Sammarco (University of Catanzaro, Catanzaro, Italy); Gilda de Paola (University of Milano, Milano, Italy); Salvatore Pucciarelli, Francesco Marchegiani, Gaya Spolverato, Gianluca Buzzi (Azienda Ospedale-Università di Padova, Padova, Italy); Salomone Di Saverio, Paola Meroni, Cristiano Parise, Elisa I. Bottazzoli (University of Insubria, University Hospital of Varese, ASST Sette Laghi, Regione Lombardia, Varese, Italy); Pierfrancesco Lapolla, Gioia Brachini, Bruno Cirillo, Andrea Mingoli (“P. Valdoni”, Policlinico Umberto I University Hospital, Sapienza University of Rome, Rome, Italy); Giuseppe Sica, Leandro Siragusa, Vittoria Bellato, Daniele Cerbo (University of Rome “Tor Vergata”, Rome, Italy); Carlo A. de Pasqual, Giovanni de Manzoni, Maria A. di Cosmo (University of Verona, Verona, Italy); Bourhan M.H. Alrayes, Mahmoud W. M. Qandeel (Islamic Hospital Amman, Amman, Jordan); Mohammad Bani Hani (King Abdullah University Hospital, Ar-Ramtha, Jordan); Alexander Rabadi, Mohammad S. el Muhtaseb, Basel Abdeen, Fahed Karmi (The University of Jordan, Amman, Jordan); Justas Žilinskas, Tadas Latkauskas, Algimantas Tamelis, Ingrida Pikūnienė, Vygintas Šlenfuktas (Hospital of Lithuanian University of Health Sciences Kaunas Clinics, Kaunas, Lithuania); Tomas Poskus, Marius Kryzauskas, Matas Jakubauskas, Saulius Mikalauskas, Lina Jakubauskiene (Vilnius University, Vilnius, Lithuania); Soha Y. Hassan, Amani Altrabulsi (Benghazi Medical Center, Benghazi, Libya); Eman Abdulwahed, Reem Ghmagh, Abdulqudus Deeknah, Entisar Alshareea (Tripoli Central Hospital, Tripoli, Libya); Muhammed Elhadi, Saleh Abujamra, Ahmed A. Msherghi, Osama W.E. Tababa (Tripoli University Hospital, Tripoli, Libya); Mohammed A. Majbar, Amine Souadka, Amine Benkabbou, Raouf Mohsine, Sabrillah Echiguer (National Institute of Oncology, University Mohammed V in Rabat, Rabat, Morocco); Paulina Moctezuma-Velázquez, Noel Salgado-Nesme, Omar Vergara-Fernández, Juan C. Sainz-Hernández, Francisco E. Alvarez-Bautista (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico); Andee D. Zakaria, Zaidi Zakaria, Michael P.K. Wong, Razif Ismail (Universiti Sains Malaysia, Kubang Kerian, Kelantan, Malaysia); Aini F. Ibrahim, Nik A.N. Abdullah, Rokayah Julaihi (Universiti Malaysia Sarawak, Kota Samarahan, Sarawak); Sameer Bhat, Greg O’Grady, Ian Bissett (University of Auckland, Auckland, New Zealand); Bas Lamme, Gijsbert D. Musters, Anne M. Dinaux (Albert Schweitzer Hospital, Dordrecht, The Netherlands); Brechtje A. Grotenhuis, Ernst J. Steller Arend G.J. Aalbers, Marjolein M. Leeuwenburgh (Netherlands Cancer Institute-Antoni van Leeuwenhoek, Amsterdam, The Netherlands); Harm J.T. Rutten, Jacobus W.A. Burger, Johanne G. Bloemen, Stijn H.J. Ketelaers (Catharina Hospital, Eindhoven, The Netherlands); Usama Waqar, Tabish Chawla, Hareem Rauf, Pallavi Rani (Aga Khan University, Karachi City, Pakistan); Aaldert K. Talsma, Lieke Scheurink, Jasper B. van Praagh (Deventer Hospital, Deventer, The Netherlands); Josefin Segelman, Jonas Nygren, Kajsa Anderin, Marit Tiefenthal (Ersta Hospital, Stockholm, Sweden); Beatriz de Andrés, Juan P. Beltrán de Heredia, Andrea Vázquez, Tania Gómez (University Clinical Hospital of Valladolid, Valladolid, Spain); Parisa Golshani, Rawaz Kader, Abudi Mohamed (Gävle Hospital, Gävle, Sweden); Marinke