Abstract
α-Amylase from the antarctic psychrophile Alteromonas haloplanktis is synthesized at 0 ± 2°C by the wild strain. This heat-labile α-amylase folds correctly when overexpressed in Escherichia coli, providing the culture temperature is sufficiently low to avoid irreversible denaturation. In the described expression system, a compromise between enzyme stability and E. coli growth rate is reached at 18°C.
Psychrophilic enzymes possess specific properties, such as high activity at low temperatures and weak thermal stability, which promise to allow the use of these enzymes as industrial biocatalysts, as biotechnological tools, or for fundamental research (6, 8, 11). For instance, substantial energy savings can be obtained if heating is not required during large-scale processes which take advantage of the efficient catalytic capacity of cold-adapted enzymes in the range 0 to 20°C. The pronounced heat lability of psychrophilic enzymes also allows their selective inactivation in a complex mixture, as illustrated by an antarctic bacterial alkaline phosphatase which is available for molecular biology research (7). Finally, psychrophilic enzymes represent the lower natural limit of protein stability (3) and are useful tools for studies in the field of protein folding.
Large-scale fermentation of psychrophilic microorganisms suffers from two main drawbacks, however: the low production levels of wild strains and the prohibitive cost of growing wild strains at low temperatures. A possible alternative is to overexpress the gene coding for a psychrophilic protein in a mesophilic host for which efficient expression systems have been designed. In this context, two crucial questions remain to be solved: (i) what is the folding state of an enzyme normally synthesized at 0°C when it is expressed by the mesophilic genetic machinery at higher temperatures, and (ii) is there a temperature at which a compromise can be reached between the stability of the psychrophilic enzyme and the mesophilic growth rate? To address these questions, the heat-labile α-amylase from the antarctic psychrophile Alteromonas haloplanktis (2, 4) was expressed in Escherichia coli at various temperatures.
Construction of the expression vector and α-amylase production.
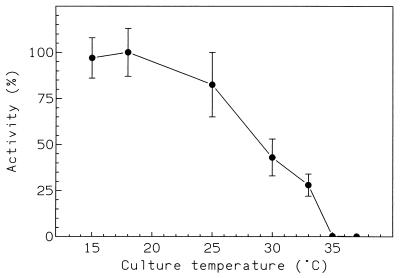
The α-amylase gene (2) was cloned downstream from the lacZ promoter in pUC12 by ligating the SmaI site of the polylinker to the HpaI site located 60 nucleotides upstream from the formylmethionine codon. This construction is devoid of the C-terminal peptide cleaved by the wild strain following α-amylase secretion. The recombinant enzyme was expressed in E. coli RR1 with the constitutive assistance of lacZ (without IPTG [isopropyl-β-d-thiogalactopyranoside] induction) in a medium containing 16 g of bactotryptone, 16 g of yeast extract, 5 g of NaCl, 2.5 g of K2HPO4, 0.1 μM CaCl2, and 100 mg of ampicillin per liter. The effect of the culture temperature on α-amylase production by E. coli is illustrated in Fig. 1. Within the range of temperatures used, maximal enzyme production was reached below 18°C, whereas higher temperatures induced a gradual decrease of α-amylase activity in cultures. Three independent cultures were pooled for the purification of the recombinant enzymes produced at 18 and 25°C.
FIG. 1.
Temperature dependence of α-amylase production by E. coli. Results are expressed as percent mean maximal activity recorded at 18°C.
α-Amylase purification.
The gram-negative A. haloplanktis was cultivated at 4°C, and α-amylase was purified from the culture supernatants by ion-exchange chromatography on DEAE-agarose followed by gel filtration on Sephadex G-100 and Ultrogel AcA54 as previously described (2, 4). The recombinant α-amylases were purified by the protocol developed for the wild-type enzyme except that concentration by ammonium sulfate precipitation at 70% saturation was required before the first chromatographic step. Recombinant enzyme production at 18 and 25°C ranged between 60 and 100 mg/liter of culture, which corresponds to a 10-fold improvement over production by the wild strain.