Westerterp, Andreas Marinelli, Quirine Niemer (Medical Center Haaglanden, Westeinde, Den Haag, Netherlands); Pascal G. Doornebosch, Joël Shapiro, Maarten Vermaas, Eelco J.R. de Graaf (Jsselland Hospital, Capelle Aan Den IJssel, The Netherlands); Hendrik L. van Westreenen, Marije Zwakman, Annette D. van Dalsen (Isala Hospital, Zwolle, The Netherlands); Wouter J. Vles, Joost Nonner, Boudewijn R. Toorenvliet, Paul T.J. Janssen (Ikazia Hospital, Rotterdam, the Netherlands); Emiel G.G. Verdaasdonk, Femke J. Amelung (Jeroen Bosch Hospital, ‘s-Hertogenbosch, The Netherlands); Koen C.M.J. Peeters Renu R. Bahadoer, Fabian A. Holman (Leiden University Medical Center, Leiden, Netherlands); Jeroen Heemskerk, Noortje Vosbeek, Jeroen W.A. Leijtens, Sophie B.M. Taverne (Laurentius Hospital, Roermond, the Netherlands); Bob H.M. Heijnen, Youssef El-Massoudi, Irene de Groot-van Veen (LangeLand Hospital, Zoetermeer, The Netherlands); Christiaan Hoff, Daniela Jou-Valencia (Medical Centre Leeuwarden, Leeuwarden, the Netherlands); Esther C.J. Consten Thijs A. Burghgraef, Ritch Geitenbeek, Lorenzo G.W.L. Hulshof (Meander Medical Centre, Amersfoort, Netherlands); Gerrit D. Slooter, Muriël Reudink (Máxima Medical Centre, Veldhoven, Netherlands); Nicole D. Bouvy, Aurelia C. L. Wildeboer, Sonja Verstappen, Alexander J. Pennings (Maastricht University Medical Centre, Maastricht, The Netherlands); Berber van den Hengel, Allard G. Wijma, Jael de Haan (Martini Hospital, Groningen, The Netherlands); Lindsey C.F. de Nes, Vera Heesink (Maasziekenhuis Pantein, Boxmeer, The Netherlands); Tom Karsten, Charlotte M. Heidsma, Willem J. Koemans (Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands); Jan-Willem T. Dekker, Charlène J. van der Zijden, Daphne Roos (Reinier de Graaf Gasthuis, Delft, The Netherlands); Ahmet Demirkiran, Sjirk van der Burg (Red Cross Hospital, Beverwijk, The Netherlands); Steven J. Oosterling, Tijs J. Hoogteijling (Spaarne Gasthuis, Haarlem, The Netherlands); Bastiaan Wiering, Diederik P.J. Smeeing (Slingeland Ziekenhuis, Doetinchem, Netherlands); Klaas Havenga, Hamid Lutfi, Esther C.J. Consten (University Medical Centre Groningen, Groningen, The Netherlands); Konstantinos Tsimogiannis, Filip Sköldberg, Joakim Folkesson (Uppsala University, Uppsala, Sweden); Frank den Boer, Ted G. van Schaik , Pieter van Gerven (Zaans Medical Center, Zaandam, the Netherlands); Colin Sietses, Jeroen C. Hol (Gelderse Vallei Hospital Ede, Ede, The Netherlands); Evert-Jan G. Boerma, Davy M.J. Creemers (Zuyderland Medical Center, Sittard/Heerlen, The Netherlands); Johannes K. Schultz, Tone Frivold, Rolf Riis (Akershus University Hospital, Lørenskog, Norway); Hilde Gregussen, Sondre Busund (Hospital innland Hamar, Hamar, Norway); Ole H. Sjo, Maria Gaard, Nina Krohn, Amanda L. Ersryd (Ullevål Oslo University Hospital, Oslo, Norway); Edmund Leung (Hereford County Hospital, Hereford, United Kingdom); Usama Waqar, Tabish Chawla, Hareem Rauf, Pallavi Rani (Aga Khan University, Karachi City, Pakistan); Hytham Sultan, Baraa Nabil Hajjaj, Ahmed Jehad Alhisi, Ahmed A.E. Khader (Al-Shifa Hospital, Gaza City, Palestine); Ana Filipa Dias Mendes, Miguel Semião, Luis Queiroz Faria, Constança Azevedo (Centro Hospitalar Universitário Cova da Beira, Covilha, Portugal); Helena M. da Costa Devesa, Sónia Fortuna Martins, Aldo M. Rodrigues Jarimba, Sónia M. Ribeiro Marques (Hospital Distrital de Santarém, Santarém, Portugal); Rita Marques Ferreira, António Oliveira, Cátia Ferreira, Ricardo Pereira (Centro Hospitalar de Trás-os-Montes e Alto Douro EPE, Vila Real, Portugal); Valeriu M. Surlin, Giorgiana M. Graure, Stefan Patrascu Sandu D. Ramboiu (Clinical County Emergency Hospital of Craiova, University of Medicine and Pharmacy of Craiova, Romania); Ionut Negoi, Cezar Ciubotaru, Bogdan Stoica, Ioan Tanase (Carol Davila University of Medicine and Pharmacy Bucharest, Bucharest, Romania); Bogdan Stoica, Cezar Ciubotaru, Valentina M. Negoita (Clinical Emergency Hospital Bucharest, Bucharest, Romania); Sabrina Florea, Florin Macau, Mihai Vasile, Victor Stefanescu (Central Military Emergency Hospital Dr. Carol Davila, Bucharest, Romania); Gabriel-Mihail Dimofte, Sorinel Luncă, Cristian-Ene Roată, Ana-Maria Mușină (Regional Oncology Institute, Iasi, Romania); Tatiana Garmanova, Mikhail N. Agapov, Daniil G. Markaryan, Galliamov Eduard (Lomonosov Moscow State University, Moscow, Russia); Alexey Yanishev, Alexander Abelevich, Andrey Bazaev (Privolzhsky Research Medical University, Nizhny Novgorod, Russia); Sergey V. Rodimov, Victor B. Filimonov, Andrey A. Melnikov, Igor A. Suchkov (Ryazan State Medical University, Ryazan, Russia); EvgeniyS. Drozdov, Dmitriy N. Kostromitskiy (Siberian State Medical University, Tomsk, Russia); Olle Sjöström (Östersund Hospital, Östersund, Sweden); Peter Matthiessen, Bayar Baban, Soran Gadan, Kaveh Dehlaghi Jadid (chool of Medical Sciences, Örebro University, Örebro, Sweden); Maria Staffan (Region Dalarna Hospital, Dalarna University, Falun, Sweden); Jennifer M. Park, Daniel Rydbeck (Scandinavian Surgical Outcomes Research Group, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, Region Västra Götaland, Sahlgrenska University Hospital/Östra, Gothenburg, Sweden); Marie-Louise Lydrup, Pamela Buchwald, Henrik Jutesten, Lotten Darlin, Ebba Lindqvist (Skåne Univeristy Hospital, Malmö, Sweden); Karl Nilsson, Per-Anders Larsson (Skaraborgs Hospital, Skövde, Sweden); 186 Staffan Jangmalm (Växjö Hospital, Växjö, Sweden); Jurij A. Košir, Aleš Tomažič, Jan Grosek, Tajda Košir Božič (Ljubljana University Medical Center, Ljubljana, Slovenia); Aya Zazo, Rama Zazo, Hala Fares, Kusay Ayoub (University of Aleppo, Aleppo, Syria); Ammar Niazi, Ali Mansour, Ayman Abbas, Mohammad Tantoura (The Arabic Medicine Hospital, Aleppo, Syria); Alaa Hamdan, Naya Hassan, Bassam Hasan, Ahmad Saad (Tishreen University, Latakia, Syria); Amine Sebai, Anis Haddad, Houcine Maghrebi, Montasser Kacem (La Rabta Hospital, Tunis, Tunisia); Ömer Yalkın, Mehmet Veysi Samsa, İbrahim Atak (Ali Osman Sönmez Oncology Hospital, Bursa, Turkiye); Bengi Balci, Elifcan Haberal, Lütfi Dogan (Ankara Oncology Training and Research Hospital, Ankara, Turkiye); Ibrahim E. Gecim, Cihangir Akyol, Mehmet A. Koc (Ankara University Medical School, Ankara, Turkiye); Emre Sivrikoz, Deniz Piyadeoğlu (Bahçeşehir University, Istanbul, Turkiye); John O. Larkin, Dara O. avanagh (St. James’s, Hospital, Dublin, Ireland); Selman Sökmen, Tayfun Bişgin, Erşan Günenç, Melek Güzel (Dokuz Eylul University, Balcova, Izmir, Turkiye); Sezai Leventoğlu, Osman Yüksel, Ramazan