Characterization of the recombinant α-amylases.
N- and C-terminal amino acid sequences (determined on an Applied Biosystems Procise analyzer and by carboxypeptidase Y digestion, respectively) of α-amylase produced at 18 and 25°C indicated that the signal peptide is correctly cleaved in E. coli and that no additional posttranslational cleavage occurred. The isoelectric point (5.5) and the molecular mass (49,340 Da as determined from the sequence and 49,342 ± 8 Da as determined from electrospray mass spectroscopy measurements) were identical to the values recorded for the wild-type enzyme. Dynamic light scattering (DynaPro-801; DLS Instruments) also showed that the purified recombinant enzymes are homogeneous, without any evidence of aggregated forms.
Comparison of the wild-type and recombinant α-amylases.
Several properties of the wild-type enzyme produced at 4°C and the recombinant α-amylase expressed in E. coli at 18°C were compared (Table 1).
TABLE 1.
Kinetic parameters, dissociation constants, and free thiol groups for the wild-type and recombinant α-amylases
| α-Amylase | kcat (s−1) | Km (μM) | kcat/Km (s−1 · μM−1) |
Kd
|
Cysteinesa (mol−1) | Free thiol (mol−1) | |
|---|---|---|---|---|---|---|---|
| Cl− (mM) | Ca (M) | ||||||
| Wild-type (produced at 4°C) | 780 ± 25 | 174 ± 8 | 4.6 | 5.9 ± 0.2 | 2.10−8 | 8 | 0.03 |
| Recombinant (produced at 18°C) | 792 ± 34 | 168 ± 14 | 4.7 | 6.1 ± 0.2 | 2.10−8 | 8 | 0.05 |
| Recombinant (produced at 25°C) | 609 ± 29 | 186 ± 22 | 3.3 | 6.0 ± 0.3 | 2.10−8 | 8 | 0.05 |
From the amino acid sequence.
(i) Kinetic and ion binding parameters.
4-Nitrophenyl-α-d-maltoheptaoside-4,6-O-ethylidene (EPS) was used as the substrate in a coupled assay with α-glucosidase at 25°C. The absorption coefficient for 4-nitrophenol was 8,990 M−1 · cm−1 at 405 nm, and a stoichiometric factor of 1.25 was applied for kcat (turnover number) calculation. Dissociation constants were determined by activation kinetics following Cl− or Ca2+ titration of the apoenzyme obtained by dialysis against 25 mM HEPES-NaOH (pH 7.2) and 25 mM HEPES-NaOH–5 mM EGTA (pH 8.0), respectively. The saturation curves were computer fitted by a nonlinear regression analysis of the Hill equation in the form v = kcat [I]h/Kd + [I]h where [I] is the ion concentration and h is the Hill coefficient. The free calcium concentrations were set by calcium titration in the presence of 5 mM EGTA at pH 8.0. Kinetic parameters (kcat, Km and kcat/Km) for the hydrolysis of EPS as well as dissociation constants (Kd) for calcium and chloride ions were found to be identical in the wild-type and recombinant enzymes produced at 18°C (Table 1). Owing to the stringent structural requirements for functional active site and ion binding site conformation, it can be safely concluded that the recombinant enzyme is properly folded at 18°C.
(ii) Disulfide bond integrity.
Free thiol content was determined by DTNB (5,5′-dithiobis-2-nitrobenzoic acid) titration of the unfolded enzyme in 8 M urea in order to promote −SH group accessibility. The eight cysteine residues of A. haloplanktis α-amylase are engaged in disulfide linkages (4). Thus, the lack of free sulfhydryl groups, as detected by DTNB titration of both the native and the unfolded enzymes (Table 1), indicates that the four disulfide bonds are formed in the recombinant α-amylase samples.