Kozan, Hüseyin Göbüt (Gazi University Medical School, Ankara, Turkiye); Fevzi Cengiz, Kemal Erdinc, Nihan Coşgun Acar, Erdinc Kamer (Izmir Katip Celebi University, İzmir, Turkiye); İlker Özgür, Oguzhan Aydın, Metin Keskin, Mehmet Türker Bulut, Cemil B. Kulle (Istanbul University, Istanbul Faculty of Medicine, Istanbul, Turkiye); Yasin Kara, Osman Sıbıç (University of Health Sciences, Kanuni Sultan Suleyman Training and Research Hospital, Istanbul, Turkiye); İbrahim H. Özata, Dursun Buğra, Emre Balık, Cemil B. Kulle (Koç University Hospital, Istanbul, Turkiye); Murat Çakır, Anas Alhardan (Meram Tip Faculty Hospital, Meram/Konya, Turkiye); Elif Colak, Ahmet B. CiftciEngin Aybar, Ahmet Can Sari (University of Samsun, Samsun Training and Research Hospital, Samsun, Turkiye); Semra Demirli Atici, Tayfun Kaya, Ayberk Dursun, Bulent Calik (University of Health Sciences, Tepecik Training and Research Hospital, Izmir, Turkiye); Ömer Faruk Özkan, Hanife Şeyda Ülgür, Özgül Düzgün (University of Health Sciences Turkiye, Ümraniye Training and Research Hospital, Istanbul, Turkiye); John Monson, Sarah George, Kayla Woods (AdventHealth Orlando, Orlando, Florida, United States of America); Fatima Al-Eryani, Rudaina Albakry (Al-Kuwait Hospital, Sana’a, Yemen); Emile Coetzee (Life St. George’s Hospital, Port Elizabeth, Eastern Cape, South Africa); Adam Boutall, Ayesiga Herman, Claire Warden, Naser Mugla (Groote Schuur Hospital and University of Cape Town, Cape Town, South Africa); Tim Forgan, Imraan Mia, Anton Lambrechts (Tygerberg Academic Hospital, Parow, Cape Town, South Africa).
Funding
The TENTACLE-Rectum study was funded by Medtronic. Medtronic had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
Nynke Greijdanus (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—original draft, Writing—review & editing), Kiedo Wienholts (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—original draft, Writing—review & editing), Sander Ubels (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Kevin Talboom (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Gerjon Hannink (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Albert Wolthuis (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), F. Borja de Lacy (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Jérémie Lefevre (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Michael Solomon (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Matteo Frasson (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Nicolas Rotholtz (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Quentin Denost (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), R. Perez (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Tsuyoshi Konishi (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Yves Panis (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Martin Rutegård (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Roel Hompes (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Camiel Rosman (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing—review & editing), Frans van Workum (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—review & editing), Pieter Tanis (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), and Johannes De Wilt (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing).