(iii) Conformational stability.
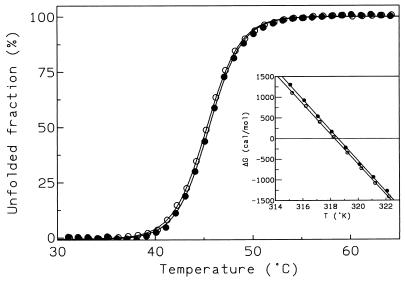
Fluorescence intensity of α-amylases (50 μg/ml) was recorded in 30 mM MOPS (morpholinepropanesulfonic acid)–50 mM NaCl–1 mM CaCl2 (pH 7.2) at a scanning rate of 1°C/min and at an excitation wavelength of 280 nm and an emission wavelength of 347 nm with a Perkin-Elmer LS 50 spectrofluorimeter. Raw data were corrected for thermal dependence of the fluorescence by using the slopes of the pre- and posttransition regions as described elsewhere (10). The conformational stability (ΔGN⇔U) was determined by reversible, thermally induced unfolding recorded by fluorescence. Both the wild-type and the recombinant α-amylases have melting point (Tm) values of 45 ± 0.2°C and display the same cooperative transition (Fig. 2). Consequently, plots of ΔG as a function of T (constructed by using the relation ΔG = −RTlnK, where K = fraction unfolded/fraction folded) are similar (Fig. 2, inset). These results indicate that the weak interactions stabilizing the folded state of the wild-type and recombinant α-amylases are quantitatively identical.
FIG. 2.
Heat-induced unfolding transitions of the wild-type α-amylase (•) and the recombinant enzyme produced at 18°C (○). The fraction of protein in the unfolded state (fU) was calculated as follows: fU = (yF − y)/(yF − yU), where yF and yU are the fluorescence intensities of the native and the fully unfolded states, respectively, and y is the fluorescence intensity at a given temperature. The inset shows a plot of ΔG as a function of the temperature around the melting point (Tm), where ΔG = 0.
Expression at 25 and 37°C.
When cultures of the recombinant E. coli are carried out at 25°C, all parameters determined by activation kinetics and independent of the enzyme concentration, such as Km and Kd, remain constant, as does the free sulfhydryl content (Table 1). This indicates that the native enzyme fraction is correctly folded. By contrast, the kcat of the recombinant α-amylase is reduced by about 20%, suggesting the occurrence of a corresponding inactive fraction. When expressed at 37°C, no α-amylase activity is recorded; the recombinant heat-labile enzyme could fail to fold at this high temperature, or its denaturation rate could exceed its synthesis rate. Furthermore, Western blotting with rabbit polyclonal antibodies to α-amylase detects only trace amounts of the recombinant gene product, suggesting that the denatured enzyme is quickly degraded by the E. coli cell.
Conclusions.
We have previously shown that cloning of a psychrophilic gene in E. coli and detection of the gene product can be achieved by careful control of the culture conditions: overnight incubation at 25°C of transformed cells followed by 1 to 2 days of incubation at 4°C produced halos of substrate hydrolysis on agar plates (5). The folding state of the recombinant psychrophilic enzymes (e.g., fully or partly active, native or marginal stability, etc.), however, was unknown. The results presented here demonstrate that the genuine properties of a psychrophilic enzyme are preserved when it is expressed in a mesophilic host, providing the culture temperature is sufficiently low to allow correct folding and to avoid irreversible denaturation. In our expression system, a compromise is reached between the stability of the psychrophilic enzyme and the growth rate of the mesophilic host by cultivating the recombinant E. coli at 18°C. It should be noted that commonly used E. coli strains have different growing capacities at that temperature. We found E. coli RR1, HB101, or XL1-Blue (Stratagene) suitable for these culture conditions (the generation times are about 3 h, and stationary phase is reached after approximately 30 h), whereas E. coli DH5α grows twice as slowly at 18°C.