Disclosure
The TENTACLE-Rectum study was funded by Medtronic External Research Program. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Study data are available on reasonable request
References
- 1. Chiarello MM, Fransvea P, Cariati M, Adams NJ, Bianchi V, Brisinda G. Anastomotic leakage in colorectal cancer surgery. Surg Oncol 2022;40:101708. [DOI] [PubMed] [Google Scholar]
- 2. Bakker IS, Snijders HS, Wouters MW, Havenga K, Tollenaar RA, Wiggers Tet al. High complication rate after low anterior resection for mid and high rectal cancer; results of a population-based study. Eur J Surg Oncol 2014;40:692–698 [DOI] [PubMed] [Google Scholar]
- 3. Bostrom P, Haapamaki MM, Rutegard J, Matthiessen P, Rutegard M. Population-based cohort study of the impact on postoperative mortality of anastomotic leakage after anterior resection for rectal cancer. BJS Open 2019;3:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Snijders HS, Bakker IS, Dekker JW, Vermeer TA, Consten EC, Hoff Cet al. High 1-year complication rate after anterior resection for rectal cancer. J Gastrointest Surg 2014;18:831–838 [DOI] [PubMed] [Google Scholar]
- 5. Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ; Dutch Snapshot Research Group . Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross-sectional study. Ann Surg 2017;266:870–877 [DOI] [PubMed] [Google Scholar]
- 6. Hammond J, Lim S, Wan Y, Gao X, Patkar A. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg 2014;18:1176–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ashraf SQ, Burns EM, Jani A, Altman S, Young JD, Cunningham Cet al. The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: are we adequately remunerating them? Colorectal Dis 2013;15:e190–e198 [DOI] [PubMed] [Google Scholar]
- 8. Arron MNN, Greijdanus NG, Bastiaans S, Vissers PAJ, Verhoeven RHA, Ten Broek RPGet al. Long-term oncological outcomes after colorectal anastomotic leakage; a retrospective Dutch population based study. Ann Surg 2022;276:882–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindgren R, Hallbook O, Rutegard J, Sjodahl R, Matthiessen P. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum 2011;54:41–47 [DOI] [PubMed] [Google Scholar]
- 10. Jutesten H, Buchwald PL, Angenete E, Rutegård M, Lydrup ML. High risk of low anterior resection syndrome in long-term follow-up after anastomotic leakage in anterior resection for rectal cancer. Dis Colon Rectum 2022;65:1264–1273 [DOI] [PubMed] [Google Scholar]
- 11. Daams F, Slieker JC, Tedja A, Karsten TM, Lange JF. Treatment of colorectal anastomotic leakage: results of a questionnaire amongst members of the Dutch Society of Gastrointestinal Surgery. Dig Surg 2012;29:516–521 [DOI] [PubMed] [Google Scholar]
- 12. Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis 2017;32:549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas MS, Margolin DA. Management of colorectal anastomotic leak. Clin Colon Rectal Surg 2016;29:138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blumetti J, Abcarian H. Management of low colorectal anastomotic leak: preserving the anastomosis. World J Gastrointest Surg 2015;7:378–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg 2007;245:254–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borstlap WAA, Musters GD, Stassen LPS, van Westreenen HL, Hess D, van Dieren Set al. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc 2018;32:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Workum F, Talboom K, Hannink G, Wolthuis A, de Lacy BF, Lefevre JHet al. Treatment of anastomotic leakage after rectal cancer resection: the TENTACLE-Rectum study. Colorectal Dis 2021;23:982–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sparreboom CL, Wu ZQ, Ji JF, Lange JF. Integrated approach to colorectal anastomotic leakage: communication, infection and healing disturbances. World J Gastroenterol 2016;22:7226–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Musters GD, Borstlap WA, Bemelman WA, Buskens CJ, Tanis PJ. Intersphincteric completion proctectomy with omentoplasty for chronic presacral sinus after low anterior resection for rectal cancer. Colorectal Dis 2016;18:147–154 [DOI] [PubMed] [Google Scholar]
- 20. Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ. Treatment of chronic presacral sinus after low anterior resection. Colorectal Dis 2013;15:727–732 [DOI] [PubMed] [Google Scholar]