The lack of α-amylase expression at 37°C is not an isolated case: under the same conditions, lipases and proteases (1, 5, 9) from antarctic psychrophiles were not expressed in an active form. This illustrates the general heat lability of psychrophilic enzymes, which is thought to arise from their flexible conformation, allowing high catalytic activity at temperatures close to 0°C (3).
Acknowledgments
This work was supported by the E.U. under the form of a network contract, ERBCHT 940521, a concerted action, BIO4-CT95-0017, and a Biotech program, BIO4-CT96-0051.
The Institut Français de Recherche et de Technologie Polaire is acknowledged for the support and facilities offered at the antarctic station Dumont d’Urville. We thank N. Gerardin-Otthiers and R. Marchand for expert technical assistance.
REFERENCES
- 1.Davail S, Feller G, Narinx E, Gerday C. Sequence of the subtilisin-encoding gene from an antarctic psychrotroph Bacillus TA41. Gene. 1992;119:143–144. doi: 10.1016/0378-1119(92)90080-9. [DOI] [PubMed] [Google Scholar]
- 2.Feller G, Lonhienne T, Deroanne C, Libioulle C, Van Beeumen J, Gerday C. Purification, characterization and nucleotide sequence of the thermolabile α-amylase from the antarctic psychrotroph Alteromonas haloplanctis A23. J Biol Chem. 1992;267:5217–5221. [PubMed] [Google Scholar]
- 3.Feller G, Narinx E, Arpigny J L, Aittaleb M, Baise E, Genicot S, Gerday C. Enzymes from psychrophilic organisms. FEMS Microbiol Rev. 1996;18:189–202. [Google Scholar]
- 4.Feller G, Payan F, Theys F, Qian M, Haser R, Gerday C. Stability and structural analysis of α-amylase from the antarctic psychrophile Alteromonas haloplanctis A23. Eur J Biochem. 1994;222:441–447. doi: 10.1111/j.1432-1033.1994.tb18883.x. [DOI] [PubMed] [Google Scholar]
- 5.Feller G, Thiry M, Arpigny J L, Gerday C. Cloning and expression in Escherichia coli of three lipase-encoding genes from the psychrotrophic antarctic strain Moraxella TA144. Gene. 1991;102:111–115. doi: 10.1016/0378-1119(91)90548-p. [DOI] [PubMed] [Google Scholar]
- 6.Gounot A M. Bacterial life at low temperature: physiological aspects and biotechnological implications. J Appl Bacteriol. 1991;71:386–397. doi: 10.1111/j.1365-2672.1991.tb03806.x. [DOI] [PubMed] [Google Scholar]
- 7.Kobori H, Sullivan C W, Shizuya H. Heat-labile alkaline phosphatase from antarctic bacteria: rapid 5′ end-labeling of nucleic acids. Proc Natl Acad Sci USA. 1984;81:6691–6695. doi: 10.1073/pnas.81.21.6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margesin R, Schinner F. Properties of cold-adapted microorganisms and their potential role in biotechnology. J Biotechnol. 1994;33:1–14. [Google Scholar]
- 9.Narinx E, Davail S, Feller G, Gerday C. Nucleotide and derived amino acid sequence of the subtilisin from the antarctic psychrotroph Bacillus TA39. Biochim Biophys Acta. 1992;1131:111–113. doi: 10.1016/0167-4781(92)90108-c. [DOI] [PubMed] [Google Scholar]
- 10.Pace C N, Shirley B A, Thomson J A. Measuring the conformational stability of a protein. In: Creighton T E, editor. Protein structure, a practical approach. Oxford, England: IRL Press; 1989. pp. 311–330. [Google Scholar]
- 11.Russel N J. Physiology and molecular biology of psychrophilic microorganisms. In: Herbert R A, Sharp R J, editors. Molecular biology and biotechnology of extremophiles. London, England: Blackie; 1992. pp. 203–224. [Google Scholar]