- 21. Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 2008;22:1818–1825 [DOI] [PubMed] [Google Scholar]
- 22. Talboom K, Greijdanus NG, Ponsioen CY, Tanis PJ, Bemelman WA, Hompes R. Endoscopic vacuum-assisted surgical closure (EVASC) of anastomotic defects after low anterior resection for rectal cancer; lessons learned. Surg Endosc 2022;36:8280–8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shalaby M, Emile S, Elfeki H, Sakr A, Wexner SD, Sileri P. Systematic review of endoluminal vacuum-assisted therapy as salvage treatment for rectal anastomotic leakage. BJS Open 2019;3:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuehn F, Janisch F, Schwandner F, Alsfasser G, Schiffmann L, Gock Met al. Endoscopic vacuum therapy in colorectal surgery. J Gastrointest Surg 2016;20:328–334 [DOI] [PubMed] [Google Scholar]
- 25. Kühn F, Janisch F, Schwandner F, Gock M, Wedermann N, Witte Met al. Comparison between endoscopic vacuum therapy and conventional treatment for leakage after rectal resection. World J Surg 2020;44:1277–1282 [DOI] [PubMed] [Google Scholar]
- 26. Daams F, Luyer M, Lange JF. Colorectal anastomotic leakage: aspects of prevention, detection and treatment. World J Gastroenterol 2013;19:2293–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Talboom K, Greijdanus NG, van Workum F, Ubels S, Rosman C, Hompes Ret al. International expert opinion on optimal treatment of anastomotic leakage after rectal cancer resection: a case-vignette study. Int J Colorectal Dis 2022;37:2049–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JPet al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499 [DOI] [PubMed] [Google Scholar]
- 29. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich Aet al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339–351 [DOI] [PubMed] [Google Scholar]
- 30. D’Souza N, de Neree Tot Babberich MPM, d’Hoore A, Tiret E, Xynos E, Beets-Tan RGHet al. Definition of the rectum: an international, expert-based Delphi consensus. Ann Surg 2019;270:955–959 [DOI] [PubMed] [Google Scholar]
- 31. Buuren SV, Taylor, Francis. Flexible Imputation of Missing Data (2nd edn). Boca Raton: CRC Press, 2018 [Google Scholar]
- 32. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MGet al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greijdanus NG, Wienholts K, Ubels S, Talboom K, Hannink G, Wolthuis Aet al. Stoma-free survival after rectal cancer resection with anastomotic leakage: development and validation of a prediction model in a large international cohort. Ann Surg 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010;172:1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012;21:69–80 [DOI] [PubMed] [Google Scholar]
- 36. Bemelman WA, Arezzo A, Banasiewicz T, Brady R, Espin-Basany E, Faiz Oet al. Use of sponge-assisted endoluminal vacuum therapy for the treatment of colorectal anastomotic leaks: expert panel consensus. BJS Open 2022;6:zrac123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuhn F, Schardey J, Wirth U, Schiergens T, Crispin A, Beger Net al. Endoscopic vacuum therapy for the treatment of colorectal leaks—a systematic review and meta-analysis. Int J Colorectal Dis 2022;37:283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Lacy FB, Talboom K, Roodbeen SX, Blok R, Curell A, Tanis PJet al. Endoscopic vacuum therapy and early surgical closure after pelvic anastomotic leak: meta-analysis of bowel continuity rates. Br J Surg 2022;109:822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdalla S, Cotte E, Epin A, Karoui M, Lefevre JH, Berger Aet al. Short-term and long-term outcome of endoluminal vacuum therapy for colorectal or coloanal anastomotic leakage: results of a nationwide multicenter cohort study from the French GRECCAR Group. Dis Colon Rectum 2020;63:371–380 [DOI] [PubMed] [Google Scholar]
- 40. Eriksen JD, Emmertsen KJ, Madsen AH, Iversen LH. Anastomotic leakage following restorative rectal cancer resection: treatment and impact on stoma presence 1 year after surgery-a population-based study. Int J Colorectal Dis 2022;37:1161–1172 [DOI] [PubMed] [Google Scholar]
- 41. Slooter MD, Talboom K, Sharabiany S, van Helsdingen CPM, van Dieren S, Ponsioen CYet al. IMARI: multi-interventional program for prevention and early management of anastomotic leakage after low anterior resection in rectal cancer patients: rationale and study protocol. BMC Surg 2020;20:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data are available on reasonable request